Abstract
Background:
The objective of this study is to determine the outcomes and toxicities of patients with malignant pleural mesothelioma (MPM) treated with stereotactic body radiotherapy (SBRT).
Materials and Methods:
Data were extracted from an institutional tumor registry for patients diagnosed with mesothelioma and treated with SBRT. Kaplan-Meier and Cox regression analyses were employed to determine local control (LC) and overall survival (OS).
Results:
Forty-four patients with 59 total treated tumors from December 2006 to April 2022 were identified. Fifty-one (86.4%) cases had oligoprogressive disease (five sites or less). The median prescription dose delivered was 3000 cGy in 5 fractions (range: 2700-6000 cGy in 3-8 fractions). Fifty-one (86.4%) tumors were in the pleura, 4 (6.8%) spine, 2 (3.4%) bone, 1 (1.7%) brain, and 1 (1.7%) pancreas.
The median follow-up from SBRT completion for those alive at last follow-up was 28 months (range: 14-52 months). The most common toxicities were fatigue (50.8%), nausea (22.0%), pain flare (15.3%), esophagitis (6.8%), dermatitis (6.8%), and pneumonitis (5.1%). There were no grade ≥3 acute or late toxicities. There were 2 (3.4%) local failures, one of the pleura and another of the spine. One-year LC was 92.9% (95% CI: 74.6-98.2%) for all lesions and 96.3% (95% CI: 76.5-99.5%) for pleural tumors. One-year LC was 90.9% (95% CI: 68.1-97.6%) for epithelioid tumors and 92.1% (95% CI: 72.1-98.0%) for oligoprogressive tumors. One-year OS from time of SBRT completion was 36.4% (95% CI: 22.6-50.3%). On multivariable analysis, KPS was the lone significant predictor for OS (p=0.029).
Conclusions:
Our single-institutional experience on patients with MPM suggests that SBRT is safe with a low toxicity profile and potentially achieve good local control.
INTRODUCTION
Malignant pleural mesothelioma (MPM) is an aggressive tumor with about 3,000 new cases diagnosed each year.1 Prognosis is poor, and the 5-year relative survival rate is 12%.2
Treatment options for those with resectable disease usually incorporate multimodality therapy, including systemic therapy, macroscopic complete resection, and consideration of external beam radiation therapy.3-6 The majority of patients, however, present with unresectable disease, and first-line treatment for patients with unresectable MPM has historically been limited to chemotherapy alone. Recent progress made in immune checkpoint inhibitors, however, has ushered in a new era of MPM treatment, significantly improving overall survival (OS) for certain patients.7-9 Nevertheless, symptoms resulting from local disease commonly include pain and/or respiratory symptoms, significantly affecting quality of life. Usually, MPM progresses in a diffuse pattern, but oligoprogression or isolated nodules are occasionally observed.10 As a result, there is a continued need to find ways to improve local disease control and its durability.
Stereotactic body radiation therapy (SBRT) has emerged as a noninvasive treatment option precisely delivering ablative radiation dose to lesions.11-13 This technique allows for the delivery of a high radiation biologically effective dose (BED) to a targeted volume in a highly conformal manner. Higher BED for different cancers has been associated with excellent palliation, excellent local control, and improved patient survival,14-16 and can overcome intrinsic radioresistance to treat radioresistant histologies.17 SBRT for early-stage and oligometastatic non-small cell lung cancer (NSCLC) has demonstrated excellent long-term local control rates with low risk for significant toxicity and is the standard of care for inoperable patients.18-22 However, data regarding SBRT for MPM are very limited.11,23 In this single-institutional retrospective analysis, we investigate the outcomes of patients diagnosed with MPM and treated with SBRT.
MATERIALS AND METHODS
Patients
This institutional retrospective study was approved by the Institutional Review Board. The records of 44 patients with 59 total tumors treated with SBRT between December 2006 to April 2022 were reviewed. Thirteen patients were treated on a previously published phase I trial combining avelumab and SBRT.11 All patients had pathologic diagnosis of malignant pleural mesothelioma (MPM) and radiographically confirmed disease on pre-treatment computed tomography (CT) and/or positron emission tomography (PET) scan.
Radiotherapy
All patients underwent CT-guided simulation for SBRT planning, and all treatment plans were generated using Eclipse (Varian Medical Systems, Palo Alto, CA). Patients were immobilized in supine position with their arms raised in an alpha-cradle. Gross tumor volume (GTV) was delineated on the basis of the CT scan at simulation, and a four-dimensional CT was performed to account for respiratory motion and generate an internal target volume (ITV) from the GTV. The clinical target volume (CTV) was generally defined as the ITV plus a 2 mm margin. A planning target volume (PTV) was generated generally using a 5 mm margin. SBRT was delivered in three to eight fractions (minimum total prescription dose of 30 Gy) with 6 mV photons using the volumetric arc technique with the goal of delivering the prescription dose to greater than or equal to 95% of the planning target volume while respecting normal tissue constraints.
