Abstract
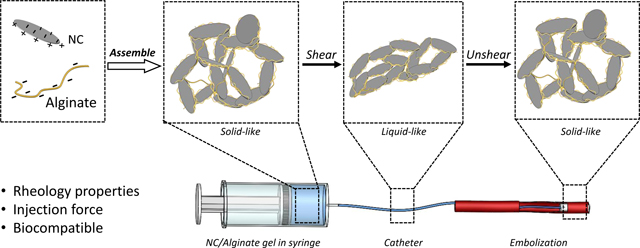
Shear-thinning materials have held considerable promise as embolic agents due to their capability of transition between solid and liquid state. In this study, a laponite nanoclay (NC)/ alginate gel embolic agent was developed, characterized, and studied for transcatheter based minimally invasive procedures. Both NC and alginate are biocompatible and FDA-approved. Due to electrostatic interactions, the NC/alginate gels exhibit shear-thinning properties that are desirable for transcatheter delivery. The unique shear-thinning nature of the NC/alginate gel allows it to function as a fluid-like substance during transcatheter delivery and as a solid-like embolic agent once deployed. To ensure optimal performance and safety in clinical applications, the rheological characteristics were thoroughly investigated to optimize the mechanical properties of the NC/alginate gel, including storage modulus, yield stress/strain, and thixotropy. To improve physicians’ experience and enhance the predictability of gel delivery, a combination of experimental and theoretical approaches was used to assess the injection force required for successful delivery of the gel through clinically employed catheters. Overall, NC/alginate gel exhibited excellent stability and tunable injectability by optimizing the composition of each component. These findings highlight the gel’s potential as a robust embolic agent for a wide range of minimally invasive procedures.
Keywords: Minimally invasive procedure, Gel embolic agent, Shear-thinning, Transcatheter injectability
Graphical Abstract
1. Introduction
Minimally invasive surgery has gained recognition as a reliable technique for its notable safety and effectiveness. This surgical approach can achieve outcomes comparable to traditional open surgery, while minimizing patient trauma and expediting postoperative recovery. Vascular embolization, among the various applications of minimally invasive techniques, stands out as a notable procedure that offers precise and localized treatment. By selectively obstructing blood flow to targeted sites, embolization has demonstrated its efficacy in addressing a diverse range of conditions such as arteriovenous malformations, brain aneurysms and tumors [1–5].
Solid and liquid embolic agents have been developed and widely utilized in clinical practice. However, these agents come with certain limitations. Solid embolic agents (e.g., coils and particulates) [1], have predetermined sizes that cannot fully match with the diverse human vasculature sizes (8μm to 25mm) [1,6,7]. The size-mismatch results in complications such as migration or fragmentation of deployed embolics, leading to recanalization or stroke. Besides, the cost of solid embolic agent is expensive, ranging from $599 to $172,179 depending on the type of material and the quantity [8]. On the other hand, liquid embolic agents have the advantage in distal infiltration into fine vasculature beds (i.e., tumors) where solid embolic agents cannot reach. However, they also carry their own limitations. For instance, cyanoacrylates generate heat during solidification and result in toxic degradation byproducts, and Onyx® contains potential neurotoxic solvents (i.e., DMSO) [9]. Additionally, the price of liquid embolic agent can also be high, which starts from $5950 [8].
To overcome the shortcomings, shear-thinning gel embolic agents offer a promising solution by combining the desirable characteristics of both solid and liquid agents. Shear-thinning gels exhibit decreased viscosity under shear stress, allowing easy catheter delivery like a liquid and occlusion stably like a solid [5]. To ensure their safety and effectiveness, several specific characteristics are crucial. Firstly, the gel embolics should possess appropriate rheological properties to enable catheter delivery without obstruction. The gels should own an acceptable yield stress to withstand physiological forces such as blood pressure, ensuring stability and durability during occlusion. Additionally, the recoverability of shear-thinning embolic gel enables multiple injections during a procedure [9]. It ensures the effectiveness of the embolic agent remains sustained in response to the changes in the body’s environment [1]. Moreover, the embolic agent should be biocompatible and non-toxic to the human body, eliciting no immune response or adverse reactions [9–11].
Catheter deliverability, or transcatheter injectability, is a critical factor in the precise and smooth delivery of the embolic agent to the targeted site by physicians [12]. It can be quantified by measuring the injection force required for delivery. The prediction of injection force has been extensively researched on needle-based injections [13–15]. However, there is a lack of investigation into the specific prediction of injection force in the context of transcatheter-based delivery. This is particularly important considering that the required force for catheter-based delivery is significantly amplified compared to needle injection due to the increased contact area between the embolic agent and the lumen of the catheters. Consequently, the quantification and prediction of injection force are essential for improved understanding of embolization procedures and optimizing the formulation of embolic agents.
Both laponite nanoclay (NC) and alginate are FDA approved materials, which have been extensively studied for biomedical applications as they do not provoke significant immune responses [16–18]. NC is a synthetic material with formula of Na+0.7[(Mg5.5Li0.3)Si8O20(OH)4]−0.7.
It exhibits a 2D disc-like shape with a diameter of 25 nm and a thickness of 1 nm. NC particle is negatively charged (OH−) on its faces and positively charged (Na+) on the edges [19]. The anisotropic distribution of charges contributes to its shear-thinning properties, forming a “house of cards” structure [19]. Alginate, a naturally occurring linear copolymer, shares structural similarities with the extracellular matrices [18]. It consists of repeating units of D-mannuronic acid and L-guluronic acid, resulting in an overall negative charge and shear-thinning property [20]. It has gained attention in the field of biomedical applications due to its excellent biocompatibility, regenerative properties, and the ability to serve as a therapeutic carrier [18]. Due to the electrostatic interaction between NC and alginate in water, a promising shear-thinning embolic agent can be potentially obtained by integrating these two FDA-approved materials.
In this study, the transcatheter injectability of the NC/alginate embolic agents was investigated, showing reasonable correlation between experimental measurements and theoretical predictions. Their rheological behavior, including shear-thinning characteristics, mechanical strength, and recoverability, was further optimized to achieve effective occlusion. Sterility, hemolysis and cell biocompatibility tests were carried out, showing in-vitro safety of the engineered NC/alginate embolic. This work highlights the potential of NC/alginate system as a safe and promising embolic agent for transcatheter embolization procedures.
2. Material and Methods
2.1. Material
For this study, medium viscosity alginic acid sodium salt derived from brown algae (Sigma-Aldrich, St. Louis, MO, USA) and Laponite-XLG nanoclay (NC) (BYK USA Inc, Gonzales, Texas, United States) were used. Molecular-grade water was purchased from Intermountain Life Sciences (WFIMGW20L, Intermountain Life Sciences, UT, USA). Luria-Bertani (LB) broth was obtained from Sigma-Aldrich (Miller, Sigma-Aldrich, St. Louis, MO, USA).
2.2. Dynamics light scattering and zeta-potential
All measurements in this study were conducted at 25 °C. The hydrodynamic diameter and polydispersity index (PDI) of NC at 0.0625 wt. % in ultrapure water were obtained using dynamic light scattering (DLS) (Litesizer 500, Anton Paar Ltd., Graz, Austria). Zeta potential measurements of NC and alginate were performed by diluting the particle suspension with water solution using the same particle size analyzer. DLS result is the average result of 3 independent samples with 6 repetitive measurements of each sample. The number of repetitive measurements was determined according to ISO 22412 [21].
2.3. Preparation of NC/alginate gel
To investigate the interaction between NC and alginate, a group of NC/alginate gels were engineered by combining NC, alginate and molecular-grade water using a specialized mixer (DAC 330–100 SE, FlackTek SpeedMixer, Landrum, SC, USA). The formulations are shown in Table 1.The gels were labeled as xNCyA, where is NC percentage, and represents alginate percentage.
Table 1.
Composition of the NC/alginate gel. The percentage of solid content was kept constant at 4, 5, 6, 7 wt.% respectively.
| Total Solid wt.% | Sample Name | NC (wt.%) | Alginate (wt.%) | Water (wt.%) |
|---|---|---|---|---|
| 7% | 7NC0A | 7 | 0 | 93 |
| 6.5NC0.5A | 6.5 | 0.5 | 93 | |
| 6NC1A | 6 | 1 | 93 | |
| 6% | 6NC0A | 6 | 0 | 94 |
| 5.5NC0.5A | 5.5 | 0.5 | 94 | |
| 5.25NC0.75A | 5.25 | 0.75 | 94 | |
| 5NC1A | 5 | 1 | 94 | |
| 4.5NC1.5A | 4.5 | 1.5 | 94 | |
| 5% | 5NC0A | 5 | 0 | 95 |
| 4.5NC0.5A | 4.5 | 0.5 | 95 | |
| 4NC1A | 4 | 1 | 95 | |
| 3.5NC1.5A | 3.5 | 1.5 | 95 | |
| 4% | 4NC0A | 4 | 0 | 96 |
| 3.5NC0.5A | 3.5 | 0.5 | 96 | |
| 3NC1A | 3 | 1 | 96 | |
| 2.5NC1.5A | 2.5 | 1.5 | 96 | |
| 2NC2A | 2 | 2 | 96 |
2.4. Scanning Electron Microscopy (SEM)
The morphology of 5NC0A and 4.5NC0.5A embolic agent was imaged using a scanning electron microscope (Hitachi SU3900, Japan) at an accelerating voltage of 20 kV. Before imaging, the gels were frozen at −80°C for 24 hours, followed by a subsequent 24-hour lyophilization process to ensure thorough water removal.
