Abstract
Background
Partial enteral nutrition (PEN) is a well-established treatment for children with Crohn’s disease (CD). However, its efficacy in adults with CD remains uncertain. We aimed to assess the effectiveness of PEN as an add-on to escalated biological therapy in adults with CD who have lost response to biologics.
Methods
We conducted a retrospective observational study including patients who had lost response to biologics and received PEN in combination with escalated treatment, compared to those treated only with escalated therapy. The primary endpoint was steroid-free clinical remission (CR) at 24 weeks. Secondary endpoints included transmural healing (TH) and response (TR) rates along with selected clinical outcomes.
Results
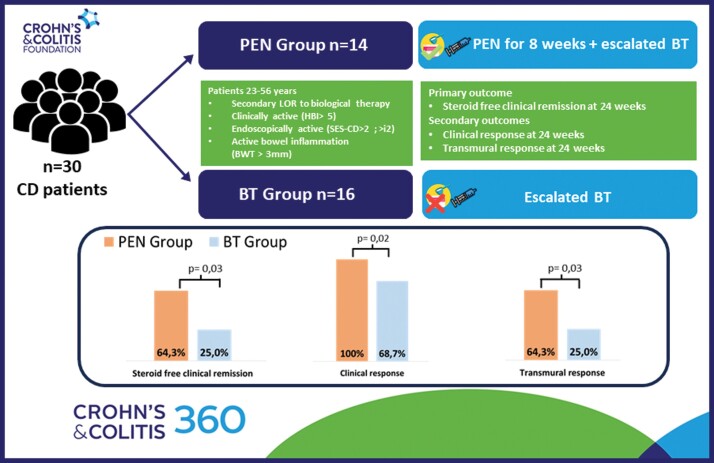
Forty-two patients were screened; 12 (28.6%) were excluded for complicated disease and 30 (71.4%) were included in the final analysis. Fourteen (46.7%) patients completed PEN treatment at 8 weeks, while 16 patients (53.3%) discontinued treatment due to intolerance and continued with escalation of biologic (BT group). At 24 weeks, 9 patients (64.3%) in the PEN group achieved CR, compared to 4 patients (25%) in the BT group (P = .03). The TR rate was 64.9% in the PEN group and 25% in the BT group (P = .03). Patients receiving PEN exhibited an increase in albumin levels compared to those in the BT group (Δ = 0.5; P = .02). A higher rate of therapy changes (68.7%) was observed in the BT group compared to 14.2% in the PEN group (P = .004). Prior failure to 2 lines of biological therapy was associated with adherence to PEN (OR = 1.583; CI = 1.06-2.36; P = .01).
Conclusions
In patients who had lost response to biologics, PEN in combination with escalated biologics was associated with CR and TR and improved nutritional status. Hence, the addition of PEN should be considered for patients with difficult-to-treat CD.
Keywords: Crohn’s disease, partial enteral nutrition, loss of response, difficult to treat
Graphical Abstract
Graphical Abstract.
Introduction
Inflammatory bowel diseases (IBD) arise from a persistent alteration in mucosal immune responses, determined by genetic variations and environmental factors, including diet and gut microbiota.1 Despite the recent advancement in the management of IBD and the increased wealth of treatment options, a substantial percentage of patients with IBD do not reach or maintain the response, representing a crucial unmet need.2,3 Much effort has been made to find predictors of clinical relapse and therapeutic response, but the biological mechanisms of these diseases remain uncertain. Indeed, the more biologics patients are exposed to, the greater the difficulty in choosing a new treatment and the lower the likelihood that they will respond to the next therapy.4
Based on a recent survey with the support of the International Organization for Inflammatory Bowel Disease (IOIBD) almost all (96%) respondents agreed that difficult-to-treat IBD can be defined by the failure of biologics and advanced small molecules with at least two different mechanisms of action.4–6 In this context, combination therapy with immune modulators and dose escalation has shown to be effective in recapturing clinical response and achieving higher rates of remission in some patients with signs of loss of response.7,8
Nutritional therapies represent an additional treatment strategy for Crohn’s disease (CD) due to the implications on inflammatory pathways, gut mucosal immune responses, and gut microbiota.9,10 Enteral nutrition has been traditionally used for induction of remission in pediatric patients with active (luminal) mild-to-moderate CD and it is usually used for 6–8 weeks.11 The mode of action of exclusive enteral nutrition (EEN) involves anti-inflammatory functions of the gut microbiota, a mere reduction of (dietary or microbial) antigen load or a reduction of nutrient-induced immune responses.9 In recent years, there have been several attempts to implement partial enteral nutrition (PEN) in the adult population. A recent pilot trial12 has demonstrated the feasibility of using dietary monotherapy with the Crohn’s disease exclusion diet (CDED), both with and without PEN, to induce clinical and endoscopic remission in adults with CD.
