Abstract
Background & Aims:
Primary sclerosing cholangitis (PSC) is a chronic liver disease characterized by progressive inflammation that can result in hepatic decompensation. The definitive treatment for this disease is liver transplantation. This study investigates the effect of the sex of the donor and recipient as a prognostic risk factor for adverse outcomes after liver transplant in patients with PSC.
Methods:
The UNOS-STAR registry was used to select patients who received liver transplant grafts in patients with PSC from 1987 to 2019. The study cohort was stratified based on the sex of the liver transplant recipient and further subdivided based on the sex of the donor. The primary endpoints of this study were all-cause mortality and graft failure, which were evaluated using a sequential Cox regression analysis. Specific causes of mortality were also analyzed in this study.
Results:
This study ultimately included 2829 patients; 906 female recipients were transplanted from 441 male donors and 465 female donors. 1923 male recipients were transplanted from 1194 male donors and 729 female donors. Within the mismatch analyses, the male-to-male recipients also had a significantly reduced hazard ratio of graft failure compared to female-to-male transplants (aHR 0.51, 95% CI 0.33-0.79, p=0.003). No difference in graft failure was observed in the mismatched female recipient subgroup. The mismatched male recipient group also showed a decreased hazard ratio of mortality from graft rejection and respiratory causes. No differences in specific mortality causes were identified in the mismatched female recipient group.
Conclusions:
This study demonstrated a significant increase in the risk of graft failure and mortality secondary to graft failure in male recipients of female donor livers. No significant differences in mortality or graft failure were identified in female recipients of male livers.
Keywords: primary sclerosing cholangitis, liver transplant, sex mismatch, UNOS-STAR, post-liver transplantation outcomes, donor sex, recipient sex
Introduction
In patients with primary sclerosing cholangitis (PSC), there are inflammation-induced cytoarchitectural distortions of the hepatobiliary structures of the liver that result in interstitial collagen deposition and fibrosis (1,2). The culminating effects of these histological changes include cirrhosis and end-stage liver disease, which can propagate the syndromes of hepatic decompensation, including findings of portal hypertension, ascites, hepatic encephalopathy, jaundice, and variceal bleeding. Ultimately, decompensated cirrhosis and end-stage liver disease can result in complications such as sepsis, shock, and death (3,4). In PSC, while temporary interventions exist to alleviate these manifestations, the definitive treatment consists of liver transplantation (LT), which removes the diseased liver to replace it with a viable graft (5,6), thereby effectively restoring the quality of life of the patient (7,8).
Multiple risk factors are associated with LT, some of which pertain to donor and recipient characteristics. PSC and liver transplant studies thus far have primarily focused on recipient risk factors as etiologic causes of adverse post-LT prognosis (9,10), with only a minority of papers directing their focus to donor profiles as potential prognostic risk factors (11,12). However, there have been strides in the current understanding of the host-graft interface, which elucidates the pivotal role of donor risk profiles in mediating the recipient’s post-transplant prognosis (13,14). In particular, several non-PSC liver transplant studies have identified recipient-donor sex mismatch to confer a modified risk to post-LT prognosis, potentiating early demise of grafted livers and causing intrinsic graft complications (15,16). Given that PSC has been associated with differential prognostic and disease-development patterns based on sex (17,18), it is essential to investigate the differences in post-LT prognosis due to sex differences or sex mismatch between recipients and donors.
In this study, we use a global transplant database to delineate the sex mismatch-related complications and prognosis of PSC patients that undergo transplants.
Methods
Database
Patient data was taken from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) database, which stores patient data curated from accessible UNOS data and maintains a history of health-related information in the UNOS registry from 1987 through June 2019. The database itself is rigorously protected through extensive data use agreement (DUA) agreements certifying confidentiality and de-identification status and is supported by the Health Resources and Services Administration Contract 234-2005-370011C. This paper’s material is the collective creation of the authors only and does not reflect the Department of Health and Human Services’ views and policies. Furthermore, all references to trade names, organizations, or commercial products have no implication pointing to the U.S. government’s support or endorsement.
Study population and covariates
The team employed a step-by-step method to select patients from a raw, predetermined total to capture the unique PSC population while excluding large confounders or comorbidities. To begin, we chose 99,987 database patients, including individuals who had undergone liver transplantation between 1987 and 2019. Within this population group, a series of exclusions were applied; these exclusions included those who were lost to follow-up (n=3445), under 18 years of age (n=6872), and those with unlikely laboratory values (n=5). We also excluded subjects who underwent retransplantation (n = 4310) or non-whole liver transplantation (n=1012), those who received organs through living donors (n=3081) or non-heart-beating donors (n=4427), and those receiving dual-organ transplants (n=6462). Individuals who underwent non-heart-beating organ donation were excluded due to its known association with poorer post-transplant outcomes such as ischemic cholangiopathy, hepatic artery thrombosis, and overall graft loss (19). Finally, those without a formal diagnosis of PSC (n=62,107) were also excluded. Histopathological data related to the formal diagnosis of PSC in this study cohort was not available. However, UNOS registration protocol requires that verified institutional diagnoses are registered at the time of LT evaluation. Therefore, PSC diagnosis was established as individuals having a diagnosis of PSC as the primary indication for liver transplantation (20). The remaining cohort sum included 2829 patients. This patient exclusion process is shown in Figure 1. The recipient and donor groups were further divided into sub-groups by reported sex. There were 906 female recipients transplanted from 441 male donors and 465 female donors. There were 1923 male recipients, transplanted from 1194 male donors and 729 female donors.
Figure 1.
This figure shows the patient selection process of this study
Study Variables and Outcomes
The relationship between donor and recipient sex was investigated in this study; donor sex and sex mismatch were the exposures of interest. The primary outcomes measured were graft failure and all-cause mortality. All endpoint markers were taken from UNOS, specifically the variables for primary outcome as well as censoring events, meaning that if the primary outcome was graft failure, the subject was then censored from inclusion in the all-cause mortality group to prevent overlap.
Statistical methods
The baseline characteristics were noted through mean-based, nominal-based statistics, specifically through Fisher’s or the Chi-Square test. Non-nominal variables were analyzed for kurtosis, skewness, and parametricity and were further interpreted using non-parametric and parametric testing through the Whitney U Test. Models of iteration were created with comorbidities and run through multiple sequential iterations (all variations of the forward selection), for which multivariate Cox regression was carried out through predefined outcome variables representative of the dependent variable, regression endpoints. The variable iterations included four models: Model 1 includes VOI (variable of interest) and demographics; Model 2 includes Model 1 terms with the addition of comorbidities, and liver disease etiologies; Model 3 includes Model 2 terms with the addition of hepatic variables, MELD score, and liver laboratory markers; Model 4 includes Model 3 terms with the addition of donor demographics. Standard 95% confidence intervals (with two-tails and a p-value of 0.05) were statistically defined as showing significance. Kaplan-Meier hazard-event and survival analysis were run to calculate log-rank statistics and identify all-cause mortality, utilizing prespecified strata to analyze all comparative outcomes. Cumulative hazard curves were produced and demonstrated the relationship between sex and the primary endpoints in the PSC cohort. Further analysis was conducted to produce cumulative hazard curves subsetted by specific time period (2005-2009, 2010-2014, and 2015-2019). Forest plots identifying data from the Cox regression model were produced and evaluated associations for both all-cause mortality and graft failure.
Given that mortality and graft failure may occur as competing events, we utilized a competing risk regression analysis defined by Fine and Gray (21) to determine regression representations for each hazard. This was also formulated through the sequential iterative model outlined previously, extending in the range from the unadjusted model to model 5. Interaction plots were utilized to analyze relationships before the regression analyses were applied. In addition to exclusionary variables, variable terms were interpreted on a graph to determine missing patterns and run with the multiple imputations using the chained equations (MICE) method to improve the validity and statistical power (22).
All tests were performed using RStudio version 1.2.5042 with R code version 3.6.3.
Results
Baseline Characteristics
The recipient demographics for donor cohorts stratified by sex can be seen in Table 1a. A higher proportion of recipients in the male donor cohort were male than in the female donor cohort (73.00 vs. 61.10 %, p<0.001). There was a significantly different racial profile between the male and female donor cohort (White: 78.00 vs. 72.90; Black: 15.70 vs. 19.00; Hispanic: 4.34 vs. 4.02; Asian: 0.98 vs. 2.93; Other: 0.98 vs. 1.09 %, p<0.001). Additionally, the male donor cohort had a higher mean BMI than the female donor cohort (25.70 ± 4.73 vs. 25.30 ± 4.94 kg/m2, p=0.006). Regarding medical comorbidities, the only significant difference lay in the occurrence of hepatitis B infection, which was lower for the male cohort recipients than females (0.80 vs 2.01%, p=0.008). There were no differences in the presence of hepatic decompensations such as ascites or encephalopathy, use of immunosuppressants, and laboratory markers. The primary inotropic agent profiles between the male and female donor cohorts were significantly different (dobutamine: 1.96 vs. 1.84; dopamine: 17.50 vs. 16.80; epinephrine: 1.71 vs. 1.09; levophed: 14.30 vs. 18.80; neosynephrine: 14.60 vs. 17.30; none: 47.80 vs. 41.40; other: 2.14 vs 2.76 %, p=0.002). Finally, in terms of donor demographics, the male donor cohort generally was comprised of younger donors (39.40 ± 16.90 vs. 45.50 ± 17.00 years, p<0.001) with lower BMI (27.00 ± 5.73 vs. 28.50 ± 7.90 kg/m2, p<0.001), higher creatinine (1.70 ± 1.69 vs. 1.44 ± 1.62 mg/dL, p<0.001), and higher total bilirubin (0.94 ± 0.80 vs. 0.79 ± 0.66 mg/dL, p<0.001), compared to the female donor cohort. The racial profiles between the male and female donor cohorts were also statistically different (White: 67.90 vs. 71.40; Black: 17.10 vs. 17.20; Hispanic: 11.80 vs. 7.79; Asian: 1.65 vs. 2.18; Other: 1.53 vs. 1.42 %, p=0.010). The remaining data regarding baseline characteristics between the sexes can be identified in Table 1a.