2.5. Rheology of NC/alginate gels
Rheological tests were carried out on NC/alginate gels to characterize their shear-thinning, viscoelastic, and recovery properties. The measurements were conducted at a temperature of 25 °C, which is the ambient temperature an injection would be performed in clinics [22], using an MCR 302e rheometer (Anton Paar USA Inc., Torrance, CA). For all experiments, a 25 mm diameter sandblasted upper plate and a sandblasted lower plate were used. The gap between the upper and lower plates was maintained at 1 mm for all measurements. A solvent trap was used to maintain the chamber humidity and prevent materials from drying. Shear rate sweeps were conducted ranging from 10−3 s−1 to 103 s−1 to investigate the shear-thinning behavior of the NC/alginate gel. Large-amplitude oscillatory shear (LAOS) tests were performed at a fixed angular frequency of 10 rad s−1. Both the shear rate sweeps, and LAOS tests were carried out in triplicate. Frequency sweeps were conducted at a fixed strain of 0.1% within the linear viscoelastic region as obtained from LAOS. The angular frequency was varied between 0.1 and 100 rad s−1 to analyze the viscoelastic properties of the NC/alginate gel as a function of frequency. Lastly, thixotropic tests were performed to assess the recoverability of the NC/alginate gels at an angular frequency of 10 rad s−1 under strain oscillation between 0.1 % (low strain) for 2 minutes each and 100 % (high strain) for 2 minutes each for a total of 16 minutes.
2.5.1. Power Law Fitting
To quantitatively assess the shear-thinning behavior of the NC/alginate gels, power law (Eq. 1) was used to describe the flow behavior of non-Newtonian fluids, as described by [23]:
| Eq. 1 |
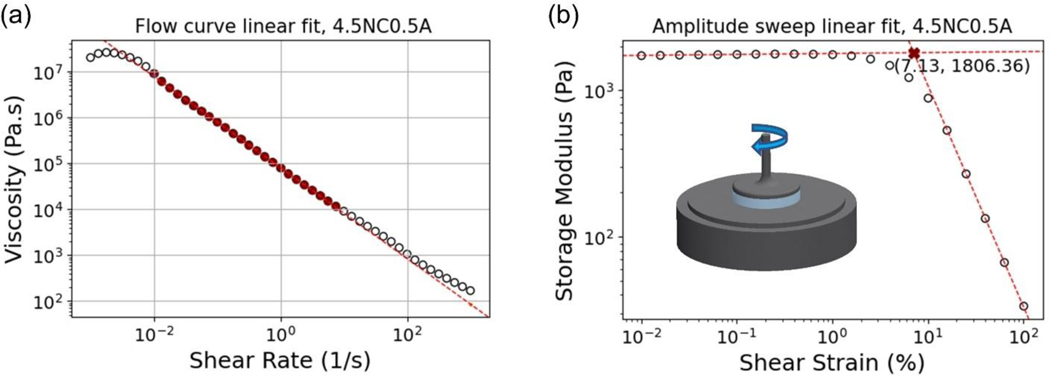
stands for flow consistency index, stands for the viscosity of the gel and is the shear rate. The flow behavior index () represents the extent to which the fluid deviates from Newtonian flow [23]. The linear segment of the shear rate sweeps was fitted as shown in Figure 1 (a).
Figure 1.
Example for the linear fit representative of (a) shear rate sweeps and (b) amplitude sweeps.
2.5.2. Yield stress/strain
In this study, the yield stress and strain of the NC/alginate gel were assessed through the LAOS curves following a previously developed protocol [24]. Specifically, two linear segments in the storage modulus data were fitted as shown in Figure 1 (b). The identification of the yield strain was first determined as the intersection of the two fitting lines, represented by the red cross mark. Yield stress was then extrapolated as the stress value corresponding to yield strain. Despite many methods available for yield stress analysis [25,26], we selected to measure the crossover of G’ and G” as a well-defined point in amplitude sweep. This method in general shows excellent reproducibility and especially useful when measuring yield stress for high-viscosity systems [26].
2.6. Transcatheter injectability
The injectability of NC/alginate gels through clinical catheters was measured. The gels were first loaded into 3 mL BD syringes (BD Luer-LokTM Syringe sterile, BD, USA). Then, the force required to inject NC/alginate gels was measured as they passed through a 5 F, 100 cm Infiniti® diagnostic catheter (Cordis Corporation, Miami, FL, USA) at a flow rate of 2 mL min−1 using a mechanical testing system (UniVert, CellScale, Waterloo, Ontario, Canada). In this study, injection force is calculated as the mean value of the stable plateau within a certain range of piston displacement.
2.7. In-Vitro Occlusion-Displacement Test
The ability of the 5NC0A and 4.5NC0.5A gels to withstand physiologically relevant pressure was evaluated using an in-vitro occlusion model. Gels were loaded into PVC tubes with inner diameters of 2.38 mm (3/32”) and 4.76mm (3/16”) tubes respectively (Line 6516T18 and 6516t52, McMasterCarr, Atlanta, GA, USA). 1X Phosphate-buffered saline (PBS) (Sigma-Aldrich, St. Louis, MO, USA) was then pumped at a rate of 50 mL min−1 using a syringe pump (Legato 100, KD Scientific, Holliston, MA, USA) to displace selected gels inside the tube. The pressure required to displace 1 mL of the material was measured using a pressure sensor (model PX409030DWUUSBH, Omega Engineering Inc., Norwalk, CT, USA), and this value was recorded as the displacement pressure. Three tests were conducted for each sample.
2.8. Sterility
To assess the sterility of 5NC0A and 4.5NC0.5A, 1 mL of each material was placed in 10 mL LB broth in centrifuge tubes. Plain LB broth was used as negative control, and LB broth inoculated with Escherichia coli (E. coli, ATCC, 25922) was used as positive control. Subsequently, the tubes were placed in an orbital shaker (C24 Incubator/Shaker, New Brunswick Scientific, Edison, NJ, USA) overnight at 37°C at a speed of 100 rpm. The optical density of the suspension was then measured at 600 nm using a cell density meter (Ultrospec 10 cell density meter; Amersham Biosciences, Little Chalfont, UK). Three independent tests were run for each sample, and a total of six readings were conducted for each test.
2.9. Hemocompatibility
The hemocompatibility of NC/alginate gels were assessed through hemolysis test for 5NC0A and 4.5NC0.5A, following ISO-10993–4 [27]. First, 1 mL of each gel was incubated with 9 mL of 1X PBS in centrifuge tubes, and the tubes were pre-warmed at 37 ºC for 30 minutes. Porcine whole blood (anticoagulated with sodium citrate, Lampire Biological Laboratories, Inc., Pipersville, PA, USA) was diluted with 1X PBS at a ratio of 4:5. Then, 0.2 mL of diluted blood was added into each tube and incubated at 37 ºC for 1 hour. As negative and positive controls, 10 mL PBS and 10 mL deionized water were incubated with 0.2 mL of diluted blood, respectively. After incubation, the samples were centrifuged at 3000 rpm for 5 minutes and the supernatant was transferred to a 96-well plate for measurement. The absorbance (A) of the supernatant was measured using a microplate reader (GENios, TECAN, Crailsheim, Germany) at a wavelength of 550 nm. The hemolysis percentage was calculated according to the formula below.
| Eq. 2 |
2.10. Thrombogenicity
The thrombogenicity of selected gels was conducted. 5NC0A and 4.5NC0.5A were loaded into a 96-well plate at a volume of 100μL per well. The plate was then centrifuged at 1000 RPM to establish a uniform gel surface, ensuring a consistent interface between the gel and blood. Porcine blood was activated by gently mixing it with 10% (v/v) 0.1 M CaCl2. Then, 100 μL of the activated blood was added into the gel containing wells. The gels were allowed to interact with the blood for designated time intervals of 3, 4, 5.5, 7, and 8 minutes respectively. At selected time points, clotting was stopped by addition of sodium citrate solution (0.109 M). The liquid was immediately aspirated and washed repeatedly until the solution was clear, leaving only clotted blood. Porcine blood alone and clinically used coils (2D Helical-35 micro-coils, Boston Scientific, MA, United States) were used as controls for clotting time comparison.
2.11. Cell Viability
The biocompatibility of the gels was evaluated using extraction assay according to ISO-10993–5 using L929 fibroblasts (American Type Culture Collection (ATCC), Manassas, VA) and Human umbilical vein endothelial cells (HUVEC, Corning, Corning, NY) [28]. The culture medium for L929 was comprised of Dulbecco’s Modified Eagle Medium (DMEM, Gibco BRL, Grand Island, NY, USA), with 10% heat-inactivated fetal bovine serum (Cytiva, Marlborough, MA), and 1% penicillin/streptomycin (Thermo Fisher Scientific, Inc., Waltham, MA). The medium for HUVEC was obtained from Lonza (EGM™−2 Endothelial Cell Growth Medium-2 BulletKit, Morristown, NJ, USA). L-929 mouse fibroblasts and HUVEC cells were incubated at 37°C in an atmosphere containing 5% CO2 and 95% humidity, each in their respective culture medium.
For the viability assay, the cells were seeded in 96 well plates at a density of 5000 cells per well for 24 hours. Selected NC/alginate gels, including 5NC0A and 4.5NC0.5A, were used to prepare the extractions. Specifically, 1 mL of each gel was added in 10 mL of culture medium and incubated at 37 °C overnight. The supernatant was collected to prepare for a series of extraction dilution with the culture medium, including 100%, 50%, 25% and 12.5% (v/v). To evaluate the potential cytotoxicity of the NC/alginate, L-929 and HUVEC were then treated with different concentrations of the liquid extracts (100%, 50%, 25%, and 12.5% v/v) in their respective medium. Following a 24-hour incubation period, the viability of the treated cells was determined using the WST-1 assay (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. 10% DMSO was used as positive control and non-treated cells were used as negative control. Three independent experiments were conducted, and each experiment included four replicates for statistical analysis.
