Abstract
Introduction:
Placental phospholipid synthesis is critical for the expansion of the placental exchange surface area and for production of signaling molecules. Despite their importance, it is not yet established which enzymes involved in the de novo synthesis and remodeling of placental phospholipids are expressed and active in the human placenta.
Methods:
We identified phospholipid synthesis enzymes by immunoblotting in placental homogenates and immunofluorescence in placenta tissue sections. Primary human trophoblast (PHT) cells from term healthy placentas (n=10) were cultured and exposed to 13C labeled fatty acids (16:0, 18:1 and 18:2 n-6, 22:6 n-3) for 2 and 24 hrs. Three phospholipid classes; phosphatidic acid, phosphatidylcholine, and lysophosphatidylcholine containing 13C fatty acids were quantified by Liquid Chromatography with tandem mass spectrometry (LC/MS-MS).
Results:
Acyl transferase and phospholipase enzymes were detected in human placenta homogenate and primarily expressed in the syncytiotrophoblast. Three representative 13C fatty acids (16:0, 18:1 and 18:2 n-6) were incorporated rapidly into phosphatidic acid in trophoblasts, but 13C labeled docosahexaenoic acid (DHA; 22:6 n-3) incorporation was not detected. 13C DHA was incorporated into phosphatidylcholine. Lysophosphatidylcholine containing all four 13C labeled fatty acids were found in high abundance.
Conclusions:
Phospholipid synthesis and remodeling enzymes are present in the syncytiotrophoblast. 13C labeled fatty acids were rapidly incorporated into cellular phospholipids. 13C DHA was incorporated into phospholipids through the remodeling pathway rather than by de novo synthesis. These understudied pathways are highly active and critical for structure and function of the placenta.
Keywords: Maternal-fetal exchange, fetal development, trophoblast, pregnancy, phosphatidic acid, lysophospholipids
Introduction
The human placenta is the only transient organ in humans, which has likely contributed to the lack of detailed knowledge in multiple areas of placental biology. The syncytial nature of the transporting epithelium, or syncytiotrophoblast, is also a unique feature of this organ. The syncytiotrophoblast has two polarized plasma membranes, an apical brush border or microvillous plasma membrane (MVM) in contact with the pooled maternal blood and a basal plasma membrane (BM), toward the fetal capillary. This allows for vectorial transfer of nutrients from the maternal to the fetal circulation as well as removal of fetal waste products to the maternal circulation. The expanded surface area in the microvilli is important for adequate uptake of oxygen and nutrients to support both placental and fetal growth across gestation. Microvilli are cytoplasm-filled projections of plasma membrane comprised of a phospholipid bilayer. Microvilli are supported by a cytoskeleton and their surface area and number increases across gestation [1-3]. The synthesis of phospholipids to support expansion of the MVM surface area, placental growth and appropriate development of the fetus is an aspect of trophoblast biology that is poorly understood.
Placentas of pregnancies complicated by fetal growth restriction have reduced microvillous surface area which is thought to contribute to the impaired intrauterine growth [4-9]. Whether placentas that support accelerated fetal growth have greater MVM surface area for transport of nutrients is less clear [10] but the placentas are usually larger. We have previously shown that per μg membrane protein, nutrient transport capacity of the human placenta is reduced in pregnancies complicated by growth restriction and either increased or unchanged in pregnancies complicated by accelerated fetal growth [11]. We also found a significant correlation between placental nutrient transport capacity and birth weight suggesting a functional association between placental membrane transporter expression and/or function and fetal growth [12-14]. Trophoblast synthesis of phospholipids has not been extensively studied in healthy or pathological pregnancies.
Uniformly labeled 13C non-esterified “free” fatty acids (NEFA) enter cultured primary human trophoblasts and are rapidly and predominantly incorporated into the phospholipid fraction [15]. This was true for all four representative fatty acids species we previously tested (palmitic acid, 16:0; oleic acid, 18:1; linoleic acid, 18:2 n-6 and docosahexaenoic acid, 22:6 n-3) and suggested a rapid turnover of the cellular phospholipid pool. We found 50-60% of total cellular phospholipids contained a newly incorporated 13C fatty acid species after 24 h incubation [15]. De novo synthesis (Kennedy pathway) of phosphatidic acid is required for formation of both phospholipids and triacylglycerols. This involves sequential enzymatic esterification of fatty acids to glycerol-3-phosphate (G-3-P) which is followed by addition of the specific head group such as choline to generate phosphatidylcholine [16, 17]. Glycerol Phosphate Acyl Transferase isoform 3 (GPAT3) catalyzes the first esterification step to add a single acyl chain to the glycerol-3-phosphate backbone in the sn-1 position [18]. Enzymes responsible for the esterification of a second acyl chain to the glycerol backbone, Acyl Glycerol Phosphate Acyl Transferase (AGPAT) [19] produce the intermediate phosphatidic acid. Phosphatidic acid is converted to diacylglycerol, which is the substrate for addition of the hydrophilic head group to complete phospholipid synthesis. Hydrolysis of acyl chains from existing phospholipids to create the ‘lyso’ form is achieved by phospholipase A2 group (PLA2G) enzyme activity [20]. Additionally, the fatty acid composition of phospholipids can be modified by hydrolysis of a fatty acid chain from the phospholipid by PLA2G activity producing a lysophospholipid (single acyl chain) and re-esterification of a new acyl chain by lysophosphatidylcholine acyl transferases (LPCAT) [21], thus regenerating a phospholipid with a different fatty acid composition [22]. This phospholipid remodeling pathway is known as the Lands cycle. These critical pathways and the enzymes responsible have not been extensively studied in the human placenta.
Our goal was to characterize phospholipid synthesis and remodeling in the human placenta by identifying isoforms of acyl transferase enzymes that are responsible for esterification of acyl species to the phospholipid and phospholipases that hydrolyze acyl groups from phospholipids by using immunoblotting and immunofluorescence in healthy term placentas. In addition, we used cultured primary human trophoblasts from healthy term pregnancies to determine the incorporation of four distinct uniformly 13C labeled fatty acids. These four fatty acids; palmitic acid (16:0), oleic acid (18:1), linoleic acid (18:2 n-6) and docosahexaenoic acid (22:6 n-3) were selected to represent the main fatty acid types; saturated (SFA), monounsaturated (MUFA), omega 6 essential polyunsaturated fatty acid (PUFA) and omega 3 long chain polyunsaturated fatty acid (LCPUFA) and were used in physiological concentrations found in maternal circulation at the end of normal pregnancy [23]. We analyzed three key phospholipid forms: phosphatidic acid and lysophosphatidylcholine (intermediates in the de novo and remodeling pathways, respectively) and phosphatidylcholine.
