ABSTRACT
Objective:
Despite the standard guideline recommendations to prevent ventilator-associated pneumonia (VAP), it has remained one of the common lung infections in the intensive care unit (ICU). This clinical trial was designed to evaluate the effect of HemoHIM®, a mixture of traditional Korean medicinal plants, on preventing VAP in ICU patients.
Methods:
This randomized controlled clinical trial was conducted on mechanically ventilated adult ICU patients with a clinical pulmonary infection score of VAP ≤6 in the first 48 h of ventilation. Patients in the intervention group received a packet of HemoHIM daily and orally for 7 days in addition to standard prevention strategies. However, in the control group, only standard prevention strategies were carried out. All patients were followed daily for VAP incidence for 14 days.
Findings:
The overall VAP incidence was 36.4 and 57.4 episodes per 1000 days of mechanical ventilation in the intervention and control groups, respectively (P = 0.041; odds ratio = 0.26; 95% confidence interval = 0.070–0.944). The median length of mechanical ventilation during study follow-up was significantly lower in the intervention than in the control group (P = 0.033). The number of pneumonia-free days during the study was considerably higher in the intervention group (P value of the log-rank test = 0.023).
Conclusion:
According to the results of this study, the HemoHIM herbal supplement had beneficial effects in preventing the occurrence of VAP and significantly reduced the incidence of pneumonia in the intervention group. Further comprehensive research is required to draw more accurate conclusions.
KEYWORDS: HemoHIM, intensive care unit, ventilator-associated pneumonia
INTRODUCTION
Hospital-acquired pneumonia (HAP) is the leading cause of death from hospital-acquired infection and is one of the most common lung infections in which most refractory pathogens are involved.[1,2,3] Ventilator-associated pneumonia (VAP) is categorized as HAP, with an incidence rate of 15%–60% and a mortality rate of 25%–76%, which occurs almost 48 h after the initiation of endotracheal intubation and mechanical ventilation.[4]
Patients with comorbidities such as chronic obstructive pulmonary disease, obesity, diabetes, and alcoholism are high-risk groups for VAP.[5,6] Furthermore, the presence of underlying conditions such as sepsis, trauma, diseases of the central nervous system, and respiratory diseases increases the incidence of VAP.[7]
Research shows that endotracheal or gastric tubes are the leading cause of VAP because pathogens readily colonize them.[5] Patients receiving mechanical ventilation are vulnerable to infection, and oral care is valuable in their prognosis.[8] Lack of oral nutrition, decreased salivation, restriction of oral care, imbalance of pH, and impaired body defenses against aspiration of pharyngeal secretions to the lungs increase the risk of pneumonia in these patients.[9]
The VAP prevention guideline currently provides instructions for controlling preventable risk factors.[10] In the field of prevention, head elevation with an angle of 30°–45°, oral care with chlorhexidine daily, adjusting the use of sleeping pills, hand hygiene, modifying the gastric flora using probiotics, and preventing gastric ulcer and deep vein thrombosis, reduce the incidence of VAP.[11,12]
VAP in intensive care unit (ICU) patients significantly worsens the prognosis, increases treatment costs, and prolongs hospitalization time.[5] Hence, different interventions are being investigated to prevent VAP and its related complications.
A healthy immune system is necessary to prevent infectious diseases, including VAP. Ventilator disrupts the proper functioning of the immune system and the balance of immune cytokines, such as interferon (IFN)-g, interleukin (IL)-17F, IL-1B, IL-31, tumor necrosis factor-a, IL-6, and IL-1.[13,14] Moreover, immunosuppression following the critical condition of patients in the ICU is another challenge that makes them prone to various infections.[15,16] Hence, it seems modalities enhancing the immune system and modulating immune cytokines may help prevent VAP.
HemoHIM® is a commercial plant extract consisting of angelica radix root, the rhizome of the Korean plant Cnidium officinalis, and the root of Paeonia japonica.[17] KolmarBNH Sun BioTech Business (SBT) manufactured this product in 20 mL pocket. Numerous studies have shown the effects of increasing the immune system against cancer cells in animal models.[18,19,20,21] Other studies have shown the positive impact of HemoHIM as immunomodulatory, antidiabetic, anti-inflammatory, and antitumor effects in animal models.[19,22,23] However, there is no data regarding the clinical benefit of preventing VAP.
