Abstract
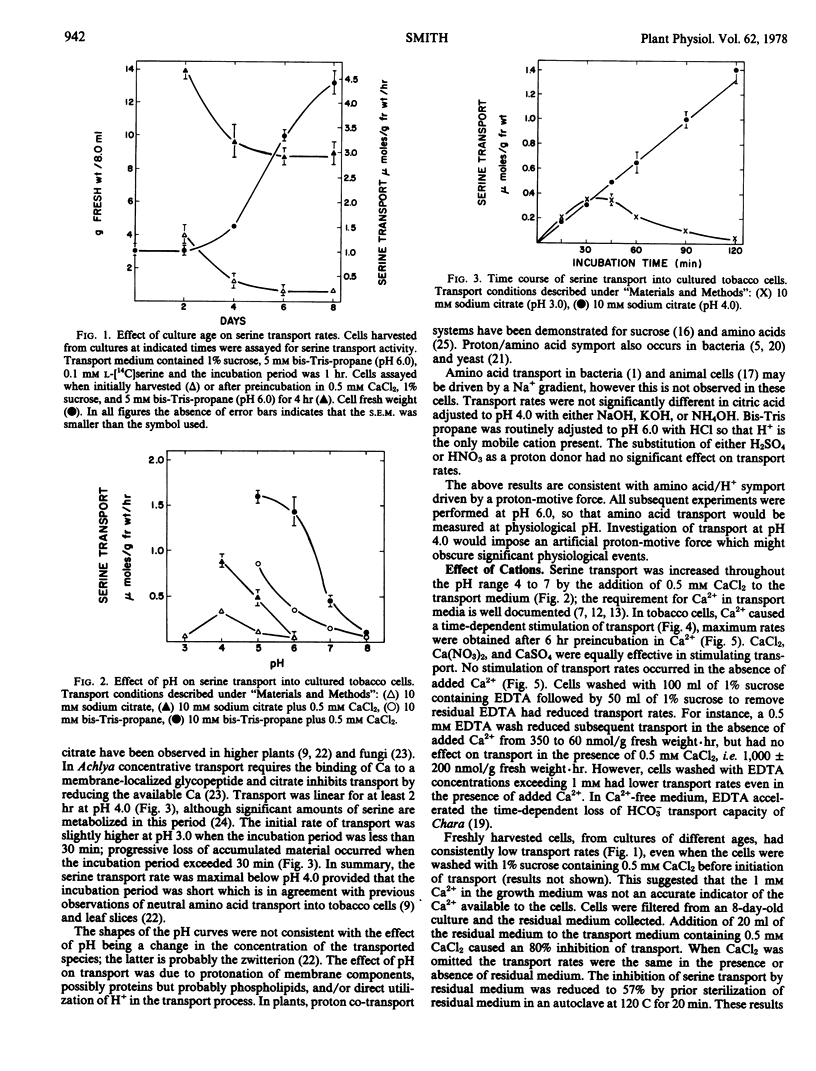
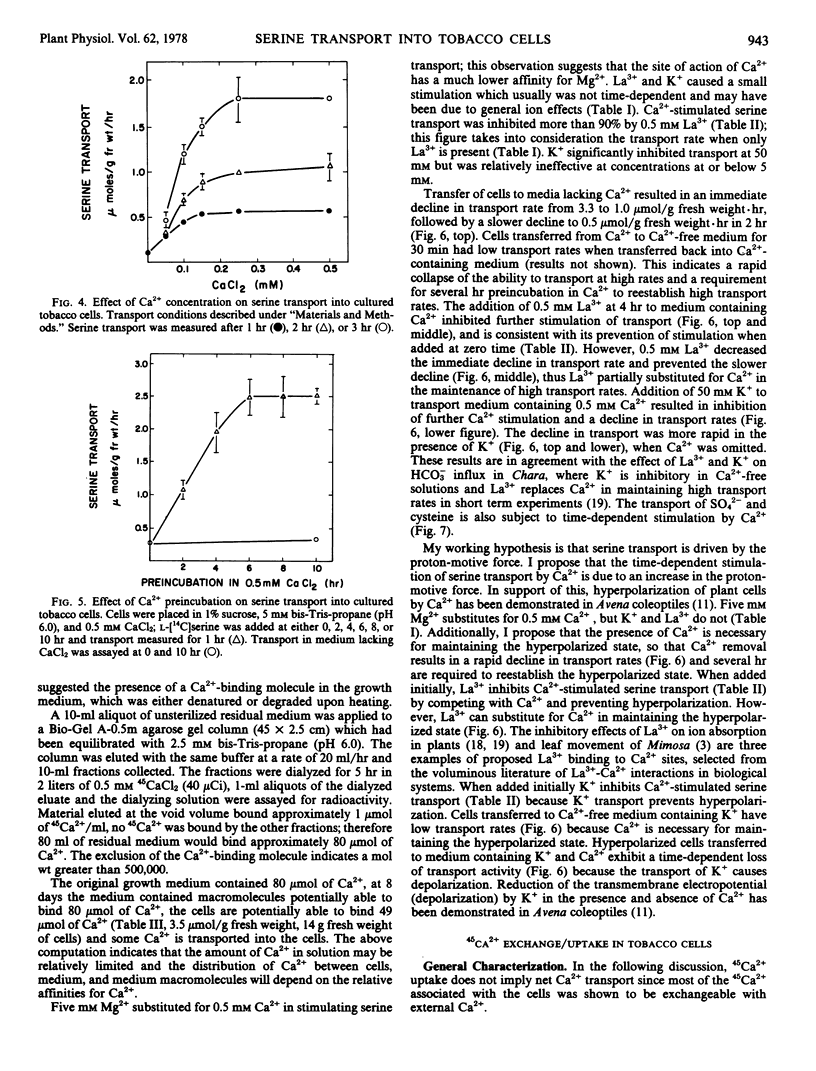
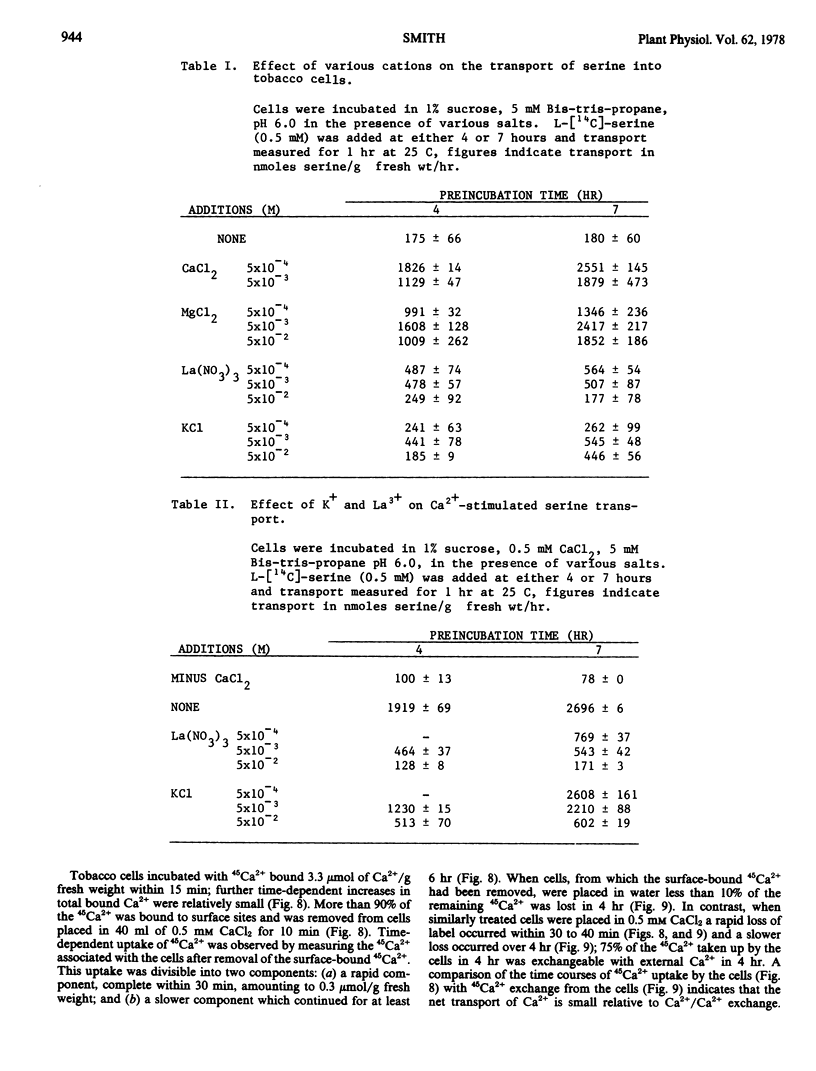
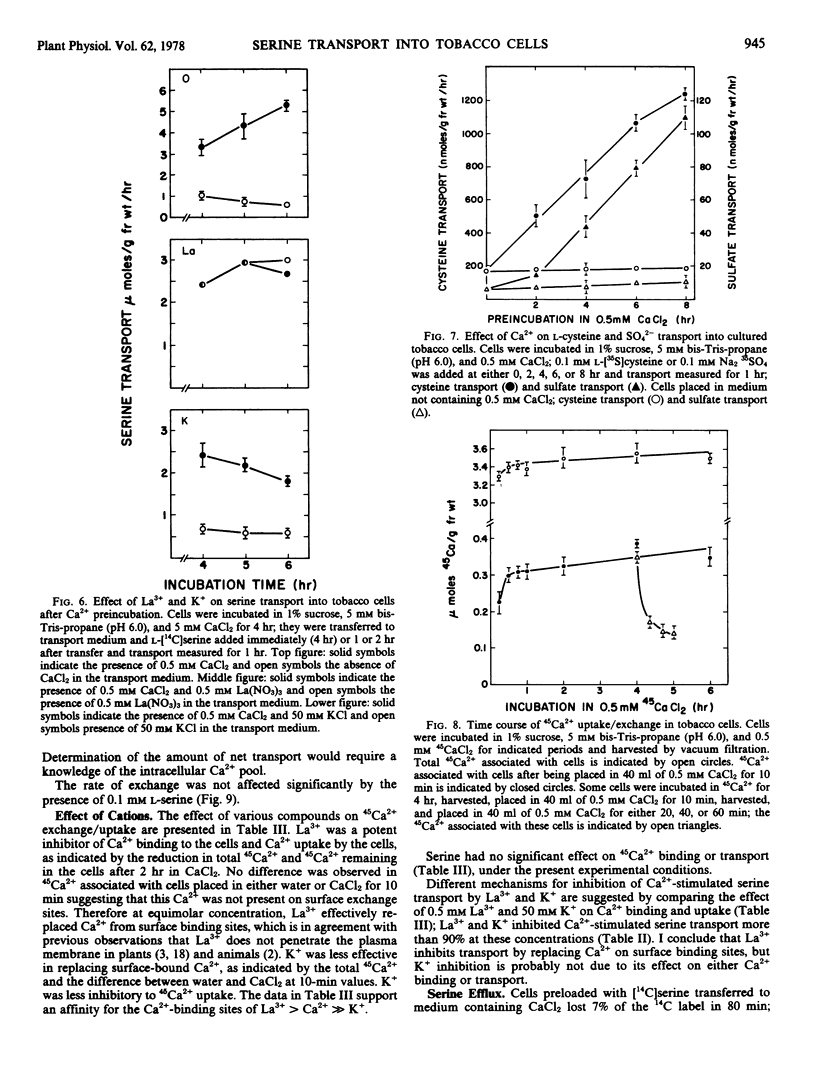
The transport of serine into tobacco (Nicotiana tabacum L. var. Xanthi) cells grown in liquid medium was studied. Serine transport was maximal below pH 4.0. A time-dependent stimulation of transport was observed when cells were incubated in medium containing 0.5 mm Ca2+. Maximum transport rates were achieved after 6 hours preincubation in Ca2+. The following three distinct roles of Ca2+ in serine transport were demonstrated: time-dependent stimulation of transport rate, maintenance of high transport rates, and retention of transported material. Stimulation occurred in the presence of either Ca2+ or Mg2+ and was inhibited by either La3+ or K+. Removal of Ca2+ from the transport medium caused a rapid decline in the rate of serine uptake. This decline was prevented by addition of La3+ after Ca2+ removal. Cells transferred to medium lacking Ca2+ lost substantial amounts of transported serine, this loss was significantly reduced by either La3+ or K+.
