Abstract
Pulmonary vasodilator treatment can improve hemodynamics, right ventricular function, symptoms, and survival in pediatric pulmonary hypertension (PH). However, clinical trial data are lacking due to many constraints. One major limitation is the lack of relevant trial endpoints reflective of hemodynamics or functional status in patients in whom standard exercise testing is impractical, unreliable, or not reproducible. The Kids Mod PAH trial (Mono‐ vs. Duo Therapy for Pediatric Pulmonary Arterial Hypertension) is an ongoing multicenter, Phase III, randomized, open‐label, pragmatic trial to compare the safety and efficacy of first‐line combination therapy (sildenafil and bosentan) to first‐line monotherapy (sildenafil alone) in 100 pediatric patients with PH across North America. Investigators will measure participants’ physical activity with a research‐grade, wrist‐worn actigraphy device at multiple time points as an exploratory secondary outcome. Vector magnitude counts per minute and activity intensity will be compared between the treatment arms. By directly and noninvasively measuring physical activity in the ambulatory setting, we aim to identify a novel, simple, inexpensive, and highly reproducible approach for quantitative assessment of exercise tolerance in pediatric PH. These data will increase the field's understanding of the effect of pulmonary vasodilator treatment on daily activity – a quantitative measure of functional status and wellbeing in pediatric PH and a potential primary outcome for future clinical trials in children with cardiopulmonary disorders.
Keywords: accelerometer, pediatric pulmonary arterial hypertension
PEDIATRIC PULMONARY HYPERTENSION
With a prevalence of 2–16 cases per million children, 1 , 2 , 3 pediatric pulmonary hypertension (PH) is a rare condition that can present in children of all ages. Common classifications of pediatric PH include World Symposium of PH Group 1 pulmonary arterial hypertension (PAH; idiopathic, heritable, or associated with congenital heart disease) and Group 3 PH due to developmental lung diseases. 4 In PH, pulmonary vascular changes including medial hypertrophy, intimal proliferation, and fibrous occlusion of distal vessels results in increased right ventricular afterload that manifests clinically as exercise intolerance and shortness of breath with exertion. Without treatment, the disease leads to right ventricular dysfunction, right ventricular failure, and often death. Although survival has improved with pharmacologic advances, 5‐year survival from diagnosis of PAH is still only about 75% and patients often suffer from reduced exercise capacity. 2 , 5 , 6 Patients with developmental lung diseases (Group 3 PH), including bronchopulmonary dysplasia and congenital diaphragmatic hernia, may be the fastest growing group of pediatric PH patients, 4 and yet neither pediatric or adult Group 3 patients have been studied in clinical trials. The ability to reliably assess exercise capacity and tolerance is limited in both Group 1 (PAH) and Group 3 (lung disease‐related PH).
CLINICAL TRIALS IN PH
Pulmonary vasodilator treatment can improve pulmonary vascular hemodynamics, right ventricular heart function, symptoms, exercise capacity and survival. While many randomized controlled clinical trials have been performed in adult PH, there have been very few in pediatric PH. 7 , 8 Consequently, only two pulmonary vasodilator medications (sildenafil and bosentan) are approved by the United States Food and Drug Administration (FDA) for treatment of children with PH. Announcement of FDA approval of sildenafil for children with PH in 2023 translated a longstanding practice trend into more common practice. Although children with PH are successfully treated off‐label with various classes of pulmonary vasodilators based on expert opinion, small single‐center studies, and extrapolation from adult data, outcomes would be enhanced by robust randomized controlled clinical trial data.
