Abstract
Introduction.
Congenital anomalies of the kidney and urinary tract (CAKUT) are the most common cause of childhood chronic kidney disease (CKD). We hypothesized that hypertension varies across CAKUT categories and increases the risk of CKD.
Methods.
This was a retrospective cohort study and included cases with a multicystic dysplastic kidney (MCDK)(n=81), unilateral kidney agenesis (UKA)(n=47), kidney hypoplasia (KH)(n=130), and posterior urethral valves (PUV)(n=75). Hypertension was defined as systolic or diastolic blood pressure ≥95th percentile for age, sex and height, and CKD as an estimated glomerular filtration rate <60 ml/min/1.73 m2, both at 2 consecutive clinic visits at least 3 months apart.
Results.
Sixty-two (19%) out of 333 cases developed hypertension, with significant difference according to CAKUT type. Patients with smaller kidney size (7.7 vs. 8.3, p=0.045), kidney anomalies in addition to the primary diagnosis (aCAKUT) (53 vs. 38%, p=0.03), proteinuria (46 vs. 12%, p<0.001), and CKD (51 vs. 23%, p<0.001) were more likely to develop hypertension. When adjusted for kidney size, the diagnoses of PUV (OR 10.9, 95% 3.0, 40.5), UKA (OR 6.4, 95% CI 1.6, 24.9) and KH (OR 4.2, 95% CI 1.1, 16.1), and aCAKUT (OR 2.1, 95% CI 1.2, 3.9) were independent risk factors for hypertension. Hypertension increased the risk of developing CKD by 2-fold (HR 1.9, 95% CI 1.19, 2.94).
Conclusion.
Hypertension is common in children with CAKUT and increases the risk of CKD. These findings will aid in the development of a standardized clinical pathway for the care of hypertensive children with CAKUT.
Keywords: CAKUT, hypertension, pediatric, risk factor, outcomes
Introduction
Congenital anomalies of the kidney and urinary tract (CAKUT) account for approximately 60% of all cases of chronic kidney disease (CKD) in children (1). CAKUT is often associated with a decrease in nephron endowment, which can lead to chronic kidney injury (2). CAKUT, however, is a heterogeneous group of anomalies that have variable phenotypes, clinical characteristics, and long-term outcomes (3), which has engendered some debate about the appropriateness of the CAKUT terminology (4, 5).
Hypertension is common in children with kidney malformations; however, its prevalence varies according to the underlying kidney anomaly. The North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry reported 19% of children with structural kidney disease had associated hypertension at registration (6), while in the Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients (ESCAPE) trial cohort, 39% of cases enrolled had hypertension (blood pressure greater than the 95th percentile for age, sex, and height). In the Chronic Kidney Disease in Children (CKiD) cohort, 26% of children with non-glomerular disease had blood pressure measurements greater than the 90th percentile at enrollment (1). Notably, only cases with pre-existing CKD were included in these cohorts. It is therefore unclear whether CAKUT malformations are associated with hypertension independent of GFR impairment. In addition, in these cohorts, children with kidney and urinary tract malformations were classified into broad categories such as ahypodysplasia or non-glomerular disease, therefore obscuring potential differences in the prevalence and risk factors among different types of CAKUT.
The risk factors associated with hypertension and the effect of hypertension on long-term kidney outcomes in children with specific kidney malformations have not been well described. In general, multiple factors have been associated with progression of kidney disease in children and adults, including hypertension, proteinuria, and underlying kidney disease (7). While the rate of decline of kidney function has been shown to be greater in hypertensive children with non-glomerular kidney disease in the CKiD cohort (8), and in children with ahypodypslasia in the ESCAPE trial, common risk factors for hypertension in specific forms of CAKUT are unknown.
The aims of this report are to 1) define the prevalence of hypertension in a contemporaneous cohort of children with various forms of CAKUT; 2) identify clinical characteristics associated with the development of hypertension that are shared across the different types of malformations; and 3) study the influence of hypertension on the progression of CKD in these children. Ultimately, the results will aid in the development of a standardized clinical pathway for the care of hypertensive children with CAKUT.
Methods
Data management.
This was a retrospective cohort quality improvement (QI) study. Ethics approval was not sought, as QI initiatives are exempt from Research Ethics Board review at our center. We identified cases from 2000-2018 with kidney malformations using our program clinical datasets. Clinical data were extracted from patient files, and information from patients meeting study inclusion criteria was entered into a QI REDCap data collection tool.
Case selection.
Cases were included if they had: 1) a primary diagnosis of MCDK, UKA, KH, or PUV, with the criteria for diagnoses confirmed as previously described (9–11); 2) blood pressure measurements; and 3) age 0-18 years. Cases were excluded if their diagnosis could not be confirmed, or there was insufficient clinical data (Figure 1).
Figure 1. Diagram of CAKUT case enrollment.
