Abstract
Background
Neoadjuvant chemoradiation and chemotherapy are recommended for the treatment of nonmetastatic esophageal cancer. The benefit of neoadjuvant treatment is mostly limited to patients who exhibit pathologic complete response (pCR). Existing estimates of pCR rates among patients receiving neoadjuvant therapy have not been synthesized and lack precision.
Methods
We conducted an independently funded systematic review and meta‐analysis (PROSPERO CRD42023397402) of pCR rates among patients diagnosed with esophageal cancer treated with neoadjuvant chemo(radiation). Studies were identified from Medline, EMBASE, and CENTRAL database searches. Eligible studies included trials published from 1992 to 2022 that focused on nonmetastatic esophageal cancer, including the gastroesophageal junction. Histology‐specific pooled pCR prevalence was determined using the Freeman–Tukey transformation and a random effects model.
Results
After eligibility assessment, 84 studies with 6451 patients were included. The pooled prevalence of pCR after neoadjuvant chemotherapy in squamous cell carcinomas was 9% (95% CI: 6%–14%), ranging from 0% to 32%. The pooled prevalence of pCR after neoadjuvant chemoradiation in squamous cell carcinomas was 32% (95% CI: 26%–39%), ranging from 8% to 66%. For adenocarcinoma, the pooled prevalence of pCR was 6% (95% CI: 1%–12%) after neoadjuvant chemotherapy, and 22% (18%–26%) after neoadjuvant chemoradiation.
Conclusions
Under one‐third of patients with esophageal cancer who receive neoadjuvant chemo(radiation) experience pCR. Patients diagnosed with squamous cell carcinomas had higher rates of pCR than those with adenocarcinomas. As pCR represents an increasingly utilized endpoint in neoadjuvant trials, these estimates of pooled pCR rates may serve as an important benchmark for future trial design.
Keywords: chemotherapy, esophageal cancer, meta‐analysis, neoadjuvant chemoradiation, pathologic complete response
This systematic review and meta‐analysis revealed that less than one‐third of nonmetastatic esophageal cancer patients achieve pathologic complete response (pCR) after neoadjuvant chemoradiation or chemotherapy, with squamous cell carcinomas exhibiting higher pCR rates than adenocarcinomas. These findings derived from a robust pool of 84 studies could offer a critical benchmark for future trial design.
1. INTRODUCTION
Esophageal cancer is a leading cause of global cancer‐specific mortality, with less than 20% of individuals surviving 5 years of past diagnosis. 1 Esophageal cancer accounts for over 16,000 deaths in the United States annually, with the burden of disease expected to increase over time as the population ages. 2 , 3 At the time of diagnosis, a plurality of individuals present with advanced disease. 4 Landmark clinical trials over the past several decades have demonstrated that neoadjuvant therapy prior to esophagectomy confers a significant survival advantage over surgery alone. 5 , 6 , 7 Consequently, current treatment guidelines reflect these findings, recommending several neoadjuvant options for treating locally advanced tumors: neoadjuvant chemotherapy and/or chemoradiation followed by esophagectomy and perioperative chemotherapy. 8 , 9 , 10
Improved survival and quality of life are key considerations when selecting treatment for esophageal cancer. 11 To nominate promising neoadjuvant therapeutic strategies, pathologic response in the primary tumor is a relatively rapid readout proposed as a surrogate for antitumor activity. Research has demonstrated that pathologic complete response (pCR) following neoadjuvant therapy often correlates with improved recurrence‐free and overall survival. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 However, most patients do not experience pCR following neoadjuvant therapy, and individuals receiving trimodality therapy without response to chemoradiation have been shown to have survivals approximating surgery alone. 21 , 22 Currently, no studies provide pooled, durable estimates of the expected pCR rate for patients with esophageal cancer receiving neoadjuvant therapy. While multiple trials have reported on this outcome, individually they are small studies with limited precision and do not examine how patient factors may predict likelihood of experiencing pCR.
In this study, we performed a systematic review and meta‐analysis to summarize the existing literature on pCR amongst individuals with nonmetastatic esophageal cancer who received either neoadjuvant chemotherapy and/or chemoradiation. We aimed to characterize the associations between certain study‐ and patient‐level factors and the outcome of pCR. Procuring precise estimates of the probability of experiencing pCR after neoadjuvant therapy can help to inform the design of trials to more efficiently prioritize new agents in esophageal cancer.
2. MATERIALS AND METHODS
2.1. Study identification
We performed a literature review in accordance with the guidance established by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 statement 23 and the JBI methodology for systematic reviews of prevalence and incidence. 24 Table S1 contains the PRISMA checklist. We identified relevant studies in the following three electronic databases: (1) Medline; (2) EMBASE; and (3) Cochrane Central Register of Controlled Trials (CENTRAL). Table S2 contains the search strategy used for study identification in each database, implemented on November 30, 2022. We pre‐registered the review protocol on PROSPERO (CRD42023397402) before eligibility assessment and data extraction occurred (but after the search was performed and locked‐in). We utilized the Covidence web‐based platform for systematic review data management.
2.2. Eligibility criteria
Prospective clinical trials (randomized, single arm, and nonrandomized) of individuals with incident, nonmetastatic esophageal carcinoma of any histology, including all sites of the esophagus and the gastroesophageal junction (GEJ), that contained at least one trial treatment arm of neoadjuvant therapy were eligible for inclusion. Nonrandomized trials were included because they constitute an interventional study design and the summary measure of our analysis (prevalence of pCR) was not being compared across arms. Neoadjuvant therapies consisted of chemotherapy (including perioperative) and chemoradiation (including induction chemotherapy followed by chemoradiation). We focused on cytotoxic chemotherapies; we did not include HER‐2 targeted therapy. Thus, if neoadjuvant chemo(radiation) strategies were coupled with other neoadjuvant treatment types, such as immunotherapy, they were excluded because we wanted to isolate the effect of only guideline‐recommended treatment strategies on pCR. However, studies could have nonchemotherapy adjuvant treatment. A minimum arm size of 20 individuals receiving resection after neoadjuvant therapy for evaluation of pCR was required to ensure inclusion of well‐powered studies. Eligible studies were required to report the prevalence of pCR in patients receiving neoadjuvant therapy. Several methods for evaluating pCR exist, such as the Mandard 25 and Chirieac 19 tumor regression grade (TRG) systems. All systems were eligible for inclusion, but if a study did not specify a TRG, we required the trial to have either explicitly labeled their response outcome as pCR or the absence of residual disease upon pathology. Studies published in English between 1992 and 2022 from any geographic location were eligible.
2.3. Study selection
After implementing the protocol search strategy in the three databases and de‐duplicating records, the titles and abstracts of all search results were screened for relevance by two independent reviewers (JS and AA). Following screening, the full‐text publications for the screen‐eligible studies were reviewed for further evaluation of eligibility by two of three potential independent reviewers (JS, AA, and EO). Any disagreement about eligibility (screening stage or full‐text stage) was resolved by a third investigator (CG).
2.4. Assessment of methodologic quality
As our study focused on pooling a percentage (rather than a contrast measure such as hazard ratio of relative risk), there was not “between‐arms” bias such as confounding to evaluate with standard risk‐of‐bias tools. Thus, we critically appraised the methodologic quality of eligible studies using a study‐specific adaptation of the JBI Critical Appraisal Checklist for Studies Reporting Prevalence Data. 24 The adapted JBI checklist contained five items for assessment (Table S2). Studies had their data extracted and included in the final analysis regardless of their assessed quality, but quality assessments for each study are included in Table S3.