Follow-up and outcomes
The cut-off date for follow-up was May 8, 2023. The Karnofsky Performance Scale (KPS) Index at time of SBRT was used as the assessment tool for functional impairment. The endpoints analyzed were treatment-related toxicity, local control (LC), and overall survival (OS). Treatment-related toxicities associated with the irradiated lesion were assessed according to Common Toxicity Criteria for Adverse Events version 5.0 (CTCAE v5.0). Palliation from pain was recorded as such per physician documentation in the patient chart, with no future recurrence of pain at treated site. Local failure was defined as progression of disease in the SBRT field, and disease was noted on diagnostic CT and/or PET scan as reviewed on imaging and documented on the radiologist report, with or without biopsy. LC was defined as the interval between date of SBRT completion for the irradiated lesion and the date of local recurrence, censored at the time of last follow-up or time of death for those without recurrence. OS was defined as the interval between the date of SBRT completion and the date of death, censored at the time of last follow-up.
Statistical Analysis
All data analyses were performed using SPSS 27.0 (Armonk, NY: IBM Corp.). Local control was assessed at the level of the individual lesion, whereas overall survival was assessed at the patient level. Kaplan-Meier plots were generated to estimate LC and OS. Cox regression analysis was used to identify associated prognostic factors and determine their hazard ratios. P-values of less than 0.05 were considered statistically significant.
RESULTS
The median age of the cohort was 73 years (range: 48-86) (Table 1). The median follow-up time from initial diagnosis was 43 months (range: 6-135) and from SBRT completion was 9 months (range: 1-52). The median follow-up from SBRT completion for those alive at last follow-up was 28 months (range: 14-52 months). At time of initial diagnosis and primary treatment, 17 of 44 patients (38.6%) presented with unresectable disease, 23 (52.3%) underwent pleural decortication, 2 (4.5%) extended pleurectomy, and 2 (4.5%) underwent extrapleural pneumonectomy (EPP). Two (4.5%) patients received intra-operative Cisplatin chemotherapy during their surgery. Forty-three (97.7%) patients had a KPS ≥70. The site of treated disease was pleura (n=51; 86.4%), spine (n=4; 6.8%), bone (n=2; 3.4%), brain (n=1; 1.7%), and pancreas (n=1; 1.7%). The median gross tumor volume (GTV) for pleural tumors was 69.4 cm3 (range: 0.9-2042.9) and for non-pleural sites 12.6 cm3 (range: 2.1-246.5). Based on initial diagnostic histology, forty-two (71.1%) tumors were of epithelioid histology, 13 (22.0%) biphasic, and 4 (6.8%) sarcomatoid. Fifty-one (86.4%) cases had oligoprogressive disease (five sites or less). Forty-five of 59 (76.3%) cases did not present with nodal disease at time of SBRT, while 14 (23.7%) did.
Table 1.
Patient, clinicopathologic, and treatment characteristics
| n | Percent | |
|---|---|---|
| Patients (n=44) | ||
| Age (years) | ||
| Median (range) | 73 (48-86) | |
| Gender | ||
| Male | 32 | 54.2% |
| Female | 12 | 45.8% |
| Race | ||
| White | 42 | 95.4% |
| Black | 1 | 2.3% |
| Other | 1 | 2.3% |
| KPS | ||
| 60 | 1 | 2.3% |
| 70 | 7 | 15.9% |
| 80 | 16 | 36.4% |
| 90 | 20 | 45.4% |
| Prior Surgery (at initial diagnosis) | ||
| Yes α | 27 | 61.4% |
| No | 17 | 38.6% |
