Abstract
Rad52 plays a pivotal role in double-strand break (DSB) repair and genetic recombination in Saccharomyces cerevisiae, where mutation of this gene leads to extreme X-ray sensitivity and defective recombination. Yeast Rad51 and Rad52 interact, as do their human homologues, which stimulates Rad51-mediated DNA strand exchange in vitro, suggesting that Rad51 and Rad52 act cooperatively. To define the role of Rad52 in vertebrates, we generated RAD52−/− mutants of the chicken B-cell line DT40. Surprisingly, RAD52−/− cells were not hypersensitive to DNA damages induced by γ-irradiation, methyl methanesulfonate, or cis-platinum(II)diammine dichloride (cisplatin). Intrachromosomal recombination, measured by immunoglobulin gene conversion, and radiation-induced Rad51 nuclear focus formation, which is a putative intermediate step during recombinational repair, occurred as frequently in RAD52−/− cells as in wild-type cells. Targeted integration frequencies, however, were consistently reduced in RAD52−/− cells, showing a clear role for Rad52 in genetic recombination. These findings reveal striking differences between S. cerevisiae and vertebrates in the functions of RAD51 and RAD52.
The many strategies that have evolved to deal with DNA damage attest to the vital importance of chromosomal integrity to all organisms. Double-strand breaks (DSBs) can lead to immediate cell death if unrepaired or to chromosomal loss or translocation if not repaired correctly. Aside from environmentally induced damage, DSBs of genomic DNA are generated during several biological processes such as meiotic recombination or the development of the vertebrate immune system. Accordingly, the enzymes and systems of DSB repair are of great interest in biology.
The RAD52 epistasis group of genes (RAD50, RAD51, RAD52, RAD54, RAD55, RAD57, RAD59, MRE11, and XRS2) has been defined by the respective Saccharomyces cerevisiae mutants, which are hypersensitive to ionizing radiation and exhibit mitotic and meiotic recombination defects. While phenotypic differences between mutants distinguish between these genes genetically, it is clear that they are constituents of a pathway for the repair of DSB damage by homologous recombination (reviewed in references 10, 22, and 27). The high degree of conservation of the RAD52 group of genes from yeast to vertebrates (4, 8, 14, 23, 25) suggests a similar role for these proteins. However, while vertebrate RAD54−/− cells reflect the yeast phenotype, namely, extreme sensitivity to γ-ray and defective recombination (5, 9), RAD51 deficiency results in the death of vertebrate cells, indicating that Rad51 is essential for cell proliferation (16, 29, 34).
Rad52 mutants show the most pronounced phenotype among RAD52 epistasis group mutants in S. cerevisiae. Rad52 is essential for an intermediate stage after the formation of DSBs but before the appearance of stable recombinants during gene conversion (26). Rad52 is also involved in single-strand annealing (13) and other RAD51-independent forms of recombination (30), which may explain the severe phenotype of rad52 mutants. Recent evidence suggests that both yeast and human Rad52 interact with the respective Rad51 protein, a structural and functional homologue of the well-characterized Escherichia coli RecA protein (25, 26), during recombination (31). This interaction facilitates the Rad51 strand exchange reaction (3, 21, 28), potentially involving Rad52 binding of DNA (3, 18, 27). To define the role of Rad52 in vertebrate cells, we generated RAD52−/− clones from the chicken B-lymphocyte line DT40, which exhibits highly efficient targeted integration following the transfection of genomic DNA constructs (7).
MATERIALS AND METHODS
Plasmid constructs.
The ∼11-kb genomic chicken RAD52 locus was cloned from DT40 genomic DNA by long-range PCR with the chicken RAD52 cDNA primers 5′-tga aag gca agg gaa gga cag tga aag cca tg-3′ and 5′-gtc tgt cca cat tta gtg ttt att ctt gtg tt-3′ (4), and the positions of the exons and introns were determined by sequencing (Fig. 1). The Hisr or Bsrr selection marker genes under the control of the β-actin promoter (7) were inserted between the left and right arms derived by PCR amplification of this construct. To make the disruption construct for the rearranged Igλ locus, Igλ-neo, the Neor cassette was inserted into the BglI site in the C region of the 13-kb BglII-XbaI fragment from the 18-D-1 clone (24). The RAD52 expression vector consisted of the EcoRI-SalI fragment of chicken cDNA (4) cloned into pApuroII (15).