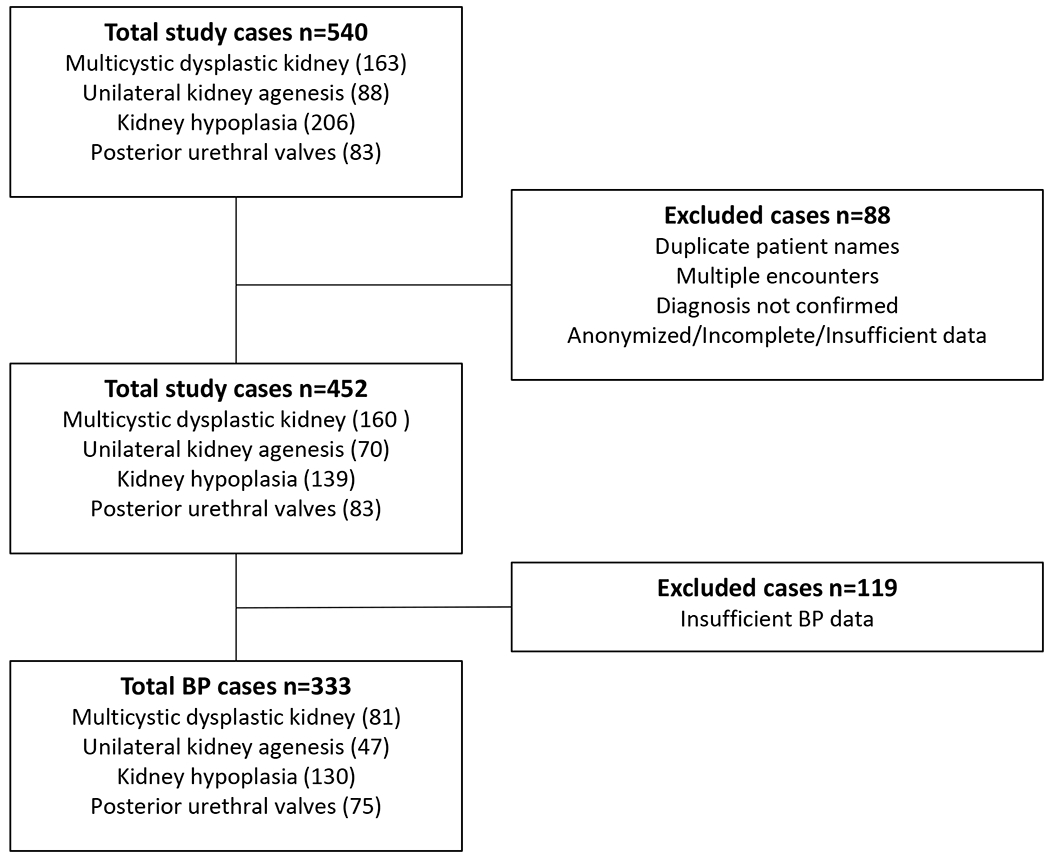
From a total cohort of 540 cases of CAKUT due to MCDK, UKA, KH, and PUV, excluding cases with duplicate records and those without a clear primary diagnosis, left 452 cases. From those, 119 were excluded because of incomplete blood pressure data, leaving a final total of 333 cases, distributed across the different diagnoses, as indicated.
Clinical characteristics.
Clinical characteristics considered as potential risk factors for developing hypertension included the type of malformation (MCDK, UKA, KH, and PUV), birthweight, gestational age at birth, body mass index (BMI) at the last clinic visit, presence of an identified genetic syndrome or associated non-kidney anomalies, baseline estimated glomerular filtration rate (eGFR), kidney size at diagnosis, proteinuria, and presence of kidney anomalies in addition to the primary diagnosis (aCAKUT). BMI was calculated using weights and heights obtained at outpatient clinic visits. Overweight was defined using BMI cut points as per published nomograms for children (12). Baseline eGFR was calculated using the first serum creatinine value available and the appropriate Schwartz formula (13, 14). Only creatinine measurements obtained after 5 days of life were used, to mitigate the influence of maternal creatinine. For PUV cases, the baseline serum creatinine used was at 1 year of age or later to allow adequate time for the urinary tract to decompress following valve ablation surgery. Kidney size metric was the average of the kidney lengths divided by the body length (and adjusted for the regression analyses by multiplying by 100) and expressed as KL:BL, as previously described and validated (10, 15). This was determined at the time of CAKUT diagnosis or at 1 year of age for PUV cases. Proteinuria was defined as protein ≥0.25 g/L on urinalysis or a urine dipstick on two consecutive outpatient visits, separated by at least 3 months (10). In PUV cases, as with eGFR, only samples obtained at 1 year of age or later were included for the reasons explained above. Additional CAKUT (aCAKUT) refers to structural or anatomical anomalies detected by kidney ultrasound in the solitary functioning kidney for MCDK and UKA cases, and in one or both kidneys for KH and PUV cases. For cases with MCDK, UKA, and KH, examples of aCAKUT included kidney cysts, increased kidney parenchymal echogenicity, dysplasia, hydroureteronephrosis, vesicoureteral reflux, ureterocele, and duplex collecting system. For cases with PUV, aCAKUT anomalies were similar, but hydroureteronephrosis or vesicoureteral reflux were excluded, as they were considered not independent from the effects of bladder outlet obstruction.
