Abstract
Objectives:
Although delirium is well described in patients with sepsis, there are limited data on other neurological complications. We aimed to systematically review the prevalence, neuromonitoring tools, and neurocognitive outcomes in sepsis patients with neurological complications.
Data Sources:
MEDLINE and six other databases (Embase, Web of Science, Cochrane CENTRAL, and ClinicalTrials.gov) were searched through January 2023.
Study Selection: Studies of adult patients with sepsis reported neurological complications, use of neuromonitoring tools, neuropathology, and cognitive outcomes.
Data Extraction:
Two independent reviewers extracted the data. Random-effect meta-analyses were used to pool data.
Data Synthesis:
Seventy-four studies (n=146,855) were included. Neurological complications were reported in 38 studies (n=142,193) including septic encephalopathy [36%, 95% confidence interval (CI)=27–46%; I2=99%], ischemic stroke (5%, 95% CI=2.1–11.5; I2=99%), intracranial hemorrhage (2%, 95% CI=1.0–4.4%; I2=96%), seizures (1%, 95%CI=0.2–7%; I2=96%), posterior reversible encephalopathy syndrome (9%), and hypoxic-ischemic brain injury (7%). In the meta-regression analysis, pulmonary infection, sepsis induced by a gram-positive organism, higher sequential organ failure assessment score, acute physiology and chronic health evaluation II score on admission, and longer intensive care unit length of stay were associated with higher risk of developing septic encephalopathy. Three studies (n=159) reported post-mortem neuropathological findings, acute brain injury was noted in 47% of patients. Twenty-six studies (n=1,358) reported the use of neuromonitoring tools, electroencephalogram was the most utilized tool for seizure detection. Transcranial Doppler and near infrared spectroscopy were used for monitoring cerebral hemodynamic changes to detect early ischemia. Six studies reported cognitive outcomes (n=415) up to 12 months post-discharge and cognitive impairment (≥ one domain) was reported in 30%.
Conclusions
In-hospital neurological complications are common in patients with sepsis. However, the mechanism and timing of those sepsis-associated complications are poorly understood and there are limited data on standardized neuromonitoring in this population.
Keywords: sepsis, septic shock, acute brain injury, neurologic complications, septic encephalopathy, stroke
INTRODUCTION
Sepsis remains a leading cause of morbidity and mortality worldwide.1 End organ injury in sepsis occurs from a dysregulated inflammatory response to an infectious insult, resulting in organ hypoperfusion and tissue hypoxia.2,3 Animal studies showed endothelial dysfunction, microglial activation, and oxidative injury in the brain within 24 hours of sepsis onset.4 Reported neurological complications of sepsis include prolonged alterations in level of consciousness, seizures, long-term cognitive impairment, and focal deficits from stroke or intracranial hemorrhage.2,3 These complications are associated with increased morbidity and mortality.5 However, treatments targeting the underlying mechanisms of acute brain injuries in sepsis are lacking, as are clear neuromonitoring strategies.
Although the association between sepsis and neurological complications, such as septic encephalopathy, has been well described, there are limited data characterizing the prevalence of other types of neurological complications, neuropathological findings, neuromonitoring strategies and long-term cognitive outcomes. In this study, we conduct a systemic review and meta-analysis to characterize the prevalence of neurological complications in patients with sepsis, neuropathological findings, and long-term cognitive outcomes in sepsis patients. The secondary objective was to investigate the existing neuromonitoring tools in this population.
MATERIALS AND METHODS
The data that support the findings of this study are available from the corresponding author upon request. The corresponding author has full access to all the data in the study and takes responsibility for its integrity and the data analysis. Institutional review board review was not required for this study as it represents a secondary analysis of aggregated datasets and did not directly involve human subject research.
Search Strategy
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.6 Electronic searches for published literature were conducted by a medical librarian (M.L.) using Ovid MEDLINE (1946 to present), Embase.com (1947 to present), Web of Science (1900 to present), Cochrane Central Register of Controlled Trials via Ovid (1991 to present), and ClinicalTrials.gov (1999 to present). The searches were run until November 4th, 2021, and an update was run on January 4th, 2023. The search strategy incorporated controlled vocabulary and free-text synonyms for the concepts of sepsis, septic shock, and neurological complications. The full database search strategies are documented in Appendix A. No restrictions on language or any other search filters were applied. All identified studies were combined and de-duplicated using a single reference manager (EndNote) then uploaded into Covidence systematic review software. These results were then reviewed by the two members of the research team (T.H.F and J.R) for eligibility. All articles that met the inclusion criteria were retrieved and the full text reviewed. References of the included studies were screened. Appendix B provides the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Inclusion and Exclusion Criteria
All randomized controlled trials (RCTs) and observational studies that reported sepsis and/or septic shock in adult patients (≥ 18 years) and acute brain injuries, neurological complications, neuromonitoring were deemed eligible. Studies of patients with sepsis diagnosis based on any of the 3 versions of sepsis definitions (1991, 2001 and 2016) were considered eligible.7,8 We excluded case series/reports, editorials, commentaries, meta-analysis articles, review articles, animal studies, articles not available in English, and articles with a pediatric population (age < 18). We also excluded articles without any description of neurological complications and if neurological complications occurred prior to the sepsis diagnosis. All articles were discussed among authors (T.H.F, and J. R.) before being excluded.
Study Selection and Data Extraction
Two reviewers (T.H.F, and J.R.) independently screened all studies based on study titles and abstracts. Disagreements were resolved through consensus or referral to a third reviewer (S.M.C.). Each study was evaluated independently. Data were extracted from eligible studies into a shared Excel spreadsheet (Microsoft®, Redmond, WA). Information collected included: patient characteristics (number of patients, age, sex, baseline co-morbidities), details of initial sepsis (cause, severity, organisms), prevalence or frequency of neurological complications, acute brain injuries, types of neuromonitoring, neurocognitive outcomes, survival rates, and predictors of neurological complications.
Definitions of Outcomes
Primary outcomes were the occurrence of neurological complications and acute brain injuries in sepsis patients. Neurological complications of interest included: 1) ischemic stroke, 2) intracranial hemorrhage, 3) seizures, 4) hypoxic-ischemic brain injury, 5) posterior reversible encephalopathy syndrome, and 6) septic encephalopathy. Acute brain injuries are histologic evidence of brain injuries including gross or micro ischemic infarct, gross or micro hemorrhagic lesions, leukoencephalopathy, micro-abscesses, and vascular injuries. Septic encephalopathy was defined as cognitive and neuropsychiatric disorders with Glasgow Coma Score (GCS) < 15 or manifestations of delirium (including inattention, disorientation, altered thinking, decreased psychomotor activity, and/or agitation) +/− confirmed by the Confusion Assessment Method for the Intensive Care Unit or Intensive Care Delirium Screening Checklist after excluding other etiologies such as metabolic abnormalities, primary central nervous system disease.9 Secondary outcome includes mortality from hospital discharge to up to 90 days, risk factors associated with development of septic encephalopathy following sepsis, and neurocognitive outcomes.