| Prior Systemic therapy (at initial diagnosis) | ||
| Yes | 41 | 93.2% |
| No | 3 | 6.8% |
| Prior Hemithoracic IMPRINT β (at initial diagnosis) | ||
| Yes | 12 | 27.3% |
| No | 32 | 72.7% |
| Expired | ||
| Yes | 40 | 90.9% |
| No | 4 | 9.1% |
| Tumors (n=59) | ||
| Histology Ω | ||
| Epithelioid | 42 | 71.2% |
| Biphasic | 13 | 22.0% |
| Sarcomatoid | 4 | 6.8% |
| Primary site of SBRT § | ||
| Pleura | 51 | 86.4% |
| Spine | 4 | 6.8% |
| Bone (non-spine) | 2 | 3.4% |
| Pancreas | 1 | 1.7% |
| Brain | 1 | 1.7% |
| Gross tumor volume (cm3) | ||
| Pleura | ||
| Median (range) | 69.4 (0.9-2042.9) | |
| Other sites | ||
| Median (range) | 12.6 (2.1-246.5) | |
| Oligoprogressive tumor | ||
| Yes | 51 | 86.4% |
| No | 8 | 13.6% |
| Treatment (n=59) | ||
| SBRT§ dose | ||
| 3000 cGy in 5 fractions | 34 | 57.6% |
| Gross tumor volume (cm3) | ||
| Median (range) | 161.2 (2.1-2042.9) | |
| 2700 – 3000 cGy in 3 fractions | 14 | 23.7% |
| Gross tumor volume (cm3) | ||
| Median (range) | 22.1 (0.9-572.2) | |
| 3500 – 4000 cGy in 5 fractions | 6 | 10.2% |
| Gross tumor volume (cm3) | ||
| Median (range) | 28.1 (11.7-246.5) | |
| 4500 – 5000 cGy in 5 fractions | 3 | 5.2% |
| Gross tumor volume (cm3) | 7.1 (3.6-19.7) | |
| Median (range) | ||
| 6000 cGy in 8 fractions | 1 | 1.7% |
| Gross tumor volume (cm3) | 49.8 | |
| 4800 cGy in 4 fractions | 1 | 1.7% |
| Gross tumor volume (cm3) | 8.0 | |
| On systemic therapy prior to SBRT | ||
| Yes | 36 | 61.0% |
| Chemotherapy | 24 | 40.6% |
| Immunotherapy | 7 | 11.9% |
| No | 23 | 39.0% |
| On systemic therapy following SBRT | ||
| Yes | 46 | 78.0% |
| Chemotherapy | 13 | 42.3% |
| Immunotherapy | 10 | 16.9% |
| No | 13 | 22.0% |
Pleurectomy and decortication, pneumonectomy, or debulking
Hemithoracic intensity-modulated pleural radiotherapy
Stereotactic body radiation therapy
Based on initial diagnostic histology
Most tumors were treated using a prescription dose of 3000 cGy in 5 fractions (n=34; 57.6%; median GTV: 161.2 cm3 (range: 2.1-2042.9); BED=48.0), whereas the remainder were treated to a BED of >50 Gy (n=25; 42.4%; median GTV: 21.7 cm3 (range: 0.9-572.2) (Table 1). Twelve (27.3%) patients had prior hemithoracic intensity-modulated pleural RT (IMPRINT). Three of these patients who had prior IMPRINT were treated with re-irradiation using SBRT to recurrent disease, while the remainder were treated to areas outside of the prior IMPRINT field, e.g. contralateral or distant metastases. The most common acute toxicities were fatigue (n=30; 50.8%), nausea (n=13; 22.0%), pain flare (n=9; 15.3%), esophagitis (n=4; 6.8%), dermatitis (n=4; 6.8%), and pneumonitis (n=3; 5.1%) (Supplementary Table 1). There were no grade 3 or higher acute or late toxicities, and the majority of toxicities were grade 1.
The majority of SBRT cases (n=36; 61.0%) occurred while the patient was on systemic therapy and not on active surveillance, with most of those patients on chemotherapy (n=24; 40.6%), including bevacizumab (n=9; 15.2%), or immunotherapy (n=7; 11.9%). Forty-six cases (78.0%) went onto receive systemic therapy following SBRT completion, with most patients receiving chemotherapy (n=25; 42.3%) or immunotherapy (n=10; 16.9%).
Thirty-five (59.3%) of the lesions in our investigation presented for treatment due to the need for pain palliation. Of these cases, with a median follow-up of 5 months (range: 1-45) status-post SBRT completion, 21 (60.0%) had palliation of symptoms.