Table 1a:
Baseline Characteristics of Liver Transplant Recipients with Primary Sclerosing Cholangitis and Donors Stratified by Donor Sex
| Characteristics | Comparison of Male vs Female | |||||
|---|---|---|---|---|---|---|
| Female Donor | Male Donor | |||||
| n = 1194 | 42.21 % | n = 1635 | 57.79 % | p-value | ||
| Recipient Demographics | ||||||
| Age (years) | 49.30 | ± 13.30 years | 48.60 | ± 13.50 years | 0.18 | |
| Male Sex (%) | 729 | 61.10 % | 1194 | 73.00 % | < 0.001 | *** |
| Race | < 0.001 | *** | ||||
| White (%) | 871 | 72.90 % | 1276 | 78.00 % | ||
| Black (%) | 227 | 19.00 % | 256 | 15.70 % | ||
| Hispanic (%) | 48 | 4.02 % | 71 | 4.34 % | ||
| Asian (%) | 35 | 2.93 % | 16 | 0.98 % | ||
| Other (%) | 13 | 1.09 % | 16 | 0.98 % | ||
| BMI (kg/m2) | 25.30 | ± 4.94 kg/m2 | 25.70 | ± 4.73 kg/m2 | 0.006 | ** |
| Comorbidities | ||||||
| Hepatitis B (%) | 24 | 2.01 % | 13 | 0.80 % | 0.008 | ** |
| Hepatitis C (%) | 53 | 4.44 % | 56 | 3.43 % | 0.20 | |
| Alcoholic Liver Disease (%) | 13 | 1.09 % | 11 | 0.67 % | 0.33 | |
| Diabetes (%) | 135 | 11.30 % | 185 | 11.30 % | 1.00 | |
| Assistance ‡ | 0.88 | |||||
| 0 (%) | 24 | 2.01 % | 38 | 2.32 % | ||
| 1 (%) | 304 | 25.50 % | 406 | 24.80 % | ||
| 2 (%) | 483 | 40.50 % | 677 | 41.40 % | ||
| 3 (%) | 383 | 32.10 % | 514 | 31.40 % | ||
| Hepatic Variables | ||||||
| Ascites | 0.46 | |||||
| Absent (%) | 391 | 32.70 % | 519 | 31.70 % | ||
| Slight (%) | 548 | 45.90 % | 788 | 48.20 % | ||
| Moderate (%) | 255 | 21.40 % | 328 | 20.10 % | ||
| Encephalopathy | 0.54 | |||||
| None (%) | 612 | 51.30 % | 839 | 51.30 % | ||
| 1 - 2 (%) | 507 | 42.50 % | 709 | 43.40 % | ||
| 3 - 4 (%) | 75 | 6.28 % | 87 | 5.32 % | ||
| MELD Scores | 22.50 | ± 9.08 | 22.60 | ± 9.21 | 0.83 | |
| Medications | ||||||
| Mycophenolate Mofetil (%) | 990 | 82.90 % | 1379 | 84.30 % | 0.33 | |
| Cyclosporine (%) | 29 | 2.43 % | 50 | 3.06 % | 0.37 | |
| Tacrolimus (%) | 1121 | 93.90 % | 1545 | 94.50 % | 0.55 | |
| Sirolimus (%) | 14 | 1.17 % | 12 | 0.73 % | 0.31 | |
| Steroids (%) | 1109 | 92.90 % | 1543 | 94.40 % | 0.12 | |
| Biomarkers | ||||||
| Albumin (mg/dL) | 2.95 | ± 0.73 mg/dL | 2.94 | ± 0.73 mg/dL | 0.28 | |
| Creatinine (mg/dL) | 1.23 | ± 0.99 mg/dL | 1.25 | ± 1.04 mg/dL | 0.31 | |
| INR | 1.85 | ± 1.27 | 1.88 | ± 1.41 | 0.64 | |
| Total Bilirubin (mg/dL) | 13.80 | ± 12.80 mg/dL | 13.50 | ± 12.00 mg/dL | 0.94 | |
| Life Support Variables | ||||||
| Primary Inotropic Agent | 0.002 | ** | ||||
| Dobutamine (%) | 22 | 1.84 % | 32 | 1.96 % | ||
| Dopamine (%) | 201 | 16.80 % | 286 | 17.50 % | ||
| Epinephrine (%) | 13 | 1.09 % | 28 | 1.71 % | ||
| Levophed (%) | 224 | 18.80 % | 234 | 14.30 % | ||
| Neosynephrine (%) | 207 | 17.30 % | 238 | 14.60 % | ||
| None (%) | 494 | 41.40 % | 782 | 47.80 % | ||
| Other (%) | 33 | 2.76 % | 35 | 2.14 % | ||
| Secondary Inotropic Agent | 0.30 | |||||
| Dobutamine (%) | 8 | 0.67 % | 7 | 0.43 % | ||
| Dopamine (%) | 13 | 1.09 % | 10 | 0.61 % | ||
| Epinephrine (%) | 6 | 0.50 % | 11 | 0.67 % | ||
| Levophed (%) | 41 | 3.43 % | 54 | 3.30 % | ||
| Neosynephrine (%) | 74 | 6.20 % | 77 | 4.71 % | ||
| None (%) | 1038 | 86.90 % | 1449 | 88.60 % | ||
| Other (%) | 14 | 1.17 % | 27 | 1.65 % | ||
| Ternary Inotropic Agent | 0.88 | † | ||||
| Dobutamine (%) | 2 | 0.17 % | 2 | 0.12 % | ||
| Dopamine (%) | 2 | 0.17 % | 2 | 0.12 % | ||
| Epinephrine (%) | 2 | 0.17 % | 1 | 0.06 % | ||
| Levophed (%) | 7 | 0.59 % | 7 | 0.43 % | ||
| Neosynephrine (%) | 5 | 0.42 % | 8 | 0.49 % | ||
| None (%) | 1173 | 98.20 % | 1607 | 98.30 % | ||
| Other (%) | 3 | 0.25 % | 8 | 0.49 % | ||
| ICU Admission | 0.64 | |||||
| ICU (%) | 97 | 8.12 % | 142 | 8.69 % | ||
| No ICU (%) | 1097 | 91.90 % | 1493 | 91.30 % | ||
| Ventilator Support (%) | 25 | 2.09 % | 36 | 2.20 % | 0.95 | |
| TIPS Procedure (%) | 78 | 6.53 % | 89 | 5.44 % | 0.26 | |
| Donor Demographics | ||||||
| Donor Age (years) | 45.50 | ± 17.00 years | 39.40 | ± 16.90 years | < 0.001 | *** |
| Donor Male Sex (%) | 0 | 0.00 % | 1635 | 100.00 % | < 0.001 † | *** |
| Donor Race | 0.010 | ** | ||||
| White (%) | 853 | 71.40 % | 1110 | 67.90 % | ||
| Black (%) | 205 | 17.20 % | 280 | 17.10 % | ||
| Hispanic (%) | 93 | 7.79 % | 193 | 11.80 % | ||
| Asian (%) | 26 | 2.18 % | 27 | 1.65 % | ||
| Other (%) | 17 | 1.42 % | 25 | 1.53 % | ||
| Donor BMI (kg/m2) | 28.50 | ± 7.90 kg/m2 | 27.00 | ± 5.73 kg/m2 | < 0.001 | *** |
| Donor Biomarkers | ||||||
| Donor Creatinine (mg/dL) | 1.44 | ± 1.62 mg/dL | 1.70 | ± 1.69 mg/dL | < 0.001 | *** |
| Donor Total Bilirubin (mg/dL) | 0.79 | ± 0.66 mg/dL | 0.94 | ± 0.80 mg/dL | < 0.001 | *** |
p < 0.05
p < 0.01
p < 0.001
Fisher's Test
Isolative sample indicates that patients had no alternative liver diagnoses except NASH; hence, patients with hepatitis B, C, alcoholic liver disease, and HCC were excluded
Assistance variable was created through combining the multileveled activity and functional status scales into three dependence categories.