Quality Assessment/Risk of Bias
Two investigators (T.H.F, and J.R.) independently reviewed and assessed each included study’s risk of bias. All RCTs were assessed with the Cochrane Risk of Bias assessment tool 1.0 for RCTs, the following domains of bias were considered: selection (random sequence generation, allocation concealment), performance, detection (blinding of participants and personnel, and outcome assessment), attrition (incomplete outcome data), and selective outcome reporting.10 We explicitly judged the risk of bias in each criterion as “low,” “high,” or “unclear”.11 If at least one of the domains was rated as high, the trial was considered at high risk of bias. If all domains were judged as low, the trial was considered at low risk of bias. Prevalence studies on neurological complications were assessed using the risk of bias tool (Rob-PrevMH) developed by Tonia et al. based on two domains: selection bias and information bias with each criteria judged as “low,” “high,” or “unclear”. 11 A study was rated as low risk if all criteria were low risk. Observational studies of neuromonitoring tools were assessed with the Newcastle-Ottawa Scale (NOS).12 The NOS scores were based on three domains: patient selection, comparability, and assessment of outcome or exposure. Scores of 0–9 points were allocated to each study. Studies scoring 6 or more points were considered to be of high quality. Any disagreement was resolved in consensus with a third investigator (S.M.C.) for discrepancies.
Statistical Analysis
The preceding analysis was developed upon consultation with all authors. Analyses were computed with R studio statistical software (version 4.2.1). Data were expressed as mean (standard deviation, SD) for continuous variables and number (percentages, %) for categorical variables. Transformations from median (interquartile range, IQR) to estimated mean (SD) for use in the meta-analysis were performed as described by Furukawa et al.13
Meta-analysis was conducted to obtain pooled prevalence estimates for acute brain injuries, neurological complications in sepsis patients, and relevant secondary outcomes. Pooled estimates were obtained using random-effects models, assuming an identity link for continuous variables and a logit link (GLM) for binary variables. Meta-analysis of relative risk (RR) was performed using the overall study effect size and study level moderator variables to estimate the risk of mortality and risk factors for septic encephalopathy. Using the ‘predict’ function, pooled and individual study estimates were adjusted for follow-up time. We used random-effects models to account for expected between-study heterogeneity. Between-study heterogeneity was assessed using the Cochrane Q test, tau, and the Higgins I2 statistic.14Confidence intervals (CIs) for binary outcomes were calculated using Wilson scores with between-study variation estimated using the Hartung–Knapp–Sidik–Jonkman method.15,16 Sensitivity analysis was performed to account for the differences in included study types, patients’ population, and impact of study published year due to changes in sepsis definition and changes in clinical practice impacting outcome.
RESULTS
The search yielded 5,345 citations after duplicates were removed. Following title and abstract screening, 391 articles were eligible for full-text review. Of these studies, 317 were excluded based on the exclusion criteria leaving the final 74 studies (n=146,855 patients). Figure 1 shows the flowchart of the selection process. The studies included 2 RCTs (n=35), 48 prospective observational cohort studies (n=5,792), and 24 retrospective observational cohort studies (n=141,025). The references of the included studies are detailed in Appendix C.
Figure 1.
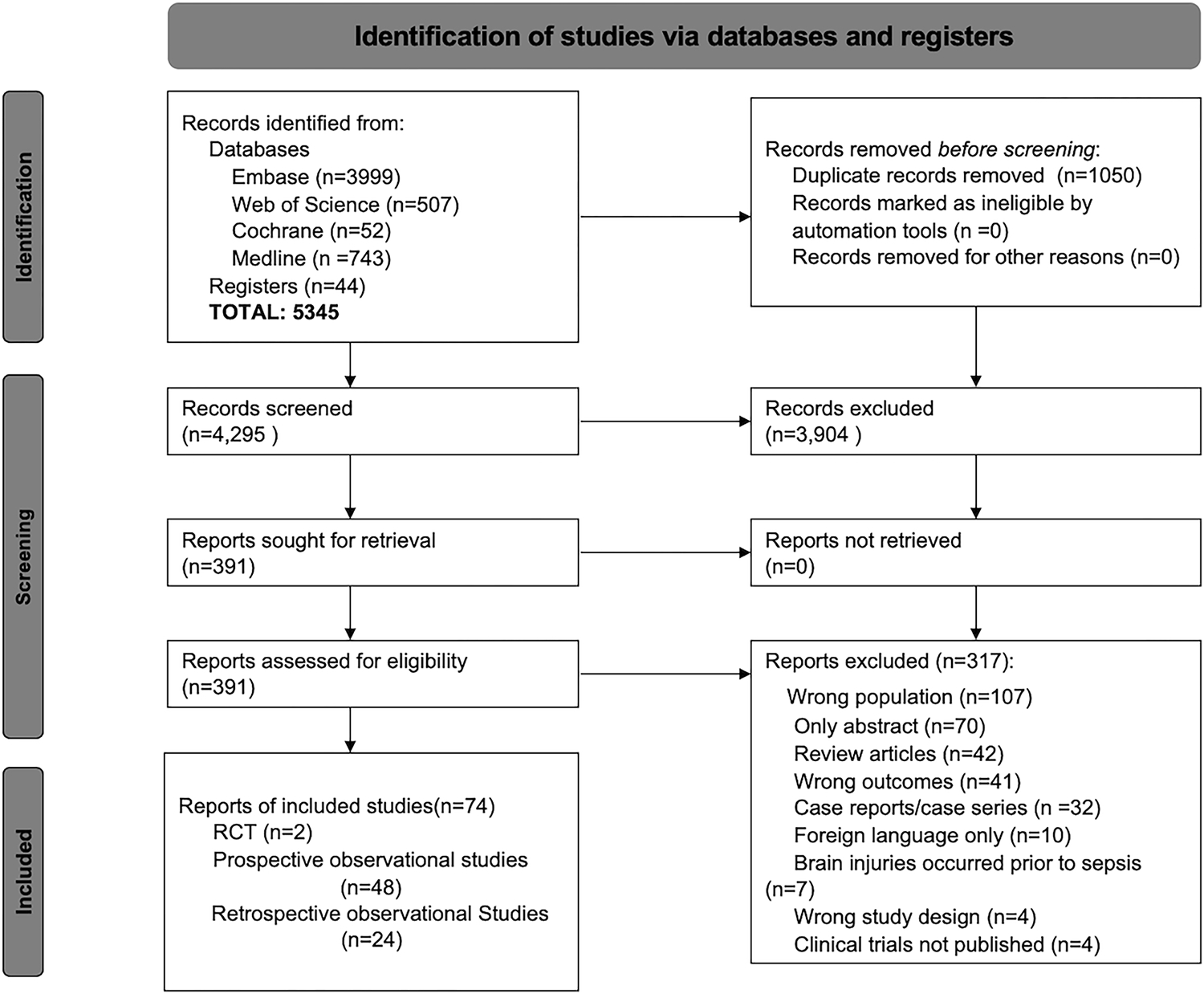
Study flowchart for literature search and selection of studies
*RCT: randomized controlled trial
Among 146,855 patients (median age=62 [57–66], 52% male), 79% suffered from severe sepsis or septic shock, and the overall survival was 73%. The most reported infection source for sepsis was pulmonary (35% of reported), followed by genitourinary infection (n=28% of reported). Table 1 summarizes the baseline characteristics of the sepsis patients included.