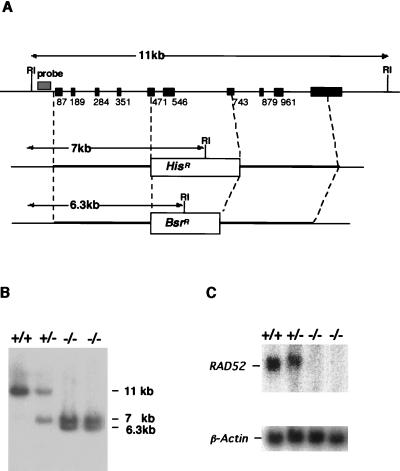
FIG. 1.
Strategy of disruption of the RAD52 gene. (A) Schematic representation of part of the RAD52 locus, the two gene disruption constructs, and the configuration of the targeted loci. Solid boxes indicate the positions of exons; numbers show the 3′ nucleotide of each exon relative to the start codon (4). Relevant EcoRI recognition sites are indicated by RI. (B) Southern blot analysis of EcoRI-digested DNA from the indicated genotypes with the probe shown in panel A. The positions and sizes of the hybridizing fragments of the wild-type and targeted loci are indicated. (C) Northern blot analysis of total RNA with the full-length chicken RAD52 cDNA as a probe. The same filter was rehybridized with a chicken β-Actin probe (7).
Cell culture, DNA transfections, and sIgM staining.
The conditions of cell culture and DNA transfections were described previously (29). To measure the rate of immunoglobulin (Ig) gene conversion, cells were subcloned and the percentage of surface IgM-positive (sIgM+) cells in 45 subclones from each genotype was measured as described previously (5).
Measurement of Rad51 foci.
Paraformaldehyde-fixed cells were permeabilized with 0.1% Nonidet P-40 (Sigma) and incubated with rabbit anti-human Rad51 antiserum (33). Staining was visualized with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (Santa Cruz), and the cells were mounted under Slow Fade solution (Molecular Probes). All the images were taken by confocal microscopy (MRC-1024; Bio-Rad) and processed with Adobe Photoshop version 4.0J.
Measurement of sensitivity of cells to γ-rays, MMS, and cisplatin.
Serially diluted cells were plated in medium containing methylcellulose and irradiated with a 137Cs source or treated with methyl methanesulfonate (MMS) (Sigma) or cis-platinum(II)diammine dichloride (cisplatin). Colonies were counted 10 days after treatment. The percent survival was determined relative to the number of colonies from untreated cells.
Measurement of targeted integration frequencies.
To analyze targeted integration events at the β-Actin and Ovalbumin loci, the disruption construct DNA for either locus described in reference 7 was transfected into cells and Southern blot analysis was performed following selection of clones resistant to the appropriate antibiotic. To analyze targeted integration events at the rearranged Igλ locus, sorter-purified sIgM+ cells were transfected with the rearranged Igλ-neo disruption construct. G418-resistant cells were selected in bulk populations, and sIgM expression was measured by flow cytometry (7).
RESULTS
Generation of RAD52−/− mutants.
Two RAD52 disruption constructs, RAD52his and RAD52bsr, were generated from an ∼11-kb genomic PCR product (4). Targeted integration of these constructs was expected to replace the chicken RAD52 coding sequence for amino acids 137 to 182 (which lie in the conserved N-terminal region implicated in DNA binding [18, 19]) with the selection markers. The disruption of the RAD52 gene in two independently isolated clones was verified by Southern and Northern blot analyses (Fig. 1B and C). These RAD52−/− clones were indistinguishable from wild-type cells with respect to growth rate and cloning efficiency (data not shown).
