Abstract
Purpose:
The Sex Differences in Radiation Research workshop addressed the role of sex as a confounder in radiation research and its implication in real-world radiological and nuclear applications.
Methods:
In April 2022, HHS-wide partners from the Radiation and Nuclear Countermeasures Program, the Office of Research on Women’s Health National Institutes of Health Office of Women’s Health, U.S. Food and Drug Administration, and the Radiological and Nuclear Countermeasures Branch at the Biomedical Advanced Research and Development Authority conducted a workshop to address the scientific implication and knowledge gaps in understanding sex in basic and translational research. The goals of this workshop were to examine sex differences in 1. Radiation animal models and understand how these may affect radiation medical countermeasure development; 2. Biodosimetry and/or biomarkers used to assess acute radiation syndrome, delayed effects of acute radiation exposure, and/or predict major organ morbidities; 3. medical research that lacks representation from both sexes. In addition, regulatory policies that influence inclusion of women in research, and the gaps that exist in drug development and device clearance were discussed. Finally, real-world sex differences in human health scenarios were also considered.
Results:
This report provides an overview of the two-day workshop, and open discussion among academic investigators, industry researchers, and U.S. government representatives.
Conclusions:
This meeting highlighted that current study designs lack the power to determine statistical significance based on sex, and much is unknown about the underlying factors that contribute to these differences. Investigators should accommodate both sexes in all stages of research to ensure that the outcome is robust, reproducible, and accurate, and will benefit public health.
Keywords: Women’s health, sex differences, radiation, medical countermeasures, biodosimetry
Introduction
Biomedical research has suffered from an inequality of female representation in both preclinical and clinical studies. Male biology has been centered as the norm with female responses either not investigated or viewed as atypical or abnormal deviations (Zucker et al. 2022); that notion is now dispelled (Prendergast et al. 2014; Becker et al. 2016; Beery 2018). Between the 1990s and early 2000s, reports highlighted the lack of sex inclusion across multiple disciplines and pushed for the use of females in research studies (Mogil and Chanda 2005; Holdcroft 2007; Prendergast et al. 2014; Becker et al. 2016). However, data were not stratified by sex because few studies included female animals, and sex as a biological variable (SABV) was not fully considered (Clayton 2016). To further enhance reproducibility and transparency, the National Institutes of Health (NIH) enacted various inclusion policy milestones to advance this area of science.1
The default to males in research studies leaves a knowledge gap and can lead to drug safety issues. A 2001 audit by the U.S. Government Accountability Office found that eight of ten approved drugs in clinical use had greater adverse effects in women than men (U.S. Government Accountability Office 2001). Until recently, NIH-funded radiation research was skewed due to the primary inclusion of males in preclinical studies; however, even with the historical lack of inclusion of females, several retrospective studies and preclinical studies have shown that SABV must be considered in this field (Gandhi et al. 2004; Holdcroft 2007; Coleman et al. 2019b; Broustas et al. 2022). For example, in 2006 the National Academy of Sciences published the Biological Effects of Ionizing Radiation (BEIR VII) report, which showed that low-level ionizing radiation effects lead to an excess relative risk for specific cancers dependent on sex (National Research Council (US) Committee on Health Effects of Exposure to Low Levels of Ionizing Radiations (BEIR VII) 1998). In addition, a lifespan study (1958–2009), which included 105,444 atomic bomb survivors, also showed sex differences in the types of cancer incidences (Sakata et al. 2019). Sex differences have also been reported in radiation preclinical studies for the development of medical countermeasures (MCMs) (Velardi et al. 2018; Daniel et al. 2020) and in animal models of irradiation injuries (Beach et al. 2021). Noting these differences early in the drug development pathway is critical as it can help prioritize resources toward developing sex-independent biomarkers and MCMs.
Meeting program overview
The Radiation and Nuclear Countermeasures Program (RNCP), within NIAID at the NIH hosted a U.S. Department of Health and Human Services (HHS)-wide workshop on April 26 & 27, 2022 titled Sex Differences in Radiation Research, in partnership with the NIH Office of Research on Women’s Health (ORWH), the Office of Women’s Health (OWH), within the U.S. Food and Drug Administration (FDA), and the Biomedical Advanced Research and Development Authority (BARDA) Radiological and Nuclear Countermeasures Branch within the Administration for Strategic Preparedness and Response (ASPR). The keynote speakers included Dr. Janine Austin Clayton, director of NIH ORWH, and Dr. Kaveeta Vasisht, director of the FDA OWH. The workshop’s goal was to examine sex differences within animal models of radiation injury and their effect on MCM development, explore sex differences in biodosimetry and/or biomarkers of acute and delayed effects of radiation exposure (DEARE), and learn about the challenges in medical research lacking representation from both sexes. These were considered in the context of regulatory policies that influence the inclusion of both sexes in research, gaps that exist, and the impact of sex differences on drug development and device clearance. Finally, sex differences in real-world human health scenarios such as nuclear incidents and space flight were also examined.
This report captures presentations and discussions from the 27 subject matter experts across 20 institutions that participated in this workshop (Table 1). The audience included investigators from global research partners, academia, and private industry, as well as U.S. government representatives from NIH, BARDA, FDA, and other federal agencies. The content of this meeting report contains comments and information shared at this workshop and was reviewed by all presenters prior to submission. A summary of the meeting sessions and and topic areas is summarized in Table 2.
Table 1.
Workshop Speakers and areas of expertise.
| Name | Affiliation | Area of Expertise |
|---|---|---|
| Sally A. Amundson, PhD | Columbia University Irving Medical Center | Radiation transcriptomics, biomarkers, cancer biology, radiation protection, biodosimetry |
| Dina A. Andrews, DVM, PhD, DACVP | Amgen, Inc. | Translational safety & bioanalytical sciences, clinical pathology, non-clinical safety, veterinary pathology |
| Tyler A. Beach, PhD | SRI International (SRI) | Toxicology and radiation biology, RNA sequencing, histology, flow cytometry, and molecular biology |
| Jeremy B. Brower, PhD | Lovelace Biomedical Research Institute | Drug development, pre-clinical CBRNE animal models, toxicology, and pulmonary disease |
| Amrita K. Cheema, PhD | Georgetown University | Oncology, molecular biology, biochemistry, DNA repair, multi-omics, and radiation research |
| Janine A. Clayton, MD | NIH Office of Research on Women’s Health (ORWH) | Ophthalmology, NIH policy, sex and gender in health and disease |
| J. Mark Cline, DVM, PhD | Wake Forest School of Medicine | Veterinary pathology, animal models of cancer and radiation effects, breast and reproductive cancer risk, non-human primates |
| Julia R. Coleman, MD, MPH | University of Colorado Health Science Center | Trauma surgery, surgical critical care, sex dimorphisms in coagulation, |
| C. Norman Coleman, MD | National Cancer Institute (NCI) | Medical and radiation oncology, global health security, radiological-nuclear threats, biomarkers |
| Vishwa Deep Dixit, DVM, PhD | Yale School of Medicine | Immunobiology, immune-metabolism and aging, immune-senescence, metabolic dysfunction |
| Anne-Marie Downey, PhD | Charles River Labs (CRL) | Radiation-related preclinical studies, pharmacology |
| Melanie L. Doyle-Eisele, PhD | Lovelace Biomedical Research Institute (LBRI) | Drug development, pre-clinical CBRNE animal models, PK/PD, toxicology, pulmonary disease, infectious disease, inflammation, and injury |
| S. Robin Elgart, PhD | NASA Human Research Program | Microbiology, DNA damage response, medical physics, and radiation biology |
| Sanchita P. Ghosh, PhD | Armed Forces Radiobiology Research Institute (AFRRI) | Radiation countermeasures, molecular markers of radiation injury, DEARE |
| Heather A. Himburg, PhD | Medical College of Wisconsin | Hematology, oncology, normal tissue radiation-induced injury, immune system, tumor radioresistance, DEARE |
| Carol J. Iddins, MD | Radiation Emergency Assistance Center/Training Site (REAC/TS) | Radiation emergency management, radiological security and safety, disaster medicine, obstetrics and gynecology, occupational medicine, military operational and aerospace medicine |
| Isabel L. Jackson, PhD | University of Maryland, School of Medicine (UMSOM) | Normal tissue radiobiology, animal models of ARS/DEARE, MCM development under the FDA Animal Rule |
| Maureen A. Kane, PhD | University of Maryland, School of Pharmacy | Analytical chemistry, targeted metabolomics, lipidomics, proteomics, mass spectrometry imaging, biomarkers |
| Evagelia C. Laiakis, PhD | Georgetown University | Human genetics, radiation biodosimetry, metabolomics, lipidomics, and space radiation effects |
| Naresh Menon, PhD | ChromoLogic, LLC | Sensor fabrication, instrumentation and novel data analytic methods, wound care and infectious disease, diagnostics/screening, drug delivery, and telehealth |
| Andrea M. Patterson, PhD | Indiana University School of Medicine | Hematology, oncology, microbiology, immunology, radioprotection and radiomitigation, age specific animal models |
| Subhrajit Saha, MTech, PhD | University of Kansas Medical Center | Radiation oncology, regenerative medicine, and organoids |
| Alla Shapiro, MD, PhD | Consultant | Pediatric hematology oncology |
| Kaveeta Parikh Vasisht, MD, Pharm.D | FDA Office of Women’s Health | Internal medicine and adult endocrinology, scientific programing in women’s health, and policy development |
| Gordana Vunjak-Novakovic, PhD | Columbia University | Biomedical engineering, regenerative medicine, and organs-on-a-chip |
| Stephanie Zalesak-Kravec, BS | University of Maryland, Baltimore, School of Pharmacy | Biochemistry, innovative analytical chemistry platforms, mass spectrometry, cardiac, and immune disorders |
Table 2.
Meeting sessions and topic areas.
| Keynotes Talks: |
| • The need for inclusive research – How far have we come? – NIH ORWH |
| • Research for women’s health – FDA OWH |
| Session I: Sex differences and model considerations |
| • Sex differences observed in a variety of nonclinical models (TBI, WTLI, and PBI) |
| • Sex differences in acute and late effects of radiation |
| Session II: Radiation research for medical countermeasures – influence of sex on efficacy |
| • Male and female differences in the effectiveness of, or response to, MCMs |
| • Sex differences in targeted mechanisms of action, or off-target effects |
| • Sex differences in animal response to irradiation |
| Session III: Sex differences in biomarker and biodosimetry development |
| • Sex differences observed in radiation biodosimetry signatures |
| • Differences in radiation-induced responses due to sex, sex-linked organ specificity (e.g. lung, GI, heart), and other sex-specific confounders |
| Session IV: Etiology of sex differences in radiation research outcomes |
| • Genetic, hormonal, and other sources of sex differences in tissues and biological systems and the response to radiation exposure |
| • Understanding the biological mechanisms underlying male and female differences that may influence radiation exposure outcomes of targets for biomarker development and therapeutic interventions |
| Session V: Real-life impacts of sex differences |
| • Impact of sex differences in a variety of real-world settings, including space flight and nuclear accidents |
| • Impact of sex and gender differences on national preparedness |
Keynotes
Day 1 Keynote: The need for inclusive research – How far have we come? (Janine Austin Clayton).
Over half of the world’s population has historically been neglected by biomedical and clinical research (Seydel 2021). In 1990, the NIH ORWH was established as the first U.S. government office to advance research for the health of women, promote women in clinical trials, and help women advance in biomedical careers. The NIH ORWH partners with various NIH institutes and centers through partnerships and signature programs.2 In addition, ORWH partners with the FDA to expand clinical trial diversity by sending out NIH Outreach Toolkits to encourage the recruitment of women in clinical research.3
Sex and gender inclusion in the research and publication process is critical. Three key inclusion-related reports have emphasized the need to analyze, interpret, report, and disseminate data in a sex-relevant manner. In 2015, a U.S. Government Accountability Office report examined NIH-funded research relating to the inclusion of women and the analysis of sex differences and recommended that the NIH examine and report more detailed data to continue the progress of women’s health research (U.S. Government Accountability Office 2015). In 2021, the EQUATOR network team demonstrated that sex and gender were consistently deficient in research design and reporting guidelines, leading to inadequate data integration in health research publications (Heidari et al. 2016). In 2022, Congress and ORWH in partnership with the National Academy of Sciences recommended standardized language to be used in survey questions to obtain information regarding sex, gender identity, and sexual orientation (National Academies of Sciences Engineering and Medicine 2022). Recently, ORWH published Guiding Principles: Sex and gender influences in COVID-19 and the health of women.4 In addition, funding opportunities have been issued to ensure studies are powered to understand the nuance of these sex differences.5 It is therefore critical to be mindful of study design and SABV data capture, as simply adding females to experiments may not reveal physiological or genetic differences that underlie the pathogenesis and diseased state.
Day 2 Keynote: Research for women’s health (Kaveeta Vasisht).
The mission of the FDA’s Office of Women’s Health (OWH)6 is to promote the inclusion of women in clinical trials, identify and monitor the progress of crosscutting and multidisciplinary initiatives, and serve as the principal government advisor on scientific, ethical, and policy issues relating to women’s health. This is achieved through the foundational principle that sex is a biological variable, and should be factored into research design, analysis, reporting, and education. FDA OWH sponsored a campaign in partnership with NIH to encourage the participation of women in clinical trials and to bring awareness to the need for diverse female involvement (e.g. participants from different races, ethnicities, ages, comorbidities, and socioeconomic backgrounds).7
The 1977 FDA guidance General Considerations for the Clinical Evaluation of Drugs excluded women with childbearing potential from participating in Phase I and early Phase II clinical trials. This was revisited in 1992 when the U.S. Government Accountability Office released a report on the need for the FDA to ensure the representation of women and investigate sex differences in clinical trials (U.S. Government Accountability Office 1992). It is important to note that sex and gender are distinct terms that, although related, are not mutually exclusive. Sex is the classification of living things, generally male or female, according to their reproductive organs and functions assigned by the chromosomal complement. Gender is defined as a person’s self-representation, or how that person is viewed by social institutions based on the individual’s gender presentation (Wizemann and Pardue 2001).