2.12. Degradation
The gel degradation kinetics was measured at varying pH values over 24 hours. 200 μL of embolic gel was loaded into individual Eppendorf tubes, and the combined weight of the gel and tube was recorded as W0. Then, 200 μL of PBS was added into each tube, and the mixtures were incubated at pH levels of 4, 7.4 and 9, respectively, at 37 °C. At selected time points, PBS was carefully removed and the weights of these tubes containing residual gel were recorded as Wt. The remaining weight percentage of the gels was calculated using the following equation:
| Eq. 3 |
2.13. Statistical Analysis
Statistical analysis was conducted utilizing GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). An unpaired t-test was conducted to analyze hemolysis. One-way analysis of variance (ANOVA) with Tukey’s comparisons method was performed for experiments containing more than two groups. p < 0.05 was defined as statistically significant. Data is reported as average ± standard deviation (s.d.).
3. Result and Discussion
3.1. Particle size and zeta potential
The size distribution of nanoclay particles was measured using DLS. NC was shown to have hydrodynamic diameter of 45.9 ± 0.9 nm with a low polydispersity index (PDI) of 0.045 ± 0.004. PDI is a measurement of the breadth of the molecular weight distribution, indicating the spread of the particle size distribution [29]. A PDI threshold of 0.1 is commonly used as a criterion to define a monodisperse system, where values below this threshold suggest a monodisperse distribution [30]. Hence, the PDI of NC indicates its monodisperse particle size distribution.
Zeta ()-potential for NC and sodium alginate in DI water were measured to be −50.9 ± 4.4 mV and −52.2 ± 9.5 mV, respectively. A higher absolute value of the -potential (> 30 mV) indicates the colloidal stability with a less tendency for particle aggregation [31]. Therefore, both materials exhibit excellent colloidal stability. NC particles have a disk-like morphology, with positive charges on the edges and negative charges on the surface, whereas alginate is a negatively charged linear copolymer. The negative charge on alginate enables its adsorption onto the edges of NC particles. The electrostatic interaction contributes to the homogeneous formation of NC/alginate gels [32–34].
3.2. Rheology of NC/alginate gels
3.2.1. Shear rate sweeps
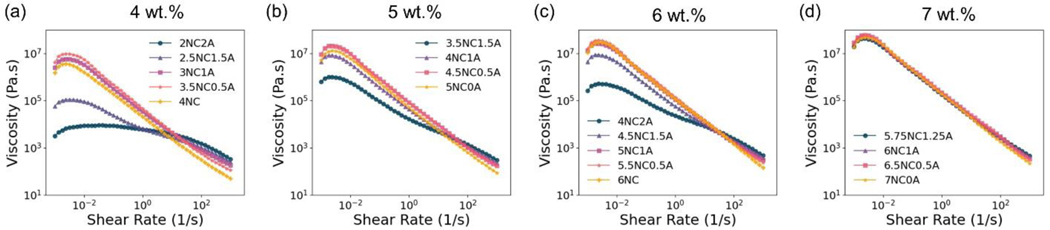
Shear rate sweeps ranging from 10−3 s−1 to 103 s−1 with a total solid content of 4 wt.%, 5 wt.%, 6 wt.% (Figure 3) and 7 wt.% (Figure S 1) were conducted to study the shear-thinning behavior of the NC/alginate gels at 25 °C. Figure 2 (a–d) shows the change in viscosity of the NC/alginate gels as a function of shear rate. In particular, viscosity decreases with increased shear rate for all gels, indicating a consistent shear-thinning behavior. When subjected to shear, both alginate chains and NC particles orient along the shear direction, resulting in a reduced flow resistance and viscosity [35–37]. The process involves both the disrupted “house of cards” structure established by the NC particles and reduced alginate chain entanglement. Thus, the shear-thinning property can benefit embolization procedures as it facilitates transcatheter delivery.
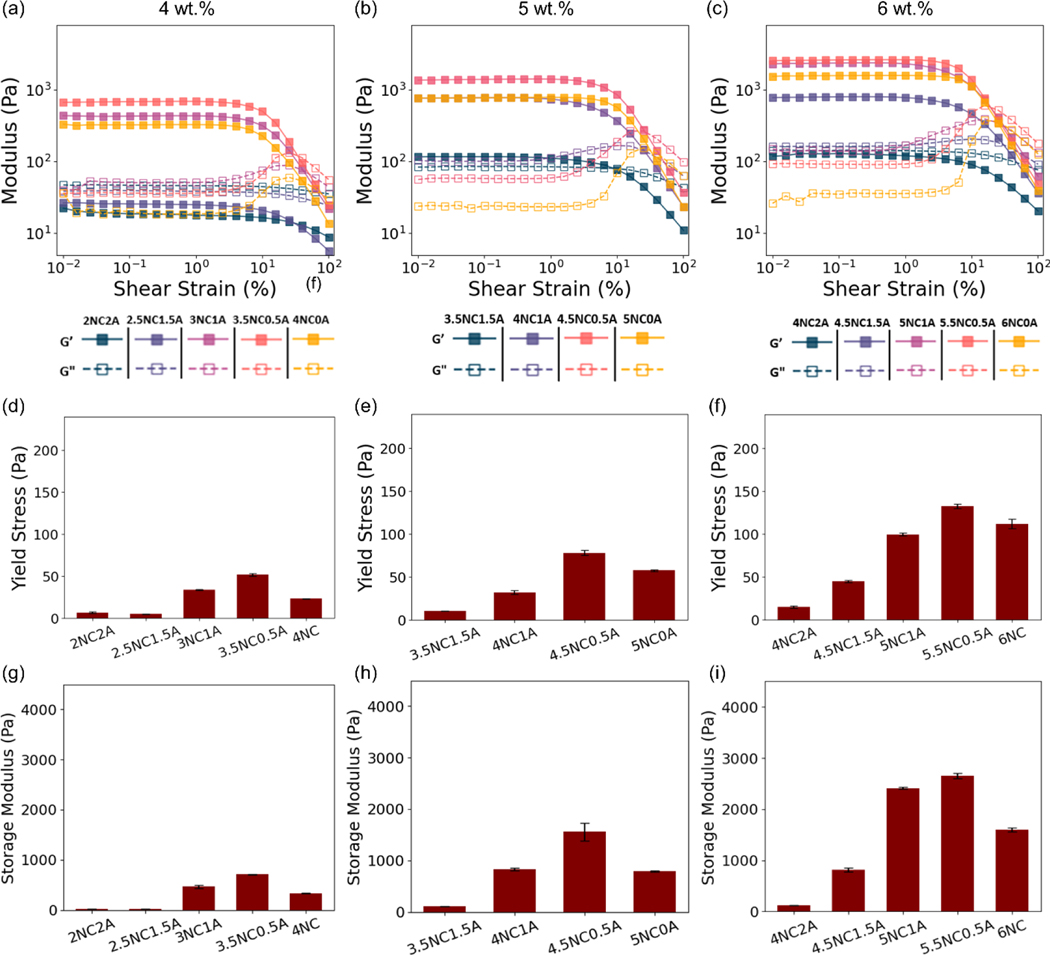
Figure 3.
Representative amplitude sweeps of (a) 4 wt.%, (b) 5 wt.%, and (c) 6 wt.% NC/alginate embolic agent. Summary of yield stress (d-f) and storage modulus of engineered NC/alginate gels (g-i). Data are presented as mean ± standard deviation (n = 3 for (d–i)).
Figure 2.
Representative flow curves of (a) 4 wt.%, (b) 5 wt.%, (c) 6 wt.% and (d) 7wt.% NC/alginate embolic agent.
The shear-thinning behavior of the NC/alginate gels was assessed using power law (Eq. 1) by fitting the linear segment of the shear rate sweeps (Figure 1(a)). For shear-thinning materials (n<1), a lower n value indicates a higher degree of shear-thinning, implying a more significant decrease in viscosity as the shear rate increases [23]. As is shown in Table 2, an increase in alginate content was associated with a continuous increase in the value of n. This suggests that the gel exhibits reduced non-Newtonian behavior as the alginate content increases, indicating a lower degree of shear-thinning [10]. The flow consistency index () characterizes the average viscosity of a non-Newtonian fluid. It describes the relationship between shear rate and the reduction in viscosity of a material. For the same n value, a higher value indicates a more obvious shear-thinning property [38]. Interestingly, despite different n values, the addition of 0.5 wt.% alginate constantly exhibited the highest value among the gels with same total solid content.