Methods and Materials
Study subjects for plasma and placenta collection
Pregnant women were enrolled to donate their placenta to a data/bio-sample repository for use in multiple research studies under a protocol approved by the Institutional Review Board at University of Colorado, Denver (COMIRB 14-1073). All participants gave informed written consent for sample collection and use of protected health information. Inclusion criteria for the repository included information on pre-pregnancy/first trimester body mass index (BMI), ultrasound confirmation of gestational age at 14-18 weeks, singleton pregnancy and maternal age 18-45 years. Exclusion criteria included concurrent inflammatory or vascular disease, current use of tobacco, street drugs, or medications, fetal malformations, history of pregnancy loss or pre-term delivery. For the current study, women with a diagnosis of any form of diabetes, gestational hypertension and preterm birth or other chronic illness were also excluded. Placentas (n=11) for the current study were from women with normal pre-gravid or first trimester BMI. At delivery we collected placental tissue which was processed by fixation, homogenization of villous tissue, or isolation of primary trophoblasts as described below. Maternal and infant characteristics for the current study are summarized in Table 1.
Table 1.
Clinical parameters for the samples used.
| Maternal & Pregnancy Data | |
|---|---|
| N | 11 |
| Maternal Age (years) | 30.64 ± 1.92 |
| Ethnicity | 7 White non Hispanic |
| 1 Black non Hispanic | |
| 3 White Hispanic | |
| Body Mass Index (BMI, kg/m2) | 22.09 ± 0.58 |
| Delivery Type | 8 C-section no labor |
| 3 vaginal with labor | |
| Gestational Age (weeks) | 39.15 ± 0.05 |
| Neonate Data | |
| Gender | 5 Female |
| 6 Male | |
| Placenta Weight (grams) | 649.30 ± 25.96 |
| Birth Weight (kilograms) | 3.47 ± 0.08 |
| Birth Weight / Placental Weight Ratio | 5.43 ± 0.24 |
| Birth Length (cm) | 50.07 ± 0.45 |
Placenta tissue homogenization
Placentas were obtained within 30 min after delivery. After removing decidua basalis and chorionic plate, villous tissue was dissected and rinsed in ice cold physiological saline. Villous tissue was transferred to ice-cold buffer D (250 mM sucrose, 10 mM hepes, pH 7.4) containing protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO) and homogenized on ice. Placental homogenates were aliquoted and rapidly frozen in liquid nitrogen and stored at −80°C until further analysis.
Immunohistochemistry and Immunoblotting for acyl transferase and phospholipase enzymes
Immunoreactive GPAT3, AGPAT4, and PLA2G6 were localized in paraffin-embedded samples from term human placental villous tissues, as previously described [24]. Antigen retrieval was performed using boiling citrate. Sections were then blocked in 10% normal goat serum for 1 hour at room temperature, sections were then incubated overnight with the following primary antibodies: rabbit anti-GPAT3 polyclonal antibody (Sigma, HPA029414; 1:50), rabbit anti-AGPAT4 polyclonal (Novus Biological, NBP1-79870; 1:250), and rabbit anti-PLA2G6 polyclonal (Sigma, HPA001171; 1:250). Each antibody was used at a dilution optimized for that antibody. Normal rabbit (EMD Milipore, Billerica, MA, USA) IgG, at a concentration equal to that for the primary IgG, was used as a negative control. Immunoreactive proteins were detected using Alexa Fluor 594-conjugated secondary antibody (Life Technologies) for 1 hour at room temperature at a dilution of 1:250. Tissue sections were then washed three times for 5 min/wash in PBS. Slides were counterstained with Prolong Gold Antifade reagent containing 4',6-diamidino-2-phenylindole (DAPI) (Life Technologies) and cover slipped. Images were taken using a Nikon Eclipse 80i microscope interfaced with a Prisme BSI Express camera.
Frozen sections of term human placental villous tissues (8μm) were used to localize LPCAT1. Using immunofluorescence previously as described [25]. Sections were fixed in methanol at −20°C and washed in PBS. Sections were then blocked with 10% normal goat serum diluted in antibody dilution buffer for 1 hour at room temperature. Rabbit anti-LPCAT1 polyclonal (Sigma, HPA012501; 1:100) was added at a dilution optimized for the antibody and incubated overnight at 4°C in a humidified chamber. Tissue sections were then washed three times for 5min/wash in PBS. Immunoreactive proteins were detected using Alexa Fluor 594-conjugated secondary antibody (Life Technologies) was added to sections and incubated for 1 hour at room temperature at a dilution of 1:250. Tissue sections were then washed three times for 5 min/wash in PBS. Slides were processed for counterstain and imaging as previously described.
Placental homogenates from healthy term pregnancies (n=3) were thawed on ice and protein content was determined using bicinchoninic acid (BCA) (Thermo Fisher). We used either automated capillary immunoblotting on Jess (Simple Western by Protein Simple) or traditional polyacrylamide gel electrophoresis (PAGE) Western blot to determine the presence of phospholipid de novo and remodeling enzymes. We ran the Jess plates based on the recommended manufacturer’s settings (separation voltage of 375 for 25 minutes) with the placental homogenates at a 1μg/μl protein concentration per capillary [26]. An equalizer sample was used on each plate to correct for variations, and positive controls were run with each antibody to confirm the presence of the target. PLAG4c and both LPCATs were detected with near infrared and converted to grey scale in photoshop.
Traditional PAGE Western blot was performed as previously described [27, 28] using a precast gel system (Bio-Rad). Ten μg total placental protein was loaded and separated on Bis-Tris gels (4-20%). Human brain cerebellum whole tissue lysate, (Novus Biologicals, catalog number NB820-59180) was used as a positive control. Electrophoresis was performed at 120V for 20 min and then at a constant 150V for 1 h. Proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories Inc.) overnight at 4°C at constant 35 V. After blocking, incubation with primary antibodies (Table 2) was carried out overnight at 4°C in 5% bovine serum albumin (BSA)/tris-buffered saline (TBS)-tween or milk. The washed membranes were incubated for 1 h at room temperature with secondary antibody (peroxidase labeled anti-Rabbit or anti mouse IgG, Cell Signaling Technology, Danvers, MA) diluted at 1:10000. Immunolabeling was made visible with SuperSignal West Pico Plus detection solution (Thermo Scientific) in a G:Box ChemiXL1.4 (SynGene, Cambridge, UK).
Table 2.