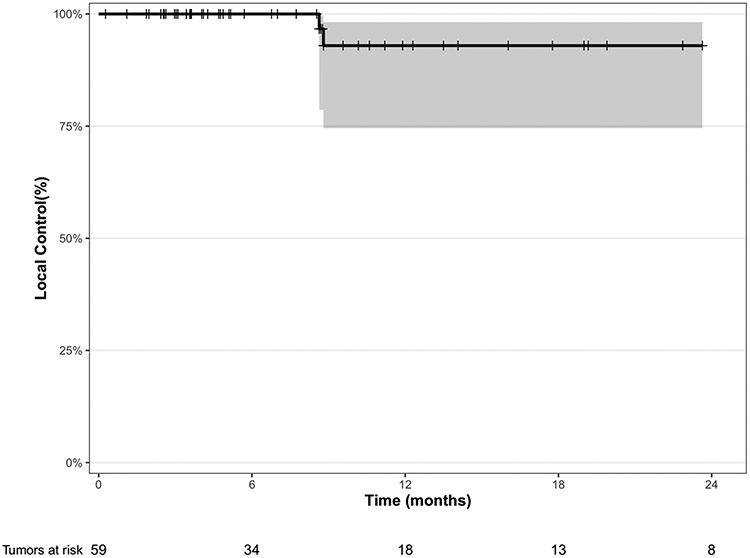
There were only 2 (3.4%) local failures, one of the pleura and another of the spine. Neither case had presented while on systemic therapy. Both presented with oligoprogressive disease at time of SBRT. The pleural case had received 2700 cGy in 3 fractions, and the spine case had received 3000 cGy in 5 fractions. Following SBRT, both cases went onto systemic therapy (pleura: nivolumab/ipilimumab; spine: gemcitabine), and then recurred 9 months following SBRT completion. One-year LC was 92.9% (95% CI: 74.6-98.2%) for all lesions (Figure 1a) and 96.3% (95% CI: 76.5-99.5%) for pleural tumors. One-year LC was 90.9% (95% CI: 68.1-97.6%) for epithelioid tumors and 92.1% (95% CI: 72.1-98.0%) for oligoprogressive tumors (Supplemental Figure 1). One-year LC was 92.3% (95% CI: 56.6-98.9%) for those with oligometastatic tumors. Of the 13 biphasic and 4 sarcomatoid cases, there were no local failures. There was no statistically significant difference in 1-year LC between SBRT cases receiving Avelumab (n=14) versus those not receiving it (n=45) (100.0% vs. 90.2%, p=0.369). Additionally, neither type of surgery at initial diagnosis nor nodal status at time of SBRT influenced LC or OS after SBRT.
Figure 1a.
Kaplan-Meier local control for all tumors (n=59)
One-year OS from time of SBRT completion was 36.4% (95% CI: 22.6-50.3%) (Figure 1b). On multivariable analysis, KPS was the lone significant predictor for OS (p=0.029) (Supplementary Table 2).
Figure 1b.
Kaplan-Meier overall survival from SBRT completion (n=44)
DISCUSSION
To the authors’ knowledge, this single-institutional retrospective analysis is the largest study to date investigating SBRT for the treatment of MPM demonstrating excellent local control. There remains limited data regarding the use of radiotherapy in MPM, mainly due to risks for pulmonary toxicity and paucity of data regarding its efficacy.24-26 Furthermore, questions regarding radiosensitivity remain, but limited data suggest that dose per fraction for MPM may be associated with disease response. In a single-institutional retrospective review on 189 MPM patients receiving palliative radiotherapy, a higher local response rate was observed in those treated to 400 cGy per fraction versus those receiving less than 400 cGy per fraction (50% vs. 39%).27 In our analysis, SBRT to the pleura or metastatic sites of disease at 600 to 1200 cGy per fraction was associated with very high rates of local control.
The safety, feasibility, and durability of SBRT for MPM patients has gained considerable interest.11-13,23 An expert consensus opinion from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation suggests that SBRT may allow for the safe delivery of high doses per fraction and, as such, may be efficacious for treating localized or isolated recurrences with relatively high rates of long-term LC.28 SBRT has emerged as a viable option for oligoprogressive MPM. In a single-institutional retrospective study on 21 patients with oligoprogressive disease with a total 50 lesions treated to a median dose per fraction of 500 cGy in a median 5 fractions, Schröder et al., found that 1-year LC was 73.5%, and median OS from first SBRT course was 29 months with only one patient experiencing grade >3 toxicity.12 In a single-institutional study on 37 patients with 43 oligoprogressive lesions treated with either SBRT (≥500 cGy per fraction) or hypo-fractionated radiotherapy (<500 cGy per fraction), Ghirardelli et al., found that 1-year LC was 76%, and six-month LC was better in patients treated with a BED >100 Gy, with no ≥ G3 acute or late toxicities were reported.13 In a third smaller study of 15 patients treated with SBRT to a median dose prescribed of 40 Gy (range, 30–50 Gy) in 5 fractions (range, 4–5 fractions) to a total of 25 distinct lesions for oligoprogressive MPM, LC was 100% at 2 years, dropping to 87.5% at 3 years. Finally, in an unpublished single-institutional series of 15 pleural SBRT patients with oligoprogressive MPM to a total of 25 distinct lesions after systemic therapy with or without surgery with a median prescribed dose of 4000 cGy in 5 fractions (range: 3000-5000 cGy in 4-5 fractions) at a median follow-up of 13.2 months, 1-year and 2-year LC were 100% and 87.5%, respectively.23 In our series, patients had excellent 1-year LC rates of approximately 92.9% for all lesions, and 92.1% for cases presenting with oligoprogressive disease (five sites or less).