Table 2.
n and K values for gels with 4wt.%, 5 wt.%, 6 wt.% and 7 wt.% total solid content.
| Total solid weight content | Sample | n | |
|---|---|---|---|
| 4% | 2NC2A | 0.78±0.00 | 5.67±0.06 |
| 2.5NC1.5A | 0.57±0.01 | 7.20±0.12 | |
| 3NC1A | 0.12±0.00 | 35.64±0.23 | |
| 3.5NC0.5A | 0.02±0.01 | 46.50±0.17 | |
| 4NC0A | 0.06±0.00 | 20.72±0.11 | |
| 5% | 3.5NC1.5A | 0.41±0.00 | 19.30±1.20 |
| 4NC1A | 0.14±0.00 | 45.12±0.94 | |
| 4.5NC0.5A | 0.04±0.01 | 83.69±0.54 | |
| 5NC0A | 0.012±0.01 | 51.94±0.93 | |
| 6% | 4NC2A | 0.51±0.01 | 24.54±0.19 |
| 4.5NC1.5A | 0.20±0.00 | 63.24±1.24 | |
| 5NC1A | 0.05±0.00 | 110.66±1.02 | |
| 5.5NC0.5A | 0.02±0.01 | 138.11±0.20 | |
| 6NC0A | 0.03±0.02 | 115.34±1.22 | |
| 7% | 5.75NC1.25A | 0.05±0.00 | 184.59±2.68 |
| 6NC1A | 0.03±0.00 | 194.50±2.32 | |
| 6.5NC0.5A | 0.05±0.00 | 221.71±3.76 | |
| 7NC0A | −0.03±0.02 | 188.52±5.52 |
As shown in Figure 2, for gels with the same solid content, the shear rate sweeps show a notable trend where first the slopes become steeper and then flatter as the content of alginate increases. Take 5 wt. % solid content gels as an example, 4.5NC0.5A has the highest value, with its n value close to zero, suggesting its enhanced shear-thinning behavior. Similar trends in shear sweeps, as well as and n values, were observed in gel groups with solid contents of 4 wt.%, 6 wt.%, and 7 wt.% (Figure 2 (a, c, d)). Furthermore, the impact of alginate addition on the shear-thinning properties becomes less obvious as the overall solid content of the gel increases. For instance, in gels with 7 wt.% solid content, the flow behavior index (n) ranges from 0.05 to −0.03, exhibiting a change of 0.08, and the flow consistency index () ranges from 221.71 to 184.59, exhibiting a change of 33.94. These changes are smaller than those observed in 4–6 wt.% gels, suggesting that the incorporation of alginate has lesser impact on the shear-thinning behavior in 7 wt.% gels. While 7NC0A has a negative n value, it is very close to zero, confirming its excellent shear-thinning property.
3.2.2. Amplitude sweep
Figure 3 (a–c) and Figure S 1 shows the amplitude-dependent response of NC/alginate to shear strain at an angular frequency of 10 rad s−1 at 25 °C. The majority of NC/alginate systems has a higher storage modulus (G′) than the loss modulus (G″), suggesting their tendency for energy storage over dissipation and a solid-like behavior [39]. On the contrary, higher alginate content such as 2NC2A, 2.5NC1.5A, and 4NC2A exhibited a loss modulus that exceeded the storage modulus at the lower shear strain, indicating a liquid-like behavior. These compositions were, therefore, excluded from further study due to the lack of mechanical stability which may cause distal migration.
For shear-thinning gels, yield stress is the minimum stress required to disrupt bonds between particles and initiate the deformation of the material to transit from a solid to a liquid-like structure [40]. A lower yield stress facilitates smoother and more efficient injection process. In contrast, a higher yield stress enhances the gel’s resistance to flow and deformation, increasing its stability and integrity as embolic agents at the target site, preventing undesired migration. Thus, yield stress is a crucial parameter for the trade-off between the injection process and embolic occlusion. In this study, gels with tunable mechanical properties were engineered, with storage modulus ranging from 113.1 ± 2.4 Pa (3.5NC1.5A) to 4136.5 ± 200.6 Pa (6NC1A), and the calculated yield stress varying between 10.7 ± 0.2 Pa (3.5NC1.5A) and 213.4 ± 6.4 Pa (6.5NC0.5A). The peak wall shear stress in the human blood vessel was reported 4.3 Pa [41], which is less than half of the lowest yield stress. This suggests that the NC/alginate embolic agent remains stable within the human body. By tuning the compositions, gels can be tailored to exhibit desired rheological properties for specific embolization needs. In addition, distinct yield points, represented by the overshoot in loss modulus (Figure 3, Figure S 1) [42], are clearly observed. Related to the plasticity and the yielding transition, the yield point may be attributed to the occurrence where shear forces induce collisions between adjacent particles, resulting in the dissipation of energy through bond breakage, as well as the amplitude dependent buildup/breakdown of the structure [42].
Figure 4 presents a schematic illustration showing possible interaction between NC particles and alginate, as well as their response upon shear. In an aqueous medium, the NC disk-like particles form edge-face structures resembling ‘house of cards’ configurations [43] due to the delicate electrostatic equilibrium [43,44]. When NC gel is under shear, the ‘house of cards’ structure undergoes deformation and reorientation. When shear is removed, the particles can revert to a ‘house of cards’ structure and restore to a solid-like behavior. The negatively charged alginate chains [18] are absorbed onto the edges of NC disks and tune [45] the gel’s rheology behaviors.
Figure 4.
Schematic illustration showing interactions between NC particles and alginate chains.
Figure 3 (d–f) and Figure S 1(b) showed that gels with identical total solid content experience a notable increase in yield stress upon the addition of 0.5 wt.% alginate. However, as the alginate addition exceeded this point, an inverse relationship emerged between the alginate concentration and the resultant gel’s yield stress. This trend can be attributed to the change in the existing bonds and microstructure in the system.
For the illustration of storage modulus, we selected the 5 wt.% gel as a representative example (Figure 3 (h)). The storage modulus showed an increase from 792.30 ± 10.87 Pa to 1561.50 ± 173.16 Pa. The yield stress demonstrated an increase from 57.72 ± 1.05 Pa to 78.38 ± 3.06 Pa, corresponding to an increase in alginate content from 0 to 0.5 wt.%. This indicates the addition of alginate to NC resulted in a development of enhanced network with increased yield stress and storage modulus. The reinforcement can be attributed to additional electrostatic interactions due to the increased amount of alginate chains. The dynamic physical crosslinking can effectively dissipate energy via destruction and reorganization [45], and thus increase the storage modulus. On the other hand, the addition of 1 wt.% of alginate resulted in a decrease in storage modulus and yield stress to 829.09 ± 29.02 Pa and 32.37 ± 2.15 Pa, respectively. This may be due to that storage modulus is primarily influenced by the interactions between the NC particles themselves in the NC/alginate network [36]. An excessive amount of alginate may tightly wrap the edges of NC disks, thus blocking the edge-face interaction of the NC discs and resulting in a reduced modulus. The smooth and even surfaces observed in the SEM images (Figure S 5) indicate the effective dispersion of alginate in the NC matrix, highlighting the homogeneity of both 5NC0A and 4.5NC0.5A embolic agents.
Similar trends were observed in gels with 4 wt.% and 6 wt.% total solid content. For NC/alginate gel with the same solid content, the yield stress (Figure 3 (d–f), Figure S 1(b)) and storage modulus (Figure 3 (g–j), Figure S 1(c)) first increase and then decrease as the concentration of NC is increased. The addition of 0.5 wt.% alginate constantly leads to the highest yield strength due to its balance between dynamic crosslinking density and the ability to maintain the NC ‘house of cards’ structure. For 7 wt.% solid gels (Figure S 1), the addition of 0.5 wt.% alginate resulted in the highest yield stress. However, there were minimal changes observed in the storage modulus of the samples 6.5NC0.5A and 6NC1A, which only differed by 1.72%. This can be attributed to the presence of high content of NC, where increased amount of alginate does not affect the clay-clay interaction significantly.
3.2.3. Loss tangents
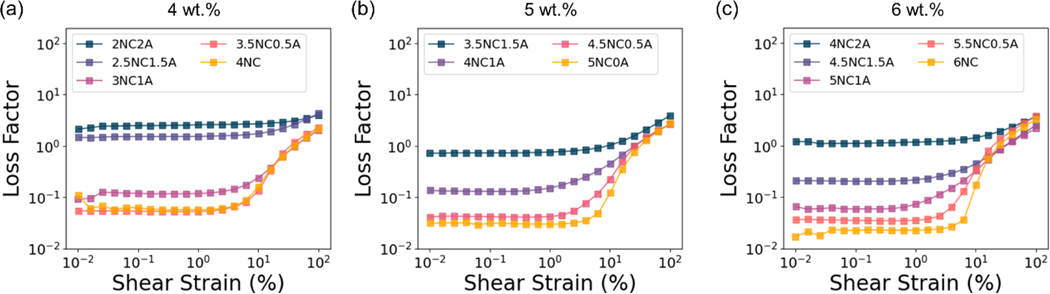
The ratio of loss modulus to storage modulus (G″/G′), known as the loss tangent or loss factor (), measures energy dissipation and expresses the degree of gel viscoelasticity [46]. For conventional gels, the values of are less than 0.1, suggesting the formation of stronger gels. While weak gels exhibit values ranging from 0.1 to 1.0, with lower strength. [47,48]. The point of which tan is defined as gel point [49], denoting a phase transition between liquid and solid states. As is shown in Figure 5 (a–c) and Figure S 2 (a), the loss tangent of 2NC2A, 2.5NC1.5A, and 4NC2A are larger than 1, indicating a liquid-like behavior. For the rest of the gels, remains below 1 when shear strain is less than 1%, indicating minimal energy dissipation and a more elastic behavior. With increasing strain, the gel-like materials () gradually transit to a liquid-like fluid () [49]. This sol-gel transit can facilitate the injection process. Moreover, for gels with same solid content, the loss tangent increases as the proportion of alginate increases within the shear strain up to 1%. This suggests gels with higher alginate content exhibit a greater energy dissipation upon deformation and a less elastic behavior.
Figure 5.
Representative loss tangent of (a) 4 wt.%, (b) 5 wt.%, and (c) 6 wt.% NC/alginate embolic agents.