However, despite PEN’s effectiveness, compliance among adults has been low, primarily due to unpalatable formulas and a lack of motivation.13,14 Due to tolerability and compliance concerns, the use of PEN could be best suited for limited time periods as an add-on treatment to standard-of-care medical management.
In the current study, we aimed to assess the effectiveness and tolerability of PEN in combination with escalated biological therapy in adults with CD who had experienced a secondary loss of response to biologics compared to escalated treatment alone. We further assess the benefit of PEN on clinical outcomes such as nutritional status, rate of hospitalizations, changes in therapy and surgery at 24 weeks.
Methods
We performed an observational analysis of patients with CD aged > 18 years in follow-up between January 2019 and May 2022 at our tertiary center who had a secondary loss of response to biological therapy. The primary endpoint was steroid-free clinical remission (CR) at 24 weeks. Secondary endpoints included transmural healing (TH)/response (TR) rate and clinical outcomes. All patients had initiated biological therapy more than 6 months before the study started. In accordance with the European Crohn’s and Colitis Organization (ECCO) and the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) guidelines,15 responses to treatment were determined by a combination of clinical parameters, endoscopy, cross-sectional imaging and laboratory markers such as fecal calprotectin (FC) and C-reactive protein (CRP).
Secondary loss of response was defined as clinical/ultrasound relapse after a response period longer than 3 months on stable dose biological therapy without the use of corticosteroids. Drug compliance was assessed through questioning by physicians.
Patients considered eligible for biological dose escalation and PEN were clinically screened according to the Harvey–Bradshaw Index (HBI), and inflammatory biomarkers such as FC and CRP. Exclusion criteria included positive stool cultures, stool tests for parasites, or Clostridioides difficile and positive blood-based test for Cytomegalovirus (CMV). At baseline assessment, patients underwent ultrasound evaluation including the assessment of bowel wall thickness (BWT), bowel wall vascularization (BWF) for each intestinal segment and the exclusion of complications (strictures, abscesses, and fistula). The entire abdomen was scanned systematically starting from the right iliac fossa. BWT was measured in longitudinal and transverse sections, from the interface between the mucosa and the lumen to the interface between the serosa and the muscle layer. A mean of 2 measurements for each section was calculated and the worst segment was taken into account. A BWT > 3 mm and/or positive Doppler signal was considered an ultrasound relapse.
Dose escalation included interval reduction [infliximab (IFX) to every 4 weeks; adalimumab (ADA) to 40 mg weekly, vedolizumab (VDZ) to 4 weeks, ustekinumab (UST) to 4 weeks]. Patients were excluded if they had received any new medication [antibiotics, steroids, and immunomodulators].
CD patients were nutritionally assessed and received an 8-week regimen of 1 kcal/1 mL enteral nutrition formula (Modulen, Nestlé) with a maximum dosage of 1000 kcal/day, depending on BMI and bioelectrical impedance analysis (BIA) in combination with a low insoluble fiber diet. The nutritional status of patients was assessed based on the Global Leadership Initiative on Malnutrition (GLIM) criteria.16 Patients were considered adherent to PEN if they completed at least 8 weeks of therapy, whereas they were considered not adherent if they stopped or did not tolerate it. Subsequently, they transitioned to a healthy mediterranean-style diet, limiting, or eliminating foods known to induce inflammation, such as dairy, animal fat, processed meats, products containing emulsifiers, canned goods, and packaged products.17 Compliance was assessed by physicians or dieticians at follow-up visits or fortnightly phone calls. Of note, nutritional monitoring, including calorie intake, was conducted throughout the follow-up period.