2.5. Data extraction
Study‐level fields were extracted by a single reviewer and consisted of authorship, year of publication, geographic location(s) where the trial was performed, and number of patients stratified by histologic subtype. Within studies, treatment arm‐specific fields extracted included neoadjuvant treatment class (nCT or nCRT), specific chemotherapy agents used, and pCR rate. Number of patients and pCR rate were stratified according to histologic subtype if the trial included both adenocarcinoma and squamous cell carcinoma and reported histology‐specific pCR rates. Data extracted from studies are available as supplemental materials.
2.6. Statistical analysis
We calculated the pooled prevalence of pCR, overall and according to tumor histology and type of neoadjuvant therapy. Neoadjuvant chemotherapy and perioperative chemotherapy were analyzed together given that chemotherapy was the only treatment delivered prior to surgery and pathological response assessment. Likewise, neoadjuvant chemoradiation and induction chemotherapy with chemoradiation were analyzed together. We used the Freeman–Tukey double arcsine transformation to calculate the pooled prevalence using a random‐effects model with inverse‐variance weighting calculated via the DerSimonian and Laird method. Results were visually displayed using forest plots. We used univariable meta‐regression models to assess whether the pCR rate was associated with patient and treatment characteristics.
3. RESULTS
We retrieved 6575 records from the preliminary database searches (Figure 1). After elimination of 2037 duplicate records, we then screened the titles and abstracts of 4538 records, 4347 of which were excluded due to not meeting eligibility criteria. The full‐text publications for the remaining 191 studies were reviewed. Of these, 84 studies 7 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 met the inclusion and exclusion criteria and were included in this systematic review and meta‐analysis, yielding a total of 6451 patients who received neoadjuvant therapy and had tumor response measured pathologically after surgery. Of the 84 included trials, 13 (15%) were published between 1992 and 2001, 31 (37%) between 2002 and 2011, and 41 (49%) between 2012 and 2022. In terms of study design, 44 (52%) of the included studies were single‐arm trials, 38 (45%) were randomized trials, and three (4%) were nonrandomized trials of multiple treatments. Histologically, 32 (38%) studies were performed amongst study populations with squamous cell carcinoma only, 15 (18%) in adenocarcinoma only, 26 (31%) in squamous cell carcinoma and adenocarcinoma, and 12 (14%) included other histologic subtypes along with squamous cell and adenocarcinoma. Geographically, 32 (38%), 28 (33%), 23 (27%), and 2 (2%) of the included trials were performed in Asia, Europe, North America, and Australia, respectively. Across 102 trial arms that delivered either neoadjuvant chemotherapy or chemoradiation, platinum, and fluorouracil‐based regimens were the most common (n = 41, 40%), followed by platinum and taxane‐based regimens (n = 28, 27%), regimens that contained platinum‐based agents, fluorouracil, and a taxane (n = 16, 16%), and other regimens (n = 17, 17%). The full distribution of regimens is presented in Figure S1.
FIGURE 1.
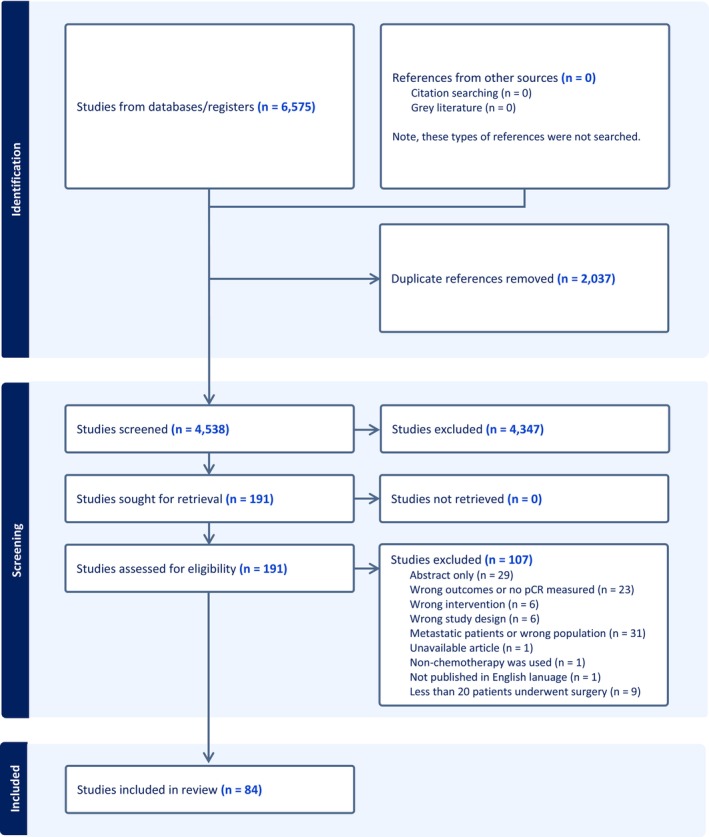
PRISMA 2020 diagram depicting identification, screening, and inclusion of studies.
3.1. Pathologic complete response
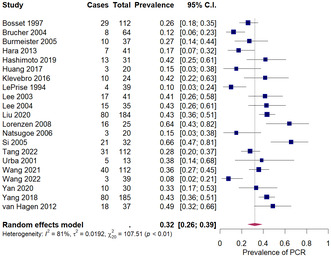
The pooled prevalence of pCR after neoadjuvant chemotherapy in squamous cell carcinoma was 9% (95% CI: 6%–14%) across 16 studies, ranging from 0% to 32% (Figure 2). The pooled prevalence of pCR after neoadjuvant chemoradiation in squamous cell carcinoma was 32% (95% CI: 26%–39%) across 21 studies, ranging from 8% to 66% (Figure 3). For adenocarcinoma, the pooled prevalence of pCR was 6% (95% CI: 1%–12%) after neoadjuvant chemotherapy, across five studies (Figure 4), and 22% (18%–26%) after neoadjuvant chemoradiation across 15 studies (Figure 5). A secondary analysis of all studies, regardless of histologic subtype found a pooled pCR prevalence of 8% (95% CI: 6%–11%) for neoadjuvant chemotherapy (Figure S2) and 29% (95% CI: 26%–32%) for neoadjuvant chemoradiation (Figure S3 ).
FIGURE 2.
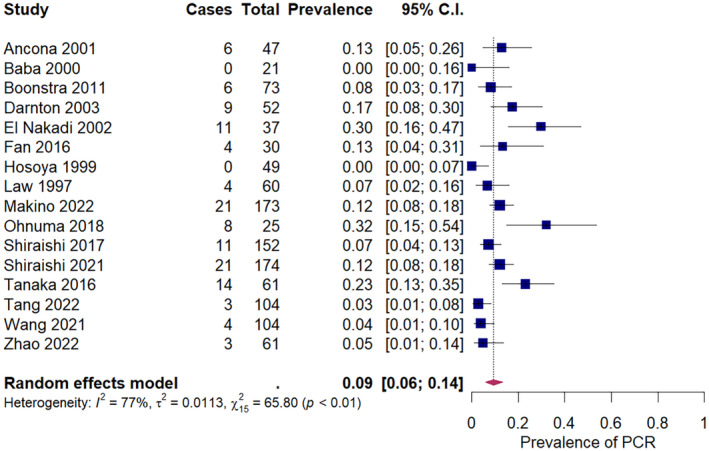
Pathologic complete response amongst squamous cell carcinoma patients receiving neoadjuvant chemotherapy.
FIGURE 3.