3.2.4. Frequency sweep
The behavior of gels within the linear viscoelastic regime (0.1% shear strain) in response to angular frequency was investigated at 25 °C. The storage and loss moduli were measured as a function of frequency ranging from 10 to 104 rad s−1. As shown in Figure 6 (a), 2NC2A exhibited a higher loss modulus compared to storage modulus at all measured angular frequencies, indicating a liquid-like behavior. On the other hand, 2.5NC1.5A showed a higher storage modulus compared to loss modulus at lower frequencies, whereas the loss modulus surpassed the storage modulus at higher frequencies, suggesting a shift from solid-like to liquid like behavior. The remaining gels all exhibited higher storage modulus than loss modulus, indicating a solid-like behavior (Figure 6 and Figure S 2). Furthermore, it is shown that the storage modulus is nearly independent of frequency, indicating stable gel networks.
Figure 6.
Representative (a-c) frequency sweep, (d-f) thixotropy test for 4 wt.%, 5 wt.%, and 6 wt.% NC/alginate embolic agent, respectively.
3.2.5. Thixotropy testing
Thixotropy testing was performed at 25 °C to evaluate the recoverability of NC/alginate gel response under alternating high-strain (i.e., break the gel network) and low-strain cycles (i.e., recover the gel structure). It simulates intermittent injection and delivery process during the intervention procedures. As shown in Figure 6 (d-f) and Figure S 2 (c), all gels underwent a marked decrease in G′ at the high strain initially but rapidly recovered (within 1 min) at 25 °C. The gels’ mechanical properties are not compromised significantly under repeated shear over time. These results suggest that NC and alginate form a stable polymer network structure that can undergo repeated breakage and recovery due to rapid, reversible electrostatic interactions.
3.3. Transcatheter Injectability
In addition to rheological characterization, transcatheter injectability of the gels was investigated as a direct measurement reflecting the physician’s experience. Different from needle-based injection, it is important to note that minimally invasive embolization employs catheters that are much longer (i.e., 100 cm or more). The increased length is crucial to be considered when developing new embolics, as it introduces considerable resistance during the injection process. Previous research [12,15,50] has indicated that the average maximum force that can be exerted during injection is 79.8 N (95.4 N for males and 64.1 N for females). Thus, this work considers gels with injection force less than 60 N injectable [12,15,50]. Combined experimental results and theoretical prediction of the transcatheter injection forces of the gels were carried out.
3.3.1. Experimental Result
As is shown in Eq. 4, the total injection force () can be divided into two components: the force necessary to push the gel embolic agent out of the syringe () and the force required for the embolic agent to traverse through the catheter (). Herein, both and were measure for NC/alginate gels with varying total solid percentage from 4 wt.% to 7 wt.%.
| Eq. 4 |
The injection force was measured as the total force by passing syringe-loaded gels through a clinically used 100 cm 5 F catheter, and was measured as the force using the same setup but without the catheter. The injection force was tested at a controlled flow rate (Q) of 2 mL min−1 [51], which is within clinical injection rates ranging from 0.2–10.75 mL min−1 [51,52].
As is shown in Figure 7 (a–c) and Figure S 3(a), the force needed to inject gels through a 3 mL syringe () was stable, indicating a smooth injection and the homogeneity of the material. Figure 7 (d–f) and Figure S 3(b), presents a summary of , which was calculated based on the mean force within the displacement range of 6 mm to 10 mm. The stable injection force shows that increases with higher total solid content, and the overall force remains relatively low, ranging from 1.03 ± 0.06 N (4NC0A) to 3.08 ± 0.11 N (6.5NC0.5A).
Figure 7.
Representative curves for (a-c) syringe force measurements using a 3 mL BD syringe and (d-f) the summary of stable force; Data are presented as mean ± standard deviation (n = 3 for (d–f)).
Figure 8 (a–c) and Figure S4 (a) shows the recorded injection force () of different gels injected through a 5 F, 100 cm catheter until 10 mm displacement was achieved to ensure that the forces reached plateau. In general, increases with increased total solid content (Figure 8 (d–f), Figure S4 (b)). With the same total solid content, the higher content of alginate leads to the higher injection force. However, for 7 wt.% gels (Figure S 4), the addition of 0.5 wt.% alginate (6NC0.5A) increased injection force compared to 7NC0A, reaching 102.58 ± 0.89 N. More than 0.5 wt.% addition of alginate did not impact the injection force. Furthermore, it is evident that there was an overshoot in the injection force, namely the break loose force, before reaching a steady state for 3.5NC0.5A, 4.5NC0.5A, 5.5NC0.5A, 6NC0A, 6.5NC0.5A, and 7NC0A. The break loose force represents an increased friction between the plunger and the barrel to be overcome to initiate the flow. For a smooth injection, this factor needs to be taken into consideration, particularly when it represents the peak force that exceeds the maximum acceptable injection force (60 N).
Figure 8.
(a-c) Representative injection curves of passing varying gels through a 5 F, 100 cm catheter with a black arrowing showing break-loose force in (c). (d-f) Summary of F for gels. Data are presented as mean ± standard deviation (n = 3 for (d–f)).
3.3.2. Theory
To further correlate gel rheological properties to its transcatheter injectability, theoretical predictions were conducted. By combining the power-law (Eq. 1) and Hagen-Poiseuille law (Eq. 5), the gliding force for non-Newtonian fluids to pass a catheter can be expressed as Eq. 6 [13]. In the power-law equation, the viscosity of a non-Newtonian fluid is described by the flow consistency index , shear rate , and the power law index . The shear rate is determined by Eq. 7 where represents the inner radius of the syringe. In the Hagen-Poiseuille law, the glide force is described as a function of dynamic viscosity described by the power law (Eq. 1), which is determined by volumetric flow rate , , and inner catheter radius . Lastly, F was calculated by Eq. 4, where is determined from experimental result. The detailed derivation can be found in the supplementary information.
| Eq. 5 |
| Eq. 6 |
| Eq. 7 |
| Eq. 8 |
3.3.3. Comparison between experimental and theoretical transcatheter injection forces
The transcatheter injection experimental setup is shown in Figure 9 (a), and the measured and predicted transcatheter injection force for each gel is shown in Figure 9 (b). As is shown in Figure 9 (c), a positive correlation between the experimental and predicted injection forces. For gels with the same solid content but less alginate content, such as 0 wt.% and 0.5 wt.%, better agreement between the predicted and experimental results was observed (red arrows). For instance, the difference between the predicted and experimental values is 4.38 ±0.07 N for 6NC0A. However, a large difference of 159.93 ± 1.83 N was observed for 4.5NC1.5A. It is important to note that the predicted forces do not perfectly match the experimental forces, as indicated by the deviation of data points from the dashed reference line (100% match).
Figure 9.
(a) Experiment setup for injection force test, where a 3 mL syringe is connected with a 5 F, 100 cm catheter; (b) Comparison of experimental and predicted injection force for different NC/ alginate formulations, red arrows indicate better agreement between experimental and predicted results; (c) Comparison between predicted and experimental injection forces, where the black dashed line indicates a perfect match between predicted and experimental data; and (d) A heatmap of experimental injection force. An orange dashed line was drawn around 60N to show desired injectability vs high injection forces.
The mismatch between the predicted and experimental forces can be attributed to various factors. In this study, power law and Hagen-Poiseuille law were used to estimate injection forces. These are models that do not fully capture the complexities of the NC/alginate system. Additionally, small deviations in slopes () can result in significant deviations in values of viscosity due to the logarithmic nature of the power law equation. For better prediction and correlation, an improved model that can better capture the flow behavior of the gels is needed for future work.
An experimental heatmap showing the injection force was plotted in Figure 9 (d). The heatmap visually represents injection force variations through color gradations, where darker colors indicate higher injection forces, with lighter shades indicating lower forces. The heatmap reveals the positive correlation between the injection force and the NC/alginate content. By drawing a dashed line around 60N on the heatmap, the injectable formulations are situated to the left of this line, indicating forces below 60N. This map allows physicians to identify formulations meeting clinical requirements as well as a smoother injection process. Considering the acceptable injection force (<60N) [12,15,50] and the yield stress to withstand the peak wall shear stress in the blood vessels (>4.3 Pa) [41], 5NC0A and 4.5NC0.5A gels were selected for further research.
3.4. Displacement pressure
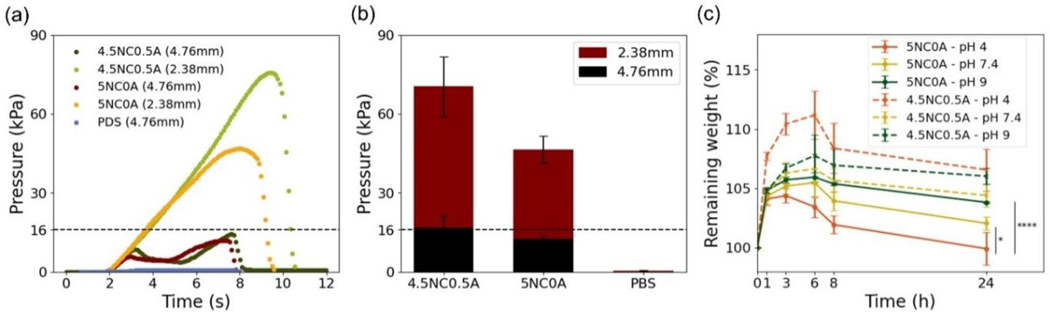
Displacement pressure plays a critical role in evaluating the capability of embolic agents to withstand hemodynamic forces within the bloodstream. It provides a quantitative measure on the embolic’s resistance to migration, as distal dislodgement can cause pulmonary embolism and stroke [9,11]. To assess the displacement pressure, 5NC0A and 4.5NC0.5A gels were tested using soft tubes with varying diameters, mimicking the sizes of the vasculature to be embolized. As an example, to simulate renal arteries with a mean diameter of 3.9mm [53], a tube with a diameter of 4.76mm (3/16”) was used. To simulate structures like left anterior descending artery which has a diameter of 2.14 mm [54,55], a tube of 2.38 mm (3/32”) in diameter was selected.
