Abstract
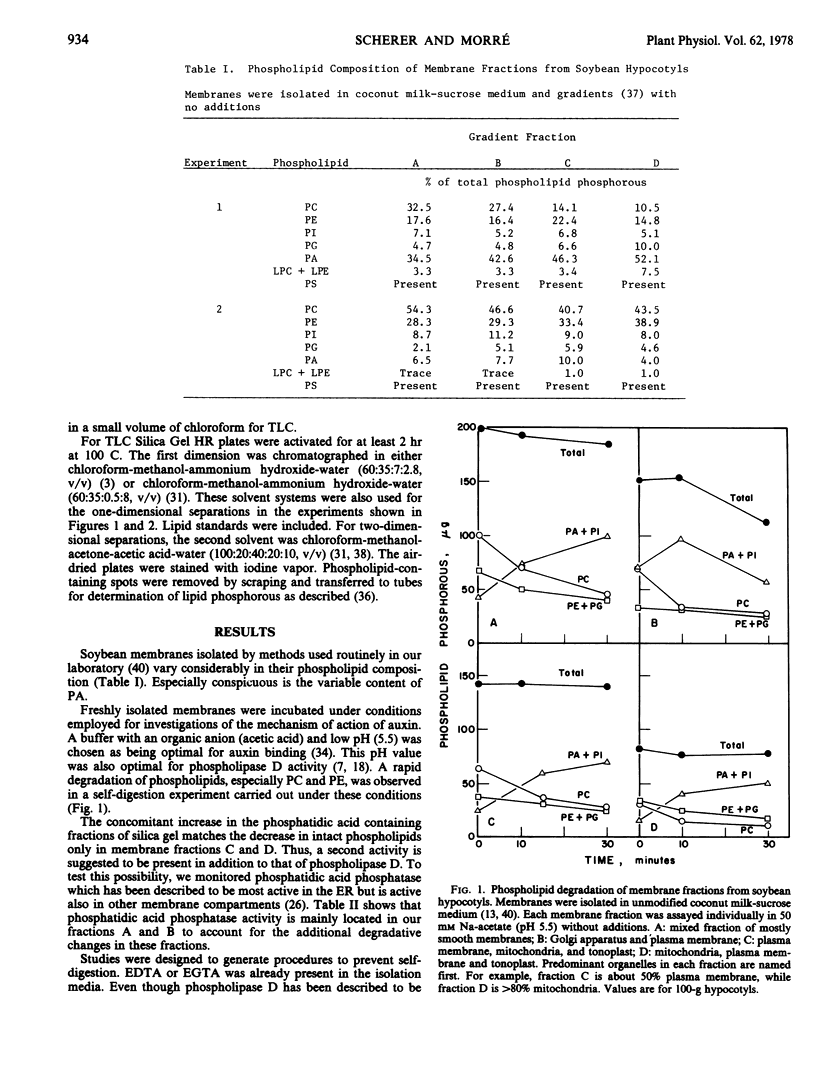
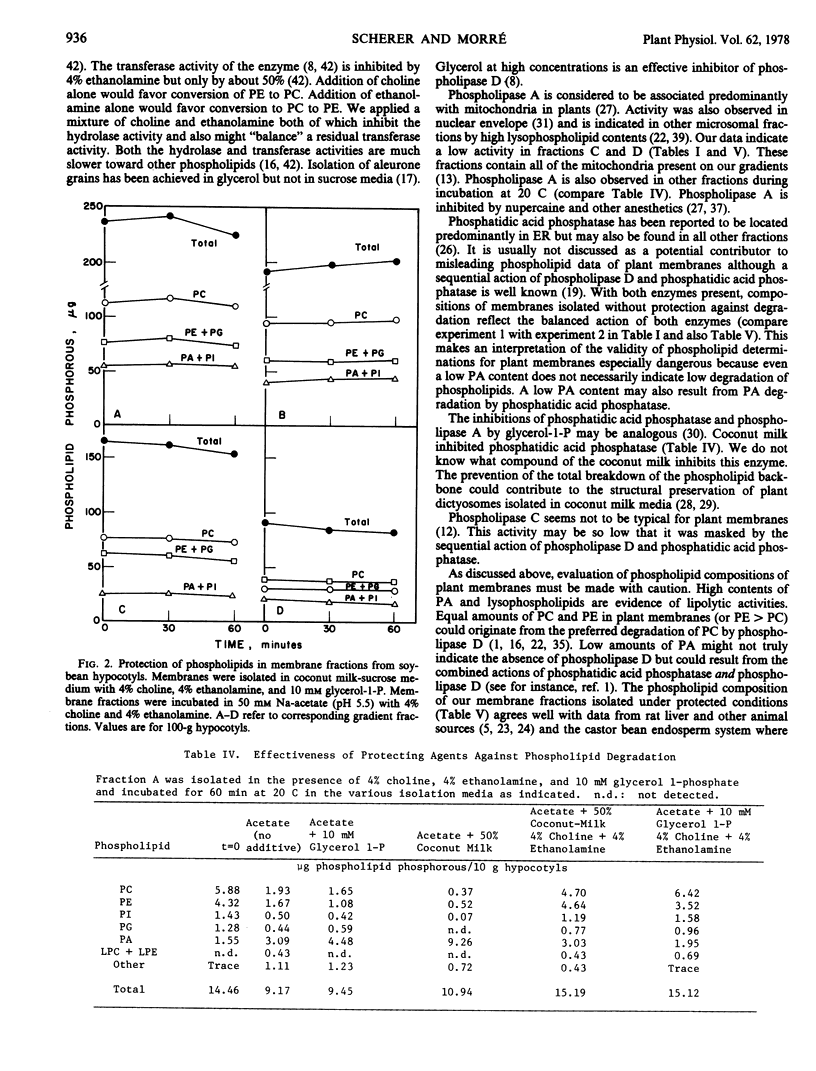
Endogenous phospholipase D and phosphatidic acid phosphatase activities were demonstrated in membrane fractions isolated from soybean (Glycine max L.) hypocotyls. Phospholipase D activity was distributed widely among different membrane fractions while phosphatidic acid phosphatase was found predominantly in membranes equilibrating in lower sucrose densities. Phospholipase D action was unaffected by ethylenediaminetetraacetic acid, sodium salt or ethylene glycol-bis(β-aminoethyl ether)-N,N′-tetraacetic acid but was prevented by a mixture of 4% choline and 4% ethanolamine. Phosphatidic acid phosphatase was inhibited by 10 millimolar glycerol 1-phosphate or by homogenization media prepared with coconut milk as a solvent instead of water. Under fully protected conditions the phospholipid composition of soybean membrane fractions remained unchanged for at least 1 hour at 20 C. Membranes prepared under protected conditions had low phosphatidic acid contents and the phospholipid compositions closely resembled those of corresponding animal membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelkader A. B., Mazliak P. Echanges de lipides entre mitochondries, microsomes et surnageant cytoplasmique de cellules de pomme de terre ou de chou-fleur. Eur J Biochem. 1970 Aug;15(2):250–262. doi: 10.1111/j.1432-1033.1970.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Bartholomew L., Mace K. D. Isolation and identification of phospholipids from root tip cell plasmalemma of Phaseolus limensis. Cytobios. 1972 Jul;5(20):241–247. [PubMed] [Google Scholar]
- Clermont H., Douce R. Localisation de l'activite phospholipasique dans les tissus vegetaux. 1) Sur l'absence d'activité phospholipasique D dans les mitochondries et les plastes isolés. FEBS Lett. 1970 Aug 31;9(5):284–286. doi: 10.1016/0014-5793(70)80378-7. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. Some properties of purified phospholipase D and especially the effect of amphipathic substances. Biochem J. 1967 Jan;102(1):76–86. doi: 10.1042/bj1020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M. The formation of phosphatidylglycerol and other phospholipids by the transferase activity of phospholipase D. Biochem J. 1967 Jan;102(1):205–210. doi: 10.1042/bj1020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P., Beevers H. Lipid composition of organelles from germinating castor bean endosperm. Plant Physiol. 