Table 1b demonstrates baseline characteristics of liver transplant recipients with PSC and donors in both sex-matched and mismatched cases. There were no significant differences in recipient demographics, comorbidities, and laboratory markers. Fewer recipients in the male mismatch donor cohort received a transjugular intrahepatic portosystemic shunt (TIPS) procedure (4.94 vs. 7.68%, p=0.02). Additionally, a statistically greater number of recipients in the male mismatch donor cohort received steroids as immunosuppressants (94.70 vs. 91.80%, p=0.01). Recipients in the female-mismatch sex cohort were more likely to have a lower BMI (25.20 ± 5.50 vs. 26.20 ± 5.53 kg/m2, p=0.005). A significantly different racial profile was observed between the two groups (White: 63.70 vs. 71.40; Black: 26.00 vs. 22.20; Hispanic: 4.73 vs. 4.31; Asian: 4.09 vs. 1.13; Other: 1.51 vs. 0.91 %, p=0.02). Additionally, these recipients in the mismatch cohort were more likely to have comorbid Hepatitis B (2.15 vs. 0.45 %, p=0.04). There were no significant differences in the severity of ascites, severity of encephalopathy, model for end-stage liver disease (MELD) scores, use of immunosuppressants, laboratory markers, and critical care and life support measures. The remaining mismatch sub-analyses regarding baseline characteristics for both female and male recipients can be identified in Table 1b.
Table 1b:
Baseline Characteristics of Liver Transplant Recipients with Primary Sclerosing Cholangitis and Donors Stratified by Donor Sex in Sex Mismatch Subanalyses
| Characteristics | Male Recipients | Female Recipients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male Donor (Sex Match) | Female Donor (Sex Mismatch) | Male Donor (Sex Mismatch) | Female Donor (Sex Match) | |||||||||
| n = 729 | 37.91 % | n = 1194 | 62.09 % | p-value | n = 465 | 51.32 % | n = 441 | 48.68 % | p-value | |||
| Recipient Demographics | ||||||||||||
| Age (years) | 49.00 | ± 13.60 years | 48.70 | ± 13.50 years | 0.61 | 49.70 | ± 12.80 years | 48.20 | ± 13.60 years | 0.12 | ||
| Race | 0.15 | 0.02 † | * | |||||||||
| White (%) | 575 | 78.90 % | 961 | 80.50 % | 296 | 63.70 % | 315 | 71.40 % | ||||
| Black (%) | 106 | 14.50 % | 158 | 13.20 % | 121 | 26.00 % | 98 | 22.20 % | ||||
| Hispanic (%) | 26 | 3.57 % | 52 | 4.36 % | 22 | 4.73 % | 19 | 4.31 % | ||||
| Asian (%) | 16 | 2.19 % | 11 | 0.92 % | 19 | 4.09 % | 5 | 1.13 % | ||||
| Other (%) | 6 | 0.82 % | 12 | 1.01 % | 7 | 1.51 % | † FM | 0.91 % | ||||
| BMI (kg/m2) | 25.30 | ± 4.55 kg/m2 | 25.50 | ± 4.39 kg/m2 | 0.21 | 25.20 | ± 5.50 kg/m2 | 26.20 | ± 5.53 kg/m2 | 0.005 | ** | |
| Comorbidities | ||||||||||||
| Hepatitis B (%) | 14 | 1.92 % | 11 | 0.92 % | 0.10 | 10 | 2.15 % | 2 | 0.45 % | 0.04 † | * | |
| Hepatitis C (%) | 33 | 4.53 % | 45 | 3.77 % | 0.49 | 20 | 4.30 % | 11 | 2.49 % | 0.19 | ||
| Alcoholic Liver Disease (%) | 10 | 1.37 % | 9 | 0.75 % | 0.27 | 3 | 0.65 % | 2 | 0.45 % | 1.00 † | ||
| Diabetes (%) | 87 | 11.90 % | 135 | 11.30 % | 0.73 | 48 | 10.30 % | 50 | 11.30 % | 0.70 | ||
| Assistance ‡ | 0.61 | 0.54 † | ||||||||||
| 0 (%) | 20 | 2.74 % | 29 | 2.43 % | † FM | 0.86 % | 9 | 2.04 % | ||||
| 1 (%) | 195 | 26.70 % | 300 | 25.10 % | 109 | 23.40 % | 106 | 24.00 % | ||||
| 2 (%) | 273 | 37.40 % | 483 | 40.50 % | 210 | 45.20 % | 194 | 44.00 % | ||||
| 3 (%) | 241 | 33.10 % | 382 | 32.00 % | 142 | 30.50 % | 132 | 29.90 % | ||||
| Hepatic Variables | ||||||||||||
| Ascites | 0.07 | 0.77 | ||||||||||
| Absent (%) | 254 | 34.80 % | 388 | 32.50 % | 137 | 29.50 % | 131 | 29.70 % | ||||
| Slight (%) | 311 | 42.70 % | 572 | 47.90 % | 237 | 51.00 % | 216 | 49.00 % | ||||
| Moderate (%) | 164 | 22.50 % | 234 | 19.60 % | 91 | 19.60 % | 94 | 21.30 % | ||||
| Encephalopathy | 0.26 | 0.76 | ||||||||||
| None (%) | 378 | 51.90 % | 611 | 51.20 % | 234 | 50.30 % | 228 | 51.70 % | ||||
| 1 - 2 (%) | 306 | 42.00 % | 528 | 44.20 % | 201 | 43.20 % | 181 | 41.00 % | ||||
| 3 - 4 (%) | 45 | 6.17 % | 55 | 4.61 % | 30 | 6.45 % | 32 | 7.26 % | ||||
| MELD Scores | 22.80 | ± 9.19 | 22.70 | ± 9.21 | 0.82 | 22.00 | ± 8.89 | 22.10 | ± 9.20 | 0.78 | ||
| Medications | ||||||||||||
| Mycophenolate Mofetil (%) | 600 | 82.30 % | 992 | 83.10 % | 0.71 | 390 | 83.90 % | 387 | 87.80 % | 0.11 | ||
| Cyclosporine (%) | 21 | 2.88 % | 36 | 3.02 % | 0.98 | 8 | 1.72 % | 14 | 3.17 % | 0.23 | ||
| Tacrolimus (%) | 681 | 93.40 % | 1132 | 94.80 % | 0.24 | 440 | 94.60 % | 413 | 93.70 % | 0.63 | ||
| Sirolimus (%) | 10 | 1.37 % | 10 | 0.84 % | 0.37 | † FM | 0.86 % | 2 | 0.45 % | 0.69 † | ||
| Steroids (%) | 669 | 91.80 % | 1131 | 94.70 % | 0.01 | * | 440 | 94.60 % | 412 | 93.40 % | 0.53 | |
| Biomarkers | ||||||||||||
| Albumin (mg/dL) | 2.97 | ± 0.70 mg/dL | 2.92 | ± 0.74 mg/dL | 0.10 | 2.93 | ± 0.78 mg/dL | 2.97 | ± 0.70 mg/dL | 0.65 | ||
| Creatinine (mg/dL) | 1.31 | ± 1.05 mg/dL | 1.33 | ± 1.12 mg/dL | 0.80 | 1.09 | ± 0.87 mg/dL | 1.05 | ± 0.76 mg/dL | 0.96 | ||
| INR | 1.86 | ± 1.03 | 1.86 | ± 1.10 | 0.38 | 1.84 | ± 1.58 | 1.95 | ± 2.03 | 0.88 | ||
| Total Bilirubin (mg/dL) | 13.80 | ± 12.70 mg/dL | 13.50 | ± 12.10 mg/dL | 0.82 | 13.80 | ± 13.00 mg/dL | 13.60 | ± 11.90 mg/dL | 0.83 | ||
| Life Support Variables | ||||||||||||
| Primary Inotropic Agent | 0.04 | * | 0.09 † | |||||||||
| Dobutamine (%) | 11 | 1.51 % | 24 | 2.01 % | 11 | 2.37 % | 8 | 1.81 % | ||||
| Dopamine (%) | 132 | 18.10 % | 208 | 17.40 % | 69 | 14.80 % | 78 | 17.70 % | ||||
| Epinephrine (%) | 9 | 1.23 % | 18 | 1.51 % | † FM | 0.86 % | 10 | 2.27 % | ||||
| Levophed (%) | 124 | 17.00 % | 160 | 13.40 % | 100 | 21.50 % | 74 | 16.80 % | ||||
| Neosynephrine (%) | 133 | 18.20 % | 177 | 14.80 % | 74 | 15.90 % | 61 | 13.80 % | ||||
| None (%) | 302 | 41.40 % | 579 | 48.50 % | 192 | 41.30 % | 203 | 46.00 % | ||||
| Other (%) | 18 | 2.47 % | 28 | 2.35 % | 15 | 3.23 % | 7 | 1.59 % | ||||
| Secondary Inotropic Agent | 0.47 | 0.42 † | ||||||||||
| Dobutamine (%) | 6 | 0.82 % | 7 | 0.59 % | 2 | 0.43 % | 0 | 0.00 % | ||||
| Dopamine (%) | 8 | 1.10 % | 8 | 0.67 % | 5 | 1.08 % | 2 | 0.45 % | ||||
| Epinephrine (%) | 5 | 0.69 % | 6 | 0.50 % | 1 | 0.22 % | 5 | 1.13 % | ||||
| Levophed (%) | 26 | 3.57 % | 36 | 3.02 % | 15 | 3.23 % | 18 | 4.08 % | ||||
| Neosynephrine (%) | 41 | 5.62 % | 49 | 4.10 % | 33 | 7.10 % | 28 | 6.35 % | ||||
| None (%) | 635 | 87.10 % | 1068 | 89.40 % | 403 | 86.70 % | 381 | 86.40 % | ||||
| Other (%) | 8 | 1.10 % | 20 | 1.68 % | 6 | 1.29 % | 7 | 1.59 % | ||||
| Ternary Inotropic Agent | 0.66 † | 0.80 † | ||||||||||
| Dobutamine (%) | 1 | 0.14 % | 1 | 0.08 % | 1 | 0.22 % | 1 | 0.23 % | ||||
| Dopamine (%) | 1 | 0.14 % | 2 | 0.17 % | 1 | 0.22 % | 0 | 0.00 % | ||||
| Epinephrine (%) | 1 | 0.14 % | 1 | 0.08 % | 1 | 0.22 % | 0 | 0.00 % | ||||
| Levophed (%) | 6 | 0.82 % | 5 | 0.42 % | 1 | 0.22 % | 2 | 0.45 % | ||||