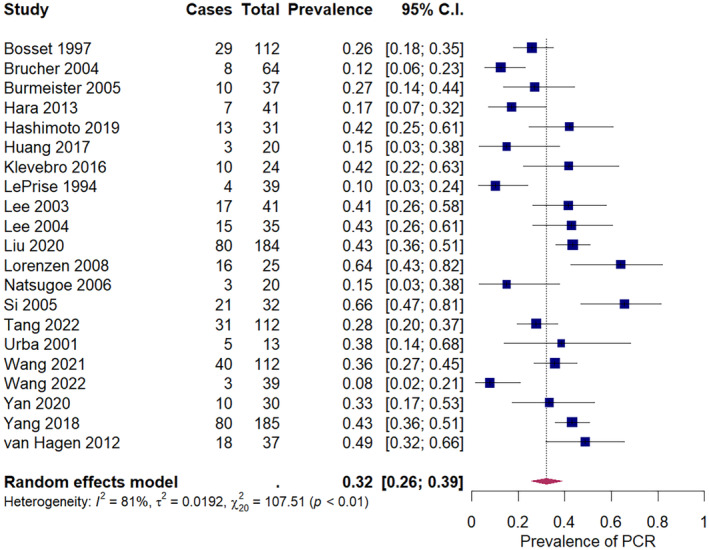
Pathologic complete response amongst squamous cell carcinoma patients receiving neoadjuvant chemoradiation.
FIGURE 4.
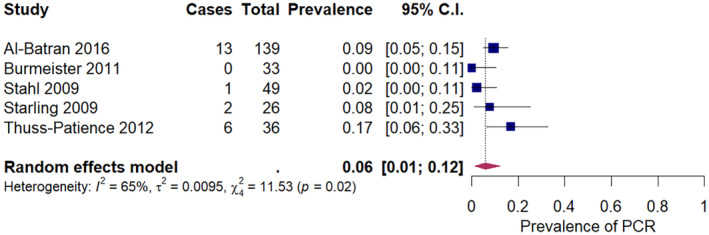
Pathologic complete response amongst adenocarcinoma patients receiving neoadjuvant chemotherapy.
FIGURE 5.
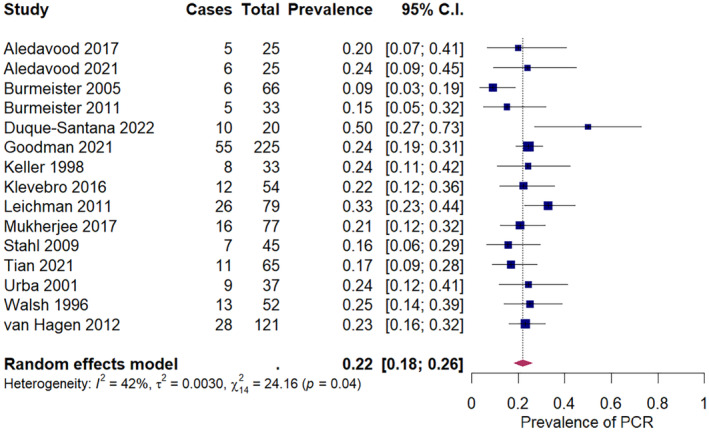
Pathologic complete response amongst adenocarcinoma patients receiving neoadjuvant chemoradiation.
3.2. Associations between study‐ and patient‐level factors and pCR
Exploratory analyses (Table 1 ) found the odds of a pCR were twice as high in single‐arm trials than in randomized trials (OR = 1.99; 95% CI: 1.47–2.70). Regimens that contained three chemotherapeutic agents were associated with higher pCR amongst nCT trial arms (OR = 2.34, 95% CI: 1.40–3.91), but the association was attenuated and compatible with the null hypothesis for nCRT trials arms (OR = 1.26, 95% CI: 0.85–1.86). For adenocarcinoma, neoadjuvant chemo(radiation) regimens that consisted of a platinum‐based chemotherapy with a taxane were associated with a higher rate of pCR than regimens that consisted of platinum‐based chemotherapy with fluorouracil (OR = 3.13; 95% CI: 1.46–6.72). No association was found between the percentage of the study population that was male and pCR (for a 10% increase in male proportion: OR = 0.97, 95% CI: 0.79–1.18). Also, pCR estimates in Asian and non‐Asian countries did not significantly differ (OR = 0.94; 95% CI: 0.66–1.32). Our results also indicated no association with pCR for average age or year of publication (Figures S4 and S5).
TABLE 1.
Results from unadjusted meta‐regression displaying relationships between study characteristics and pathologic complete response rate.
| Trial type a | Regimen count b | Regimen type c | Induction regimen d | Male sex e | Trial location f | |
|---|---|---|---|---|---|---|
| OR (95% CI) | ||||||
| Overall | 1.99 (1.47–2.70) | 0.84 (0.56–1.26) | 1.55 (0.96–2.50) | 0.73 (0.51–1.06) | 0.97 (0.79–1.18) | 0.94 (0.66–1.32) |
| Treatment modality | ||||||
| NCT | 2.70 (1.71–4.28) | 2.34 (1.40–3.91) | 2.27 (0.79–6.55) | 0.78 (0.55–1.08) | 1.17 (0.68–2.01) | |
| NCRT | 1.27 (0.97–1.66) | 1.26 (0.85–1.86) | 1.10 (0.78–1.55) | 0.73 (0.51–1.06) | 0.97 (0.83–1.13) | 1.24 (0.93–1.66) |
| Histology | ||||||
| Squamous cell carcinoma | 1.70 (0.93–3.10) | 0.86 (0.44–1.67) | 0.98 (0.35–2.72) | 0.34 (0.05–2.20) | 1.21 (0.79–1.86) | 0.65 (0.34–1.24) |
| Adenocarcinoma | 2.00 (1.18–3.41) | 0.57 (0.30–1.07) | 3.13 (1.46–6.72) | 0.99 (0.69–1.43) | 0.77 (0.53–1.12) | 1.11 (0.46–2.66) |
Abbreviations: CI, confidence interval; NCRT, neoadjuvant chemoradiation therapy; NCT, neoadjuvant chemotherapy; OR, odds ratio.
Single‐arm trials versus randomized trials (reference group).
Triplet regimen versus doublet regimen (reference group).
Platinum + taxanes versus platinum + fluorouracil regimen (reference group).
Induction versus no induction chemoradiation (reference group).
OR reported for a 10% increase in the proportion of the study population that was male.
Asian versus non‐Asian regions (reference group).
4. DISCUSSION
In this study, we demonstrated that fewer than one‐third of patients diagnosed with nonmetastatic esophageal cancer receiving neoadjuvant chemotherapy or chemoradiation experience pCR. Specifically, we found that trials reporting on patients with squamous cell carcinoma receiving neoadjuvant chemoradiation had the highest pCR rates (32%), while trials reporting on adenocarcinoma receiving neoadjuvant chemotherapy had the lowest pCR rates (6%). Our findings provide synthesized evidence to support that patients with squamous cell carcinoma may be more likely to derive benefit from chemo (radiation) than patients with adenocarcinoma. Collectively, these results support the overall concept that pCR occurs in a minority of patients with esophageal cancer.
