Abstract
The heart undergoes a dynamic maturation process following birth, in response to a wide range of stimuli, including both physiological and pathological cues. This process entails substantial re-programming of mitochondrial energy metabolism coincident with the emergence of specialized structural and contractile machinery to meet the demands of the adult heart. Many components of this program revert to a more “fetal” format during development of pathological cardiac hypertrophy and heart failure. In this review, emphasis is placed on recent progress in our understanding of the transcriptional control of cardiac maturation, encompassing the results of studies spanning from in vivo models to cardiomyocytes derived from human stem cells. The potential applications of this current state of knowledge to new translational avenues aimed at the treatment of heart failure is also addressed.
Keywords: Cardiomyocyte maturation, Postnatal development, Nuclear receptor, Gene transcription, Mitochondrial metabolism, Fetal gene program
What is cardiac maturation and why is it important?
Early cardiac development involves a cascade of orchestrated signaling and transcriptional events that drive cardiomyocyte (CM) lineage determination, proliferation, differentiation, and morphogenesis leading to the development of the cardiac chambers and the great vessels [1–3]. However, much less is known about the maturation process which begins in the late fetal period and proceeds into the postnatal period, resulting in the adult heart [1]. In contrast to early development, this process largely involves mitochondrial, structural, and functional maturation of the post-mitotic cell, although some CM proliferation occurs during the early postnatal period [1, 4–10] (Figure 1). This terminal maturation process is critical for the development of a fully functional mammalian heart that serves as a constant pump throughout the life of the organism. Given the extraordinary work and energy demands, the heart requires specialized adult contractile machinery and a high-capacity mitochondrial system to generate a tremendous ATP output using a variety of fuels. This review, which serves as a companion to the 2023 International Society for Heart Research (ISHR) Peter Harris Award Lecture, focuses on current knowledge of the mechanisms involved in driving postnatal cardiac maturation. Herein, we define “cardiomyocyte maturation” as the constellation of changes in metabolism, contractile function, cell biology, and structure that convert fetal CMs to adult CMs. Emphasis has been placed on transcriptional regulatory mechanisms given the flurry of recent advances in this area.
Figure 1. Coordinated Cardiac Structural, Contractile, and Mitochondrial Metabolic Maturation.

The illustration in Figure 1 highlights the intricate maturation processes observed during the lifespan of the mammalian heart. The fetal heart relies largely on glucose and lactate as the preferred energy substrates. However, during the perinatal period a mitochondrial biogenic response is followed by a maturation process that equips the heart with high capacity for fatty acid oxidation and a substrate preference switch from carbohydrates to fatty acids. In healthy adult hearts, fatty acids are the primary source for energy production, with over 95% of ATP synthesis occurring through oxidative phosphorylation within the mitochondria. The maturation of mitochondrial metabolism is accompanied by distinct transitions from fetal to adult isoform contractile protein expression, along with a significant augmentation of Ca2+ handling and ion transport machinery. Contractile protein isoform switching is largely completed by ~9 months in human heart and by postnatal day 21 (P21) in mouse hearts based on troponin I expression switching. During the development of heart failure, a so-called “fetal gene program” is re-activated in adult CMs, marked by a decline in adult cardiac gene expression and concurrent induction of a subset of fetal cardiac genes. The control of cell proliferative activity also undergoes substantial regulation during the developmental process, with fetal CMs displaying robust proliferative capacity. However, as development proceeds after birth, the vast majority of CMs exit the cell cycle, culminating in their transformation into fully differentiated, mature CMs.
Why is understanding the late phase of cardiac maturation important? Deciphering the regulatory components and mechanisms involved in postnatal energy maturation of the CM has translational implications. During the development of pathologic cardiac hypertrophy and heart failure (HF), the heart reverts to a more immature state (Figure 1). Whereas these changes may initially represent an adaptive response to an acute stress, over time, energetics and contractile function become constrained, likely contributing to the pathogenesis of HF. Understanding the circuitry involved in this re-programming will allow studies aimed at preventing deactivation of the maturation process in cardiac disease states to determine if this approach may lead to new therapeutic avenues for the treatment of HF. Conversely, recent studies also suggest that inhibition of cardiac maturation pathways, particularly mitochondrial fuel metabolism, enable re-entry of the postnatal CM into the cell cycle which has potential cardiac regeneration therapies after cardiac injury [11–20]. In addition, there is great excitement regarding the use of stem-cell derived CMs, such as human embryonic stem cell-derived cardiomyocytes (hESC-CMs) and human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) for in vitro interrogation of human cardiac disease and for the screening of new therapeutics [6, 21, 22]. However, this field has been significantly hampered by a lack of full maturation of hiPSC-CMs. Delineation of the mechanisms driving hiPSC-CM maturation following exit from the cell cycle may provide more effective approaches for establishing mature CMs for human cardiac “disease in a dish” studies.
Energy metabolic maturation of the cardiomyocyte: Mitochondrial biogenesis and development of a high-capacity fatty acid oxidation system
After birth, a tremendous mitochondrial biogenesis occurs in the CM to meet the high energy requirements of the postnatal heart [9, 23, 24]. This mitochondrial biogenic response is driven by transcriptional activation of nuclear- and mitochondrial-encoded genes involved in mitochondrial expansion and energy transduction pathways, equipping the mitochondria with tremendous capacity for ATP production [24–26]. The perinatal cardiac mitochondrial biogenesis is linked to mitophagy suggesting that at least a portion of the fetal mitochondria are “cleared” prior to the expansion of new mitochondria [27]. The normal adult mammalian heart is a fuel “omnivore” capable of oxidizing various energy substrates including fatty acids (FA), glucose, lactate, pyruvate, and ketone bodies to generate reducing equivalents for the generation of ATP [28–31]. However, the chief fuel for the normal adult heart is FA which are catabolized via a high-capacity mitochondrial fatty acid oxidation (FAO) pathway [28–30]. The early postnatal mitochondrial biogenesis is followed by a period of postnatal maturation that equips the CM for high rates of FAO coupled to a high-capacity oxidative phosphorylation/electron transport system [24]. The mature CM is also capable of oxidizing glucose and ketone bodies [28–31]. This fuel utilization versatility allows the heart to generate sufficient ATP in the context of limited energy stores in the context of a variety of physiological demands.
A complex transcriptional regulatory circuitry drives the postnatal biogenesis and maturation of cardiac mitochondria (Figure 2). Early studies focused on the induction of FAO enzyme genes following birth led to the identification of the nuclear receptor peroxisome proliferator–activated receptor alpha (PPARα; NR1C1) as a key player in the transcriptional control of this pathway as well as other processes involved in CM FA utilization including uptake, trafficking, and storage [32, 33]. The related NR, PPARδ (NR1C2), also regulates cardiac genes involved in lipid metabolism [32–34]. Conditional cardiac-specific PPARα knockout (KO) mice exhibit CM lipid accumulation following fasting and accelerated pressure overload-induced cardiac remodeling and contractile dysfunction [35, 36]. Cardiac-specific PPARδ KO in mouse hearts leads to cardiac dysfunction and hypertrophy along with decreased levels of FAO genes [37, 38]. Conversely, transgenic overexpression of PPARα in mouse heart results in increased CM FA uptake and lipid accumulation that is worsened during fasting [39, 40]. Taken together, mouse studies of PPAR gain- and loss-of-function demonstrate the importance of these nuclear receptors in the development and maintenance of a high-capacity FAO in the postnatal CM.
Figure 2. Regulation of PGC-1α Activity and Transcriptional Co-activation in Mitochondrial Energy Metabolism.
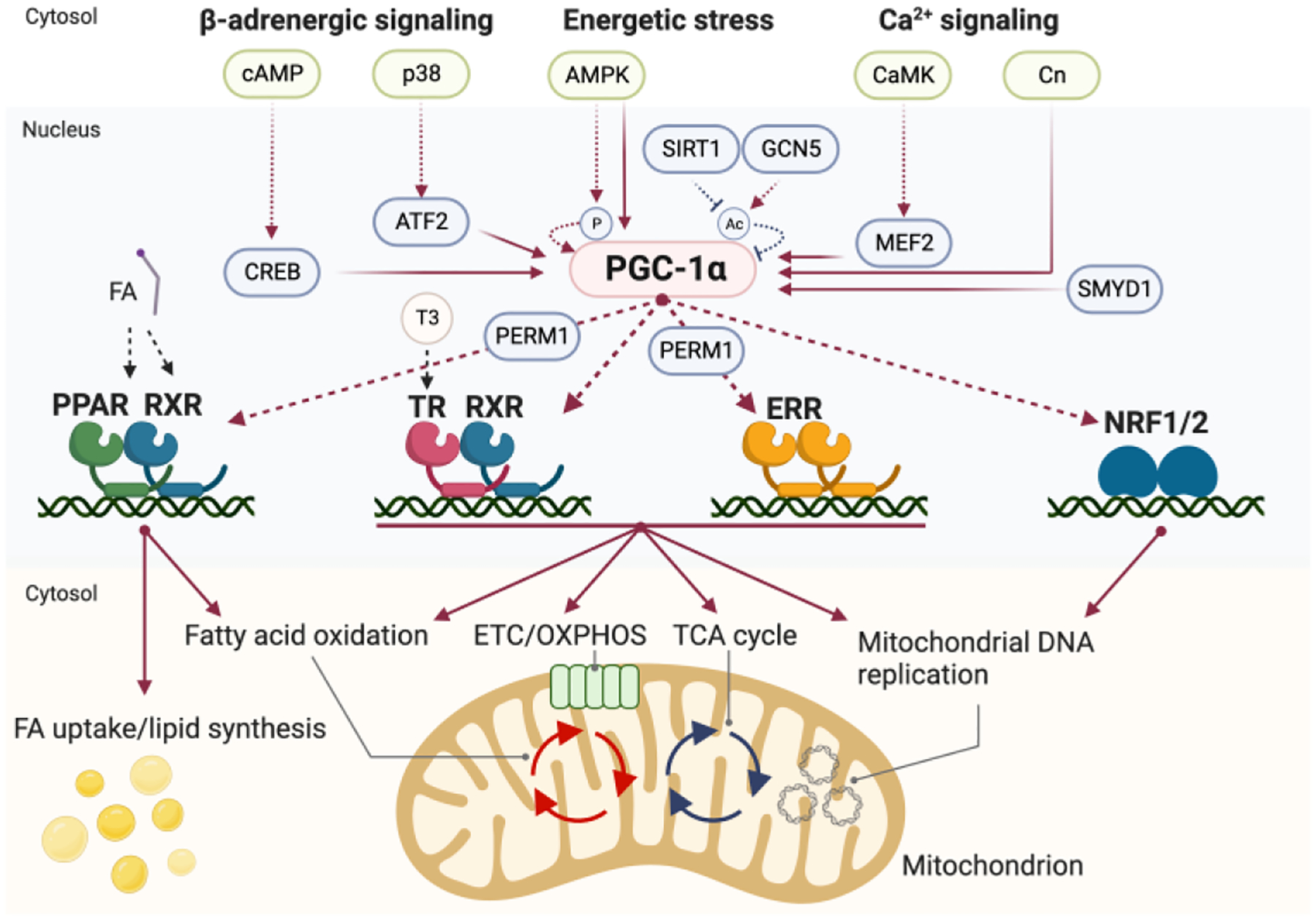
PGC-1α expression and activity are modulated by a network of signaling pathways and transcription factors as indicated. PGC-1α serves as a central co-activator by directly interacting with various transcriptional regulators, including nuclear receptors, to modulate the expression of genes involved in mitochondrial oxidative metabolism. Of particular significance, the Estrogen-Related Receptor (ERR) is highlighted as a master regulator governing multiple facets of mitochondrial energy metabolism, encompassing fatty acid oxidation, oxidative phosphorylation (OXPHOS), tricarboxylic acid (TCA) cycle, and mitochondrial biogenesis. PERM1 coactivates PGC-1α/ERR and PGC-1α/PPAR transcriptional events but it has distinct functions in mitochondria and cytoplasm to facilitate mitochondrial metabolism. In the figure, red dotted lines represent activation of activity, red solid lines signify activation of transcription, black dotted lines depict fatty acid ligands binding to the indicated nuclear receptors, and blue dotted lines depict deactivation of activity.