Table 1.
Baseline Characteristics of Included Patients
| Demographics | All sepsis patients (n=146,855) |
|---|---|
| Age | 62 (57–66) |
| Male | 76,365 (52%) |
| Hospital Admission characteristics | |
| APACHE II | 19 (15–22) |
| SOFA score | 6 (5.5–8.2) |
| SAPA II | 46 (43–58) |
| Survival at discharge up to 30 days | 15,224 (69%) * |
| Reported source of sepsis, n (%) | |
| Pulmonary | 6,963 (35%) |
| Genitourinary | 5,571 (28%) |
| Gastrointestinal/Biliary | 4,262 (21%) |
| *Other infection | 3,835 (16%) |
| Types of organism, n (%) | |
| Gram-positive organism | 5,208 (28%) |
| Gram-negative, fungal | 7,495 (41%) |
| Not identified | 5,757 (31%) |
| Types of acute brain injury, n (%) | |
| Septic encephalopathy | 10,853 (83%) |
| Ischemic Stroke | 2,294 (18%) |
| Intracranial Hemorrhage | 607 (5%) |
| Seizure | 41 (0.3%) |
| HIE, PRES | 44 (0.4%) |
Age, APACHE II, SOFA, SAPS score are reported in median with interquartile range. Number of male patients and survival at any time are reported in numbers of patients and percentage.
Number of patients survived after sepsis at discharge up to 30 days is based on numbers reported in 18 studies.
Other infection including unidentified source, skin, soft tissue, CNS, bone, surgical site.
The overall percentage does not add up to 1.0 due to some patients suffered from more than one neurological complication.
Abbreviations: APACHE, acute physiology chronic health evaluation; CNS, central nervous system; HIE, hypoxic ischemic encephalopathy; PRES, posterior reversible encephalopathy syndrome; SAPS, simplified acute physiology score; SOFA, sequential organ failure assessment.
Risk of Bias Assessment
Both RCTs were judged to be at high risk for bias due to selection and performance bias (Supplemental Table 1). Sixteen out of forty-eight studies on the prevalence of neurological complications had an overall low risk of bias. Observational studies on neuromonitoring tool have a median NOS score of 6.5 (IQR 5–8). (Supplemental Table 2, 3).
Neurological Complications
Stroke.
Twelve studies (n=123,735) reported ischemic stroke (n=2,289) in sepsis patients. In the meta-analysis, the frequency of ischemic stroke was 5% among sepsis patients (95% CI=2.1–11.5%; I2=99%). Four studies (n=67,191) reported 2% of sepsis patients suffered from intracranial hemorrhage (95% CI=1.0–4.4%; I2=96%) (Figure 2).
Figure 2.
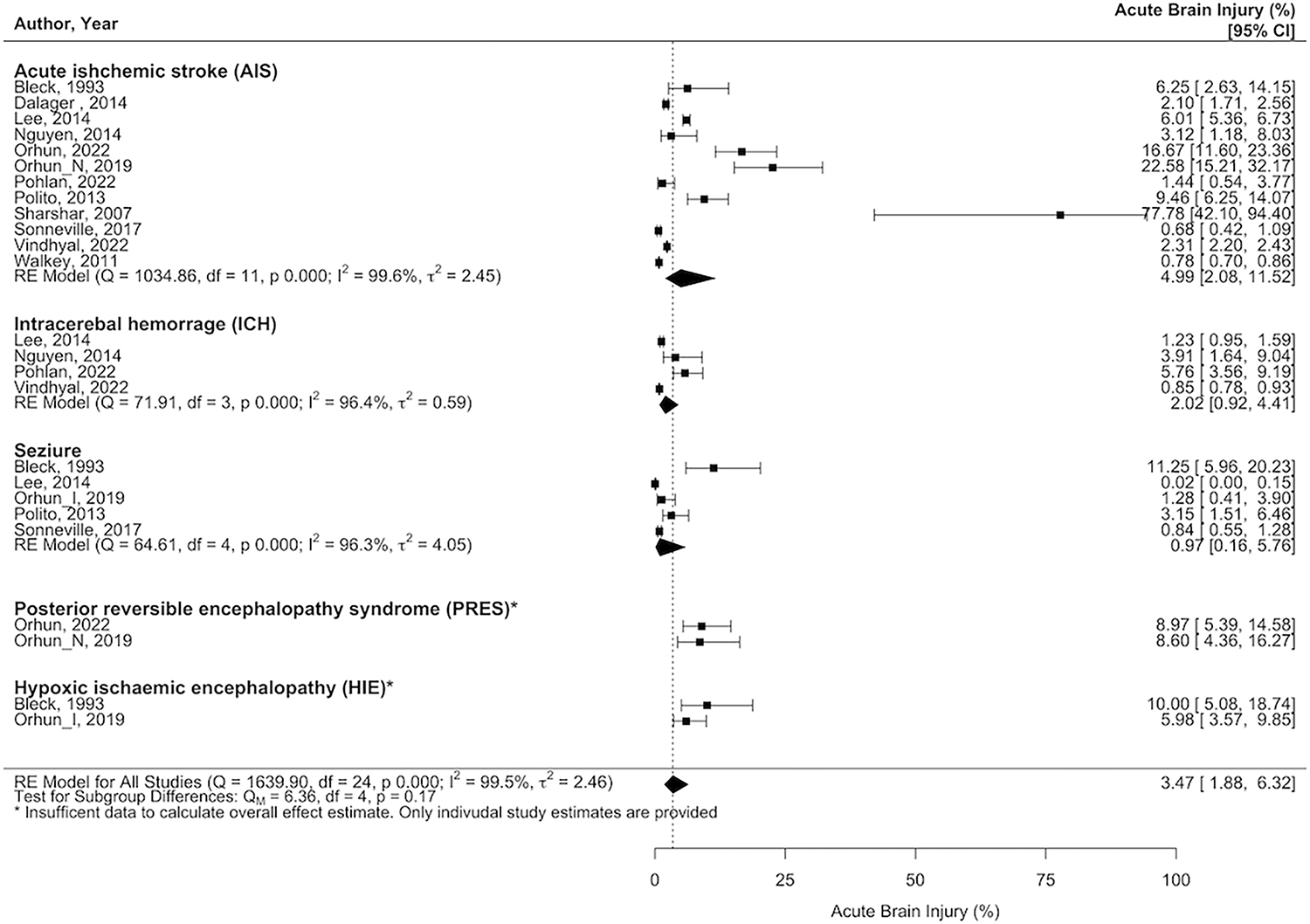
Weighted prevalence of acute ischemic stroke, intracranial hemorrhage, seizure, posterior reversible encephalopathy syndrome and hypoxic-ischemic encephalopathy among studies of patients with sepsis.