Clinical outcomes.
The primary outcome was hypertension, defined as a systolic or diastolic blood pressure (sBP/dBP) ≥95th percentile for age, sex, and height on two consecutive visits occurring at least 3 months apart (9, 16), and/or being treated with a blood pressure medication. Outpatient clinic blood pressure measurements were performed as single manual measurements by sphygmomanometer by a specialty clinic nurse. The secondary outcome was CKD, defined as an eGFR <60 mL/min/1.73 m2 on two consecutive outpatient visits separated by at least 3 months.
Statistical analyses.
All analyses were conducted using SPSS version 28 software (IBM Corp., Armonk NY) with a threshold of p<0.05 for statistical significance. Parametric and non-parametric data were expressed as means ± standard deviation (SD) and medians with interquartile range (IQR), respectively, based on visual inspection and normality testing. Categorical data were expressed as proportions (percent). Between-group comparisons were made using the student’s t test, Mann-Whitney U test, and Pearson’s chi square or Fisher’s exact test, as indicated. Multivariate binary logistic regression was used to identify clinical variables that were associated with hypertension and CKD, reported as odds ratios (OR) and 95% confidence intervals (CI). The choice of variables in the regression model was informed by the strength of their association in the bivariate analysis. For binary variables, cut points were determined by optimal sensitivity and specificities in receiver operating characteristic (ROC) curve analysis. Model performance was determined by prediction accuracy and concordance(c)-score statistic. Kaplan-Meier analysis was used to determine outcome-free survival according to age and stratified for the type of kidney malformation, the presence of CKD, and the presence of hypertension. Differences were determined by log-rank analysis. Cox regression was utilized to obtain hazard ratios (HR) and their corresponding 95% confidence intervals for developing CKD, comparing patients with and without hypertension.
Results
Clinical characteristics.
Three hundred and thirty-three patients with CAKUT were included in this analysis, of which 24% (81/333) had MCDK, 14% (47/333) had UKA, 39% (130/333) had KH, and 23% (75/333) had PUV (Figure 1, Table 1).
Table 1.
Clinical characteristics of the CAKUT cohort
| No hypertension | Hypertension | p | |
|---|---|---|---|
|
|
|||
| Number | 271/333 (81) | 62/333 (19) | |
| CAKUT diagnosis | <0.001a | ||
| Multicystic dysplastic kidney (%) | 77/81 (95) | 4/81 (5) | |
| Unilateral kidney agenesis (%) | 37/47 (79) | 10/47 (21) | |
| Kidney hypoplasia (%) | 108/130 (83) | 22/130 (17) | |
| Posterior urethral valve (%) | 49/75 (65) | 26/75 (35) | |
| Median gestational age in weeks | 36.0 (2.14) | 36.4 (3.86) | 0.13b |
| Median birth weight in kg | 3.20 (0.86) | 3.20 (1.22) | 0.77b |
| Genetic syndrome (%) | 42/271 (16) | 9/62 (15) | 1.00a |
| Non-kidney anomalies (%) | 70/271 (26) | 20/62 (32) | 0.34a |
| BMI overweight (%) | 54/240 (23) | 10/58 (17) | 0.38a |
| Mean first eGFR (ml/min/1.73 m2) | 81 ± 45 | 70 ± 40 | 0.07c |
| Median age at first eGFR in years | 2.02 (6.70) | 1.60 (5.63) | 0.68b |
| Mean KL:BL | 8.3 ± 2.2 | 7.7 ± 1.9 | 0.045c |
| Median age at KL:BL in years | 0.95 (5.54) | 1.60 (5.45) | 0.38b |
| aCAKUT (%) | 102/271 (38) | 33/62 (53) | 0.03a |
| Proteinuria (%) | 30/258 (12) | 27/59 (46) | <0.001a |
| CKD (eGFR<60) (%) | 56/245 (23) | 30/59 (51) | <0.001a |
Proportion and percentage in parentheses with differences analyzed by Pearson’s Chi-square test,
median with interquartile range in parentheses with differences analyzed by Mann-Whitney U test,
mean ± standard deviation with differences analyzed by Student’s t-test.
CAKUT= congenital anomaly of the kidney and urinary tract, BMI = body mass index, eGFR = estimated glomerular filtration rate in ml/min/1.73 m2, KL:BL = average kidney length:body length*100, aCAKUT = kidney or urinary tract malformation in addition to primary diagnosis.
Characteristics of included vs excluded cases.
When compared to the excluded group, the included group had significantly less cases of MCDK (24 vs 66%), and significantly more cases of KH (39 vs 8%) and PUV (22 vs 7%). The included cases were significantly more likely to have been born with a lower median birth weight (3.20 vs 3.90 kg), and to have non-kidney anomalies (77 vs 13%) and aCAKUT (41 vs 28%). Cases in the included group had significantly worse initial mean eGFRs (79 vs 103 ml/min/1.73 m2), and smaller standardized kidney size estimates (KL:BL 8.2 vs 9.6).
CAKUT and hypertension.
