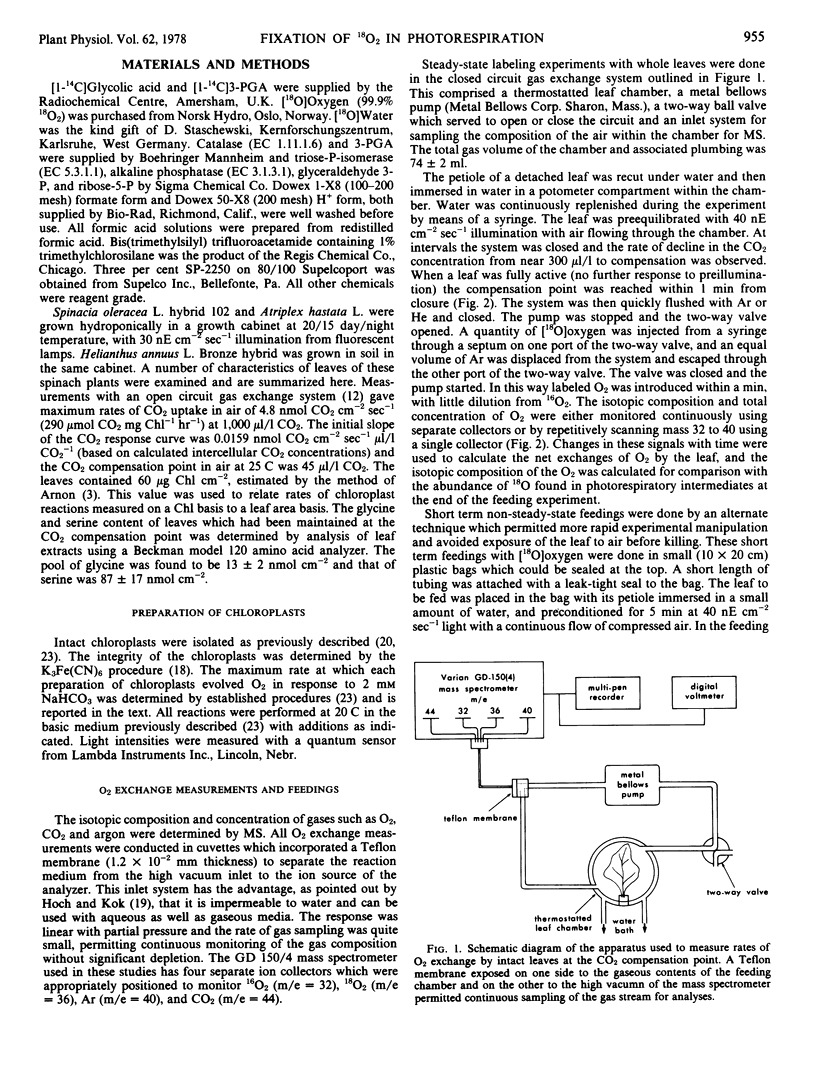
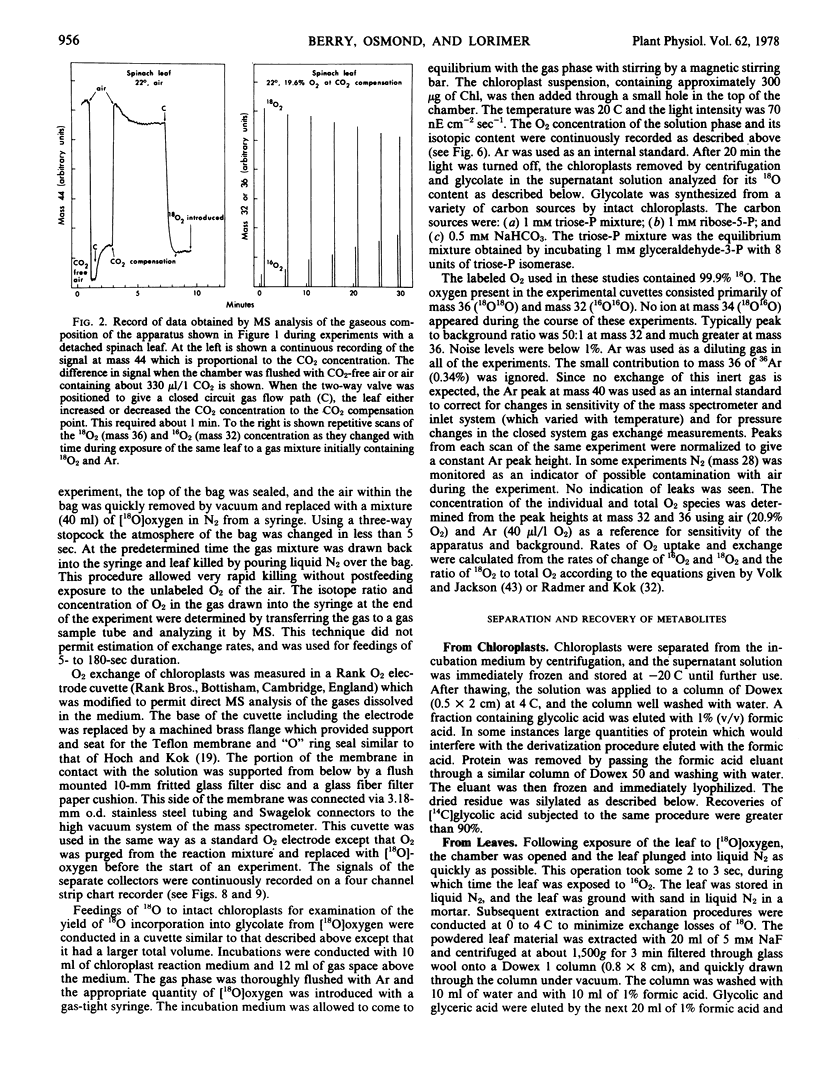
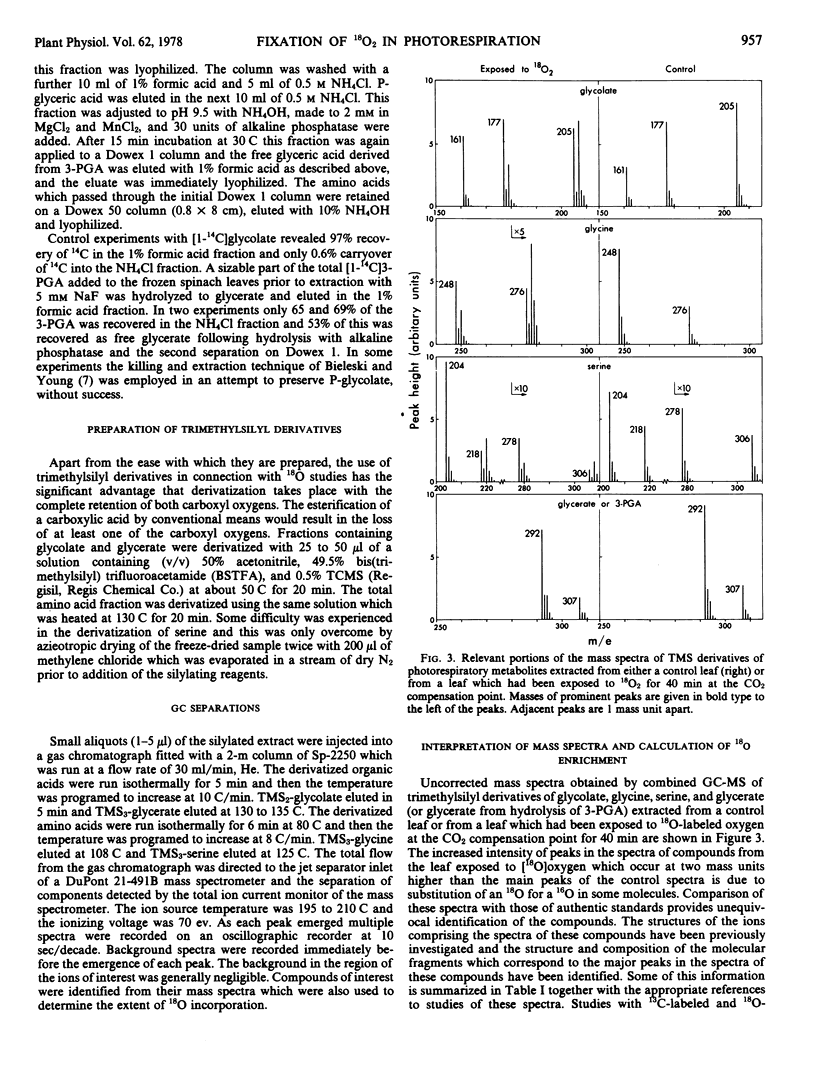
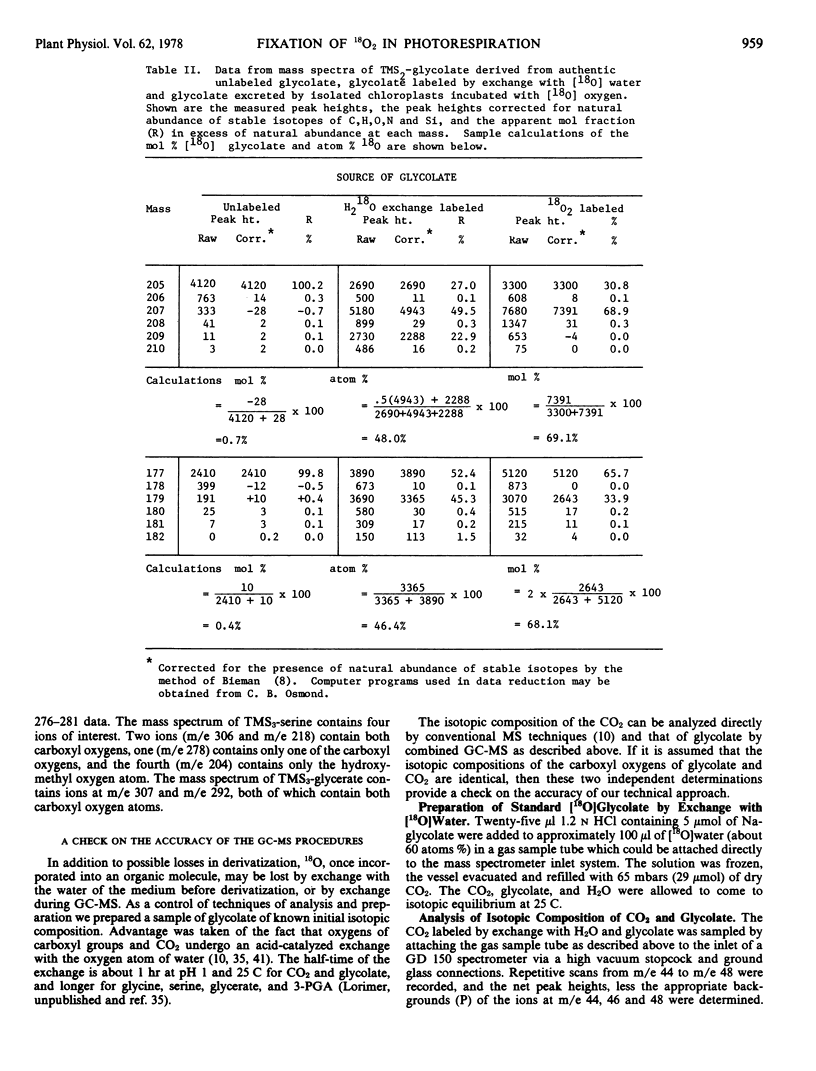
Abstract
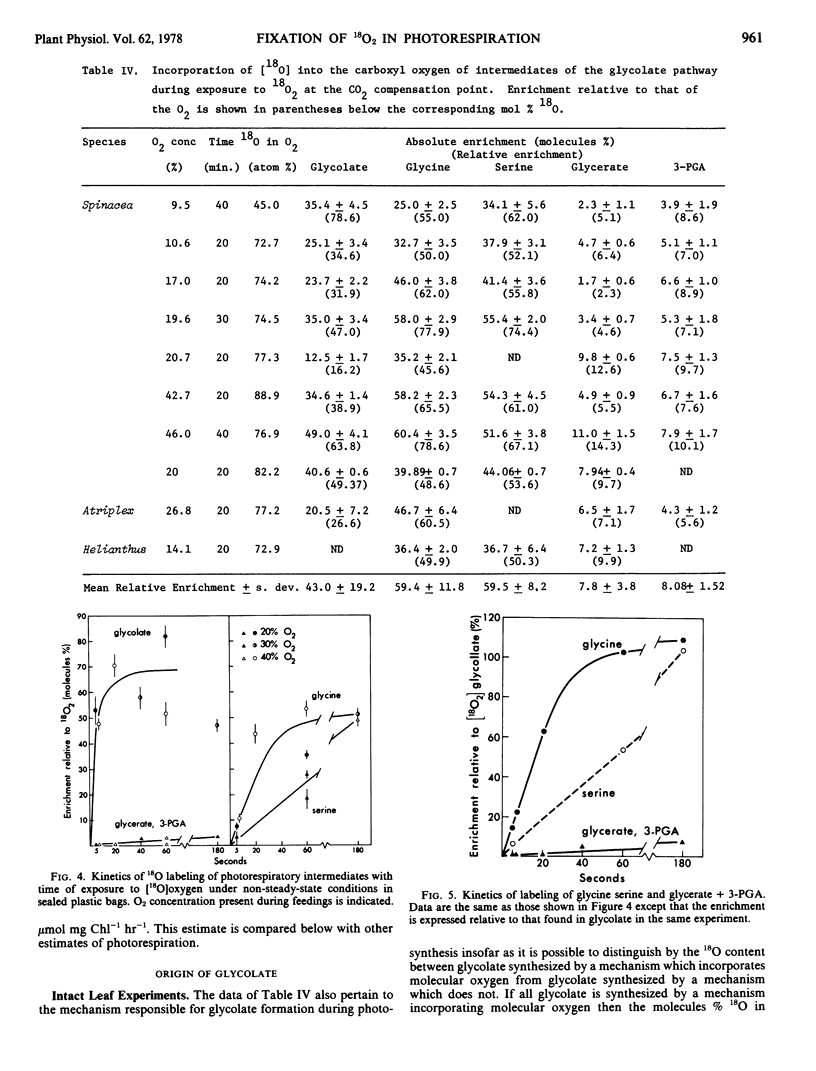
Mass spectrometric techniques were used to trace the incorporation of [18O]oxygen into metabolites of the photorespiratory pathway. Glycolate, glycine, and serine extracted from leaves of the C3 plants, Spinacia oleracea L., Atriplex hastata, and Helianthus annuus which had been exposed