The PPARs are activated by lipid ligands in vitro including medium- and long-chain fatty acid species [41–43]. The actual endogenous ligands are not well-characterized. However, phosphatidylcholine and mevalonate metabolites have been identified as endogenous activators for PPARα in liver and for PPARγ in adipose tissues, respectively [44–47]. Accordingly, the activity of the PPARs and corresponding regulation of downstream targets involved in FA uptake, storage, and oxidation is regulated, at least in part, by the levels of endogenous FA ligand which in turn likely provide a feedback mechanism to control FA utilization with metabolic demands. Some compelling evidence suggests that one important source of PPAR ligand in the CM is derived from the triglyceride storage depot. The hearts of mice rendered deficient in CM lipolysis by genetic targeting of cardiac adipose triglyceride lipase (ATGL) exhibit reduced activity of PPARα and its target gene expression along with a dramatic expansion of neutral lipid and reduced FAO associated with cardiomyopathy [48]. In addition, recent evidence suggests that the retinoid X receptor (RXRs), the obligate heterodimeric partner of PPARs, also serve as critical sensors of FAs during cardiac maturation. Specifically, γ-linolenic acid in maternal milk was shown to activate RXR in the CM contributing to the increase in mitochondrial FAO gene expression during postnatal cardiac maturation [49].
A major breakthrough in defining the upstream transcriptional control of postnatal mitochondrial biogenesis came with the discovery that the inducible transcriptional co-regulator PPARgamma coactivator 1 alpha (PGC-1α) binds to and co-activates PPARα [43]. PGC-1α was originally discovered in brown adipose tissue (BAT) where it was shown to play a key role in mitochondrial biogenesis [50]. A structurally related homolog, PGC-1β was subsequently identified [51]. Functionally, PGC-1α recruits components of the transcriptional regulatory machinery such as the histone deacetylase p300/CREB binding protein (CBP), steroid receptor coactivator 1 (SRC-1), and members of the Mediator complex to activate transcription [52–55]. The expression of PGC-1α is highest in tissue/cell types with high rates of mitochondrial oxidative metabolism such as BAT, slow-twitch skeletal muscle, kidney, and heart [50]. Importantly, the PGC-1α expression is markedly induced after birth coincident with the mitochondrial biogenic response [23, 56]. A series of PGC-1 gain-of-function and loss-of-function studies demonstrated that PGC-1α and β are both necessary and sufficient for postnatal CM mitochondrial biogenesis. Specifically, overexpression of PGC-1α in rat neonatal CMs results in a robust mitochondrial biogenic response [23, 57]. CM-specific loss of PGC-1α and β in mice results in a block in mitochondrial biogenesis and functional maturation after birth and a cardiomyopathy [23, 56]. Transgenic induction of PGC-1α in the adult mouse heart increases CM mitochondrial content but ultimately leads to an uncontrolled biogenic response that leads to dilated cardiomyopathy [57]. These collective findings defined an indispensable role for PGC-1α and β in early postnatal CM mitochondrial biogenesis.
The dramatic effects of PGC-1 loss of function studies in BAT and heart strongly suggested a master regulatory role for these inducible transcriptional activators in driving postnatal mitochondrial biogenesis and heart maturation. Studies conducted in BAT, heart and other mitochondrial-rich cell types defined downstream effectors of PGC-1, in addition to the PPARs. This includes nuclear respiratory factor 1 (NRF-1) and the nuclear receptors, thyroid hormone receptor (TR) and the estrogen-related receptors (ERRs) [50, 58] (Figure 2). The inducible nature of PGC-1α expression also led to the identification of a number of upstream cellular signaling pathways (Figure 2).
The ERRs were identified as downstream PGC-1 effectors using a two-hybrid screen with PGC-1 as “bait” [59–61]. The ERR family is composed of three members: ERRα (NR3B1) coded in ESRRA, ERRβ (NR3B2) coded in ESRRB, and ERRγ (NR3B3) coded in ESRRG, originally cloned as steroid hormone receptors closely related to estrogen receptors (ERs) [62–66]. However, ERRs are not true ERs. Despite the structural similarity between ERRs and ERs, ERRs possess a distinct ligand binding domain and estrogen does not activate ERR transcriptional activity [63]. Given that a true endogenous ligand for ERRs has not been identified, they are referred to as “orphan nuclear receptors”. The transcriptional activity of ERR is controlled, at least in part, by PGC-1 recruitment; PGC-1α/β are sometimes referred to as an ERR “protein ligand” [59, 61]. ERRα and ERRγ are enriched in the postnatal CM and the central role for these nuclear receptors in the transcriptional control of cardiac maturation will be described later.
Thyroid hormone receptors (TRs), TRα (NR1A1) and TRβ (NR1A2), members of the nuclear receptor superfamily, are known regulators of metabolic maturation including mitochondrial biogenesis and function, although the exact mechanisms involved have not been well-delineated compared with that of the PPARs and ERRs [67–69]. Cardiac-specific dominant negative TRα overexpression decreases the expression of genes coding tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS)-related enzymes [13]. Most of the downregulated metabolic genes are defined as TRα-direct targets with ChIP-seq and one of the notable targets is carnitine palmitoyltransferase 2 (Cpt2), which catalyzes the essential step of FAO [13]. As an indirect mechanism, T4 or T3 treatment induces levels of PPARα, PGC-1α, and ERRα in hearts or liver [67, 70]. Functional cooperation between TRs and other transcriptional regulators has been shown in tissues with high oxidative mitochondrial metabolism. For instance, PGC-1α directly interacts with TRβ in a T3 ligand-dependent manner in mouse brown fat [50]. The functional cooperation of TRβ and ERRα drives mitochondrial biogenesis and function in the mouse liver [70]. Therefore, the cooperation of TRs with other members of the PGC-1 circuitry likely plays an important role in the CM maturation downstream of thyroid hormone.
More recently, another level of energy metabolic regulation by PGC-1 has been discovered. PGC-1 and ERR-induced regulator in muscle 1 (Perm1) was cloned as a PGC-1α-induced gene in C2C12 myotubes [71]. Cardiac Perm1 expression is highly induced during postnatal developmental stages and enhances mitochondrial oxidative metabolism by recruiting the ERR/PGC-1α complex to a subset of nuclear-coded metabolic genes [72–74]. In addition, in mouse skeletal muscle, Perm1 activates calmodulin-dependent kinase (CaMK) II and p38MAPK during exercise, resulting in an adaptive mitochondrial biogenesis [75, 76]. Given that these kinases are upstream of PGC-1α (Figure 2), a feedforward mechanism (CaMKII-PGC-1α/ERRα-Perm1 axis) could exist in myocytes [75]. Notably, the interaction and functional cooperation between PPARα/PGC-1α complex and Perm1 has also been reported in primary CMs [77]. Global deletion of Perm1 results in decreased expression of oxidative metabolic mitochondrial genes and proteins in mouse hearts, although the impact of Perm1 deletion on cardiac function and the exact mechanism whereby it exerts control on mitochondrial metabolism in this cell type is unclear [73, 77, 78]. In addition, cardiac-specific overexpression of Perm1 protects against pressure overload-associated cardiac dysfunction and remodeling along with increased mitochondrial oxidative metabolism [79]. Interestingly, Perm1 is found in mitochondria and cytoplasm [71, 73, 78, 79]. In cardiac and skeletal mitochondria, it directly interacts with mitochondrial protein complexes to facilitate respiratory function [79, 80].
In addition to the PGC-1 circuitry, other transcription factors have been implicated in cardiac energy metabolic maturation. The Kruppel-like factor (KLF) family of transcription factors has been shown to regulate energy metabolism in the CM. KLF4 expression is increased in heart during postnatal cardiac development [81]. KLF4 was shown to cooperate with the ERRα/PGC-1α complex to drive expression of various metabolic and mitochondrial biogenesis in mouse heart [81]. In addition, KLF7 modulates cardiac energy metabolism by regulating glycolysis and FAO, and KLF15 has been shown to drive expression of FAO genes in cooperation with PPARα in mouse heart [82–84]. The TEA domain transcription factor (Tead)1, a downstream effector of the Hippo pathway, activates transcription of the genes coding mitochondrial metabolic enzymes and PGC-1α expression levels [85–88]. The transcription of a subset of electron transport chain genes is directed by Tead1, suggesting it may function in parallel with that of the PGC-1 cascade [87, 88].
The importance of nuclear receptors in cardiac maturation has led to the development of so-called “maturation medias or cocktails” for coaxing stem-cell-derived CMs in culture towards a more mature state. Addition of a mixture of T3, synthetic PPAR ligands, and FAs has been shown to increase stem cell-derived CM maturation [56, 89–96]. Despite this progress, none of the maturation cocktails have achieved fully mature adult-like CMs.
Structural and contractile maturation of the postnatal heart.
During postnatal cardiac maturation, genes encoding many cardiac-specific structural proteins, including components of the sarcomeric machinery, undergo a fetal-to-adult isoform switch to meet the contractile demands of the adult heart (Figure 3). A well-characterized example of contractile protein gene isoform switching involves the myosin heavy chain genes; Myh6 encoding α-myosin heavy chain (α-MHC) and Myh7 which codes for β-MHC. The vast majority of studies focused on Myh isoform regulation have been conducted in the rodent cardiac ventricle where the expression of Myh6 is induced during the early postnatal period concordant with reduced expression of Myh7. By postnatal day 7, Myh6 transcripts have completely replaced that of Myh7 [97]. Re-expression of the “fetal” Myh7 isoform occurs under chronic cardiac stress such as ventricular pressure overload and in the failing heart [98]. Interestingly, in human cardiac ventricle, the developmental pattern of the MHC isoform shifts is opposite to that of the rodent; MYH7 being the major adult isoform and MYH6 expressed highest during fetal stages. The mechanistic basis for this species difference is poorly understood. Despite the opposite perinatal MYH isoform shifts in rodent compared to human ventricles, the isoform response to T3 (as described below) and expression changes in the failing heart are conserved between the two species [89, 93, 99, 100].
Figure 3. The Cardiac Fetal-to-Adult Contractile Isoform Transition.

Postnatal development is marked by significant shifts and switches in the expression of sarcomere proteins isoforms, driven primarily by a combination of transcriptional and posttranscriptional regulatory processes. Well-described gene isoform changes are depicted.
In addition to the MHC isoform switches, the isoform expression patterns of an array of other cardiac contractile genes are regulated during the perinatal period (Figure 3). For example, a shift from cardiac troponin I (TNNI) 1 to 3 occurs in both the human and rodent during the fetal-adult transition [9, 101, 102]. The transition from Tnni1 to Tnni3 isoforms in mouse hearts is completed around postnatal day 21, concomitant with the completion of isoform switching for the fatty acid metabolic enzyme acyl-CoA synthetase [9, 10, 102]. In humans, TNNI3 expression commences shortly after birth, coinciding with a gradual decline in the corresponding fetal isoform, TNNI1. By approximately 9 months postnatal, TNNI3 emerges as the predominant isoform, with complete absence of TNNI1 protein and mRNA expression [101]. These collective findings suggest that human postnatal maturation is largely completed by ~9 months of age (Figure 1). Titin isoform switching is also well-described. Fetal CMs express a longer and compliant titin N2BA form whereas adult CMs express the shorter and stiffer N2B isoform, which supports the sarcomere integrity and elasticity in mature adult CMs [103, 104]. Part of this regulation involves differential transcript splicing carried out by the RNA-binding motif protein 20 (RBM20) [105]. Re-expression of fetal N2BA isoform is observed in the hearts of dilated cardiomyopathy patients [106]. ACTC1, encoding α-cardiac actin (α-CA), is induced during cardiac postnatal development, although α-skeletal actin (α-SKA;ACTA1) is also expressed in the healthy adult heart and its expression is further induced in HF [107–111]. ACTA2 (also known as α-smooth muscle actin, α-SMA) is a well-defined marker of myofibroblasts [112]. However, recent single nuclear RNA-sequencing technology identified an Acta2-positive CM population in P1 neonatal CMs with regenerative capacity [113]. Acta2 expression in mouse cardiomyocytes and hearts rapidly decreases during the postnatal maturation process [113, 114]. The ventricular form of myosin light chain 2 or MLC2v (MYL2) and atrial form, MLC2a (MYL7), are co-expressed in ventricle during early fetal development [115]. However, MLC2a expression declines in ventricle by E10, and is extinguished by E12 [115, 116]. The expression of four different isoforms of cardiac troponin T (cTnT) are regulated during postnatal cardiac development as well as during development of HF [117, 118]. The shortest cTnT isoform (cTnT4) is enriched in fetal hearts and re-expresses in failing adult hearts [117, 118]. Myomesin1, a structural component of the M-band in striated muscle, and a specific longer isoform termed EH-myomesin is expressed specifically in embryonic hearts, with rapid downregulation after birth [119]. EH-myomesin is re-expressed in the hearts of dilated cardiomyopathy in mice and humans [120]. In summary, a variety of contractile protein genes exhibit a fetal to adult isoform switch with re-expression of the fetal pattern in cardiac disease states such as pathological cardiac hypertrophy and in the failing heart. For more information on sarcomeric protein maturation, we refer you to [4, 121–123].