In all, 19% (62/333) of the cases of CAKUT had hypertension. Of the 62 cases, 31 (50%) were on an anti-hypertensive medication and 31 (50%) were not. Nine cases were on an antihypertensive medication with normal blood pressure, while 22 cases still had high blood pressure after treatment was started. Although the definition of hypertension for this report required an elevated blood pressure on two consecutive visits, 19 of 22 cases (86%) were started on anti-hypertensive treatment before the second visit. Of these 22 cases, 12 (55%) had BPs in the 95-99th percentile category before being started on an antihypertensive medication, and 16 (73%), although still elevated, had BPs the same or better after treatment.
For the whole CAKUT cohort, the mean age of developing hypertension was 5.33 ± 7.26 years. Cases with hypertension were older at last follow up compared to normotensive cases (median 9.47 IQR 10.15 vs median 7.79 IQR 8.35 years, p=0.09); however, the difference was not statistically significant.
Risk factors for hypertension.
There was a significant difference in the proportion of cases with hypertension according to CAKUT type, seen in 35% (26/75) of the PUV group, 21% (10/47) of the UKA group, 17% (22/130) of the KH group and 5% (4/81) of the MCDK group (p<0.001) (Table 1). In addition, there was a significant difference in hypertension-free survival when stratified by CAKUT diagnosis and examined by Kaplan-Meier analysis (p<0.001 by log-rank) (Figure 2).
Figure 2. Hypertension-free survival in CAKUT cases according to category.
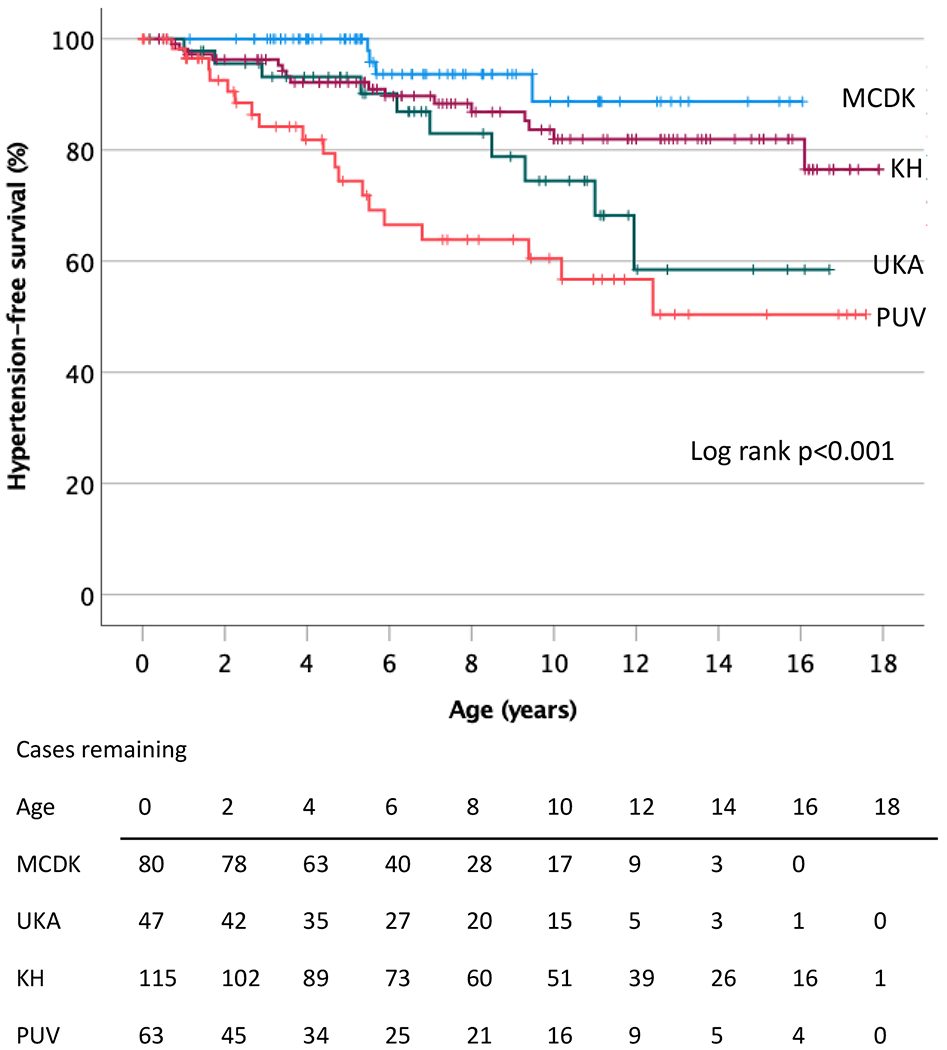
Kaplan-Meier survival analysis of CAKUT cases stratified by diagnosis, demonstrating a significant difference in the development of hypertension over time among the categories (p<0.001 by log rank analysis). MCDK = multicystic dysplastic kidney, UKA = unilateral kidney agenesis, KH = kidney hypoplasia, PUV = posterior urethral valves.