ACTIGRAPHY IN PH
One limitation to pediatric PH clinical trials is the lack of relevant endpoints indicative of hemodynamics or functional status. 9 As exercise intolerance is common in PH patients, the 6‐min walk distance (6MWD) is one of the most common primary endpoints in adult PH trials. 9 However, infants and children with PH are often too young and/or developmentally unable to perform standard cardiopulmonary exercise testing (CPET) or the 6MWD reliably. In a previous trial of sildenafil in pediatric PAH, only 49% of participants could reliably perform CPET. Reasons for not exercising included age, developmental concerns, inability to reach the pedals on the stationary bike, intolerance of the mouthpiece to measure gas exchange, and physical inactivity. 7
Actigraphy devices are wearable accelerometers that can directly and noninvasively measure physical activity in the ambulatory setting and may provide a novel, simple, and inexpensive approach for improved quantitative assessment of exercise tolerance in pediatric PH. 10 , 11 , 12 Multiple adult studies have demonstrated associations between lower activity measures and worse quality of life, higher fatigue scores, lower 6MWD, and worse performance on CPET. 13 , 14 , 15 , 16 , 17 , 18 Actigraphy devices have been used to quantify activity in infants, toddlers, and older children in diverse settings and with a range of developmental conditions. 19 , 20 , 21 , 22 , 23 Data from the Pediatric Pulmonary Hypertension Network (PPHNet) Actigraphy Working Group demonstrate lower physical activity levels in infants and children with PH compared to healthy controls. 24 , 25 Other work demonstrated that time spent in physical activity correlates positively with 6MWD and negatively with functional class in youth with PH. 26 Higher tricuspid regurgitant jet velocity by echocardiogram has been associated with fewer daily steps and greater sedentary time on commercially available fitness trackers. 27 These studies suggest that application of actigraphy in pediatric PH may meet a critical need of identifying novel measures that reflect changes in hemodynamics or functional status in response to a therapeutic intervention and could be tested in clinical trials.
KIDS MOD PAH PRIMARY STUDY DESIGN
Funded by the National Institutes of Health/National Heart, Lung, and Blood Institute, the Kids Mod PAH trial (NCT04039464) is a Phase III, randomized, open‐label, pragmatic trial to compare the safety and efficacy of first‐line combination therapy (sildenafil and bosentan) to first‐line monotherapy (sildenafil alone) in 100 pediatric patients ages 3 months to 18 years with PH from 12 participating PPHNet sites in the United States and Canada. 28 The primary outcome of this study is the World Health Organization (WHO) functional class at 12 months after initiation of study drug therapy. Consent forms for the Kids Mod PAH trial are available in English and Spanish.
Actigraphy is an exploratory secondary outcome that will be assessed at multiple time points throughout this multicenter randomized control trial. We will use the ActiGraph CentrePoint Insight Watch (CPIW; ActiGraph, LLC) to measure 7‐day physical activity for study participants at study months 0 (baseline), 3, 6, 12, 18, and 24. We will capture activity across three axes as vector magnitude counts per minute (CPM). Activity intensity will be quantified as the percentage of time spent in moderate or vigorous activity. By exploring the longitudinal association between actigraphy measures and WHO functional class, an outcome that has been used in many adult PH clinical trials, one pediatric PH clinical trial 7 and several pediatric PH retrospective observational studies, 29 , 30 we will increase understanding of actigraphy as a valid surrogate endpoint for daily functioning in future clinical trials.
ACTIGRAPHY METHODS/PROTOCOL
After informed consent, demographic information, height, weight, resting blood pressure, and resting oxygen saturation will be obtained at the screening visit. A fully charged ActiGraph CPIW device will be worn on the nondominant wrist (when identifiable) (Figure 1). Commercially available wrist straps adjust the size for small infants and children. Families will be instructed to have the child wear the device for 7 consecutive days with a goal of ≥8 h of daytime wear per day on ≥4 days. The same device is intended to be used for each participant throughout the study. The device will be programmed to record triaxial activity data at a frequency of 32 Hz. The primary caretaker will download the ActiGraph CentrePoint Connect application to their smartphone or Bluetooth‐enabled device. The primary caretaker will be asked to sync the ActiGraph daily during each 7‐day monitoring period so that the local study team can ensure transmission of data to the CentrePoint software installed on computers at the sites. In the event a family does not have access to a Bluetooth‐enabled mobile device, a syncing hub that can be plugged into an internet modem will be provided.
Figure 1.

ActiGraph CentrePoint Insight Watch (CPIW). The ActiGraph CPIW will be worn as a wristwatch on the nondominant wrist (when identifiable). Commercially available wrist straps adjust the size for small infants and children. The device is easily charged via the wireless charging dock (shown on the right).
A full data set will be downloaded to CentrePoint at the subsequent in‐person study visit. A syncing cable at the study site is required to download the entire data set. If the download demonstrates that the participant has worn the device for fewer than 4 days during the monitoring period, the team may request that the participant wear the device for additional days. In the unexpected scenario that an ActiGraph needs to be reused by a subsequent study participant, the monitor will be inspected for functionality and cleaned with a hospital‐grade disinfectant or alcohol‐based solution or wipe before charging and reusing.
During monthly check‐in phone calls for the Kids Mod PAH trial, study teams will assess for adverse events, unanticipated problems, or protocol deviations related to the ActiGraph (i.e., lack of wear) and will record in study logs.