Steroid-free clinical remission was defined as an HBI score of ≤5 and not receiving steroids at the assessment timepoint or for ≥30 days before, while clinical response was defined as a decrease of ≥3 points in the HBI and clinical relapse as HBI > 5. Baseline endoscopic disease activity was assessed using the simplified endoscopic activity score for Crohn’s disease (SES-CD) and Rutgeerts score for post-operative CD.
Based on previous study18–20 and expert consensus21 transmural healing (TH) was defined as BWT ≤ 3 mm without signs of hypervascularization, evaluated by using intestinal ultrasound. Transmural response (TR) was defined as a BWT decrease ≥ 25% from baseline and transmural activity was considered as BWT > 3 mm with signs of hypervascularisation.
Furthermore, we assessed selected clinical outcomes such as nutritional status, changes in therapy, rate of hospitalizations and surgery at 24 weeks.
All patients provided written informed consent to be included in the study. The study received a favorable opinion from our local ethical committee and was conducted in accordance wot the Helsinki Declaration (28516).
The current study protocol was conducted following the item from the STROBE statements checklist (Supplementary Table 1).
Statistical Analysis
Descriptive statistics, such as mean values with SD or median with interquartile ranges (IQR), were calculated for continuous variables, while percentages and proportions were calculated for categorical variables. Student’s t-test and chi-square test were applied for statistical analysis. A model to predict adherence to PEN was realized using continuous logistic regression (multivariate analysis) with stepwise selection criteria on significant parameters. All data were collected into an Excel spreadsheet, and statistical analysis was executed with IBM SPSS 26 software.
Results
Baseline Characteristics
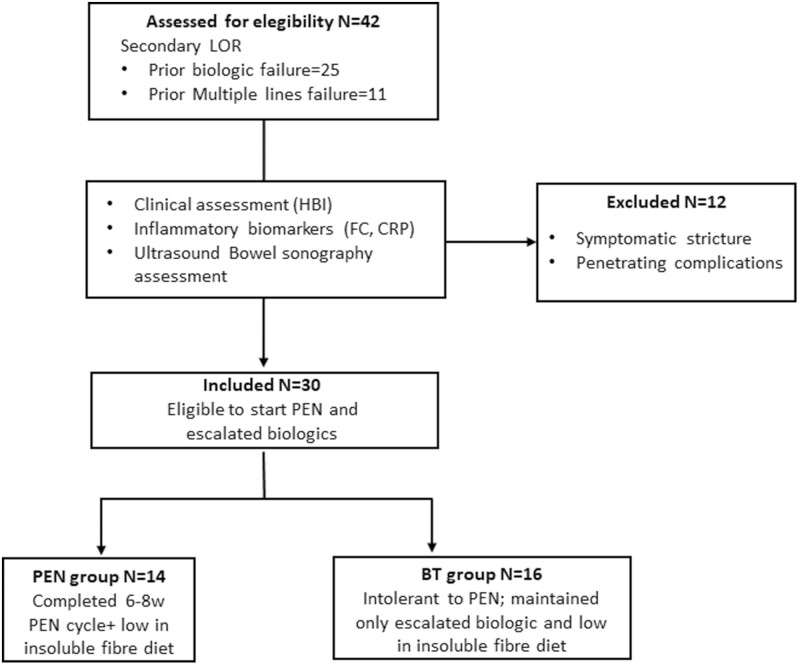
Forty-two CD patients with moderate-severe clinical activity of disease were initially screened, 30 (71.4%) being considered eligible for PEN and escalated biologics, while 12 (28.6%) patients had disease complications.
Specifically, 8 (66.6%) had symptomatic strictures and 4 (33.3%) penetrating complications that required surgery. Consequently, they were excluded from the final analysis. The flow diagram of patients is presented in Figure 1.
Figure 1.
Study flow.
The patient population had a median age of 35.5 (23.7–44.0) and included 19 (63.3%) males. The median duration of the disease was 121.0 (36–216) months. Twenty-four (80.0%) patients had an ileocolonic disease location and 15 (50.0%) patients had previously received surgery for CD. The median BMI was 19.4 kg/m2 (IQR: 18.7–21.5) and 19 (63.3%) patients met the GLIM criteria for malnutrition. The median BWT was 6.0 mm (IQR: 5–7) at baseline.