CAKUT cases with hypertension were also more likely to have smaller mean adjusted KL:BL ratios (7.7 ± 1.9 vs 8.3 ± 2.2, p=0.045), aCAKUT (53 vs 38%, p=0.03), proteinuria (46 vs 12%, p<0.001), and CKD (51 vs 23%, p<0.001) when compared to normotensive cases by bivariate analysis (Table 1).
Although the first eGFR in CAKUT cases with hypertension was lower than in normotensive cases (70 vs 81 ml/min/1.73 m2, p=0.07), the difference was not statistically significant (Table 1). There was also no significant difference in gestational age at birth (36.0 vs 36.4 weeks, p=0.13), birth weight (3.2 vs 3.2 kg, p=0.77), presence of a genetic syndrome (16 vs 15%, p=1.00), associated non-kidney anomalies (26 vs 32%, p=0.34), or overweight BMI status at last visit (23 vs 17%, p=0.38) between normotensive and hypertensive CAKUT cases, respectively.
Based on the results of the bivariate analysis (Table 1), we selected the following clinical characteristics as potential independent risk factors for hypertension in a multivariate regression model: type of CAKUT, kidney size (KL:BL), presence of aCAKUT, and whether the case had associated CKD. The diagnoses of PUV (OR 10.9, 95% 3.0, 40.5), UKA (OR 6.4, 95% CI 1.6, 24.9) and KH (OR 4.2, 95% CI 1.1,16.1) were independently associated with hypertension when compared to the diagnosis of MCDK and when adjusted for the other variables in the model (Table 2). Cases with aCAKUT had an approximately 2-fold increased odds of having hypertension (OR 2.1, 95% CI 1.2, 3.9) when compared to those cases without aCAKUT. Kidney size (KL:BL) was not found to be an independent variable in this model (OR 1.4, 95% CI 0.7, 3.9).
Table 2.
Independent predictors of hypertension and adjusted for CKD
| OR (95%CI) | Adjusted OR (95% CI) | |
|---|---|---|
|
|
||
| CAKUT diagnosisa | ||
| Unilateral kidney agenesis | 6.4 (1.6-24.9) | 6.5 (1.6-25.7) |
| Kidney hypoplasia | 4.2 (1.1-16.1) | 4.0 (1.0-15.7) |
| Posterior urethral valve | 10.9 (3.0-40.5) | 8.5 (2.2-32.8) |
| KL:BL<7.9 | 1.4 (0.7-3.9) | 1.3 (0.6-2.9) |
| aCAKUT | 2.1 (1.2-3.9) | 2.0 (1.1-3.8) |
| CKD | 2.4 (1.2-4.8) | |
| Constant | −2.29 | 0.10 |
OR= odds ratio, CI=confidence interval, CAKUT= congenital anomaly of the kidney and urinary tract, KL:BL= average kidney length:body length*100, CKD= chronic kidney disease, aCAKUT = structural or anatomical anomalies in addition to primary diagnosis (see Methods).
The “Multicystic dysplastic kidney” diagnostic group was adopted as the reference for calculating the odds ratio associated with each other specific etiology.
When the model was then adjusted for CKD, the results were similar, with comparable odds, and with PUV, UKA, KH and aCAKUT remaining as independent risk factors for hypertension (Table 2). In this model, cases with CKD had an approximately 2-fold increased odds of having hypertension (OR 2.4, 95% CI 1.2, 4.8) (Table 2), but lacked a significantly different hypertension-free survival than those cases without CKD (HR 1.8, 95% CI 0.95, 3.56) (Figure 3A). The regression model had an 83% prediction accuracy, with a c-statistic score of 0.74.
Figure 3.
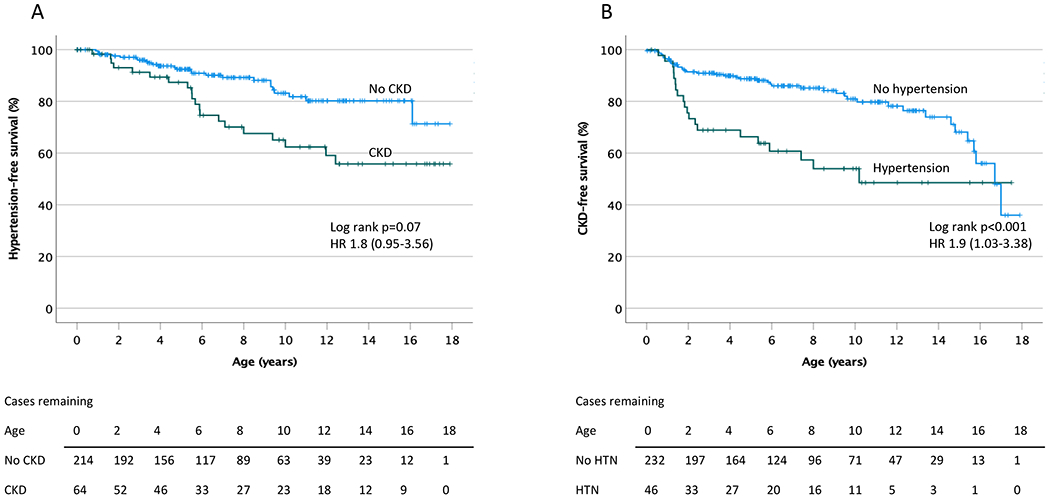
A. Development of hypertension over time in CAKUT cases. Kaplan-Meier survival analysis indicates a significance increase in hypertension over time among cases of CAKUT with associated chronic kidney disease (CKD) (p<0.001 by log rank analysis). B. Development of CKD over time in CAKUT cases. Kaplan-Meier survival analysis demonstrates a significant increase in CKD over time in cases of CAKUT with associated hypertension (HTN) (p<0.001 by log rank analysis). CKD = chronic kidney disease, HTN = hypertension.