The 2015–2019 FDA Center for Drugs Evaluation and Research Drug Trials Snapshots Summary Report included data from trials on more than 200 novel products, with ~300,000 participants. The report found significant progress in clinical trial sex inclusion from prior years, with female representation at 51% globally and 56% in the U.S. In 2020, the FDA released guidance on Enhancing the Diversity of Clinical Trial Populations – Eligibility Criteria, Enrollment Practices, and Trial Designs.8 OWH staff also created the first Women’s Health Research Roadmap – A Strategy for Science and Innovation to Improve the Health of Women.9 Another collaboration between FDA and NIH colleagues is the bench to bedside program,10 an online course to help integrate sex and gender to improve human health.
The FDA also advocates for and supports sex as a biological variable in nonclinical research and development. The FDA Guidance that outlines product development under the Animal Rule states, ‘FDA expects adequate representation of both sexes in these studies’. The male/female composition of the study groups should be justified’. Since 2013, the FDA has supported the development of acute radiation syndrome (ARS) tissue chip models at the Wyss Institute for Biologically Inspired Engineering at Harvard University (Jalili-Firoozinezhad et al. 2018). This funding enables researchers to evaluate candidate MCMs in an artificial human organ system, such as the bone marrow (Chou et al. 2020). In 2018, the OWH awarded funding for a follow-on study to create male and female human bone marrow chips to analyze differences in sex-specific responses to radiation and chemotherapy.11 In summary, the FDA advances regulatory science and promotes representation of women throughout the research pipeline by focusing on education and scientific workshops, and by supporting and reinforcing policies during sponsor meetings. With these efforts, the agency hopes to ensure adequate sex representation in all areas, so that any sex differences can be accounted for during the development of preclinical models and clinical trials.
Session I: Sex differences and model considerations
To understand sex-based differences in animal models, it is important to first understand how sex impacts the tissue and cellular responses to irradiation. Radiation destroys the most proliferative cells in the body such as hematopoietic stem cells (HSCs), resulting in hematopoietic (H)-ARS after whole-body or partial-body irradiation (i.e. 2.5–8% bone marrow shielding) with doses between 2 and 10 Gy. Death may occur within weeks due to lethal infection or hemorrhage from loss of white blood cells and platelets, respectively. DEARE can manifest in survivors including cardiovascular, renal, pulmonary, late gastrointestinal (GI) effects, residual bone marrow damage, increased risk of malignancy, changes in body composition and metabolism, and effects on the central nervous system leading to changes in cognition. Sex differences observed in a variety of preclinical models, including total body irradiation (TBI), whole thorax lung (WTLI), and partial body irradiation (PBI) as well as ‘organs on a chip’ platforms, were discussed.
Sex as a biological variable in nonclinical animal model development (Lauren Jackson).
Sex and gender are not interchangeable terms; sex is a biological attribute of cells or organisms defined by genetics, physiology, anatomy, and hormone milieu, while gender is a social construct defined by appearance, actions, thoughts, and behaviors depending on the cultural context (Woitowich and Woodruff 2019). Nonclinical animal models used for radiation injury should be well understood and predictive for human response and application because they are critical for FDA licensure under the Animal Rule (U.S. Food and Drug Administration 2015).
Sex differences in radiation dose response vary across models, with some species showing more pronounced differences in radiosensitivity between sexes. For example, female mice in a C57BL/6J WTLI mouse model of radiation pneumonitis and chronic fibrosis were more radioresistant than male mice, whereas the C57L/J WTLI mouse model showed no sex difference in survival (Dabjan et al. 2016; Jackson et al. 2017). However, the C57L/J TBI/BM2.5% DEARE-lung model showed that female mice were more radiosensitive than male mice (Gibbs et al. 2023). Interestingly, the leg-out PBI bone marrow-sparing DEARE rat model shows the reverse; males are more radiosensitive than females (Fish et al. 2021). Additional work is needed to clarify these observations.
In rhesus macaques, outcomes addressing possible sex differences are variable due to the limited volume of female non-human primate (NHP) data. In the WTLI model, a difference was not observed between male and female responses (Thrall et al. 2019); however, in a recent head-to-head study of TBI, analysis of NHP dose-radiation response curves showed that female NHPs have higher mortality than males at identical radiation doses (Beach et al. 2021).
These data highlight the importance of reporting the age and sex of animals when analyzing results (Tannenbaum et al. 2017). While it can be challenging to power a study to detect sex differences, there are statistical design methods that can be used to explore any potential sex differences in preclinical studies (Clayton 2016). When SABV is considered in the earliest stages of drug development, especially when animal models are used as surrogates for clinical trials under the FDA’s Animal Rule, clinical translation is improved.
Sex divergence in pediatric and geriatric murine models of H-ARS and DEARE (Andrea Patterson).
Researchers have studied various murine models to test radiation mitigators (Patterson et al. 2021; 2022), including several age-specific, irradiated C57BL/6J models [pediatric (3–8 weeks), young adult (10–12 weeks), and geriatric (12–24 months)] (Patterson et al. 2021; 2022). Over 500 MCM survival efficacy, confirmation studies were completed using these models in an equal number of male and female mice.
Radioresistance increases with age in C57BL/6J mouse models of H-ARS. Pediatric models are the most radiosensitive while geriatric models are the most radioresistant (Patterson et al. 2021). When age groups are separated by sex, three- and four-week-old males are more radioresistant than age-matched females (Plett et al. 2012; Patterson et al. 2020; 2021). However, after puberty, pediatric females are more radioresistant than pediatric males. The reversal of sex-specific radiosensitivity after puberty may be driven by estrogen, which increases in female C57BL/6J mice at five to six-weeks old (Hill et al. 2012; Kamimura et al. 2019). Estrogen may be protective of bone marrow; removing the primary source of estrogen by ovariectomy increases bone marrow radiosensitivity (Hui et al. 2012). Murine HSCs express high levels of estrogen receptor alpha (ER-α) and under stress, ER-α signaling increases basal HSC division and erythropoiesis (Nakada et al. 2014; Oguro et al. 2017); a similar effect is seen in humans (Chapple et al. 2018; Fananas-Baquero et al. 2021).
Young adult males and females have similar radioresistance, but a reversal of radioresistance is observed in geriatric mice, where males are more radioresistant than females. This may be due to a greater myelopoietic capacity in aged mice compared to younger mice. Traditionally viewed as a negative effect due to the increased risk of myeloid malignancy and decreased adaptive immunity, increased myeloid bias may provide a survival advantage in H-ARS. Platelet and neutrophil dynamics follow age and sex differences in radioresistance, where aged mice have higher platelet and neutrophil counts. Upon irradiation, platelet and neutrophil nadirs are higher in aged males than aged females and pediatric mice exhibit the lowest nadirs. Platelet and neutrophil counts also rebound more quickly in aged males and females compared to younger mice. Bone marrow myeloid colony-forming cells (CFCs) also follow age and sex differences in radioresistance. Before irradiation, aged male and female mice have CFC levels compared to younger mice; however, after TBI, CFC nadirs for aged males are significantly higher (p < 0.05) than that of young males.
Sex differences in DEARE mouse models are also present, but few DEARE studies have been powered to detect sex differences. Some data suggest that DEARE-kidney appears to be more severe in females than in males, with pediatric females exhibiting higher blood urea nitrogen (BUN) levels than pediatric males (Patterson et al. 2021). In summary, sex differences have been noted in H-ARS mouse models and appear to be age-dependent.
The role of ACE/ACE2 signaling in mediating sex-related differences in late radiosensitivity following PBI in WAG/RijCmcr rats (Heather Himburg).
The WAG/RijCmcr rat model of radiation injury has been used to study sex and age differences in H-ARS, GI-ARS as well as DEARE-lung and -kidney. A PBI leg-out, bone marrow-sparing model with supportive care was developed to study these four sequelae (Fish et al. 2021). Sex differences in susceptibility to GI-ARS were observed in this model, with an estimated dose of radiation expected to cause death to 50 percent of an exposed population within 7 days (LD50/7) of 13.6 Gy in males and 14.3 Gy in females. Using the same model, males have higher mortality compared to females for DEARE-lung, with a median survival time of 75 days in males compared to 142 days in females. Progression to renal failure as measured by BUN showed that female rats have a shorter latency period and progress faster to irreversible renal failure as compared to male rats.
Several biological mechanisms may play a role in sex differences in DEARE. Estrogen may have a protective effect due to its antioxidant and anti-inflammatory properties, a theory that is supported by an absence of sex differences in the survival of pre-pubertal rats with lung-DEARE (Medhora et al. 2019). Isoflavones, which act as radioprotectors and radiomitigators, have been shown to reduce DEARE-lung mortality in male rats fed a diet high in isoflavones (Moulder et al. 2019).
ACE/ACE2 signaling may also play a role in sex differences in DEARE (Sharma et al. 2023). While baseline ACE2 transcription and enzymatic activity in the lung are similar in male and female rats, following irradiation, ACE2 levels increase more in females. ACE inhibitor lisinopril mitigates DEARE-lung in both female and male rats by reducing myeloid cells in the lung, immune cell infiltration in the lung, and the expression of inflammatory cytokines during pneumonitis (Gasperetti et al. 2021).
Sex differences in TBI in various animal models (Anne Marie Downey).
Historical unpublished data from H-ARS TBI studies at Charles River Laval were compiled using four different species – NHP, Göttingen minipig, C57BL/6 mouse, and New Zealand White (NZW) rabbit. Differences in radiosensitivity occur across species and sex; Göttingen minipigs are the most radiosensitive of the four species; rhesus macaques are less radiosensitive; and C57BL/6 mice and NZW rabbits are more radioresistant.
In rhesus macaques, hematological changes occur on a similar timeline for males and females; however, cell nadirs are lower in females (Beach et al. 2021). While males and females lose weight at a similar rate following irradiation, recovery to baseline weight is slower in females than in males. While the lethality doses may be different, the timing of mortality is similar between the sexes.
In C57BL/6 mice, males are more radiosensitive than females, though the timing of mortality is similar between the sexes. There is no serial blood collection in this mouse model, so there is no hematology data across time.
In NZW rabbits, there is a biphasic sex difference in mortality. At lower irradiation doses, survival is higher in females, and at higher doses, survival is higher in males. No sex difference is noted in the timing of post-irradiation blood cell kinetics, though the cell count nadirs of female rabbits are lower than that of male rabbits across all radiation doses.
Survival data for the Göttingen minipig model is limited compared to the previous species. Converse to what was seen in NZW rabbits, Göttingen males have higher survival at lower radiation levels and females have higher survival at higher radiation levels. Hematology data is also limited for this model, but blood cell kinetics and cell count nadirs appear to be similar between males and females. Additional data is needed before conclusions can be made about sex differences in irradiated Göttingen minipigs.
Challenges associated with working with both sexes in irradiation models and how these challenges can ultimately affect the study outcome (Melanie Doyle-Eisele).
At the Lovelace Biomedical Research Institute (LBRI), TBI exposures are conducted using a 6 MV Varian 600c linear accelerator, while there are two irradiators for the cutaneous radiation injury (CRI) model-low dose X-rays from a Grenz source, and beta rays from a strontium-90 source. These models have been refined at LBRI and utilized to investigate efficacy studies using standards of care that are used in the clinic. In the TBI model for Sinclair minipigs (irradiated 1.9–2.9 Gy and observed for 45 days), no overt differences in survival, clinical observations, or circulating biomarkers were observed between males and females. This observation was similar to a TBI model of rhesus macaques focused on biomarker response in NHPs. In animals irradiated with 0–10 Gy (n=10/sex/dose), no overall differences in clinical observations or biomarkers were observed between males and females by day 14 post-exposure. Similarly, in both CRI models, no significant differences in the pathophysiology of the cutaneous injury were found between the sexes.
Sex differences in late effects of radiation in NHPs (Mark Cline).
The Wake Forest Radiation Survivor Cohort is a NIAID-supported resource to study the natural history of DEARE in NHPs (Schaaf et al. 2023). With approximately 200 NHPs housed at any given time, and over 300 total-deceased and current, most of the animals were TBI-exposed, with more recent additions of PBI-exposed animals to the colony. Given the state of previous research, with predominantly males used, the ratio of males to females in the colony was 2:1 among irradiated animals and 10:1 among controls in 2022; however, efforts are underway to bring in more female animals. All survivors are subject to a multidisciplinary assessment approach, including clinical observations, imaging (CT scans & MRI), immune tests, pathology, and sequencing.
Among these survivors, most NHPs present with multiple morbidities ranging from 2 to >12 conditions. These morbidities are directly correlated to the radiation dose and the age of the NHP (Little et al. 2022). While there is a clear distinction in lymphocyte deficits in the males based on radiation dose and age, given the small number of females in the cohort, the data for females are weak.