Effects of Rad52 deficiency on sensitivity to DNA-damaging agents, Rad51 focus formation, and Ig gene conversion.
The repair capacity of cells defective in RAD52 was analyzed in a colony survival assay with RAD54−/− cells in parallel as a control. We examined its sensitivity to ionizing radiation and to MMS, as well as to a DNA-cross-linking agent, cisplatin. Repair of DNA lesions induced by cisplatin is known to depend upon both nucleotide excision repair and recombinatorial repair in yeast (12). RAD54−/− cells showed increased sensitivity to ionizing radiation (Fig. 2A) and MMS (Fig. 2B), as reported previously. Rad54 was also required for the repair of cisplatin adducts (Fig. 2C), implicating the recombinatorial repair pathway in the repair of interstrand cross-linking in vertebrate cells. In marked contrast to rad52 mutants of S. cerevisiae, which are extremely sensitive to DNA-damaging agents, there was no significant difference between wild-type and RAD52−/− DT40 clones in sensitivity to ionizing radiation (Fig. 2A). Similarly, the sensitivity of RAD52−/− DT40 cells to MMS or to cisplatin was not significantly increased over those of wild-type (RAD52+/+) and heterozygous (RAD52+/−) cells (Fig. 2B and C). These observations indicate that although the recombinational repair pathway is involved in DSB repair in vertebrate cells, it can function in the absence of Rad52.
FIG. 2.
Sensitivity of the indicated clones to DNA-damaging agents. The fractions of colonies surviving after the indicated treatment of cells compared to nontreated controls of the same genotype are shown on the y axis on a logarithmic scale. (A) Ionizing radiation; (B) MMS; (C) cisplatin. The radiation doses and the MMS and cisplatin concentrations are displayed on the x axis on a linear scale in each graph. Data shown are the means ± standard deviations of at least three separate experiments.
To monitor the kinetics of recombinational repair, we analyzed the appearance of Rad51 foci in cell nuclei over time after application of γ-radiation (Fig. 3). Since Rad51 polymerizes on DNA and promotes in vitro strand exchange (2, 26), a Rad51 focus may reflect an intermediate structure of recombinational repair. The formation of Rad51 foci is observed during meiosis in S. cerevisiae and is abrogated in rad52 mutants (10a). Furthermore, Rad51 foci are also observed in vertebrate cells during the S phase (33), which implies that Rad51 may be responsible for repairing spontaneous DSBs during the cell cycle (29). Rad51 foci were induced in DT40 cells by γ-irradiation in a dose-dependent manner (data not shown), as previously reported for mammalian cells (11). We found no significant difference between RAD52−/− and wild-type cells with respect to the number of Rad51 foci in cycling cells and to the kinetics of Rad51 focus formation following γ-irradiation. This observation, along with the capacity of RAD52−/− cells to withstand γ-radiation, indicates that DSB repair occurs efficiently in the absence of Rad52.
FIG. 3.
Immunofluorescent visualization of Rad51. At the time indicated after 8-Gy γ-irradiation, wild-type and RAD52−/− cells were analyzed. Controls were stained with normal rabbit serum followed by FITC-conjugated anti-rabbit IgG and are overexposed relative to the experimental frames.
In a manner similar to the process of B-cell diversification in the bursa of Fabricius, DT40 continues to diversify its Ig light (λ)-chain locus by gene conversion, with pseudogenes serving as donors (6). To analyze such intrachromosomal recombination, RAD52−/− clones were generated from an sIgM− variant of DT40, clone 18, containing a frameshift in its rearranged V segment in the light-chain locus. Since this frameshift mutation can be repaired by overlapping gene conversion events leading to reexpression of sIgM, the rate of Ig gene conversion can be assessed by measuring the percentage of sIgM+ revertants (6). We measured the average percentage of sIgM+ revertants of 45 subclones each from the wild-type and the two RAD52−/− clones and found no difference between these clones (the reversion rate was 2.48 × 10−3 for the wild type and 2.40 × 10−3 and 2.89 × 10−3 for the two RAD52−/− clones, as calculated from the equation in reference 17).