ACTIVITY ANALYSES
After local downloads to CentrePoint, raw actigraphy data will be integrated into 60 s epochs. Any time period with zero vector magnitude CPM for >90 min is considered sleep or nonwear time. A valid day will be defined by a minimum of 8 h of wear time. Published pediatric cut points for wrist‐worn ActiGraph accelerometer counts will be applied to determine time spent in sedentary, light activity, moderate activity, and vigorous activity. 31 , 32 , 33 Daily averages for wear time, steps, vector magnitude CPM, and activity intensity categories will be calculated (Figure 2). Although step counts will not be available in pre‐ambulatory children, other valuable measures of physical activity including vector magnitude CPM will be analyzed. Aggregate daily wear time and activity data will be accessed via an Application Programming Interface (API) data pull from the CentrePoint cloud database to a Research Electronic Data Capture database (RedCap, Vanderbilt University).
Figure 2.
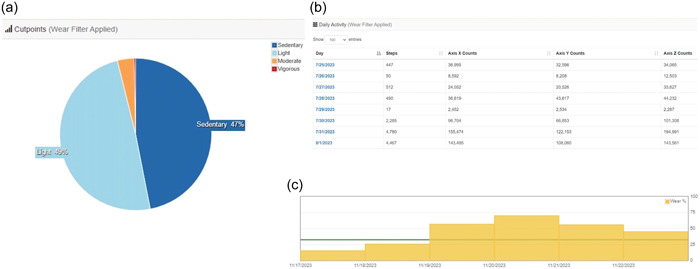
Sample ActiGraph data. Sample ActiGraph data demonstrating (a) activity intensity, (b) activity counts per day (used to compute vector magnitude counts), and (C) wear time at different time points are demonstrated. In panel C, the minimum daily wear time of 8 h is designated by the green horizonal line.
The actigraphy field is rapidly advancing. Wearable technology for monitoring of activity, sleep, and physiologic parameters in the home setting continues to evolve. As such, our approach to data collection and analysis changed from study conception to inception. We originally planned to use the ActiGraph GT3XP‐BTLE hip‐worn accelerometer and perform manual downloads of activity data from CentrePoint for analysis in a separate statistical program. However, developing a process for automated data import from the CentrePoint cloud database to RedCap via an API pull achieves a major advance for future pediatric PH clinical trials studying actigraphy and was a priority for the study team. As the ActiGraph GT3XP was not compatible with newer versions of CentrePoint required for the API connection, we updated the device to the ActiGraph CPIW and created a direct connection between CentrePoint and RedCap.
Another area of activity science that is evolving is the application of cut points to translate step or vector magnitude counts into activity intensity (sedentary, light, moderate, vigorous). Cut points are specific to device, wear location, and age group and are also derived from small samples of children within a limited age range that may not be wholly applicable to a particular study population of interest. Given the age range of participants in this study, we will apply three published wrist‐worn cutpoints to determine activity intensity in different age groups. We will apply the Johannson cutpoints 31 for infant and toddler participants <5 years, the Chandler cutpoints 32 for school children 5–12 years, and the Crouter cutpoints 33 for adolescent participants >12 years. Additionally, newer approaches such as the “MIMS‐unit algorithm” may be further refined by other investigators during the study period. MIMS is a Monitor Independent Movement Summary unit that summarizes raw accelerometer data in a device‐independent manner, accounting for differences among research and commercial devices. 34 Application of these open‐source algorithms to large actigraphy data sets such as in the National Health and Nutrition Examination Survey has led to the development of activity percentile curves, 35 eliminating the need for intensity cut points and facilitating increased standardization of data acquisition and analysis. We will apply MIMS‐unit algorithms and percentile curves to the data for this study if they become accessible in CentrePoint at the time that data analysis is undertaken.