Interestingly, we found pCR rates have not improved much over the past three decades. However, in some modality and histology pairs, such as nCRT for squamous cell carcinomas, results from trials testing state‐of‐the‐art neoadjuvant protocols outperformed the time‐pooled summary estimates. Specifically, the CROSS trial used carboplatin and paclitaxel and reported a pCR of 49% in this population, higher than the pooled average of 32%. 7
The role of pCR in guiding patient–clinician discussions about treatment decision‐making is nuanced and evolving. Prior studies have found that experiencing pCR is associated with longer overall survival than partial response or no response to neoadjuvant treatment. 12 , 13 , 15 , 17 , 18 Our work helps to highlight some of the limitations of using pCR alone as a readout for tumor response and surrogate of distant biologic activity. While patients who experience a pCR often have favorable survival outcomes, there is increasing evidence that response in the regional lymph nodes at surgical resection may be a more accurate reflection of biologic activity and presumed micrometastatic control. 109 However, this requires surgical resection to assess and does not address the increasing desire to devise strategies that may allow avoidance of surgery in some patients. In fact, this approach is being tested in the currently recruiting Neoadjuvant Chemoradiotherapy and Surgery versus Definitive Chemoradiotherapy with Salvage Surgery as Needed (NEEDS) randomized trial, with final results expected in the coming years. 110 The Surgery As Needed for Oesophageal (SANO) cancer trial is also exploring this approach, with early results suggesting that, amongst those displaying clinical complete response to chemoradiation, active surveillance is noninferior to surgery. 111 , 112
Of equal importance to identifying responder patients is the need to understand the larger nonresponder group. By analyzing a large number of trials and reporting the pooled pCR rates, we enhance confidence in the observation that a vast majority of patients do not achieve complete response in the primary tumor. There was a high degree of variability in pCR rates both across treatment modality groups and within groups. In nCT trial arms, patients receiving two chemotherapy agents instead of three were less likely to experience pCR. Notably, the pCR rates were not associated with average patient age or proportion of study population that was male, and did not improve over time in the included trials. While our exploratory analyses may explain some variability in pCR rates, other clinicopathologic features may be driving variability.
An important next step will be enhancing the ability to predict pCR at the individual‐level, both from clinical response and from entirely pretreatment variables. In practice, accurately predicting pCR based on clinical parameters has proven to be a difficult task, with existing work yielding low predictive accuracy. 113 More accurate prediction of pCR could be instrumental in (1) identifying patients with a high probability of pCR for whom salvage (instead of planned) surgery could be a viable option; (2) identifying patients unlikely to respond to chemo(radiation); and (3) defining populations where novel approaches to intensify (or de‐escalate) neoadjuvant components may be of highest yield.
Our study has several strengths. To our knowledge, this was the first and largest meta‐analysis of pCR rates in trial‐enrolled esophageal carcinoma patients receiving neoadjuvant chemotherapy or chemoradiation. Our literature search strategy was highly sensitive; the broad initial search terms were unlikely to have missed any eligible studies, with over 4500 titles and abstracts screened. The estimates of pCR rate provide context for the expected percentage of patients that are benefitting from neoadjuvant CT or CRT before their surgery. The probabilities gleaned from this meta‐analysis can be used as inputs in decision‐analysis models and cost‐effectiveness analysis. Lastly, with the advent of neoadjuvant immunotherapies, the pooled estimates of PCR from neoadjuvant chemotherapy and chemoradiation provide important benchmarks that serve as inputs into trial design development.
This study is not without limitations. First, we did not include observational studies in our review. Inclusion of observational studies would have further enhanced the sample size and precision of our estimates but concerns about selection bias prohibited their inclusion. In many observational studies, patients with a negative clinical response would be more likely to receive resection and thus also have a documented negative pathologic response. Since our study's objectives were to determine the prevalence of pooled pCR rates and explore relationships between study and patient‐level factors with pCR, we did not examine the relationship between pCR and overall survival. A number of methodologic issues would also have complicated analysis of survival. Only six of 84 studies reported survival stratified by pCR status; studies had a wide difference in length of follow‐up, reported different measures of effect, and calculated survival by randomized assignment as opposed to subset that received surgery (had pCR evaluated). Additionally, we did not extract data on regional lymph node response, an important prognostic variable that may be important to collect in addition to pCR. 109 We did not collect and analyze data on radiation dose; observational studies are conflicting on whether lower radiation doses are associated with lower pCR rates. 114 , 115 , 116 , 117
5. CONCLUSION
In conclusion, our study provides innovative findings regarding the rates and correlates of pCR among patients with nonmetastatic esophageal cancer receiving neoadjuvant chemotherapy or chemoradiation. In addition, by demonstrating variability in pCR rates according to histology, type of neoadjuvant treatment, and study‐ and patient‐level factors, we highlight the importance of considering the probability that an individual patient will experience pCR when selecting treatment. Future research should focus on developing and validating models to predict the probability of experiencing pCR based on patient‐level variables that could be collected prior to treatment selection.
AUTHOR CONTRIBUTIONS
Charles E. Gaber: Conceptualization (lead); data curation (equal); formal analysis (supporting); investigation (lead); methodology (lead); project administration (lead); supervision (lead); writing – original draft (lead); writing – review and editing (lead). Jyotirmoy Sarker: Conceptualization (supporting); data curation (lead); formal analysis (supporting); investigation (supporting); methodology (supporting); project administration (supporting); validation (supporting); visualization (supporting); writing – review and editing (supporting). Abdullah I. Abdelaziz: Conceptualization (supporting); data curation (lead); formal analysis (equal); investigation (supporting); methodology (supporting); project administration (supporting); software (lead). Ebere Okpara: Data curation (lead); formal analysis (supporting); investigation (supporting); methodology (supporting); visualization (supporting). Todd A. Lee: Conceptualization (supporting); investigation (supporting); methodology (supporting); writing – review and editing (equal). Samuel J. Klempner: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Ryan D. Nipp: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING INFORMATION
No external funding was received for this research.
CONFLICT OF INTEREST STATEMENT
Dr. Gaber: academic salary support from an educational fellowship from pharmaceutical company AbbVie Inc. Dr. Klempner has no directly relevant conflicts of interest but does perform consulting or advisory board participation for: Astellas, Merck, Bristol‐Myers Squibb, Daiichi‐Sankyo, AstraZeneca, Eli Lilly, Sanofi‐Aventis, Exact Sciences, Servier, Novartis, Coherus Biosciences, Natera, Amgen, and Mersana, reports stock/equity in: Turning Point Therapeutics (ended 6/2022) and Nuvalent (ended 11/2022), has received honoraria from Merck Serono, and research funding from Leap Therapeutics, BeiGene, and Silverback Therapeutics.
ETHICS STATEMENT
This study was granted exemption from the University of Illinois Chicago Institutional Review Board.
Supporting information
Data S1.
Gaber CE, Sarker J, Abdelaziz AI, et al. Pathologic complete response in patients with esophageal cancer receiving neoadjuvant chemotherapy or chemoradiation: A systematic review and meta‐analysis. Cancer Med. 2024;13:e7076. doi: 10.1002/cam4.7076
DATA AVAILABILITY STATEMENT
Datasets generated and analyzed during the current study are available via supplemental materials online.