Reduced targeted integration frequencies in RAD52−/− cells.
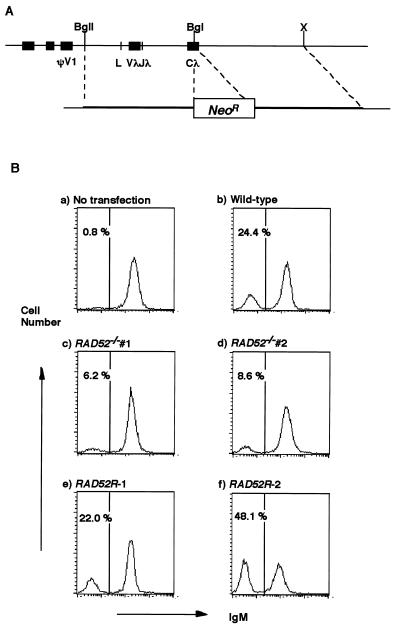
We next examined targeted integration frequencies at the Igλ, β-Actin, and Ovalbumin loci, comparing wild-type cells, two RAD52−/− clones, and two RAD52−/− clones reconstituted with chicken RAD52 cDNA. To measure targeted integration frequencies at the Igλ locus, we sorted sIgM+ cells from each of the five clones, transfected them with the Igλ-neo construct (Fig. 4A), and measured the expression of sIgM in the bulk population of G418-resistant cells. Since integration of the Igλ-neo construct at the Igλ locus results in sIgM− cells, the fraction of sIgM− cells among transfectants reflects the frequency of the targeted integration events at this locus. Spontaneous reversion caused by Ig gene conversion was ≤1%. Representative data are shown in Fig. 4B. While transfection efficiencies during these experiments were comparable, the averages of targeted integration frequencies at this locus in triplicate experiments were consistently lower (ca. threefold) in RAD52−/− clones than in the wild type. The expression of RAD52 cDNA restored targeted integration to wild-type levels in the two RAD52−/− clones (Table 1). Similarly, the disruption of RAD52 caused a four- to eightfold reduction of the targeted integration frequency at the β-Actin locus when a β-Actin targeting construct was used (7). This frequency was partially restored in a reconstituted clone (RAD52R-1), which expressed the RAD52 cDNA transcript at a level ca. 10-fold higher than that in wild-type DT40 cells (data not shown), confirming that Rad52 is involved in the targeted integration of transfected genomic DNA constructs (Table 1). While RAD52−/− clones showed a marked reduction in targeted integration frequencies at the β-Actin and Igλ loci, the targeted integration frequency at the Ovalbumin locus was reduced only to ∼85% of the wild-type levels (with some variation depending on the construct and locus in question). Taking all loci together, there is a significant reduction in targeted integration frequency between wild-type and RAD52−/− clones (P < 0.05, Dunnett’s multicomparison test). This reduced efficiency of targeted integration events has been also observed in murine embryonic stem (ES) cells deficient in RAD52 (24a).
FIG. 4.
Measurement of targeted integration frequencies at the Igλ locus. (A) Schematic representation of part of the rearranged Igλ locus and the disruption construct (Igλ-neo). ΨV1, first pseudogene; L, leader sequence; Vλ, variable gene segment; Jλ, joining gene segment; Cλ, constant gene segment. BglI (BgI), BglII (BgII), and XbaI (X) restriction sites are indicated. Not all BglI sites in this region are shown. (B) Histograms of sIgM expression of wild-type (a and b), RAD52−/− (c and d), and RAD52 cDNA-reconstituted RAD52−/− (RAD52R) (e and f) clones after no transfection (a) or transfection of Igλ-neo and G418 selection of transfectants of each clone (b to f). The x and y axes show the fluorescence intensity from an FITC-conjugated anti-IgM polyclonal antibody on a logarithmic scale and the cell number on a linear scale in each graph, respectively. The percentage of cells losing sIgM expression is shown in each panel.