As is shown in Figure 10 (a–b), for 2.38 mm diameter tubes, 4.5NC0.5A gel showed a displacement pressure of 70.51 ± 11.35 kPa, which corresponds to approximately 4.4 times the normal blood pressure in humans (16 kPa or 120 mmHg). For the same tube, the pressure for the 5NC0A gel was recorded at 46.42 ± 5.14 kPa, corresponding to approximately 2.9 times the human blood pressure. These values indicate that both gels have a high resistance to dislodgment, as they require pressures significantly higher than the threshold of physiological pressure [6]. For large 4.76mm tubes, the displacement pressure for the 4.5NC0.5A gel was 16.73±4.44 kPa, which barely exceeds the 16 kPa threshold. Whereas the displacement pressure for the 5NC0A gel was 12.67±1.03 kPa, which is below the physiological pressure. The actual displacement pressure depends on many factors, including the actual amount of embolic agent being injected, the vasculature geometry and embolic-tissue interactions. Additional in-vitro and in-vivo work is needed to evaluate the mechanical stability of the embolic agent.
Figure 10.
(a) Representative displacement curves and (b) summary of displacement pressure of 5NC0A and 4.5NC0.5A. PBS was used as control and (c) Degradation kinetics of 5NC0A and 4.5NC0.5A over 24 hours. Data are presented as mean ± standard deviation (n = 3 for (b) and n=4 for (c)). *p<0.5 and ****p<0.0001.
These results demonstrate that both the 4.5NC0.5A and 5NC0A gels exhibit the necessary characteristics to withstand the shear forces and the pressures experienced within blood vessels. Moreover, their ability to resist displacement suggests that they have the potential to effectively prevent distal migration after being delivered to the target site. These findings highlight the suitability of the 4.5NC0.5A and 5NC0A gels as embolic agents in minimally invasive procedures, where their capacity to withstand physiological forces and maintain stability is crucial for successful occlusion. It is important to note that the displacement pressure serves as a final combined measurement to evaluate gel’s stability to resist physiological pressure. The individual contributing components, such as adhesion force (i.e. between the gel and tube wall), a determinate factor attributing to the final displacement pressure, requires further investigation in future work.
3.5. Degradation
Blood and normal tissues usually maintain a constant pH of 7.4, whereas tumors exhibit a mildly acidic environment ranging between pH of 6.4–7.0 [56]. Hence, assessing the stability of the developed embolic agent across various pH environments is beneficial.
As is shown in Figure 10 (c), the remaining weight of all gels at different pH levels initially increased before declining. A minimal weight change was observed for embolic agent 5NC0A and 4.5NC0.5A across three distinct pH levels (4, 7.4 and 9) at 24 hours. At the 24-hour mark, no statistically significant difference in weight was observed for 4.5NC0.5A across all pH levels (p > 0.645). Additionally, 5NC0A demonstrated no significant difference between pH = 7.4 and pH = 9 (p = 0.1169). Instead, a statistically significant difference was detected in 5NC0A between pH 4 and 7.4 (p = 0.0178), and also pH 4 and 9 (p < 0.0001), respectively. These findings suggest initial stability with a marginal weight increase within the initial 24 hours, potentially due to moisture absorption.
3.6. In-Vitro Characterization of NC/alginate gels
3.6.1. Sterility
Inherent sterility is crucial to ensure the safety and suitability of embolic agents in a biological environment. The sterility of 5NC0A and 4.5NC0.5A was evaluated as shown in Figure 11 (a). The sterility test was conducted by measuring the optical density at 600 nm (OD600) for each sample, including the negative control (LB broth) as well as the gels. The OD600 values for the negative control and both gels were 0. Notably, no significant differences were observed among the negative control (LB broth), 5NC0A, and 4.5NC0.5A. In contrast, a significant difference was evident when compared to E-coli (p < 0.0001). These results confirmed the inherent sterility of the embolic gels.
Figure 11.
(a) Sterility test results demonstrate inherent sterility of 5NC0A and 4.5NC0.5A, using LB broth (negative control) and E. coli (positive control); (b) Hemolysis result reveals hemocompatibility of both gels, and (c) Thrombogenicity result indicates gel’s clotting capability being comparable to clinically utilized coils. Statistical analysis was done using one-way ANOVA with multiple comparisons for (a) and unpaired t-tests for (b). ns – not significant; ****p<0.0001. Data was reported as mean ± standard deviation (s.d.) in (a) (n=3) and (b) (n=3).
3.6.2. Hemocompatibility and Thrombogenicity
Blood hemocompatibility and thrombogenicity are critical biological characteristics to study for any embolic materials on account of their direct contact with blood. Hemolysis assays have been widely used as a reliable measure for hemocompatibility.[57]. This evaluation serves as a measure against possible complications such as kidney damage [58] and anemia [59]. Some of the potential consequences of hemolysis include red blood cell disorder thrombosis and infections [60]. In this study, the hemocompatibility of the 5NC0A and 4.5NC0.5A was assessed according to standard ISO-10993–4 [27]. The hemolysis rates were measured to be 1.5% ± 0.9% for 5NC0A and 1.3% ± 0.4% for 4.5NC0.5A, as shown in Figure 11 (b). Statistical analysis indicated no significant difference in hemolysis rate between the two gels (p=0.76). The hemolysis rate of 5NC0A and 4.5NC0.5A are both well below 5%, which are considered permissible [27]. The result indicates the excellent hemocompatibility for both gels.
Thrombogenicity refers to a material’s capability to promote clotting [61]. For instance, clot formation helps reduce the risk of insufficient volumetric packing and organization of thrombus during aneurysm embolization, reducing the chances of recanalization [62]. The thrombogenicity assay, shown in Figure 11 (c), demonstrated the progression of blood clot formation on gels and clinically used coils. Blood in contact with 5NC0A and 4.5NC0.5A showed complete coagulation at 7 minutes, similar to that of coils. These results suggest their comparable clotting capability compared to clinically used coils.
3.6.3. Cell viability
Cell viability assessments are essential to determine the biocompatibility of embolic gels. The biocompatibility of 5NC0A and 4.5NC0.5A gels was evaluated using L-929 fibroblast cells, following ISO-10993–5 [28], where cell viability larger than 70 % is considered non-cytotoxic. In addition, HUVEC cells were included in the study since they represent vascular endothelial cells which gel embolic will be in direct contact with upon deployment.
As shown in Figure 12 (a), by subjecting gel extractions to L-929 fibroblast cells, all cell viabilities were above 70%, indicating biocompatibility of 5NC0A and 4.5NC0.5A. Statistical analysis indicated no significant difference in cell viability between different extraction dilutions of the gels (p ≥ 0.17). On the other hand, cell viability of HUVEC showed that 100% extraction of both 5NC0A and 4.5NC0.5A gels resulted in cell viability of 55.4% ± 2.6% and 67.1% ± 5.5%, respectively. These values fall within the cytotoxic range of 50% to 70%, indicating a potential acute toxic effect on HUVEC [28]. Significant differences were observed in cell viability between different extraction dilutions of the gels. For 5NC0A, there were significant differences (p<0.0001) in cell viability between the 100% extraction and 50%, 25%, and 12.5% dilutions, indicating concentration-dependent cell viability. For 4.5NC0.5A, there was no significant difference in cell viability between 100% and 50% extractions, but between the 100% and 25% (p=0.0005), as well as 12.5% (p<0.0001) dilutions.
Figure 12.
Cell viability of (a) L-929 and (b) HUVEC cells after incubating with 100%, 50%, 25%, and 12.5% extraction from 5NC0A and 4.5NC0.5A respectively. Statistical significance was determined using one-way ANOVA with multiple comparison. ns, not significant; **P < 0.01, ***P < 0.001, and ****P < 0.0001. Data is reported as average ± standard deviation (s.d.) (n=4).
Despite the potential toxicity to HUVEC invitro, it is important to highlight that NC/alginate gels may exhibit better biocompatibility when compared to liquid embolic agents like Onyx®. Onyx® is a commonly used liquid embolic agent in clinic and the major limitation of Onyx® system is associated with the use of an organic solvent DMSO [63,64]. Onyx® usually contains over 30% of DMSO [65], which has exhibited both local and systemic cardiovascular toxicity [66]. The positive control in this study contained 10% DMSO, which is one third of the amount in Onyx®. Therefore, in-vivo studies are clearly needed to further our understanding on the biocompatibility of NC/alginate.
4. Conclusion
This study successfully developed embolic agents through the electrostatic interactions between NC particles and alginate. The shear-thinning property of these NC/alginate embolic agents allows them to shift between a liquid and a solid state, enabling smooth catheter delivery and stable occlusion at the target site. Rheology tests enabled formulation optimization, maintaining a high yield stress to withstand physiological pressure. Moreover, the gels’ ability to recover during intermittent injections or changes in the body’s environment enhances their efficacy throughout the procedure. A reasonable correlation was obtained for transcatheter injectability between experimental results and theoretical studies, allowing for predictive selection of desired gels with optimum deliverability. NC/alginate system further demonstrated inherent sterility, hemocompatibility, thrombogenicity and cell biocompatibility. In conclusion, this study presents a versatile, safe, and effective shear-thinning embolic platform that can be tailored for transcatheter embolization procedures.