Seizure.
Five studies (n=7,536) reported seizures (n=41). In a meta-analysis, the frequency of seizures was 1% (95% CI=0.2–7%; I2=96%) (Figure 2).
Hypoxic-Ischemic Brain Injury.
Two studies (n=314) reported hypoxic-ischemic brain injury (n=22) in patients suffering from sepsis, with a frequency of 7% (Figure 2).
Posterior Reversible Encephalopathy Syndrome.
Only two studies (n=249) reported finding patients with posterior reversible encephalopathy syndrome following sepsis, with a frequency of 9% (n=22) (Figure 2).
The weighted analysis was not performed for the prevalence of posterior reversible encephalopathy syndrome and hypoxic-ischemic brain injury in sepsis patients due to small number of studies (<3 studies).
Septic Encephalopathy.
Thirty studies (n=21,401) reported 10,853 patients suffered from septic encephalopathy following sepsis diagnosis. In a meta-analysis, the frequency of septic encephalopathy was 36% (95%CI=27–46%; I2=99%; Figure 3). Supplemental table 4 provided the detailed description of all the included studies on neurological complications, acute brain injuries, and cognitive outcomes.
Figure 3.
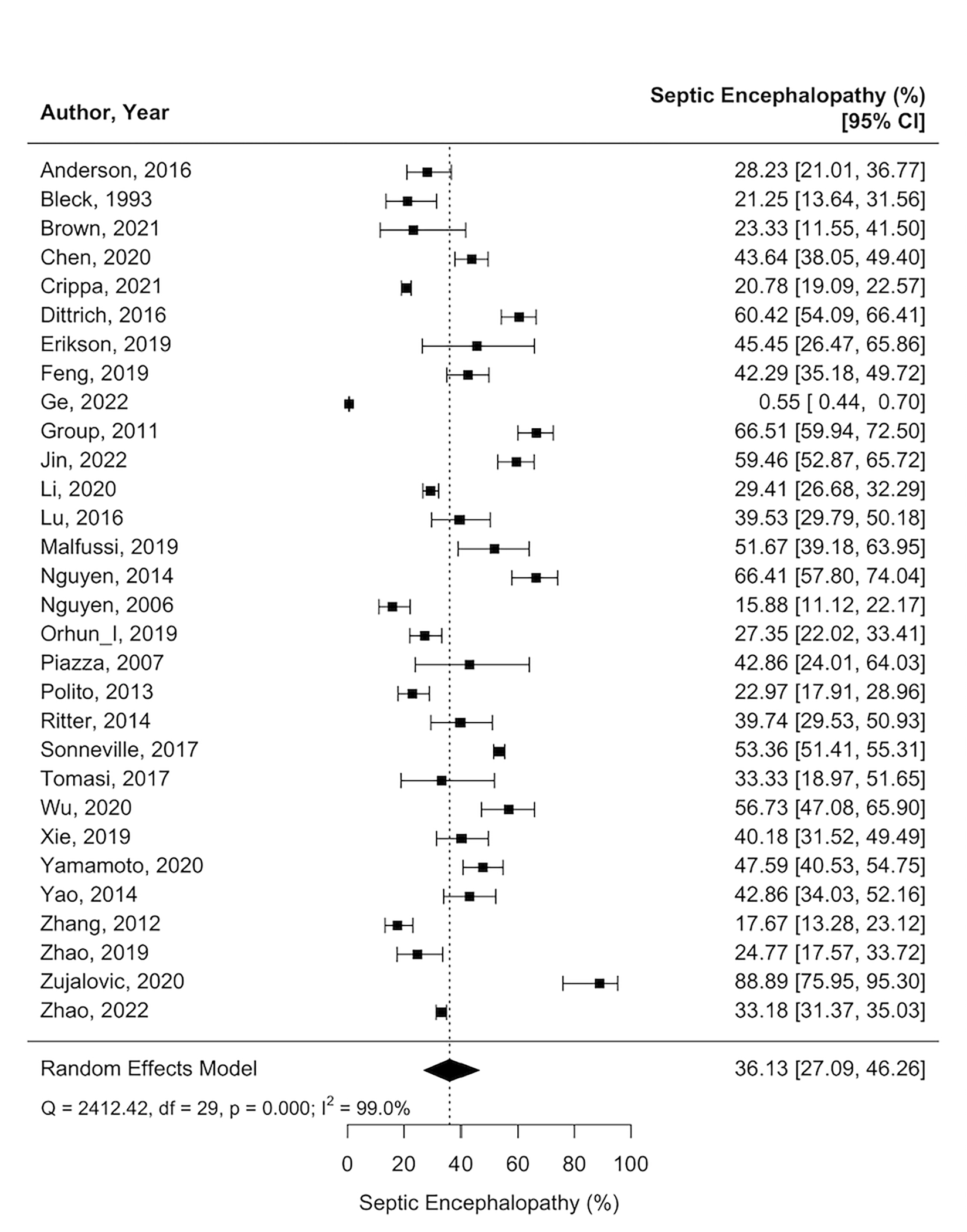
Weighted prevalence of septic encephalopathy among studies of patients with sepsis.
Exploratory Analysis of Mortality Outcome and Risk Factors for Septic Encephalopathy
Nineteen studies (n=20,108) reported characteristics of patients with septic encephalopathy vs. those without. A meta-regression was performed and showed that patients who had pulmonary infection (relative risk [RR]=1.12, 95% CI=1.04–1.20), or sepsis from a gram-positive organism (RR=1.06, 95% CI=1.02–1.09) were associated with a higher risk of developing septic encephalopathy (Supplemental Figure 1). Studies of patients with septic encephalopathy had a higher mean SOFA score (7 vs. 5, p=0.002), and APACHE II score (21 vs. 17, p=0.016) on admission and longer ICU length of stay (10 days vs. 5 days, p<0.001). However, the admission SAPS II, length of sedation, and mechanical ventilation did not differ between the two cohorts (Figure 4). Studies of patients who suffered from septic encephalopathy are at a higher risk of death compared to those without septic encephalopathy (78% vs. 55%, RR=1.64, 95% CI=1.1–2.4) after adjusting for follow-up time (Figure 5).
Figure 4.