From the outset, several morphological and body composition differences, and gonadal differences have been observed between male and female NHPs (Cupp and Uemura 1981). Further, rhesus macaques are seasonal breeders and are likely to demonstrate seasonal effects in radiation response. In these long-term survivors, NHPs present with testicular damage that persists at 6.5 Gy and higher but maintain a physiological level of testosterone, since the hormone is produced by the interstitial cells rather than the germ cells. In contrast, female NHP survivors present with normal, cyclic morphology up to 7.2 Gy of exposure, but show a total loss of oocytes at 10 Gy PBI. In females, since oocyte maturation and follicular development are required for estrogen production, a complete absence of oocytes results in estrogen deficiency (radiation-induced menopause). In general, females present lower morbidities than males in relation to radiation dose; however, this difference is not statistically significant. Female NHPs had a significantly greater risk of being underweight (<12% body fat) weights than age-matched males (p = 0.0013). Irradiated males also present with a higher incidence of diabetes compared to females (p = 0.0007), and osteopenia occurs at significantly higher rates in irradiated females than in irradiated males (p < 0.0001). While radiation effects were evident for cardiovascular, pulmonary, renal, brain, ocular, and neoplastic endpoints, no sex differences were apparent. In conclusion, some sex differences are noted; however, females are severely underrepresented, and these initial data indicate the need for more female subjects to develop a balanced view of these effects.
‘Organs on a chip’ platforms for modeling sex-specific differences in human pathology and physiology (Gordana Vunjak-Novakovic).
Organs-on-a-chip (OOC) represent a practical approach to screen and interrogate the Mechanism of Action (MOA) of MCMs. OOC approaches intend to help address challenges in translating drug studies from pre-clinical models to patients. Small rodent models often fail to fully recapitulate human physiology since they cannot capture all the individual differences due to sex, age, ethnicity, state of disease, and other biological variables. By converging stem cell biology with advances in tissue engineering and material science to simulate organ physiology in vivo, OOC provides an experimental platform to investigate human pathophysiology (Leung et al. 2022). This approach can be individualized by using induced pluripotent stem cells (iPSC) derived from somatic cells obtained from a blood sample, which can be differentiated into any lineage to capture organ function and tissue interactions. For MCM testing, desirable characteristics for an OOC platform include a modular and configurable platform (to assemble the tissues of interest and link them in a desired order), biological specificity (to conduct individualized studies), stable and mature phenotypes for the duration of a study (to recapitulate human physiology), tissue connectivity by vascular perfusion (to allow tissue communication), and functional readouts in real-time (to conduct longitudinal and dynamic studies) (Ronaldson-Bouchard and Vunjak-Novakovic 2018). Using a heart muscle OOC, researchers have conducted sex-specific studies that recapitulate the clinically observed differences in males and females in response to cardiac injury and treatments (Ronaldson-Bouchard et al. 2018; Tamargo et al. 2021; Lock et al. 2022; Tavakol et al. 2022).
Studies are underway to increase the stability and duration of a heart-brain-bone marrow OOC with vascular profusion to demonstrate functional tissue viability for up to at least 6 months. While great strides have been made in combining biological principles with engineering designs to address complex biological questions, some of the greatest challenges lie in benchmarking of OOC readouts against clinical data, as well as the interpretation and integration of different types of data.
Session II: Radiation research for MCMs – influence of sex on efficacy
Understanding the sex differences that are innate to a model and those that are present in the natural history of the disease is critical to the foundation of nonclinical testing. In this session, researchers examined the differences observed between males and females in the effectiveness of or response to MCMs.
Is sex a factor in MCM screening? Lessons learned from murine ARS models at the Armed Forces Radiobiology Research Institute (AFRRI) (Sanchita Ghosh).
AFRRI recapitulated the sex differences discussed during Session 1 in their C57BL/6 TBI model of H-ARS; females were more radioresistant with an LD70/30 of 8.4 Gy versus 8.1 Gy in males with a dose rate of ~0.6 Gy/min. Using this model, several test articles (TA) have been tested for efficacy as therapeutics for H-ARS. Sex differences were noted in the efficacy of TAs across sexes. For several TAs (not identified due to pre-publication status), females treated at 24 hours after an 8 Gy exposure had higher survival rates compared to their male counterparts. In another study, males treated with a different TA at 24 hours post-TBI had higher survival than females. It is unclear what accounts for the TA sex differences since no differences were noted in complete blood counts, bone marrow proliferative capacity, or serum chemistry between males and females.
To explore this phenomenon, AFRRI conducted pharmacokinetic (PK) studies with a TA that displayed a sex difference in efficacy. Nonirradiated females had a higher plasma concentration of the TA and cleared the compound faster than their nonirradiated male counterparts. Upon irradiation, drug clearance was delayed to the same level in both male and female mice. Metabolism affects sex-based PK variability and is disrupted in humans exposed to ionizing irradiation (Gandhi et al. 2004; Menon et al. 2016). AFRRI hypothesized that the PK sex difference in nonirradiated controls may be due to faster bloodstream absorption in females than males and the difference between nonirradiated and irradiated females may be due to metabolism disruptions from injury.
Sex differences in the search for effective radiation countermeasures (Tyler Beach).
Efficacy studies for Nplate® and other, test articles (names censored) were conducted in a C57BL/6J TBI model at SRI International. Similar to other laboratories, SRI found that untreated female mice were more radioresistant compared to males at equivalent TBI radiation doses. Nplate originally had an indication that covered only idiopathic thrombocytopenia. In 2021, the FDA expanded approval to treat patients acutely exposed to myelosuppressive doses of radiation.12 The product was evaluated in a C57BL/6J TBI (LD70/30) model of H-ARS beginning 24 h after TBI for 1–5 days (Bunin et al. 2020). Females had higher survival than their male counterparts across most dosing schedules; however, the three-day dosing schedule had a lower survival improvement in females. This sex-specific distinction between the dosing schedules was not apparent in the combined sex analysis, where all dosing schedules appeared to confer nearly the same level of survival benefit. Using this same model, another potential MCM conferred a slight, but not statistically significant, survival benefit in the combined sex analysis, but in the sex-specific analysis, the female survival benefit was statistically significant (p < 0.05).
At SRI, the C57L/6J PBI with 2.5% BM-sparing also demonstrated sex differences in MCM efficacy studies where survival was higher in males compared to females; in contrast, C57BL/6J TBI H-ARS model did not show a sex difference. This male-over-female survival benefit also translated to efficacy studies with MCMs, where males had better survival than females in studies testing different dose levels or different dose schedules.
Several hypotheses to explain observed sex differences in MCM efficacy involve inherent sex-specific attributes of the animal (e.g. body size differences) or sex differences observed in response to irradiation rather than the MCM mechanism of action. Only ~10% of all radiation MCMs tested by SRI have shown a survival benefit in only one sex, and combined sex analysis tends to represent the protection benefit for both sexes. However, inclusion of both male and female animals and independent analysis is still critical for MCM testing as it is important to rule out any confounding differences and to select doses and regimens that are optimized across sexes.
Navigating sex differences in a pivotal 60-day survival study in irradiated rhesus monkeys administered Nplate (Dina Andrews).
In 2021 Nplate received FDA approval with adequate and well-controlled efficacy studies conducted in two models, the C57BL/6J mouse and a rhesus macaque TBI model (Bunin et al. 2020; 2023). The FDA stipulated that efficacy for Nplate be demonstrated with and without Neulasta®, since Neulasta was approved in 2015 to treat H-ARS by stimulating recovery of bone marrow cells that develop into neutrophils13 and is now considered, among the other licensed products for H-ARS that may constitute the standard of care for a lethally irradiated patient. To complement the mouse studies described above, Nplate efficacy studies were conducted using an NHP TBI model, in keeping with FDA guidance to use a staggered, two-phase design to ensure alignment with replacement, reduction, and refinement considerations (Hubrecht and Carter 2019). A slight trend of lower female survival was observed in irradiated Nplate-treated groups; however, there were no sex differences detected in the secondary study endpoints, which included recovery from irradiation-induced depletion of platelets, neutrophils, and other hematopoietic cells.
After reviewing the data, the FDA acknowledged the sex difference in survival for the C57BL/6J TBI model, but these data were not incorporated into the final clinical pharmacology modeling, and it did not impact the development timeline. Nplate conferred a statistically significant (p = 0.0003) survival benefit from H-ARS, with or without Neulasta, and it was approved by the FDA in 2021 to increase survival in adult and pediatric patients exposed to myelosuppressive doses of radiation. This approval was a collaborative effort between Amgen Inc., SRI International, Charles River Laboratories, NIAID, and BARDA.
CRI-ARS studies in Yorkshire swine – Wound area kinetics and scores, and cytokines (Jeremy Brower and Stephanie Zalesak-Kravec).
BP-C2 is an immunomodulatory drug in preclinical development by Meabco Inc. as a radioprotective and radiomitigative agent with application in oncology and public health preparedness (Bykov et al. 2018). Sex differences in the effectiveness of BP-C2 were not observed in previous TBI and PBI mouse model studies. LBRI performed CRI studies of BP-C2 effectiveness using 8–10-week-old Yorkshire-Landrace swine. CRI was induced in eight distinct areas of the skin by applying 80 Gy per wound with a Grenz X-ray instrument (dose rate of 49.5 Gy/min) (Eggleston et al. 2000; O’Brien et al. 2014). BP-C2 was applied to wounds post-irradiation daily for 90 days and wounds were imaged every three days to track wound repair. Female and male swine responded differently to the topical application of two BP-C2 dose levels. Moist desquamation is a consequence of skin radiation exposure, where the loss of epithelial barrier integrity and exposure of the dermis results in serious fluid drainage. Females treated with BP-C2 had lower moist desquamation scores, and by day 50 post-irradiation only ~70% of females had scores of ≥ 2, while 100% of males had a score of ≥ 2 within 50 days. In a combined sex analysis, higher wound contraction was seen with 90 days of treatment with the high dose of BP-C2; however, when analyzed separately, females in this group had markedly more wound contraction compared to males. The opposite trend was observed in vehicle-treated controls, where males had greater wound contraction than females.
In parallel, serum cytokine responses from CRI swine were evaluated at the University of Maryland, Baltimore. In the swine, significant sex differences were observed at 90 days for GMCSF (p = 0.0005), IFNγ (p = 0.0003), IL-1α (p = 0.0356), IL-1β (p = 0.0289), IL-1Ra (p = 0.0445), IL-2 (p = 0.0194), and IL-4 (p = 0.0344) in vehicle-treated controls; however, no major sex differences were observed in BP-C2 treated groups when compared to baseline.
Session III: Sex differences in biomarker and biodosimetry development
This session examined male and female differences observed in biodosimetry signatures or assays currently under study to detect biological consequences of radiation exposures. Of specific interest are differences observed in radiation-induced responses due to sex, sex-linked organ specificity (e.g. lung, GI, heart), and other sex-specific confounders. The focused discussion explored these differences in more detail to inform researchers on how to consider and address these differences when advancing a biomarker for biodosimetry.
Sex-based modulation of metabolic response to radiation exposure (Amrita Cheema).
Ionizing radiation can dysregulate physiological processes in the metabolome (small molecules, lipids, and peptides) in a dose-dependent manner, and organ-specific metabolomic biomarkers may predict DEARE before symptoms manifest. Collaborative efforts across several laboratories showed a sex-dependent effect of the upregulation of Activated Protein C (APC) for mitigation of DEARE (Sridharan et al. 2021). Cardiac outcome measures of DEARE (e.g. ejection fraction, mitral valve velocity, collagen deposition, and capillary density) also showed a decrease in female cardiac function. However, microvessel density changes equally across all sexes and strains. All other cardiac function tests corresponded to increased severity of heart dysfunction in female irradiated animals compared to male counterparts.
Urine samples analyzed using high-resolution mass spectrometry also showed sex differences, where male mice were found to have high levels of antioxidants and acetyl-CoA, a primary substrate for cardiomyocyte function (Li et al. 2023). Female mice showed dysregulated sugar metabolism, which can potentially be correlated to mitochondrial impairment. Common changes, such as dysregulation of free fatty acids or hydroxyl cholesterols, were also observed; however, geranyl-PP, a metabolite involved in inflammation and cardiovascular disease, increased in male and female mice 24 hours post-PBI; it remained high in females but dropped to baseline in male mice after one month. Therefore, mitochondrial dysfunction and other dysregulated cardiovascular-specific metabolites may be responsible for the increased severity of cardiovascular-induced injury in females. Urine and blood samples were also collected from Wag/RijCmcr juvenile rats exposed to PBI (hind leg-out model) (Medhora et al. 2019; Fish et al. 2021). Female rats were less radiosensitive than male rats to radiation dose (juvenile rat data is currently under manuscript preparation). Both sexes experienced kidney and lung dysfunction post-irradiation but only kidney injury was correlated with a sex-differential response. Male juvenile BUN values were more elevated than female juvenile rats at death. Metabolomic and lipidomic changes in female rats exhibited metabolism defects similar to mice. Male rats showed changes in nitrogen and nucleotide metabolism, specifically in purine metabolism. Common pathways upregulated 14 days post-PBI were vitamin E and A, glycerophospholipid metabolism, and bile acid biosynthesis. Future studies will aim to increase radiation dose levels for lung studies and validate predictive biomarkers in NHPs and humans.
Sex differences in biomarker discovery and development (Maureen Kane).
A multi-omics approach to dissect the sex differences in irradiated NHPs was conducted at the University of Maryland School of Pharmacy. NHPs (rhesus macaque) were subjected to TBI (cobalt-60; 5–7.5 Gy) in a survival experiment using equal numbers of females and males (Beach et al. 2021). Male survival was higher when compared to females at similar radiation doses. Samples collected were subjected to a global, label-free proteomics approach using liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Jones et al. 2019; Huang et al. 2020). Few sex differences were observed between female-to-male control comparisons. In contrast, female-to-male irradiated samples yielded some upregulated proteins in females which included KIFC1, KCN36, KRT2, HRG, and CRISP3. Comparing control-grouped males and females to irradiated-grouped females and males revealed many upregulated proteins such as CDH5, B2M, BRM, NCAM, SERPINA5 as well as downregulated proteins such as ICAM1, LAMP2, and CNDP1. The pathways influenced by sex and irradiation were acute phase response signaling, complement system, phagosome maturation, IL-6 signaling, and L-DOPA degradation. These NHP data complement similar pathway activation seen in other rodent studies (Jones et al. 2019). Biomarkers that were dysregulated were ITIH3, CP, CRP, and SAA1, consistent with plasma pathways altered in other radiation studies (Huang et al. 2020).