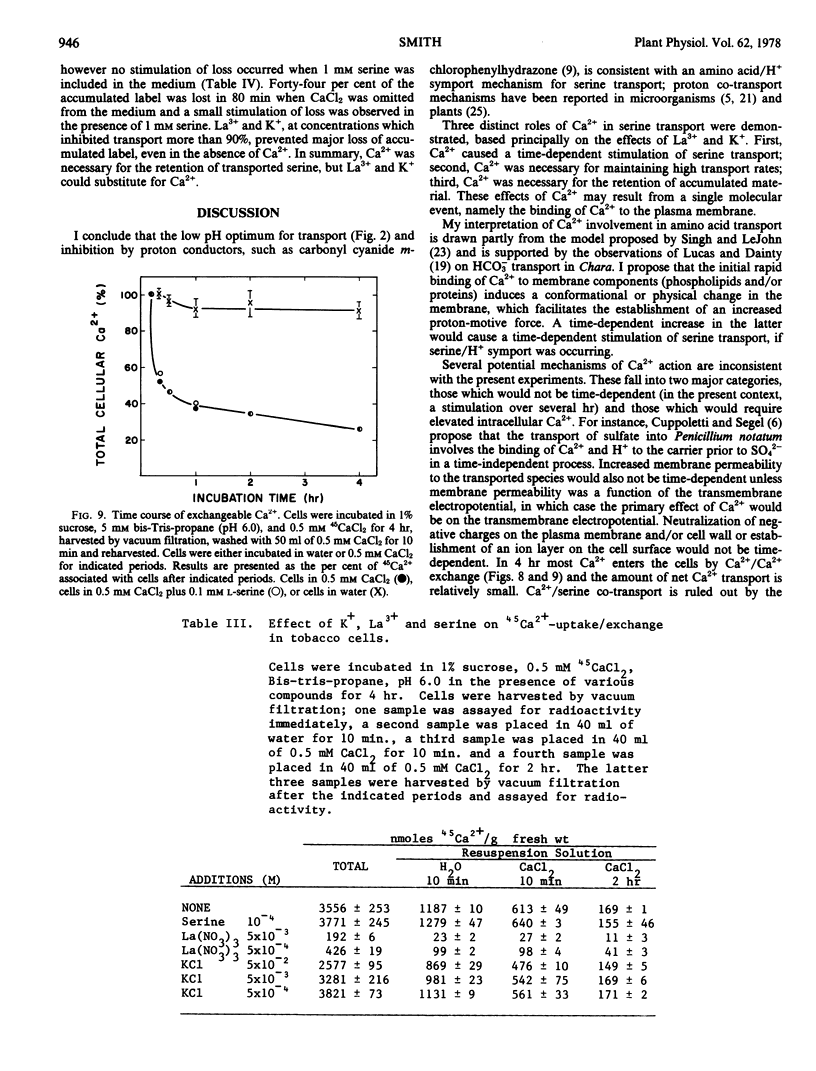
Cells placed in 45Ca2+ rapidly bound more than 3 micromoles of Ca2+/gram fresh weight, which was exchangeable within 10 minutes with medium Ca2+. Seventy-five per cent of the 45Ca2+ transported into the cells in 4 hours could be exchanged with medium Ca2+ in the same period. The amount of net Ca2+ transport into tobacco cells is insignificant relative to the total exchangeable Ca2+.