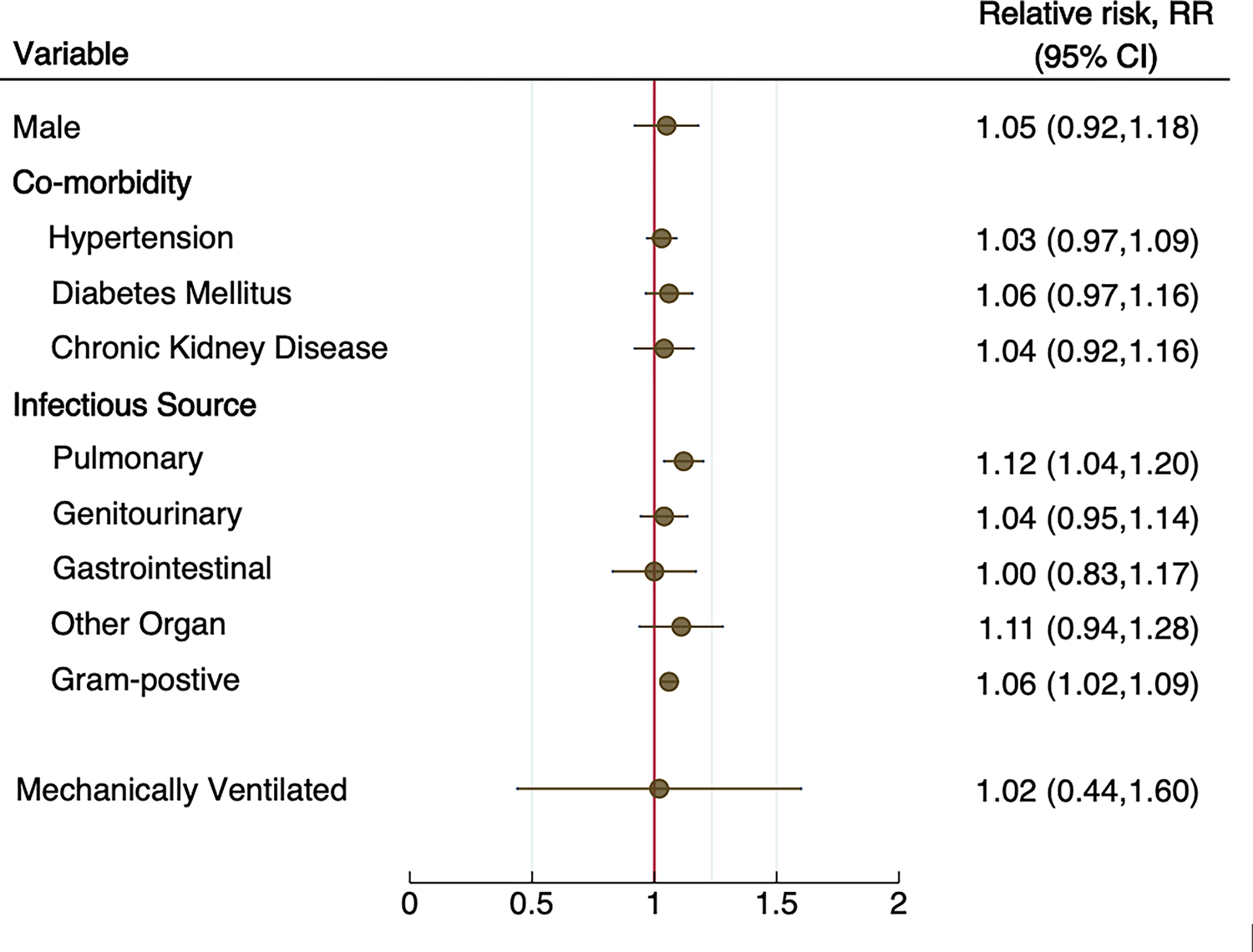
Meta-regression analysis of the risk factors associated with septic encephalopathy among studies of patients with sepsis.
Figure 5.
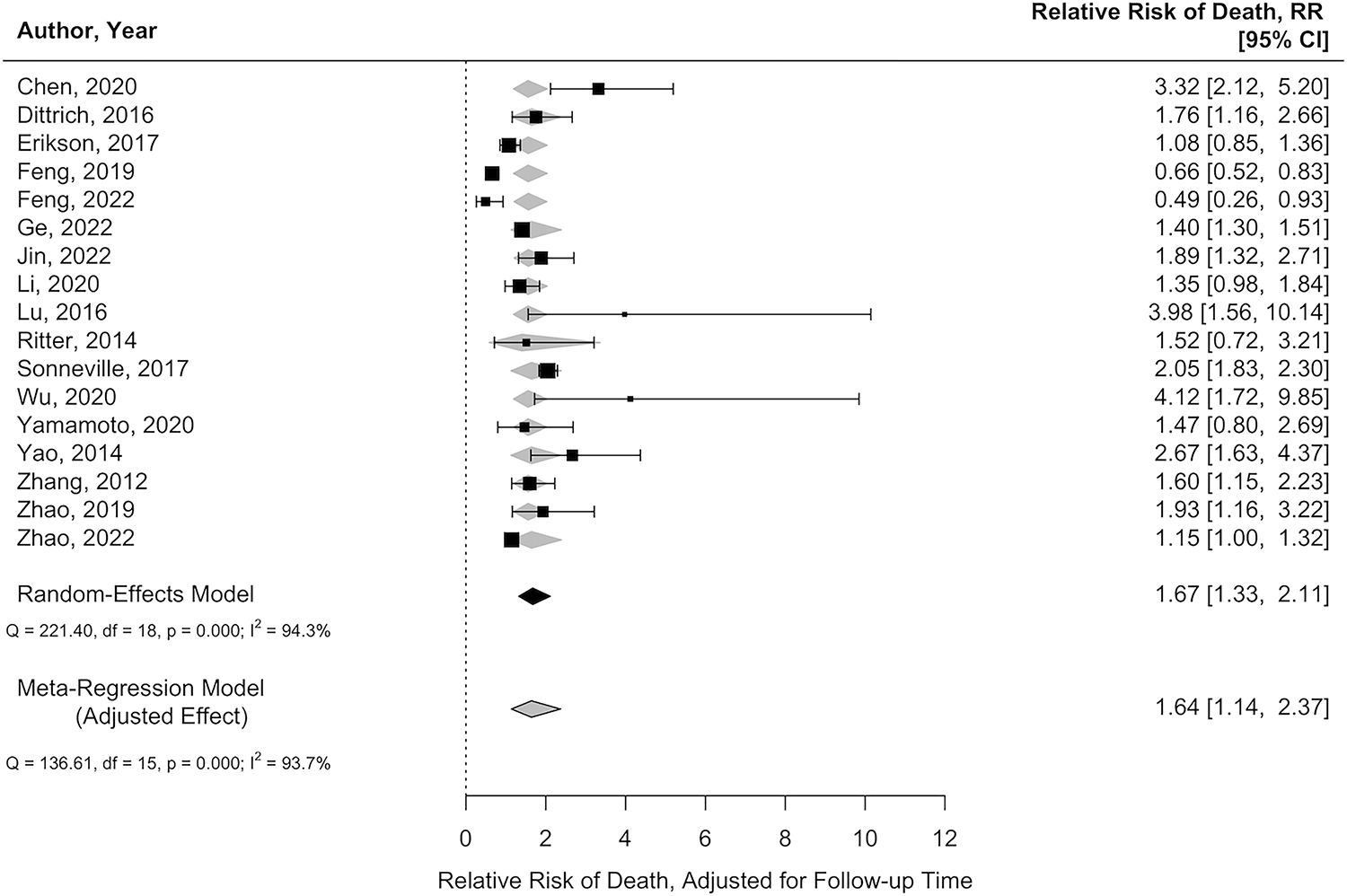
Comparison of relative risk of death of patients with and without septic encephalopathy adjusted for follow-up time (grey diamond) vs. unadjusted estimate (black square).