Overall, 15 (50.0%) patients had previously failed the first line of biologic treatment, and 6 (20.0%) were multi-failure (having failed 2 or more lines of treatment). At the time of loss of response, 17 (56.6%) were under treatment with anti-TNF-alpha, 5 (16.7%) on VDZ therapy and 8 (26.7%) on UST.
Adherence to PEN
At the end of the follow-up [median 24 weeks (22–26)], 14 (46.7%) patients completed 8-week therapy with PEN (PEN group) along with a low insoluble fiber diet, while 16 (53.3%) patients interrupted PEN (average PEN duration of 5 ± 2 days) and maintained only therapy with escalated biologic agents (BT group). Demographic and clinical characteristics of the PEN group and BT group at baseline are summarized in Table 1
Table 1.
Baseline disease characteristics of PEN group and BT group.
| PEN group (n = 14) | BT group (n = 16) | P -value | |
|---|---|---|---|
| Female sex, n (%) | 5 (35.7%) | 6 (37.5) | .61 |
| Age, median (IQR) | 31 (23.7–39.8) | 39.5 (24.5–56.5) | .22 |
| Smoking status, n (%) | 3 (21.4%) | 4 (25.0%) | .81 |
| Disease duration, median (IQR) | 60 (20–132) | 154 (72–252) | .47 |
| BMI, kg/m2 (IQR) | 19.5 (17.4–22.1) | 19.4 (19.0–20.6) | .95 |
| Malnutrition according to GLIM, n (%) | 8 (57.1%) | 11 (68.7%) | .39 |
| CD Montreal disease localization, n (%) | |||
| Ileal | 4 (28.6%) | 2 (12.5%) | .27 |
| Colonic | 0 | 0 | – |
| Ileocolonic | 10 (71.4%) | 14 (87.5%) | .27 |
| CD Montreal disease behaviour, n (%) | |||
| Inflammatory | 3 (21.4%) | 4 (25.0%) | .84 |
| Stricturing | 6 (42.9%) | 6 (37.5%) | .77 |
| Penetrating | 5 (35.7%) | 6 (37.5%) | .92 |
| Perianal disease, n (%) | 2 (14.3%) | 1 (6.3%) | .46 |
| SES-CD, median (IQR) | 10 (6–24) | 12 (4–14) | .95 |
| Rutgeerts score, median (IQR) | 3 (0.5–4) | 4 (2.5–4) | .48 |
| Median BWT (mm), median (IQR) | 5.5 (4.7–6.3) | 6 (5–7) | .47 |
| Previous surgery for IBD, n (%) | 6 (42.9%) | 9 (56.3%) | .71 |
| Extraintestinal manifestations, n (%) | 5 (35.6%) | 2 (12.6%) | .13 |
| Current biological therapy, n (%) | |||
| Anti-TNF-alpha | 8 (57.1%) | 9 (56.3%) | .96 |
| Vedolizumab | 2 (14.3%) | 3 (18.8%) | .74 |
| Ustekinumab | 4 (28.6%) | 4 (25.0%) | .82 |
| Time under biological therapy, median (IQR) | 14 (14–15.5) | 12.5 (12.5–16) | .98 |
| Treatment failed | |||
| First-line biologic therapy, n (%) | 6 (42.9%) | 9 (56.25%) | .46 |
| Multiple lines of biologics, n (%) | 2 (14.3%) | 4 (25.0%) | .46 |
Abbreviations: BWT, bowel wall thickness; CD, Crohn’s disease; GI, gastrointestinal tract; GLIM, global leadership initiative on malnutrition; IBD, inflammatory bowel disease; PEN, partial enteral nutrition; SES-CD, simple endoscopic score for Crohn’s disease.
The most common reasons of interruption were nausea or vomiting, abdominal pain and lack of palatability, which respectively occurred in 5 (31.2%), 9 (56.2%), and 2 (12.5%) cases. Noteworthy, multivariate analysis showed that prior failure to 2 lines of biological therapy was associated with adherence to PEN (OR = 1.583; CI = 1.06–2.36; P = .01).