to [18O]oxygen at the CO2 compensation point were heavily labeled with 18O. In each case one, and only one of the carboxyl oxygens was labeled. The abundance of 18O in this oxygen of glycolate reached 50 to 70% of that of the oxygen provided after only 5 to 10 seconds exposure to [18O]oxygen. Glycine and serine attained the same final enrichment after 40 and 180 seconds, respectively. This confirms that glycine and serine are synthesized from glycolate.
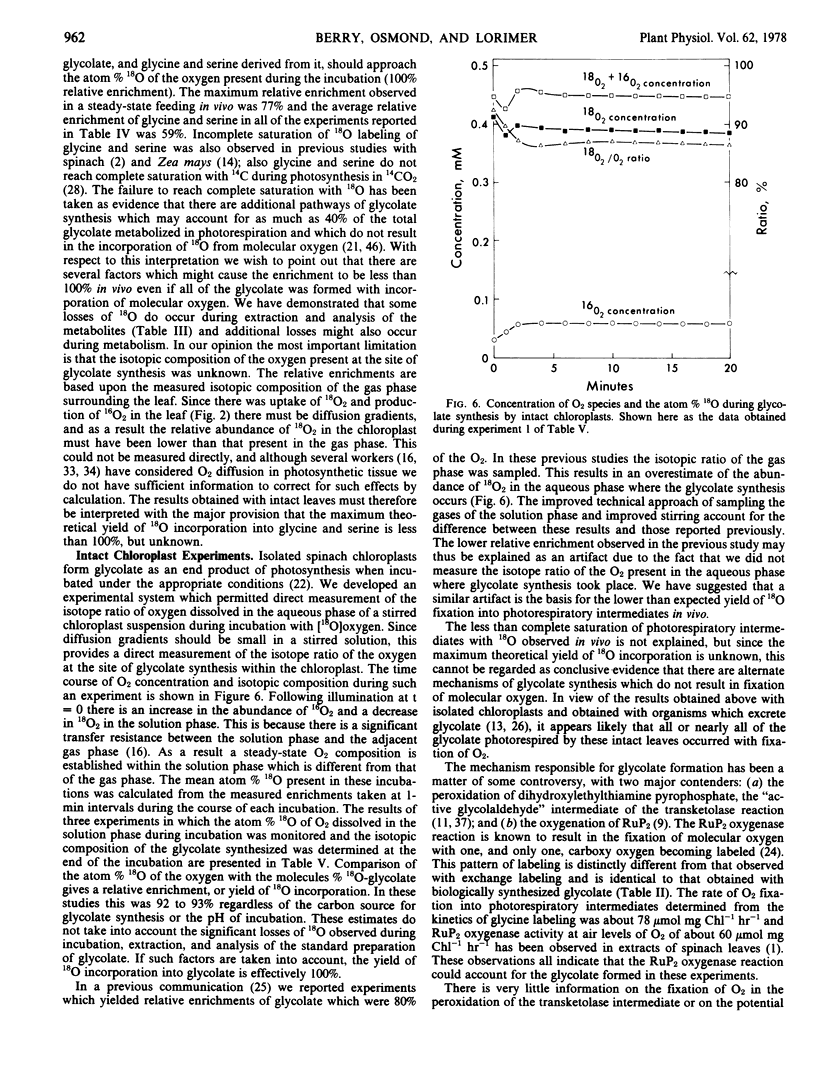