To explore changes in these novel activity metrics, longitudinal data analyses will be performed examining actigraphy measures over 2 years of follow‐up. To compare treatment groups, we will implement unadjusted and covariate‐adjusted generalized estimating equations or categorical mixed effect models accounting for repeated measures of actigraphy (baseline, 3, 6, 12, 18, and 24 months) using the Poisson link function. We will adjust for age, gender, and World Symposium of PH diagnostic group 1 or 3. The primary analysis will be conducted according to the principle of intention‐to‐treat with participants analyzed and outcomes attributed according to the treatment arm to which the participants were randomized, regardless of subsequent crossover or postrandomization medical care. We will select the appropriate working covariance matrix based on the data to account for within patient correlation. To account for possible crossovers, we will conduct additional as‐treated analyses with participants analyzed according to the treatment actually received for all comparisons. All models will include study arm, time point for each visit, and their interactions. This will be done to assess for differences in longitudinal trajectories in actigraphy measures by study arm as well to compare outcomes between arms at pre‐specified time points, such as 12 and 24 months. We will also assess for early changes in actigraphy measures that might precede changes in WHO functional class and will model the relationships between treatment received, actigraphy, and other exploratory outcomes as independent variables and WHO functional class status as the dependent variable using longitudinal proportional odds models. This analysis will explore the association between actigraphy measures and WHO functional class at different time points and can potentially reveal if early changes in actigraphy measures are associated with WHO functional class outcomes at 12 and 24 months.
CONCLUSION
The Kids Mod PAH trial will explore the use of actigraphy to measure physical activity in a large sample of clinically well‐characterized children with a new diagnosis of PH receiving two different pulmonary vasodilator treatment strategies. By directly and noninvasively measuring physical activity in the ambulatory setting, we aim to identify a novel, simple, and inexpensive approach for quantitative assessment of exercise tolerance in pediatric PH that can be applied in future trials.
AUTHOR CONTRIBUTIONS
Catherine M. Avitabile developed the actigraphy methods and crafted the manuscript. Usha S. Krishnan, Delphine Yung, Stephanie S. Handler, Nidhy Varghese, Angela Bates, Jeff Fineman, Rachel Sullivan, Grace Friere, Eric Austin, Mary P. Mullen, Eric J. Christensen, and Joseph M. Collaco provided site‐specific support in study design and execution, and edited the manuscript. Carol Pereira developed the ActiGraph to REDCap API connection and edited the manuscript. Gayane Yenokyan provided statistical methods and edited the manuscript. Steven H. Abman, Lew Romer, D. Dunbar Ivy, and Erika B. Rosenzweig provided senior leadership in study design and execution and edited the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Pediatric Pulmonary Hypertension Network (PPHNet) for its collaboration and commitment to the pediatric pulmonary hypertension community. Finding sources: NIH [UG3HL151458, UH3HL151458 (to Lew Romer, Erika B. Rosenzweig, Steven H. Abman, Joseph M. Collaco), and U24HL151457 (to Gayane Yenokyan). 100% of the project support was from federal funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. ClinTrials.gov #NCT04039464.
Avitabile CM, Krishnan US, Yung D, Handler SS, Varghese N, Bates A, Fineman J, Sullivan R, Friere G, Austin E, Mullen MP, Pereira C, Christensen EJ, Yenokyan G, Collaco JM, Abman SH, Romer L, Dunbar Ivy D, Rosenzweig EB. Actigraphy methodology in the Kids Mod PAH trial: physical activity as a functional endpoint in pediatric clinical trials. Pulm Circ. 2024;14:e12339. 10.1002/pul2.12339
REFERENCES
- 1. Fraisse A, Jais X, Schleich JM, di Filippo S, Maragnès P, Beghetti M, Gressin V, Voisin M, Dauphin C, Clerson P, Godart F, Bonnet D. Characteristics and prospective 2‐year follow‐up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis. 2010;103(2):66–74. [DOI] [PubMed] [Google Scholar]