Outcomes at the End of Follow-Up
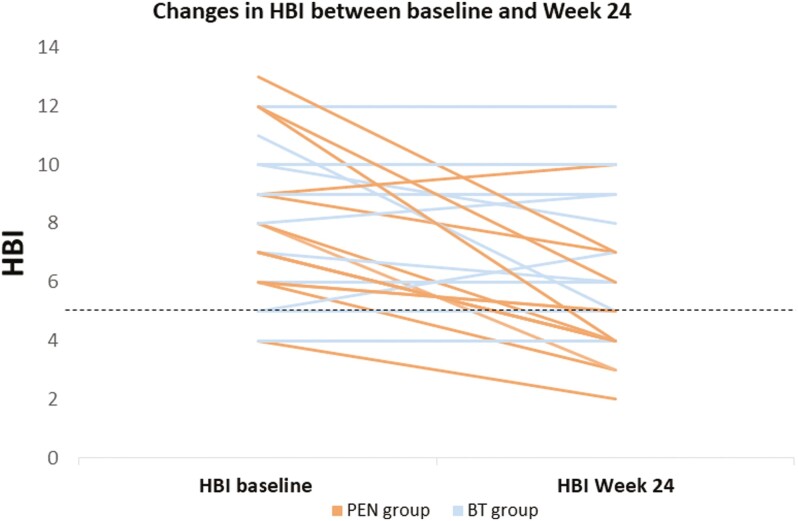
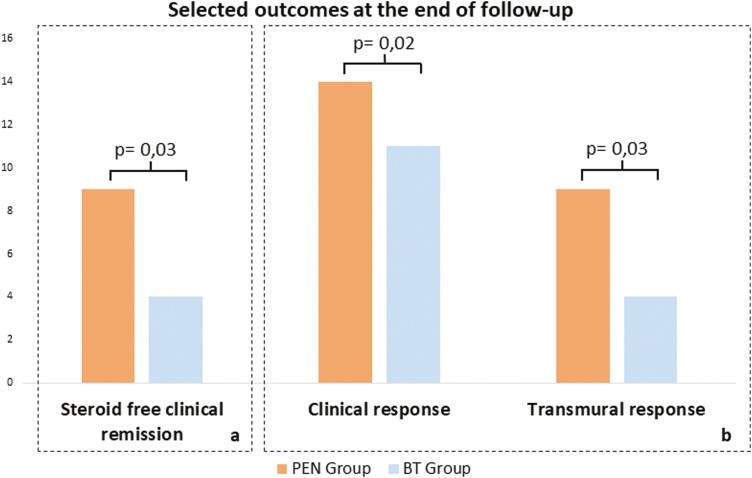
At 24 weeks, steroid-free clinical remission was achieved by 9 patients (64.3%) in the PEN group, with a median HBI of 4 (IQR: 3.7–6.3), compared to 4 patients (25.0%) in the BT group (P = .03), in whom the median HBI was 6.5 (IQR: 5–9) (Δ = 2.5; P = .01). [Table 2 and —Figure 2].
Table 2.
Differences in CD clinical activity at T0 and T1 between the PEN group and the BT group.
| T 0 | T 1 | P -value | |
|---|---|---|---|
| HBI PEN group (n = 14), median (IQR) | 7.5 (6.0-9.8) | 4 (3.8-6.3) | .001 |
| HBI BT group (n = 16), median (IQR) | 7.5 (5.0-10.0) | 6.5 (5,0-9.9) | .25 |
| P -value | .69 | .001 |
Figure 2.
Clinical activity changes between the PEN group and the BT group over a 24-week follow-up period.
All 14 patients in the PEN group achieved a clinical response, while only 11 individuals (68.7%) in the BT group exhibited such a response (P = .02).
Nine patients (64.3%) in the PEN group reached a TR, with a median BWT of 4.4 mm (IQR: 3.9–5.0), whereas only 4 patients (25.0%) treated with escalated biologics achieved the same outcome (P = .03) with a median BWT of 5.5 (IQR: 5.0–6.5). Among the patients who achieved TR, 92.3% were also in steroid-free clinical remission. However, none of the patients reached TH in either group. [Figure 3].