REFERENCES
- 1. Abbas G, Krasna M. Overview of esophageal cancer. Ann Cardiothorac Surg. 2017;6(2):131‐136. doi: 10.21037/acs.2017.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA A Cancer J Clinicians. 2022;72(1):7‐33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 3. Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol. 2017;112(8):1247‐1255. doi: 10.1038/ajg.2017.155 [DOI] [PubMed] [Google Scholar]
- 4. Then EO, Lopez M, Saleem S, et al. Esophageal cancer: an updated surveillance epidemiology and end results database analysis. World J Oncol. 2020;11(2):55‐64. doi: 10.14740/wjon1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062‐5067. doi: 10.1200/JCO.2009.22.2083 [DOI] [PubMed] [Google Scholar]
- 6. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for Resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11‐20. doi: 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 7. Van Hagen P, Hulshof MCCM, Van Lanschot JJB, et al. Preoperative Chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074‐2084. doi: 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 8. Shah MA, Kennedy EB, Catenacci DV, et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol. 2020;38(23):2677‐2694. doi: 10.1200/JCO.20.00866 [DOI] [PubMed] [Google Scholar]
- 9. Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17(7):855‐883. doi: 10.6004/jnccn.2019.0033 [DOI] [PubMed] [Google Scholar]
- 10. Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27:v50‐v57. doi: 10.1093/annonc/mdw329 [DOI] [PubMed] [Google Scholar]
- 11. Noordman BJ, De Bekker‐Grob EW, Coene PPLO, et al. Patients' preferences for treatment after neoadjuvant chemoradiotherapy for oesophageal cancer. Br J Surg. 2018;105(12):1630‐1638. doi: 10.1002/bjs.10897 [DOI] [PubMed] [Google Scholar]
- 12. Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23(19):4330‐4337. doi: 10.1200/JCO.2005.05.017 [DOI] [PubMed] [Google Scholar]
- 13. Soror T, Kho G, Zhao KL, Ismail M, Badakhshi H. Impact of pathological complete response following neoadjuvant chemoradiotherapy in esophageal cancer. J Thorac Dis. 2018;10(7):4069‐4076. doi: 10.21037/jtd.2018.06.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blum Murphy M, Xiao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival‐the University of Texas MD Anderson Cancer Center experience: pathological response in esophageal cancer. Cancer. 2017;123(21):4106‐4113. doi: 10.1002/cncr.30953 [DOI] [PubMed] [Google Scholar]
- 15. Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol. 2010;17(4):1159‐1167. doi: 10.1245/s10434-009-0862-1 [DOI] [PubMed] [Google Scholar]
- 16. Lin JW, Hsu CP, Yeh HL, Chuang CY, Lin CH. The impact of pathological complete response after neoadjuvant chemoradiotherapy in locally advanced squamous cell carcinoma of esophagus. J Chin Med Assoc. 2018;81(1):18‐24. doi: 10.1016/j.jcma.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 17. Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87(2):392‐399. doi: 10.1016/j.athoracsur.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32(27):2983‐2990. doi: 10.1200/JCO.2014.55.9070 [DOI] [PubMed] [Google Scholar]
- 19. Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103(7):1347‐1355. doi: 10.1002/cncr.20916 [DOI] [PubMed] [Google Scholar]
- 20. Meguid RA, Hooker CM, Taylor JT, et al. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg. 2009;138(6):1309‐1317. doi: 10.1016/j.jtcvs.2009.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chevrollier GS, Giugliano DN, Palazzo F, et al. Patients with non‐response to neoadjuvant chemoradiation for esophageal cancer have no survival advantage over patients undergoing primary esophagectomy. J Gastrointest Surg. 2020;24(2):288‐298. doi: 10.1007/s11605-019-04161-9 [DOI] [PubMed] [Google Scholar]
- 22. Den Bakker CM, Smit JK, Bruynzeel AME, et al. Non responders to neoadjuvant chemoradiation for esophageal cancer: why better prediction is necessary. J Thorac Dis. 2017;9(S8):S843‐S850. doi: 10.21037/jtd.2017.06.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178‐189. doi: 10.1016/j.jclinepi.2021.03.001 [DOI] [PubMed] [Google Scholar]
- 24. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123‐128. doi: 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic Correlations. Cancer. 1994;73(11):2680‐2686. doi: [DOI] [PubMed] [Google Scholar]
- 26. Gabrielson S, Sanchez‐Crespo A, Klevebro F, et al. 18F FDG‐PET/CT evaluation of histological response after neoadjuvant treatment in patients with cancer of the esophagus or gastroesophageal junction. Acta Radiol. 2019;60(5):578‐585. doi: 10.1177/0284185118791204 [DOI] [PubMed] [Google Scholar]
- 27. Anvari K, Aledavood SA, Toussi MS, et al. A clinical trial of neoadjuvant concurrent chemoradiotherapy followed by resection for esophageal carcinoma. J Res Med Sci. 2015;20(8):751‐756. doi: 10.4103/1735-1995.168377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TPJ. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462‐467. doi: 10.1056/NEJM199608153350702 [DOI] [PubMed] [Google Scholar]
- 29. Ajani JA, Xiao L, Roth JA, et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2013;24(11):2844‐2849. doi: 10.1093/annonc/mdt339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Starling N, Okines A, Cunningham D, et al. A phase II trial of preoperative chemotherapy with epirubicin, cisplatin and capecitabine for patients with localised gastro‐oesophageal junctional adenocarcinoma. Br J Cancer. 2009;100(11):1725‐1730. doi: 10.1038/sj.bjc.6605070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klevebro F, Alexandersson von Döbeln G, Wang N, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro‐oesophageal junction. Ann Oncol. 2016;27(4):660‐667. doi: 10.1093/annonc/mdw010 [DOI] [PubMed] [Google Scholar]
- 32. Le Prise E, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73(7):1779‐1784. doi: [DOI] [PubMed] [Google Scholar]
- 33. Lee JL, Park SI, Kim SB, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15(6):947‐954. doi: 10.1093/annonc/mdh219 [DOI] [PubMed] [Google Scholar]
- 34. Horgan AM, Darling G, Wong R, et al. Adjuvant sunitinib following chemoradiotherapy and surgery for locally advanced esophageal cancer: a phase II trial: adjuvant sunitinib for esophageal cancer. Dis Esophagus. 2016;29(8):1152‐1158. doi: 10.1111/dote.12444 [DOI] [PubMed] [Google Scholar]
- 35. Zhao J, He M, Li J, et al. Apatinib combined with paclitaxel and cisplatin neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma. Cancer Biother Radiopharm. 2022;37(4):324‐331. doi: 10.1089/cbr.2021.0086 [DOI] [PubMed] [Google Scholar]
- 36. Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous‐cell cancer of the esophagus. N Engl J Med. 1997;337(3):161‐167. doi: 10.1056/nejm199707173370304 [DOI] [PubMed] [Google Scholar]
- 37. Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339(27):1979‐1984. doi: 10.1056/nejm199812313392704 [DOI] [PubMed] [Google Scholar]
- 38. Boonstra JJ, Kok TC, Wijnhoven BP, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long‐term results of a randomized controlled trial. BMC Cancer. 2011;11:181. doi: 10.1186/1471-2407-11-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vollenbrock SE, Voncken FEM, Lambregts DMJ, et al. Clinical response assessment on DW‐MRI compared with FDG‐PET/CT after neoadjuvant chemoradiotherapy in patients with oesophageal cancer. Eur J Nucl Med Mol Imaging. 2021;48(1):176‐185. doi: 10.1007/s00259-020-04917-5 [DOI] [PubMed] [Google Scholar]