Supplementary Material
Highlights.
A group of nanoclay-alginate gels was developed as shear-thinning embolic agents.
The gels exhibited tunable rheological properties for varying embolization conditions.
Transcatheter injectability was assessed for improved physician experience.
The embolic gels showed good stability, sterility, hemo- and bio-compatibility.
Acknowledgement
The authors are grateful to North Carolina State University, the Ralph E. Powe Junior Faculty Enhancement Award, North Carolina Biotechnology Center, and the National Institutes of Health (NIBIB 1R03EB033633-01A1 and NIA 1R21AG083692-01) for financial support.
Abbreviations:
- NC
Laponite nanoclay
- DLS
Dynamic light scattering
- LAOS
Large-amplitude oscillatory shear
- LB
Luria-Bertani
- PBS
Phosphate-buffered saline
- E. coli
Escherichia coli
- HUVEC
Human umbilical vein endothelial cells
- A
Absorbance at a wavelength of 550 nm
Footnotes
CRediT authorship contribution statement
Keren Zhao - Conceptualization, Investigation, Formal Analysis, Validation, Writing; George Varghese P J - Investigation, Formal Analysis, Validation, Review & Editing; Peng Chen – Investigation, Formal Analysis, Validation, Review & Editing; Jingjie Hu - Conceptualization, Writing, Review & Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Hu J, Albadawi H, Chong BW, Deipolyi AR, Sheth RA, Khademhosseini A, Oklu R, Advances in biomaterials and technologies for vascular embolization, Advanced Materials 31 (2019) 1901071. 10.1002/adma.201901071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alifano M, Gaucher S, Rabbat A, Brandolini J, Guinet C, Damotte D, Regnard JF, Alternatives to Resectional Surgery for Infectious Disease of the Lung. From Embolization to Thoracoplasty, Thorac Surg Clin 22 (2012) 413–429. 10.1016/j.thorsurg.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [3].Lopera J, Embolization in Trauma: Principles and Techniques, Semin Intervent Radiol 27 (2010) 014–028. 10.1055/s-0030-1247885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Topol EJ, Yadav JS, Recognition of the Importance of Embolization in Atherosclerotic Vascular Disease, Circulation 101 (2000) 570–580. 10.1161/01.CIR.101.5.570. [DOI] [PubMed] [Google Scholar]
- [5].Li X, Ullah MW, Li B, Chen H, Recent Progress in Advanced Hydrogel-Based Embolic Agents: From Rational Design Strategies to Improved Endovascular Embolization, Adv Healthc Mater 12 (2023). 10.1002/adhm.202202787. [DOI] [PubMed] [Google Scholar]
- [6].Robinson SC, Range of normal blood pressure, Arch Intern Med 64 (1939) 409. 10.1001/archinte.1939.00190030002001. [DOI] [Google Scholar]
- [7].Müller B, Lang S, Dominietto M, Rudin M, Schulz G, Deyhle H, Germann M, Pfeiffer F, David C, Weitkamp T, High-resolution tomographic imaging of microvessels, in: Developments in X-Ray Tomography VI, SPIE, 2008: p. 70780B. 10.1117/12.794157. [DOI] [Google Scholar]
- [8].Simon SD, Reig AS, James RF, Reddy P, Mericle RA, Relative cost comparison of embolic materials used for treatment of wide-necked intracranial aneurysms, J Neurointerv Surg 2 (2010) 163–167. 10.1136/jnis.2009.001719. [DOI] [PubMed] [Google Scholar]
- [9].Ko G, Choi JW, Lee N, Kim D, Hyeon T, Kim HC, Recent progress in liquid embolic agents, Biomaterials 287 (2022). 10.1016/j.biomaterials.2022.121634. [DOI] [PubMed] [Google Scholar]
- [10].Leyon JJ, Littlehales T, Rangarajan B, Hoey ET, Ganeshan A, Endovascular Embolization: Review of Currently Available Embolization Agents, Curr Probl Diagn Radiol 43 (2014) 35–53. 10.1067/j.cpradiol.2013.10.003. [DOI] [PubMed] [Google Scholar]
- [11].Vaidya S, Tozer K, Chen J, An Overview of Embolic Agents, Semin Intervent Radiol 25 (2008) 204–215. 10.1055/s-0028-1085930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Astin AD, Finger force capability: measurement and prediction using anthropometric and myoelectric measures, Virginia Tech, 1999. http://hdl.handle.net/10919/30923 (accessed June 21, 2023). [Google Scholar]
- [13].Allmendinger A, Fischer S, Huwyler J, Mahler H-C, Schwarb E, Zarraga IE, Mueller R, Rheological characterization and injection forces of concentrated protein formulations: An alternative predictive model for non-Newtonian solutions, European Journal of Pharmaceutics and Biopharmaceutics 87 (2014) 318–328. 10.1016/j.ejpb.2014.01.009. [DOI] [PubMed] [Google Scholar]
- [14].Rathore N, Pranay P, Bernacki J, Eu B, Ji W, Walls E, Characterization of protein rheology and delivery forces for combination products., J Pharm Sci 101 (2012) 4472–80. 10.1002/jps.23297. [DOI] [PubMed] [Google Scholar]
- [15].Watt RP, Khatri H, Dibble ARG, Injectability as a function of viscosity and dosing materials for subcutaneous administration, Int J Pharm 554 (2019) 376–386. 10.1016/j.ijpharm.2018.11.012. [DOI] [PubMed] [Google Scholar]
- [16].Ahmad Raus R, Wan Nawawi WMF, Nasaruddin RR, Alginate and alginate composites for biomedical applications, Asian J Pharm Sci 16 (2021) 280–306. 10.1016/j.ajps.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Das SS, Neelam K. Hussain S. Singh A. Hussain A. Faruk M. Tebyetekerwa, Laponite-based Nanomaterials for Biomedical Applications: A Review, Curr Pharm Des 25 (2019) 424–443. 10.2174/1381612825666190402165845. [DOI] [PubMed] [Google Scholar]
- [18].Lee KY, Mooney DJ, Alginate: Properties and biomedical applications, Prog Polym Sci 37 (2012) 106–126. 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Afghah F, Altunbek M, Dikyol C, Koc B, Preparation and characterization of nanoclayhydrogel composite support-bath for bioprinting of complex structures, Sci Rep 10 (2020). 10.1038/s41598-020-61606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rezende RA, Bártolo PJ, Mendes A, Filho RM, Rheological behavior of alginate solutions for biomanufacturing, J Appl Polym Sci 113 (2009) 3866–3871. 10.1002/app.30170. [DOI] [Google Scholar]
- [21].Paar Anton, The principles of dynamic light scattering, (n.d.). https://wiki.anton-paar.com/en/the-principles-of-dynamic-light-scattering/ (accessed July 9, 2023).