The labeling of photorespiratory intermediates in intact leaves reached a mean of 59% of that of the oxygen provided in the feedings. This indicates that at least 59% of the glycolate photorespired is synthesized with the fixation of molecular oxygen. This estimate is certainly conservative owing to the dilution of labeled oxygen at the site of glycolate synthesis by photosynthetic oxygen. We examined the yield of 18O in glycolate synthesized in vitro by isolated intact spinach chloroplasts in a system which permitted direct sampling of the isotopic composition of the oxygen at the site of synthesis. The isotopic enrichment of glycolate from such experiments was 90 to 95% of that of the oxygen present during the incubation.
The carboxyl oxygens of 3-phosphoglycerate also became labeled with 18O in 20- and 40-minute feedings with [18O]oxygen to intact leaves at the CO2 compensation point. Control experiments indicated that this label was probably due to direct synthesis of 3-phosphoglycerate from glycolate during photorespiration. The mean enrichment of 3-phosphoglycerate was 14 ± 4% of that of glycine or serine, its precursors of the photorespiratory pathway, in 10 separate feeding experiments. It is argued that this constant dilution of label indicates a constant stoichiometric balance between photorespiratory and photosynthetic sources of 3-phosphoglycerate at the CO2 compensation point.
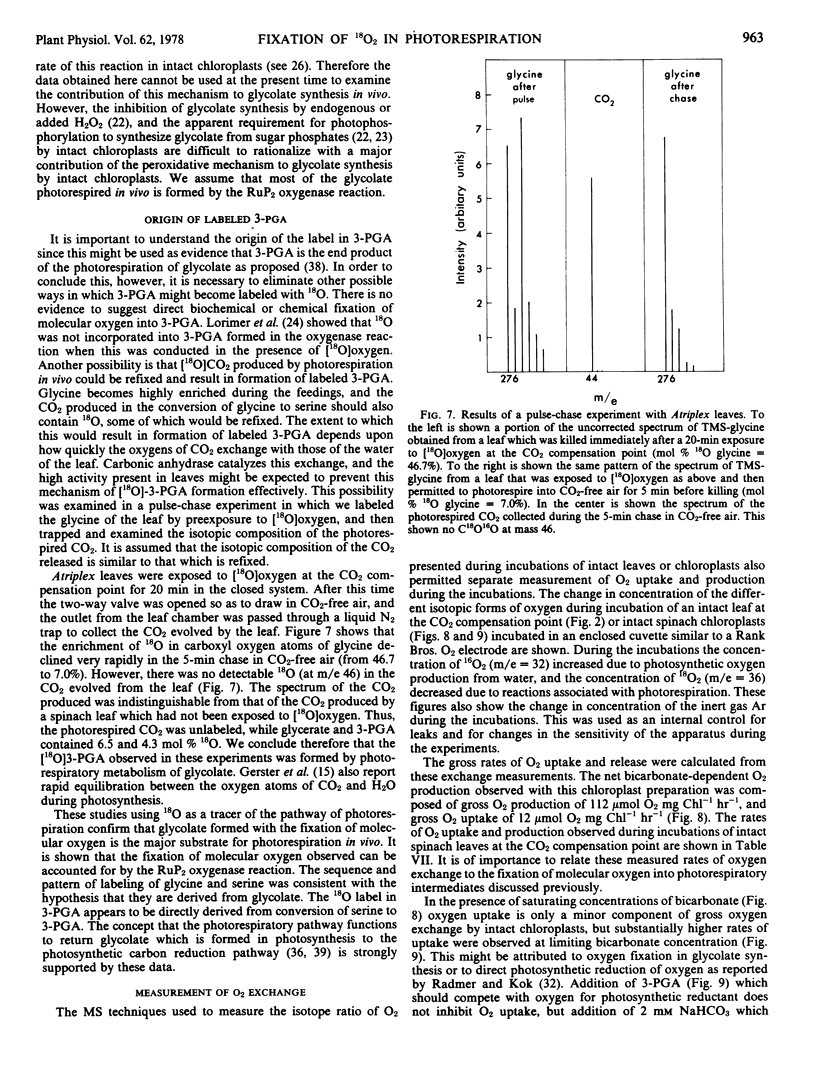
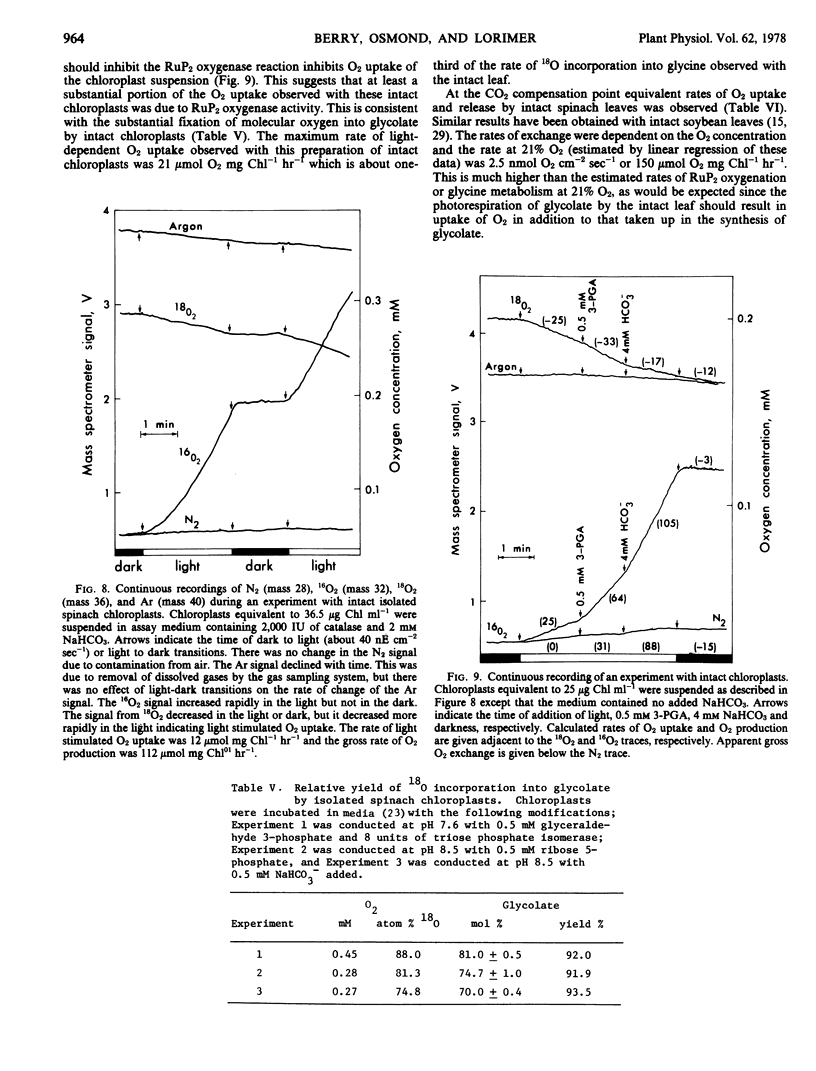
Oxygen uptake sufficient to account for about half of the rate of 18O fixation into glycine in the intact leaves was observed with intact spinach chloroplasts. Oxygen uptake and production by intact leaves at the CO2 compensation point indicate about 1.9 oxygen exchanged per glycolate photorespired. The fixation of molecular oxygen into glycolate plus the peroxisomal oxidation of glycolate to glyoxylate and the mitochondrial conversion of glycine to serine can account for up to 1.75 oxygen taken up per glycolate.