- 40. Stahl M, Wilke H, Fink U, et al. Combined preoperative chemotherapy and radiotherapy in patients with locally advanced esophageal cancer. Interim analysis of a phase II trial. J Clin Oncol. 1996;14(3):829‐837. doi: 10.1200/jco.1996.14.3.829 [DOI] [PubMed] [Google Scholar]
- 41. Cheraghi A, Barahman M, Hariri R, Nikoofar A, Fadavi P. Comparison of the pathological response and adverse effects of oxaliplatin and capecitabine versus paclitaxel and carboplatin in the neoadjuvant chemoradiotherapy treatment approach for esophageal and gastroesophageal junction cancer: a randomized control trial study. Med J Islam Repub Iran. 2021;35:140. doi: 10.47176/mjiri.35.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Posner MC, Gooding WE, Lew JI, Rosenstein MM, Lembersky BC. Complete 5‐year follow‐up of a prospective phase II trial of preoperative chemoradiotherapy for esophageal cancer. Surgery. 2001;130(4):620‐626, 628. doi: 10.1067/msy.2001.116673 [DOI] [PubMed] [Google Scholar]
- 43. Lin CC, Hsu CH, Cheng JC, et al. Concurrent chemoradiotherapy with twice weekly paclitaxel and cisplatin followed by esophagectomy for locally advanced esophageal cancer. Ann Oncol. 2007;18(1):93‐98. doi: 10.1093/annonc/mdl339 [DOI] [PubMed] [Google Scholar]
- 44. Urba SG, Orringer MB, Ianettonni M, Hayman JA, Satoru H. Concurrent cisplatin, paclitaxel, and radiotherapy as preoperative treatment for patients with locoregional esophageal carcinoma. Cancer. 2003;98(10):2177‐2183. doi: 10.1002/cncr.11759 [DOI] [PubMed] [Google Scholar]
- 45. Duque‐Santana V, López‐Campos F, Martin M, et al. Dose‐escalated neoadjuvant chemoradiotherapy for locally advanced oesophageal or oesophagogastric junctional adenocarcinoma. Rep Pract Oncol Radiother. 2022;27(3):500‐508. doi: 10.5603/RPOR.a2022.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee JL, Kim SB, Jung HY, et al. Efficacy of neoadjuvant chemoradiotherapy in resectable esophageal squamous cell carcinoma—a single institutional study. Acta Oncol. 2003;42(3):207‐217. doi: 10.1080/02841860310010736 [DOI] [PubMed] [Google Scholar]
- 47. Aledavood SA, Shahid Sales S, Anvari K, Forghani MN, Memar B, Emadi TA. Evaluation of tumor Resectability rate and pathologic response to preoperative concurrent chemoradiotherapy in locally advanced proximal gastric and esophagogastric junction adenocarcinomas: a clinical trial. Int J Cancer Manag. 2017;10(5):e7473. doi: 10.5812/ijcm.7473 [DOI] [Google Scholar]
- 48. Si YS, Kim JH, Jin SR, et al. FDG‐PET in the prediction of pathologic response after neoadjuvant chemoradiotherapy in locally advanced, resectable esophageal cancer. Int J Radiat Oncol Biol Phys. 2005;63(4):1053‐1059. doi: 10.1016/j.ijrobp.2005.03.033 [DOI] [PubMed] [Google Scholar]
- 49. Shiraishi O, Yamasaki M, Makino T, et al. Feasibility of preoperative chemotherapy with docetaxel, cisplatin, and 5‐fluorouracil versus adriamycin, cisplatin, and 5‐fluorouracil for resectable advanced esophageal cancer. Oncology. 2017;92(2):101‐108. doi: 10.1159/000452765 [DOI] [PubMed] [Google Scholar]
- 50. Keller SM, Ryan LM, Coia LR, et al. High dose chemoradiotherapy followed by esophagectomy for adenocarcinoma of the esophagus and gastroesophageal junction: results of a phase II study of the eastern cooperative oncology group. Cancer. 1998;83(9):1908‐1916. doi: [DOI] [PubMed] [Google Scholar]
- 51. Pasini F, De Manzoni G, Pedrazzani C, et al. High pathological response rate in locally advanced esophageal cancer after neoadjuvant combined modality therapy: dose finding of a weekly chemotherapy schedule with protracted venous infusion of 5‐fluorouracil and dose escalation of cisplatin, docetaxel and concurrent radiotherapy. Ann Oncol. 2005;16(7):1133‐1139. doi: 10.1093/annonc/mdi207 [DOI] [PubMed] [Google Scholar]
- 52. Al‐Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4‐AIO): results from the phase 2 part of a multicentre, open‐label, randomised phase 2/3 trial. Lancet Oncol. 2016;17(12):1697‐1708. doi: 10.1016/S1470-2045(16)30531-9 [DOI] [PubMed] [Google Scholar]
- 53. Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47(3):354‐360. doi: 10.1016/j.ejca.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 54. Tian Y, Wang J, Qiao X, et al. Long‐term efficacy of neoadjuvant concurrent chemoradiotherapy for potentially resectable advanced Siewert type II and III adenocarcinomas of the esophagogastric junction. Front Oncol. 2021;11:756440. doi: 10.3389/fonc.2021.756440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Choong NW, Mauer AM, Haraf DC, et al. Long‐term outcome of a phase II study of docetaxel‐based multimodality chemoradiotherapy for locally advanced carcinoma of the esophagus or gastroesophageal junction. Med Oncol. 2011;28(Suppl 1):S152‐S161. doi: 10.1007/s12032-010-9658-1 [DOI] [PubMed] [Google Scholar]
- 56. Swisher SG, Ajani JA, Komaki R, et al. Long‐term outcome of phase II trial evaluating chemotherapy, chemoradiotherapy, and surgery for locoregionally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 2003;57(1):120‐127. doi: 10.1016/s0360-3016(03)00522-4 [DOI] [PubMed] [Google Scholar]
- 57. Lew JI, Gooding WE, Ribeiro U Jr, Safatle‐Ribeiro AV, Posner MC. Long‐term survival following induction chemoradiotherapy and esophagectomy for esophageal carcinoma. Arch Surg. 2001;136(7):737‐742. doi: 10.1001/archsurg.136.7.737 [DOI] [PubMed] [Google Scholar]
- 58. Kleinberg L, Knisely JP, Heitmiller R, et al. Mature survival results with preoperative cisplatin, protracted infusion 5‐fluorouracil, and 44‐Gy radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2003;56(2):328‐334. doi: 10.1016/s0360-3016(02)04598-4 [DOI] [PubMed] [Google Scholar]
- 59. Wang H, Tang H, Fang Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical Trial. JAMA Surg. 2021;156(5):444‐451. doi: 10.1001/jamasurg.2021.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chiarion‐Sileni V, Innocente R, Cavina R, et al. Multi‐center phase II trial of chemo‐radiotherapy with 5‐fluorouracil, leucovorin and oxaliplatin in locally advanced esophageal cancer. Cancer Chemother Pharmacol. 2009;63(6):1111‐1119. doi: 10.1007/s00280-008-0834-3 [DOI] [PubMed] [Google Scholar]
- 61. Ruhstaller T, Widmer L, Schuller JC, et al. Multicenter phase II trial of preoperative induction chemotherapy followed by chemoradiation with docetaxel and cisplatin for locally advanced esophageal carcinoma (SAKK 75/02). Ann Oncol. 2009;20(9):1522‐1528. doi: 10.1093/annonc/mdp045 [DOI] [PubMed] [Google Scholar]