The perinatal shift in fetal-to-adult contractile protein gene isoforms likely impacts cardiac contractile properties. For example, the α-MHC and β-MHC proteins differ in their ATPase activities and actomyosin interaction kinetics; the ATPase activity and the velocity of actin displacement of the β-MHC protein are several-fold lower than those of the α-MHC protein. The ratio of α-MHC / β-MHC levels in the CM also correlates with contractile rate in vitro in hiPSC-CMs and in vivo across species [124, 125]. The resting heart rate in adult mouse hearts (500~700 bpm) with dominant α-MHC levels is markedly higher than that of human hearts (50~70 bpm) which have a greater proportion of β-MHC. The MLC2a to MLC2v switch during perinatal development matches increases in ventricular contractile force [126]. Compared to TNNI1, TNNI3 has an amino-terminal extension that harbors phosphorylation sites for PKA phosphorylation and other kinases [127]. PKA-mediated phosphorylation of the specific serine residues on TNNI3, downstream of β-adrenergic signaling, leads to enhanced relaxation (diastolic function) of cardiac muscle due to attenuation of the troponin C and troponin I interaction [127]. Postnatal titin isoform shift results in a change in cardiac muscle stiffness as evidenced by decreased passive tension and stiffness in failing hearts with increased N2BA (fetal): adult N2B (adult) ratio [106].
In parallel with contractile maturation, the CM undergoes electrophysiological maturation during postnatal cardiac development. This maturation process is characterized by precise perinatal induction in the expression profiles of various ion channels and Ca2+ handling proteins, including the well-studied ryanodine receptor 2 (RYR2) and ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 or SERCA2 coded in ATP2A2 [56]. The postnatal expression of these genes occurs coincident with that of other ion transporters such as KCNJ2 and KCNJ12, the latter preserving inward rectifying current and maintaining a negative resting membrane potential, typically around −85 to −90 mV in the adult CM [128, 129]. In contrast, immature CMs, such as hiPSC-CMs, exhibit automaticity due to an immature pattern of ion channel expression levels [130]. Recent research has identified elevated and sustained expression levels of HCN4, CACNA1H, and SLC8A1, coupled with the absence of KCNJ2 expression, as key contributors to automaticity in immature hiPSC-CMs [130]. The expression of HCN4, encoding hyperpolarization-activated cyclic nucleotide-gated potassium channel, is dramatically downregulated in cardiac ventricles during development and its expression is induced in the hypertrophied heart [131–133]. A hallmark of cardiac maturation is the formation of transverse tubules (T-tubules), specialized invaginations of the cell membrane primarily found in striated muscle cells [4, 134]. The specialized membranous structures of the T-tubules are located transversely into the central regions of CMs, housing a substantial complement of ion channels and regulatory proteins integral to the orchestration of excitation-contraction coupling processes. These distinctive membrane features facilitate the rapid propagation of electrical signals, ultimately culminating in the prompt release of Ca2+ from the sarcoplasmic reticulum [134]. Stem cell-derived CMs typically do not form T-tubules under conventional two-dimensional culture conditions [135, 136]. However, several experimental manipulations such as engineered heart tissue (EHT) with concomitant electrical stimulation, a combined regimen of T3 and dexamethasone (Dex) treatment, or the treatment with the ERRγ modulator T112 has been shown to induce T-tubule development in hiPSC-CMs [94, 137, 138].
Fetal-to-adult isoform switching in contractile proteins involves transcriptional regulatory mechanisms. The murine Myh6 promoter region contains binding motifs for a number of cardiogenic transcription factors, including GATA and MEF2, suggesting that activating these transcription factors is key for Myh6 expression [139–141]. Neonatal inactivation of GATA4 and GATA6 disrupts the proper fetal Myh7 to adult Myh6 isoform switching in postnatal mouse hearts [142, 143]. Serum response factor (SRF) has also been shown to regulate Myh7 to Myh6 isoform switches in mouse ventricular myocytes along with others including Tnni1 to Tnni3, Myl7 to Myl2, and Myl4 to Myl3 [144]. SRF ChIP-seq data in mouse ventricles demonstrates that SRF occupancy regions often contain MEF2 and GATA binding motifs suggesting functional cooperation between SRF, MEF2, and GATA [144]. More recently, a super-enhancer region located upstream of the MYH6/7 cluster has been implicated in a shift in these isoforms during perinatal development [124]. Deleting this enhancer region results in the reversal of MYH6/7 switching during hiPSC-CM differentiation [124].
Thyroid hormone also regulates fetal-to-adult contractile protein gene isoform switching. Most of this work has focused on the rodent Myh6/7 switch [97, 145, 146], but is also relevant to Tnni1/3 [102]. TRs are thought to be activated after birth when a surge of T3 concentration occurs [13, 147]. TRβ1 has been shown to upregulate the promoter activity and transcript level of Myh6, while downregulating Myh7 in neonatal rat ventricular myocytes [148]. The Myh6 promoter contains thyroid hormone receptor response element (TRE) adjacent to MEF2 binding motifs, and the functional cooperation between TRs and MEF2 has been shown to be required for the full activation of the Myh6 promoter activity [149]. Cardiac-specific transgenic expression of the dominant negative TRα transgene significantly increases the fetal contractile protein levels (Myh7 and Tnni1) and decreases the levels of the adult forms (Myh6 and Tnni3) [13]. Recent genomic interrogation identifies that TR recruitment is essential for activating postnatal cardiac enhancers defined by p300 occupation in mouse hearts [150].
Coordinate control of cardiac mitochondrial and structural maturation: The role of ERRα/γ and its transcriptional coactivator PGC-1
A key question relates to the mechanism whereby maturation of the ATP-producing machinery is matched with energy-utilizing processes such as contractile function and ion transport. In other words, how is maturation of the mitochondrial and structural components of the heart coordinately regulated? A clue came with the delineation of the key role of the transcriptional coactivator PGC-1 in the early postnatal CM mitochondrial biogenic response. Accordingly, one or several downstream effectors in the PGC-1 regulatory circuitry seemed likely to play a central role in the coordinate energy metabolic and structural/functional maturation of the postnatal heart. Particular focus was given to the ERR family of nuclear receptors given that they regulate a wide array of targets downstream of PGC-1 (Figure 2). Generalized ERRα-deficient mice are viable with normal cardiac structure and function at baseline [151]. However, upon short-term pressure overload stress, they undergo rapid pathological cardiac remodeling and develop HF [151]. Mice with global ERRγ deficiency die soon after birth of HF and multi-system failure including brain, pancreas, skeletal muscle, and kidney [152–156]. Taken together, these results with generalized KO models suggest that ERR signaling is essential for the development and maintenance of heart and cardiac mitochondrial metabolism and function.
Studies of cardiac-specific ERRα and ERRγ knockouts provided evidence for the critical function played by these orphan nuclear receptors in cardiac maturation. A series of cardiac-specific ERR loss-of-function mice were generated, and the results of these studies illuminated the partially redundant roles of ERRα and ERRγ in heart, as well as the importance of the cooperative function of the two nuclear receptors in postnatal energetic, structural, and contractile cardiac maturation. Whereas cardiac-specific targeting of either ERRα or ERRγ alone does not result in a phenotype at baseline, prenatal ERRα/γ KO achieved with an Nkx2–5-Cre recombinase driver results in a cardiomyopathy with non-compacted ventricles and death right after birth [9, 157]. In order to probe the role of ERRα/γ signaling in cardiac maturation following birth, temporally-controlled postnatal ERRα/γ knockdown (KD) in mouse heart was achieved by injection of AAV9 expressing Cre in a cardiac selective manner into mice with LoxP sites integrated into both the Esrra and Esrrg loci [9]. Postnatal cardiac targeting of ERRα and ERRγ results in a multitude of cardiac immaturity signatures including abnormal mitochondrial morphology, and a broad gene expression pattern indicative of a block in postnatal maturation including reduced expression of a wide array of genes involved in mitochondrial energy metabolism including FAO, adult-type sarcomere gene isoform expression, and increased fetal-type sarcomere gene expression [9, 158]. Specifically, postnatal loss of ERRα/γ leads to decreased expression of Tnni3, Myh6, and Myl2 in addition to a global reduction in genes involved in energy transduction, and increased expression of fetal isoform sarcomeric genes including Tnni1, Myl7, and Myh7 [9, 158]. Whole genome ChIP-seq studies with hiPSC-CMs confirmed that ERRγ directly occupies promoter and enhancer regions of both energy metabolic and adult cardiac-specific sarcomeric and ion channel genes confirming that this broad coordinate regulation is mediated by ERRs. Additionally, ERRs were shown to functionally and physically cooperate with GATA4 in the control of adult-isoform contractile genes such as TNNI3 and MYH7 expression levels in hiPSC-CMs [159].
The cardiac actions of ERRβ, another member of the ERR family, were also investigated using mouse loss-of-function approaches. Cardiac-specific ERRβ knockout mice develop dilated cardiomyopathy with aging [160]. Notably, ERRβ deficiency in mouse hearts at 6 months of age leads to minimal changes in many known ERR target genes, particularly those related to OXPHOS [160]. Interestingly, the perinatal hearts of ERRα/γ knockout mice at E17.5 exhibit a significant induction of Esrrb, the gene coding for ERRβ [9]. However, this induction does not rescue the phenotypes resulting from the cardiac loss of ERRα/γ, indicating distinct functions of ERRβ compared to ERRα and ERRγ in the CM [9].
Further genomic interrogation demonstrated that ERRγ occupies many cardiac promoters and enhancer regions defined by H3K27ac deposition in hiPSC-CMs [159]. Depletion of ERRγ via CRISPR/Cas9 genome editing results in reduced H3K27ac deposition around many genes coding mitochondrial metabolism as well as cardiac structural components, suggesting that ERRγ occupation is required to maintain cardiac enhancer activity [159]. Moreover, more than half of cardiac super-enhancer regions (essential for cardiac-specific gene expression) defined by MED1 deposition contain ERRγ binding regions [159, 161]. In the cardiac super-enhancer regions, ERRγ co-occupies and directly cooperates with the cardiac pioneering factor GATA4 (and likely other cardiogenic transcription factors) in enhancers controlling maturation of primarily cardiac-specific genes such as MYH6/7 and NPPA/NPPB clusters [124, 159, 161, 162]. Notably, the functional cooperation of ERRγ and GATA4 is mostly found on genes involved in cardiac-specific functions such as the sarcomere, ion transport, and natriuretic peptides. In contrast, regulatory elements controlling expression of canonical target genes involved in mitochondrial energy metabolism are typically regulated by ERR independent of cardiogenic transcription factors such as GATA4 (Figure 4) [159]. PGC-1α serves as a coactivator for both mechanisms [159]. Taken together, the ERR loss-of-function and genomic interrogation studies indicate that PGC-1/ERR serve to coordinately regulate energy metabolism and contractile function during cardiac maturation via both GATA4-dependent and -independent mechanisms (Figure 4).
Figure 4. Coordinate transcriptional control of energy metabolic and contractile maturation by the ERR/PGC-1α complex.

ERR has emerged as a significant regulator of cardiac gene transcription in the adult heart. ERRα and γ occupy numerous cardiac promoters and enhancer regions in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), characterized by H3K27ac deposition. The functional collaboration between ERR and GATA4 is prominently evident in the regulation of cardiac-specific genes that encode components of the cardiac sarcomere, ion transport proteins, calcium handling proteins, and natriuretic peptides. In contrast, ERRs regulate canonical energy metabolic genes independent of cardiogenic transcription factors. PGC-1 serves as a coactivator of both mechanisms. This mechanism serves to regulate mitochondrial ATP-producing and downstream contractile and ion transport processes in a coordinate manner during cardiomyocyte maturation.
The indispensable role of ERR signaling in driving CM maturation has been further confirmed by independent unbiased genomic interrogation studies. Single-cell RNA-sequencing across distinct developmental time points in murine hearts and stem cell-derived CMs, identified a role of the ERR-PGC-1α axis as a fundamental driver of postnatal cardiac maturation [163, 164]. Additionally, an unbiased enhancer screening using massive parallel reporter assays in mouse heart identified a key role for ERR in adult ventricular myocyte enhancers, suggesting that ERRs may play a role in cardiac ventricular myocyte differentiation [158]. Lastly, the ERRγ agonist T112 was identified as a key CM gene regulator by unbiased screening employing hiPSC-CMs expressing dual fluorescence reporters under endogenous TNNI3 and TNNI1 promoters [138]. T112 activates mitochondrial oxidative metabolism in an ERRγ-dependent manner in hiPSC-CMs and induces the T-tubule formation [138].