| Neosynephrine (%) | 3 | 0.41 % | 3 | 0.25 % | 2 | 0.43 % | 5 | 1.13 % | ||||
| None (%) | 716 | 98.20 % | 1176 | 98.50 % | 457 | 98.30 % | 431 | 97.70 % | ||||
| Other (%) | 1 | 0.14 % | 6 | 0.50 % | 2 | 0.43 % | 2 | 0.45 % | ||||
| ICU Admission | 0.95 | 0.35 | ||||||||||
| ICU (%) | 56 | 7.68 % | 94 | 7.87 % | 41 | 8.82 % | 48 | 10.90 % | ||||
| No ICU (%) | 673 | 92.30 % | 1100 | 92.10 % | 424 | 91.20 % | 393 | 89.10 % | ||||
| Ventilator Support (%) | 12 | 1.65 % | 21 | 1.76 % | 1.00 | 13 | 2.80 % | 15 | 3.40 % | 0.74 | ||
| TIPS Procedure (%) | 56 | 7.68 % | 59 | 4.94 % | 0.02 | * | 22 | 4.73 % | 30 | 6.80 % | 0.23 | |
| Donor Demographics | ||||||||||||
| Donor Age (years) | 45.70 | ± 16.20 years | 39.80 | ± 16.50 years | < 0.001 | *** | 45.20 | ± 18.30 years | 38.30 | ± 17.90 years | < 0.001 | *** |
| Donor Male Sex (%) | 0 | 0.00 % | 1194 | 100.00 % | < 0.001 † | *** | 0 | 0.00 % | 441 | 100.00 % | < 0.001 † | *** |
| Donor Race | 0.01 | * | 0.65 | |||||||||
| White (%) | 529 | 72.60 % | 820 | 68.70 % | 324 | 69.70 % | 290 | 65.80 % | ||||
| Black (%) | 128 | 17.60 % | 198 | 16.60 % | 77 | 16.60 % | 82 | 18.60 % | ||||
| Hispanic (%) | 51 | 7.00 % | 143 | 12.00 % | 42 | 9.03 % | 50 | 11.30 % | ||||
| Asian (%) | 13 | 1.78 % | 17 | 1.42 % | 13 | 2.80 % | 10 | 2.27 % | ||||
| Other (%) | 8 | 1.10 % | 16 | 1.34 % | 9 | 1.94 % | 9 | 2.04 % | ||||
| Donor BMI (kg/m2) | 29.50 | ± 8.47 kg/m2 | 27.50 | ± 5.71 kg/m2 | < 0.001 | *** | 27.00 | ± 6.64 kg/m2 | 25.70 | ± 5.62 kg/m2 | 0.02 | * |
| Donor Biomarkers | ||||||||||||
| Donor Creatinine (mg/dL) | 1.48 | ± 1.52 mg/dL | 1.68 | ± 1.60 mg/dL | < 0.001 | *** | 1.38 | ± 1.76 mg/dL | 1.76 | ± 1.92 mg/dL | < 0.001 | *** |
| Donor Total Bilirubin (mg/dL) | 0.77 | ± 0.63 mg/dL | 0.96 | ± 0.80 mg/dL | < 0.001 | *** | 0.82 | ± 0.72 mg/dL | 0.91 | ± 0.77 mg/dL | 0.01 | * |
p < 0.05
p < 0.01
p < 0.001
Fisher's Test
Isolative sample indicates that patients had no alternative liver diagnoses except NASH; hence, patients with hepatitis B, C, alcoholic liver disease, and HCC were excluded
Assistance variable was created through combining the multileveled activity and functional status scales into three dependence categories.
Clinical outcomes
For the male vs female donor analysis, the total donor median follow-up time was 4.27 years (interquartile range [IQR]: 1.85-8.32) with male donor median follow-up time at 4.23 years (IQR: 1.80-8.32) and female donor median follow-up time at 4.41 years (IQR: 1.89-8.31). For the male recipient subanalysis, the male donor, female donor, and total donor median follow-up time was 4.35 years (IQR: 1.86-8.55), 4.46 years (IQR: 1.86-8.07), and 4.35 years (1.86-8.30) respectively. For the female recipient subanalysis, the male donor, female donor, and total donor median follow-up time was 4.01 years (IQR: 1.59-8.04), 4.35 years (1.96-8.77), and 4.10 years (1.78-8.36) respectively.
Table 2 demonstrates the primary outcomes of all-cause mortality and graft failure, stratified by matched and mismatched donor sex. No differences in all-cause mortality were observed between any of the comparison groups. Male donors as a group had a decreased hazard ratio of graft failure (aHR 0.63, 95% CI 0.44-0.91, p=0.01). For the mismatch analyses, the male-to-male recipients also had a significantly reduced hazard ratio of graft failure compared to female-to-male transplants (aHR 0.51, 95% CI 0.33-0.79, p=0.003). No similar difference in graft failure was observed in the mismatched female recipient subgroup. The survival curves for all-cause mortality and graft failure are illustrated in Figure 2, with analysis of outcomes between the donor and recipient sex.
Table 2:
Sequential Cox Regression Analysis Using Donor Sex as a Prognostic Risk Factor for All-Cause Mortality and Graft Failure in Liver Transplant Recipients with Primary Sclerosing Cholangitis
| Male vs Female Donor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) All-Cause Mortality | (B) Graft Failure | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 32.38 | (28.73 - 36.36) | Male | 6.57 | (4.97 - 8.52) | ||||||
| Female | 35.18 | (30.76 - 40.04) | Female | 10.98 | (8.56 - 13.88) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 221 | Male | 69 | ||||||||
| Female | 276 | Female | 56 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.33 | 0.92 | (0.77 - 1.09) | 1 | 0.004 ** | 0.60 | (0.42 - 0.85) | ||||
| 2 | 0.31 | 0.91 | (0.76 - 1.09) | 2 | 0.005 ** | 0.60 | (0.42 - 0.86) | ||||
| 3 | 0.31 | 0.91 | (0.76 - 1.09) | 3 | 0.004 ** | 0.60 | (0.42 - 0.85) | ||||
| † FM | 0.47 | 0.94 | (0.78 - 1.12) | † FM | 0.01 * | 0.63 | (0.44 - 0.91) | ||||
| Mismatch Subanalysis: Female (Sex Mismatch) vs Male Donor in Male Recipients | |||||||||||
| (A) All-Cause Mortality | (B) Graft Failure | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 31.74 | (27.55 - 36.37) | Male | 6.35 | (4.54 - 8.63) | ||||||
| Female | 36.90 | (31.15 - 43.38) | Female | 12.56 | (9.28 - 16.62) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 141 | Male | 48 | ||||||||
| Female | 200 | Female | 40 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.16 | 0.86 | (0.69 - 1.06) | 1 | 0.002 ** | 0.51 | (0.33 - 0.77) | ||||
| 2 | 0.14 | 0.85 | (0.68 - 1.06) | 2 | 0.002 ** | 0.51 | (0.33 - 0.78) | ||||
| 3 | 0.13 | 0.84 | (0.68 - 1.05) | 3 | 0.002 ** | 0.50 | (0.33 - 0.77) | ||||
| † FM | 0.17 | 0.86 | (0.69 - 1.07) | † FM | 0.003 ** | 0.51 | (0.33 - 0.79) | ||||
| Mismatch Subanalysis: Male (Sex Mismatch) vs Female Donor in Female Recipients | |||||||||||
| (A) All-Cause Mortality | (B) Graft Failure | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 34.21 | (27.05 - 42.64) | Male | 7.20 | (4.12 - 11.67) | ||||||
| Female | 32.51 | (25.86 - 40.30) | Female | 8.53 | (5.29 - 13.02) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 80 | Male | 21 | ||||||||
| Female | 76 | Female | 16 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.94 | 1.01 | (0.74 - 1.39) | 1 | 0.50 | 0.80 | (0.41 - 1.54) | ||||
| 2 | 0.97 | 1.01 | (0.73 - 1.39) | 2 | 0.65 | 0.86 | (0.44 - 1.67) | ||||
| 3 | 0.99 | 1.00 | (0.72 - 1.38) | 3 | 0.64 | 0.85 | (0.44 - 1.67) | ||||
| † FM | 0.72 | 1.06 | (0.76 - 1.48) | † FM | 0.99 | 1.00 | (0.50 - 2.02) | ||||
p < 0.05
p < 0.01
p < 0.001
FM indicates Final Model
Model 1 includes VOI (variable of interest) and demographics; Model 2 includes Model 1 terms with the addition of comorbidities, and liver disease etiologies; Model 3 includes Model 2 terms with the addition of hepatic variables, MELD score, and liver laboratory markers; Model 4 includes Model 3 terms with the addition of donor demographics
Figure 2: Comparison of Cumulative Events With All-Cause Mortality and Graft Failure as Endpoints Male vs Female Donor.