Therefore, this clinical trial was designed to evaluate the effect of HemoHIM on preventing VAP in ICU patients.
METHODS
In two parallel groups, this randomized controlled clinical trial evaluated the HemoHIM supplement’s effect on preventing VAP among patients admitted to the ICU of (deleted for blinded article file).
The research committee of “Isfahan University of Medical Sciences” approved all procedures involving human patients. in this study, IR.MUI.RESEARCH. REC.1399.767. Moreover, written informed consent was obtained for study recruitment from all patients or their legal guardians.
This study was registered at the Iranian Registry of Clinical Trials, with the registration identification number IRCT20150221021159N5.
Inclusion criteria were gaining informed consent and adult patients (18 years and above) undergoing mechanical ventilation for more than 48 h without suspected pneumonia.
Exclusion criteria included acute respiratory distress syndrome diagnosis, pneumonia at ICU admission, immunodeficiency, or leukopenia. Moreover, patients with a follow-up period of <3 days were excluded.
The formula for determining the sample size of the study.
(Z1-α/2 + Z1-β) 2 [p1 (1-p1) + p2 (1-p2)]/(p1-p2)2
At the level of type 1 error α (5%) and type 2 error β (20%), considering the 30% prevalence of VAP in patients hospitalized in ICU in the placebo group (P2 = 30%) and reducing its prevalence by 25% in the drug group (P1 = 5%), 15 patients were considered in each group.
After gaining informed consent, the pharmacist conveniently recruited eligible patients and randomly allocated them to the intervention and control groups (15 patients in each group). Restricted randomization with the permuted block method was used to create the random sequence and balance the number of allocated samples in each group (control and intervention). The computer program was used for a random series in blocks 2, 4, and 6. Each block has an equal number of patients in the control and intervention groups.
In the intervention group, in addition to standard prevention strategies, a packet of HemoHIM supplement containing 20 cc of herbal extract (manufactured by KolmarBNH SBT) is used orally or by a nasogastric tube daily for 7 days. However, in the control group, only standard prevention strategies were conducted.
In our research, all patients received preventive strategies for VAP, including head elevation (angle of 30°–45°), oral care with daily chlorhexidine, adjusting the use of sleeping pills, hand hygiene, preventing contact with contaminated equipment, and preventing stomach ulcers.
All patients were followed daily for VAP diagnosis based on clinical pulmonary infection score (CPIS) criteria for 14 days. The research team recorded and meticulously assessed parameters such as temperature, white blood cell (WBC) count, amount and purulence of tracheal secretions, chest radiography, and PaO2/FiO2 to evaluate this criterion.
Finally, the physician diagnosed VAP based on radiological findings, laboratory parameters, and microbiological test results.
Other demographic and clinical data were collected, including age, sex, reasons for hospitalization and admission to the ICU, chronic diseases, and past medical history.
Glasgow Coma Scale, Acute Physiology and Chronic Health Evaluation, and Sequential Organ Failure Assessment scores were also calculated and reported in the study.
The rate of early-onset (within 4 days of intubation), late-onset, and overall VAP, VAP duration, the length of intubation, and ICU stay were evaluated in both the groups.
One-sample Kolmogorov–Smirnov was used to test the normal distribution of data. Categorical variables were presented as numbers (percent), and continuous variables were reported as median (interquartile range) or mean ± standard deviation. The statistical comparison was performed by Student’s t-test or Mann–Whitney U-test in continuous variables and Chi-square or Fisher’s exact test for categorical variables. Kaplan–Meier curves and the log-rank test were used to compare the number of pneumonia-free days between the two groups in the first 2 weeks.
Binary logistic regression analysis was applied to assess the effect of HemoHIM prophylaxis on VAP development.
Statistical analyses were performed using the statistical software package SPSS, version 20 (SPSS Inc., Chicago, IL, USA). P <0.05 was considered statistically significant for all analyses.