TABLE 1.
Targeted integration frequencies in RAD52−/− cells
| Locus | Targeted integration frequency ina:
|
||||
|---|---|---|---|---|---|
| Wild type | RAD52−/− (clone 1) | RAD52−/− (clone 2) | RAD52R-1d | RAD52R-2d | |
| Igλ | |||||
| Expt 1 | 24% | 6.2% | 8.6% | 48% | 22% |
| Expt 2 | 39% | 15% | NDc | 51% | 23% |
| Expt 3 | 35% | 13% | 19% | 39% | ND |
| Expt 4 | 44% | ND | 17% | ND | 59% |
| Relative ratiob | 1 | 0.34 ± 0.067 | 0.42 ± 0.1 | 1.5 ± 0.47 | 0.94 ± 0.35 |
| β-Actin | 48% (45/93) | 6.6% (4/61) | 12.5% (3/24) | 31% (22/72) | 32% (23/72) |
| Relative ratio | 1 | 0.14 | 0.26 | 0.65 | 0.67 |
| Ovalbumin | 89% (31/35) | 70% (19/27) | 83% (24/29) | ND | ND |
| Relative ratio | 1 | 0.79 | 0.93 | ||
Targeted integration frequencies indicated are, for Igλ, the percentage of cells losing sIgM expression measured by flow cytometry as shown in Fig. 4. For β-Actin and Ovalbumin, the percentages of targeted clones are shown relative to the total number of G418-resistant clones analyzed by Southern analysis, with absolute numbers (number of targeted integration events/number of clones analyzed) given in parentheses.
The ratios relative to the wild type are the frequency of targeted integration in the respective clone divided by that of the wild-type cells; for Igλ, the mean ± standard deviation calculated from at least three separate experiments is indicated.
ND, not determined.
RAD52R-1 and RAD52R-2 are RAD52−/− cells expressing the chicken RAD52 cDNA.
DISCUSSION
The above data demonstrate that Rad52 is not required for the repair of induced DSBs, Rad51 focus formation, or intragenic Ig gene conversion. Similarly, X-ray sensitivity was not increased in RAD52-deficient ES cells or mutant mice (24a). These results are perhaps surprising, given the severe phenotype associated with mutation of the RAD52 gene in S. cerevisiae. Furthermore, while the rad51, rad52, and rad54 mutants of S. cerevisiae exhibit similar defects in both DNA repair and recombination, we and other groups have revealed that RAD51-, RAD52-, and RAD54-deficient DT40 cells display quite distinct phenotypes, as do the corresponding murine ES cell mutants (5, 9, 16, 29, 34). A further example of such differences is found in Schizosaccharomyces pombe, where mutation of the RAD51 and RAD54 homologues, rhp51+ and rhp54+, leads to similar hypersensitivity to radiation and targeted integration deficiency, whereas deficiency in the RAD52 homologue, rad22+, has a less severe effect (20).
There are a number of possible explanations for the difference in phenotypes resulting from the deficiency of RAD52 homologues between S. cerevisiae and vertebrate cells, and these explanations are not mutually exclusive. There may be as yet undescribed vertebrate homologues of RAD52, which might compensate for the absence of Rad52. The recent description of S. cerevisiae RAD59, a homologue of RAD52, and its role in intrachromosomal recombination (1) suggests that other vertebrate RAD52 homologues remain to be defined. However, neither low-stringency Southern hybridization nor PCR with degenerate primers encompassing the most highly conserved N-terminal region identified any RAD52 homologue in chicken genomic DNA or cDNA, respectively. Nevertheless, a complex of Rad55 and Rad57 has been shown to act similarly to Rad52 in facilitating strand exchange by Rad51 in the presence of replication protein A in vitro (32), suggesting that any putative Rad52 homologue which is responsible for this Rad51-dependent function of Rad52 may be a functional but not necessarily a structural homologue.