Upstream signals regulating cardiomyocyte maturation.
While the precise interconnections between well-established upstream regulators and the transcriptional control of cardiac maturation remain poorly understood, several noteworthy findings have emerged. Notably, PGC-1α expression levels are induced by physiological stimuli such as cold exposure and exercise [50, 165–168]. These stimuli activate a cascade of signaling events including β-adrenergic signaling-mediated cAMP/CREB, p38MAPK-ATF2, and AMP-activated kinase (AMPK) signaling pathways in brown adipose, skeletal muscle, and heart [165, 167, 169–171] (Figure 2). Additionally, PGC-1α activity is modulated by post-translational modifications such as acetylation and phosphorylation (Figure 2) [172–178]. The histone methyltransferase SET and MYND domain containing 1 (Smyd1) has also been identified as a regulator of PGC-1α expression by modifying H3K4me3 active marks around the PGC-1α promoter in mouse hearts [179, 180].
ERR expression levels are induced during the postnatal cardiac maturation period and the hiPSC-CM differentiation process [9, 56, 72, 159, 164]. Upstream factors governing ERRγ expression have been elucidated in several non-cardiac tissues (e.g. liver) triggered by inflammation, fasting/feeding, hypoxia, and ER stress [181]. It remains uncertain whether these regulatory cascades are active and operative to drive ERRγ expression in the CM. The RNA binding protein muscleblind-like 1 (Mbnl1) has recently been shown to serve an upstream activator of ERR expression through stabilizing its mRNA in CMs [10].
Signals originating from the cellular microenvironment have also garnered significant attention relevant to CM maturation. Among these, nutrients that influence CM substrate metabolism play a role. As described above, FAs can serve as ligands, activating the PPAR to substantially activate FAO capacity and CM maturation [41–43]. Ketone bodies, another substrate utilized in adult hearts, have been shown to influence metabolic maturation. Deficiency of 3-hydroxymethylglutaryl-CoA synthase 2 (Hmgcs2) results in a reduction of β-hydroxybutyrate content in the mouse heart, resulting in impaired postnatal metabolic maturation and diminished FAO capacity [31, 182]. Interestingly, overexpression of Hmgcs2 also results in CM de-differentiation and enable CM proliferation [183]. Glucose also exerts an inhibitory effect on CM maturation. Glucose uptake, as monitored by 18F-FDG accumulation, markedly diminishes from embryonic day E10.5 to postnatal day P7 mouse hearts [184], with a concurrent reduction in hexokinase activity, the first and rate-limiting step in glycolysis [185]. High glucose exposure inhibits cardiac maturation in hES-CMs and mouse hearts by activating biosynthesis pathways such as the pentose phosphate pathway [184]. Inhibition of lactate dehydrogenase A (LDHA) converting pyruvate to lactate with a small compound (GSK 2837808A) along with HIF1α inhibition has also been shown to enhance hiPSC-CM maturation [185].
Signaling molecules and physiologic biomechanical cues from surrounding CMs and non-CMs such as cardiac fibroblasts and neurons likely also contribute to CM maturation [186, 187]. hiPSC-CM maturation occurs by in vivo transplantation [130, 188], although the precise mechanisms and connection to the known transcriptional cascades related to CM maturation are unknown. Although the techniques, materials, and cell types of non-CMs to generate a three-dimensional culture are varied, the field of stem cell-derived CMs recognizes the special culture conditions such as EHT and patterned culture substrate profoundly affect the CM maturation process [91, 137, 189–191]. Fetal-to-adult gene isoform switching in hiPSC-CMs occurs during the development of EHT such as MYH6 to 7 transition and MYL2 and TNNI3 inductions [137, 192]. The ratio of MYH6/7 in EHT approaches the ratio in the fetal CMs after a three-week culture period, but it is still far from the reported ratio in adult myocardium [192]. The combination of EHT and consistent electrical stimulation allows the MYH6 to MYH7 transcriptional transition more pronounced and makes it closer to the ratio of the human adult ventricles [137]. hiPSC-CM mitochondrial proteomic analysis comparing EHT with two-dimensional culture conditions highlight the noteworthy induction of mitochondrial proteins associated with OXPHOS, TCA cycle, and FAO coupled with significant mitochondrial biogenesis in the more mature EHT condition [193]. The induction of PGC-1α and ERRα expression represents one potential mechanism underlying the greater structural and metabolic maturation observed with EHT [137, 193]. However, the upstream signaling pathways and associated paracrine factors involved in the maturation associated with the EHT condition remain incompletely understood but mechanical stress would appear to be important [6]. Even though complete maturation does not occur with EHT, the current EHT techniques allow for functional assessment and recapitulate the disease-related phenotypes with hiPSC-CMs harboring disease-causing mutations such MYBPC3 truncation variants [194, 195] and other mutations of sarcomere genes and ion channels [196].
Future directions for translational cardiac maturation research
Re-activating the maturation process in the failing heart.
A loss of cardiac maturation signatures are well-described in the hypertrophied and failing heart [197–200]. The expression of genes involved in energy metabolism is reduced and a subset of adult sarcomeric genes switch to a fetal format as discussed above. The corresponding energetic and contractile consequences of the fetal shift are likely to contribute to the development and severity of HF. It is, therefore, tempting to speculate that strategies to re-activate energy metabolic and contractile maturation could serve as a therapeutic strategy for HF although this fundamental premise needs to be rigorously evaluated. Previous studies using mouse transgenic overexpression strategies for PGC-1α or its downstream effectors such as ERRγ or PPARα have led to a cardiac dysfunction due to exuberant activation of downstream pathways [39, 57, 201, 202]. However, recent studies focused on the transcriptional co-repressor RIP140 targeting PPAR and ERR [203] have provided early evidence that “re-activation of the adult gene program” can be protective for HF. This strategy involves removing an endogenous “brake” on ERR signaling to allow activation of the target transcription factor (e.g. ERR) activity within the physiological range as opposed to the massive overexpression that occurs with transgenic overexpression of the target nuclear receptors. Striated muscle- and cardiac-specific RIP140 knockouts are relatively resistant to the development of cardiac hypertrophy and HF caused by chronic pressure overload [204]. Notably, gene expression profiling demonstrated that metabolic and contractile signatures of cardiac maturation are significantly less downregulated in the context of the HF stimulus in the RIP140-deficient hearts. In addition, myocardial palmitate utilization and triacylglyceride turnover is enhanced in the RIP140 knockout hearts at baseline in the context of chronic pressure overload. These results suggest that future studies aimed at targeting the upstream regulatory circuitry involved in postnatal cardiac maturation as a strategy to prevent or treat HF are warranted.
Targeting ligands for the nuclear receptors involved in cardiac maturation should also be considered as candidate therapeutics. PPARα agonists have been shown to exert triglyceride-lowering effects largely by acting upon the liver, but direct effects in human heart are poorly understood [205–210]. An early-stage study assessing thyroid hormone supplementation for heart failure has been launched (ClinicalTrials.gov Identifier: NCT04112316). Lastly, small molecule activators of ERRs have now been developed and show promise for heart failure in preclinical studies [211].
Maturation of stem cell-derived CMs.
The promise of hiPSC-CMs studies for “disease in dish” platforms to probe pathogenesis of genetic forms of heart disease and to screen for new therapeutics is clear [21, 22]. The experimental systems continue to be hampered by incomplete CM maturation [6], but some significant progress has been made to improve their immaturity phenotypes. For instance, the combination treatment of ERRγ agonist T112 and mechanical load with EHT further improves the hiPSC-CM maturation status and helps replicate the disease phenotypes caused by MYH7 or MYBPC3 mutation in the EHT platform [212]. In addition, advances in stem-cell derived CM replacement have been challenged by incomplete maturation of the transferred cells causing untoward effects such as cardiac arrhythmias [213]. One of the recent key findings is that knocking out depolarization-associated genes HCN4, CACNA1H, and SLC8A1, along with forced expression of the hyperpolarization-associated gene KCNJ2 establishes quiescent but excitable hESC-CMs, that reduce automaticity [130]. The transplantation of the gene-edited “matured” CMs successfully prevents ventricular rhythm disturbances in pig hearts [130]. Accordingly, future advanced approaches targeting the circuitry driving CM maturation could prove useful in human cardiac regeneration. The ERRγ T112 agonist and the recent identification of new ERR modulators may be useful along with other “maturation-driving approaches” including the media cocktails described above [138, 211, 212].
Reversing cardiac maturation to enhance cardiac regeneration.
Immature mouse CMs, such as fetal and postnatal CMs (within 7 days), have a significant proliferative capacity, allowing for restoration of resected myocardium in day 1 postnatal hearts [8]. During the postnatal maturation process, CMs exit the cell cycle. Evidence is emerging that prevention or reversal of CM maturation enables re-entry into the cell cycle. Therefore, in addition to activating the maturation program, under certain circumstances inhibiting cardiac maturation signaling may prove to be an attractive pathway to revoke cardiac regeneration capacity. In this case, a transient period of unlocking maturation to promote CM regeneration could prove useful for cardiac diseases in which there is significant myocyte loss such as ischemic injury. The proliferation activity of CMs are regulated by the common cell cycle regulators such as cyclins (CCNs) and cyclin-dependent kinases (CDKs) as evidenced by studies involving introduction of these cell cycle regulators or manipulating their upstream pathway such as Hippo-YAP signaling pathway in adult CMs to activate proliferative activity and restore cardiac function following cardiac injuries [214–219]. Several lines of evidence support the notion that reversal of the upstream regulator of the CM maturation process, particularly the energy metabolic component, provides a permissive environment for driving CM proliferation and regeneration. First, pharmacological, or genetic inhibition of the β-adrenergic signaling pathway, upstream signaling for PGC-1α, and mitochondrial oxidative metabolism, induces CM proliferation in parallel with downregulated mitochondrial oxidative metabolism in mouse hearts [11, 17, 18]. Ablation of β-adrenergic receptor signaling contributes to the YAP activation through the RhoA activation [11, 18]. Second, dominant negative TRα transgenic expression driven by αMHC promoter results in decreased mitochondrial oxidative metabolism and significantly increased CM proliferation in adult mouse hearts [13]. TRα mutant mice have improved cardiac function and less fibrosis after cardiac injury [13]. The combined inhibition of both TR signaling and β-adrenergic signaling pathways cooperatively increases CM proliferation and regeneration capacity after cardiac injury [17]. Third, loss of Ppargc1a coding PGC-1α has been shown to increase CM proliferation in zebrafish hearts following cardiac injury [15]. Lastly, deletion of Mbnl1, which has recently been shown to stabilize all ERR transcripts, increases CM proliferation in neonatal hearts and rescues the insulted hearts [10].
Re-activation of the fetal pattern of CM fuel metabolism also promotes CM proliferation. CM proliferative activity is reactivated in adult mouse hearts by re-activating glycolysis or inhibiting mitochondrial FAO and TCA cycle either genetically or pharmacologically [12, 14–16, 19, 20, 220]. For example, pyruvate dehydrogenase kinase 4 (Pdk4), a prominent target of PPAR and ERR, is responsible for switching cardiac substrate utilization to FAO from glucose oxidation [221, 222]. Cardiac-specific inducible knockout of Pdk4 resulted in increased CM proliferative activity, improved left ventricular function, and decreased cardiac remodeling following myocardial infarction [14]. Recently, inhibition of FAO by pharmacologic or genetic inactivation of carnitine palmitoyltransferase b (Cpt1b) was shown to result in a dramatic increase in CM proliferation in vitro and in vivo in mice [12, 20]. Lastly, it should be noted that activation of CM proliferation with forced expression of cell cycle regulators including CCNB1, CCND1, CDK1, and CDK4 significantly increases CM proliferation and reprograms cellular metabolism to drive glycolysis, hexosamine, phospholipid, and serine biosynthetic pathways which support their proliferative activity [214, 223]. Taken together, modulation of the CM maturation program, particularly metabolic pathways, show promise for unlocking the exit of adult CMs from the cell cycle, a “holy grail” for achieving cardiac regeneration.