However, the OS of our cohort was relatively lower than the aforementioned studies. Twenty of 44 (45.4%) patients in our investigation developed distant disease progression with median time to progression of 3 months (range: 1-28), while 19 of 44 (43.2%) developed either locoregional or locoregional and distant disease progression with median time to progression of 3 months (range: 1-28), and all but 3 of these patients having subsequently expired by time of last follow-up. The higher locoregional and distant failure rates within a relatively short amount of time following SBRT completion is an important factor for OS outcomes in these patients, and there remains a clear need for more optimal systemic therapies for better locoregional and distant control.
Importantly, SBRT in our investigation was safe, with no patients experiencing grade 3 or higher acute toxicities. Most toxicities were grade 1, and there were only 3 cases of pneumonitis (each grade 1). Notably, while the majority of cases were treated to 3000 cGy in 5 fractions, 42.4% of cases were treated to a higher BED. The majority (61.0%) of our patients presented while on systemic therapy, and an even greater number of patients were on systemic therapy following SBRT completion. Thus, our data suggests that SBRT can be delivered in a safe manner in between systemic therapy cycles with low rates of toxicity.
Historically, MPM has been considered as having a local pattern of disease spread but growing evidence has shown that it can also present with a more distant pattern of progression. In a retrospective study on 164 patients aiming to describe identified metastatic sites, Collins et al., found that 67% of patients were diagnosed with distant metastatic disease, including 19% of disease in bone, 14% visceral, 35% contralateral lung, and 22% peritoneal metastases.29 Smaller case reports have described disease in the oral cavity, muscle, scalp, axilla, liver, and heart.30,31 An increasingly studied option for those with oligometastatic disease is SBRT. Long-term results from the SABR-COMET trial, an international randomized phase II trial, studying 99 patients with a controlled primary malignancy and 1-5 metastatic lesions all amenable to SBRT, found that the impact of SBRT on OS was relatively large in magnitude and durable over time without any significant effect on quality of life.32 Five-year OS was 42.3% in those receiving SBRT plus standard of care treatment, whereas it was 17.7% for those receiving standard of care palliative treatment alone (p=0.006). In our investigation, oligometastatic patients had an excellent 1-year LC rate of 92.3%. Treatment sites in our population included spine, non-spine bone, brain, and pancreas. As systemic treatment options expand with hope for continued improvement in prognosis,7 it can be expected that patients presenting with oligoprogressive and oligometastatic disease increase and, subsequently, a growing number of potential candidates for SBRT.
The emergence of immunotherapy and targeted therapies have ushered in a new era of investigating possibilities in conjunction with radiotherapy, taking advantage of enhanced and complimentary antitumor effects.33 The majority (79.7%; n=44) of our cases were treated from 2017, when the preliminary data from KEYNOTE-028, a non-randomized, open-label, phase 1b trial, suggested that pembrolizumab might confer anti-tumor activity in patients with PD-L1 positive MPM and appeared to be well-tolerated.34 Seven cases in our analysis were on immunotherapy prior to SBRT (4 on nivolumab/ipilimumab and 3 on pembrolizumab) and 10 cases were on immunotherapy after SBRT completion (7 on nivolumab/ipilimumab and 3 on pembrolizumab), and none experienced any grade ≥3 toxicity. In a single-arm, investigator-initiated phase I safety trial including 13 MPM patients who progressed on prior chemotherapy, Rimner et al. investigated avelumab (monoclonal antibody therapy against programmed death-ligand 1(PD-L1)) delivered every other week with SBRT in 3 to 5 five fractions (minimum of 3000 cGy) followed by continued avelumab for up to 24 months.11 There was only one grade 3 toxicity, and no grade 4 or 5 SBRT-related adverse events. Median follow-up was 8.9 months (range: 4.3-32.7). In 9 of 13 assessable patients, all irradiated lesions were locally controlled at the end of follow-up. The MESO-PRIME phase I trial in the United Kingdom is ongoing and investigating SBRT to 3000 cGy in 3 fractions in combination with pembrolizumab.35
The main limitations of our investigation are inherent to a single-institutional analysis, and our results need to be interpreted with caution due to the potential for selection bias. Additionally, an important limitation is the lack of long-term follow-up and survival. Finally, SBRT may not be appropriate for certain patients, including those with low KPS and/or limited life expectancy. However, our data supports the growing literature suggesting that treating MPM using SBRT is safe and effective as a management option for maximizing both palliation and local control, without putting patients at risk for significant toxicity. Further studies are warranted to determine optimal dose parameters for efficacy and constraints for limiting toxicity. And with continued progress in systemic therapies and hopeful improvement in overall survival, the durability of SBRT efficacy, ideally within controlled prospective trials, will need to be reassessed.