(A) and (B) represent the cumulative hazards for all-cause mortality and graft failure in liver transplant recipients, who receivedtheir grafts from male versus female donors. (C) and (D) represent the cumulative hazards for all-cause mortality and graft failurein female liver transplant recipients who received their grafts from male versus female donors. (E) and (F) represent thecumulative hazards for all-cause mortality and graft failure in male liver transplant recipients who received their grafts from maleversus female donors.The p-value indicates the respective log-rank p-value for each curve.
Investigation of secondary causes of mortality, including general cardiac, respiratory, infectious, renal, and recurrent hepatic etiologies, was conducted. Analysis of death secondary to graft-related complications (infections, vascular, biliary) and graft failure were also performed. The above review of secondary causes of mortality stratified by donor sex and matched and mismatched recipient sex are demonstrated in Table 3. No differences between male and female donor groups were found in any of the analyses mentioned above. Within the sex mismatch analysis, the mismatched male recipient group showed a decreased hazard ratio of mortality from graft rejection (aHR 0.17, 95% CI 0.04-0.72, p=0.02) and respiratory causes (aHR 0.38, 95% CI 0.15-0.96, p=0.04). No differences in specific mortality causes were identified in the mismatched female recipient group.
Table 3:
Sequential Cox Regression Analysis Using Donor Sex as a Prognostic Risk Factor for Specific Causes of Death in Liver Transplant Recipients with Primary Sclerosing Cholangitis
| Male vs Female Donor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) Death due to General Cardiac Causes | (B) Death due to Graft Biliary Complications | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 2.82 | (1.80 - 4.19) | Male | 0.12 | (0.00 - 0.65) | ||||||
| Female | 3.50 | (2.20 - 5.30) | Female | 0.80 | (0.26 - 1.86) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 22 | Male | 5 | ||||||||
| Female | 24 | Female | 1 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.48 | 0.81 | (0.45 - 1.45) | 1 | 0.07 | 0.13 | (0.02 - 1.15) | ||||
| 2 | 0.48 | 0.81 | (0.45 - 1.45) | 2 | 0.07 | 0.13 | (0.02 - 1.16) | ||||
| 3 | 0.47 | 0.81 | (0.45 - 1.45) | 3 | 0.06 | 0.12 | (0.01 - 1.08) | ||||
| † FM | 0.35 | 0.75 | (0.41 - 1.36) | † FM | 0.07 | 0.10 | (0.01 - 1.19) | ||||
| (C) Death due to Graft Infections | (D) Death due to Recurrent Liver Disease | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 0.00 | (0.00 - 0.43) | Male | 1.06 | (0.48 - 2.00) | ||||||
| Female | 0.32 | (0.04 - 1.15) | Female | 1.43 | (0.66 - 2.72) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 2 | Male | 9 | ||||||||
| Female | 0 | Female | 9 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 1.00 | 0.00 | (0.00 - Inf) | 1 | 0.48 | 0.72 | (0.28 - 1.82) | ||||
| 2 | 1.00 | 0.00 | (0.00 - Inf) | 2 | 0.49 | 0.72 | (0.28 - 1.82) | ||||
| 3 | 1.00 | 0.00 | (0.00 - Inf) | 3 | 0.49 | 0.72 | (0.28 - 1.82) | ||||
| † FM | 0.98 | 0.00 | (0.00 - Inf) | † FM | 0.56 | 0.75 | (0.29 - 1.96) | ||||
| (E) Death due to Graft Rejection | (F) Death due to Graft Vascular Complications | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 0.82 | (0.33 - 1.69) | Male | 0.23 | (0.03 - 0.85) | ||||||
| Female | 1.59 | (0.76 - 2.93) | Female | 0.64 | (0.17 - 1.63) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 10 | Male | 4 | ||||||||
| Female | 7 | Female | 2 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.15 | 0.49 | (0.19 - 1.29) | 1 | 0.26 | 0.37 | (0.07 - 2.06) | ||||
| 2 | 0.16 | 0.50 | (0.19 - 1.31) | 2 | 0.28 | 0.39 | (0.07 - 2.14) | ||||
| 3 | 0.13 | 0.47 | (0.17 - 1.25) | 3 | 0.24 | 0.35 | (0.06 - 1.98) | ||||
| † FM | 0.07 | 0.38 | (0.13 - 1.08) | † FM | 0.15 | 0.26 | (0.04 - 1.65) | ||||
| (G) Death due to General Respiratory Causes | (H) Death due to General Infectious Causes | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 1.17 | (0.56 - 2.16) | Male | 7.51 | (5.79 - 9.58) | ||||||
| Female | 2.39 | (1.34 - 3.94) | Female | 7.80 | (5.78 - 10.30) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 15 | Male | 49 | ||||||||
| Female | 10 | Female | 64 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.08 | 0.49 | (0.22 - 1.09) | 1 | 0.89 | 0.97 | (0.67 - 1.42) | ||||
| 2 | 0.10 | 0.51 | (0.23 - 1.14) | 2 | 0.90 | 0.98 | (0.67 - 1.42) | ||||
| 3 | 0.11 | 0.51 | (0.23 - 1.15) | 3 | 0.90 | 0.98 | (0.67 - 1.42) | ||||
| † FM | 0.12 | 0.52 | (0.23 - 1.18) | † FM | 0.87 | 1.03 | (0.70 - 1.51) | ||||
| (I) Death due to General Renal Causes | |||||||||||
| Incidence Rates per 1000 Person-Years | |||||||||||
| Male | 1.29 | (0.64 - 2.31) | |||||||||
| Female | 1.59 | (0.76 - 2.93) | |||||||||
| Raw Incidence | |||||||||||
| Male | 10 | ||||||||||
| Female | 11 | ||||||||||
| Sequential Cox Regression Analysis | |||||||||||
| Model | p-value | aHR | 95% CI | ||||||||
| 1 | 0.53 | 0.76 | (0.32 - 1.79) | ||||||||
| 2 | 0.56 | 0.77 | (0.33 - 1.84) | ||||||||
| 3 | 0.53 | 0.76 | (0.32 - 1.81) | ||||||||
| † FM | 0.55 | 0.76 | (0.31 - 1.86) | ||||||||
| Mismatch Subanalysis: Female (Sex Mismatch) vs Male Donor in Male Recipients | |||||||||||
| (A) Death due to Graft Rejection | (B) Death due to General Respiratory Causes | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 0.63 | (0.17 - 1.62) | Male | 1.27 | (0.55 - 2.50) | ||||||
| Female | 2.09 | (0.90 - 4.12) | Female | 3.14 | (1.62 - 5.48) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 8 | Male | 12 | ||||||||
| Female | 4 | Female | 8 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.05 | 0.30 | (0.09 - 1.00) | 1 | 0.05 | 0.41 | (0.17 - 1.00) | ||||
| 2 | 0.04 | * | 0.28 | (0.08 - 0.96) | 2 | 0.07 | 0.43 | (0.18 - 1.07) | |||
| 3 | 0.03 | * | 0.26 | (0.07 - 0.90) | 3 | 0.06 | 0.42 | (0.17 - 1.03) | |||
| † FM | 0.02 | * | 0.17 | (0.04 - 0.72) | † FM | 0.04 | * | 0.38 | (0.15 - 0.96) | ||
| (C) Death due to Graft Infections | (D) Death due to Recurrent Liver Disease | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 0.00 | (0.00 - 0.59) | Male | 1.11 | (0.45 - 2.29) | ||||||
| Female | 0.52 | (0.06 - 1.89) | Female | 1.57 | (0.58 - 3.41) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 2 | Male | 6 | ||||||||
| Female | 0 | Female | 7 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 1.00 | 0.00 | (0.00 - Inf) | 1 | 0.57 | 0.73 | (0.24 - 2.17) | ||||
| 2 | 1.00 | 0.00 | (0.00 - Inf) | 2 | 0.54 | 0.71 | (0.24 - 2.12) | ||||
| 3 | 1.00 | 0.00 | (0.00 - Inf) | 3 | 0.55 | 0.72 | (0.24 - 2.17) | ||||
| † FM | 1.00 | 0.00 | (0.00 - Inf) | † FM | 0.42 | 0.62 | (0.20 - 1.98) | ||||
| (E) Death due to General Cardiac Causes | (F) Death due to Graft Vascular Complications | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 3.02 | (1.82 - 4.70) | Male | 0.32 | (0.04 - 1.15) | ||||||
| Female | 3.40 | (1.81 - 5.81) | Female | 0.79 | (0.16 - 2.29) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 13 | Male | 3 | ||||||||
| Female | 19 | Female | 2 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.75 | 0.89 | (0.44 - 1.80) | 1 | 0.31 | 0.39 | (0.06 - 2.36) | ||||
| 2 | 0.76 | 0.90 | (0.44 - 1.82) | 2 | 0.38 | 0.45 | (0.07 - 2.73) | ||||
| 3 | 0.67 | 0.86 | (0.42 - 1.74) | 3 | 0.32 | 0.40 | (0.06 - 2.49) | ||||
| † FM | 0.65 | 0.84 | (0.40 - 1.76) | † FM | 0.35 | 0.39 | (0.06 - 2.75) | ||||
| (G) Death due to Graft Biliary Complications | (H) Death due to General Infectious Causes | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 0.16 | (0.00 - 0.88) | Male | 6.66 | (4.81 - 9.00) | ||||||