Targeted LC-MS/MS metabolomics was also used to assess changes in plasma, an approach that has been employed in lung, GI, and MCM studies comparing male and female mouse plasma, as well as NHP plasma and heart samples. The initial comparison of male and female control plasma yielded baseline sex differences with greater triacylglycerol (TG) levels in females and greater phosphatidylcholine (PC) levels in males. Fewer differences were observed in the irradiated female and male plasma metabolites, where the differences were potentially muted by irradiation effects. In general, proteomic and metabolomic data showed limited sex differences in control and irradiated animal, which may be promising for a sex-agnostic biomarker approach.
Sex differences and outcomes to radiation: How to address them in biomarker development (Naresh Menon).
ChromoLogic, LLC aims to develop a micro-RNA (miRNA) transcriptomic biomarker diagnostic approach for radiation exposure (Menon et al. 2016; Rogers et al. 2020; 2021a, 2021b). These miRNAs are present in most organisms and are precursors to transcriptomic modulation following irradiation (Jacob et al. 2013; Dinh et al. 2016; Fendler et al. 2016; Menon et al. 2016; Fendler et al. 2017; Aryankalayil et al. 2018; Ostheim et al. 2020). C3H and C57BL/6 mouse strains were used to assess miRNA induction after WTLI (LD50/180). Equally powered numbers for males and females were used, and radiation dose selection was based on a male mice probit (Rogers et al. 2020). Post-irradiation blood samples were drawn to assess circulating miRNA composition, blood counts, respiratory rates, and micro-CT scans. In both strains, micro-CT scans showed irradiation damage in the lung. Irradiation led to significant lung volume decreases (p < 0.05), but no effects were observed based on sex or strain. The survival curves between the mouse strains were slightly different, but no statistical differences within strain sexes were observed, even though the pre-irradiation weight was higher in male mice.
WTLI NHP studies were performed at AltaSciences at LD30/180 (9.8 Gy) and LD50/180 (10.7 Gy) with 6 MV X-ray Linac (Rogers et al. 2021a). The NHP data collection mirrored the parameters collected in mice, except for monthly CT scans. Similarly, NHP lung volume assessed via CT imaging and aerated lung volume decreased with increasing irradiation dose from 9.8 to 10.7 Gy, but no sex differences were observed. While the weight of the animals was not statistically different; females were slightly more radioresistant than males (Rogers et al. 2021b). miR-202–5p and miR-672–3p were more abundant in females, while miR-484 was more abundant in males. Interestingly, miR-202–5p is associated with egg production (Gay et al. 2018) and was differentially expressed in both CH3 and C57BL/6 female mice. miR-672–5p was only expressed in C57BL/6 animals, while miR-34c-3p and miR-672–3p were only expressed in C3H female mice. miR-34c-5p was expressed post-irradiation in both mouse strains, and sex differences were observed on day 2 post-irradiation. In NHPs, no sex differences in circulating miRNA were identified. Future work will assess longitudinal time courses to better assess miRNA sex differences and radiation doses.
Designing small molecule-based biodosimetry assays for early assessment of radiation exposure: what does sex have to do with it? (Evagelia C. Laiakis).
Small molecule metabolomic biomarkers are being studied for early assessment of different types of radiation exposure: gamma or X-ray exposures, internal emitters, neutrons, and very high or low dose rates. In addition, they are also under evaluation for systemic responses, organ- and sex-specific injury. Radiation dose-response studies in male and female C57BL/6 mice exposed to 1–8 Gy was used to develop a 29-biomarker panel in urine and 18 serum biomarkers 24 h post-irradiation (unpublished results). Urine and serum biomarker signatures from serum data were combined (over 270 data points) and analyzed to rank predictors of dose. The outcome was that sex and day after exposure did not outperform baseline variables, suggesting that the same radiation-responsive metabolite signature can be used for both sexes, potentially up to 30 days post-irradiation.
Using Ultra Performance Liquid Chromatography coupled to time-of-flight mass spectrometry and a neutron source for irradiations that mimics the Hiroshima spectrum, 13 urine metabolites and 18 serum metabolites were identified in spot urine samples and serum from cardiac punctures collected at the time of euthanasia (day 1 or 7 post-irradiation) in C57BL/6 male and female mice (Laiakis et al. 2021). While the signatures were distinct in the males in terms of separation of the quality of radiation, the same signature was dampened in females, requiring biosignature enrichment and refinement.
Similarly, in NHPs irradiated with 0–10 Gy, metabolic signatures were different in males and females at 7 days post-exposure (Pannkuk et al. 2015). Although the signal was dampened in females, the trend was in the same direction in both females and males. In patients undergoing TBI protocols, urine collected after a cumulative dose of 3.75 Gy (three fractions of 1.25 Gy in 24 hours) but prior to stem cell transplantation yielded metabolic signatures in two major pathways – the carnitine pathway in the β-oxidation of fatty acids and purine catabolism (Laiakis et al. 2014). Higher levels were found in males and blunted in females similar to observations in rodents and NHPs. Rather than developing different biosignatures for males and females, data were reanalyzed, and it was found that sex is NOT a confounder when a signature or panel is used rather than individual biomarkers. Although there are sex differences in global profiles of radiation biomarkers, these can be eliminated by selection. Similarly, there are differences in expression of individual radiation biomarkers, with females exhibiting dampened responses. Hence, with careful selection of biomarkers, sex differences in metabolic signatures can be avoided and yield a strong predictive model.
Similarities and differences in sex responses for genomic and transcriptomic-based biodosimetry (Sally Amundson).
Cytogenetics approaches focusing on dose rate effects such as fallout scenarios and ultra-high dose rates, with a secondary emphasis on age and sex effects on micronuclei and dicentric expression have been explored. The cytokinesis-blocked micronucleus assay (CBMN) is a triage biodosimetry tool used to measure DNA ejected from the cell during division following chromosomal damage in mitogen-stimulated human lymphocytes (Pujol-Canadell et al. 2020). This assay produces sex-independent dose-response data which fit a linear-quadratic model for exposures to low linear energy transfer radiation and for doses up to 5 Gy, with limited accuracy at higher doses.
Another cytogenetic approach in radiation biodosimetry, the dicentric chromosome assay (DCA) is considered the gold standard for radiation biodosimetry. DCA measures mis-repaired DNA damage and has a quadratic dose-response, like the CBMN assay. The assay is fully automated in multi-well plates and requires only a small fingerstick of blood for analysis (Royba et al. 2019). Similar to the CBMN assay, DCA analysis revealed that dose had a significant effect (p = 0027) on dose reconstruction, indicating the robustness of the cytogenetic approaches, but sex did not.
Transcriptomic studies have also been done using human peripheral blood irradiated ex vivo, with equal numbers of male and female blood donors. When analyzed by sex and dose effects small, differences were found in the lower dose range (Paul and Amundson 2011). For radiation doses <2 Gy, 14 genes were found to be differentially modulated by both radiation dose and sex, with APOBEC3H having the most robust radiation dose-response, and a slightly higher, but non-significant induction in females (p = 0.16). The performance of the previously defined 74-gene signature was unaffected by sex. Using data from multiple microarray studies, an 11-gene signature, validated by qRT-PCR, showed no sex effect.
Rodent biodosimetry studies were originally conducted using irradiated (0–8 Gy) samples from C57BL/6 male mice collected 24 h post-exposure and are now being validated in female mice. To eliminate sex as a confounder, up and down-regulated genes are combined for self-normalization. Using these approaches, genes with the highest correlation to dose showed no effect on sex and had a robust radiation dose construction (Ghandhi et al. 2022). If age is included as a confounder in addition to sex, gene expression in 2-month-old irradiated (4 Gy) males and females are highly similar, while dose reconstruction for older females (24 months) was less accurate, but not significantly different (Broustas et al. 2022).
Session IV: Etiology of sex differences in radiation research outcomes
Genetic, hormonal, and other sources of sex differences influence tissue function and response to radiation exposure. Understanding the biological mechanisms underlying sex differences in tissues and biological systems may inform research on novel targets for biomarker development and therapeutic interventions.
Sex-dependent differences in radiosensitivity of intestinal stem cells (Subhrajit Saha).
Intestinal stem cells (ISCs) are the building block for mucosal epithelial homeostasis and regeneration. In preclinical studies, researchers have shown that the growth of ISCs is enhanced in females, but this does not appear to be estrogen-dependent (Zhou et al. 2018). Studies were undertaken to help rescue Lgr5+ cells from radiation toxicity and provide a benefit after lethal irradiation in both in vivo mouse and ex vivo intestinal organoids (Bhanja et al. 2018). Lgr5+ cells derived from female mice subjected to abdominal irradiation had better regenerative responses than male mice (unpublished results). DNA damage assays using measurement of γ H2AX levels in the C57BL/6 organoids were dependent on sex, with female-derived organoids showing less damage post-irradiation. These sex-dependent differences in intestinal radiosensitivity were consistent with age, with both four- and 12-week females being less radiosensitive following exposure to PBI (2.5% bone marrow-sparing model). To translate these findings, a patient-derived, normal small bowel organoid system was developed to assess radiation sensitivity and for MCM screening. As seen with animals, the female human intestinal organoids (HIOs), derived from surgical resections, exhibited a greater organoid area post-irradiation and had a higher oxygen consumption rate (unpublished). On a molecular level, RNAseq analysis carried out on Lgr5+ cells derived from the HIOs also showed sex-specific differences in the metabolic signature of Lgr5+ ISCs. Moreover, mRNAs related to mitochondrial function are differentially regulated between male and female ISCs suggesting a possible role of mitochondrial biology in sex-specific differences in intestinal epithelial radiosensitivity.
Previous reports showed that maternal input influences radiosensitivity, in which offspring from a C57BL/6 radioresistant maternal lineage had better survival outcomes, whereas offspring from BALB/c radiosensitive maternal lineage were more sensitive to radiation (Zhang et al. 2014). Given that inherited mitochondria (mt) are from the maternal line, their role in radiation resistance was suspected. In summary, pre-clinical in vivo and ex vivo studies show sex-dependent differences in ISC radiosensitivity expression, of mitochondrial function genes, and metabolic signature attributable to mitochondria, which may be possible targets for MCMs. However, more studies are needed to determine the underlying mitochondria biology involved in the radiosensitivity of ISCs. One aspect to explore includes RNA seq data on Lgr5+ human ISCs, which demonstrate sex differences in mitochondrial function.
Researchers have examined the nature of different radiation sensitivities across mouse strains: for example, C57BL/6 mice are radioresistant, and BALB/c are more radiation sensitive (Kallman and Kohn 1956; Grahn and Hamilton 1957; Grahn 1958; Roderick 1963a; 1963b). Survival analysis and intestinal histopathology demonstrated the differences in mucosal radiosensitivity in these mouse strains (unpublished results). Maternal input influences survival where offspring from a C57BL/6 radioresistant maternal lineage had better survival outcomes, whereas offspring from BALB/c radiosensitive maternal lineage were more sensitive to radiation (Zhang et al. 2014). To understand mt effects on GI-ARS, a hybrid embryo containing mt and other cytoplasmic material from one strain and the nucleus from another was created. In these studies, BALBc, C57BL/6, and the very radioresistant FVB mice were crossbred. If BALBc or C57BL/6 mt were introduced, the FVB hybrid progeny became less radioresistant in a PBI 2.5% bone marrow-sparing model. On the other hand, FVB mt reduced intestinal stem cell (ISC) radiosensitivity in C57BL/6 mice exposed to TBI, as demonstrated by increased survival and histology (unpublished). Furthermore, ex vivo GI organoids recapitulated the in vivo work, with progeny organoids derived from combinations of the three strains showing growth patterns consistent with the radioresistance of the female parent strain. In summary, pre-clinical in vivo and ex vivo studies showed ISC sex-dependent radiosensitivity and regenerative response differences. Metabolic signatures also differed between the sexes, with mts representing a novel target for MCMs.
Sex specific hallmarks of age-related inflammation (Vishwa deep Dixit).
The size of the elderly population in the U.S. is increasing, with large differences in the overall life span of males and females (Fund UNP 2012). With an increase in lifespan, low-grade inflammation also increases in a sex-independent manner (Ferrucci et al. 2005). A meta-analysis consisting of 300 studies revealed that adipose tissue is an immunological organ separate from the endocrine and metabolic organs (Tchernof and Despres 2013). In addition, a binary loss of certain cell types (innate cells and macrophages) and expansion in resident B cells happens with increasing age. B cell expansion is increased more drastically in female mice than male mice but is unrelated to endocrine ovarian hormones (Jackson-Jones et al. 2016; Perez-Shibayama et al. 2018; Camell et al. 2019). Overall, proinflammatory B cells increase with age, preferentially in female mice. The role of this inflammation on the incidence of autoimmune disease in females is still undetermined and may impact the radiosensitivity of geriatric female mice.
The role of the thymus in aging and the loss of T cell immunity was also discussed. Adipocytes begin to take over the thymic space with aging at an accelerated pace compared to other organs (Dixit 2010). A clinical trial looking at the extension of life revealed that 14% calorie restriction led to enhancement of thymic T cell production and the response was more prominent in females than males (Spadaro et al. 2022). However, the population size was not powered enough, hence more research is needed to understand these sex differences.
Sex dimorphism in coagulation and platelet biology: implication for radiation (Julia Coleman).