Supplementary Material
Highlights.
SBRT can achieve high local control rates
SBRT is effective for oligoprogressive disease
SBRT is safe with low risk for toxicity
Disclosure of funding:
This research was supported, in part, by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748. Thirteen patients received Avelumab, and funding and drug were provided by Pfizer as part of an alliance between Pfizer and the health care business of Merck KGaA, Darmstadt, Germany.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Statement
Jacob Y. Shin: Conceptualization; Data curation; Formal analysis; Investigation; Roles/Writing - original draft; Writing - review & editing
Michael Offin: Conceptualization; Investigation; Writing - review & editing
Charles B. Simone II: Investigation; Writing - review & editing
Zhigang Zhang: Formal analysis; Writing - review & editing
Annemarie F. Shepherd: Investigation; Writing - review & editing
Abraham J. Wu: Investigation; Writing - review & editing
Daniel R. Gomez: Investigation; Writing - review & editing
Narek Shaverdian: Investigation; Writing - review & editing
Daphna Y. Gelblum: Investigation; Writing - review & editing
Jennifer L. Sauter: Investigation; Writing - review & editing
Michelle S. Ginsberg: Investigation; Writing - review & editing
Prasad S. Adusumilli: Investigation; Writing - review & editing
Valerie W. Rusch: Investigation; Writing - review & editing
Marjorie G. Zauderer: Conceptualization; Investigation; Writing - review & editing
Andreas Rimner: Conceptualization; Data curation; Investigation; Roles/Writing - original draft; Writing - review & editing
Conflicts of Interest statement: This study was presented as an oral communication at the 16th International Conference of the International Mesothelioma Interest Group in Lille, France, on June 28, 2023.
Jacob Y. Shin-No conflicts of interest to disclose.
Michael Offin-Dr. Offin has consulted regarding oncology drug development with Novartis, Jazz, and PharmaMar; and has received honorarium from Targeted Oncology, OncLive, and the American Society for Radiation Oncology.
Charles B. Simone II-Dr. Simone II reported receiving honoraria from Varian Medical Systems outside the submitted work.
Zhigang Zhang-No conflicts of interest to disclose.
Annemarie F. Shepherd-Dr. Shepherd reported having stock or stock options in Doximity and ArcellX.
Abraham J. Wu-Dr. Wu reported receiving grants from CivaTech Oncology; receiving personal fees from MoreHealth, AstraZeneca, and Nanovi; receiving travel expenses from AlphaTau; and serving on the scientific advisory board of Simphotek outside the submitted work.
Narek Shaverdian-Dr. Shaverdian reported receiving research funding from Novartis outside the submitted work.
Daphna Y. Gelblum-No conflicts of interest to disclose.
Daniel R. Gomez-Dr. Gomez reported receiving grants from Merck, AstraZeneca, Varian Medical Systems, and Bristol Myers Squibb during the conduct of the study and receiving personal fees from Bristol Myers Squibb, Reflexsion, Merck, Medscape, Vindico, US Oncology, MedLearning Group, AstraZeneca, GRAIL, Medtronic, Johnson & Johnson, and Varian Medical Systems outside the submitted work.
Jennifer L. Sauter-Dr. Sauter reported having stock or stock options in Chemed Corporation, Merck & Co., Inc., Pfizer, Inc., and Thermo Fisher Scientific.
Michelle S. Ginsberg-No conflicts of interest to disclose.
Prasad S. Adusumilli-Dr. Adusumilli is a scientific advisory board member and consultant for ATARA Biotherapeutics, Bayer, Carisma Therapeutics, Imugene, ImmPACT Bio, Johnson & Johnson, and Outpace Bio; declares having patents, royalties, and intellectual property on mesothelin-targeted CAR and other T-cell therapies, which have been licensed to ATARA Biotherapeutics, issued patent method for detection of cancer cells using virus, and pending patent applications on PD-1–dominant negative receptor, wireless pulse oximetry device, and on an ex vivo malignant pleural effusion culture system. Memorial Sloan Kettering Cancer Center (MSK) has licensed intellectual property related to mesothelin-targeted CARs and T-cell therapies to ATARA Biotherapeutics and has associated financial interests.
Valerie W. Rusch-Dr. Rusch reported receiving grants from the National Institutes of Health (NIH)/National Cancer Institute, during the conduct of the study; grants from Genelux, Inc.; grants from Genentech, other from DaVinci Surgery; nonfinancial support from Bristol Myers Squibb; and personal fees from the NIH/Coordinating Center For Clinical Trials, outside the submitted work.