It is proposed that serine transport into tobacco cells involves H+ cotransport and that the stimulation by Ca2+ is due to an increase in the proton-motive force.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belliveau J. W., Lanyi J. K. Analogies between respiration and a light-driven proton pump as sources of energy for active glutamate transport in Halobacterium holobium. Arch Biochem Biophys. 1977 Jan 15;178(1):308–314. doi: 10.1016/0003-9861(77)90196-5. [DOI] [PubMed] [Google Scholar]
- Bieger W., Peter S., Völkl A., Kern H. F. Amino acid transport in the rat exocrine pancreas. II. Inhibition by lanthanum and tetracaine. Cell Tissue Res. 1977 May 10;180(1):45–62. doi: 10.1007/BF00227029. [DOI] [PubMed] [Google Scholar]
- Campbell N. A., Thomson W. W. Effects of lanthanum and ethylenediaminetetraacetate on leaf movements of mimosa. Plant Physiol. 1977 Oct;60(4):635–639. doi: 10.1104/pp.60.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Y. N., Nobel P. S. Amino Acid uptake by pea leaf fragments: specificity, energy sources, and mechanism. Plant Physiol. 1973 Dec;52(6):633–637. doi: 10.1104/pp.52.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. H., Jarvis A. W., Lindsay R. J., Hamilton W. A. Proton movements coupled to lactate and alanine transport in Escherichia coli: isolation of mutants with altered stoichiometry in alanine transport. J Bacteriol. 1976 Jun;126(3):1232–1244. doi: 10.1128/jb.126.3.1232-1244.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppoletti J., Segel I. H. Kinetics of sulfate transport by Penicillium notatum. Interactions of sulfate, protons, and calcium. Biochemistry. 1975 Oct 21;14(21):4712–4718. doi: 10.1021/bi00692a023. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L. The effects of amino acids and ammonium on the growth of plant cells in suspension culture. Plant Physiol. 1970 Apr;45(4):372–375. doi: 10.1104/pp.45.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Smith I. K. Cysteine transport into cultured tobacco cells. Plant Physiol. 1977 Dec;60(6):807–811. doi: 10.1104/pp.60.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J. W., Filner P. Regulation of sulfate uptake by amino acids in cultured tobacco cells. Plant Physiol. 1969 Sep;44(9):1253–1259. doi: 10.1104/pp.44.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cam A., Freychet P. Neutral amino acid transport. Characterization of the A and L systems in isolated rat hepatocytes. J Biol Chem. 1977 Jan 10;252(1):148–156. [PubMed] [Google Scholar]
- Leonard R. T., Nagahashi G., Thomson W. W. Effect of lanthanum on ion absorption in corn roots. Plant Physiol. 1975 Mar;55(3):542–546. doi: 10.1104/pp.55.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J., Dainty J. HCO(3) Influx Across the Plasmalemma of Chara corallina: Divalent Cation Requirement. Plant Physiol. 1977 Dec;60(6):862–867. doi: 10.1104/pp.60.6.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaston A., Carr G., Eddy A. A. The concentration of glycine by preparations of the yeast Saccharomyces Carlsbergensis depleted of adenosine triphosphate: Effects of proton gradients and uncoupling agents. Biochem J. 1976 Mar 15;154(3):669–676. doi: 10.1042/bj1540669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D. P., LéJohn H. B. Amino acid transport in a water-mould: the possible regulatory roles of calcium and N6-(delta2-isopentenyl)adenine. Can J Biochem. 1975 Sep;53(9):975–988. doi: 10.1139/o75-134. [DOI] [PubMed] [Google Scholar]