Hypertension as a risk factor for CKD.
A significantly greater proportion of CAKUT cases with hypertension developed CKD compared to those without hypertension (51 vs 23%, p<0.001, respectively), while the median age at developing CKD was not statistically different between the groups (2.39, IQR 9.20 vs 5.87, IQR 12.4 years, p=0.50). The CKD-free survival was significantly worse in the CAKUT group with hypertension (Figure 3B) with an approximately 2-fold increase in CKD over the period of follow up (HR 1.9, 95% CI 1.19, 2.94).
To determine the influence of hypertension as a risk factor for CKD, we developed a regression model that included the type of CAKUT, kidney size as determined by KL:BL, the presence of aCAKUT, and hypertension, then further adjusted for proteinuria in a separate model. When adjusting for the other variables, the odds of developing CKD were significantly higher in patients with hypertension (OR 2.4, 95% CI 1.2, 4.9) (Table 3). In addition, the odds of developing CKD were highest in PUV patients when compared to patients with MCDK (OR 6.4, 95% CI 2.1, 19.8). However, UKA and KH were not found to be statistically significant independent variables for developing CKD (OR 0.96, 95% CI 0.22, 4.07 and OR 2.8, 95% CI 0.9, 8.7, respectively). Cases with a KL:BL <7.9 had an approximately 4-fold increased odds of CKD (OR 3.6, 95% CI 1.7, 7.7).
Table 3.
Independent predictors of CKD and adjusted for proteinuria
| OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
|
|
||
| CAKUT diagnosisa | ||
| Unilateral kidney agenesis | 0.96 (0.22-4.07) | 0.5 (0.1-2.6) |
| Kidney hypoplasia | 2.8 (0.9-8.7) | 2.8 (0.9-9.2) |
| Posterior urethral valve | 6.4 (2.1-19.8) | 4.5 (1.4-14.9) |
| KL:BL<7.9 | 3.6 (1.7-7.7) | 2.6 (1.1-5.9) |
| aCAKUT | 1.4 (0.7-2.5) | 1.7 (0.9-3.4) |
| Hypertension | 2.4 (1.2-4.9) | 1.6 (0.7-3.6) |
| Proteinuria | 6 (2.7-13.3) | |
| Constant | 0.09 | 0.07 |
OR= odds ratio, CI=confidence interval, CAKUT= congenital anomaly of the kidney and urinary tract, KL:BL= average kidney length:body length*100, aCAKUT = structural or anatomical anomalies in addition to primary diagnosis (see Methods), CKD= chronic kidney disease.
The “Multicystic dysplastic kidney” diagnostic group was adopted as the reference for calculating the odds ratio associated with each other specific etiology.
The influence of proteinuria on the progression of CKD in children has been described (17), however this is not independent from that of hypertension. Nonetheless, we adjusted the model to include proteinuria, and found it to be a significant independent risk factor for CKD (OR 6, 95% CI 2.7,13.3), when adjusted for the other variables (Table 3). While PUV and kidney size continued to be independent risk factors, the inclusion of proteinuria attenuated the effect of hypertension in the model (Table 3).
Discussion
CAKUT is the major cause of CKD in children and is commonly associated with hypertension. Our study is the first to assess the shared clinical risk factors associated with hypertension in a contemporaneous cohort of four major types of CAKUT, including MCDK, UKA, KH, and PUV. In our cohort, 19% of cases developed hypertension. The prevalence of hypertension varied among the various categories of CAKUT, being most common in cases with PUV, and least common in those with MCDK. After adjusting for co-variates, the PUV and UKA groups had the highest odds ratio for having hypertension. In cases with a solitary functioning kidney, those with MCDK were less likely to develop hypertension when compared to those with UKA. The reason for this difference is unknown but may be due to fundamental embryologic differences between the two.
Beside type of CAKUT, having aCAKUT was also an independent risk factor for hypertension. Decreased nephron endowment is a possible link among these otherwise independent risk factors. The relationship between decreased nephron endowment, hyperfiltration injury, and hypertension has been substantiated in preclinical models (18, 19). Middle-aged adults with primary hypertension have been shown to have half the number of nephrons in comparison to normotensive subjects matched for age, weight, height, and sex (20).