1977 Feb;59(2):259–263. doi: 10.1104/pp.59.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Galliard T. Techniques for overcoming problems of lipolytic enzymes and lipoxygenases in the preparation of plant organelles. Methods Enzymol. 1974;31:520–528. doi: 10.1016/0076-6879(74)31056-7. [DOI] [PubMed] [Google Scholar]
- Hardin J. W., Cherry J. H., Morré D. J., Lembi C. A. Enhancement of RNA polymerase activity by a factor released by auxin from plasma membrane. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3146–3150. doi: 10.1073/pnas.69.11.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A., Morré D. J. Evidence for an increase in microviscosity of plasma membranes from soybean hypocotyls induced by the plant hormone, indole-3-acetic Acid. Plant Physiol. 1976 Oct;58(4):548–551. doi: 10.1104/pp.58.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Arad R. Properties of the phospholipase D from peanut seeds. Biochim Biophys Acta. 1970 Jul 14;210(2):276–286. doi: 10.1016/0005-2760(70)90172-4. [DOI] [PubMed] [Google Scholar]
- KATES M. Hydrolysis of glycerolphosphatides by plastid phosphatidase C. Can J Biochem Physiol. 1956 Sep;34(5):967–980. [PubMed] [Google Scholar]
- KATES M. Hydrolysis of lecithin by plant plastid enzymes. Can J Biochem Physiol. 1955 Jul;33(4):575–589. [PubMed] [Google Scholar]
- KATES M. Lecithinase systems in sugar beet, spinach, cabbage, and carrot. Can J Biochem Physiol. 1954 Sep;32(5):571–583. [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J. Phospholipid class and fatty acid composition of golgi apparatus isolated from rat liver and comparison with other cell fractions. Biochemistry. 1970 Jan 6;9(1):19–25. doi: 10.1021/bi00803a003. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Nyquist S. E., Mollenhauer H. H. Lipid composition of subcellular fractions from rat testis. Biochim Biophys Acta. 1972 Aug 11;270(4):433–443. doi: 10.1016/0005-2760(72)90108-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H. ISOLATION OF THE GOLGI APPARATUS FROM PLANT CELLS. J Cell Biol. 1964 Nov;23:295–305. doi: 10.1083/jcb.23.