| Female | 0.79 | (0.16 - 2.29) | Female | 8.38 | (5.74 - 11.80) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 3 | Male | 32 | ||||||||
| Female | 1 | Female | 42 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.16 | 0.20 | (0.02 - 1.93) | 1 | 0.37 | 0.81 | (0.51 - 1.29) | ||||
| 2 | 0.16 | 0.19 | (0.02 - 1.89) | 2 | 0.37 | 0.81 | (0.51 - 1.29) | ||||
| 3 | 0.10 | 0.14 | (0.01 - 1.44) | 3 | 0.34 | 0.80 | (0.50 - 1.27) | ||||
| † FM | 0.30 | 0.25 | (0.02 - 3.45) | † FM | 0.47 | 0.84 | (0.52 - 1.35) | ||||
| (I) Death due to General Renal Causes | |||||||||||
| Incidence Rates per 1000 Person-Years | |||||||||||
| Male | 1.59 | (0.76 - 2.92) | |||||||||
| Female | 1.83 | (0.74 - 3.77) | |||||||||
| Raw Incidence | |||||||||||
| Male | 7 | ||||||||||
| Female | 10 | ||||||||||
| Sequential Cox Regression Analysis | |||||||||||
| Model | p-value | aHR | 95% CI | ||||||||
| 1 | 0.77 | 0.86 | (0.33 - 2.27) | ||||||||
| 2 | 0.99 | 1.01 | (0.38 - 2.69) | ||||||||
| 3 | 0.86 | 0.91 | (0.34 - 2.50) | ||||||||
| † FM | 0.84 | 0.90 | (0.32 - 2.53) | ||||||||
| Mismatch Subanalysis: Male (Sex Mismatch) vs Female Donor in Female Recipients | |||||||||||
| (A) Death due to General Cardiac Causes | (B) Death due to Graft Biliary Complications | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 2.25 | (0.73 - 5.24) | Male | 0.00 | (0.00 - 1.66) | ||||||
| Female | 3.66 | (1.67 - 6.93) | Female | 0.81 | (0.10 - 2.93) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 9 | Male | 2 | ||||||||
| Female | 5 | Female | 0 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.32 | 0.57 | (0.19 - 1.73) | 1 | 1.00 | 0.00 | (0.00 - Inf) | ||||
| 2 | 0.29 | 0.55 | (0.18 - 1.67) | 2 | 1.00 | 0.00 | (0.00 - Inf) | ||||
| 3 | 0.27 | 0.53 | (0.18 - 1.61) | 3 | 1.00 | 0.00 | (0.00 - Inf) | ||||
| † FM | 0.16 | 0.44 | (0.14 - 1.40) | † FM | 0.99 | 0.00 | (0.00 - Inf) | ||||
| (C) Death due to Recurrent Liver Disease | (D) Death due to Graft Rejection | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 0.90 | (0.11 - 3.25) | Male | 1.35 | (0.28 - 3.94) | ||||||
| Female | 1.22 | (0.25 - 3.56) | Female | 0.81 | (0.10 - 2.93) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 3 | Male | 2 | ||||||||
| Female | 2 | Female | 3 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.68 | 0.68 | (0.11 - 4.20) | 1 | 0.77 | 1.30 | (0.21 - 7.97) | ||||
| 2 | 0.69 | 0.70 | (0.11 - 4.25) | 2 | 0.54 | 1.83 | (0.26 - 12.64) | ||||
| 3 | 0.79 | 1.30 | (0.19 - 9.10) | 3 | 0.61 | 1.70 | (0.22 - 13.02) | ||||
| † FM | 0.32 | 2.99 | (0.34 - 26.12) | † FM | 0.69 | 1.47 | (0.22 - 9.90) | ||||
| (E) Death due to Graft Vascular Complications | (F) Death due to General Respiratory Causes | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 0.00 | (0.00 - 1.66) | Male | 0.90 | (0.11 - 3.25) | ||||||
| Female | 0.41 | (0.01 - 2.26) | Female | 1.22 | (0.25 - 3.56) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 1 | Male | 3 | ||||||||
| Female | 0 | Female | 2 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 1.00 | 0.00 | (0.00 - Inf) | 1 | 0.79 | 0.78 | (0.13 - 4.75) | ||||
| 2 | 1.00 | 0.00 | (0.00 - Inf) | 2 | 0.77 | 0.77 | (0.13 - 4.65) | ||||
| 3 | 1.00 | 0.00 | (0.00 - Inf) | 3 | 0.83 | 0.81 | (0.13 - 5.25) | ||||
| † FM | 1.00 | 0.00 | (0.00 - Inf) | † FM | 0.94 | 0.93 | (0.13 - 6.85) | ||||
| (G) Death due to General Infectious Causes | (H) Death due to Sepsis | ||||||||||
| Incidence Rates per 1000 Person-Years | Incidence Rates per 1000 Person-Years | ||||||||||
| Male | 9.90 | (6.22 - 14.96) | Male | 7.65 | (4.46 - 12.22) | ||||||
| Female | 6.91 | (4.03 - 11.04) | Female | 4.47 | (2.23 - 7.98) | ||||||
| Raw Incidence | Raw Incidence | ||||||||||
| Male | 17 | Male | 11 | ||||||||
| Female | 22 | Female | 17 | ||||||||
| Sequential Cox Regression Analysis | Sequential Cox Regression Analysis | ||||||||||
| Model | p-value | aHR | 95% CI | Model | p-value | aHR | 95% CI | ||||
| 1 | 0.34 | 1.37 | (0.72 - 2.59) | 1 | 0.23 | 1.60 | (0.75 - 3.45) | ||||
| 2 | 0.31 | 1.39 | (0.73 - 2.64) | 2 | 0.19 | 1.68 | (0.77 - 3.64) | ||||
| 3 | 0.36 | 1.35 | (0.71 - 2.57) | 3 | 0.21 | 1.64 | (0.75 - 3.58) | ||||
| † FM | 0.29 | 1.43 | (0.74 - 2.77) | † FM | 0.16 | 1.76 | (0.79 - 3.92) | ||||
| (I) Death due to General Renal Causes | |||||||||||
| Incidence Rates per 1000 Person-Years | |||||||||||
| Male | 0.45 | (0.01 - 2.51) | |||||||||
| Female | 1.22 | (0.25 - 3.56) | |||||||||
| Raw Incidence | |||||||||||
| Male | 3 | ||||||||||
| Female | 1 | ||||||||||
| Sequential Cox Regression Analysis | |||||||||||
| Model | p-value | aHR | 95% CI | ||||||||
| 1 | 0.30 | 0.29 | (0.03 - 2.92) | ||||||||
| 2 | 0.29 | 0.28 | (0.03 - 3.02) | ||||||||
| 3 | 0.24 | 0.20 | (0.01 - 2.88) | ||||||||
| † FM | 0.61 | 0.55 | (0.06 - 5.35) | ||||||||
p < 0.05
p < 0.01
p < 0.001
FM indicates Final Model
Model 1 includes VOI (variable of interest) and demographics; Model 2 includes Model 1 terms with the addition of comorbidities, and liver disease etiologies; Model 3 includes Model 2 terms with the addition of hepatic variables, MELD score, and liver laboratory markers; Model 4 includes Model 3 terms with the addition of donor demographics
Supplementary Table (ST) 1 depicts competing risk regression analyses for all-cause mortality and graft failure. When comparing male and female donors, a significant difference was found for graft failure (aHR 0.62, 95% CI 0.43-0.89, p=0.01) but not for all-cause mortality (aHR 1.09, 95% CI 0.88-1.35, p=0.43). Further sub-analysis of mismatched sex groups, specifically in male recipients, showed a significant difference for graft failure (aHR 0.50, 95% CI 0.33-0.77, p=0.001), as similarly identified in the above analyses. No significant findings were identified in this female recipient mismatch analysis for graft failure or all-cause mortality.
Additional analyses were conducted by stratifying by years: 2005-2009, 2010-2014, and 2015-2019. ST 2a-2i parallel Table 1a and 1b by depicting the baseline characteristics of liver transplant recipients and recipients with donors in both sex-matched and mismatched cases. Furthermore, ST 3a-3i demonstrate the primary outcomes (all-cause mortality and graft failure) while ST 4a-4i demonstrate the secondary outcomes (specific causes of death). Furthermore, competing risk regression analyses for male vs female donor and the mismatch subanalyses was performed for each year bracket: 2005-2019 (ST 5-7), 2010-2014 (ST 8-10), and 2015-2019 (ST 11-13). Figure 3-5 demonstrates the primary outcomes stratified by both donor sex, as well as time period (2005-2009, 2010-2014, and 2015-2019, respectively) with corresponding Forest Plots (Supplementary Figure 1-6).
Figure 3: Comparison of Cumulative Events With All-Cause Mortality and Graft Failure as Endpointswith Subsetted Timeframes.
(A) and (B) represent the cumulative hazards for all-cause mortality and graft failure in liver transplant recipients, who received their grafts from male versus female donors in 2005-2009. (C) and (D) represent the cumulative hazards for all-cause mortality andgraft failure in liver transplant recipients, who received their grafts from male versus female donors in 2010-2014. (E) and(F) represent the cumulative hazards for all-cause mortality and graft failure in liver transplant recipients, who received their grafts from male versus female donors in 2015-2019.The p-value indicates the respective log-rank p-value for each curve.