Chronic kidney disease was also found to be a significant independent risk factor for hypertension in our study when adjusted for the other variables. This relationship is complex, as CKD has been described as both a risk factor for and an outcome of hypertension. Our cohort, and CAKUT cases in general, represents a mix of these influences. Postnatally, hypertension and CKD independently emerge as consequences of altered kidney development, but it is unclear why one becomes clinically apparent first. Despite this, CKD increases the likelihood of developing hypertension (21). When adjusted for CKD, the primary diagnosis, and the presence of aCAKUT continued to be independent risk factors for hypertension.
In our CAKUT cohort, cases with hypertension were more likely to have proteinuria. However, proteinuria was not included in the multivariate model for developing hypertension, due to its confounding effect on and lack of independence from hypertension. Little evidence is available to evaluate the relationship between proteinuria and hypertension in children (22).
Birth weight, gestational age at birth, and presence of a genetic syndrome were not significant risk factors for hypertension despite their known associations in children without CAKUT (23, 24). This may be explained by the fact that CAKUT is also associated with low birth weight and premature birth (25, 26) thereby obscuring differences in these risk factors between the hypertensive and non-hypertensive groups, which might be more obvious in a larger cohort.
The progression of CKD in children with kidney disease is influenced by several non-independent risk factors including underlying diagnosis, medication use, episodes of acute kidney injury, and the covariates of proteinuria and hypertension (21). In our CAKUT cohort, hypertension was found to be a significant independent risk factor for CKD when adjusted for the effects of the other independent variables including primary diagnosis and kidney size. This association has also been described in CKiD study patients with non-glomerular disease, where the average GFR decline was 1.7 ml/min per 1.73 m2 per year in children with blood pressure at the 90th percentile, compared to 1.2 ml/min per 1.73 m2 per year in children with blood pressure at the 50th percentile (27). Hypertensive children with kidney dysplasia also had a faster decline in kidney function compared to normotensive children with kidney dysplasia (28). While not a primary objective of this report, we also found proteinuria to be a significant independent risk factor for the development of CKD in CAKUT cases. Interestingly, adjusting the CKD prediction model for proteinuria attenuated the effect of hypertension. This most likely reflects the fact that the effects of the two variables are not independent (21).
A strength of our study is the novelty of including all four major types of CAKUT in the analyses, which allows the comparison of their distinct risk of developing hypertension and the role of hypertension in developing CKD. The cohort is also contemporaneous meaning the criteria for diagnosis, diagnostic labels, and outcome measures were similar across groups. Additional strengths include the reporting of data from subjects with CAKUT prior to GFR decline (distinguishing this work from the NAPRTCS registry and other prospective studies such as CKiD and ESCAPE) and the use of early postnatal ultrasound data including aCAKUT and kidney length to predict hypertension.
Despite the aforementioned strengths, several limitations to this study warrant mentioning. As our study design was retrospective, it resulted in missing data points over time for the variables studied. For example, missing consecutive blood pressure measurements resulted in a smaller final sample size. The retrospective design also introduced a follow up bias of cases with a less favorable course. Had the excluded cases been included we might have expected there to be an even stronger association of the risk factors to hypertension and a larger effect of hypertension on the CKD outcome. Being a single center study may have limited generalizability and introduced sampling and measurement bias, due to center-specific protocols for measuring hypertension and CKD outcomes. Although the inclusion criteria for hypertension was stringent (blood pressures at or above the 95th percentile on consecutive visits at least 3 months apart), the use of ambulatory blood pressure monitoring (ABPM) might be a better way to assess hypertension and identify masked hypertension, which is more common in CKD (29, 30).
Lastly, while our sample size is large and over a decade long, more cases would potentially help discern other predictors of hypertension in patients with CAKUT. A larger cohort would also enable the inclusion of other types of CAKUT in the same types of outcome analyses, for example primary/isolated vesicoureteral reflux and ureteropelvic obstruction. Further multicenter, prospective studies with other types of CAKUT are required to improve and validate the multivariate model.
In conclusion, this study has evaluated the common risk factors for developing hypertension (and the role of hypertension in developing CKD) in a large contemporaneous cohort of CAKUT cases. Results reinforce the importance of timely recognition and treatment of high blood pressure. As part of the development of a clinical pathway for the management of children with kidney anomalies, findings will help inform frequency of follow up and monitoring for the development of hypertension and CKD at pediatric nephrology centers.