RESULTS
We evaluated about 700 patients regarding inclusion and exclusion criteria, and 49 were eligible for clinical study. Finally, 42 patients (19 in the intervention and 23 in the control group) completed the study [Figure 1].
Figure 1.
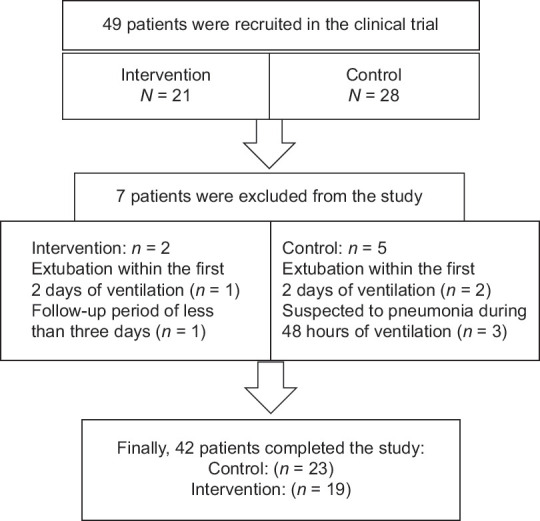
Flowchart illustrating the participation of patients in the study
Demographic and baseline clinical characteristics are illustrated in Table 1. Age, gender distribution, and other laboratory and clinical data were similar between the groups except for the history of diabetes, which was significantly higher in the intervention group (P = 0.02) [Table 1].
Table 1.
Demographic and clinical parameters of patients at the beginning of the study
| Parameters | Control group (n=23) | Intervention group (n=19) | P |
|---|---|---|---|
| Sex* | |||
| Male | 15 (65.2) | 10 (52.6) | 0.408 |
| Female | 8 (34.8) | 9 (47.4) | |
| Age# | 62 (36–74) | 71 (46–76) | 0.306 |
| APACHE II** | 18.48±5.52 | 19±4.58 | 0.72 |
| SOFA# | 7 (6–8) | 6 (5–7) | 0.421 |
| CRP# | 21 (6–79) | 23 (9–77) | 0.985 |
| ESR# | 25 (6–63) | 22 (13–60) | 0.865 |
| GCS# | 8 (5–9) | 7 (5–8) | 0.69 |
| Albumin# | 3.2 (3–3.6) | 3.4 (3.1–3.5) | 0.228 |
| MAP** | 97±7.8 | 97.27±9.75 | 0.92 |
| Smoking history* | 3 (13) | 1 (5.3) | 0.393 |
| Diabetes* | 8 (34.8) | 1 (5.3) | 0.02 |
| Hypertension* | 11 (47.8) | 12 (63.2) | 0.32 |
| Chronic cardiac disease* | 5 (21.7) | 5 (26.3) | 0.729 |
| Chief complaint* | |||
| Medical | 8 (34.8) | 5 (26.3) | 0.45 |
| Trauma | 3 (13) | 2 (10.5) | |
| Neurosurgery | 5 (21.7) | 9 (47.4) | |
| Other surgery | 6 (26.1) | 2 (10.5) | |
| Head trauma | 1 (4.3) | 1 (5.3) |
*n (%),**Mean±SD, #Median (IQR1–IQR3). IQR=Interquartile range, SD=Standard deviation, APACHE II=Acute Physiology and Chronic Health Evaluation II, CRP=C-reactive protein, ESR=Erythrocyte sedimentation rate, GCS=Glasgow Coma Scale, MAP=Mean arterial pressure, SOFA=Sequential Organ Failure Assessment
Antibiotics prescribed for any reason during the first 2 days of the study, except pneumonia, were not significantly different between groups (P > 0.05) [Table 2].
Table 2.
Antibiotics received for any reason except pneumonia in the first 2 days of the study
| Antibiotic | Control group (n=23) | Intervention group (n=19) | P |
|---|---|---|---|
| Antibiotics effective on MRSA | 15 (65.2) | 17 (89.5) | 0.066 |
| Fluoroquinolone | 4 (17.4) | 1 (5.3) | 0.227 |
| Third generation of cephalosporin | 13 (56.5) | 7 (36.8) | 0.204 |
| Tazocin | 1 (4.3) | 4 (21.1) | 0.096 |
| Metronidazole | 2 (8.7) | 1 (5.3) | 0.667 |
| Carbapenems | 10 (43.5) | 12 (63.2) | 0.204 |
Data are presented as n (%). MRSA=Methicillin-resistant Staphylococcus aureus
Table 3 results indicated that the CPIS criteria were similar between study groups at the beginning of the study. However, significant differences in outcomes emerged after intervention between the groups, as demonstrated in Table 4.