- 2. Moledina S, Hislop AA, Foster H, Schulze‐Neick I, Haworth SG. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart. 2010;96(17):1401–1406. [DOI] [PubMed] [Google Scholar]
- 3. van Loon RLE, Roofthooft MTR, Hillege HL, ten Harkel ADJ, van Osch‐Gevers M, Delhaas T, Kapusta L, Strengers JLM, Rammeloo L, Clur SAB, Mulder BJM, Berger RMF. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124(16):1755–1764. [DOI] [PubMed] [Google Scholar]
- 4. Abman SH, Mullen MP, Sleeper LA, Austin ED, Rosenzweig EB, Kinsella JP, Ivy D, Hopper RK, Raj JU, Fineman J, Keller RL, Bates A, Krishnan US, Avitabile CM, Davidson A, Natter MD, Mandl KD; Pediatric Pulmonary Hypertension Network . Characterisation of paediatric pulmonary hypertensive vascular disease from the PPHNet registry. Eur Respir J. 2021;59(1):2003337. 10.1183/13993003.03337-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barst RJ, McGoon MD, Elliott CG, Foreman AJ, Miller DP, Ivy DD. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long‐term pulmonary arterial hypertension disease management. Circulation. 2012;125(1):113–122. [DOI] [PubMed] [Google Scholar]
- 6. Zijlstra WMH, Douwes JM, Rosenzweig EB, Schokker S, Krishnan U, Roofthooft MTR, Miller‐Reed K, Hillege HL, Ivy DD, Berger RMF. Survival differences in pediatric pulmonary arterial hypertension. J Am Coll Cardiol. 2014;63(20):2159–2169. [DOI] [PubMed] [Google Scholar]
- 7. Barst RJ, Ivy DD, Gaitan G, Szatmari A, Rudzinski A, Garcia AE, Sastry BKS, Pulido T, Layton GR, Serdarevic‐Pehar M, Wessel DL. A randomized, double‐blind, placebo‐controlled, dose‐ranging study of oral sildenafil citrate in treatment‐naive children with pulmonary arterial hypertension. Circulation. 2012;125(2):324–334. [DOI] [PubMed] [Google Scholar]
- 8. Barst RJ, Beghetti M, Pulido T, Layton G, Konourina I, Zhang M, Ivy DD. STARTS‐2: long‐term survival with oral sildenafil monotherapy in treatment‐naive pediatric pulmonary arterial hypertension. Circulation. 2014;129(19):1914–1923. [DOI] [PubMed] [Google Scholar]
- 9. Ivy DD, Abman S. Beyond the 6‐minute walk test for assessing pediatric pulmonary hypertension. Chest. 2015;148(3):576–577. [DOI] [PubMed] [Google Scholar]
- 10. Pugh ME, Buchowski MS, Robbins IM, Newman JH, Hemnes AR. Physical activity limitation as measured by accelerometry in pulmonary arterial hypertension. Chest. 2012;142(6):1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi L, Liu Z, Matthews CE, BUCHOWSKI MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26(14):1557–1565. [DOI] [PubMed] [Google Scholar]
- 13. Saxer S, Lichtblau M, Berlier C, Hasler ED, Schwarz EI, Ulrich S. Physical activity in incident patients with pulmonary arterial and chronic thromboembolic hypertension. Lung. 2019;197(5):617–625. [DOI] [PubMed] [Google Scholar]
- 14. Mainguy V, Provencher S, Maltais F, Malenfant S, Saey D. Assessment of daily life physical activities in pulmonary arterial hypertension. PLoS ONE. 2011;6(11):e27993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okumus G, Aslan GK, Arseven O, Ongen G, Issever H, Kiyan E. The role of an activity monitor in the objective evaluation of patients with pulmonary hypertension. Clin Respir J. 2018;12(1):119–125. [DOI] [PubMed] [Google Scholar]
- 16. Ulrich S, Fischler M, Speich R, Bloch KE. Wrist actigraphy predicts outcome in patients with pulmonary hypertension. Respiration. 2013;86(1):45–51. [DOI] [PubMed] [Google Scholar]
- 17. Matura LA, Shou H, Fritz JS, Smith KA, Vaidya A, Pinder D, Archer‐Chicko C, Dubow D, Palevsky HI, Sommers MS, Kawut SM. Physical activity and symptoms in pulmonary arterial hypertension. Chest. 2016;150(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. González‐Saiz L, Santos‐Lozano A, Fiuza‐Luces C, Sanz‐Ayán P, Quezada‐Loaiza CA, Ruiz‐Casado A, Alejo LB, Flox‐Camacho A, Morán M, Lucia A, Escribano‐Subías P. Physical activity levels are low in patients with pulmonary hypertension. Ann Transl Med. 2018;6(11):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Babirekere‐Iriso E, Rytter MJH, Namusoke H, Mupere E, Michaelsen KF, Stark KD, Lauritzen L, Briend A, Friis H, Brage S, Faurholt‐Jepsen D. Physical activity level among children recovering from severe acute malnutrition. Trop Med Int Health. 2018;23(2):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ketcheson L, Pitchford EA, Kwon HJ, Ulrich DA. Physical activity patterns in infants with and without Down syndrome. Pediatr Phys Ther. 2017;29(3):200–206. [DOI] [PubMed] [Google Scholar]