In addition, the precise mechanism for recombinational repair may differ between vertebrate and yeast species. Accordingly, the relative contributions of Rad51, Rad52, and Rad54 homologues to recombinational repair may not be identical, explaining the observed weaker phenotype of the RAD52-deficient vertebrate cells compared to RAD51- and RAD54-deficient cells.
RAD52−/− DT40 clones showed a significant decrease in the frequency of targeted integration at the Igλ and β-Actin loci while showing normal sensitivity to γ-rays and MMS, in agreement with observations on RAD52-deficient murine ES cells. DSB repair in DT40 cells relies on recombinational repair, as shown by the severe effects of RAD54 deficiency (5). While the absence of such hypersensitivity to DSBs implies a secondary role for Rad52 in recombinational repair, recombination of transfected DNA with genomic sequences may be a more subtle method to define the involvement of Rad52 in homologous recombination than is a survival assay. Recent in vitro studies of strand exchange reactions have indicated that Rad52 is required during the critical, early steps in genetic recombination (3, 21, 28). Since the DNA structure is known to play a role in determining the genetic requirements for yeast recombination in vivo (30), the in vitro reaction may reflect the homologous recombination of transfected plasmid DNA rather than the recombinational repair of chromosomal DNA. Rad52 may facilitate targeted integration by specifically interacting with transfected targeting construct DNA, which might explain the results obtained in the gene-targeting experiments above. Further genetic studies with knockout mouse and DT40 technology may help to clarify the relationships in the complex mechanism(s) of recombination and chromosomal maintenance.
ACKNOWLEDGMENTS
We would like to thank T. Shibata (Riken, Wako, Japan), T. Ogawa (Institute of Genetics, Mishima, Japan), and A. Pastink (University of Leiden, Leiden, The Netherlands) for critically reading the manuscript; Y. Kubota (Caltech, Pasadena, Calif.) for statistical analysis; and M. Hashishin, Y. Sato, O. Koga, and M. Hirao for their excellent technical assistance.
C.M. is the recipient of a JSPS Postdoctoral Fellowship. The Bayer-chair of the Department of Molecular Immunology and Allergology is supported by Bayer Yakuhin, Kyoto, Japan. This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science and Culture of Japan and by a grant from The Mochida Memorial Foundation for Medical and Pharmaceutical Research. The Basel Institute for Immunology was founded and is supported by F. Hoffmann La-Roche Ltd., Basel, Switzerland.
REFERENCES
- 1.Bai Y, Symington L S. A Rad52 homolog is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae. Genes Dev. 1996;10:2025–2037. doi: 10.1101/gad.10.16.2025. [DOI] [PubMed] [Google Scholar]
- 2.Baumann P, Benson F E, West S C. Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell. 1996;87:757–766. doi: 10.1016/s0092-8674(00)81394-x. [DOI] [PubMed] [Google Scholar]
- 3.Benson F E, Baumann P, West S C. Synergistic actions of Rad51 and Rad52 in recombination and DNA repair. Nature. 1998;391:401–404. doi: 10.1038/34937. [DOI] [PubMed] [Google Scholar]
- 4.Bezzubova O Y, Schmidt H, Ostermann K, Heyer W-D, Buerstedde J-M. Identification of a chicken RAD52 homologue suggests conservation of the RAD52 recombination pathway throughout the evolution of higher eukaryotes. Nucleic Acids Res. 1993;21:5945–5949. doi: 10.1093/nar/21.25.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezzubova O Y, Silbergleit A, Yamaguchi-Iwai Y, Takeda S, Buerstedde J-M. Reduced X-ray resistance and homologous recombination frequencies in a RAD54−/− mutant of the chicken DT40 cell line. Cell. 1997;89:185–193. doi: 10.1016/s0092-8674(00)80198-1. [DOI] [PubMed] [Google Scholar]