Neuropathological Findings
Three studies (n=159)17–19 evaluated septic patients’ brains through post-mortem autopsy. Forty-seven percent of the patients in these studies had histologic evidence of acute brain injuries, of which ischemic infarct was the most common finding (45%), followed by hemorrhagic lesion (26%), multifocal leukoencephalopathy (8%), and micro-abscesses (8%). One study assessed the association of sepsis with moderate-severe microvascular brain injury (defined as more than 2 microinfarcts [focal ischemic lesions found only on microscopic examination] anywhere in the brain). The authors found that 30% of included sepsis patients had evidence of microvascular brain injuries and are twice more likely to have microvascular brain injuries than patients without sepsis, which is strongly and independently associated with dementia.20 Erikson et al. found that 38% of sepsis patients included in the study had no expression of occludin in the endothelium of cerebral microvasculature, an evidence of damaged blood-brain barrier (BBB).17 Evidence of diffuse intravascular coagulation with multiple fibrinous microthrombi causing diffuse small microinfarcts and hemorrhages was reported in the study by Sharshar et al.19
Cognitive Outcomes
Six studies (n=415)21–26 evaluated cognitive outcomes in sepsis survivors between up to 1-year post-discharge. Among those, nearly one-third of the patients in these studies (n=124) reported cognitive impairment in at least one domain between 3 months up to 1-year post-discharge. The meta-regression analysis suggested a lower prevalence of cognitive impairment on longer-term follow-up (Supplemental Figure 2). Cognitive tests included the Hayling Sentence Completion test (measurement of executive function), Repeatable Battery for the Assessment of Neuropsychological Status, and the Neurocognitive effects test. Four studies (n=197)21–24 had volumetric brain magnetic resonance imaging (MRIs) performed between 1–6 months following the sepsis admission and found reduced brain volume in sepsis survivors compared to healthy controls. These changes were most notable in the hippocampus, caudate nuclei, putamen, thalamus, superior frontal lobe, and cerebellum regions. Longer duration of encephalopathy during index hospitalization was also associated with smaller brain volume on MRI and long-term cognitive impairment at 12 months.24 One study followed sepsis patients after discharge for up to 9 years and found 14% patients were subsequently diagnosed with various forms of dementia.27
Neuromonitoring
Twenty-six studies (n=1,358) reported the use of neuromonitoring tools in septic patients including near Infrared spectroscopy (NIRS) in 8 studies (n=124), electroencephalogram (EEG) in 7 studies (n=727), transcranial doppler (TCD) in 7 studies (n=214), optic nerve sheath diameter ultrasonography (ONSD) in one study (n=10), magnetic resonance angiogram (MRA) in one study (n=22), pupillometry in 2 studies (n=167), acoustocerebrography in one study(n=20) and somatosensory evoked potentials (SSEP) in one study (n=68). See Supplemental Table 5 for the detailed description of each neuromonitoring study.
EEG and TCDs were the most frequently utilized monitoring tools for sepsis patients. EEGs were helpful in identifying nonclinical seizures and those who are at risk for seizure based on certain EEG patterns such as lack of reactivity and the presence of periodic discharges, all of which were reported to be associated with higher mortality and worse outcome.28–32 Both TCD and NIRS were used in sepsis patients to assess changes in cerebral autoregulation /hemodynamic,33–36 vasomotor reactivity, 37 and cerebral emboli monitoring (TCD only).38 More than half of sepsis patients were found to have impaired cerebral autoregulation, which was an independent risk factor of septic encephalopathy,33,34 and 3-month mortality.39 Similarly, Masse et al. found significantly increased cerebral blood flow in sepsis patients compared to controls with chronic hypertensions using MRA.40 Septic encephalopathy patients also had impaired cerebral vasomotor reactivity when compared to healthy controls.37 Regional cerebral desaturation detected on cerebral NIRS was associated with an increased risk of death but not the risk for delirium in sepsis patients.41 Neuromonitoring tools such as SSEP,42 ONSD,43 pupillometry-derived neurologic pupil index44 were used to assess cerebral autoregulation impairment and predicted mortality in septic encephalopathy patients. Acoustocerebrography is a tool using multifrequency transcranial ultrasound to measure molecular acoustic changes in brain tissues to diagnose septic encephalopathy with a specificity of 89% and a sensitivity of 75%.45
DISCUSSION
We performed a systematic review and meta-analysis to determine the prevalence and characteristics of neurological complications in sepsis. We included 38 studies involving 142,193 sepsis patients, representing the largest systematic review and meta-analysis carried out for this purpose. Septic encephalopathy was the most reported neurological complication (36%) in patients with sepsis. In addition, patients with sepsis also suffered from ischemic stroke (5%), intracranial hemorrhage (2%), seizure (1%), posterior reversible encephalopathy syndrome, (9%) and hypoxic-ischemic brain injury (7%). Nearly half of patients in the included neuropathological studies had histological evidence of acute brain injuries (the majority being ischemic infarct). Despite the common occurrence of neurological complications and acute brain injuries in sepsis population, studies on understanding the mechanisms, and risk factors associated with neurological complications and acute brain injuries are scare. The standardized neuromonitoring strategies for early detection and prevention of acute brain injuries in this population is not currently established.
Although the exact mechanism of acute brain injuries associated with sepsis is unknown, the frequent occurrence of ischemic acute brain injuries raises questions about the impairment of cerebral perfusion in this population. A previous study noted impaired cerebral perfusion, decreased cerebral blood flow and alterations in the regulation of cerebral perfusion, including impaired CO2-reactivity and cerebrovascular pressure autoregulation among patients suffered from sepsis.46 Another study reported a high prevalence of watershed infarcts suggesting that significant hypotension might contribute to the occurrence of stroke.19,47 The risk of ischemic stroke or hypoxic-ischemic brain injury would be increased in sepsis patients with hypotension in conjunction with altered cerebral autoregulation capacity. These mechanisms may also explain the high prevalence of posterior reversible encephalopathy syndrome reported in this population as the result of blood brain barrier breakdown and the loss of cerebral autoregulation function.