Table 3.
Clinical pulmonary infection score parameters on the first day of the study
| CPIS criterion parameters on the first day of the study | Control group (n=23) | Intervention group (n=19) | P |
|---|---|---|---|
| CPIS criterion on the first day of the study# | 3 (2–4) | 3 (3–4) | 1 |
| Pulmonary infiltration on the first day of the study* | |||
| No infiltrate | 12 (52.2) | 9 (47.4) | 0.951 |
| Diffuse | 10 (43.5) | 9 (47.4) | |
| Localized | 1 (4.3) | 1 (5.3) | |
| Temperature* | |||
| 36.5–38.4 | 22 (95.7) | 17 (89.5) | 0.445 |
| 36.01–36.49 or 38.5–38.99 | 1 (4.3) | 2 (10.5) | |
| Quality of pulmonary secretions* | |||
| None or scant | 4 (17.4) | 5 (26.3) | 0.483 |
| Nonpurulent | 19 (82.6) | 14 (73.7) | |
| Oxygenation PaO2/FiO2* | |||
| ARDS or >240 | 6 (26.1) | 4 (21.1) | 0.71 |
| <240 | 17 (73.9) | 15 (78.9) | |
| WBC count* | |||
| 4000–11,000 | 18 (78.3) | 17 (89.5) | 0.338 |
| <4000 or >11,000 | 5 (21.7) | 2 (10.5) |
*n (%), #Median (IQR1–IQR3). IQR=Interquartile range, CPIS=Clinical pulmonary infection score, ARDS=Acute respiratory distress syndrome, WBC=White blood cell
Table 4.
Clinical outcomes and prevalence of pathogens causing ventilator-associated pneumonia in the study population of patients after follow-up
| Parameters | Control group (n=23) | Intervention group (n=19) | P |
|---|---|---|---|
| Incidence of global VAP* | 17 (73.9) | 8 (42.1) | 0.037 |
| Duration of VAP** | 7.53±2.82 | 7.87±2.75 | 0.78 |
| Culture of lung secretions* | |||
| Positive TC | 6 (35.3) | 4 (50) | 0.457 |
| Positive TC and BC | 8 (47.1) | 4 (50) | |
| None | 3 (17.6) | 0 | |
| Type of microorganism* | |||
| Gram-negative Cocci | 0 | 2 (25) | 0.429 |
| Acinetobacter baumannii | 4 (28.6) | 3 (37.5) | |
| Klebsiella spp. | 1 (7.1) | 1 (12.5) | |
| Staphylococcus aureus | 3 (21.4) | 1 (12.5) | |
| Pseudomonas aeruginosa | 3 (21.4) | 1 (12.5) | |
| Enterobacter aerogenes | 2 (14.3) | 0 | |
| Fungi | 1 (7.1) | 0 | |
| Pulmonary infiltration* | |||
| No infiltrate | 2 (11.8) | 0 | 0.482 |
| Diffuse | 2 (11.8) | 2 (25) | |
| Localized | 13 (76.4) | 6 (75) | |
| Length of ICU stay# | 23 (15–30) | 13 (10–27) | 0.146 |
| Duration of ventilation# | 14 (14–14) | 12 (10–14) | 0.033 |
| Outcome* | |||
| Alive | 14 (60.9) | 6 (31.6) | 0.024 |
| Dead | 5 (21.7) | 2 (10.5) | |
| Discharge | 4 (17.4) | 11 (57.9) |
*n (%),**Mean±SD, #Median (IQR1–IQR3). IQR=Interquartile range, SD=Standard deviation, BC=Blood culture, ICU=intensive care unit, TC=Tracheal culture, VAP=Ventilator-associated pneumonia
The incidences of early VAP were 0 and 6.8 episodes per 1000 days of mechanical ventilation in the intervention and control groups, respectively. The incidences of late VAP were 36.4 and 50.6 episodes per 1000 days of mechanical ventilation in the intervention and control groups, respectively (P = 0.14; odds ratio [OR] =0.39; 95% confidence interval [CI] =0.11–1.36). The overall VAP incidence was 36.4 and 57.4 episodes per 1000 days of mechanical ventilation in the intervention and control groups, respectively (P = 0.041; OR = 0.26; 95% CI = 0.070–0.944).