Dimorphisms exist in nature that allow for the determination of sex phenotypically. These drastic dimorphisms also exist in coagulation, where females are more hypercoagulable than males in clot formation, propagation, and strength (Coleman et al. 2019a). A recent study has shown that radiation-induced coagulopathy is very similar to trauma-induced coagulopathy (Kennedy et al. 2016). Exposure to estradiol shortened the time to clot formation (reaction time), increased clot strength, decreased fibrinolysis, increased functional fibrinogen, increased platelet reactivity, and increased peak thrombin in females; similar effects are seen in vivo (Coleman et al. 2019b). Estradiol was also associated with increases in several procoagulant and antifibrinolytic proteins (Coleman et al. 2023). These studies may have important clinical implications for transfusions of female platelets during trauma and understanding the potential survival advantage is critical to ensuring safe and effective treatments during a mass casualty incident.
Session V: Real-life impacts of sex differences
Sex differences can impact radiation exposure outcomes in real-world settings, occupational exposures, and nuclear accidents. Speakers covered what is currently known regarding sex differences in these settings, and how these differences are being considered.
Impact of sex on environmental exposures during space exploration (Robin Elgart).
The National Aeronautics and Space Administration (NASA) Human Research Program studies the effects of different spaceflight stress exposures on humans, such as radiation, isolation and confinement, distance from the earth, microgravity, and austere environments. HRP has already studied simulated spaceflight hazards on Earth, followed by human studies in low earth orbit on the International Space Station, and the next step will be lunar missions. The studies are being done on a limited population, with an equal ratio of men and women, to examine the effects of sex on bone and muscle composition, the immune system, space-associated neuro-ocular syndrome, sensorimotor, cardiovascular, and orthostatic intolerance, carcinogenesis, and MCM efficacy. A sex difference has been observed with orthostatic intolerance, where females are more affected upon return to Earth’s gravity. Hence, research is being done to see if it is possible to mitigate this observed sex difference.
Unfortunately, due to the composition of space radiation (heavy particles), the materials that can be used to shield are limited, therefore radiation exposure is inevitable. In the past, NASA career radiation risk limit prevented female astronauts from serving on spaceflight missions for the same duration as their male counterparts, due to the sex difference in cancer risk integrated into the NASA Space Cancer Risk model. To meet ethical obligations and respect the astronaut’s autonomy, NASA recently changed this number to a dose limit that is the same across sexes and ages. Astronauts are now enabled to decide their own risk tolerance and are not limited by their sex or age. Furthermore, the potential impact of space irradiation on reproductive health and fertility must be considered. NASA currently ensures astronauts are aware of the risk and provides opportunities for them to preserve eggs or sperm before spaceflight to manage the risk of infertility, making sure to encourage sex impartiality and health equality.
Sex disparities in late health effects of Chernobyl (Alla Shapiro).
The Chernobyl disaster resulted in multiple late health implications for survivors related to their radiation exposure. Rates of anxiety, depression, suicide, and alcoholism doubled among Chernobyl evacuees, with even higher levels reported in mothers than their children (United Nations 2006). Among survivors, nonmalignant diseases included cataracts, psychological effects, cardiovascular, and respiratory diseases. Circulatory system diseases such as heart attack, stroke, heart failure, valve disease, and atherosclerosis were noted in clean-up workers (Cardis and Hatch 2011). Risk factors were dependent on the dose and duration of radiation exposure; 6 out of 18 survivors died of sudden cardiac death 20 years later.
Malignant diseases such as thyroid, leukemia, esophageal, lung, and urinary bladder cancers, as well as solid organ tumors were present, especially in Belarus, Russia, and Ukraine, which were severely impacted (Marino and Nunziata 2018; Samet et al. 2018; Leung et al. 2019). Esophageal, lung, and urinary bladder cancer were diagnosed five times more often in males than females. Urinary bladder cancer increased in men with benign prostatic hyperplasia who lived in contaminated areas and were subjected to low doses of radiation. There was an unprecedented increase in thyroid cancer rates in exposed children and adolescents starting eight years after the accident. Thyroid cancer rates were 3 to 4 times higher among females, although males presented a more aggressive form of the disease (Shobab et al. 2022). Finally, chronic lymphocytic leukemia, which was previously not considered to be linked to irradiation, was shown to correlate with radiation exposure (Vrijheid et al. 2008). It is obvious from this small but important set of human data that sex differences are observable in humans following the kind of radiation exposure that is the focus of the meeting. Therefore, even older epidemiological data must be re-analyzed to tease out any other potential sex-determining health outcomes.
An overview of sex differences in non-occupational (i.e. civilian) and occupational radiation exposures (Carol Iddins).
Non-occupational, radiation exposure data is usually derived from medical radiotherapy and medical imaging, unintentional radiological exposures, nuclear incidents, or exposure due to environmental radiological contamination. Occupational exposure data is obtained from worker radiation exposures received during employment in areas including nuclear medicine, nuclear power plants, and radiography. In short, exposure to radiation at low doses or at intentional, targeted doses is not uncommon. Also, acceptable dose guidelines can differ based on the type of worker as well as the targeted body area (e.g. eyes, skin, and hands).14 Ultimately, it is critical to understand potential sex differences to secure the safety of workers and enable informed decision-making, as well as to ensure preparedness for a radiological or nuclear incident.
Reproductive issues after radiation exposure are sex specific. Maternal radiation exposure is of concern since it can impact a developing fetus; however, the effects largely depend on the type of exposure (external, internal radionuclides, etc.). In the case of internal exposure, the effect on the embryo or fetus can differ if the radionuclide passes through the placenta, or if it remains localized to a nearby organ such as the bladder. Timing can also play a role; the first two weeks post-conception are the most critical time, and the embryo or fetus is highly sensitive to radiation. Studies looking at medical imaging of pregnant people with less than 50 mGy have shown no risk for fetal anomalies, growth restriction, or spontaneous abortions (mICRP 2000). An increased incidence of breast cancer in women was shown in the Techa River residents from protracted low-to-moderate doses of radiation (Ostroumova et al. 2008). While there is concern about the potential to pass on germline mutations to children, current evidence from the atomic bomb survival cohort and from Chernobyl survivors suggests that there is no relationship between parental exposure to radiation and the frequency of genetic mutations in offspring (Yeager et al. 2021). Epidemiological follow-ups in these cohorts have not found an increased risk of cancer or non-cancer disease mortality (Ozasa et al. 2018). The testes are also highly radiosensitive, with direct radiation exposure resulting in decreased spermatogenesis, germ cell loss, and Leydig cell dysfunction (De Felice et al. 2019). Decreased spermatogenesis can occur at doses as low as 0.1 Gy, with irreversible gonadal damage occurring at 4 Gy. The testes do not need to be directly in the radiation field to experience spermatogenesis dysfunction; proximal radiation fields can diminish spermatogenesis even if the testes are shielded.
While sex-specific reproductive effects of radiation are reasonably well-defined, the long-term impacts of radiation are unclear. Data from the atomic bomb survivor cohort largely involves cancer risk, which shows a linear increase in solid cancer and mortality in a person at age 70 exposed at age 30. Some sex differences in atomic bomb survivors include an increased risk of female breast and ovarian cancer, but not uterine. However, the atomic bomb survivor cohort lacks data for males between the ages of 20 and 40 at the time of exposure since males of military age were less likely to be at the bombing sites (Thomas et al. 1994). The Million Person Study found an increased risk of lung cancer in male and female workers (Boice et al. 2018). The Mayak workers cohort comprised of individuals who worked at the Mayak nuclear reactors and plutonium production plant showed increased ischemic heart disease in male workers as compared to females, though mortality risk was only significant for females from 1991 to 1995 (Azizova et al. 2015). Overall, there is still a deficit of statistically powered gender/sex studies focused on the non-cancerous effects of ionizing radiation. Nevertheless, there are clear and known radiation-linked reproductive effects and issues that must be taken into consideration.
Discussion
In this workshop, research was presented on sex differences in humans, animal models, and in vitro after radiation exposure. Some underlying pathways such as the Wnt/β-catenin pathway may play a role in these sex-specific etiologies. A recent paper demonstrated that the Wnt/β-catenin pathway is inhibited by estrogen-1 receptor (ESR1), which leads to a lower incidence and progression of liver cancer in females (Bhat et al. 2021). Similarly, a feedforward loop exists where Wnt activation regulates mitochondrial function, which in turn drives Wnt/β-catenin signaling (Delgado-Deida et al. 2020). Since β-catenin transcription is critical in intestinal mucosal crypts, discussions emerged regarding the effect of mitochondria and Lgr5+ stem cell response in females compared to males, given the difference in ESR1 expression levels. Understanding these underlying differences may help unravel H-ARS and GI-ARS sex differences.
In addition, the effects of adipose tissue and calorie restriction (CR) on sex-dependent thymus function were also discussed. Mouse studies show that lifelong CR leads to increased thymic architecture and output as well as improved T-cell reconstitution (Dixit 2010); however, these effects have not been studied in humans. Sex differences are observed in aged male vs. female mice, and older females tend to have higher fat/lean ratios and better survival outcomes (Patterson et al. 2022). As a result, a discussion about how adipose tissue may contribute to radiation protection ensued. Ideas centered on whether adipose cells secrete immune-associated cytokines, but further research is required.
Research has also shown sex-specific benefits to receiving blood or platelet transfusions in severe life-threatening injuries, but this concept has not been demonstrated in humans to date (Coleman et al. 2019a, 2019b). In addition, the effects of progesterone and testosterone on coagulation were also discussed. Progesterone has not shown an effect, but it does have a positive impact on limiting neurotrauma and brain injury inflammation. Testosterone has not been fully evaluated, but studies are underway.
All these underlying effects can have an impact on survival after radiation exposure. As we study the effects of radiation using animal models it is important to understand changes that can impact survival and the study power needed for statistical significance. Since Rhesus macaques are seasonal breeders, hormonal changes (e.g. estrogen) may impact sensitivity to radiation. Interestingly, differences have been noted based on study start; for instance, when comparing control animals, the first Nplate irradiation study with a November start date resulted in an LD50/60 whereas the second cohort with a July start date resulted in an LD15/60. All animals were sourced from the same vendor and quarantined in the same location; all things appeared equal at the study start for each cohort. In general, it is important to know the details of any model and ensure the controls are appropriate to have a successful outcome. Sex differences in cutaneous pig models are also observed, but once again, the underlying nature is unknown. Understanding these differences can be complicated since it may be necessary to obtain serum samples to document the hormone status (e.g. progesterone) of female animals at the time of irradiation. For NHPs, daily vaginal swabs may be needed to determine ovulation status (which can be challenging), so it may be best to use serum samples at the time of irradiation.
The idea of managing sex differences by determining the optimal dose and dosing schedule for each sex was discussed, but this study design could be cost-prohibitive. While sex differences are readily noted in rodents, the data can still be supportive to show overall efficacy. Furthermore, sex differences are less pronounced in NHPs, and it is reasonable to expect that human responses may be more like NHP responses. Therefore, these findings emphasize the importance of having two independent animal models (i.e. small and large) when determining the efficacy of an MCM under the Animal Rule.
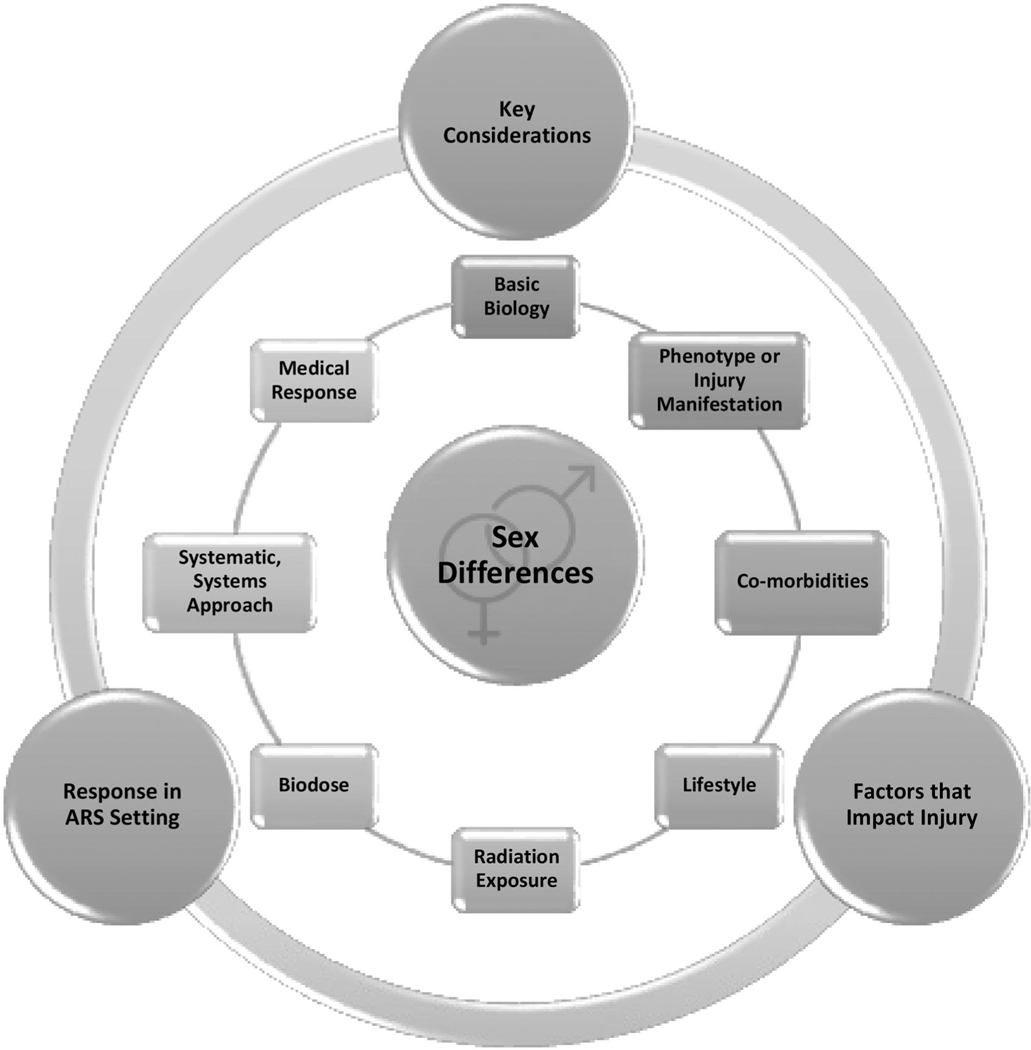
Conclusions
Overview and final thoughts (C. N. Coleman). To summarize a workshop of this complexity and excellence required focusing on a certain perspective. Dr. C. Norman Coleman centered his comments on his radiological and nuclear incidents responsibilities at the ASPR, on preparedness, planning, operations, and response.15 He also discussed biomarkers of radiation injury at the doses applicable for nuclear and radiological incidents.16 Thus, the interest herein is how the information from this workshop is viewed from the key question of ‘What do underline?’ The ASPR team endeavors to answer this question with science-based, ‘just-in-time’ information for those involved in the preparedness and operational response for the potentially no-notice nuclear/radiological mass casualty incident (Coleman et al. 2022).