Figure 3.
Twenty-four weeks outcomes in PEN group vs. BT group. A, Primary outcome: steroid-free clinical remission. B, Secondary outcomes: clinical response and transmural response.
Median FC and CRP values were lower in the PEN group compared to the BT group, though the differences were not statistically significant (FC Δ = 5 mcg/g; P = .63) (CRP Δ = 1.6 mg/L; P = .6). The mean BMI value was slightly higher in the PEN group (Δ = 0.8 kg/m2; P = .16) compared to the BT group, although this difference did not reach statistical significance. Yet, the albumin value in the PEN group was significantly higher (Δ = 0.5; P = .02) than in the BT group.
At the end of the follow-up, 3 patients (18.7%) treated with solely escalated biologics, required surgical intervention, while none of the patients treated with PEN required surgery (P = .14). Additionally, 11 (68.7%) patients in the BT group changed their treatment at 24 weeks, compared to only 2 patients (14.2%) among those receiving PEN (P = .004). All outcomes were summarized in Table 3.
Table 3.
Clinical outcomes at 24 weeks in patients treated with PEN plus fiber-free diet combined with escalated biological therapy (PEN group) vs. patients who interrupted PEN and maintained escalated biological therapy plus fiber-free diet (BT group).
| PEN group (n = 14) | BT group (n = 16) | P-value | |
|---|---|---|---|
| HBI, median (IQR) | 4 (3.7-6.3) | 6.5 (5-9) | .01 |
| FC (μg/g), median (IQR) | 152 (82-245) | 157 (103-541) | .63 |
| CRP (mg/L), median (IQR) | 5.4 (3.7-9.0) | 7.0 (3.3-14.3) | .6 |
| Albumin (g/L), median (IQR) | 3.9 (3.6-4.2) | 3.4 (3.0-4.0) | .02 |
| BMI (kg/m2), median (IQR) | 20.9 (18,8-22,0) | 19,7 (17,9-21,0) | .17 |
| Clinical response, n (%) | 14 (100%) | 11 (68.7%) | .02 |
| Steroid-free clinical remission, n (%) | 9 (64.3%) | 4 (25.0%) | .03 |
| Transmural response, n (%) | 9 (64.3%) | 4 (25.0%) | .03 |
| Surgery, n (%) | 0 | 3 (18.7%) | .14 |
| Change in therapy n (%) | 2 (14.2%) | 11 (68.7%) | .004 |
Abbreviations: BMI, body mass index; CRP, C-reactive Protein; FC, faecal calprotectin; HBI, Harvey–Bradshaw Index; PEN, partial enteral nutrition.
Discussion
The treatment response rate in IBD and specifically in CD varies widely, ranging between 30 and 60% depending on the population considered. A possible explanation for this heterogeneity resides in the multitude and complexity of the inflammatory pathways involved in IBD.2 As the unmet needs of patient’s refractory to therapy become more pressing, new therapeutic approaches are evaluated including a combination of advanced therapies, microbiota modulation and dietary approaches.22,23
In the current study, our cohort included complex patients with challenging disease characteristics. These individuals had previously undergone surgery, exhibiting perianal disease, extraintestinal manifestations, and had experienced treatment failures with current biological therapies, hence difficult to treat.6 Most patients (70.0%) had experienced treatment failure with at least 1 line of biologics, while 20.0% had encountered failure with multiple lines. Dose escalation is a well-established approach to regain response after LOR.7,8 However, it might not be sufficient on its own.
The effect of diet has been already established in children with CD.24 Yet, there are limited studies in adults and none testing PEN effectiveness in recapturing and maintaining clinical remission and TH/TR in adults with CD who had failed different classes of biological therapies. Our pilot study was the first to show how dietary therapy can be a valuable adjunct to optimized biological therapy for regaining response in patients with CD including difficult-to-treat CD. Remarkably, we found that PEN in addition to escalated biologics can induce clinical remission and TR more significantly than escalated biologics alone. Despite the small number of patients, the effect size of PEN addition (around 40% difference in both clinical and ultrasonographic endpoints) was considerable and statistically significant for all analyses except calprotectin and CRP. These results are even more noteworthy taking into account the refractoriness of the population.