- [22].Chen MH, Wang LL, Chung JJ, Kim Y-H, Atluri P, Burdick JA, Methods To Assess Shear-Thinning Hydrogels for Application As Injectable Biomaterials, ACS Biomater Sci Eng 3 (2017) 3146–3160. 10.1021/acsbiomaterials.7b00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang L, Application of fractal theory in transient pressure properties of hydrocarbon reservoir, in: Modelling of Flow and Transport in Fractal Porous Media, Elsevier, 2021: pp. 193–249. 10.1016/B978-0-12-817797-6.00008-7. [DOI] [Google Scholar]
- [24].Hu J, Altun I, Zhang Z, Albadawi H, Salomao MA, Mayer JL, Hemachandra LPMP, Rehman S, Oklu R, Bioactive-tissue-derived nanocomposite hydrogel for permanent arterial embolization and enhanced vascular healing, Advanced Materials 32 (2020). 10.1002/adma.202002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Caballero-Hernandez J, Gomez-Ramirez A, Duran JDG, Gonzalez-Caballero F, Zubarev AY, Lopez-Lopez MT, On the effect of wall slip on the determination of the yield stress of magnetorheological fluids, Applied Rheology 27 (2017). 10.3933/ApplRheol-27-15001. [DOI] [Google Scholar]
- [26].Dinkgreve M, Paredes J, Denn MM, Bonn D, On different ways of measuring “the” yield stress, J Nonnewton Fluid Mech 238 (2016) 233–241. 10.1016/j.jnnfm.2016.11.001. [DOI] [Google Scholar]
- [27].International Organization for Standardization (ISO), ISO 10993–4: Biological evaluation of medical devices - Part 4: Selection of tests for interactions with blood, in: ISO, 2017. [Google Scholar]
- [28].International Organization for Standardization (ISO), ISO 10993–5: Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity, in: 2009. [Google Scholar]
- [29].Liang M, Lin I-C, Whittaker MR, Minchin RF, Monteiro MJ, Toth I, Cellular uptake of densely packed polymer coatings on gold nanoparticles, ACS Nano 4 (2010) 403–413. 10.1021/nn9011237. [DOI] [PubMed] [Google Scholar]
- [30].Raval N, Maheshwari R, Kalyane D, Youngren-Ortiz SR, Chougule MB, Tekade RK, Importance of physicochemical characterization of nanoparticles in pharmaceutical product development, in: Basic Fundamentals of Drug Delivery, Elsevier, 2019: pp. 369–400. 10.1016/B978-0-12-817909-3.00010-8. [DOI] [Google Scholar]
- [31].Kumar A, Dixit CK, Methods for characterization of nanoparticles, in: Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids, Elsevier, 2017: pp. 43–58. 10.1016/B978-0-08-100557-6.00003-1. [DOI] [Google Scholar]
- [32].Norde W, Colloids and interfaces in life sciences and bionanotechnology, 2011. [Google Scholar]
- [33].Cosgrove T, Colloid science: Principles, methods and applications, 2010. [Google Scholar]
- [34].Kim Y-H, Yang X, Shi L, Lanham SA, Hilborn J, Oreffo ROC, Ossipov D, Dawson JI, Bisphosphonate nanoclay edge-site interactions facilitate hydrogel self-assembly and sustained growth factor localization., Nat Commun 11 (2020) 1365. 10.1038/s41467-020-15152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sperling LH, Introduction to Physical Polymer Science, Wiley, 2005. 10.1002/0471757128. [DOI] [Google Scholar]
- [36].Nishida T, Endo H, Osaka N, Li H, Haraguchi K, Shibayama M, Deformation mechanism of nanocomposite gels studied by contrast variation small-angle neutron scattering, Phys Rev E 80 (2009) 030801. 10.1103/PhysRevE.80.030801. [DOI] [PubMed] [Google Scholar]
- [37].Dávila JL, d’Ávila MA, Laponite as a rheology modifier of alginate solutions: Physical gelation and aging evolution, Carbohydr Polym 157 (2017) 1–8. 10.1016/j.carbpol.2016.09.057. [DOI] [PubMed] [Google Scholar]
- [38].Pang B, Wang S, Chen W, Hassan M, Lu H, Effects of flow behavior index and consistency coefficient on hydrodynamics of power-law fluids and particles in fluidized beds, Powder Technol 366 (2020) 249–260. 10.1016/j.powtec.2020.01.061. [DOI] [Google Scholar]
- [39].Hu J, Albadawi H, Zhang Z, Salomao MA, Gunduz S, Rehman S, D’Amone L, Mayer JL, Omenetto F, Oklu R, Silk embolic material for catheter-directed endovascular drug delivery, Advanced Materials 34 (2022) 2106865. 10.1002/adma.202106865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pnas S, Transition between solid and liquid state of yield-stress fluids under purely extensional deformations, 117 (2020) 12611–12617. 10.1073/pnas.1922242117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Samijo SK, Willigers JM, Barkhuysen R, Kitslaar PJEHM, Reneman RS, Brands PJ, Hoeks APG, Wall shear stress in the human common carotid artery as function of age and gender, Cardiovasc Res 39 (1998) 515–522. 10.1016/S0008-6363(98)00074-1. [DOI] [PubMed] [Google Scholar]
- [42].Koumakis N, Petekidis G, Two step yielding in attractive colloids: transition from gels to attractive glasses, Soft Matter 7 (2011) 2456. 10.1039/c0sm00957a. [DOI] [Google Scholar]
- [43].Dijkstra M, Hansen JP, Madden PA, Gelation of a Clay Colloid Suspension, n.d. [DOI] [PubMed] [Google Scholar]
- [44].Kensbock P, Demco DE, Singh S, Rahimi K, Fechete R, Walther A, Schmidt AM, Möller M, Peptizing Mechanism at the Molecular Level of Laponite Nanoclay Gels, Langmuir 33 (2017) 66–74. 10.1021/acs.langmuir.6b03592. [DOI] [PubMed] [Google Scholar]
- [45].Xu J, Liu X, Ren X, Gao G, The role of chemical and physical crosslinking in different deformation stages of hybrid hydrogels, Eur Polym J 100 (2018) 86–95. 10.1016/j.eurpolymj.2018.01.020. [DOI] [Google Scholar]
- [46].Fuss FK, The Loss Tangent of Visco-Elastic Models, in: Nonlinear Approaches in Engineering Applications, Springer International Publishing, Cham, 2015: pp. 137–157. 10.1007/978-3-319-09462-5_6. [DOI] [Google Scholar]
- [47].Ross-Murphy SB, Structure–property relationships in food biopolymer gels and solutions, J Rheol (N Y N Y) 39 (1995) 1451–1463. 10.1122/1.550610. [DOI] [Google Scholar]
- [48].Ikeda S, Nishinari K, “Weak gel”-type rheological properties of aqueous dispersions of nonaggregated kappa-carrageenan helices., J Agric Food Chem 49 (2001) 4436–41. 10.1021/jf0103065. [DOI] [PubMed] [Google Scholar]
- [49].Murata H, Rheology - Theory and Application to Biomaterials, in: Polymerization, InTech, 2012. 10.5772/48393. [DOI] [Google Scholar]
- [50].Vo A, Doumit M, Rockwell G, The biomechanics and optimization of the needle-syringe system for injecting triamcinolone acetonide into keloids, J Med Eng 2016 (2016) 1–8. 10.1155/2016/5162394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jernigan SR, Osborne JA, Buckner GD, Gastric artery embolization: studying the effects of catheter type and injection method on microsphere distributions within a benchtop arterial model, Biomed Eng Online 19 (2020) 54. 10.1186/s12938-020-00794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Becker TA, Kipke DR, Flow properties of liquid calcium alginate polymer injected through medical microcatheters for endovascular embolization, J Biomed Mater Res 61 (2002) 533–540. 10.1002/jbm.10202. [DOI] [PubMed] [Google Scholar]
- [53].Douglas PS, Fiolkoski J, Berko B, Reichek N, Echocardiographic visualization of coronary artery anatomy in the adult, J Am Coll Cardiol 11 (1988) 565–571. 10.1016/0735-1097(88)91532-X. [DOI] [PubMed] [Google Scholar]
- [54].Zhou F-F, Liu Y, Ge P-C, Chen Z-H, Ding X-Q, Liu J-Y, Jia Q-W, An F-H, Li L-H, Wang L-S, Ma W-Z, Yang Z-J, Jia E-Z, Coronary Artery Diameter is Inversely Associated with the Severity of Coronary Lesions in Patients Undergoing Coronary Angiography, Cellular Physiology and Biochemistry 43 (2017) 1247–1257. 10.1159/000481765. [DOI] [PubMed] [Google Scholar]
- [55].Giri S, Hwang I, Alsafwah S, A case of left main coronary artery embolus further embolising to the left anterior descending artery, Case Reports 2014 (2014) bcr2013203159–bcr2013203159. 10.1136/bcr-2013-203159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hao G, Xu ZP, Li L, Manipulating extracellular tumour pH: An effective target for cancer therapy, RSC Adv 8 (2018) 22182–22192. 10.1039/c8ra02095g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Weber M, Steinle H, Golombek S, Hann L, Schlensak C, Wendel HP, Avci-Adali M, Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility, Front Bioeng Biotechnol 6 (2018). 10.3389/fbioe.2018.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Qian Q, Nath KA, Wu Y, Daoud TM, Sethi S, Hemolysis and acute kidney failure, American Journal of Kidney Diseases 56 (2010) 780–784. 10.1053/j.ajkd.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gehrs BC, Friedberg RC, Autoimmune hemolytic anemia, Am J Hematol 69 (2002) 258–271. 10.1002/ajh.10062. [DOI] [PubMed] [Google Scholar]
- [60].Fattizzo B, Barcellini W, Autoimmune hemolytic anemia: causes and consequences, Expert Rev Clin Immunol 18 (2022) 731–745. 10.1080/1744666X.2022.2089115. [DOI] [PubMed] [Google Scholar]
- [61].Braune S, Lendlein A, Jung F, Developing standards and test protocols for testing the hemocompatibility of biomaterials, in: Hemocompatibility of Biomaterials for Clinical Applications: Blood-Biomaterials Interactions, Elsevier, 2017: pp. 51–76. 10.1016/B978-0-08-100497-5.00004-5. [DOI] [Google Scholar]
- [62].Cornelissen SA, Verhagen HJM, van Herwaarden JA, Vonken E-JPA, Moll FL, Bartels LW, Lack of thrombus organization in nonshrinking aneurysms years after endovascular abdominal aortic aneurysm repair, J Vasc Surg 56 (2012) 938–942. 10.1016/j.jvs.2012.03.015. [DOI] [PubMed] [Google Scholar]
- [63].Vollherbst DF, Chapot R, Bendszus M, Möhlenbruch MA, Glue, Onyx, Squid or PHIL? Liquid Embolic Agents for the Embolization of Cerebral Arteriovenous Malformations and Dural Arteriovenous Fistulas, Clin Neuroradiol 32 (2022) 25–38. 10.1007/s00062-021-01066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Vollherbst DF, Sommer CM, Ulfert C, Pfaff J, Bendszus M, Möhlenbruch MA, Liquid Embolic Agents for Endovascular Embolization: Evaluation of an Established (Onyx) and a Novel (PHIL) Embolic Agent in an In Vitro AVM Model, American Journal of Neuroradiology 38 (2017) 1377–1382. 10.3174/ajnr.A5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Guimaraes M, Wooster M, Onyx (Ethylene-vinyl Alcohol Copolymer) in Peripheral Applications, Semin Intervent Radiol 28 (2011) 350–356. 10.1055/s-0031-1284462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kuroda K, Komori T, Ishibashi K, Uto T, Kobayashi I, Kadokawa R, Kato Y, Ninomiya K, Takahashi K, Hirata E, Non-aqueous, zwitterionic solvent as an alternative for dimethyl sulfoxide in the life sciences, Commun Chem 3 (2020) 163. 10.1038/s42004-020-00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.