Put in context, both activation and inhibition of cardiac maturation emerge as promising research avenues for therapeutics aimed at heart disease. However, temporal considerations are important. For example, as noted above, cardiac YAP activation may enable CM regeneration following injury [216, 218, 219]. However, persistent activation of CM YAP signaling via cardiac-specific deletion of WW45, results in detrimental effects in mouse hearts under pressure overload, despite an increase in CM cycle re-entry [224]. Hence, transient activation of regenerative capacity in the context of CM loss from insults such as acute myocardial infarction may be required. In stages of chronic heart failure following such injury, the strategy of long-term re-activation of CM maturation pathways may prove useful. Future studies aimed at assessing the efficacy and timing of these new therapeutic avenues should prove interesting and fruitful.
ACKNOWLEDGEMENTS
Special thanks to Teresa Leone for an expert review, proofing, and formatting of the manuscript. Work described in this review was supported by NIH R01 HL058493 (D.P.K.), R01 HL151345 (D.P.K.), R01 HL128349 (D.P.K.) and a postdoctoral fellowship from the American Heart Association #14POST20490309 (T.S.). All figures were created with BioRender.com. The illustration in Figure 4 was adapted from “Genomic Architecture” by BioRender.com. The original template was retrieved from https://app.biorender.com/biorender-templates/figures/all/t-6254ceb3fc98fe4c9f4ddf93-genomic-architecture-layout.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest.
DECLARATION OF GENERATIVE AI AND AI-ASSISTED TECHNOLOGIES IN THE WRITING PROCESS
D.P.K. wrote the first draft of this manuscript. T.S edited the manuscript and used ChatGPT 3.5 to proof English grammar and word usage. After using this tool/service, T.S. and D.P.K. reviewed and edited the final text and take full responsibility for the content of the publication.
REFERENCES
- [1].Galdos FX, Guo Y, Paige SL, VanDusen NJ, Wu SM, Pu WT, Cardiac Regeneration: Lessons From Development, Circ Res 120(6) (2017) 941–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Van Vliet P, Wu SM, Zaffran S, Puceat M, Early cardiac development: a view from stem cells to embryos, Cardiovasc Res 96(3) (2012) 352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Paige SL, Plonowska K, Xu A, Wu SM, Molecular regulation of cardiomyocyte differentiation, Circ Res 116(2) (2015) 341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guo Y, Pu WT, Cardiomyocyte Maturation: New Phase in Development, Circ Res 126(8) (2020) 1086–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kannan S, Kwon C, Regulation of cardiomyocyte maturation during critical perinatal window, J Physiol 598(14) (2020) 2941–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X, Murry CE, Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine, Nat Rev Cardiol 17(6) (2020) 341–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maroli G, Braun T, The long and winding road of cardiomyocyte maturation, Cardiovasc Res 117(3) (2021) 712–726. [DOI] [PubMed] [Google Scholar]
- [8].Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA, Transient regenerative potential of the neonatal mouse heart, Science 331(6020) (2011) 1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sakamoto T, Matsuura TR, Wan S, Ryba DM, Kim JU, Won KJ, et al. , A Critical Role for Estrogen-Related Receptor Signaling in Cardiac Maturation, Circ Res 126(12) (2020) 1685–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bailey LRJ, Bugg D, Reichardt IM, Ortac CD, Gunaje J, Johnson R, et al. , MBNL1 regulates programmed postnatal switching between regenerative and differentiated cardiac states, bioRxiv (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu H, Zhang CH, Ammanamanchi N, Suresh S, Lewarchik C, Rao K, et al. , Control of cytokinesis by beta-adrenergic receptors indicates an approach for regulating cardiomyocyte endowment, Sci Transl Med 11(513) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cao T, Liccardo D, LaCanna R, Zhang X, Lu R, Finck BN, et al. , Fatty Acid Oxidation Promotes Cardiomyocyte Proliferation Rate but Does Not Change Cardiomyocyte Number in Infant Mice, Front Cell Dev Biol 7 (2019) 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, et al. , Evidence for hormonal control of heart regenerative capacity during endothermy acquisition, Science 364(6436) (2019) 184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cardoso AC, Lam NT, Savla JJ, Nakada Y, Pereira AHM, Elnwasany A, et al. , Mitochondrial Substrate Utilization Regulates Cardiomyocyte Cell Cycle Progression, Nat Metab 2(2) (2020) 167–178. [PMC free article] [PubMed] [Google Scholar]
- [15].Fukuda R, Marin-Juez R, El-Sammak H, Beisaw A, Ramadass R, Kuenne C, et al. , Stimulation of glycolysis promotes cardiomyocyte proliferation after injury in adult zebrafish, EMBO Rep 21(8) (2020) e49752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Magadum A, Singh N, Kurian AA, Munir I, Mehmood T, Brown K, et al. , Pkm2 Regulates Cardiomyocyte Cell Cycle and Promotes Cardiac Regeneration, Circulation 141(15) (2020) 1249–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Payumo AY, Chen X, Hirose K, Chen X, Hoang A, Khyeam S, et al. , Adrenergic-Thyroid Hormone Interactions Drive Postnatal Thermogenesis and Loss of Mammalian Heart Regenerative Capacity, Circulation 144(12) (2021) 1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sakabe M, Thompson M, Chen N, Verba M, Hassan A, Lu R, Xin M, Inhibition of beta1-AR/Galphas signaling promotes cardiomyocyte proliferation in juvenile mice through activation of RhoA-YAP axis, Elife 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li Y, Yang M, Tan J, Shen C, Deng S, Fu X, et al. , Targeting ACSL1 promotes cardiomyocyte proliferation and cardiac regeneration, Life Sci 294 (2022) 120371. [DOI] [PubMed] [Google Scholar]
- [20].Li X, Wu F, Gunther S, Looso M, Kuenne C, Zhang T, et al. , Inhibition of fatty acid oxidation enables heart regeneration in adult mice, Nature (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paik DT, Chandy M, Wu JC, Patient and Disease-Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics, Pharmacol Rev 72(1) (2020) 320–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sayed N, Liu C, Wu JC, Translation of Human-Induced Pluripotent Stem Cells: From Clinical Trial in a Dish to Precision Medicine, J Am Coll Cardiol 67(18) (2016) 2161–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, et al. , Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart, Genes Dev 22(14) (2008) 1948–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dorn GW 2nd, Vega RB, Kelly DP, Mitochondrial biogenesis and dynamics in the developing and diseased heart, Genes Dev 29(19) (2015) 1981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vega RB, Kelly DP, Cardiac nuclear receptors: architects of mitochondrial structure and function, J Clin Invest 127(4) (2017) 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kelly DP, Scarpulla RC, Transcriptional regulatory circuits controlling mitochondrial biogenesis and function, Genes Dev 18(4) (2004) 357–68. [DOI] [PubMed] [Google Scholar]
- [27].Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, Dorn GW 2nd, Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice, Science 350(6265) (2015) aad2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stanley WC, Recchia FA, Lopaschuk GD, Myocardial substrate metabolism in the normal and failing heart, Physiol Rev 85(3) (2005) 1093–129. [DOI] [PubMed] [Google Scholar]
- [29].Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED, Cardiac Energy Metabolism in Heart Failure, Circ Res 128(10) (2021) 1487–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. , Comprehensive quantification of fuel use by the failing and nonfailing human heart, Science 370(6514) (2020) 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Matsuura TR, Puchalska P, Crawford PA, Kelly DP, Ketones and the Heart: Metabolic Principles and Therapeutic Implications, Circ Res 132(7) (2023) 882–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Madrazo JA, Kelly DP, The PPAR trio: regulators of myocardial energy metabolism in health and disease, J Mol Cell Cardiol 44(6) (2008) 968–975. [DOI] [PubMed] [Google Scholar]
- [33].Huss JM, Kelly DP, Nuclear receptor signaling and cardiac energetics, Circ Res 95(6) (2004) 568–78. [DOI] [PubMed] [Google Scholar]
- [34].Barish GD, Narkar VA, Evans RM, PPAR delta: a dagger in the heart of the metabolic syndrome, J Clin Invest 116(3) (2006) 590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fillmore N, Hou V, Sun J, Springer D, Murphy E, Cardiac specific knock-down of peroxisome proliferator activated receptor alpha prevents fasting-induced cardiac lipid accumulation and reduces perilipin 2, PLoS One 17(3) (2022) e0265007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang X, Zhu XX, Jiao SY, Qi D, Yu BQ, Xie GM, et al. , Cardiomyocyte peroxisome proliferator-activated receptor alpha is essential for energy metabolism and extracellular matrix homeostasis during pressure overload-induced cardiac remodeling, Acta Pharmacol Sin 43(5) (2022) 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang P, Liu J, Li Y, Wu S, Luo J, Yang H, et al. , Peroxisome proliferator-activated receptor delta is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart, Circ Res 106(5) (2010) 911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, et al. , Cardiomyocyte-restricted peroxisome proliferator-activated receptor-δ deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy, Nature Medicine 10(11) (2004) 1245–1250. [DOI] [PubMed] [Google Scholar]
- [39].Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, et al. , The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus, J Clin Invest 109(1) (2002) 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, et al. , Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart, J Clin Invest 117(12) (2007) 3930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. , Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma, Proc Natl Acad Sci U S A 94(9) (1997) 4318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Forman BM, Chen J, Evans RM, Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta, Proc Natl Acad Sci U S A 94(9) (1997) 4312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vega RB, Huss JM, Kelly DP, The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes, Mol Cell Biol 20(5) (2000) 1868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF, Identification of a physiologically relevant endogenous ligand for PPARalpha in liver, Cell 138(3) (2009) 476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Goto T, Nagai H, Egawa K, Kim YI, Kato S, Taimatsu A, et al. , Farnesyl pyrophosphate regulates adipocyte functions as an endogenous PPARgamma agonist, Biochem J 438(1) (2011) 111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yeh YS, Jheng HF, Iwase M, Kim M, Mohri S, Kwon J, et al. , The Mevalonate Pathway Is Indispensable for Adipocyte Survival, iScience 9 (2018) 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kwon J, Yeh YS, Kawarasaki S, Minamino H, Fujita Y, Okamatsu-Ogura Y, et al. , Mevalonate biosynthesis pathway regulates the development and survival of brown adipocytes, iScience 26(3) (2023) 106161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, et al. , ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1, Nat Med 17(9) (2011) 1076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Paredes A, Justo-Mendez R, Jimenez-Blasco D, Nunez V, Calero I, Villalba-Orero M, et al. , gamma-Linolenic acid in maternal milk drives cardiac metabolic maturation, Nature 618(7964) (2023) 365–373. [DOI] [PubMed] [Google Scholar]
- [50].Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM, A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis, Cell 92(6) (1998) 829–839. [DOI] [PubMed] [Google Scholar]
- [51].Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM, Peroxisome Proliferator-activated Receptor γ Coactivator 1β (PGC-1β), A Novel PGC-1-related Transcription Coactivator Associated with Host Cell Factor, Journal of Biological Chemistry 277(3) (2002) 1645–1648. [DOI] [PubMed] [Google Scholar]
- [52].Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, Spiegelman BM, Activation of PPARgamma coactivator-1 through transcription factor docking, Science 286(5443) (1999) 1368–71. [DOI] [PubMed] [Google Scholar]
- [53].Wallberg AE, Yamamura S, Malik S, Spiegelman BM, Roeder RG, Coordination of p300-Mediated Chromatin Remodeling and TRAP/Mediator Function through Coactivator PGC-1α, Molecular Cell 12(5) (2003) 1137–1149. [DOI] [PubMed] [Google Scholar]