- 62. Makino T, Yamasaki M, Tanaka K, et al. Multicenter randomised trial of two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for locally advanced oesophageal squamous cell carcinoma. Br J Cancer. 2022;126(11):1555‐1562. doi: 10.1038/s41416-022-01726-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang YJ, Li KK, Xie XF, et al. Neoadjuvant anlotinib and chemotherapy followed by minimally invasive esophagectomy in patients with locally advanced esophageal squamous cell carcinoma: short‐term results of an open‐label, randomized, phase II trial. Front Oncol. 2022;12:908841. doi: 10.3389/fonc.2022.908841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, Open‐Label Clinical Trial. J Clin Oncol. 2018;36(27):2796‐2803. doi: 10.1200/jco.2018.79.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yan MH, Hou XB, Cai BN, Qu BL, Dai XK, Liu F. Neoadjuvant chemoradiotherapy plus surgery in the treatment of potentially resectable thoracic esophageal squamous cell carcinoma. World J Clin Cases. 2020;8(24):6315‐6321. doi: 10.12998/wjcc.v8.i24.6315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tang H, Wang H, Fang Y, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multi‐center randomized clinical trial. Ann Oncol. 2022;34:163‐172. doi: 10.1016/j.annonc.2022.10.508 [DOI] [PubMed] [Google Scholar]
- 67. Ruhstaller T, Thuss‐Patience P, Hayoz S, et al. Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: a randomized, open‐label, phase III trial (SAKK 75/08). Ann Oncol. 2018;29(6):1386‐1393. doi: 10.1093/annonc/mdy105 [DOI] [PubMed] [Google Scholar]
- 68. von Döbeln GA, Klevebro F, Jacobsen AB, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long‐term results of a randomized clinical trial. Dis Esophagus. 2019;32(2). doi: 10.1093/dote/doy078 [DOI] [PubMed] [Google Scholar]
- 69. Ohnuma H, Sato Y, Hayasaka N, et al. Neoadjuvant chemotherapy with docetaxel, nedaplatin, and fluorouracil for resectable esophageal cancer: a phase II study. Cancer Sci. 2018;109(11):3554‐3563. doi: 10.1111/cas.13772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alderson D, Cunningham D, Nankivell M, et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open‐label, randomised phase 3 trial. Lancet Oncol. 2017;18(9):1249‐1260. doi: 10.1016/s1470-2045(17)30447-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van Meerten E, Muller K, Tilanus HW, et al. Neoadjuvant concurrent chemoradiation with weekly paclitaxel and carboplatin for patients with oesophageal cancer: a phase II study. Br J Cancer. 2006;94(10):1389‐1394. doi: 10.1038/sj.bjc.6603134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lorenzen S, Brücher B, Zimmermann F, et al. Neoadjuvant continuous infusion of weekly 5‐fluorouracil and escalating doses of oxaliplatin plus concurrent radiation in locally advanced oesophageal squamous cell carcinoma: results of a phase I/II trial. Br J Cancer. 2008;99(7):1020‐1026. doi: 10.1038/sj.bjc.6604659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dipetrillo T, Suntharalingam M, Ng T, et al. Neoadjuvant paclitaxel poliglumex, cisplatin, and radiation for esophageal cancer: a phase 2 trial. Am J Clin Oncol. 2012;35(1):64‐67. doi: 10.1097/COC.0b013e318201a126 [DOI] [PubMed] [Google Scholar]
- 74. Mukherjee S, Hurt CN, Gwynne S, et al. NEOSCOPE: a randomised phase II study of induction chemotherapy followed by oxaliplatin/capecitabine or carboplatin/paclitaxel based pre‐operative chemoradiation for resectable oesophageal adenocarcinoma. Eur J Cancer. 2017;74:38‐46. doi: 10.1016/j.ejca.2016.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91(11):2165‐2174. doi: [DOI] [PubMed] [Google Scholar]
- 76. Jatoi A, Martenson JA, Foster NR, et al. Paclitaxel, carboplatin, 5‐fluorouracil, and radiation for locally advanced esophageal cancer: phase II results of preliminary pharmacologic and molecular efforts to mitigate toxicity and predict outcomes: North Central cancer treatment group (N0044). Am J Clin Oncol. 2007;30(5):507‐513. doi: 10.1097/COC.0b013e31805c139a [DOI] [PubMed] [Google Scholar]
- 77. Thuss‐Patience PC, Hofheinz RD, Arnold D, et al. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro‐oesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Ann Oncol. 2012;23(11):2827‐2834. doi: 10.1093/annonc/mds129 [DOI] [PubMed] [Google Scholar]
- 78. Ilson DH, Minsky BD, Ku GY, et al. Phase 2 trial of induction and concurrent chemoradiotherapy with weekly irinotecan and cisplatin followed by surgery for esophageal cancer. Cancer. 2012;118(11):2820‐2827. doi: 10.1002/cncr.26591 [DOI] [PubMed] [Google Scholar]
- 79. Knox JJ, Wong R, Visbal AL, et al. Phase 2 trial of preoperative irinotecan plus cisplatin and conformal radiotherapy, followed by surgery for esophageal cancer. Cancer. 2010;116(17):4023‐4032. doi: 10.1002/cncr.25349 [DOI] [PubMed] [Google Scholar]
- 80. Spigel DR, Greco FA, Meluch AA, et al. Phase I/II trial of preoperative oxaliplatin, docetaxel, and capecitabine with concurrent radiation therapy in localized carcinoma of the esophagus or gastroesophageal junction. J Clin Oncol. 2010;28(13):2213‐2219. doi: 10.1200/jco.2009.24.8773 [DOI] [PubMed] [Google Scholar]
- 81. Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104(11):1455‐1460. doi: 10.1111/cas.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hashimoto J, Kato K, Ito Y, et al. Phase II feasibility study of preoperative concurrent chemoradiotherapy with cisplatin plus 5‐fluorouracil and elective lymph node irradiation for clinical stage II/III esophageal squamous cell carcinoma. Int J Clin Oncol. 2019;24(1):60‐67. doi: 10.1007/s10147-018-1336-x [DOI] [PubMed] [Google Scholar]
- 83. Fan Y, Jiang Y, Zhou X, et al. Phase II study of neoadjuvant therapy with nab‐paclitaxel and cisplatin followed by surgery in patients with locally advanced esophageal squamous cell carcinoma. Oncotarget. 2016;7:50624‐50634. doi: 10.18632/oncotarget.9562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huang JW, Yeh HL, Hsu CP, et al. Phase II study of preoperative concurrent chemoradiotherapy with oxaliplatin for locally advanced esophageal cancer. J Chin Med Assoc. 2017;80(7):401‐407. doi: 10.1016/j.jcma.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 85. Kim DW, Blanke CD, Wu H, et al. Phase II study of preoperative paclitaxel/cisplatin with radiotherapy in locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 2007;67(2):397‐404. doi: 10.1016/j.ijrobp.2006.08.062 [DOI] [PubMed] [Google Scholar]
- 86. Jatoi A, Soori G, Foster NR, et al. Phase II study of preoperative pemetrexed, carboplatin, and radiation followed by surgery for locally advanced esophageal cancer and gastroesophageal junction tumors. J Thorac Oncol. 2010;5(12):1994‐1998. doi: 10.1097/JTO.0b013e3181fb5c3e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tanaka Y, Yoshida K, Yamada A, et al. Phase II trial of biweekly docetaxel, cisplatin, and 5‐fluorouracil chemotherapy for advanced esophageal squamous cell carcinoma. Cancer Chemother Pharmacol. 2016;77(6):1143‐1152. doi: 10.1007/s00280-016-2985-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tanaka Y, Yoshida K, Tanahashi T, Okumura N, Matsuhashi N, Yamaguchi K. Phase II trial of neoadjuvant chemotherapy with docetaxel, nedaplatin, and S1 for advanced esophageal squamous cell carcinoma. Cancer Sci. 2016;107(6):764‐772. doi: 10.1111/cas.12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27(6):851‐856. doi: 10.1200/jco.2008.17.0506 [DOI] [PubMed] [Google Scholar]