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. S., Lord J. M., Kagawa T., Beevers H. Enzymes of phospholipid metabolism in the endoplasmic reticulum of castor bean endosperm. Plant Physiol. 1973 Jul;52(1):50–53. doi: 10.1104/pp.52.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuizen W., Kunze H., de Haas G. H. Phospholipase A2 (phosphatide acylhydrolase, EC 3.1.1.4) from porcine pancreas. Methods Enzymol. 1974;32:147–154. doi: 10.1016/0076-6879(74)32018-6. [DOI] [PubMed] [Google Scholar]
- Philipp E. I., Franke W. W., Keenan T. W., Stadler J., Jarasch E. D. Characterization of nuclear membranes and endoplasmic reticulum isolated from plant tissue. J Cell Biol. 1976 Jan;68(1):11–29. doi: 10.1083/jcb.68.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privett O. S., Dougherty K. A., Erdahl W. L., Stolyhwo A. Studies on the lipid composition of developing soybeans. J Am Oil Chem Soc. 1973 Dec;50(12):516–520. doi: 10.1007/BF02640523. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. The distribution of phospholipase D in developing and mature plants. Biochem J. 1969 May;112(5):787–794. doi: 10.1042/bj1120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R. Is phospholipase D really an enzyme? A comparison of in situ and in vitro activities. Biochim Biophys Acta. 1976 Apr 22;431(1):86–95. doi: 10.1016/0005-2760(76)90262-9. [DOI] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- Singh H., Privett O. S. Incorporation of 33P in soybean phosphatides. Biochim Biophys Acta. 1970 Feb 10;202(1):200–202. doi: 10.1016/0005-2760(70)90236-5. [DOI] [PubMed] [Google Scholar]
- Williamson F. A., Morré D. J. Association of Phytochrome with Rough-surfaced Endoplasmic Reticulum Fractions from Soybean Hypocotyls. Plant Physiol. 1975 Dec;56(6):738–743. doi: 10.1104/pp.56.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Phospholipids in the developing soybean seed. Plant Physiol. 1974 Nov;54(5):744–747. doi: 10.1104/pp.54.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. F., Freer S., Benson A. A. Transphosphatidylation by phospholipase D. J Biol Chem. 1967 Feb 10;242(3):477–484. [PubMed] [Google Scholar]