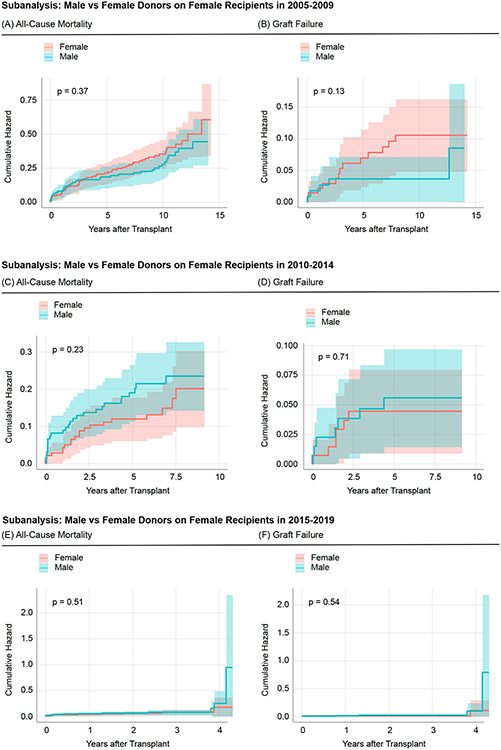
Figure 5: Comparison of Cumulative Events With All-Cause Mortality and Graft Failure as Endpoints with Subsetted Timeframes-Subanalysis of Female Recipients.
(A) and (B) represent the cumulative hazards for all-cause mortality and graft failure in female liver transplant recipients, who received their grafts from male versus female donors in 2005-2009. (C) and (D) represent the cumulative hazards for all-cause mortality and graft failure in female liver transplant recipients, who received their grafts from male versus female donors in 2010-2014. (E) and (F) represent the cumulative hazards for all-cause mortality and graft failure in female liver transplant recipients, who received their grafts from male versus female donors in 2015-2019.The p-value indicates the respective log-rank p-value for each curve.
From 2005-2009, the male vs female donor analysis exhibited these median follow-up times: male donor - 10.62 years (IQR: 8.59-12.01); female donor - 10.35 years (8.00-11.97); total donor - 10.52 years (IQR: 8.26-12.00). The male recipient and female recipient subanalysis demonstrated the following median follow-up times respectively: male donor - 10.72 and 10.45 years (IQR: 8.49-12.01 and 8.88-11.98); female donor - 10.93 and 9.98 years (IQR: 8.25-12.06 and 7.27-11.75); total donor - 10.81 and 10.01 years (IQR: 8.37-12.01 and 8.11-11.91).
From 2010-2014, the male vs female donor analysis exhibited these median follow-up times: male donor - 5.99 years (IQR: 4.81-7.38); female donor - 5.96 years (4.71-7.37); total donor - 5.98 years (IQR: 4.79-7.38). The male recipient and female recipient subanalysis demonstrated the following median follow-up times respectively: male donor - 5.99 and 5.98 years (IQR: 4.83-7.50 and 4.75-7.03); female donor - 5.93 and 6.01 years (IQR: 4.66-7.21 and 4.87-7.64); total donor - 5.97 and 6.00 years (IQR: 4.81-7.41 and 4.76-7.20).
From 2015-2019, the male vs female donor analysis exhibited these median follow-up times: male donor - 1.87 years (IQR: 0.89-2.91); female donor - 1.89 years (0.82-2.88); total donor - 1.88 years (IQR: 0.85-2.90). The male recipient and female recipient subanalysis demonstrated the following median follow-up times respectively: male donor - 1.90 and 1.80 years (IQR: 0.83-2.92 and 0.95-2.87); female donor - 1.88 and 1.91 years (IQR: 0.79-2.82 and 0.86-2.96); total donor - 1.89 and 1.86 years (IQR: 0.81-2.86 and 0.92-2.92).
The Supplementary Tables 2-4 shows the primary tables for the year-delineated subanalyses (stratified by 2005-2009, 2010-2014, and 2014-2019). The corresponding competing risk regression analyses that include the complete cohort and the recipient and donor sex-matching and mismatching subcohorts are shown in the Supplementary Tables 5-13.
Discussion
This study explores the impact of donor and recipient sex on post-LT outcomes in patients with PSC. The interaction between sexes in general LT studies has been shown to affect post-transplant results, particularly in graft survival (23). However, no studies have been conducted to determine the relationship between donor and recipient sexes after LT in patients with PSC, where sex is known to play a role in the epidemiology of this disease (17, 18).
The UNOS STAR Database was used to investigate several post-transplantation outcomes among PSC patients, including various causes of mortality and graft failure. The most notable finding of interest was a statistically significant difference in graft failure in male recipients of female livers. Interestingly, there was no statistical difference in graft failure in female recipients of male donor livers and no notable differences in all-cause mortality across all the groups. As mentioned previously, sex, as it pertains to the donor-recipient relationship, has been well-studied as a factor influencing transplantation outcomes (23, 24). Early studies in graft outcomes showed female liver donation was associated with poorer outcomes (23). Since then, there has been a multitude of literature demonstrating an increased risk of graft loss, specifically in female donor-to-male recipient liver transplants, which is recapitulated in our present study, though with a specific focus on the PSC population (25, 26). In one systematic review by Lai et al., general LT patients with a mismatched sex donor were found to have as high as a 30-fold increased risk of graft loss. When divided into sex subgroups, male-to-female LT conferred no increased risk, while female-to-male recipient liver transplants were associated with the risk of graft loss (25).
All-cause mortality was the second primary endpoint evaluated in this study. When controlling for demographic variables, patient comorbidities, hepatic complications, MELD scores, and liver laboratory markers, no differences were identified in all-cause mortality between any of the sex groups. When further divided into specific causes of mortality, the mismatch sub-analysis showed a significant increase in mortality risk due to graft rejection in male recipients of female donor livers. In contrast, there was no increase in mortality risk in the group of female recipients of male livers. This finding supports prior studies that show a worse survival rate due to graft failure in female-to-male liver transplants (26). Ultimately, female-to-male transplantation was found to have an increased hazard ratio of graft failure as well as mortality from graft failure. Clinical studies in general organ transplants have previously shown that recipients of organs from female donors have not only a greater risk of rejection but a higher risk of mortality from rejection (27,28), which is demonstrated in our study of liver transplantation in the PSC population.
The definitive etiology of the differences in transplant outcomes between male and female sexes is not well elucidated. Existing research indicates that hormonal disparities may play a role in these findings. Sex hormones exhibit metabolic differences between males and females after liver transplantation. Transplanted livers from donor females have been shown to have an increased rate of acidosis through transplant-mediated ischemia, a response suspected to be mediated by estrogen (25,28). A study by Fairweather et al. reported that females are known to be more “immunogenic” than males due to the action of estrogen as an immune response regulator (29). Specifically, it is postulated that estrogen works to impair negative selection of B cells, leading to enhanced antibody production and Th2 response, thereby resulting in a more robust immune response to transplanted organs (29, 30). In contrast, testosterone has been associated with immunosuppressive effects by moderating the synthesis of extracellular signaling cascades involved in anti-inflammatory signaling (30). However, patients, particularly women, whose immune system is highly active may face greater difficulty suppressing the immune response in transplantation. This idea is further supported by the high rates of autoimmune diseases in women and may also explain why both female recipients and recipients of female donors have higher graft failure rates (31, 32).
As mentioned previously, PSC has been considered an atypical autoimmune disease, as it predominantly affects males; however, it is associated with worse outcomes than females (33). The cause of PSC is not well-defined, but it is believed that underlying inflammation can lead to injury of cholangiocytes, which can cause an abnormal immune response in genetically vulnerable individuals resulting in progressive sclerosis of the biliary system (34). PSC is thought to involve dysfunction of adaptive immunity and regulatory T cells, which may be exacerbated by an estrogen-driven enhanced immune response from a female donor to a male recipient (35).
The results of this study demonstrate an association between female liver donation to male recipients with both an increased risk of graft loss and an increased risk of mortality due to graft rejection. Given that PSC is known to be a male-predominant disease, this study suggests that female liver donation in male recipients may serve as a poor prognostic indicator. This study is among the first to describe post-LT outcomes using donor sexes as the prognostic risk factor in PSC. Notably, this study did not include the concurrence rate of IBD in PSC patients. One study evaluating risk factors in the development of PSC found that female sex may have a marginally protective effect (36). However, when matched for the IBD phenotype, females had a lower rate of ulcerative colitis (UC) compared to males. Given that PSC is known to be highly associated with UC, this may suggest that there is a disproportionate incidence of UC-associated PSC rather than a higher incidence of PSC in males alone (36). In addition, PSC has a relatively high rate of recurrence, thereby requiring re-transplantation (37). Further investigation into the interplay between sex donor-recipient relationships in the context of co-existing co-occurring IBD in PSC would provide more insight into rates of recurrence, retransplantation, and other post-transplant outcomes.
There were several limitations to this study. Primarily, this was a retrospective study using a UNOS STAR database, limiting the analysis to broad causes of mortality as recorded in the database. Therefore, specific causes of death could not be investigated in detail. Furthermore, the UNOS STAR database provides information regarding immunosuppressive medications which was utilized as a covariate in our study model. However, the database does not delineate between the use of these medications for induction versus maintenance immunosuppressive therapy, and which modality a patient is receiving at the time of data collection, all of which may have a confounding effect on transplant outcomes in our study population. Secondly, the comorbidities listed were limited to donor demographics while recipient demographics were not available, which may impact outcomes. Finally, IBD-phenotypes and non-IBD-phenotypes of PSC were not included in the demographics in this study. The co-occurrence of IBD could also be a significant factor in the outcomes of this cohort.