Marjorie G. Zauderer-In the last 3 years, Dr. Zauderer has received consulting fees from Curis, Ikena, Takeda, GlaxoSmithKline, and Novocure and honoraria for CME content from PER, Medscape, and Research to Practice. Memorial Sloan Kettering receives research funding from the Department of Defense, the National Institutes of Health, MedImmune, Precog, GlaxoSmithKline, Epizyme, Polaris, Sellas Life Sciences, Bristol Myers Squibb, Millenium/Takeda, Curis, and Atara for research conducted by Dr. Zauderer. Dr. Zauderer serves as Chair of the Board of Directors of the Mesothelioma Applied Research Foundation, uncompensated.
Andreas Rimner-Dr. Rimner has consulted regarding oncology drug development with AstraZeneca, Merck, Boehringer Ingelheim, and Cybrexa; has received honorarium from MoreHealth and ResearchToPractice; has served on a scientific advisory board of Merck; and has received grants from Varian Medical Systems, Boehringer Ingelheim, Pfizer, AstraZeneca, and Merck.
References
- 1.Key Statistics About Malignant Mesothelioma. American Cancer Society. Last Revised: 9 January 2019. Accessed 20 March 2023. <https://www.cancer.org/cancer/malignant-mesothelioma/about/key-statistics.html>. 2019; [Google Scholar]
- 2.Survival Rates for Malignant Mesothelioma. American Cancer Society. Last Revised: 2 March 2023. Accessed 20 March 2023. <https://www.cancer.org/cancer/malignant-mesothelioma/detection-diagnosis-staging/survival-statistics.html>. 2023; [Google Scholar]
- 3.Rimner A, Zauderer MG, Gomez DR, et al. Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma. J Clin Oncol. Aug 10 2016;34(23):2761–8. doi: 10.1200/JCO.2016.67.2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thieke C, Nicolay NH, Sterzing F, et al. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol. Dec 30 2015;10:267. doi: 10.1186/s13014-015-0575-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindler HL, Ismaila N, Armato SG, 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. May 1 2018;36(13):1343–1373. doi: 10.1200/JCO.2017.76.6394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsao AS, Lindwasser OW, Adjei AA, et al. Current and Future Management of Malignant Mesothelioma: A Consensus Report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J Thorac Oncol. Nov 2018;13(11):1655–1667. doi: 10.1016/j.jtho.2018.08.2036 [DOI] [PubMed] [Google Scholar]
- 7.Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. Jan 30 2021;397(10272):375–386. doi: 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 8.Peters S, Scherpereel A, Cornelissen R, et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol. May 2022;33(5):488–499. doi: 10.1016/j.annonc.2022.01.074 [DOI] [PubMed] [Google Scholar]
- 9.Hu ZI, Ghafoor A, Sengupta M, Hassan R. Malignant mesothelioma: Advances in immune checkpoint inhibitor and mesothelin-targeted therapies. Cancer. Apr 1 2021;127(7):1010–1020. doi: 10.1002/cncr.33433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchevsky AM, Khoor A, Walts AE, et al. Localized malignant mesothelioma, an unusual and poorly characterized neoplasm of serosal origin: best current evidence from the literature and the International Mesothelioma Panel. Mod Pathol. Feb 2020;33(2):281–296. doi: 10.1038/s41379-019-0352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimner A, Adusumilli PS, Offin MD, et al. A Phase 1 Safety Study of Avelumab Plus Stereotactic Body Radiation Therapy in Malignant Pleural Mesothelioma. JTO Clin Res Rep. Jan 2023;4(1):100440. doi: 10.1016/j.jtocrr.2022.100440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroder C, Opitz I, Guckenberger M, et al. Stereotactic Body Radiation Therapy (SBRT) as Salvage Therapy for Oligorecurrent Pleural Mesothelioma After Multi-Modality Therapy. Front Oncol. 2019;9:961. doi: 10.3389/fonc.2019.00961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghirardelli P, Franceschini D, D'Aveni A, et al. Salvage radiotherapy for oligo-progressive malignant pleural mesothelioma. Lung Cancer.Feb 2021;152:1–6. doi: 10.1016/j.lungcan.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 14.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. Jul 2007;2(7 Suppl 3):S94–100. doi: 10.1097/JTO.0b013e318074de34 [DOI] [PubMed] [Google Scholar]