Details of Antibodies used.
| Name of Antibody |
Species Raised in; Monoclonal or Polyclonal |
Manufacturer and Catalog Number |
Positive Control | Protein Assay |
Dilation Used |
IF Dilution Used |
|---|---|---|---|---|---|---|
| AGPAT1 | Rabbit; Polyclonal | Sigma HPA073355 | Human Cerebellum | Jess | 1:50 | |
| AGPAT2 | Rabbit; Monoclonal | Cell Signaling CST 14937 | Human Cerebellum | Jess | 1:100 | |
| AGPAT4 | Rabbit; Polyclonal | Novus NBP1-79870 | Mouse Brain | Western Blot | 1:1000 | 1:250 |
| GPAT3 | Rabbit; Polyclonal | Sigma HPA029414 | Human Cerebellum | Jess | 1:100 | 1:50 |
| LPCAT1 | Rabbit; Polyclonal | Sigma HPA012501 | Human Cerebellum | Jess | 1:25 | 1:100 |
| LPCAT4 | Rabbit; Polyclonal | Invitrogen PA5-50544 | Human Cerebellum | Jess | 1:50 | |
| PLA2G4c | Rabbit; Polyclonal | Sigma HPA043083 | Human Intestine | Jess | 1:150 | |
| PLA2G6 | Rabbit; Polyclonal | Sigma HPA001171 | Human Intestine | Western Blot | 1:1000 | 1:250 |
Culture of primary human trophoblasts and uptake and metabolism of uniformly labeled 13C fatty acids
Villous cytotrophoblasts were isolated and purified as previously described [15, 29, 30]. Briefly, cells were isolated after DNAse/trypsin digestion and then purified on a Percoll gradient. Primary human trophoblasts (PHTs) were plated at a density of 4 x 106 per 60 mm dish and cultured in media containing Ham’s F-12 and high glucose Dulbecco’s Modified Eagle Medium (DMEM) (1:1, v/v), 10% fetal bovine serum (FBS; S11550, Atlanta Biologicals, Lawrenceville, GA), and antibiotics (penicillin, gentamicin and streptomycin) according to a well-established protocol [27]. Production of human Chorionic Gonadotropin (hCG) in the culture media was measured daily by ELISA (IBL-America, Minneapolis, MN, catalogue number IB19115) to demonstrate differentiation into syncytiotrophoblast.
At 66 or 88 h culture, trophoblasts were treated with a combination of uniformly labeled 13C palmitic acid (95 μM, 16:0), 13C oleic acid (95 μM, 18:1), 13C linoleic acid (95 μM, 18:2 n-6) and 13C docosahexaenoic acid (15 μM, 22:6 n-3) (Cambridge Isotope Laboratories, Tewksbury, MA) in 10% fatty acid free-BSA by adding them to culture media containing 10% lipoprotein depleted fetal bovine serum (Kalen Biomedical, LCC, Germantown, MD) to remove the lipids in FBS. Untreated cells were cultured in the same conditions by adding an equal volume of fatty acid free-BSA to the culture media containing lipoprotein depleted media. Cells were incubated at 37°C with 5% CO2 and harvested at 90 h culture after carefully washing the cells four times in ice-cold PBS to remove the extracellular 13C labeled fatty acids. Cells were scraped using a rubber tipped spatula into 0.9 % NaCl and were stored at −80°C until analysis.
Lipids were extracted from PHT (250 μL) with a combination of water (750 μL), methanol (900 μL) and methyl-tert-butyl ether (3 mL) [31] after the addition of an internal standard cocktail containing phosphatidylcholine (PC)-19:0/19:0 (2000 pmol), d7-PC-18:1/OH (200 pmol), and phosphatidic acid-17:0/17:0 (20 pmol). For phospholipid analysis, samples were injected into a High Pressure Liquid Chromatography (HPLC) system connected to a triple quadrupole mass spectrometer (Sciex 4000 QTRAP, Framingham, MA) and normal phase chromatography was performed using a silica column (150x2 mm, Luna Silica 5 μm, Phenomenex). The mobile phase system consisted of solvent A (isopropanol/hexane/water (58/40/2, v/v)) and 35% solvent B (hexane/isopropanol/water (300/400/84, v/v/v)) both containing 10 mM ammonium acetate. Mass spectrometric analysis was performed in the negative ion mode using multiple reaction monitoring (MRM). The precursor ions monitored were the molecular ions [M−H]− for phosphatidic acid and the acetate adducts [M+CH3COO]− for phosphatidylcholine and lysophosphatidylcholine species. The product ions analyzed after collision-induced decomposition were the carboxylate anions corresponding to 16:0, 18:1, 18:2, and 22:6 (m/z 225.1, 281.1, 279.1, and 327.1) and the uniformly labeled 13C acyl chains of 16:0, 18:1, 18:2, and 22:6 (m/z 271.1, 299.1, 297.1, and 349.1). The MRM transitions for phosphatidylcholine and phosphatidic acid lipid species monitored in this study are listed in Supplemental Tables 1 and 2, respectively. Percent incorporation was determined by dividing the area of the uniformly labeled 13C phospholipid peak by the summed area of the endogenous phospholipid peak and the uniformly labeled 13C phospholipid peak. The percent incorporation was calculated as the phospholipid (PL) species containing the 13C labeled fatty acid as a fraction of the total (13C fatty acid PL / 13C fatty acid PL + unlabeled fatty acid PL * 100).
Acid labile plasmalogens were hydrolyzed by exposure to 12N HCl fumes [32]. This was achieved by drying down the placenta total lipid extract under a stream of nitrogen followed by exposure to hydrochloric acid fumes for 1 h. Upon acid hydrolysis, the vinyl ether bond of the plasmalogens was hydrolyzed but the 1-O-alkyl or 1-acyl phospholipid species remained intact. The lipids were reconstituted in initial HPLC conditions for LC-MS/MS analysis and plasmalogen identity was determined by disappearance of the molecular species by comparing pre- vs. post-HCl exposed samples.
Statistical Methods
Demographic and clinical variables were summarized and compared using two-sample t-tests and Fisher's exact tests. Significance was set at 0.05. R software (R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
Results
Enzymes responsible for phospholipid de novo synthesis and remodeling
We identified acyl transferase enzyme isoforms in human placenta that are likely involved in the Kennedy pathway and demonstrated localization of several isoforms in human placenta syncytiotrophoblast by immunofluorescence and immunoblotting (Figure 1). Glycerol Phosphate Acyl Transferase isoform 3 (GPAT3) was identified in human placenta and catalyzes the initial first esterification of G-3-P [18]. Acyl Glycerol Phosphate Acyl Transferase enzyme isoforms 1, 2 and 4 (AGPAT 1, 2 and 4) were identified and are responsible for the esterification of a second acyl chain to form phosphatidic acid. The positive control for AGPAT4 gave a band at a slightly higher molecular weight in multiple tissues and species we tested suggesting a post translational modification that is tissue and species specific. Hydrolysis of acyl chains from existing phospholipids in human placenta is by cytosolic PLA2G enzymes and two isoforms were identified PLA2G4c and PLA2G6. Re-esterification of acyl chains by lysophosphatidylcholine acyl transferases (LPCAT1 and 3) were also localized to human placenta.
Figure 1. Identification of phospholipid de novo synthesis and remodeling enzymes in human placenta.
Traditional Western blotting or capillary immunoblotting using Jess and immunofluorescence were used to demonstrate isoforms of de novo phospholipid synthesis pathway.