The median length of mechanical ventilation during study follow-up was 14 (14–14) days in the control group and 12 (10–14) in the intervention group (P = 0.033). The number of pneumonia-free days during the study was significantly higher in the intervention group (P value of the log-rank test = 0.023).
We could not find any significant relationship between HemoHIM administration and the duration of VAP and ICU stay (P = 0.78 and P = 0.146, respectively) [Table 2]. By evaluating the results of cultured samples at the time of VAP occurrence, there was no significant difference between the control and intervention groups regarding reported microorganisms (P = 0.429) [Table 4].
Hospital mortality during the study was 21.7% in the control group and 10.5% in the intervention group, while 57.9% in the intervention group and 17.4% in the intervention group were discharged from the ICU during the study follow-up (P = 0.024).
The prevalence of pathogens causing VAP in the study population was similar between study groups (P = 0.429) [Table 4].
DISCUSSION
According to the results of this study, the HemoHIM herbal supplement had beneficial effects in preventing the occurrence of VAP. It significantly reduced the incidence of pneumonia in the intervention group compared to the control.
The significant benefits of HemoHIM in enhancing the immune system, safeguarding the respiratory system from inflammation, and regulating immune responses may be the key mechanisms behind its advantageous effect in preventing VAP. HemoHIM increased the secretion of IFN-γ and IL-2, decreased secretion of IL-4, and regulation of the Th1/Th2 balance.[24,25,26,27] In addition, clinical studies performed on this supplement also confirm the use of faith in it in the clinic, so no side effects or drug interactions have been recorded so far.[28,29,30]
A clinical study evaluated the immune system enhancement and antioxidant effects of HemoHIM in people with WBC counts between 5000 and 10,000/L. No noticeable differences in WBC and lymphocyte levels were observed in groups that consumed HemoHIM. However, Natural killer (NK) cell activity was increased in HemoHIM intake groups, and IFN-γ and IL-12, the biomarkers of immune cell functions, were raised in proportion to the dose and intake period of HemoHIM.[28]
In a preliminary clinical study in India, 85 patients diagnosed with breast or cervical cancer were administered HemoHIM for 12 weeks during radiation and/or chemotherapy. In the HemoHIM group, fewer cases of severe leucopenia were shown compared with the control group. In conclusion, HemoHIM may be a beneficial supplement during radiotherapy and chemotherapy for enhancing the antitumor efficacy and reducing the side effects.[29]
The subhealthy volunteers with peripheral WBC counts below 5000/μL were recruited in a double-blind and placebo-controlled human study. A trend was observed that the dose and duration of HemoHIM administration were correlated to the increased number of immune cells, such as WBCs and lymphocytes. The cytokines involved in immune activation (IL-2, IFN-γ, IL-6) and NK cell activity were significantly increased or showed trends of increases in HemoHIM-administered groups.[30]
However, to achieve more precise conclusions and diagnoses, it is advised to carry out similar research with a bigger sample size, more frequent culture tests from secretions, and radiographs.
The findings of this study indicate that the HemoHIM herbal supplement exhibited positive effects in preventing VAP and significantly decreased pneumonia incidence in the intervention group. However, additional thorough research is necessary to arrive at more precise conclusions.
AUTHORS’ CONTRIBUTIONS
S. Farsaei played a pivotal role in conceptualizing the study, contributing to developing research methodologies and conducting formal analyses and investigations essential for interpreting the findings.
G. Khamooshpour actively participated in data collection, and her expertise and insights significantly shaped the initial draft of the manuscript. H. Mahjobipoor contributed significantly to the technical aspects of the study.
All authors actively participated in securing funding for the project and utilized available resources effectively.