- 15.Machtay M, Bae K, Movsas B, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. Jan 1 2012;82(1):425–34. doi: 10.1016/j.ijrobp.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung IH, Yoon SM, Kwak J, et al. High-dose radiotherapy is associated with better local control of bone metastasis from hepatocellular carcinoma. Oncotarget. Feb 28 2017;8(9):15182–15192. doi: 10.18632/oncotarget.14858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moding EJ, Mowery YM, Kirsch DG. Opportunities for Radiosensitization in the Stereotactic Body Radiation Therapy (SBRT) Era. Cancer J. Jul-Aug 2016;22(4):267–73. doi: 10.1097/PPO.0000000000000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmerman RD, Hu C, Michalski JM, et al. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol. Sep 1 2018;4(9):1287–1288. doi: 10.1001/jamaoncol.2018.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezjak A, Paulus R, Gaspar LE, et al. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol. May 20 2019;37(15):1316–1325. doi: 10.1200/JCO.18.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. Sep-Oct 2017;7(5):295–301. doi: 10.1016/j.prro.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 21.Modh A, Rimner A, Williams E, et al. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. Dec 1 2014;90(5):1168–76. doi: 10.1016/j.ijrobp.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Reibnitz D, Shaikh F, Wu AJ, et al. Stereotactic body radiation therapy (SBRT) improves local control and overall survival compared to conventionally fractionated radiation for stage I non-small cell lung cancer (NSCLC). Acta Oncol. Nov 2018;57(11):1567–1573. doi: 10.1080/0284186X.2018.1481292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barsky AR, Yegya-Raman N, Katz SI, Simone CB, 2nd, Cengel KA. Managing oligoprogressive malignant pleural mesothelioma with stereotactic body radiation therapy. Lung Cancer. Jul 2021;157:163–164. doi: 10.1016/j.lungcan.2021.02.033 [DOI] [PubMed] [Google Scholar]
- 24.Hanna GG, John T, Ball DL. Controversies in the role of radiotherapy in pleural mesothelioma. Transl Lung Cancer Res. Apr 2021;10(4):2079–2087. doi: 10.21037/tlcr-20-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacRae RM, Ashton M, Lauk O, et al. The role of radiation treatment in pleural mesothelioma: Highlights of the 14th International Conference of the International mesothelioma interest group. Lung Cancer. Jun 2019;132:24–27. doi: 10.1016/j.lungcan.2019.03.023 [DOI] [PubMed] [Google Scholar]
- 26.Patel R, Ludmir EB, Miccio JA, et al. Disease-Related Outcomes and Toxicities of Intensity Modulated Radiation Therapy After Lung-Sparing Pleurectomy for Malignant Pleural Mesothelioma: A Systematic Review. Pract Radiat Oncol. Nov-Dec 2020;10(6):423–433. doi: 10.1016/j.prro.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 27.de Graaf-Strukowska L, van der Zee J, van Putten W, Senan S. Factors influencing the outcome of radiotherapy in malignant mesothelioma of the pleura--a single-institution experience with 189 patients. Int J Radiat Oncol Biol Phys. Feb 1 1999;43(3):511–6. doi: 10.1016/s0360-3016(98)00409-x [DOI] [PubMed] [Google Scholar]
- 28.Gomez DR, Rimner A, Simone CB 2nd, et al. The Use of Radiation Therapy for the Treatment of Malignant Pleural Mesothelioma: Expert Opinion from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J Thorac Oncol. Jul 2019;14(7):1172–1183. doi: 10.1016/j.jtho.2019.03.030 [DOI] [PubMed] [Google Scholar]
- 29.Collins DC, Sundar R, Constantinidou A, et al. Radiological evaluation of malignant pleural mesothelioma - defining distant metastatic disease. BMC Cancer. Dec 9 2020;20(1):1210. doi: 10.1186/s12885-020-07662-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerpel SM, Freedman PD. Metastatic mesothelioma of the oral cavity. Report of two cases. Oral Surg Oral Med Oral Pathol. Dec 1993;76(6):746–51. doi: 10.1016/0030-4220(93)90046-7 [DOI] [PubMed] [Google Scholar]
- 31.Tertemiz KC, Ozgen Alpaydin A, Gurel D, Savas R, Gulcu A, Akkoclu A. Multiple distant metastases in a case of malignant pleural mesothelioma. Respir Med Case Rep. 2014;13:16–8. doi: 10.1016/j.rmcr.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. Sep 1 2020;38(25):2830–2838. doi: 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alley EW, Katz SI, Cengel KA, Simone CB, 2nd. Immunotherapy and radiation therapy for malignant pleural mesothelioma. Transl Lung Cancer Res. Apr 2017;6(2):212–219. doi: 10.21037/tlcr.2017.04.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. May 2017;18(5):623–630. doi: 10.1016/S1470-2045 [DOI] [PubMed] [Google Scholar]
- 35.Pembrolizumab and Hypofractionated Stereotactic Radiotherapy in Patients With Malignant Pleural Mesothelioma (MESO-PRIME). Royal Marsden NHS Foundation Trust. Last Update Posted:12 April 2021. Accessed 20 March 2023. <https://clinicaltrials.gov/ct2/show/NCT04166734>. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.