A) Immunofluorescence Glycerol Phosphate Acyl Transferase (GPAT3), AcylGlycerol Acyl Transferase (AGPAT4), Phospholipase A2 group (PLA2G6), Lysophosphatidylcholine Acyl Tranferase (LPCAT1) and IgG control section. No significant autofluorescence was observed in these sections.
B) Immunoblotting for GPAT3, AGPAT1, 2 and 4, PLA2G4c, PLA2G6, LPCAT1 and 4. Immunofluorescence localization: GPAT3 appears weaker in the syncytiotrophoblast compared to the strong signal in the capillary smooth muscle but immunoblotting results indicate expression in isolated PHT. AGPAT4 and LPCAT1 are both strong in the syncytiotrophoblast with no staining the stroma. AGPAT4 appears to be localized to an intracellular compartment and the enzyme is known to be localized in the mitochondria. PLA2G6 has a strong signal in the capillary endothelium and red blood cells, the signal is present in the trophoblast but less intense. PLA2G6 expression was found in isolated PHT cells supporting trophoblast localization. Immunoblotting included positive control for all gels, human cerebellum, placental homogenate (PH) and PHY. Each antibody gave a band near the predicted molecular weight for each protein as indicated in the respective blots. When the PH signal is stronger than the PHT cell lysate this suggests other cell types in the placenta may be expressing this isoform.
Abbreviations: N, Nuclei; ST, Syncytiotrophoblast; E, Endothelium; SM, Smooth Muscle
Phospholipid synthesis studies using 13C labeled fatty acids
Secretion of hCG into the culture media increased more than 4-fold between 18 and 66 h confirming differentiation, syncytialization and cell viability (Supplemental Figure 1). Treatment with 13C labeled fatty acids did not impact hCG secretion between 66 and 90 h culture (data not shown).
We measured phosphatidic acid containing 13C labeled fatty acids in PHT cells after 2 and 24 h incubation. Incorporation of 13C fatty acids is presented as % incorporation for totals of each lipid. The relative levels of both 13C and unlabeled phospholipid species at 2 and 24 h in three phospholipid forms can be found in Supplemental Tables 1-3. Figure 2 demonstrates that 13C Palmitic (16:0), 13C Oleic (18:1) and 13C Linoleic acid (18:2, n-6) are rapidly incorporated into phosphatidic acid in cultured trophoblasts and the levels of incorporation increased significantly from 2 to 24 h for all phosphatidic acid species measured. The levels of 13C labeled phosphatidic acid in PHT cells reached 30-50% for most species by 24 h incubation. Interestingly, we were not able to detect 13C DHA incorporated into phosphatidic acid at either 2 or 24 h. Therefore, 13C DHA in phosphatidic acid was either not present or was lower than our detection limit. Both of these explanations would support de novo synthesis not being the primary means of incorporating DHA into phospholipids in trophoblasts.
Figure 2. Incorporation of 13C labeled fatty acids into phosphatidic acid in cultured primary human trophoblasts.
After 2 and 24 h incubation with physiological levels of Palmitic (16:O), Oleic (18:1), Linoleic (18:2, n-6) and docosahexaenoic (22:6, n-3) fatty acids we measured phosphatidic acid (PA). Nomenclature is the two fatty acid species on the G-3-P backbone and the first listed is the uniformly 13C labelled fatty acid in the PA. Two h incorporation is the black portion of the bar and 24 h incorporation is the grey portion of the bar. Incorporation of 13C labeled fatty acids was greater at 24 h compared to 2 h by T-Test, n=10 unique PHT isolations.
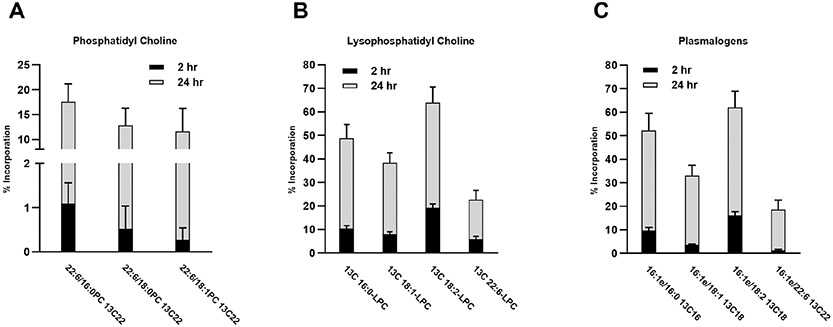
We analyzed the phosphatidylcholine fraction in the same cells after 2 and 24 h exposure to 13C labeled fatty acids and found significant increases with time. Many species of phosphatidylcholine achieved a high level (40-70%) of newly incorporated 13C fatty acids by 24 h incubation, suggesting rapid de novo phospholipid biosynthesis by trophoblasts (Figure 3). We previously found similar results of rapid incorporation of 13C fatty acids into the total cellular phospholipid fraction [15]. Our current data allows us, for the first time, to examine individual phospholipid species incorporating a 13C labeled acyl chain. Notably, we found low levels of 13C DHA incorporated into phosphatidylcholine at 2 h, and by 24 h nearly 40% of the DHA in phosphatidylcholine lipids was 13C labeled (Figure 4A). Incorporation of DHA appears to be accomplished through the remodeling pathway rather than the de novo synthesis pathway due to lack of measurable labeled DHA in the phosphatidic acid fraction (Figure 2).
Figure 3. Incorporation of 13C labeled fatty acids into phosphatidylcholine in cultured primary human trophoblasts.
Phosphatidylcholine species were analyzed after 2 and 24 h incubation with physiological levels of Palmitic (16:O), Oleic (18:1), and Linoleic (18:2, n-6) fatty acids. Nomenclature is the two fatty acids species in the phosphatidylcholine (PC) and which fatty acid is 13C labelled (13C16,18, or 22). Ether linked fatty acids are denoted with a small “e” after the fatty acid. All others are ester linkages to the G-3-P. Two h incorporation is the black portion of the bar and 24 h incorporation is the grey portion of the bar which were significantly greater at except for 18:0e/18:1 13C18, analyzed by T-Test, n=10 unique PHT isolations.
Figure 4. Incorporation of 13C labeled DHA into phosphatidylcholine and lysophosphatidylcholine in cultured primary human trophoblasts.
A) Phosphatidylcholine containing 13C labeled DHA (22:6, n-3) fatty acids as a percent of total. The 24 h incorporation is the grey portion of the bar which is significantly greater than the 2 h incorporation (black portion of the bar), analyzed by T-Test, n=10 PHT isolations.
B) Lysophosphatidylcholine species 2 and 24 h after incubation with physiological levels of Palmitic (16:O), Oleic (18:1), Linoleic (18:2, n-6) and docosahexaenoic (22:6, n-3) fatty acids. The 24 h levels are significantly higher than the 2 h, analyzed by T-Test, n=10 PHT isolations.