Although no patient achieved TH, this is considered a very stringent endpoint, challenging to reach, and probably requiring longer follow-up, particularly given the baseline characteristics of the disease and its long duration. It is worth noting that among the patients who achieved TR, 92.3% were also in steroid-free clinical remission. This data is consistent with the results of our previous study25 in which patients who reached TH had a higher rate of steroid-free clinical remission and better clinical outcomes 2 years after anti-TNF treatment. Hence, PEN could potentially contribute to delaying the need for surgery.
The improvement in nutritional status, and particularly albumin concentration, observed in our pilot study deserves attention. Nutritional status is strongly correlated with post-surgical clinical outcomes,26 frailty, risk of infection and other metrics. In particular, albumin levels are known to be directly correlated with response to biological treatments. Because the majority of our patients (63.3%) were malnourished at baseline, it is possible that some of the response recaptures observed were due to restored albumin levels and improved nutrition, rather than a direct anti-inflammatory effect of the diet per se.
At the end of the follow-up, no patients in the PEN group underwent surgery. Notably, most patients (68.7%) were treated with biologics and PEN maintained the treatment with escalated biologics. This implies that PEN could improve the persistence of maintenance treatment with biologics. Consistently, a meta-analysis by Nguyen et al.,27 found that IFX coupled with PEN and low-fat or regular diet was more likely to have better-sustained remission at 1 year than biological therapy alone. Yet, no data is available concerning different types of biological therapies.
Despite the promising efficacy results, compliance and adherence pose challenges for dietary therapies. Data on adults utilizing enteral nutrition are contradictory, and some studies suggest that poor compliance might contribute to an inadequate response.28–30 We found that adherence to PEN was achieved in 46.6% of patients who completed the follow-up, while the remaining discontinued it after an average duration of 5 ± 2 days due to intolerable side effects such as nausea, vomiting, and palatability issues. In accordance with the existing literature, our data highlight the challenges in completing a full therapy cycle with PEN and provide insight into the reasons for its limited utilization. Achieving efficacy with PEN typically requires more than just a few days. Therefore, the relatively short duration of PEN is unlikely to have a significant impact on outcomes. A previous analysis31 demonstrated that patients who received exclusive enteral nutrition (EEN) for less than 4 weeks experienced lower rates of clinical remission and response compared to those treated for over 6 weeks. However, at multivariate analysis, multiple previous failures with biological therapy were associated with better adherence to PEN. This finding suggests that multi-failure patients might have been more motivated to tolerate PEN as they are likely aware of the limited therapeutic options available.
To the best of our knowledge, this is the first experience evaluating the effect of PEN as an add-on to optimized biologics on TR/TH in difficult-to-treat CD patients. Although this was only a pilot study, our findings suggest that PEN combined with biologics could be used in adults with CD and represent a valid and safe therapeutic alternative for steroids in patients with secondary loss of response. It is also important to stress how adverse events related to PEN were all mild and self-limiting, with no serious safety concerns recorded.
Consistently, a recent pediatric study has shown an emerging trend toward EEN combined with biologics as induction treatment without any significant differences in remission or response rates between the first and repeated courses with a favorable side effect profile.29Therefore, combination treatment may represent a valid strategy, providing additional benefits for enhancing long-term responses to biologics and improving disease outcomes.
The primary limitations of our study include its retrospective design and the relatively limited patient cohort. However, we selected complex patients with a long-standing and disabling course of the disease, the majority of whom had previously undergone surgery. Although there was a difference in disease duration observed between the PEN and BT groups, it did not reach statistical significance, which may be attributed to the small sample size. A further limitation is that we did not have trough levels and antibodies for most of the patients who lost response to biologics, as this was not covered by our Institution. Besides, the clinical application of TDM can be challenging due to heterogeneity in settings for drug level measurements, and therapeutic ranges vary. A recent paper32 has also suggested that TDM should be performed following dose intensification, rather than at the time of secondary loss of response.