- [54].Chen W, Yang Q, Roeder RG, Dynamic interactions and cooperative functions of PGC-1alpha and MED1 in TRalpha-mediated activation of the brown-fat-specific UCP-1 gene, Mol Cell 35(6) (2009) 755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rambout X, Cho H, Blanc R, Lyu Q, Miano JM, Chakkalakal JV, et al. , PGC-1alpha senses the CBC of pre-mRNA to dictate the fate of promoter-proximally paused RNAPII, Mol Cell 83(2) (2023) 186–202 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Murphy SA, Miyamoto M, Kervadec A, Kannan S, Tampakakis E, Kambhampati S, et al. , PGC1/PPAR drive cardiomyocyte maturation at single cell level via YAP1 and SF3B2, Nat Commun 12(1) (2021) 1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP, Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis, J Clin Invest 106(7) (2000) 847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. , Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1, Cell 98(1) (1999) 115–24. [DOI] [PubMed] [Google Scholar]
- [59].Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A, The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha), J Biol Chem 278(11) (2003) 9013–8. [DOI] [PubMed] [Google Scholar]
- [60].Huss JM, Kopp RP, Kelly DP, Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha, J Biol Chem 277(43) (2002) 40265–74. [DOI] [PubMed] [Google Scholar]
- [61].Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, et al. , PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity, Proc Natl Acad Sci U S A 100(21) (2003) 12378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Giguere V, Transcriptional control of energy homeostasis by the estrogen-related receptors, Endocr Rev 29(6) (2008) 677–96. [DOI] [PubMed] [Google Scholar]
- [63].Giguere V, Yang N, Segui P, Evans RM, Identification of a new class of steroid hormone receptors, Nature 331(6151) (1988) 91–4. [DOI] [PubMed] [Google Scholar]
- [64].Hong H, Yang L, Stallcup MR, Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3, J Biol Chem 274(32) (1999) 22618–26. [DOI] [PubMed] [Google Scholar]
- [65].Heard DJ, Norby PL, Holloway J, Vissing H, Human ERRgamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult, Mol Endocrinol 14(3) (2000) 382–92. [DOI] [PubMed] [Google Scholar]
- [66].Eudy JD, Yao S, Weston MD, Ma-Edmonds M, Talmadge CB, Cheng JJ, et al. , Isolation of a gene encoding a novel member of the nuclear receptor superfamily from the critical region of Usher syndrome type IIa at 1q41, Genomics 50(3) (1998) 382–4. [DOI] [PubMed] [Google Scholar]
- [67].Goldenthal MJ, Weiss HR, Marin-Garcia J, Bioenergetic remodeling of heart mitochondria by thyroid hormone, Mol Cell Biochem 265(1–2) (2004) 97–106. [DOI] [PubMed] [Google Scholar]
- [68].Goldenthal MJ, Ananthakrishnan R, Marin-Garcia J, Nuclear-mitochondrial cross-talk in cardiomyocyte T3 signaling: a time-course analysis, J Mol Cell Cardiol 39(2) (2005) 319–26. [DOI] [PubMed] [Google Scholar]
- [69].Portman MA, Thyroid hormone regulation of heart metabolism, Thyroid 18(2) (2008) 217–25. [DOI] [PubMed] [Google Scholar]
- [70].Singh BK, Sinha RA, Tripathi M, Mendoza A, Ohba K, Sy JAC, et al. , Thyroid hormone receptor and ERRalpha coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function, Sci Signal 11(536) (2018). [DOI] [PubMed] [Google Scholar]
- [71].Cho Y, Hazen BC, Russell AP, Kralli A, Peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1)- and estrogen-related receptor (ERR)-induced regulator in muscle 1 (Perm1) is a tissue-specific regulator of oxidative capacity in skeletal muscle cells, J Biol Chem 288(35) (2013) 25207–25218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cho Y, Tachibana S, Lam K, Arita Y, Khosrowjerdi S, Zhang O, et al. , Perm1 promotes cardiomyocyte mitochondrial biogenesis and protects against hypoxia/reoxygenation-induced damage in mice, J Biol Chem 297(1) (2021) 100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Aravamudhan S, Turk C, Bock T, Keufgens L, Nolte H, Lang F, et al. , Phosphoproteomics of the developing heart identifies PERM1 - An outer mitochondrial membrane protein, J Mol Cell Cardiol 154 (2021) 41–59. [DOI] [PubMed] [Google Scholar]
- [74].Oka SI, Sabry AD, Horiuchi AK, Cawley KM, O’Very SA, Zaitsev MA, et al. , Perm1 regulates cardiac energetics as a downstream target of the histone methyltransferase Smyd1, PLoS One 15(6) (2020) e0234913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Cho Y, Tachibana S, Hazen BC, Moresco JJ, Yates JR 3rd, Kok B, et al. , Perm1 regulates CaMKII activation and shapes skeletal muscle responses to endurance exercise training, Mol Metab 23 (2019) 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cho Y, Hazen BC, Gandra PG, Ward SR, Schenk S, Russell AP, Kralli A, Perm1 enhances mitochondrial biogenesis, oxidative capacity, and fatigue resistance in adult skeletal muscle, FASEB J 30(2) (2016) 674–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Huang CY, Oka SI, Xu X, Chen CF, Tung CY, Chang YY, et al. , PERM1 regulates genes involved in fatty acid metabolism in the heart by interacting with PPARalpha and PGC-1alpha, Sci Rep 12(1) (2022) 14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Oka SI, Sreedevi K, Shankar TS, Yedla S, Arowa S, James A, et al. , PERM1 regulates energy metabolism in the heart via ERRalpha/PGC-1alpha axis, Front Cardiovasc Med 9 (2022) 1033457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tachibana S, Yu NK, Li R, Fernandez-Costa C, Liang A, Choi J, et al. , Perm1 Protects the Heart From Pressure Overload-Induced Dysfunction by Promoting Oxidative Metabolism, Circulation 147(11) (2023) 916–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bock T, Turk C, Aravamudhan S, Keufgens L, Bloch W, Rozsivalova DH, et al. , PERM1 interacts with the MICOS-MIB complex to connect the mitochondria and sarcolemma via ankyrin B, Nat Commun 12(1) (2021) 4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Liao X, Zhang R, Lu Y, Prosdocimo DA, Sangwung P, Zhang L, et al. , Kruppel-like factor 4 is critical for transcriptional control of cardiac mitochondrial homeostasis, J Clin Invest 125(9) (2015) 3461–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang C, Qiao S, Zhao Y, Tian H, Yan W, Hou X, et al. , The KLF7/PFKL/ACADL axis modulates cardiac metabolic remodelling during cardiac hypertrophy in male mice, Nat Commun 14(1) (2023) 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Prosdocimo DA, Anand P, Liao X, Zhu H, Shelkay S, Artero-Calderon P, et al. , Kruppel-like factor 15 is a critical regulator of cardiac lipid metabolism, J Biol Chem 289(9) (2014) 5914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Prosdocimo DA, John JE, Zhang L, Efraim ES, Zhang R, Liao X, Jain MK, KLF15 and PPARalpha Cooperate to Regulate Cardiomyocyte Lipid Gene Expression and Oxidation, PPAR Res 2015 (2015) 201625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Liu R, Lee J, Kim BS, Wang Q, Buxton SK, Balasubramanyam N, et al. , Tead1 is required for maintaining adult cardiomyocyte function, and its loss results in lethal dilated cardiomyopathy, JCI Insight 2(17) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Liu R, Jagannathan R, Sun L, Li F, Yang P, Lee J, et al. , Tead1 is essential for mitochondrial function in cardiomyocytes, Am J Physiol Heart Circ Physiol 319(1) (2020) H89–H99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Liu J, Wen T, Dong K, He X, Zhou H, Shen J, et al. , TEAD1 protects against necroptosis in postmitotic cardiomyocytes through regulation of nuclear DNA-encoded mitochondrial genes, Cell Death Differ 28(7) (2021) 2045–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Akerberg BN, Gu F, VanDusen NJ, Zhang X, Dong R, Li K, et al. , A reference map of murine cardiac transcription factor chromatin occupancy identifies dynamic and conserved enhancers, Nat Commun 10(1) (2019) 4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Funakoshi S, Fernandes I, Mastikhina O, Wilkinson D, Tran T, Dhahri W, et al. , Generation of mature compact ventricular cardiomyocytes from human pluripotent stem cells, Nat Commun 12(1) (2021) 3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yang X, Rodriguez ML, Leonard A, Sun L, Fischer KA, Wang Y, et al. , Fatty Acids Enhance the Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells, Stem Cell Reports 13(4) (2019) 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Knight WE, Cao Y, Lin YH, Chi C, Bai B, Sparagna GC, et al. , Maturation of Pluripotent Stem Cell-Derived Cardiomyocytes Enables Modeling of Human Hypertrophic Cardiomyopathy, Stem Cell Reports 16(3) (2021) 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Feyen DAM, McKeithan WL, Bruyneel AAN, Spiering S, Hormann L, Ulmer B, et al. , Metabolic Maturation Media Improve Physiological Function of Human iPSC-Derived Cardiomyocytes, Cell Rep 32(3) (2020) 107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Yang X, Rodriguez M, Pabon L, Fischer KA, Reinecke H, Regnier M, et al. , Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells, Journal of Molecular and Cellular Cardiology 72 (2014) 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K, et al. , Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes, Circ Res 121(12) (2017) 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wang L, Wada Y, Ballan N, Schmeckpeper J, Huang J, Rau CD, et al. , Triiodothyronine and dexamethasone alter potassium channel expression and promote electrophysiological maturation of human-induced pluripotent stem cell-derived cardiomyocytes, J Mol Cell Cardiol 161 (2021) 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wickramasinghe NM, Sachs D, Shewale B, Gonzalez DM, Dhanan-Krishnan P, Torre D, et al. , PPARdelta activation induces metabolic and contractile maturation of human pluripotent stem cell-derived cardiomyocytes, Cell Stem Cell 29(4) (2022) 559–576 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lompre AM, Nadal-Ginard B, Mahdavi V, Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated, J Biol Chem 259(10) (1984) 6437–46. [PubMed] [Google Scholar]
- [98].Dorn GW 2nd, Robbins J, Ball N, Walsh RA, Myosin heavy chain regulation and myocyte contractile depression after LV hypertrophy in aortic-banded mice, Am J Physiol 267(1 Pt 2) (1994) H400–5. [DOI] [PubMed] [Google Scholar]
- [99].Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA, Myosin heavy chain gene expression in human heart failure., Journal of Clinical Investigation 100(9) (1997) 2362–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Miyata S, Minobe W, Bristow MR, Leinwand LA, Myosin Heavy Chain Isoform Expression in the Failing and Nonfailing Human Heart, Circulation Research 86(4) (2000) 386–390. [DOI] [PubMed] [Google Scholar]
- [101].Sasse S, Brand NJ, Kyprianou P, Dhoot GK, Wade R, Arai M, et al. , Troponin I gene expression during human cardiac development and in end-stage heart failure, Circ Res 72(5) (1993) 932–8. [DOI] [PubMed] [Google Scholar]
- [102].Bedada Fikru B., Chan Sunny S.-K., Metzger Stefania K., Zhang L, Zhang J, Garry Daniel J., et al. , Acquisition of a Quantitative, Stoichiometrically Conserved Ratiometric Marker of Maturation Status in Stem Cell-Derived Cardiac Myocytes, Stem Cell Reports 3(4) (2014) 594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lahmers S, Wu Y, Call DR, Labeit S, Granzier H, Developmental Control of Titin Isoform Expression and Passive Stiffness in Fetal and Neonatal Myocardium, Circulation Research 94(4) (2004) 505–513. [DOI] [PubMed] [Google Scholar]
- [104].Opitz CA, Leake MC, Makarenko I, Benes V, Linke WA, Developmentally Regulated Switching of Titin Size Alters Myofibrillar Stiffness in the Perinatal Heart, Circulation Research 94(7) (2004) 967–975. [DOI] [PubMed] [Google Scholar]
- [105].Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, et al. , RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing, Nat Med 18(5) (2012) 766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, et al. , Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy, Circulation 110(2) (2004) 155–62. [DOI] [PubMed] [Google Scholar]
- [107].Carrier L, Boheler KR, Chassagne C, De La Bastie D, Wisnewsky C, Lakatta EG, Schwartz K, Expression of the sarcomeric actin isogenes in the rat heart with development and senescence., Circulation Research 70(5) (1992) 999–1005. [DOI] [PubMed] [Google Scholar]
- [108].Boheler KR, Carrier L, De La Bastie D, Allen PD, Komajda M, Mercadier JJ, Schwartz K, Skeletal actin mRNA increases in the human heart during ontogenic development and is the major isoform of control and failing adult hearts., Journal of Clinical Investigation 88(1) (1991) 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Driesen RB, Verheyen FK, Debie W, Blaauw E, Babiker FA, Cornelussen RNM, et al. , Re-expression of alpha skeletal actin as a marker for dedifferentiation in cardiac pathologies, Journal of Cellular and Molecular Medicine 13(5) (2009) 896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ilkovski B, Clement S, Sewry C, North KN, Cooper ST, Defining alpha-skeletal and alpha-cardiac actin expression in human heart and skeletal muscle explains the absence of cardiac involvement in ACTA1 nemaline myopathy, Neuromuscul Disord 15(12) (2005) 829–35. [DOI] [PubMed] [Google Scholar]