C) The labeled and unlabeled plasmalogen species containing Palmitic (16:O), Oleic (18:1), Linoleic (18:2, n-6) and docosahexaenoic (22:6, n-3) fatty acid. The ether linkage is denoted by a small e after the fatty acid. For all plasmalogen species measured, the percent incorporation of 13C labeled fatty acids was greater at 24 h than at 2 h, analyzed by T-Test, n=10 PHT isolations.
Phospholipid remodeling
We examined lysophosphatidylcholine species with 13C labeled fatty acid incorporation and all four labeled fatty acids were found at measurable levels after 2 h with significantly higher levels at 24 h (Figure 4B). Finding this intermediate form of the Lands cycle in high levels with up to 60% 13C labeled fatty acid incorporation by 24 h suggests a robust remodeling of existing phospholipids in human trophoblasts. The lysophosphatidylcholines containing 13C DHA were found in lower abundance (approximately 20% at 24 h) which may reflect the lower levels of 13C DHA in the incubation media.
Plasmalogens and ether linked fatty acids in phospholipids
In our analysis of phospholipids in human primary trophoblasts we found robust levels of phospholipids with ether linked fatty acids. We identified a total of 15 phosphatidylcholine species with ether linked 13C fatty acids in the human placenta (Figure 3). All ether linked forms containing 13C fatty acids increased from 2 to 24 hours. We tested for the presence of vinyl ether (plasmalogen) linkages and found that 4 of the 15 ether linked fatty acids were plasmalogens. The lipids with a C16 ether linkage in the sn-1 position and a 13C fatty acid in the sn-2 position were plasmalogens. The synthesis of the plasmalogens, 16:1e/13C16:0 and 16:1e/13C 18:2, were both significantly higher at 24 h compared to 2 h (Figure 4C).
Discussion
The ability of the placental syncytiotrophoblast to synthesize phospholipids is critical to support the rapidly expanding microvillous surface area across gestation. The MVM is in direct contact with maternal blood and is essential for adequate oxygen and nutrient delivery to the developing fetus [3]. However, little research exists on the role of phospholipid synthesis in either healthy pregnancy or in pregnancy complications of known placental origin. Placental phospholipid synthesis was first described in 1959 [33] and again in 1973 [34]. Detailed phospholipid composition of the human placenta was reported in 1993 [35] as predominantly phosphatidylcholine and phosphatidylethanolamine and having a high content of LCPUFA, including in plasmalogens (sn-1 position with a vinyl ether linkage rather than ester linkage to G-3-P). Placental phospholipid content and fatty acid composition change with moderate maternal malnutrition [36]. Glycemic state of the mother may also modulate placental phospholipid synthesis [37]. The fatty acid composition of placental plasma membrane phospholipids differs in pregnancies complicated by growth restriction [38] and placental phospholipid content has been suggested to be increased in placentas of pre-eclamptic pregnancies [39]. Gestational diabetes and obesity also influence placental phospholipids with an increase in arachidonic acid and docosahexaenoic acid in the phosphatidylcholine fraction [40, 41]. However, the biosynthetic pathway responsible for the synthesis and remodeling of trophoblast phospholipids was not studied in these reports. Likewise, detailed studies of phospholipid synthesis in placenta using stable isotope labeled fatty acids and identifying the enzymes responsible for phospholipid synthesis is lacking.
In this report we demonstrate rapid incorporation of four unique 13C labeled NEFA into phosphatidylcholine species in cultured trophoblasts from healthy term pregnancies. Our data show that palmitic acid (16:0), oleic acid (18:1 n-9) and linoleic acid (18:2 n-6) are incorporated through the ‘de novo synthesis pathway by finding high levels of these fatty acids in phosphatidic acid, the Kennedy pathway intermediate. Similarly, we also found approximately 50% of all phosphatidylcholine species had incorporated 13C labeled fatty acids by 24 h incubation. The percent incorporation of saturated, monounsaturated and n-6 essential fatty acids, all highly abundant in maternal plasma, was similar. While 13C DHA was also incorporated into phosphatidylcholine in human trophoblasts, it appears DHA is added predominantly through the remodeling pathway since we found no labeled 13C DHA in the phosphatidic acid fraction. We identified multiple acyl transferase and phospholipase enzyme isoforms that are responsible for phospholipid synthesis and remodeling in the human placenta. This data supports the importance of phospholipid synthesis for the maintenance and expansion of MVM which is essential for adequate nutrient transport and gas exchange for the growing fetus.
Our study is the first to demonstrate that highly abundant fatty acid species, with uniform 13C labeling (16:0, 18:1, 18:2) are incorporated into phosphatidylcholine through the Kennedy pathway. Enzymes responsible include GPAT3 and AGPAT1, 2 and 4 that were identified by immunoblotting and immunofluorescence (Figure 1). One of the difficulties associated with lipid related enzymes is their overlapping functional specificity. Glycerol Phosphate Acyl Transferases (GPAT) have the lowest specific activity of enzymes in the de novo pathway but have been demonstrated to have a preference for palmitic and oleic acids [42]. This initial step in de novo phospholipid synthesis has been considered to be rate limiting [19]. Fatty acid binding proteins (FABP) and the intermediate product, lysophosphatidic acid, are known positive and negative regulators of this initial esterification step. Nomenclature can be difficult as exemplified by the multiple names for each enzyme. GPAT3 is also referred to as AGPAT 9,10 and Lysophosphatidic acid acyl transferase (LPAAT)-theta, indicative of its many roles as a non-specific acyl transferase enzyme.
The AGPAT enzymes esterify a fatty acid to lysophosphatidic acid to generate phosphatidic acid [42], the intermediate step in both phospholipid and triacylglycerol synthesis. These enzymes also have broad specificity. Isoform AGPAT1 has been suggested to have acceptor specificity for lysophosphatidic acid and broad fatty acid donor specificity with a preference for fatty acid species C12:0–16:0, C16:1, C18:2, and C18:3, followed by C18:0, C18:1, and C20:4. Isoform AGPAT2 has strict acceptor specificity for lysophosphatidic acids and broad fatty acid specificity with a preference for C14:0, C16:0, C18:1, and C18:2 fatty acids, and lower preference for C18:0 and C20:4 [50]. Lysophosphatidic acid is the preferred acceptor substrate for AGPAT4 with unsaturated fatty acids C18:1, C22:6, C20:4 followed by C16:0 being the preferred acyl chain substrates for this enzyme [43]. AGPAT 1, 2 and 4 are also referred to as LPAAT- alpha, beta and delta respectively indicating they possess the ability to esterify a fatty acid at the sn-2 position of a lysophosphatidic acid.