- 6.Buerstedde J M, Reynaud C A, Humphries E H, Olson W, Ewert D L, Weill J C. Light chain gene conversion continues at high rate in an ALV-induced cell line. EMBO J. 1990;9:921–927. doi: 10.1002/j.1460-2075.1990.tb08190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buerstedde J M, Takeda S. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell. 1991;67:179–188. doi: 10.1016/0092-8674(91)90581-i. [DOI] [PubMed] [Google Scholar]
- 8.Dolganov G M, Maser R S, Novikov A, Tosto L, Chong S, Bressan D A, Petrini J H J. Human Rad50 is physically associated with human Mre11: identification of a conserved multiprotein complex implicated in recombinational DNA repair. Mol Cell Biol. 1996;16:4832–4841. doi: 10.1128/mcb.16.9.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Essers J, Hendriks R W, Swagemakers S M A, Troelstra C, de Wit J, Bootsma D, Hoeijmakers J H J, Kanaar R. Disruption of mouse RAD54 reduces ionizing radiation resistance and homologous recombination. Cell. 1997;89:195–204. doi: 10.1016/s0092-8674(00)80199-3. [DOI] [PubMed] [Google Scholar]
- 10.Game J C. DNA double-strand breaks and the RAD50-RAD57 genes in Saccharomyces. Semin Cancer Biol. 1993;4:73–83. [PubMed] [Google Scholar]
- 10a.Gasior S L, Wong A K, Kora Y, Shinohara A, Bishop D K. Rad52 associates with RPA and functions with Rad55 and Rad57 to assemble meiotic recombination complexes. Genes Dev. 1998;12:2208–2221. doi: 10.1101/gad.12.14.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haaf T, Golub E I, Reddy G, Radding C M, Ward D C. Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proc Natl Acad Sci USA. 1995;92:2298–2302. doi: 10.1073/pnas.92.6.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannan M A, Zimmer S G, Hazle J. Mechanisms of cisplatin (cis-diamminodichloroplatinum II)-induced cytotoxicity and genotoxicity in yeast. Mutat Res. 1984;127:23–30. doi: 10.1016/0027-5107(84)90136-2. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov E L, Sugawara N, Fishman-Lobell J, Haber J E. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics. 1996;142:693–704. doi: 10.1093/genetics/142.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanaar R, Troelstra C, Swagemakers S M A, Essers J, Smit B, Franssen J-H, Pastink A, Bezzubova O, Buerstedde J-M, Clever B, Heyer W-D, Hoeijmakers J H J. Human and mouse homologs of the Saccharomyces cerevisiae RAD54 DNA repair gene: evidence for functional conservation. Curr Biol. 1996;6:828–838. doi: 10.1016/s0960-9822(02)00606-1. [DOI] [PubMed] [Google Scholar]
- 15.Kurosaki T, Takata M, Yamanashi Y, Inazu T, Taniguchi T, Yamamoto T, Yamamura H. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med. 1994;179:1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim D-S, Hasty P. A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol Cell Biol. 1996;16:7133–7143. doi: 10.1128/mcb.16.12.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luria S E, Delbrück M. Mutation of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortensen U H, Bendixen C, Sunjevaric I, Rothstein R. DNA strand annealing is promoted by the yeast Rad52 protein. Proc Natl Acad Sci USA. 1996;93:10729–10734. doi: 10.1073/pnas.93.20.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muris D F, Bezzubova O, Buerstedde J-M, Vreeken K, Balajee A S, Osgood C J, Troelstra C, Hoeijmakers J H, Ostermann K, Schmidt H, Natarajan A T, Eeken J C J, Lohman P H M, Pastink A. Cloning of human and mouse genes homologous to RAD52, a yeast gene involved in DNA repair and recombination. Mutat Res. 1994;315:295–305. doi: 10.1016/0921-8777(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 20.Muris D F R, Vreeken K, Schmidt H, Ostermann K, Clever B, Lohman P H M, Pastink A. Homologous recombination in the fission yeast Schizosaccharomyces pombe: different requirements for the rhp51+, rhp54+, and rad22+ genes. Curr Genet. 1997;31:248–254. doi: 10.1007/s002940050202. [DOI] [PubMed] [Google Scholar]