- 90. Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086‐1092. doi: 10.1200/jco.2007.12.9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Aledavood SA, Anvari K, Shahidsales S, Hosseini S, Emadi Torghabeh A, Masudian M. Post‐neoadjuvant chemoradiotherapy tumor resectability following induction chemotherapy in locally advanced proximal gastric and adenocarcinoma of the esophagogastric junction: a clinical trial. Caspian J Intern Med. 2021;12(3):256‐262. doi: 10.22088/cjim.12.3.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hosoya Y, Shibusawa H, Nagai H, et al. Preoperative chemotherapy for advanced esophageal cancer and relation with histological effect. Surg Today. 1999;29(8):689‐694. doi: 10.1007/s005950050493 [DOI] [PubMed] [Google Scholar]
- 93. Keresztes RS, Port JL, Pasmantier MW, Korst RJ, Altorki NK. Preoperative chemotherapy for esophageal cancer with paclitaxel and carboplatin: results of a phase II trial. J Thorac Cardiovasc Surg. 2003;126(5):1603‐1608. doi: 10.1016/s0022-5223(03)00710-4 [DOI] [PubMed] [Google Scholar]
- 94. Law S, Fok M, Chow S, Chu KM, Wong J. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;114(2):210‐217. doi: 10.1016/s0022-5223(97)70147-8 [DOI] [PubMed] [Google Scholar]
- 95. Darnton SJ, Archer VR, Stocken DD, Mulholland PJ, Casson AG, Ferry DR. Preoperative mitomycin, ifosfamide, and cisplatin followed by esophagectomy in squamous cell carcinoma of the esophagus: pathologic complete response induced by chemotherapy leads to long‐term survival. J Clin Oncol. 2003;21(21):4009‐4015. doi: 10.1200/JCO.2003.01.236 [DOI] [PubMed] [Google Scholar]
- 96. Meluch AA, Greco FA, Gray JR, et al. Preoperative therapy with concurrent paclitaxel/carboplatin/infusional 5‐FU and radiation therapy in locoregional esophageal cancer: final results of a Minnie pearl cancer research network phase II trial. Cancer J. 2003;9(4):251‐260. doi: 10.1097/00130404-200307000-00007 [DOI] [PubMed] [Google Scholar]
- 97. Baba M, Natsugoe S, Shimada M, et al. Prospective evaluation of preoperative chemotherapy in resectable squamous cell carcinoma of the thoracic esophagus. Dis Esophagus. 2000;13(2):136‐141. doi: 10.1046/j.1442-2050.2000.00101.x [DOI] [PubMed] [Google Scholar]
- 98. Natsugoe S, Okumura H, Matsumoto M, et al. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Dis Esophagus. 2006;19(6):468‐472. doi: 10.1111/j.1442-2050.2006.00615.x [DOI] [PubMed] [Google Scholar]
- 99. Goodman KA, Ou FS, Hall NC, et al. Randomized phase II study of PET response‐adapted combined modality therapy for esophageal cancer: mature results of the CALGB 80803 (Alliance) Trial. J Clin Oncol. 2021;39(25):2803‐2815. doi: 10.1200/jco.20.03611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19(2):305‐313. doi: 10.1200/jco.2001.19.2.305 [DOI] [PubMed] [Google Scholar]
- 101. Liu S, Wen J, Yang H, et al. Recurrence patterns after neoadjuvant chemoradiotherapy compared with surgery alone in oesophageal squamous cell carcinoma: results from the multicenter phase III trial NEOCRTEC5010. Eur J Cancer. 2020;138:113‐121. doi: 10.1016/j.ejca.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 102. Brücher BL, Stein HJ, Zimmermann F, et al. Responders benefit from neoadjuvant radiochemotherapy in esophageal squamous cell carcinoma: results of a prospective phase‐II trial. Eur J Surg Oncol. 2004;30(9):963‐971. doi: 10.1016/j.ejso.2004.06.008 [DOI] [PubMed] [Google Scholar]
- 103. Leichman LP, Goldman BH, Bohanes PO, et al. S0356: a phase II clinical and prospective molecular trial with oxaliplatin, fluorouracil, and external‐beam radiation therapy before surgery for patients with esophageal adenocarcinoma. J Clin Oncol. 2011;29(34):4555‐4560. doi: 10.1200/jco.2011.36.7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nusrath S, Thammineedi SR, Raju KVVN, et al. Short‐term outcomes in patients with carcinoma of the esophagus and gastroesophageal junction receiving neoadjuvant chemotherapy or chemoradiation before surgery. A prospective study. Rambam Maimonides Med J. 2019;10(1):e0002. doi: 10.5041/RMMJ.10339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. El Nakadi I, Van Laethem JL, Houben JJ, et al. Squamous cell carcinoma of the esophagus: multimodal therapy in locally advanced disease. World J Surg. 2002;26(1):72‐78. doi: 10.1007/s00268-001-0184-3 [DOI] [PubMed] [Google Scholar]
- 106. Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6(9):659‐668. doi: 10.1016/s1470-2045(05)70288-6 [DOI] [PubMed] [Google Scholar]
- 107. Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416‐2422. doi: 10.1200/jco.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 108. Shiraishi O, Makino T, Yamasaki M, et al. Two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for treating locally advanced esophageal cancer: short‐term outcomes of a multicenter randomized phase II trial. Esophagus. 2021;18(4):825‐834. doi: 10.1007/s10388-021-00831-3 [DOI] [PubMed] [Google Scholar]
- 109. Moore JL, Green M, Santaolalla A, et al. Pathologic lymph node regression after Neoadjuvant chemotherapy predicts recurrence and survival in esophageal adenocarcinoma: a multicenter study in the United Kingdom. J Clin Oncol. 2023;41(28):4522‐4534. doi: 10.1200/JCO.23.00139 [DOI] [PubMed] [Google Scholar]
- 110. Nilsson M, Olafsdottir H, Alexandersson Von Döbeln G, et al. Neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma versus definitive chemoradiotherapy with salvage surgery as needed: the study protocol for the randomized controlled NEEDS Trial. Front Oncol. 2022;12:917961. doi: 10.3389/fonc.2022.917961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Noordman BJ, Spaander MCW, Valkema R, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018;19(7):965‐974. doi: 10.1016/S1470-2045(18)30201-8 [DOI] [PubMed] [Google Scholar]
- 112. Van Der Wilk BJ, Eyck BM, Wijnhoven BPL, et al. LBA75 Neoadjuvant chemoradiotherapy followed by surgery versus active surveillance for oesophageal cancer (SANO‐trial): a phase‐III stepped‐wedge cluster randomised trial. Ann Oncol. 2023;34:S1317. doi: 10.1016/j.annonc.2023.10.076 [DOI] [Google Scholar]
- 113. Ajani JA, Correa AM, Hofstetter WL, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2012;23(10):2638‐2642. doi: 10.1093/annonc/mds210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yang Y, Xu X, Zhou X, et al. Impact of radiation dose on survival for esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Front Oncol. 2020;10:1431. doi: 10.3389/fonc.2020.01431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mantziari S, Farinha HT, Messier M, et al. Low‐dose radiation yields lower rates of pathologic response in esophageal cancer patients. Ann Surg Oncol. 2024;10. doi: 10.1245/s10434-023-14810-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Li R, Shinde A, Glaser S, et al. Analyzing the impact of neoadjuvant radiation dose on pathologic response and survival outcomes in esophageal and gastroesophageal cancers. J Gastrointest Oncol. 2019;10(4):712‐722. doi: 10.21037/jgo.2019.02.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Thomas M, Borggreve AS, Van Rossum PSN, et al. Radiation dose and pathological response in oesophageal cancer patients treated with neoadjuvant chemoradiotherapy followed by surgery: a multi‐institutional analysis. Acta Oncol. 2019;58(10):1358‐1365. doi: 10.1080/0284186X.2019.1646432 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
Datasets generated and analyzed during the current study are available via supplemental materials online.