- [111].Suurmeijer AJ, Clement S, Francesconi A, Bocchi L, Angelini A, Van Veldhuisen DJ, et al. , Alpha-actin isoform distribution in normal and failing human heart: a morphological, morphometric, and biochemical study, J Pathol 199(3) (2003) 387–97. [DOI] [PubMed] [Google Scholar]
- [112].Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, et al. , Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart, Journal of Clinical Investigation 128(5) (2018) 2127–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Cui M, Atmanli A, Morales MG, Tan W, Chen K, Xiao X, et al. , Nrf1 promotes heart regeneration and repair by regulating proteostasis and redox balance, Nat Commun 12(1) (2021) 5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Woodcock-Mitchell J, Mitchell JJ, Low RB, Kieny M, Sengel P, Rubbia L, et al. , Alpha-smooth muscle actin is transiently expressed in embryonic rat cardiac and skeletal muscles, Differentiation 39(3) (1988) 161–6. [DOI] [PubMed] [Google Scholar]
- [115].Kubalak SW, Miller-Hance WC, O’Brien TX, Dyson E, Chien KR, Chamber specification of atrial myosin light chain-2 expression precedes septation during murine cardiogenesis, J Biol Chem 269(24) (1994) 16961–70. [PubMed] [Google Scholar]
- [116].O’Brien TX, Lee KJ, Chien KR, Positional specification of ventricular myosin light chain 2 expression in the primitive murine heart tube, Proc Natl Acad Sci U S A 90(11) (1993) 5157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Anderson PAW, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, et al. , Molecular Basis of Human Cardiac Troponin T Isoforms Expressed in the Developing, Adult, and Failing Heart, Circulation Research 76(4) (1995) 681–686. [DOI] [PubMed] [Google Scholar]
- [118].Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD, Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle., Circulation Research 69(5) (1991) 1226–1233. [DOI] [PubMed] [Google Scholar]
- [119].Agarkova I, Auerbach D, Ehler E, Perriard JC, A novel marker for vertebrate embryonic heart, the EH-myomesin isoform, J Biol Chem 275(14) (2000) 10256–64. [DOI] [PubMed] [Google Scholar]
- [120].Schoenauer R, Emmert MY, Felley A, Ehler E, Brokopp C, Weber B, et al. , EH-myomesin splice isoform is a novel marker for dilated cardiomyopathy, Basic Res Cardiol 106(2) (2011) 233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ahmed RE, Tokuyama T, Anzai T, Chanthra N, Uosaki H, Sarcomere maturation: function acquisition, molecular mechanism, and interplay with other organelles, Philosophical Transactions of the Royal Society B: Biological Sciences 377(1864) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yang X, Pabon L, Murry CE, Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes, Circ Res 114(3) (2014) 511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Yin Z, Ren J, Guo W, Sarcomeric protein isoform transitions in cardiac muscle: a journey to heart failure, Biochim Biophys Acta 1852(1) (2015) 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gacita AM, Fullenkamp DE, Ohiri J, Pottinger T, Puckelwartz MJ, Nobrega MA, McNally EM, Genetic Variation in Enhancers Modifies Cardiomyopathy Gene Expression and Progression, Circulation 143(13) (2021) 1302–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Janssen PM, Biesiadecki BJ, Ziolo MT, Davis JP, The Need for Speed: Mice, Men, and Myocardial Kinetic Reserve, Circ Res 119(3) (2016) 418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sheikh F, Lyon RC, Chen J, Functions of myosin light chain-2 (MYL2) in cardiac muscle and disease, Gene 569(1) (2015) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sheng JJ, Jin JP, TNNI1, TNNI2 and TNNI3: Evolution, regulation, and protein structure-function relationships, Gene 576(1 Pt 3) (2016) 385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL, The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes, J Physiol 533(Pt 3) (2001) 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Grant AO, Cardiac ion channels, Circ Arrhythm Electrophysiol 2(2) (2009) 185–94. [DOI] [PubMed] [Google Scholar]
- [130].Marchiano S, Nakamura K, Reinecke H, Neidig L, Lai M, Kadota S, et al. , Gene editing to prevent ventricular arrhythmias associated with cardiomyocyte cell therapy, Cell Stem Cell 30(4) (2023) 396–414 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Yasui K, Liu W, Opthof T, Kada K, Lee JK, Kamiya K, Kodama I, I(f) current and spontaneous activity in mouse embryonic ventricular myocytes, Circ Res 88(5) (2001) 536–42. [DOI] [PubMed] [Google Scholar]
- [132].Kuratomi S, Kuratomi A, Kuwahara K, Ishii TM, Nakao K, Saito Y, Takano M, NRSF regulates the developmental and hypertrophic changes of HCN4 transcription in rat cardiac myocytes, Biochem Biophys Res Commun 353(1) (2007) 67–73. [DOI] [PubMed] [Google Scholar]
- [133].Fernandez-Velasco M, Goren N, Benito G, Blanco-Rivero J, Bosca L, Delgado C, Regional distribution of hyperpolarization-activated current (If) and hyperpolarization-activated cyclic nucleotide-gated channel mRNA expression in ventricular cells from control and hypertrophied rat hearts, J Physiol 553(Pt 2) (2003) 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hong T, Shaw RM, Cardiac T-Tubule Microanatomy and Function, Physiol Rev 97(1) (2017) 227–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L, Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes, Am J Physiol Heart Circ Physiol 285(6) (2003) H2355–63. [DOI] [PubMed] [Google Scholar]
- [136].Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, et al. , Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes, Stem Cells Dev 18(10) (2009) 1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, et al. , Advanced maturation of human cardiac tissue grown from pluripotent stem cells, Nature 556(7700) (2018) 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Miki K, Deguchi K, Nakanishi-Koakutsu M, Lucena-Cacace A, Kondo S, Fujiwara Y, et al. , ERRgamma enhances cardiac maturation with T-tubule formation in human iPSC-derived cardiomyocytes, Nat Commun 12(1) (2021) 3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Molkentin JD, Kalvakolanu DV, Markham BE, Transcription factor GATA-4 regulates cardiac muscle-specific expression of the alpha-myosin heavy-chain gene, Mol Cell Biol 14(7) (1994) 4947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Adolph EA, Subramaniam A, Cserjesi P, Olson EN, Robbins J, Role of myocyte-specific enhancer-binding factor (MEF-2) in transcriptional regulation of the alpha-cardiac myosin heavy chain gene, J Biol Chem 268(8) (1993) 5349–52. [PubMed] [Google Scholar]
- [141].Molkentin JD, Markham BE, Myocyte-specific enhancer-binding factor (MEF-2) regulates alpha-cardiac myosin heavy chain gene expression in vitro and in vivo, J Biol Chem 268(26) (1993) 19512–20. [PubMed] [Google Scholar]
- [142].Prendiville TW, Guo H, Lin Z, Zhou P, Stevens SM, He A, et al. , Novel Roles of GATA4/6 in the Postnatal Heart Identified through Temporally Controlled, Cardiomyocyte-Specific Gene Inactivation by Adeno-Associated Virus Delivery of Cre Recombinase, PLoS One 10(5) (2015) e0128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].VanDusen NJ, Lee JY, Gu W, Butler CE, Sethi I, Zheng Y, et al. , Massively parallel in vivo CRISPR screening identifies RNF20/40 as epigenetic regulators of cardiomyocyte maturation, Nat Commun 12(1) (2021) 4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Guo Y, Jardin BD, Zhou P, Sethi I, Akerberg BN, Toepfer CN, et al. , Hierarchical and stage-specific regulation of murine cardiomyocyte maturation by serum response factor, Nat Commun 9(1) (2018) 3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ojamaa K, Klemperer JD, MacGilvray SS, Klein I, Samarel A, Thyroid hormone and hemodynamic regulation of beta-myosin heavy chain promoter in the heart, Endocrinology 137(3) (1996) 802–8. [DOI] [PubMed] [Google Scholar]
- [146].Morkin E, Regulation of myosin heavy chain genes in the heart, Circulation 87(5) (1993) 1451–60. [DOI] [PubMed] [Google Scholar]
- [147].Hadj-Sahraoui N, Seugnet I, Ghorbel MT, Demeneix B, Hypothyroidism prolongs mitotic activity in the post-natal mouse brain, Neurosci Lett 280(2) (2000) 79–82. [DOI] [PubMed] [Google Scholar]
- [148].Kinugawa K, Yonekura K, Ribeiro RC, Eto Y, Aoyagi T, Baxter JD, et al. , Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy, Circ Res 89(7) (2001) 591–8. [DOI] [PubMed] [Google Scholar]
- [149].Lee Y, Nadal-Ginard B, Mahdavi V, Izumo S, Myocyte-specific enhancer factor 2 and thyroid hormone receptor associate and synergistically activate the alpha-cardiac myosin heavy-chain gene, Mol Cell Biol 17(5) (1997) 2745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zhou P, VanDusen NJ, Zhang Y, Cao Y, Sethi I, Hu R, et al. , Dynamic changes in P300 enhancers and enhancer-promoter contacts control mouse cardiomyocyte maturation, Dev Cell 58(10) (2023) 898–914 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Huss JM, Imahashi K-I, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, et al. , The Nuclear Receptor ERRα Is Required for the Bioenergetic and Functional Adaptation to Cardiac Pressure Overload, Cell Metabolism 6(1) (2007) 25–37. [DOI] [PubMed] [Google Scholar]
- [152].Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, et al. , ERRγ Directs and Maintains the Transition to Oxidative Metabolism in the Postnatal Heart, Cell Metabolism 6(1) (2007) 13–24. [DOI] [PubMed] [Google Scholar]
- [153].Pei L, Mu Y, Leblanc M, Alaynick W, Barish GD, Pankratz M, et al. , Dependence of hippocampal function on ERRgamma-regulated mitochondrial metabolism, Cell Metab 21(4) (2015) 628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Yoshihara E, Wei Z, Lin CS, Fang S, Ahmadian M, Kida Y, et al. , ERRgamma Is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive beta Cells, Cell Metab 23(4) (2016) 622–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Gan Z, Rumsey J, Hazen BC, Lai L, Leone TC, Vega RB, et al. , Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism, J Clin Invest 123(6) (2013) 2564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Zhao J, Lupino K, Wilkins BJ, Qiu C, Liu J, Omura Y, et al. , Genomic integration of ERRγ-HNF1β regulates renal bioenergetics and prevents chronic kidney disease, Proceedings of the National Academy of Sciences 115(21) (2018) E4910–E4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wang T, McDonald C, Petrenko NB, Leblanc M, Wang T, Giguere V, et al. , Estrogen-related receptor alpha (ERRalpha) and ERRgamma are essential coordinators of cardiac metabolism and function, Mol Cell Biol 35(7) (2015) 1281–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Cao Y, Zhang X, Akerberg BN, Yuan H, Sakamoto T, Xiao F, et al. , In Vivo Dissection of Chamber-Selective Enhancers Reveals Estrogen-Related Receptor as a Regulator of Ventricular Cardiomyocyte Identity, Circulation 147(11) (2023) 881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Sakamoto T, Batmanov K, Wan S, Guo Y, Lai L, Vega RB, Kelly DP, The nuclear receptor ERR cooperates with the cardiogenic factor GATA4 to orchestrate cardiomyocyte maturation, Nat Commun 13(1) (2022) 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Rowe GC, Asimaki A, Graham EL, Martin KD, Margulies KB, Das S, et al. , Development of dilated cardiomyopathy and impaired calcium homeostasis with cardiac-specific deletion of ESRRbeta, Am J Physiol Heart Circ Physiol 312(4) (2017) H662–H671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Ang YS, Rivas RN, Ribeiro AJS, Srivas R, Rivera J, Stone NR, et al. , Disease Model of GATA4 Mutation Reveals Transcription Factor Cooperativity in Human Cardiogenesis, Cell 167(7) (2016) 1734–1749 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Man JCK, van Duijvenboden K, Krijger PHL, Hooijkaas IB, van der Made I, de Gier-de Vries C, et al. , Genetic Dissection of a Super Enhancer Controlling the Nppa-Nppb Cluster in the Heart, Circ Res 128(1) (2021) 115–129. [DOI] [PubMed] [Google Scholar]
- [163].Uosaki H, Cahan P, Lee DI, Wang S, Miyamoto M, Fernandez L, et al. , Transcriptional Landscape of Cardiomyocyte Maturation, Cell Rep 13(8) (2015) 1705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Kannan S, Miyamoto M, Zhu R, Lynott M, Guo J, Chen EZ, et al. , Trajectory reconstruction identifies dysregulation of perinatal maturation programs in pluripotent stem cell-derived cardiomyocytes, Cell Rep 42(4) (2023) 112330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I, Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle, Biochem Biophys Res Commun 296(2) (2002) 350–4. [DOI] [PubMed] [Google Scholar]
- [166].Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, et al. , Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1, FASEB J 16(14) (2002) 1879–86. [DOI] [PubMed] [Google Scholar]