The high prevalence of microvascular infarct and microhemorrhages in septic patients18,19 suggests the possible role of cerebral microvascular injury in the pathophysiology of sepsis associated acute brain injuries. Microvascular injury can occur in the setting of sepsis-related coagulation derangement such as disseminated intravascular coagulation and endothelial activation. Microvascular brain injury and microinfarct are also one of the common pathologic changes seen in the aging brain and are associated with cognitive decline. Multiple studies have demonstrated that microinfarct burden is an independent risk factor for dementia.48,49 In our study, nearly one third of the sepsis survivors in the studies examined cognitive outcome suffered from long-term cognitive impairment. The high prevalence of microvascular injury may account for this finding.43 Prospective research on MRI brain imaging of patients with sepsis may better characterize the impact of cerebral small vessel disease on cognitive outcomes.
Although septic encephalopathy was previously thought to be a reversible condition, recent studies report up to 40% of these patients experienced long-term cognitive impairment.50,51 This raises concern that the duration of septic shock, the severity of sepsis, and ICU critical illness may also contribute to long-term cognitive impairment. Animal studies showed evidence of increased accumulation of Aβ and phosphorylated tau (p-tau) 30 days after the induction of sepsis in the brain of survivor animals as a result of neuroinflammation, which results in impaired behavioral learning and memory function.52–55These studies suggest sepsis-related neuroinflammation likely plays a direct role in long-term cognitive outcome and may provide a potential therapeutical approach to prevent or treat long-term cognitive impairments.
A strength of this study is the large number of cohorts included in the analysis which provided the latest information on the neuromonitoring and autopsy data in the sepsis population. However, our study has several limitations that need to be mentioned. First, we found substantial heterogeneity (I2>90%) in estimating the prevalence of neurological complications owing to the variabilities in the included studies. These variabilities include: 1) the differences in definition of neurological complications and included patient population; 2) potential selection bias as patients who undergone imaging studies, autopsy likely sicker compared to those did not; 3) observer bias between clinician’s interpretation of imaging and data while making diagnosis of a neurological complication; 4) the included studies have a wide range of timeframes, which may result in difference in prevalence of neurological complications due to changes in clinical practice; 5) a lack of detail on certain neurological complications in some of the studies can lead to inaccuracies in prevalence estimation. Sensitivity analyses adjusting for the study published year and study types did not alter our findings (Supplemental Table 6). Second, two studies Orhun et al.56 and Sharshar et al.57 included only patients with septic encephalopathy and patients with neurological signs that underwent MRI brain respectively. Sensitivity analysis excluding either study did not impact our results (Supplemental Table 6). Orhun et al. also reported frequencies of posterior reversible encephalopathy syndrome, this may result in overestimation of prevalence of posterior reversible encephalopathy syndrome in general population. Third, although our study summarized current available evidence on neuromonitoring technology in sepsis population, the relative value of these tools needs further validation before being incorporated into routine practice. Lastly, the retrospective nature of the study prevents us from inferring causality, and we are not able to determine the timing and risk factors associated with each neurological complication.
CONCLUSIONS
In-hospital neurological complications were common in patients with sepsis and neuropathological studies demonstrated almost half of septic patients have evidence of acute brain injuries, the majority of which are ischemic injuries. The mechanism and timing of sepsis associated acute brain injuries is poorly understood, partly due to the lack of standardized neuromonitoring and imaging protocol in this population. Despite the significant heterogeneity, our study shed some light on future research directions on understanding the mechanisms of sepsis associated acute brain injuries, and better diagnostic/neuromonitoring strategies for early detection and making prevention possible.
Supplementary Material
Key points:
• Question:
How common are in-hospital neurological complications in patients suffered from sepsis and what are the long-term cognitive outcomes in sepsis survivors?
• Findings:
In this systematic review and meta-analysis of patients with sepsis, septic encephalopathy was the most reported neurological complications (36%). In addition, septic patients also suffered from ischemic stroke (5%), intracranial hemorrhage (2%), seizure (1%), posterior reversible encephalopathy syndrome (9%) and hypoxic-ischemic brain injury (7%). Nearly half (45%) of the sepsis patients among the neuropathological studies had histological evidence of acute brain injuries (the majority being ischemic infarct) and one third of the patients in the studies that examined cognitive outcome also reported long-term neurocognitive impairment.
• Meaning:
In-hospital neurological complications and acute brain injuries are common in patients with sepsis. However, the mechanism and timing of sepsis-associated complications are poorly understood.
Funding:
S.M.C. is funded by NHLBI 1K23HL157610.
Footnotes
Disclosures: The authors declare that they have no conflicts of interest.
Availability of data and material:
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1.Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304(16):1833–1834. [DOI] [PubMed] [Google Scholar]
- 2.Pytel P, Alexander JJ. Pathogenesis of septic encephalopathy. Curr Opin Neurol. 2009;22(3):283–287. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Ely EW, Smith DM, et al. Long-Term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. [DOI] [PMC free article] [PubMed]
- 4.Sekino N, Selim M, Shehadah A. Sepsis-associated brain injury: underlying mechanisms and potential therapeutic strategies for acute and long-term cognitive impairments. Journal of Neuroinflammation 2022 19:1. 2022;19(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eidelman LA, Putterman D, Putterman C, Sprung CL. The spectrum of septic encephalopathy. Definitions, etiologies, and mortalities - PubMed. JAMA. February 1996:470–473. [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. In: Journal of Clinical Epidemiology. Vol 62.; 2009:e1–e34. [DOI] [PubMed] [Google Scholar]
- 7.Gül F, Arslantaş MK, Cinel İ, Kumar A. Changing Definitions of Sepsis. Turk J Anaesthesiol Reanim. 2017;45(3):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour C, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhry N, Duggal AK. Sepsis Associated Encephalopathy. Adv Med. 2014;2014:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonia T, Buitrago-Garcia D, Peter N, et al. A tool to assess risk of bias in studies estimating the prevalence of mental health disorders (RoB-PrevMH). medRxiv. February 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos PT M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- 13.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59(1):7–10. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 15.Sidik K, Jonkman JN. A comparison of heterogeneity variance estimators in combining results of studies. Stat Med. 2007;26(9):1964–1981. [DOI] [PubMed] [Google Scholar]