The utility of this information from an operational response has much broader implications. First and foremost, the need for studying sex differences in biology is important, as presented by Janine Austin Clayton, NIH17 and Kaveeta Vasisht, FDA,18 which can be summarized in a few words: ‘It’s about time this was done’. The workshop presentations provide robust details about sex differences in radiation response and the general impression is that there is: a) much to learn in understanding the impact of sex differences on radiation injury, b) a need for developing biomarkers for triage, c) a potential benefit to developing interventions that incorporate this knowledge, d) the need to understand how the many confounders of life may lead to a sex difference, and e) an opportunity to use the improved knowledge so that sex differences are appropriately used – or not – in the medical management of whole-body, single-exposure to radiation. Rigorous studies were presented across various animal species and systems, demonstrating differences in animal models, biomarkers, and real-world scenarios.
Radiation is complex and results in multi-organ injuries, so the differences can be dependent on the dose, source, and type of radiation exposure. Hence, it is best to examine how the research can ultimately impact the medical response (METREPOL H; N; G; C severity grading system) (Fliedner et al. 2008). Figure 1 breaks down the information into 8 topic areas and 3 ways to consider each one: 1. key considerations; 2. factors that impact injury; and 3. response in the ARS setting or how this state-of-the-science would be used.
Figure 1.
Sex difference considerations can impact the outcome of radiation effects and response in many ways, from basic biology (i.e. genetics, pathways), phenotype (natural history of the disease), co-morbidities, lifestyle, radiation exposure, biodose, systematic/systems approach (i.e. individualized response), through medical response. Overall, these areas can be broken down into subcategories: 1) key considerations; 2) factors that impact injury; and 3) response in an ARS setting - how this state-of-the-science would be used today. Ultimately, this information can be used to inform radiation research and medical response to radiation exposure.
For basic biology, it is important to understand the underlying genetic, epigenetic, biochemical, and structural pathways that affect cellular and tissue mechanisms that then lead to sex differences. This information can enable targeted investigations to improve medical management. The phenotype is a physical manifestation of the disease that allows for early diagnosis of an injury or early intervention before an injury cascade begins. It is important to understand the natural history of the disease in animal models and how it impacts the medical treatment decision process. Added factors such as co-morbidities and/or lifestyle choices can influence the extent of these sex differences and affect decision-making. These can include the influence of obesity, smoking, diet, stress, auto-immune diseases, exercise, etc.
The type of radiation exposure depends on the dose, type of irradiation, dose rate, and shielding. This can be further impacted by a combined injury and the ARS setting (e.g. mass casualty, small or industrial incident). Ultimately, biodose or biomarker kinetics must be considered when elucidating the biology of sex differences. The time course over which the biomarker is useful in medical management will define the type of assay needed. A biomarker that can be examined days or weeks after exposure could be sent to a central or specialized laboratory. Small-sized incidents are managed much differently than in a mass casualty setting, where specialized laboratory tests would be limited in the short term due to overwhelming numbers of samples to be tested. Ultimately, the utility of any biological difference based on sex differences will be subject to use by a medical decision-maker. For example, certain dose ranges, such as low and very high doses, will likely lead to immediate medical management decisions based on dose estimates alone, thus sex differences may not likely be a significant factor in the initial decision-making.
For the dose ranges where MCMs and other interventions may be critical for mitigating life-threatening radiation injuries (e.g. bone marrow cytokines and possibly bone marrow transplantation), decisions will likely be made using a systematic/systems approach applied to each individual as the clinical course plays out. Ultimately, a medical response for a nuclear or large-sized radiological incident depends on preparedness (Koerner et al. 2014). For a nuclear detonation, an immediate robust response is critical within the first hour; hence, the effectiveness of the incident management will depend on the preparedness of the public, healthcare systems, and the response of governmental agencies. The availability of resources and a timely operational approach will help determine if and where resource scarcity will lead to crisis standards of care situations (Coleman et al. 2022) and how rapidly one could emerge from such a challenging situation.
While uncertainty in estimating an individual’s biodose is expected, biodose will be used for deciding triage and medical decisions (Koerner et al. 2014). Therefore, reliable laboratory assays that account for sex differences will be essential for optimal decision-making, but it must be emphasized that these data only augment clinical decision-making. Therefore, these assays should be as informative as possible, recognizing that the medical management for each person will change as their medical course evolves and as available resources improve. This workshop showcased potential areas that need further exploration, such as the lack of complete understanding about underlying reasons for sex differences and the need for well-powered studies. It is the hope of the authors that this meeting report provides an important contribution to radiation biology, increasing awareness and knowledge of the impact of sex on radiation injury and management.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Department of Health and Human Services or its components, including NIH, BARDA, or FDA. Many thanks to RNCP/NIAID colleagues David Cassatt, Carmen Rios, and Thomas Winters for your support and review of this manuscript. Thank you to the speakers that made this meeting a success and for their review of the report.
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 USC. 105, no copyright protection is available for such works under US Law.
Funding
This research received no external funding.
Footnotes
Disclosure statement
The authors report there are no competing interests to declare.
References
- Aryankalayil MJ, Chopra S, Makinde A, Eke I, Levin J, Shankavaram U, MacMillan L, Vanpouille-Box C, Demaria S, Coleman CN. 2018. Microarray analysis of miRNA expression profiles following whole body irradiation in a mouse model. Biomarkers. 23(7):689–703. eng. doi: 10.1080/1354750X.2018.1479771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizova TV, Grigoryeva ES, Haylock RG, Pikulina MV, Moseeva MB. 2015. Ischaemic heart disease incidence and mortality in an extended cohort of Mayak workers first employed in 1948–1982. Br J Radiol. 88(1054):20150169. doi: 10.1259/bjr.20150169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach T, Authier S, Javitz HS, Wong K, Bakke J, Gahagen J, Bunin DI, Chang PY. 2021. Total body irradiation models in NHPs – consideration of animal sex and provision of supportive care to advance model development. Int J Radiat Biol. 97(2):126–130. doi: 10.1080/09553002.2021.1844335 [DOI] [PubMed] [Google Scholar]
- Becker JB, Prendergast BJ, Liang JW. 2016. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 7(1):34. doi: 10.1186/s13293-016-0087-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK. 2018. Inclusion of females does not increase variability in rodent research studies. Curr Opin Behav Sci. 23:143–149. doi: 10.1016/j.cobeha.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanja P, Norris A, Gupta-Saraf P, Hoover A, Saha S. 2018. BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury. Stem Cell Res Ther. 9(1):26. eng. doi: 10.1186/s13287-017-0763-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M, Pasini E, Pastrello C, Angeli M, Baciu C, Abovsky M, Coffee A, Adeyi O, Kotlyar M, Jurisica I. 2021. Estrogen receptor 1 inhibition of WNT/beta-catenin signaling contributes to sex differences in hepatocarcinogenesis. Front Oncol. 11:777834. doi: 10.3389/fonc.2021.777834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice JD Jr., Ellis ED, Golden AP, Girardi DJ, Cohen SS, Chen H, Mumma MT, Shore RE, Leggett RW. 2018. The past informs the future: An overview of the million worker study and the Mallinckrodt Chemical Works cohort. Health Phys. 114(4):381–385. doi: 10.1097/HP.0000000000000825 [DOI] [PubMed] [Google Scholar]
- Broustas CG, Shuryak I, Duval AJ, Amundson SA. 2022. Effect of age and sex on gene expression-based radiation biodosimetry using mouse peripheral blood. bioRxiv.2022.2010.2027.514053. [DOI] [PMC free article] [PubMed]
- Bunin DI, Bakke J, Green CE, Javitz HS, Fielden M, Chang PY. 2020. Romiplostim (Nplate; R) as an effective radiation countermeasure to improve survival and platelet recovery in mice. Int J Radiat Biol. 96(1):145–154. doi: 10.1080/09553002.2019.1605465 [DOI] [PubMed] [Google Scholar]
- Bunin DI, Javitz HS, Gahagen J, Bakke J, Lane JH, Andrews DA, Chang PY. 2023. Survival and hematologic benefits of romiplostim after acute radiation exposure supported FDA approval under the animal rule. Int J Radiat Oncol Biol Phys. 117(3):705–717. doi: 10.1016/j.ijrobp.2023.05.008 [DOI] [PubMed] [Google Scholar]
- Bykov VN, Drachev IS, Kraev SY, Maydin MA, Gubareva EA, Pigarev SE, Anisimov VN, Baldueva IA, Fedoros EI, Panchenko AV. 2018. Radioprotective and radiomitigative effects of BP-C2, a novel lignin-derived polyphenolic composition with ammonium molybdate, in two mouse strains exposed to total body irradiation. Int J Radiat Biol. 94(2):114–123. doi: 10.1080/09553002.2018.1416204 [DOI] [PubMed] [Google Scholar]
- Camell CD, Gunther P, Lee A, Goldberg EL, Spadaro O, Youm YH, Bartke A, Hubbard GB, Ikeno Y, Ruddle NH, et al. 2019. Aging induces an NLRP3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 30(6):1024–1039 e1026. doi: 10.1016/j.cmet.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardis E, Hatch M. 2011. The Chernobyl accident–an epidemiological perspective. Clin Oncol (R Coll Radiol). 23(4):251–260. doi: 10.1016/j.clon.2011.01.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple RH, Hu T, Tseng Y-J, Liu L, Kitano A, Luu V, Hoegenauer KA, Iwawaki T, Li Q, Nakada D. 2018. ERα promotes murine hematopoietic regeneration through the Ire1α-mediated unfolded protein response. Elife. 7:e31159. doi: 10.7554/eLife.31159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou DB, Frismantas V, Milton Y, David R, Pop-Damkov P, Ferguson D, MacDonald A, Vargel Bolukbasi O, Joyce CE, Moreira Teixeira LS, et al. 2020. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat Biomed Eng. 4(4): 394–406. doi: 10.1038/s41551-019-0495-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA. 2016. Studying both sexes: a guiding principle for biomedicine. Faseb J. 30(2):519–524. doi: 10.1096/fj.15-279554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CN, Cliffer KD, DiCarlo AL, Homer MJ, Moyer BR, Loelius SG, Tewell AW, Bader JL, Koerner JF. 2022. Preparedness for a ‘no-notice’ mass-casualty incident: a nuclear detonation scenario. Int J Radiat Biol. 98(5):873–877. doi: 10.1080/09553002.2021.2013573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JR, Moore EE, Kelher MR, Samuels JM, Cohen MJ, Sauaia A, Banerjee A, Silliman CC, Peltz ED. 2019a. Female platelets have distinct functional activity compared with male platelets: Implications in transfusion practice and treatment of trauma-induced coagulopathy. J Trauma Acute Care Surg. 87(5):1052–1060. doi: 10.1097/TA.0000000000002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JR, Moore EE, Samuels JM, Cohen MJ, Sauaia A, Sumislawski JJ, Ghasabyan A, Chandler JG, Banerjee A, Silliman CC, et al. 2019b. Trauma resuscitation consideration: Sex matters. J Am Coll Surg. 228(5):760–768 e761. doi: 10.1016/j.jamcollsurg.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JR, Moore EE, Sauaia A, Samuels JM, Moore HB, Ghasabyan A, Chandler JG, Swope ML, Fleming CD, Banerjee A, et al. 2020. Untangling sex dimorphisms in coagulation: Initial steps toward precision medicine for trauma resuscitation. Ann Surg. 271(6):e128–e130. doi: 10.1097/SLA.0000000000003726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JR, Moore EE, Schmitt L, Hansen K, Dow N, Freeman K, Cohen MJ, Silliman CC. 2023. Estradiol provokes hypercoagulability and affects fibrin biology: a mechanistic exploration of sex dimorphisms in coagulation. J Trauma Acute Care Surg. 94(2):179–186. doi: 10.1097/TA.0000000000003822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp CJ, Uemura E. 1981. Body and organ weights in relation to age and sex in Macaca mulatta. J Med Primatol. 10(2–3):110–123. eng. doi: 10.1159/000460061 [DOI] [PubMed] [Google Scholar]