Financial support and sponsorship
This study was financially supported by the Isfahan University of Medical Sciences, Isfahan, I.R. Iran through Grant No. 399994.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lyons PG, Kollef MH. Prevention of hospital-acquired pneumonia. Curr Opin Crit Care. 2018;24:370–8. doi: 10.1097/MCC.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 2.Colombo SM, Palomeque AC, Li Bassi G. The zero-VAP sophistry and controversies surrounding prevention of ventilator-associated pneumonia. Intensive Care Med. 2020;46:368–71. doi: 10.1007/s00134-019-05882-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohd Sazlly Lim S, Zainal Abidin A, Liew SM, Roberts JA, Sime FB. The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: A systematic review and meta-analysis. J Infect. 2019;79:593–600. doi: 10.1016/j.jinf.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Fernando SM, Tran A, Cheng W, Klompas M, Kyeremanteng K, Mehta S, et al. Diagnosis of ventilator-associated pneumonia in critically ill adult patients-a systematic review and meta-analysis. Intensive Care Med. 2020;46:1170–9. doi: 10.1007/s00134-020-06036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kózka M, Sega A, Wojnar-Gruszka K, Tarnawska A, Gniadek A. Risk factors of pneumonia associated with mechanical ventilation. Int J Environ Res Public Health. 2020;17:656. doi: 10.3390/ijerph17020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Dong Y, Zhou P. Investigation on risk factors of ventilator-associated pneumonia in acute cerebral hemorrhage patients in intensive care unit. Can Respir J 2017. 2017:7272080. doi: 10.1155/2017/7272080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wałaszek M, Kosiarska A, Gniadek A, Kołpa M, Wolak Z, Dobroś W, et al. The risk factors for hospital-acquired pneumonia in the intensive care unit. Przegl Epidemiol. 2016;70:15–20. 107-10. [PubMed] [Google Scholar]
- 8.Landgraf AC, Reinheimer A, Merlin JC, Couto SA, Souza PH. Mechanical ventilation and cytopathological changes in the oral mucosa. Am J Crit Care. 2017;26:297–302. doi: 10.4037/ajcc2017218. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Hou Y, Zhang J, Wang B, Zhang J, Yang A, et al. Comparison of the effect of oral care with four different antiseptics to prevent ventilator-associated pneumonia in adults: Protocol for a network meta-analysis. Syst Rev. 2017;6:103. doi: 10.1186/s13643-017-0496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. Summary of the international clinical guidelines for the management of hospital-acquired and ventilator-acquired pneumonia. ERJ Open Res. 2018;4:00028–2018. doi: 10.1183/23120541.00028-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppadoro A, Bellani G, Foti G. Non-pharmacological interventions to prevent ventilator-associated pneumonia: A literature review. Respir Care. 2019;64:1586–95. doi: 10.4187/respcare.07127. [DOI] [PubMed] [Google Scholar]
- 12.Wei HP, Yang K. Effects of different oral care scrubs on ventilator-associated pneumonia prevention for machinery ventilates patient: A protocol for systematic review, evidence mapping, and network meta-analysis. Medicine (Baltimore) 2019;98:e14923. doi: 10.1097/MD.0000000000014923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huebinger RM, Smith AD, Zhang Y, Monson NL, Ireland SJ, Barber RC, et al. Variations of the lung microbiome and immune response in mechanically ventilated surgical patients. PLoS One. 2018;13:e0205788. doi: 10.1371/journal.pone.0205788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Winter FH, Jongers B, Bielen K, Mancuso D, Timbermont L, Lammens C, et al. Mechanical ventilation impairs IL-17 cytokine family expression in ventilator-associated pneumonia. Int J Mol Sci. 2019;20:5072. doi: 10.3390/ijms20205072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duggal NA, Snelson C, Shaheen U, Pearce V, Lord JM. Innate and adaptive immune dysregulation in critically ill ICU patients. Sci Rep. 2018;8:10186. doi: 10.1038/s41598-018-28409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim M, Kim M, Jeong H, Chae JS, Kim YS, Lee JG, et al. Hyporesponsiveness of natural killer cells and impaired inflammatory responses in critically ill patients. BMC Immunol. 