We did not detect 13C DHA in the phosphatidic acid fraction after 2 or 24 h incubation as seen in Figure 2 using 13C labeled fatty acids at concentrations similar to late pregnancy maternal plasma. However, 13C labeled DHA was incorporated into phosphatidylcholine species (Figure 4A) and into lysophosphatidylcholine (Figure 4B) over the same incubation time. This data suggests that DHA is predominantly esterified to phosphatidylcholine through the remodeling pathway. We identified two intracellular localized phospholipase enzymes (PLA2G6 and PLA2G4c) that could be responsible for hydrolyzing a fatty acid from an existing phospholipid. We also detected two enzymes that can reesterify fatty acids to the resulting lysophosphatidylcholine, LPCAT1 and 3. Enzymes of the Lands cycle have overlapping specificity and nomenclature. PLA2G4c is also known as cPLA2-gamma and has multiple other names including PLA2, iPLA2 beta, NBIA2, PARK14 and PNPLA9. Both group IV and VI PLA2 enzymes are cytoplasmic and have broad specificity for cleaving fatty acids from phospholipids [44]. LPCAT1 is also referred to as AGPAT 9,10 AYTL9 and FLJ12443. LPCAT4 is also referred to as AGPAT7, AYTL3, FLJ10257, LPAAT-eta and LPEAT2. Both LPCAT enzymes have broad specificity for lysophosphatidic acid and lysophospholipids as substrates and they can esterify a broad range of fatty acids with some indication that LPCAT1 has a preference for saturated fatty acids [45]. The Lands cycle has been shown in other cell types to be the primary mechanism for adding LCPUFA to the phospholipid pool [22]. Based on our data, this appears to be the case for trophoblasts as well.
DHA is critically important in the phospholipid pool of plasma membranes. The incorporation of fatty acids with multiple double bonds is critical for membrane fluidity [46] which is important for functionality of embedded proteins, transporters and enzymes. DHA in phospholipids is also critical for the formation of lysophosphatidylcholine containing DHA through the action of phospholipase enzymes. This unique form of phospholipid is transported across cell membranes by a specific carrier known as Major Facilitated Superfamily Domain containing 2a (MFSD2a) which has been studied extensively in the blood brain barrier as critical for supplying DHA for brain phospholipid synthesis [47]. Our previous studies have reported that this unique lipid carrier is present in the BM of the human placenta and its expression is highly correlated with cord blood levels for lysophosphatidylcholine containing DHA [28], suggesting a role for MFSD2a in the delivery of DHA to the fetus. Levels of lysophosphatidylcholine containing DHA have previously been shown to be quite high in human placenta and we demonstrate in this study that after 24 h nearly 40% of lysophosphatidylcholine containing DHA contain the newly acquired 13C DHA fatty acid, indicating a high turnover rate. This pathway in syncytiotrophoblast may provide a specific mechanism for DHA to be incorporated into lysophospholipids and transported to the fetus across the BM. It has long been known that when measured as a percent of total fatty acids DHA is higher in the fetus than in the mother [48]. However, non-esterified DHA is transported down its concentration gradient and this does not provide a concentrating mechanism. This is important since most reports suggest a mechanism for preferential transfer of DHA over other fatty acids, but the nature of this preferential transfer has not been fully delineated. Rapid formation and accumulation of lysophosphatidylcholine containing DHA in trophoblasts and the presence of a specific transporter (MFSD2a) in the BM suggest this mechanism for DHA transport could contribute to preferential transfer of DHA to the fetal compartment compared to other fatty acids in the maternal circulation [28].
Ether linked phospholipids were measured in newly formed phospholipids and constitute a significant fraction of the phospholipid pool in PHT cells [35]. Ether linked phospholipids have been previously identified in human placenta across gestation [49], however, their exact role is not well defined. Plasmalogens are a critical feature of plasma membranes that influence the physicochemical properties such as hydrophilicity of the headgroup, packing density, and conformational order of the phospholipids within the membranes [50]. In addition, ether linked phospholipids represent a pool of LCPUFA in plasma membranes that are protected from oxidation [51]. The vinyl ether (plasmalogen) linkage at the sn-1 position makes that fatty acid more susceptible to oxidation which protects the LCPUFA in the sn-2 position. While the exact role for the ether and vinyl ether linkages in phospholipids in human placental function is not yet clear, we found robust levels of plasmalogens with all four of the 13C labeled fatty acids in PHT. Additional work is needed to clarify the role of these lipids in human placenta.
This study provides some clarity on the lipase and acyl transferase enzyme isoforms which are probably responsible for synthesis and remodeling of phospholipids in human placenta. Challenges with nomenclature of these enzymes are well known. Likewise, these enzymes have overlapping specificity in the esterification of fatty acids to G-3-P or lysophospholipid forms. Uptake specificity and maternal dietary intake will also impact phospholipid synthesis and composition. Fatty acid uptake transporters have previously been described in human placenta and have overlapping specificity for a variety of fatty acids [14]. A limitation of the study was the reliance on commercially available antibodies, which may not have adequate isoform specificity or strong enough binding efficiency to demonstrate low level expression. While phosphatidylcholine is the predominant phospholipid in placenta, other species such as phosphatidylethanolamine are highly abundant but have not been studied in detail. Plasma membrane phospholipids, in particular phosphatidylserines, are also known to be important for fusion events and studies in trophoblast suggest they are critical for the establishment of the syncytium [52]. We consider this study to be an initial attempt to identify critical enzymes in the biosynthetic pathway for phospholipids in human placenta. However, interconversion between phospholipid types is well established and likely contributes to the phospholipid composition of trophoblasts, but this was not quantified in these experiments. Trophoblast phospholipid synthesis and their functions in human pregnancy deserves greater attention.
Supplementary Material
Highlights.
Human placenta trophoblasts express de novo phospholipid synthesis and phospholipid remodeling enzymes
Uniformly labelled 13C fatty acids are rapidly incorporated into cellular phospholipid lipids. Unsaturated, monounsaturated and essential fatty acids are incorporated through de novo phospholipid synthesis and found in the intermediate form phosphatidic acid in syncytialized trophoblasts.
Docosahexaenoic acid is incorporated into trophoblast phospholipids through the remodeling pathway rather than de novo synthesis as evidenced by a lack of 13C DHA in the phosphatidic acid form.
Acknowledgements
We are grateful to staff at Labor & Delivery and the Clinical and Translational Research Center (CTRC) at the University of Colorado Hospital for collection of placental tissue. We thank Kate Erickson for her assistance with manuscript preparation.