- Dabjan MB, Buck CM, Jackson IL, Vujaskovic Z, Marples B, Down JD. 2016. A survey of changing trends in modelling radiation lung injury in mice: bringing out the good, the bad, and the uncertain. Lab Invest. 96(9):936–949. doi: 10.1038/labinvest.2016.76 [DOI] [PubMed] [Google Scholar]
- Daniel AR, Lee CL, Oh P, Luo L, Ma Y, Kirsch DG. 2020. Inhibiting glycogen synthase kinase-3 mitigates the hematopoietic acute radiation syndrome in a sex- and strain-dependent manner in mice. Health Phys. 119(3):315–321. doi: 10.1097/HP.0000000000001243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F, Marchetti C, Marampon F, Cascialli G, Muzii L, Tombolini V. 2019. Radiation effects on male fertility. Andrology. 7(1):2–7. doi: 10.1111/andr.12562 [DOI] [PubMed] [Google Scholar]
- Delgado-Deida Y, Alula KM, Theiss AL. 2020. The influence of mitochondrial-directed regulation of Wnt signaling on tumorigenesis. Gastroenterol Rep (Oxf). 8(3):215–223. doi: 10.1093/gastro/goaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TK, Fendler W, Chałubińska-Fendler J, Acharya SS, O’Leary C, Deraska PV, D’Andrea AD, Chowdhury D, Kozono D. 2016. Circulating miR-29a and miR-150 correlate with delivered dose during thoracic radiation therapy for non-small cell lung cancer. Radiat Oncol. 11(1):61. eng. doi: 10.1186/s13014-016-0636-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit VD. 2010. Thymic fatness and approaches to enhance thymopoietic fitness in aging. Curr Opin Immunol. 22(4):521–528. doi: 10.1016/j.coi.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleston TA, Roach WP, Mitchell MA, Smith K, Oler D, Johnson TE. 2000. Comparison of two porcine (Sus scrofa domestica) skin models for in vivo near-infrared laser exposure. Comp Med. 50(4):391–397. [PubMed] [Google Scholar]
- Fananas-Baquero S, Orman I, Becerra Aparicio F, Bermudez de Miguel S, Garcia Merino J, Yanez R, Fernandez Sainz Y, Sanchez R, Dessy-Rodriguez M, Alberquilla O, et al. 2021. Natural estrogens enhance the engraftment of human hematopoietic stem and progenitor cells in immunodeficient mice. Haematologica. 106(6):1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendler W, Madzio J, Kozinski K, Patel K, Janikiewicz J, Szopa M, Tracz A, Borowiec M, Jarosz-Chobot P, Mysliwiec M, et al. 2016. Differential regulation of serum microRNA expression by HNF1β and HNF1α transcription factors. Diabetologia. 59(7):1463–1473. eng. doi: 10.1007/s00125-016-3945-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendler W, Malachowska B, Meghani K, Konstantinopoulos PA, Guha C, Singh VK, Chowdhury D. 2017. Evolutionarily conserved serum microRNAs predict radiation-induced fatality in nonhuman primates. Sci Transl Med. 9(379):eaal2408. doi: 10.1126/scitranslmed.aal2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. 2005. The origins of age-related proinflammatory state. Blood. 105(6):2294–2299. doi: 10.1182/blood-2004-07-2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish BL, MacVittie TJ, Gao F, Narayanan J, Gasperetti T, Scholler D, Sheinin Y, Himburg HA, Hart B, Medhora M. 2021. Rat models of partial-body irradiation with bone marrow-sparing (leg-out PBI) designed for FDA approval of countermeasures for mitigation of acute and delayed injuries by radiation. Health Phys. 121(4):419–433. doi: 10.1097/HP.0000000000001444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliedner TM, Powles R, Sirohi B, Niederwieser D. 2008. Radiologic and nuclear events: the METREPOL severity of effect grading system. Blood. 111(12):5757–5758. author reply 5758–5759. doi: 10.1182/blood-2008-04-150243 [DOI] [PubMed] [Google Scholar]
- Fund UNP. 2012. Ageing in the twenty-first century: A celebration and a challenge. United Nations Population Fund [Google Scholar]
- Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. 2004. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 44(1):499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453 [DOI] [PubMed] [Google Scholar]
- Gasperetti T, Miller T, Gao F, Narayanan J, Jacobs ER, Szabo A, Cox GN, Orschell CM, Fish BL, Medhora M. 2021. Polypharmacy to mitigate acute and delayed radiation syndromes. Front Pharmacol. 12:741485. doi: 10.3389/fphar.2021.741485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay S, Bugeon J, Bouchareb A, Henry L, Delahaye C, Legeai F, Montfort J, Le Cam A, Siegel A, Bobe J, et al. 2018. MiR-202 controls female fecundity by regulating medaka oogenesis. PLoS Genet. 14(9):e1007593. eng. doi: 10.1371/journal.pgen.1007593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandhi SA, Shuryak I, Ponnaiya B, Wu X, Garty G, Morton SR, Kaur SP, Amundson SA. 2022. Cross-platform validation of a mouse blood gene signature for quantitative reconstruction of radiation dose. Sci Rep. 12(1):14124. eng. doi: 10.1038/s41598-022-18558-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs A, Gupta P, Mali B, Poirier Y, Gopalakrishnan M, Newman D, Zodda A, Down JD, Serebrenik AA, Kaytor MD, et al. 2023. A C57L/J mouse model of the delayed effects of acute radiation exposure in the context of evolving multi-organ dysfunction and failure after total-body irradiation with 2.5% bone marrow sparing. Radiat Res. 199(4):319–335. doi: 10.1667/RADE-22-00178.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn D. 1958. Acute radiation response of mice from a cross between radiosensitive and radioresistant strains. Genetics. 43(5):835–843. doi: 10.1093/genetics/43.5.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn D, Hamilton KF. 1957. Genetic variation in the acute lethal response of four inbred mouse strains to whole body x-irradiation. Genetics. 42(3):189–198. doi: 10.1093/genetics/42.3.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari S, Babor TF, De Castro P, Tort S, Curno M. 2016. Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 1(1):2. doi: 10.1186/s41073-016-0007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Wu YW, Kwek P, van den Buuse M. 2012. Modulatory effects of sex steroid hormones on brain-derived neurotrophic factor-tyrosine kinase B expression during adolescent development in C57Bl/6 mice. J Neuroendocrinol. 24(5):774–788. doi: 10.1111/j.1365-2826.2012.02277.x [DOI] [PubMed] [Google Scholar]
- Holdcroft A. 2007. Gender bias in research: how does it affect evidence based medicine? J R Soc Med. 100(1):2–3. doi: 10.1177/014107680710000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yu J, Liu T, Defnet AE, Zalesak S, Farese AM, MacVittie TJ, Kane MA. 2020. Proteomics of non-human primate plasma after partial-body radiation with minimal bone marrow sparing. Health Phys. 119(5):621–632. eng. doi: 10.1097/HP.0000000000001350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubrecht RC, Carter E. 2019. The 3Rs and humane experimental technique: Implementing change. Animals (Basel). 9(10):754. doi: 10.3390/ani9100754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui SK, Sharkey L, Kidder LS, Zhang Y, Fairchild G, Coghill K, Xian CJ, Yee D. 2012. The influence of therapeutic radiation on the patterns of bone marrow in ovary-intact and ovariectomized mice. PLoS One. 7(8):e42668. doi: 10.1371/journal.pone.0042668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson IL, Baye F, Goswami CP, Katz BP, Zodda A, Pavlovic R, Gurung G, Winans D, Vujaskovic Z. 2017. Gene expression profiles among murine strains segregate with distinct differences in the progression of radiation-induced lung disease. Dis Model Mech. 10(4): 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Jones LH, Duncan SM, Magalhaes MS, Campbell SM, Maizels RM, McSorley HJ, Allen JE, Benezech C. 2016. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat Commun. 7(1):12651. doi: 10.1038/ncomms12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob NK, Cooley JV, Yee TN, Jacob J, Alder H, Wickramasinghe P, Maclean KH, Chakravarti A. 2013. Identification of sensitive serum microRNA biomarkers for radiation biodosimetry. PLoS One. 8(2): e57603. eng. doi: 10.1371/journal.pone.0057603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili-Firoozinezhad S, Prantil-Baun R, Jiang A, Potla R, Mammoto T, Weaver JC, Ferrante TC, Kim HJ, Cabral JMS, Levy O, et al. 2018. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis. 9(2): 223. doi: 10.1038/s41419-018-0304-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Alloush J, Sellamuthu R, Chua HL, MacVittie TJ, Orschell CM, Kane MA. 2019. Effect of sex on biomarker response in a mouse model of the hematopoietic acute radiation syndrome. Health Phys. 116(4):484–502. doi: 10.1097/HP.0000000000000961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallman RF, Kohn HI. 1956. The influence of strain on acute X-ray lethality in the mouse: LD50 and death rate studies. Radiat Res. 5(4):309–317. doi: 10.2307/3570420 [DOI] [PubMed] [Google Scholar]
- Kamimura I, Watarai A, Takamura T, Takeo A, Miura K, Morita H, Mogi K, Kikusui T. 2019. Gonadal steroid hormone secretion during the juvenile period depends on host-specific microbiota and contributes to the development of odor preference. Dev Psychobiol. 61(5): 670–678. doi: 10.1002/dev.21827 [DOI] [PubMed] [Google Scholar]
- Kennedy AR, Maity A, Sanzari JK. 2016. A review of radiation-induced coagulopathy and new findings to support potential prevention strategies and treatments. Radiat Res. 186(2):121–140. doi: 10.1667/RR14406.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner JF, Coleman CN, Murrain-Hill P, FitzGerald DJ, Sullivan JM. 2014. The medical decision model and decision maker tools for management of radiological and nuclear incidents. Health Phys. 106(6):645–651. doi: 10.1097/HP.0000000000000053 [DOI] [PubMed] [Google Scholar]
- Laiakis EC, Canadell MP, Grilj V, Harken AD, Garty GY, Brenner DJ, Smilenov L, Fornace AJ. 2021. Small molecule responses to sequential irradiation with neutrons and photons for biodosimetry applications: An initial assessment. Radiat Res. 196(5):468–477. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Mak TD, Anizan S, Amundson SA, Barker CA, Wolden SL, Brenner DJ, Fornace AJ. Jr. 2014. Development of a metabolomic radiation signature in urine from patients undergoing total body irradiation. Radiat Res. 181(4):350–361. eng. doi: 10.1667/RR13567.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CM, de Haan P, Ronaldson-Bouchard K, Kim G-A, Ko J, Rho HS, Chen Z, Habibovic P, Jeon NL, Takayama S, et al. 2022. A guide to the organ-on-a-chip. Nat Rev Methods Primers. 2(1):33. doi: 10.1038/s43586-022-00118-6 [DOI] [Google Scholar]
- Leung KM, Shabat G, Lu P, Fields AC, Lukashenko A, Davids JS, Melnitchouk N. 2019. Trends in solid tumor incidence in ukraine 30 years after chernobyl. J Glob Oncol. 5:1–10. doi: 10.1200/JGO.19.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bansal S, Sridharan V, Bansal S, Jayatilake MM, Fernandez JA, Griffin JH, Boerma M, Cheema AK. 2023. Urinary metabolomics for the prediction of radiation-induced cardiac dysfunction. Metabolites. 13(4):525. doi: 10.3390/metabo13040525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP, Brenner AV, Grant EJ, Sugiyama H, Preston DL, Sakata R, Cologne J, Velazquez-Kronen R, Utada M, Mabuchi K, et al. 2022. Age effects on radiation response: summary of a recent symposium and future perspectives. Int J Radiat Biol. 98(11):1673–1683. doi: 10.1080/09553002.2022.2063962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock R, Al Asafen H, Fleischer S, Tamargo M, Zhao Y, Radisic M, Vunjak-Novakovic G. 2022. A framework for developing sex-specific engineered heart models. Nat Rev Mater. 7(4):295–313. doi: 10.1038/s41578-021-00381-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino F, Nunziata L. 2018. Long-term consequences of the chernobyl radioactive fallout: An exploration of the aggregate data. Milbank Q. 96(4):814–857. doi: 10.1111/1468-0009.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Gasperetti T, Narayanan J, Khan AH, Jacobs ER, Fish BL. 2019. Delayed effects of acute radiation exposure (DEARE) in juvenile and old rats: Mitigation by lisinopril. Health Phys. 116(4):529–545. doi: 10.1097/HP.0000000000000920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon N, Rogers CJ, Lukaszewicz AI, Axtelle J, Yadav M, Song F, Chakravarti A, Jacob NK. 2016. Detection of acute radiation sickness: A feasibility study in non-human primates circulating miRNAs for triage in radiological events. PLoS One. 11(12):e0167333. eng. doi: 10.1371/journal.pone.0167333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon SS, Uppal M, Randhawa S, Cheema MS, Aghdam N, Usala RL, Ghosh SP, Cheema AK, Dritschilo A. 2016. Radiation metabolomics: Current status and future directions. Front Oncol. 6:20. doi: 10.3389/fonc.2016.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- mICRP. 2000. Pregnancy and medical radiation. ICRP Publication, 84 Ann. ICRP. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chanda ML. 2005. The case for the inclusion of female subjects in basic science studies of pain. Pain. 117(1–2):1–5. doi: 10.1016/j.pain.2005.06.020 [DOI] [PubMed] [Google Scholar]
- Moulder JE, Fish BL, Cohen EP, Flowers JB, Medhora M. 2019. Effects of diet on late radiation injuries in rats. Health Phys. 116(4):566–570. doi: 10.1097/HP.0000000000000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, Morrison SJ. 2014. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 505(7484):555–558. doi: 10.1038/nature12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences Engineering and Medicine. 2022. Measuring sex, gender identity, and sexual orientation. Washington (DC): The National Academies Press. p. 2022937869. [PubMed] [Google Scholar]