2017;18:48. doi: 10.1186/s12865-017-0231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SJ, Kim JJ, Kang KY, Hwang YH, Jeong GY, Jo SK, et al. Herbal preparation (HemoHIM) enhanced functional maturation of bone marrow-derived dendritic cells mediated toll-like receptor 4. BMC Complement Altern Med. 2016;16:67. doi: 10.1186/s12906-016-1045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SK, Kwon DA, Lee HS, Kim HK, Kim WK. Preventive effect of the herbal preparation, HemoHIM, on cisplatin-induced immune suppression. Evid Based Complement Alternat Med 2019. 2019:3494806. doi: 10.1155/2019/3494806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park HR, Ju EJ, Jo SK, Jung U, Kim SH, Yee ST. Enhanced antitumor efficacy of cisplatin in combination with HemoHIM in tumor-bearing mice. BMC Cancer. 2009;9:85. doi: 10.1186/1471-2407-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HR, Ju EJ, Jo SK, Jung U, Kim SH. HemoHIM enhances the therapeutic efficacy of ionizing radiation treatment in tumor-bearing mice. J Med Food. 2010;13:47–53. doi: 10.1089/jmf.2009.1049. [DOI] [PubMed] [Google Scholar]
- 21.Park HR, Jo SK, Cho HH, Jung U. Synergistic anti-cancer activity of MH-30 in a murine melanoma model treated with cisplatin and its alleviated effects against cisplatin-induced toxicity in mice. In Vivo. 2020;34:1845–56. doi: 10.21873/invivo.11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo SK, Lee HJ, Kim SR, Kim JC, Bae CS, Jung U, et al. Antiinflammatory activity of an herbal preparation (HemoHIM) in rats. Phytother Res. 2007;21:625–8. doi: 10.1002/ptr.2068. [DOI] [PubMed] [Google Scholar]
- 23.Kim JJ, Choi J, Lee MK, Kang KY, Paik MJ, Jo SK, et al. Immunomodulatory and antidiabetic effects of a new herbal preparation (HemoHIM) on streptozotocin-induced diabetic mice. Evid based complement Alternat Med 2014. 2014:461685. doi: 10.1155/2014/461685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park HR, Jo SK, Jung U, Yee ST. Restoration of the immune functions in aged mice by supplementation with a new herbal composition, HemoHIM. Phytother Res. 2008;22:36–42. doi: 10.1002/ptr.2255. [DOI] [PubMed] [Google Scholar]
- 25.Kim JJ, Cho HW, Park HR, Jung U, Jo SK, Yee ST. Preventative effect of an herbal preparation (HemoHIM) on development of airway inflammation in mice via modulation of Th1/2 cells differentiation. PLoS One. 2013;8:e68552. doi: 10.1371/journal.pone.0068552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin NR, Kim SH, Ko JW, Park SH, Lee IC, Ryu JM, et al. HemoHIM, a herbal preparation, alleviates airway inflammation caused by cigarette smoke and lipopolysaccharide. Lab Anim Res. 2017;33:40–7. doi: 10.5625/lar.2017.33.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park HR, Jo SK, Choi NH, Jung U. HemoHIM ameliorates the persistent down-regulation of Th1-like immune responses in fractionated g-irradiated mice by modulating the IL-12p70-STAT4 signaling pathway. Radiat Res. 2012;177:676–84. doi: 10.1667/rr2768.1. [DOI] [PubMed] [Google Scholar]
- 28.Kim KS, Lee IK, Kwon SG. Clinical Evaluation of Immune-Promoting Functions of the Developed Product (HemoHIM). Korea, Republic of. 2007 [Google Scholar]
- 29.Jo SK, Park HR, Jung U, Choi SY, Kim SH. A herbal composition (hemohim) as a complementary agent for cancer radiotherapy and chemotherapy. J Radiat Cancer Res. 2016;7:8. [Google Scholar]
- 30.Choi HJ, Park JN, Jeon SH. Human Study of the Herbal Preparation (HemoHIM) on Enhancement of Immune and Hematopoietic Functions. Korea, Republic of. 2006 [Google Scholar]