Funding
This study was supported by grants from NIH (HD104644 to TLP) and by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Abbreviations
- AGPAT
Acyl Glycerol Phosphate Acyl Transferase
- BM
basal plasma membrane
- BMI
body mass index
- BCA
bicinchoninic acid
- BSA
bovine serum albumin
- DAPI
4',6-diamidino-2-phenylindole
- DHA
docosahexaenoic acid
- DMEM
Dulbecco’s Modified Eagle Medium
- FABP
fatty acid binding protein
- FBS
fetal bovine serum
- G-3-P
glycerol-3-phosphate
- GPAT
Glycerol Phosphate Acyl Transferase
- hCG
human chorionic gonadotropin
- HPLC
High Pressure Liquid Chromatography
- LCPUFA
long chain polyunsaturated fatty acid
- LPAAT
lysophosphatidic Acid Acyl Transferase
- LPCAT
lysophosphatidylcholine acyl transferase
- MFSD2a
Major Facilitated Superfamily Domain containing 2a
- MRM
multiple reaction monitoring
- MUFA
monosaturated fatty acids
- MVM
microvillous plasma membrane
- NEFA
non-esterified “free” fatty acids
- OA
oleic acid
- OCT
optimal cutting temperature
- PBS
phosphate-buffered saline
- PC
phosphatidylcholine
- PHT
Primary human trophoblast
- PFA
paraformaldehyde
- PL
phospholipid
- PLA2G
phospholipase A2 group
- PUFA
polyunsaturated fatty acid
- PVDF
polyvinylidene difluoride
- SFA
saturated fatty acids
- TBS
tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare that they have no known competing financial interests to declare for this manuscript.
I hereby declare that the disclosed information is correct and that no other situation of real, potential, or apparent conflict of interest is known to me. I will undertake to inform you of any change in these circumstances.
References
- [1].Teasdale F, Jean-Jacques G, Morphometric evaluation of the microvillous surface enlargement factor in the human placenta from mid-gestation to term., Placenta 6 (1985) 375–381. [DOI] [PubMed] [Google Scholar]
- [2].Mayhew TM, Wadrop E, Simpson RA, Proliferative versus hypertrophic growth in tissue subcompartments of human placental villi during gestation, J Anat 184 (Pt 3)(Pt 3) (1994) 535–43. [PMC free article] [PubMed] [Google Scholar]
- [3].Mayhew TM, Joy CF, Haas JD, Structure-function correlation in the human placenta: the morphometric diffusing capacity for oxygen at full term, J Anat 139 (Pt 4)(Pt 4) (1984) 691–708. [PMC free article] [PubMed] [Google Scholar]
- [4].Fox H, The histopathology of placental insufficiency, J Clin Pathol Suppl (R Coll Pathol) 10 (1976) 1–8. [PMC free article] [PubMed] [Google Scholar]
- [5].Teasdale F, Gestational changes in the functional structure of the human placenta in relation to fetal growth: A morphometric study, Am J Obstet Gynecol 137 (1980) 560–568. [DOI] [PubMed] [Google Scholar]
- [6].Kingdom JC, Macara LM, Whittle MJ, Fetoplacental circulation in health and disease, Arch Dis Child Fetal Neonatal Ed 70(3) (1994) F161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mayhew TM, A stereological perspective on placental morphology in normal and complicated pregnancies, J Anat 215(1) (2009) 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mayhew TM, Allometric studies on growth and development of the human placenta: growth of tissue compartments and diffusive conductances in relation to placental volume and fetal mass, J Anat 208(6) (2006) 785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Biswas S, Ghosh SK, Chhabra S, Surface area of chorionic villi of placentas: an index of intrauterine growth restriction of fetuses, J Obstet Gynaecol Res 34(4) (2008) 487–93. [DOI] [PubMed] [Google Scholar]
- [10].Mayhew TM, Sorensen FB, Klebe JG, Jackson MR, The effects of mode of delivery and sex of newborn on placental morphology in control and diabetic pregnancies, J Anat 183 (Pt 3)(Pt 3) (1993) 545–52. [PMC free article] [PubMed] [Google Scholar]
- [11].Dumolt JH, Powell TL, Jansson T, Placental Function and the Development of Fetal Overgrowth and Fetal Growth Restriction, Obstet Gynecol Clin North Am 48(2) (2021) 247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Acosta O, Ramirez VI, Lager S, Gaccioli F, Dudley DJ, Powell TL, Jansson T, Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers, Am J Obstet Gynecol 212(2) (2015) 227 e1–7. [DOI] [PubMed] [Google Scholar]
- [13].Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T, Powell TL, Activation of Placental mTOR Signaling and Amino Acid Transporters in Obese Women Giving Birth to Large Babies, J Clin Endocrinol Metab 98 (2013) 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lager S, Ramirez VI, Gaccioli F, Jang B, Jansson T, Powell TL, Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women, Placenta 40 (2016) 60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ferchaud-Roucher V, Barner K, Jansson T, Powell TL, Maternal obesity results in decreased syncytiotrophoblast synthesis of palmitoleic acid, a fatty acid with anti-inflammatory and insulin-sensitizing properties, FASEB J 33(5) (2019) 6643–6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ridgway NL, Phospholipid synthesis in mammalian cells, in: Ridgway NL, McLeod RS (Eds.), Biochemistry of Lipids, Lipoproteins and Membranes Elsevier; 2021, pp. 227–258. [Google Scholar]
- [17].Gibellini F, Smith TK, The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine, IUBMB Life 62(6) (2010) 414–28. [DOI] [PubMed] [Google Scholar]
- [18].Yu J, Loh K, Song ZY, Yang HQ, Zhang Y, Lin S, Update on glycerol-3-phosphate acyltransferases: the roles in the development of insulin resistance, Nutr Diabetes 8(1) (2018) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yamashita A, Hayashi Y, Matsumoto N, Nemoto-Sasaki Y, Oka S, Tanikawa T, Sugiura T, Glycerophosphate/Acylglycerophosphate acyltransferases, Biology (Basel) 3(4) (2014) 801–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chakraborti S, Phospholipase A(2) isoforms: a perspective, Cell Signal 15(7) (2003) 637–65. [DOI] [PubMed] [Google Scholar]
- [21].Shindou H, Shimizu T, Acyl-CoA:lysophospholipid acyltransferases, J Biol Chem 284(1) (2009) 1–5. [DOI] [PubMed] [Google Scholar]
- [22].O'Donnell VB, New appreciation for an old pathway: the Lands Cycle moves into new arenas in health and disease, Biochem Soc Trans 50(1) (2022) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Al MD, van Houwelingen AC, Kester AD, Hasart TH, de Jong AE, Hornstra G, Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. , Br J Nutr 74 (1995) 55–68. [DOI] [PubMed] [Google Scholar]
- [24].Kramer AC, Erikson DW, McLendon BA, Seo H, Hayashi K, Spencer TE, Bazer FW, Burghardt RC, Johnson GA, SPP1 expression in the mouse uterus and placenta: implications for implantationdagger, Biol Reprod 105(4) (2021) 892–904. [DOI] [PubMed] [Google Scholar]