- National Research Council (US) Committee on Health Effects of Exposure to Low Levels of Ionizing Radiations (BEIR VII). 1998. Health effects of exposure to low levels of ionizing radiations: Time for reassessment. Washington (DC): National Academies Press (US). [PubMed] [Google Scholar]
- O’Brien K, Bhatia A, Tsen F, Chen M, Wong AK, Woodley DT, Li W. 2014. Identification of the critical therapeutic entity in secreted Hsp90alpha that promotes wound healing in newly re-standardized healthy and diabetic pig models. PLoS One. 9(12):e113956. doi: 10.1371/journal.pone.0113956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro H, McDonald JG, Zhao Z, Umetani M, Shaul PW, Morrison SJ. 2017. 27-Hydroxycholesterol induces hematopoietic stem cell mobilization and extramedullary hematopoiesis during pregnancy. J Clin Invest. 127(9):3392–3401. doi: 10.1172/JCI94027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostheim P, Haupt J, Herodin F, Valente M, Drouet M, Majewski M, Port M, Abend M. 2020. miRNA expression patterns differ by total- or partial-body radiation exposure in baboons. Radiat Res. 194(6): 579–588. doi: 10.1667/RR15450.1 [DOI] [PubMed] [Google Scholar]
- Ostroumova E, Preston DL, Ron E, Krestinina L, Davis FG, Kossenko M, Akleyev A. 2008. Breast cancer incidence following low-dose rate environmental exposure: Techa River Cohort, 1956–2004. Br J Cancer. 99(11):1940–1945. doi: 10.1038/sj.bjc.6604775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozasa K, Grant EJ, Kodama K. 2018. Japanese legacy cohorts: The life span study atomic bomb survivor cohort and survivors’ offspring. J Epidemiol. 28(4):162–169. doi: 10.2188/jea.JE20170321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Authier S, Wong K, Fornace AJ. Jr. 2015. Global metabolomic identification of long-term dose-dependent urinary biomarkers in nonhuman primates exposed to ionizing radiation. Radiat Res. 184(2):121–133. eng. doi: 10.1667/rr14091.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AM, Plett PA, Chua HL, Sampson CH, Fisher A, Feng H, Unthank JL, Miller SJ, Katz BP, MacVittie TJ, et al. 2020. Development of a model of the acute and delayed effects of high dose radiation exposure in jackson diversity outbred mice; comparison to inbred C57BL/6 mice. Health Phys. 119(5):633–646. doi: 10.1097/HP.0000000000001344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AM, Sellamuthu R, Plett PA, Sampson CH, Chua HL, Fisher A, Vemula S, Feng H, Katz BP, Tudor G, et al. 2021. Establishing pediatric mouse models of the hematopoietic acute radiation syndrome and the delayed effects of acute radiation exposure. Radiat Res. 195(4):307–323. [DOI] [PubMed] [Google Scholar]
- Patterson AM, Vemula S, Plett PA, Sampson CH, Chua HL, Fisher A, Wu T, Sellamuthu R, Feng H, Katz BP, et al. 2022. Age and sex divergence in hematopoietic radiosensitivity in aged mouse models of the hematopoietic acute radiation syndrome. Radiat Res. 198(3): 221–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Amundson SA. 2011. Gene expression signatures of radiation exposure in peripheral white blood cells of smokers and non-smokers. Int J Radiat Biol. 87(8):791–801. doi: 10.3109/09553002.2011.568574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Shibayama C, Gil-Cruz C, Cheng HW, Onder L, Printz A, Morbe U, Novkovic M, Li C, Lopez-Macias C, Buechler MB, et al. 2018. Fibroblastic reticular cells initiate immune responses in visceral adipose tissues and secure peritoneal immunity. Sci Immunol. 3(26):eaar4539. doi: 10.1126/sciimmunol.aar4539 [DOI] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, Johnson CS, Katz BP, Farese AM, Parker J, et al. 2012. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 103(4):343–355. doi: 10.1097/HP.0b013e3182667309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. 2014. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 40:1–5. doi: 10.1016/j.neubiorev.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Pujol-Canadell M, Perrier JR, Cunha L, Shuryak I, Harken A, Garty G, Brenner DJ. 2020. Cytogenetically-based biodosimetry after high doses of radiation. PLoS One. 15(4):e0228350. eng. doi: 10.1371/journal.pone.0228350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick T. 1963a. Selection for radiation resistance in mice. Genetics. 48(2):205–216. eng. doi: 10.1093/genetics/48.2.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick TH. 1963b. The response of twenty-seven inbred strains of mice to daily doses of whole-body x-irradiation. Radiat Res. 20(4): 631–639. doi: 10.2307/3571354 [DOI] [PubMed] [Google Scholar]
- Rogers CJ, Kyubwa EM, Lukaszewicz AI, Starbird MA, Nguyen M, Copeland BT, Yamada-Hanff J, Menon N. 2021a. Observation of unique circulating miRNA signatures in non-human primates exposed to total-body vs. whole thorax lung irradiation. Radiat Res. 196(5):547–559. eng. [DOI] [PubMed] [Google Scholar]
- Rogers CJ, Kyubwa EM, Lukaszewicz AI, Yamada-Hanff J, Starbird MA, Miller TA, Phelps AA, Wallack S, Mahendra S, Thrall K, et al. 2021b. Identification of miRNA associated with reduced survival after whole-thorax lung irradiation in non-human primates. Radiat Res. 196(5):510–522. 513. doi: 10.1667/RADE-20-00031.1 [DOI] [PubMed] [Google Scholar]
- Rogers CJ, Lukaszewicz AI, Yamada-Hanff J, Micewicz ED, Ratikan JA, Starbird MA, Miller TA, Nguyen C, Lee JT, Olafsen T, et al. 2020. Identification of miRNA signatures associated with radiation-induced late lung injury in mice. PLoS One. 15(5):e0232411. eng. doi: 10.1371/journal.pone.0232411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G. 2018. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 556(7700):239–243. doi: 10.1038/s41586-018-0016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson-Bouchard K, Vunjak-Novakovic G. 2018. Organs-on-a-Chip: A fast track for engineered human tissues in drug development. Cell Stem Cell. 22(3):310–324. eng. doi: 10.1016/j.stem.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royba E, Repin M, Pampou S, Karan C, Brenner DJ, Garty G. 2019. RABiT-II-DCA: A fully-automated dicentric chromosome assay in multiwell plates. Radiat Res. 192(3):311–323. eng. doi: 10.1667/RR15266.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata R, Preston DL, Brenner AV, Sugiyama H, Grant EJ, Rajaraman P, Sadakane A, Utada M, French B, Cahoon EK, et al. 2019. Radiation-related risk of cancers of the upper digestive tract among japanese atomic bomb survivors. Radiat Res. 192(3):331–344. doi: 10.1667/RR15386.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Berrington de Gonzalez A, Dauer LT, Hatch M, Kosti O, Mettler FA Jr., Satyamitra MM. 2018. Gilbert w. Beebe symposium on 30 years after the chernobyl accident: Current and future studies on radiation health effects. Radiat Res. 189(1):5–18. doi: 10.1667/RR14791.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf GW, Justice JN, Quillen EE, Cline JM. 2023. Resilience, aging, and response to radiation exposure (RARRE) in nonhuman primates: a resource review. Geroscience. 45(6):3371–3379. doi: 10.1007/s11357-023-00812-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydel C. 2021. The missing sex. Nat Biotechnol. 39(3):260–265. doi: 10.1038/s41587-021-00844-4 [DOI] [PubMed] [Google Scholar]
- Sharma GP, Frei A, Fish B, Gasperetti T, Veley D, Szalewski N, Nissen A, Himburg HA. 2023. Biological sex differences in renin angiotensin system enzymes ACE and ACE2 regulate normal tissue response to radiation injury. Front Physiol. 14:1191237. doi: 10.3389/fphys.2023.1191237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobab L, Burman KD, Wartofsky L. 2022. Sex differences in differentiated thyroid cancer. Thyroid. 32(3):224–235. doi: 10.1089/thy.2021.0361 [DOI] [PubMed] [Google Scholar]
- Spadaro O, Youm Y, Shchukina I, Ryu S, Sidorov S, Ravussin A, Nguyen K, Aladyeva E, Predeus AN, Smith SR, et al. 2022. Caloric restriction in humans reveals immunometabolic regulators of health span. Science. 375(6581):671–677. doi: 10.1126/science.abg7292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan V, Johnson KA, Landes RD, Cao M, Singh P, Wagoner G, Hayar A, Sprick ED, Eveld KA, Bhattacharyya A, et al. 2021. Sex-dependent effects of genetic upregulation of activated protein C on delayed effects of acute radiation exposure in the mouse heart, small intestine, and skin. PLoS One. 16(5):e0252142. eng. doi: 10.1371/journal.pone.0252142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamargo MA, Nash TR, Fleischer S, Kim Y, Vila OF, Yeager K, Summers M, Zhao Y, Lock R, Chavez M, et al. 2021. milliPillar: A platform for the generation and real-time assessment of human engineered cardiac tissues. ACS Biomater Sci Eng. 7(11):5215–5229. doi: 10.1021/acsbiomaterials.1c01006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum C, Day D, Matera A. 2017. Age and sex in drug development and testing for adults. Pharmacol Res. 121:83–93. doi: 10.1016/j.phrs.2017.04.027 [DOI] [PubMed] [Google Scholar]
- Tavakol DN, Brown J, Graney P, Palomero T, Ferrando A, Vunjak-Novakovic G. 2022. 3018 – bioengineered human microtissue model of healthy and leukemic bone marrow. Exp Hematol. 111:S53. doi: 10.1016/j.exphem.2022.07.074 [DOI] [Google Scholar]
- Tchernof A, Despres JP. 2013. Pathophysiology of human visceral obesity: an update. Physiol Rev. 93(1):359–404. doi: 10.1152/physrev.00033.2011 [DOI] [PubMed] [Google Scholar]
- Thomas DB, Rosenblatt K, Jimenez LM, McTiernan A, Stalsberg H, Stemhagen A, Thompson WD, Curnen MG, Satariano W, Austin DF. 1994. Ionizing radiation and breast cancer in men (United States). Cancer Causes Control. 5(1):9–14. doi: 10.1007/BF01830721 [DOI] [PubMed] [Google Scholar]
- Thrall KD, Mahendra S, Jackson MK, Jackson W, Farese AM, MacVittie TJ. 2019. A comparative dose-response relationship between sexes for mortality and morbidity of radiation-induced lung injury in the rhesus macaque. Health Phys. 116(3):354–365. doi: 10.1097/HP.0000000000000925 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. 2015. Product development under the animal rule – guidance for industry. Silver Spring (MD): Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. [Google Scholar]
- U.S. Government Accountability Office. 1992. Women’s health: FDA needs to ensure more study of gender differences in prescription drug testing. Washington (DC: ): Government Accountability Office. HRD-93–17. [Google Scholar]
- U.S. Government Accountability Office. 2001. Drug safety: Most drugs withdrawn in recent years had greater health risks for women. Washington (DC): Government Accountability Office. GAO-01–286R. [Google Scholar]
- U.S. Government Accountability Office. 2015. Better oversight needed to help ensure continued progress including women in health research. Washington (DC): Government Accountability Office. [Google Scholar]
- United Nations. 2006. UN report: Chernobyl, the true scale of the accident. Manag Environ Qual. 17(1). doi: 10.1108/meq.2006.08317aaf.001 [DOI] [Google Scholar]
- Velardi E, Tsai JJ, Radtke S, Cooper K, Argyropoulos KV, Jae-Hung S, Young LF, Lazrak A, Smith OM, Lieberman S, et al. 2018. Suppression of luteinizing hormone enhances HSC recovery after hematopoietic injury. Nat Med. 24(2):239–246. doi: 10.1038/nm.4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Cardis E, Ashmore P, Auvinen A, Gilbert E, Habib RR, Malker H, Muirhead CR, Richardson DB, Rogel A, et al. 2008. Ionizing radiation and risk of chronic lymphocytic leukemia in the 15-country study of nuclear industry workers. Radiat Res. 170(5): 661–665. doi: 10.1667/RR1443.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizemann TM, Pardue ML. 2001. Exploring the biological contributions to human health: Does sex matter? Washington (DC): National Academies Press (US). (Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender Differences. [PubMed] [Google Scholar]
- Woitowich NC, Woodruff TK. 2019. Implementation of the NIH sex-inclusion policy: Attitudes and opinions of study section members. J Womens Health (Larchmt). 28(1):9–16. doi: 10.1089/jwh.2018.7396 [DOI] [PubMed] [Google Scholar]
- Yeager M, Machiela MJ, Kothiyal P, Dean M, Bodelon C, Suman S, Wang M, Mirabello L, Nelson CW, Zhou W, et al. 2021. Lack of transgenerational effects of ionizing radiation exposure from the Chernobyl accident. Science. 372(6543):725–729. doi: 10.1126/science.abg2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SB, Maguire D, Zhang M, Tian Y, Yang S, Zhang A, Casey-Sawicki K, Han D, Ma J, Yin L, et al. 2014. Mitochondrial DNA and functional investigations into the radiosensitivity of four mouse strains. Int J Cell Biol. 2014:850460–850468. eng. doi: 10.1155/2014/850460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Davis EA, Li K, Nowak RA, Dailey MJ. 2018. Sex differences influence intestinal epithelial stem cell proliferation independent of obesity. Physiol Rep. 6(13):e13746. eng. doi: 10.14814/phy2.13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker I, Prendergast BJ, Beery AK. 2022. Pervasive neglect of sex differences in biomedical research. Cold Spring Harb Perspect Biol. 14(4):a039156. doi: 10.1101/cshperspect.a039156 [DOI] [PMC free article] [PubMed] [Google Scholar]