- 16.Inthout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erikson K, Tuominen H, Vakkala M, et al. Brain tight junction protein expression in sepsis in an autopsy series. Crit Care. 2020;24(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehlenbach WJ, Sonnen JA, Montine TJ, Larson EB. Association Between Sepsis and Microvascular Brain Injury. Crit Care Med. 2019;47(11):1531–1538. [DOI] [PubMed] [Google Scholar]
- 19.Sharshar T, Annane D, de La Grandmaison GL, Brouland JP, Hopkinson NS, Gray F. The neuropathology of septic shock. Brain Pathol. 2004;14(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehlenbach WJ, Sonnen JA, Montine TJ, Larson EB. Association Between Sepsis and Microvascular Brain Injury. Crit Care Med. 2019;47(11):1531–1538. [DOI] [PubMed] [Google Scholar]
- 21.Orhun G, Tüzün E, Ergin Özcan P, et al. Association Between Inflammatory Markers and Cognitive Outcome in Patients with Acute Brain Dysfunction Due to Sepsis. Noro Psikiyatr Ars. 2019;56(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan M, Yan DY, Xu FS, et al. Effects of sepsis on hippocampal volume and memory function. World J Emerg Med. 2020;11(4):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semmler A, Widmann CN, Okulla T, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry. 2013;84(1):62–70. [DOI] [PubMed] [Google Scholar]
- 24.Gunther ML, Morandi A, Krauskopf E, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2022–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown SM, Beesley SJ, Stubben C, et al. Postseptic Cognitive Impairment and Expression of APOE in Peripheral Blood: The Cognition After SepsiS (CASS) Observational Pilot Study. J Intensive Care Med. 2021;36(3):262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Needham DM, Colantuoni E, Dinglas VD, et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med. 2016;4(3):203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters Van Ton AM, Meijer-Van Leijsen EMC, Bergkamp MI, et al. Risk of Dementia and Structural Brain Changes Following Nonneurological Infections During 9-Year Follow-Up. Crit Care Med. 2022;50(4):554–564. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen RM, Urdanibia-Centelles O, Vedel-Larsen E, et al. Continuous EEG Monitoring in a Consecutive Patient Cohort with Sepsis and Delirium. Neurocrit Care. 2020;32(1):121–130. [DOI] [PubMed] [Google Scholar]
- 29.Bryan Young G, Bolton CF, Archibald YM, et al. The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol. 1992;9(1):145–152. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore EJ, Gaspard N, Choi HA, et al. Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med. 2015;41(4):686–694. [DOI] [PubMed] [Google Scholar]
- 31.Azabou E, Magalhaes E, Braconnier A, et al. Early Standard Electroencephalogram Abnormalities Predict Mortality in Septic Intensive Care Unit Patients. PLoS One. 2015;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oddo M, Carrera E, Claassen J, et al. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37(6):2051–2056. [DOI] [PubMed] [Google Scholar]
- 33.Crippa IA, Subirà C, Vincent JL, et al. Impaired cerebral autoregulation is associated with brain dysfunction in patients with sepsis. Crit Care. 2018;22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfister D, Siegemund M, Dell-Kuster S, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierrakos C, Attou R, Decorte L, et al. Transcranial Doppler to assess sepsis-associated encephalopathy in critically ill patients. BMC Anesthesiol. 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straver JS, Keunen RWM, Stam CJ, et al. Transcranial Doppler and systemic hemodynamic studies in septic shock. Neurol Res. 1996;18(4):313–318. [DOI] [PubMed] [Google Scholar]
- 37.Szatmári S, Végh T, Csomós Á, et al. Impaired cerebrovascular reactivity in sepsis-associated encephalopathy studied by acetazolamide test. Crit Care. 2010;14(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunfeld M, Remmers M, Hoogenboezem R, et al. Late septic encephalopathy and septic shock are not associated with ongoing cerebral embolism. Perspectives in Medicine. 2012;1(1–12):224–227. [Google Scholar]
- 39.Bindra J, Pham P, Chuan A, et al. Is impaired cerebrovascular autoregulation associated with outcome in patients admitted to the ICU with early septic shock? Crit Care Resusc. 2016;18(2):95–101. [PubMed] [Google Scholar]
- 40.Masse MH, Richard MA, D’Aragon F, et al. Early Evidence of Sepsis-Associated Hyperperfusion-A Study of Cerebral Blood Flow Measured With MRI Arterial Spin Labeling in Critically Ill Septic Patients and Control Subjects. Crit Care Med. 2018;46(7):e663–e669. [DOI] [PubMed] [Google Scholar]
- 41.Funk DJ, Kumar A, Klar G. Decreases in cerebral saturation in patients with septic shock are associated with increased risk of death: a prospective observational single center study. J Intensive Care. 2016;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zauner C, Gendo A, Kramer L, et al. Impaired subcortical and cortical sensory evoked potential pathways in septic patients. Crit Care Med. 2002;30(5):1136–1139. [DOI] [PubMed] [Google Scholar]
- 43.Czempik PF, Gąsiorek J, Bąk A, et al. Ultrasonic Assessment of Optic Nerve Sheath Diameter in Patients at Risk of Sepsis-Associated Brain Dysfunction: A Preliminary Report. Int J Environ Res Public Health. 2020;17(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quispe Cornejo A, Fernandes Vilarinho CS, Crippa IA, et al. The use of automated pupillometry to assess cerebral autoregulation: a retrospective study. J Intensive Care. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauer M, Sievert A, Wrobel M, et al. Acoustocerebrography in septic patients: A randomized and controlled pilot study. Front Med Technol. 2022;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burkhart CS, Siegemund M, Steiner LA. Cerebral perfusion in sepsis. Crit Care. 2010;14(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polito A, Eischwald F, Maho ALL, et al. Pattern of brain injury in the acute setting of human septic shock. Crit Care. 2013;17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrovitch H, Ross GW, Steinhorn SC, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57(1):98–103. [DOI] [PubMed] [Google Scholar]
- 49.Hilal S, Sikking E, Shaik MA, et al. Cortical cerebral microinfarcts on 3T MRI: A novel marker of cerebrovascular disease. Neurology. 2016;87(15):1583–1590. [DOI] [PubMed] [Google Scholar]
- 50.Catarina AV, Branchini G, Bettoni L, et al. Sepsis-Associated Encephalopathy: from Pathophysiology to Progress in Experimental Studies. Mol Neurobiol. 2021;58(6):2770–2779. [DOI] [PubMed] [Google Scholar]
- 51.Annane D, Sharshar T. Cognitive decline after sepsis. Lancet Respir Med. 2015;3(1):61–69. [DOI] [PubMed] [Google Scholar]
- 52.Brown GC. The endotoxin hypothesis of neurodegeneration. J Neuroinflammation. 2019;16(1):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirk RA, Kesner RP, Wang LM, et al. Lipopolysaccharide exposure in a rat sepsis model results in hippocampal amyloid-β plaque and phosphorylated tau deposition and corresponding behavioral deficits. Geroscience. 2019;41(4):467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milioli MVM, Burger H, Olivieri R, et al. The impact of age on long-term behavioral and neurochemical parameters in an animal model of severe sepsis. Neurosci Lett. 2019;708. [DOI] [PubMed] [Google Scholar]
- 55.Gasparotto J, Girardi CS, Somensi N, et al. Receptor for advanced glycation end products mediates sepsis-triggered amyloid-β accumulation, Tau phosphorylation, and cognitive impairment. J Biol Chem. 2018;293(1):226–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orhun G, Sencer S, Tüzün E, et al. Posterior Reversible Encephalopathy in Sepsis-Associated Encephalopathy: Experience from a Single Center. Neurocrit Care. 2022;36(2):372–386. [DOI] [PubMed] [Google Scholar]
- 57.Sharshar T, Carlier R, Bernard F, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33(5):798–806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