- [167].Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ, Acute endurance exercise increases the nuclear abundance of PGC-1alpha in trained human skeletal muscle, Am J Physiol Regul Integr Comp Physiol 298(4) (2010) R912–7. [DOI] [PubMed] [Google Scholar]
- [168].Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, et al. , Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency, J Biol Chem 280(39) (2005) 33588–98. [DOI] [PubMed] [Google Scholar]
- [169].Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, et al. , An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation, Endocrinology 148(7) (2007) 3441–8. [DOI] [PubMed] [Google Scholar]
- [170].Watson PA, Birdsey N, Huggins GS, Svensson E, Heppe D, Knaub L, Cardiac-specific overexpression of dominant-negative CREB leads to increased mortality and mitochondrial dysfunction in female mice, Am J Physiol Heart Circ Physiol 299(6) (2010) H2056–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, et al. , Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway, J Biol Chem 280(20) (2005) 19587–93. [DOI] [PubMed] [Google Scholar]
- [172].Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P, Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways, FEBS Lett 582(1) (2008) 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P, GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha, Cell Metab 3(6) (2006) 429–38. [DOI] [PubMed] [Google Scholar]
- [174].Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P, Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1, Nature 434(7029) (2005) 113–8. [DOI] [PubMed] [Google Scholar]
- [175].Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, et al. , Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha, EMBO J 26(7) (2007) 1913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Kusuhara K, Madsen K, Jensen L, Hellsten Y, Pilegaard H, Calcium signalling in the regulation of PGC-1alpha, PDK4 and HKII mRNA expression, Biol Chem 388(5) (2007) 481–8. [DOI] [PubMed] [Google Scholar]
- [177].Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS, Regulation of mitochondrial biogenesis in skeletal muscle by CaMK, Science 296(5566) (2002) 349–52. [DOI] [PubMed] [Google Scholar]
- [178].Schaeffer PJ, Wende AR, Magee CJ, Neilson JR, Leone TC, Chen F, Kelly DP, Calcineurin and calcium/calmodulin-dependent protein kinase activate distinct metabolic gene regulatory programs in cardiac muscle, J Biol Chem 279(38) (2004) 39593–603. [DOI] [PubMed] [Google Scholar]
- [179].Warren JS, Tracy CM, Miller MR, Makaju A, Szulik MW, Oka SI, et al. , Histone methyltransferase Smyd1 regulates mitochondrial energetics in the heart, Proc Natl Acad Sci U S A 115(33) (2018) E7871–E7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Szulik MW, Valdez S, Walsh M, Davis K, Bia R, Horiuchi E, et al. , SMYD1a protects the heart from ischemic injury by regulating OPA1-mediated cristae remodeling and supercomplex formation, Basic Res Cardiol 118(1) (2023) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Misra J, Kim DK, Choi HS, ERRgamma: a Junior Orphan with a Senior Role in Metabolism, Trends Endocrinol Metab 28(4) (2017) 261–272. [DOI] [PubMed] [Google Scholar]
- [182].Chong D, Gu Y, Zhang T, Xu Y, Bu D, Chen Z, et al. , Neonatal ketone body elevation regulates postnatal heart development by promoting cardiomyocyte mitochondrial maturation and metabolic reprogramming, Cell Discov 8(1) (2022) 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Cheng YY, Gregorich Z, Prajnamitra RP, Lundy DJ, Ma TY, Huang YH, et al. , Metabolic Changes Associated With Cardiomyocyte Dedifferentiation Enable Adult Mammalian Cardiac Regeneration, Circulation 146(25) (2022) 1950–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Nakano H, Minami I, Braas D, Pappoe H, Wu X, Sagadevan A, et al. , Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis, Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Hu D, Linders A, Yamak A, Correia C, Kijlstra JD, Garakani A, et al. , Metabolic Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes by Inhibition of HIF1alpha and LDHA, Circ Res 123(9) (2018) 1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Hortells L, Valiente-Alandi I, Thomas ZM, Agnew EJ, Schnell DJ, York AJ, et al. , A specialized population of Periostin-expressing cardiac fibroblasts contributes to postnatal cardiomyocyte maturation and innervation, Proc Natl Acad Sci U S A 117(35) (2020) 21469–21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Kowalski WJ, Garcia-Pak IH, Li W, Uosaki H, Tampakakis E, Zou J, et al. , Sympathetic Neurons Regulate Cardiomyocyte Maturation in Culture, Front Cell Dev Biol 10 (2022) 850645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Cho GS, Lee DI, Tampakakis E, Murphy S, Andersen P, Uosaki H, et al. , Neonatal Transplantation Confers Maturation of PSC-Derived Cardiomyocytes Conducive to Modeling Cardiomyopathy, Cell Rep 18(2) (2017) 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Hirt MN, Hansen A, Eschenhagen T, Cardiac tissue engineering: state of the art, Circ Res 114(2) (2014) 354–67. [DOI] [PubMed] [Google Scholar]
- [190].Carson D, Hnilova M, Yang X, Nemeth CL, Tsui JH, Smith AS, et al. , Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells, ACS Appl Mater Interfaces 8(34) (2016) 21923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Tsan YC, DePalma SJ, Zhao YT, Capilnasiu A, Wu YW, Elder B, et al. , Physiologic biomechanics enhance reproducible contractile development in a stem cell derived cardiac muscle platform, Nat Commun 12(1) (2021) 6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Leonard A, Bertero A, Powers JD, Beussman KM, Bhandari S, Regnier M, et al. , Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues, J Mol Cell Cardiol 118 (2018) 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Ulmer BM, Stoehr A, Schulze ML, Patel S, Gucek M, Mannhardt I, et al. , Contractile Work Contributes to Maturation of Energy Metabolism in hiPSC-Derived Cardiomyocytes, Stem Cell Reports 10(3) (2018) 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Wijnker PJ, Friedrich FW, Dutsch A, Reischmann S, Eder A, Mannhardt I, et al. , Comparison of the effects of a truncating and a missense MYBPC3 mutation on contractile parameters of engineered heart tissue, J Mol Cell Cardiol 97 (2016) 82–92. [DOI] [PubMed] [Google Scholar]
- [195].Ramachandran A, Livingston CE, Vite A, Corbin EA, Bennett AI, Turner KT, et al. , Biomechanical Impact of Pathogenic MYBPC3 Truncation Variant Revealed by Dynamically Tuning In Vitro Afterload, J Cardiovasc Transl Res 16(4) (2023) 828–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Tani H, Tohyama S, Human Engineered Heart Tissue Models for Disease Modeling and Drug Discovery, Front Cell Dev Biol 10 (2022) 855763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Dirkx E, da Costa Martins PA, De Windt LJ, Regulation of fetal gene expression in heart failure, Biochim Biophys Acta 1832(12) (2013) 2414–24. [DOI] [PubMed] [Google Scholar]
- [198].Taegtmeyer H, Sen S, Vela D, Return to the fetal gene program: a suggested metabolic link to gene expression in the heart, Ann N Y Acad Sci 1188 (2010) 191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H, Metabolic gene expression in fetal and failing human heart, Circulation 104(24) (2001) 2923–31. [DOI] [PubMed] [Google Scholar]
- [200].Spurrell CH, Barozzi I, Kosicki M, Mannion BJ, Blow MJ, Fukuda-Yuzawa Y, et al. , Genome-wide fetalization of enhancer architecture in heart disease, Cell Rep 40(12) (2022) 111400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Kwon DH, Eom GH, Kee HJ, Nam YS, Cho YK, Kim DK, et al. , Estrogen-related receptor gamma induces cardiac hypertrophy by activating GATA4, J Mol Cell Cardiol 65 (2013) 88–97. [DOI] [PubMed] [Google Scholar]
- [202].Lasheras J, Pardo R, Velilla M, Poncelas M, Salvatella N, Simo R, et al. , Cardiac-Specific Overexpression of ERRgamma in Mice Induces Severe Heart Dysfunction and Early Lethality, Int J Mol Sci 22(15) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Nautiyal J, Christian M, Parker MG, Distinct functions for RIP140 in development, inflammation, and metabolism, Trends Endocrinol Metab 24(9) (2013) 451–9. [DOI] [PubMed] [Google Scholar]
- [204].Yamamoto T, Maurya SK, Pruzinsky E, Batmanov K, Xiao Y, Sulon SM, et al. , RIP140 deficiency enhances cardiac fuel metabolism and protects mice from heart failure, Journal of Clinical Investigation 133(9) (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. , Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease, N Engl J Med 317(20) (1987) 1237–45. [DOI] [PubMed] [Google Scholar]
- [206].Auwerx J, Schoonjans K, Fruchart JC, Staels B, Regulation of triglyceride metabolism by PPARs: fibrates and thiazolidinediones have distinct effects, J Atheroscler Thromb 3(2) (1996) 81–9. [DOI] [PubMed] [Google Scholar]
- [207].Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC, Mechanism of action of fibrates on lipid and lipoprotein metabolism, Circulation 98(19) (1998) 2088–93. [DOI] [PubMed] [Google Scholar]
- [208].Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. , Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial, Lancet 366(9500) (2005) 1849–61. [DOI] [PubMed] [Google Scholar]
- [209].d’Emden MC, Jenkins AJ, Li L, Zannino D, Mann KP, Best JD, et al. , Favourable effects of fenofibrate on lipids and cardiovascular disease in women with type 2 diabetes: results from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, Diabetologia 57(11) (2014) 2296–303. [DOI] [PubMed] [Google Scholar]
- [210].Jakob T, Nordmann AJ, Schandelmaier S, Ferreira-Gonzalez I, Briel M, Fibrates for primary prevention of cardiovascular disease events, Cochrane Database Syst Rev 11(11) (2016) CD009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Xu W, Billon C, Li H, Wilderman A, Qi L, Graves A, et al. , Novel Pan-ERR Agonists Ameliorate Heart Failure Through Enhancing Cardiac Fatty Acid Metabolism and Mitochondrial Function, Circulation (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Fujiwara Y, Miki K, Deguchi K, Naka Y, Sasaki M, Sakoda A, et al. , ERRgamma agonist under mechanical stretching manifests hypertrophic cardiomyopathy phenotypes of engineered cardiac tissue through maturation, Stem Cell Reports (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, et al. , Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates, Nat Biotechnol 36(7) (2018) 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, et al. , Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration, Cell 173(1) (2018) 104–116 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Sun J, Wang L, Matthews RC, Walcott GP, Lu YA, Wei Y, et al. , CCND2 Modified mRNA Activates Cell Cycle of Cardiomyocytes in Hearts With Myocardial Infarction in Mice and Pigs, Circ Res (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF, Hippo signaling impedes adult heart regeneration, Development 140(23) (2013) 4683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Monroe TO, Hill MC, Morikawa Y, Leach JP, Heallen T, Cao S, et al. , YAP Partially Reprograms Chromatin Accessibility to Directly Induce Adult Cardiogenesis In Vivo, Dev Cell 48(6) (2019) 765–779 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Leach JP, Heallen T, Zhang M, Rahmani M, Morikawa Y, Hill MC, et al. , Hippo pathway deficiency reverses systolic heart failure after infarction, Nature 550(7675) (2017) 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Liu S, Li K, Wagner Florencio L, Tang L, Heallen TR, Leach JP, et al. , Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction, Sci Transl Med 13(600) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Bae J, Salamon RJ, Brandt EB, Paltzer WG, Zhang Z, Britt EC, et al. , Malonate Promotes Adult Cardiomyocyte Proliferation and Heart Regeneration, Circulation 143(20) (2021) 1973–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Zhao G, Jeoung NH, Burgess SC, Rosaaen-Stowe KA, Inagaki T, Latif S, et al. , Overexpression of pyruvate dehydrogenase kinase 4 in heart perturbs metabolism and exacerbates calcineurin-induced cardiomyopathy, Am J Physiol Heart Circ Physiol 294(2) (2008) H936–43. [DOI] [PubMed] [Google Scholar]
- [222].Chambers KT, Leone TC, Sambandam N, Kovacs A, Wagg CS, Lopaschuk GD, et al. , Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response, J Biol Chem 286(13) (2011) 11155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Abouleisa RRE, McNally L, Salama ABM, Hammad SK, Ou Q, Wells C, et al. , Cell cycle induction in human cardiomyocytes is dependent on biosynthetic pathway activation, Redox Biol 46 (2021) 102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Ikeda S, Mizushima W, Sciarretta S, Abdellatif M, Zhai P, Mukai R, et al. , Hippo Deficiency Leads to Cardiac Dysfunction Accompanied by Cardiomyocyte Dedifferentiation During Pressure Overload, Circ Res 124(2) (2019) 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]