Supplementary Material
Funding
B.B. is supported by 5R01DK125469 (U.S. National Institutes of Health, NIDDK).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References:
- 1.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, et al. Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. American Journal of Kidney Diseases. 2015;65(6):878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlton JR, Baldelomar EJ, Hyatt DM, Bennett KM. Nephron number and its determinants: a 2020 update. Pediatric Nephrology. 2021;36(4):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walawender L, Becknell B, Matsell DG. Congenital anomalies of the kidney and urinary tract: defining risk factors of disease progression and determinants of outcomes. Pediatric Nephrology. 2023. 10.1007/s00467-023-05899-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf AS. The term CAKUT has outlived its usefulness: the case for the prosecution. Pediatric Nephrology. 2022;37(11):2785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knoers N The term CAKUT has outlived its usefulness: the case for the defense. Pediatric Nephrology. 2022;37(11):2793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VanDeVoorde RG, Mitsnefes MM. Hypertension and CKD. Advances in Chronic Kidney Disease. 2011;18(5):355–61. [DOI] [PubMed] [Google Scholar]
- 7.Harada R, Hamasaki Y, Okuda Y, Hamada R, Ishikura K. Epidemiology of pediatric chronic kidney disease/kidney failure: learning from registries and cohort studies. Pediatric Nephrology. 2022;37(6):1215–29. [DOI] [PubMed] [Google Scholar]
- 8.Gabriele MM, Koch Nogueira PC. Management of Hypertension in CAKUT: Protective Factor for CKD. Frontiers in Pediatrics. 2019;7:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsell DG, Bao C, Po White T, Chan E, Matsell E, Cojocaru D, et al. Outcomes of solitary functioning kidneys-renal agenesis is different than multicystic dysplastic kidney disease. Pediatric Nephrology. 2021;36(11):3673–80. [DOI] [PubMed] [Google Scholar]
- 10.Matsell DG, Cojocaru D, Matsell EW, Eddy AA. The impact of small kidneys. Pediatric Nephrology. 2015;30(9):1501–9. [DOI] [PubMed] [Google Scholar]
- 11.Matsell DG, Yu S, Morrison SJ. Antenatal Determinants of Long-Term Kidney Outcome in Boys with Posterior Urethral Valves. Fetal Diagnosis and Therapy. 2016;39(3):214–21. [DOI] [PubMed] [Google Scholar]
- 12.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. British Medical Journal. 2000;320(7244):1240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New Equations to Estimate GFR in Children with CKD. Journal of the American Society of Nephrology. 2009;20(3):629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Work DF. Measurement and Estimation of GFR in Children and Adolescents. Clinical Journal of the American Society of Nephrology. 2009;4(11):1832–43. [DOI] [PubMed] [Google Scholar]
- 15.Matsell DG, Bao C, White TP, Chan E, Matsell E, Cojocaru D, et al. Kidney length standardized to body length predicts outcome in infants with a solitary functioning kidney. Pediatric Nephrology. 2023;38(1):173–80. [DOI] [PubMed] [Google Scholar]
- 16.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3). [DOI] [PubMed] [Google Scholar]
- 17.Ardissino G, Testa S, Dacco V, Vigano S, Taioli E, Claris-Appiani A, et al. Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project. Pediatric Nephrology. 2004;19(2):172–7. [DOI] [PubMed] [Google Scholar]
- 18.Luyckx VA, Brenner BM. The clinical importance of nephron mass. Journal of the American Society of Nephrology : JASN. 2010;21(6):898–910. [DOI] [PubMed] [Google Scholar]
- 19.Wlodek ME, Westcott K, Siebel AL, Owens JA, Moritz KM. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney International. 2008;74(2):187–95. [DOI] [PubMed] [Google Scholar]
- 20.Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. The New England Journal of Medicine. 2003;348(2):101–8. [DOI] [PubMed] [Google Scholar]
- 21.Dionne JM, Jiang S, Ng DK, Flynn JT, Mitsnefes MM, Furth SL, et al. Mean Arterial Pressure and Chronic Kidney Disease Progression in the CKiD Cohort. Hypertension. 2021;78(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn JT. Microalbuminuria in Children With Primary Hypertension. The Journal of Clinical Hypertension. 2016;18(10):962–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughson MD. Low birth weight and kidney function: is there a relationship and is it determined by the intrauterine environment? American Journal of Kidney Diseases. 2007;50(4):531–4. [DOI] [PubMed] [Google Scholar]
- 24.Keijzer-Veen MG, Kleinveld HA, Lequin MH, Dekker FW, Nauta J, de Rijke YB, et al. Renal function and size at young adult age after intrauterine growth restriction and very premature birth. American Journal of Kidney Diseases. 2007;50(4):542–51. [DOI] [PubMed] [Google Scholar]
- 25.Hoy WE, Rees M, Kile E, Mathews JD, Wang Z. A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney International. 1999;56(3):1072–7. [DOI] [PubMed] [Google Scholar]
- 26.Argeri R, Thomazini F, Lichtenecker DCK, Thieme K, do Carmo Franco M, Gomes GN. Programmed Adult Kidney Disease: Importance of Fetal Environment. Frontiers of Physiology. 2020;11:586290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, et al. Progression of Pediatric CKD of Nonglomerular Origin in the CKiD Cohort. Clinical Journal of the American Society of Nephrology. 2015;10(4):571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González Celedón C, Bitsori M, Tullus K. Progression of chronic renal failure in children with dysplastic kidneys. Pediatric Nephrology. 2007;22(7):1014–20. [DOI] [PubMed] [Google Scholar]
- 29.Dionne JM. Evidence-based guidelines for the management of hypertension in children with chronic kidney disease. Pediatric Nephrology. 2015;30(11):1919–27. [DOI] [PubMed] [Google Scholar]
- 30.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, et al. Update: Ambulatory Blood Pressure Monitoring in Children and Adolescents. Hypertension. 2014;63(5):1116–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