- 21.New J H, Sugiyama T, Zaitseva E, Kowalczykowski S C. Rad52 protein stimulates DNA strand exchange by Rad51 and replication protein A. Nature. 1998;391:407–410. doi: 10.1038/34950. [DOI] [PubMed] [Google Scholar]
- 22.Petes T D, Malone R E, Symington L S. Recombination in yeast. In: Broach J R, Pringle J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 407–522. [Google Scholar]
- 23.Petrini J H J, Walsh M E, DiMare C, Korenberg J R, Chen X N, Weaver D T. Isolation and characterization of the human MRE11 homologue. Genomics. 1995;29:80–86. doi: 10.1006/geno.1995.1217. [DOI] [PubMed] [Google Scholar]
- 24.Reynaud C A, Anquez V, Grimal H, Weill J C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987;48:379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- 24a.Rijkers T, Van Den Ouweland J, Morolli B, Rolink A G, Baarends W M, Van Sloun P P H, Lohman P H M, Pastink A. Targeted inactivation of mouse RAD52 reduces homologous recombination but not resistance to ionizing radiation. Mol Cell Biol. 1998;18:6423–6429. doi: 10.1128/mcb.18.11.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinohara A, Ogawa H, Matsuda Y, Ushio N, Ikeo K, Ogawa T. Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat Genet. 1993;4:239–243. doi: 10.1038/ng0793-239. . (Erratum, 5:312, 1993.) [DOI] [PubMed] [Google Scholar]
- 26.Shinohara A, Ogawa H, Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992;69:457–470. doi: 10.1016/0092-8674(92)90447-k. . (Erratum, 71:180, 1992.) [DOI] [PubMed] [Google Scholar]
- 27.Shinohara A, Ogawa T. Homologous recombination and the roles of double-strand breaks. Trends Biochem Sci. 1995;20:387–391. doi: 10.1016/s0968-0004(00)89085-4. [DOI] [PubMed] [Google Scholar]
- 28.Shinohara A, Ogawa T. Stimulation by Rad52 of yeast Rad51-mediated recombination. Nature. 1998;391:404–407. doi: 10.1038/34943. [DOI] [PubMed] [Google Scholar]
- 29.Sonoda E, Sasaki M S, Buerstedde J-M, Bezzubova O, Shinohara A, Ogawa H, Takata M, Yamaguchi-Iwai Y, Takeda S. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 1998;17:598–608. doi: 10.1093/emboj/17.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugawara N, Ivanov E L, Fishman-Lobell J, Ray B L, Wu X, Haber J E. DNA structure-dependent requirements for yeast RAD genes in gene conversion. Nature. 1995;373:84–86. doi: 10.1038/373084a0. [DOI] [PubMed] [Google Scholar]
- 31.Sung P. Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J Biol Chem. 1997;272:28194–28197. doi: 10.1074/jbc.272.45.28194. [DOI] [PubMed] [Google Scholar]
- 32.Sung P. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 1997;11:1111–1121. doi: 10.1101/gad.11.9.1111. [DOI] [PubMed] [Google Scholar]
- 33.Tashiro S, Kotomura N, Shinohara A, Tanaka K, Ueda K, Kamada N. S phase specific formation of the human Rad51 protein nuclear foci in lymphocytes. Oncogene. 1996;12:2165–2170. [PubMed] [Google Scholar]
- 34.Tsuzuki T, Fujii Y, Sakumi K, Tominaga Y, Nakao K, Sekiguchi M, Matsushiro A, Yoshimura Y, Morita T. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc Natl Acad Sci USA. 1996;93:6236–6240. doi: 10.1073/pnas.93.13.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]