- 21. Prioreschi A, Brage S, Westgate K, Micklesfield LK. Describing the diurnal relationships between objectively measured mother and infant physical activity. Int J Behav Nutr Phys Act. 2018;15(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stone MR, Houser NE, Cawley J, Kolen AM, Rainham D, Rehman L, Turner J, Kirk SFL. Accelerometry‐measured physical activity and sedentary behaviour of preschoolers in Nova Scotia, Canada. Appl Physiol Nutr Metab. 2019;44(9):1005–1011. [DOI] [PubMed] [Google Scholar]
- 23. Veldman SLC, Jones RA, Santos R, Sousa‐Sá E, Pereira JR, Zhang Z, Okely AD. Associations between gross motor skills and physical activity in Australian toddlers. J Sci Med Sport. 2018;21(8):817–821. [DOI] [PubMed] [Google Scholar]
- 24. Sun H, Lawrence JP, Stockbridge N, Bossart S, Taylor A, Ariagno R, Abman S, Murphy D, Soreth J, Kleppinger C, Ivy DD. Combined physical activity and heart rate variability as measured by actigraphy as a potential assessment endpoint in children with pulmonary hypertension. Pediatric Academic Societies 2018 Meeting. Toronto, Canada. May 2018. [Google Scholar]
- 25. Avitabile CM, Yung D, Handler S, Hopper RK, Fineman J, Freire G, Varghese N, Mullen MP, Krishnan US, Austin E, Silveira L, Ivy DD. Measurement of physical activity by actigraphy in infants and young children with pulmonary arterial hypertension. J Pediatr. 2023;262:113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zijlstra WMH, Ploegstra MJ, Vissia‐Kazemier T, Roofthooft MTR, Sarvaas GM, Bartelds B, Rackowitz A, van den Heuvel F, Hillege HL, Plasqui G, Berger RMF. Physical activity in pediatric pulmonary arterial hypertension measured by accelerometry. A candidate clinical endpoint. Am J Respir Crit Care Med. 2017;196(2):220–227. [DOI] [PubMed] [Google Scholar]
- 27. Woo JL, DiLorenzo MP, Rosenzweig E, Pasumarti N, Villeda GV, Berman‐Rosenzweig E, Krishnan U. Correlation between right ventricular echocardiography measurements and functional capacity in patients with pulmonary arterial hypertension. Tex Heart Inst J. 2022;49(6):e217719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collaco JM, Abman SH, Austin ED, Avitabile CM, Bates A, Fineman JR, Freire GA, Handler SS, Ivy DD, Krishnan US, Mullen MP, Varghese NP, Yung D, Nies MK, Everett AD, Zimmerman KO, Simmons W, Chakraborty H, Yenokyan G, Newell‐Sturdivant A, Christensen E, Eyzaguirre LM, Hanley DF, Rosenzweig EB, Romer LH. Kids Mod PAH trial: a multicenter trial comparing mono‐ versus duo‐therapy for initial treatment of pediatric pulmonary hypertension. Pulm Circ. 2023;13(4):e12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ivy DD, Rosenzweig EB, Lemarié JC, Brand M, Rosenberg D, Barst RJ. Long‐term outcomes in children with pulmonary arterial hypertension treated with bosentan in real‐world clinical settings. Am J Cardiol. 2010;106(9):1332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takatsuki S, Rosenzweig EB, Zuckerman W, Brady D, Calderbank M, Ivy DD. Clinical safety, pharmacokinetics, and efficacy of ambrisentan therapy in children with pulmonary arterial hypertension. Pediatr Pulmonol. 2013;48(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johansson E, Larisch LM, Marcus C, Hagströmer M. Calibration and validation of a wrist‐ and hip‐worn actigraph accelerometer in 4‐year‐old children. PLoS One. 2016;11(9):e0162436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chandler JL, Brazendale K, Beets MW, Mealing BA. Classification of physical activity intensities using a wrist‐worn accelerometer in 8‐12‐year‐old children. Pediatr Obes. 2016;11(2):120–127. [DOI] [PubMed] [Google Scholar]
- 33. Crouter SE, Flynn JI, Bassett DR Jr.. Estimating physical activity in youth using a wrist accelerometer. Med Sci Sports Exerc. 2015;47(5):944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. John D, Tang Q, Albinali F, Intille S. An open‐source monitor‐independent movement summary for accelerometer data processing. J Measur Phys Behav. 2019;2(4):268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Belcher BR, Wolff‐Hughes DL, Dooley EE, Staudenmayer J, Berrigan D, Eberhardt MS, Troiano RP. US population‐referenced percentiles for wrist‐worn accelerometer‐derived activity. Med Sci Sports Exerc. 2021;53(11):2455–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]