- [25].Burghardt RC, Burghardt JR, Taylor JD 2nd, Reeder AT, Nguen BT, Spencer TE, Bayless KJ, Johnson GA, Enhanced focal adhesion assembly reflects increased mechanosensation and mechanotransduction at maternal-conceptus interface and uterine wall during ovine pregnancy, Reproduction 137(3) (2009) 567–82. [DOI] [PubMed] [Google Scholar]
- [26].Castillo-Castrejon M, Yang IV, Davidson EJ, Borengasser SJ, Jambal P, Westcott J, Kemp JF, Garces A, Ali SA, Saleem S, Goldenberg RL, Figueroa L, Hambidge KM, Krebs NF, Powell TL, Preconceptional Lipid-Based Nutrient Supplementation in 2 Low-Resource Countries Results in Distinctly Different IGF-1/mTOR Placental Responses, J Nutr 151(3) (2021) 556–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ferchaud-Roucher V, Kramer A, Silva E, Pantham P, Weintraub ST, Jansson T, Powell TL, A potential role for lysophosphatidylcholine in the delivery of long chain polyunsaturated fatty acids to the fetal circulation, Biochim Biophys Acta Mol Cell Biol Lipids 1864(3) (2019) 394–402. [DOI] [PubMed] [Google Scholar]
- [28].Powell TL, Barner K, Madi L, Armstrong M, Manke J, Uhlson C, Jansson T, Ferchaud-Roucher V, Sex-specific responses in placental fatty acid oxidation, esterification and transfer capacity to maternal obesity, Biochim Biophys Acta Mol Cell Biol Lipids 1866(3) (2021) 158861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF 3rd, Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae, Endocrinology 118 (1986) 1567–1582. [DOI] [PubMed] [Google Scholar]
- [30].Ferchaud-Roucher V, Rudolph MC, Jansson T, Powell TL, Fatty acid and lipid profiles in primary human trophoblast over 90h in culture, Prostaglandins Leukot Essent Fatty Acids 121 (2017) 14–20. [DOI] [PubMed] [Google Scholar]
- [31].Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D, Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics, J Lipid Res 49(5) (2008) 1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Murphy EJ, Anderson DK, Horrocks LA, Phospholipid and phospholipid fatty acid composition of mixed murine spinal cord neuronal cultures, J Neurosci Res 34(4) (1993) 472–7. [DOI] [PubMed] [Google Scholar]
- [33].Porcellati G, Curti B, Luciani S, Phospholipid phosphoric esters in the human placenta, Nature 184(Suppl 24) (1959) 1870–1. [DOI] [PubMed] [Google Scholar]
- [34].Karp W, Sprecher H, Robertson A, Human placental phospholipid synthesis, Biol Neonate 22(5) (1973) 398–406. [DOI] [PubMed] [Google Scholar]
- [35].Bayon Y, Croset M, Chirouze V, Tayot JL, Lagarde M, Phospholipid molecular species from human placenta lipids, Lipids 28(7) (1993) 631–6. [DOI] [PubMed] [Google Scholar]
- [36].Araya J, A.M. A, Soto C, Molina R, Placental phospholipid composition, impact of maternal nutritional status, Nutr Res 5 (1985) 1067–1075. [Google Scholar]
- [37].Watkins OC, Selvam P, Appukuttan Pillai R, Cracknell-Hazra VKB, Yong HEJ, Sharma N, Cazenave-Gassiot A, Bendt AK, Godfrey KM, Lewis RM, Wenk MR, Chan SY, Placental (13)C-DHA metabolism and relationship with maternal BMI, glycemia and birthweight, Mol Med 27(1) (2021) 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Powell TL, Jansson T, Illsley NP, Korotkova M, Strandvik B, Composition and permeability to water and small solutes of syncytiotrophoblast plasma membranes in pregnancies complicated by intrauterine growth restriction Biochim Biophys Acta 1420 (1999) 86–94. [DOI] [PubMed] [Google Scholar]
- [39].Huang X, Jain A, Baumann M, Korner M, Surbek D, Butikofer P, Albrecht C, Increased placental phospholipid levels in pre-eclamptic pregnancies, Int J Mol Sci 14(2) (2013) 3487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bitsanis D, Crawford MA, Moodley T, Holmsen H, Ghebremeskel K, Djahanbakhch O, Arachidonic acid predominates in the membrane phosphoglycerides of the early and term human placenta, J Nutr 135(11) (2005) 2566–71. [DOI] [PubMed] [Google Scholar]
- [41].Uhl O, Demmelmair H, Segura MT, Florido J, Rueda R, Campoy C, Koletzko B, Effects of obesity and gestational diabetes mellitus on placental phospholipids, Diabetes Res Clin Pract 109(2) (2015) 364–71. [DOI] [PubMed] [Google Scholar]
- [42].Takeuchi K, Reue K, Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis, Am J Physiol Endocrinol Metab 296(6) (2009) E1195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhukovsky MA, Filograna A, Luini A, Corda D, Valente C, The Structure and Function of Acylglycerophosphate Acyltransferase 4/ Lysophosphatidic Acid Acyltransferase Delta (AGPAT4/LPAATdelta), Front Cell Dev Biol 7 (2019) 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Khan SA, Ilies MA, The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles, Int J Mol Sci 24(2) (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang B, Tontonoz P, Phospholipid Remodeling in Physiology and Disease, Annu Rev Physiol 81 (2019) 165–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Valentine RC, Valentine DL, Omega-3 fatty acids in cellular membranes: a unified concept, Prog Lipid Res 43(5) (2004) 383–402. [DOI] [PubMed] [Google Scholar]
- [47].Hachem M, Belkouch M, Lo Van A, Picq M, Bernoud-Hubac N, Lagarde M, Brain targeting with docosahexaenoic acid as a prospective therapy for neurodegenerative diseases and its passage across blood brain barrier, Biochimie 170 (2020) 203–211. [DOI] [PubMed] [Google Scholar]
- [48].Larque E, Demmelmair H, Gil-Sanchez A, Prieto-Sanchez MT, Blanco JE, Pagan A, Faber FL, Zamora S, Parrilla JJ, Koletzko B, Placental transfer of fatty acids and fetal implications, Am J Clin Nutr 94(6 Suppl) (2011) 1908S–1913S. [DOI] [PubMed] [Google Scholar]
- [49].Bidne KL, Uhlson C, Palmer C, Zemski-Berry K, Powell TL, Human placental lipid content and lipid metabolic enzyme abundance in obesity and across gestation, Clin Sci (Lond) 136(19) (2022) 1389–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Almsherqi ZA, Potential Role of Plasmalogens in the Modulation of Biomembrane Morphology, Front Cell Dev Biol 9 (2021) 673917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Messias MCF, Mecatti GC, Priolli DG, de Oliveira Carvalho P, Plasmalogen lipids: functional mechanism and their involvement in gastrointestinal cancer, Lipids Health Dis 17(1) (2018) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang Y, Liang P, Yang L, Shan KZ, Feng L, Chen Y, Liedtke W, Coyne CB, Yang H, Functional coupling between TRPV4 channel and TMEM16F modulates human trophoblast fusion, Elife 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.