Conclusion
This study attempted to investigate the role of donor sex mismatch on post-transplantation outcomes in patients with PSC. Our analysis demonstrated a significant increase in the risk of both graft failure and mortality secondary to graft failure in male recipients of female donor livers. No significant differences in mortality or graft failure were identified in female recipients of male livers.
Supplementary Material
Supplementary Figure 1. This is a multivariate analysis Forest Plot exhibited all-cause mortality with donor sex as the exposure variable.
Supplementary Figure 2. This is a multivariate analysis Forest Plot exhibited graft failure with donor sex as the exposure variable.
Supplementary Figure 3. This is a multivariate analysis Forest Plot exhibited all-cause mortality with donor sex as the exposure variable in male recipients.
Supplementary Figure 4. This is a multivariate analysis Forest Plot exhibited all-cause mortality with donor sex as the exposure variable in female recipients.
Supplementary Figure 5. This is a multivariate analysis Forest Plot exhibited graft failure with donor sex as the exposure variable in male recipients.
Supplementary Figure 6. This is a multivariate analysis Forest Plot exhibited graft failure with donor sex as the exposure variable in female recipients.
Figure 4: Comparison of Cumulative Events With All-Cause Mortality and Graft Failure as Endpoints with Subsetted Timeframes-Subanalysis of Male Recipients.
(A) and (B) represent the cumulative hazards for all-cause mortality and graft failure in male liver transplant recipients, who received their grafts from male versus female donors in 2005-2009. (C) and (D) represent the cumulative hazards for all-cause mortality and graft failure in male liver transplant recipients, who received their grafts from male versus female donors in 2010-2014. (E) and (F) represent the cumulative hazards for all-cause mortality and graft failure in male liver transplant recipients, who received their grafts from male versus female donors in 2015-2019.The p-value indicates the respective log-rank p-value for each curve.
Footnotes
Conflict of Interest Statement:
The authors of this manuscript certify they share no affiliation or involvement with any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. None declared.
References
- 1.Penz-Österreicher M, Österreicher CH, Trauner M. Fibrosis in autoimmune and cholestatic liver disease. Best Pract Res Clin Gastroenterol. 2011;25(2):245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Downey M, O’Brien C, Frauenhoffer E, Ahmadpour N, Riley T, Schreibman I. Primary Sclerosing Cholangitis in Association with Multiple Myeloma. American Journal of Gastroenterology. 2007;102:S299. [PMC free article] [PubMed] [Google Scholar]
- 3.Shi TY, Zhang LN, Chen H, et al. Risk factors for hepatic decompensation in patients with primary biliary cirrhosis. World J Gastroenterol. 2013;19(7):1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OFW. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004;53(6):865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallo C, Howardson BO, Cristoferi L, Carbone M, Gershwin ME, Invernizzi P. An Update on Novel Pharmacological Agents for Primary Sclerosing Cholangitis. Expert Opin Ther Targets. Published online January 18, 2022. [DOI] [PubMed] [Google Scholar]
- 6.Wiesner RH, Porayko MK, Dickson ER, et al. Selection and timing of liver transplantation in primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 1992;16(5):1290–1299. [PubMed] [Google Scholar]
- 7.Wiesner RH, Porayko MK, Hay JE, et al. Liver transplantation for primary sclerosing cholangitis: impact of risk factors on outcome. Liver Transpl Surg. 1996;2(5 Suppl 1):99–108. [PubMed] [Google Scholar]
- 8.Andersen IM, Fosby B, Boberg KM, et al. Indications and Outcomes in Liver Transplantation in Patients With Primary Sclerosing Cholangitis in Norway. Transplant Direct. 2015;1(9):e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steenstraten IC, Sebib Korkmaz K, Trivedi PJ, et al. Systematic review with meta-analysis: risk factors for recurrent primary sclerosing cholangitis after liver transplantation. Aliment Pharmacol Ther. 2019;49(6):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueda Y, Kaido T, Okajima H, et al. Long-term Prognosis and Recurrence of Primary Sclerosing Cholangitis After Liver Transplantation: A Single-Center Experience. Transplant Direct. 2017;3(12):e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon FD, Goldberg DS, Goodrich NP, et al. Recurrent primary sclerosing cholangitis in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study: Comparison of risk factors between living and deceased donor recipients. Liver Transpl. 2016;22(9):1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egawa H, Ueda Y, Ichida T, et al. Risk factors for recurrence of primary sclerosing cholangitis after living donor liver transplantation in Japanese registry. Am J Transplant. 2011;11(3):518–527. [DOI] [PubMed] [Google Scholar]
- 13.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790. [DOI] [PubMed] [Google Scholar]
- 14.Halldorson JB, Bakthavatsalam R, Fix O, Reyes JD, Perkins JD. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9(2):318–326. [DOI] [PubMed] [Google Scholar]
- 15.Burra P, De Martin E, Gitto S, Villa E. Influence of Age and Gender Before and After Liver Transplantation. Liver Transplantation. 2013;19(2):122–134. [DOI] [PubMed] [Google Scholar]
- 16.Wu EM, Wong LL, Hernandez BY, et al. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Invernizzi F, Cilla M, Trapani S, et al. Gender and Autoimmune Liver Diseases: Relevant Aspects in Clinical Practice. J Pers Med. 2022;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alabraba E, Nightingale P, Gunson B, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15(3):330–340. [DOI] [PubMed] [Google Scholar]
- 19.Croome KP, Taner CB. The changing landscapes in DCD liver transplantation. Curr Transplant Rep. 2020;7(3):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Organ Procurement and Transplantation Network. Policies. Updated June 18, 2020. Accessed October 8, 2023.https://optn.transplant.hrsa.gov/media/eavh5bf3/optn_policies.pdf
- 21.Austin PC, Steyerberg EW, Putter H. Fine-Gray subdistribution hazard models to simultaneously estimate the absolute risk of different event types: Cumulative total failure probability may exceed 1. Statistics in Medicine. 2021;40(19):4200–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4(2):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks BK, Levy MF, Jennings LW, Abbasoglu O, Vodapally M, Goldstein RM, Husberg BS, Gonwa TA, Klintmalm GB. Influence of donor and recipient gender on the outcome of liver transplantation. Transplantation. 1996;62(12):1784–1787. [DOI] [PubMed] [Google Scholar]
- 24.Yoshizumi T, Shirabe K, Taketomi A, Uchiyama H, Harada N, Ijichi H, Yoshimatsu M, Ikegami T, Soejima Y, Maehara Y. Risk factors that increase mortality after living donor liver transplantation. Transplantation. 2012;93(1):93–98. [DOI] [PubMed] [Google Scholar]
- 25.Lai Q, Giovanardi F, Melandro F, et al. Donor-to-recipient gender match in liver transplantation: A systematic review and meta-analysis. World J Gastroenterol. 2018;24(20):2203–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marino IR, Doyle HR, Aldrighetti L, et al. Effect of donor age and sex on the outcome of liver transplantation. Hepatology. 1995;22(6):1754–1762. [PMC free article] [PubMed] [Google Scholar]
- 27.Zeier M, Döhler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002;13(10):2570–2576. [DOI] [PubMed] [Google Scholar]
- 28.Puoti F, Ricci A, Nanni-Costa A, Ricciardi W, Malorni W, Ortona E. Organ transplantation and gender differences: a paradigmatic example of intertwining between biological and sociocultural determinants. Biol Sex Differ. 2016;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. 2008;173(3):600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taneja V. Sex Hormones Determine Immune Response. Front Immunol. 2018;9:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- 32.Dobson R. Transplanted organs from women are more likely to be rejected. BMJ. 2002;325(7372):1057. [Google Scholar]
- 33.Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med. 2016;375(12):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabiee A, Silveira MG. Primary sclerosing cholangitis. Transl Gastroenterol Hepatol. 2021;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarcognato S, Sacchi D, Grillo F, et al. Autoimmune biliary diseases: primary biliary cholangitis and primary sclerosing cholangitis. Pathologica. 2021. Jun;113(3):170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weismüller TJ, Trivedi PJ, Bergquist A, Imam M, Lenzen H, Ponsioen CY, et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology. 2017;152(8):1975–1984.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visseren T, Erler NS, Polak WG, Adam R, Karam V, European Liver and Intestine Transplantation Association (ELITA), et al. Recurrence of primary sclerosing cholangitis after liver transplantation - analysing the European Liver Transplant Registry and beyond. Transpl Int. 2021;34(8):1455–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. This is a multivariate analysis Forest Plot exhibited all-cause mortality with donor sex as the exposure variable.
Supplementary Figure 2. This is a multivariate analysis Forest Plot exhibited graft failure with donor sex as the exposure variable.
Supplementary Figure 3. This is a multivariate analysis Forest Plot exhibited all-cause mortality with donor sex as the exposure variable in male recipients.
Supplementary Figure 4. This is a multivariate analysis Forest Plot exhibited all-cause mortality with donor sex as the exposure variable in female recipients.
Supplementary Figure 5. This is a multivariate analysis Forest Plot exhibited graft failure with donor sex as the exposure variable in male recipients.
Supplementary Figure 6. This is a multivariate analysis Forest Plot exhibited graft failure with donor sex as the exposure variable in female recipients.