These studies provide new evidence which supports the current formulation of the pathway of photorespiration and its relation to photosynthetic metabolism. The experiments described also suggest new approaches using stable isotope techniques to study the rate of photorespiration and the balance between photorespiration and photosynthesis in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Badger M. R., Lorimer G. H. Factors affecting interconversion between kinetic forms of ribulose diphosphate carboxylase-oxygenase from spinach. Arch Biochem Biophys. 1975 Nov;171(1):93–103. doi: 10.1016/0003-9861(75)90011-9. [DOI] [PubMed] [Google Scholar]
- Andrews T. J., Lorimer G. H., Tolbert N. E. Incorporation of molecular oxygen into glycine and serine during photorespiration in spinach leaves. Biochemistry. 1971 Dec 7;10(25):4777–4782. doi: 10.1021/bi00801a027. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström K., Gürtler J., Blomstrand R. Trimethylsilylation of amino acids. I. Study of glycine and lysine TMS derivatives with gas-liquid chromatography-mass spectrometry. Anal Biochem. 1970 Mar;34:74–87. doi: 10.1016/0003-2697(70)90088-6. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Coombs J., Wittingham C. P. The mechanism of inhibition of photosynthesis by high partial pressures of oxygen in Chlorella. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):511–520. doi: 10.1098/rspb.1966.0046. [DOI] [PubMed] [Google Scholar]
- Ehleringer J., Björkman O. Quantum Yields for CO(2) Uptake in C(3) and C(4) Plants: Dependence on Temperature, CO(2), and O(2) Concentration. Plant Physiol. 1977 Jan;59(1):86–90. doi: 10.1104/pp.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOOD N. E., BROWN A. H. The contribution of endogenous oxygen to the respiration of photosynthesizing Chlorella cells. Biochim Biophys Acta. 1961 Jul 8;50:544–554. doi: 10.1016/0006-3002(61)90014-2. [DOI] [PubMed] [Google Scholar]
- HOCH G., KOK B. A mass spectrometer inlet system for sampling gases dissolved in liquid phases. Arch Biochem Biophys. 1963 Apr;101:160–170. doi: 10.1016/0003-9861(63)90546-0. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G. H., Thorne S. W., Lorimer G. H. Glycolate synthesis by intact chloroplasts. Studies with inhibitors of photophosphorylation. Arch Biochem Biophys. 1977 Oct;183(2):471–479. doi: 10.1016/0003-9861(77)90382-4. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Andrews T. J., Tolbert N. E. Ribulose diphosphate oxygenase. II. Further proof of reaction products and mechanism of action. Biochemistry. 1973 Jan 2;12(1):18–23. doi: 10.1021/bi00725a004. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Krause G. H., Berry J. A. The incorporation of (18O)oxygen into glycolate by intact isolated chloroplasts. FEBS Lett. 1977 Jun 15;78(2):199–202. doi: 10.1016/0014-5793(77)80305-0. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Osmond C. B., Akazawa T., Asami S. On the mechanism of glycolate synthesis by Chromatium and Chlorella. Arch Biochem Biophys. 1978 Jan 15;185(1):49–56. doi: 10.1016/0003-9861(78)90142-x. [DOI] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain Y., Gibbs M. Formation of glycolate by a reconstituted spinach chloroplast preparation. Plant Physiol. 1971 Sep;48(3):325–330. doi: 10.1104/pp.48.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenheuvel W. J., Cohen J. S. Gas-liquid chromatography-mass spectrometry of carbon-13 enriched amino acids as trimethylsilyl derivatives. Biochim Biophys Acta. 1970 May 12;208(2):251–259. doi: 10.1016/0304-4165(70)90243-6. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Pathways of carbon fixation in green plants. Annu Rev Biochem. 1975;44:123–145. doi: 10.1146/annurev.bi.44.070175.001011. [DOI] [PubMed] [Google Scholar]