Finally, given the retrospective nature of the study, we lacked endoscopic follow-up data. Yet, TH is now acknowledged as an important adjunctive treatment measure in CD33 associated with better long‐term clinical outcomes than MH25 despite it may be more challenging to achieve and might necessitate a more extended therapy duration. However, TR can be observed as early as 4 weeks after the initiation of treatment, with sustained improvement over the long term.34 In this context ultrasound bowel sonography can be a valuable non-invasive objective tool for detecting early treatment response, thereby potentially allowing for early treatment optimization and potential therapy modification.
In conclusion, the promising results of our pilot study encourage to implementation of the use of PEN in adults with refractory CD disease. We believe that PEN could represent a new tool for strengthening the therapeutic equipment for IBD patients and might hold the potential to reduce the use of steroids. However, randomized-control trials are awaited to assess the effectiveness of PEN on large numbers of patients with difficult-to-treat CD.
Supplementary Material
Contributor Information
Olga Maria Nardone, Gastroenterology, Department of Public Health, University of Naples Federico II, Naples, Italy.
Giulio Calabrese, Gastroenterology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy.
Alessia La Mantia, Gastroenterology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy.
Anna Testa, Gastroenterology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy.
Antonio Rispo, Gastroenterology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy.
Lucia Alfonsi, Internal Medicine and Clinical Nutrition Unit, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy.
Fabrizio Pasanisi, Internal Medicine and Clinical Nutrition Unit, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy.
Fabiana Castiglione, Gastroenterology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy.
Author Contributions
All authors made substantial contributions to this work and approved the final version of the work. O.M.N: study design, manuscript writing, and manuscript reviewing for important intellectual content; G.C.: data collection and analysis, writing the manuscript, and draft reviewing; A.L.M.: data collection and draft reviewing; A.T.: draft reviewing and reviewing for important intellectual content; A.R.: draft reviewing and reviewing for important intellectual content; L.A.: reviewing for important intellectual content; F.P. reviewing for important intellectual content; F.C.: study design and supervision, manuscript reviewing for important intellectual content. F.C.: guarantor of the article.
Funding
This research work was conducted without financial support from any public, private, or not-for-profit funding agency.
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Approval
The study protocol received approval from our local ethical committee in accordance with the Helsinki Ethical Declaration.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S.. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. 2019;157(3):647-659.e4. doi: 10.1053/j.gastro.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 2. Raine T, Danese S.. Breaking through the therapeutic ceiling: what will it take? Gastroenterology. 2022;162(5):1507-1511. doi: 10.1053/j.gastro.2021.09.078 [DOI] [PubMed] [Google Scholar]
- 3. Reves J, Ungaro RC, Torres J.. Unmet needs in inflammatory bowel disease. Curr Res Pharmacol Drug Discov. 2021;2:100070. doi: 10.1016/j.crphar.2021.100070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Danese S, Parigi TL, Peyrin-Biroulet L, Ghosh S.. Defining difficult-to-treat inflammatory bowel disease: why and how. Lancet Gastroenterol Hepatol. 2021;6(7):520-522. doi: 10.1016/S2468-1253(21)00141-2 [DOI] [PubMed] [Google Scholar]
- 5. Parigi TL, D’Amico F, Abreu MT, et al. Difficult-to-treat inflammatory bowel disease: results from a global IOIBD survey. Lancet Gastroenterol Hepatol. 2022;7(5):390-391. doi: 10.1016/S2468-1253(22)00085-1 [DOI] [PubMed] [Google Scholar]
- 6. Parigi TL, D’Amico F, Abreu MT, et al. Difficult-to-treat inflammatory bowel disease: results from an international consensus meeting. Lancet Gastroenterol Hepatol. 2023;8(9):853-859. doi: 10.1016/S2468-1253(23)00154-1 [DOI] [PubMed] [Google Scholar]
- 7. Ben-Horin S, Mao R, Chen M.. Optimizing biologic treatment in IBD: objective measures, but when, how and how often? BMC Gastroenterol. 2015;15:178. doi: 10.1186/s12876-015-0408-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ollech JE, Normatov I, Peleg N, et al. Effectiveness of Ustekinumab dose escalation in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2021;19(1):104-110. doi: 10.1016/j.cgh.2020.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levine A, Sigall Boneh R, Wine E.. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut. 2018;67(9):1726-1738. doi: 10.1136/gutjnl-2017-315866 [DOI] [PubMed] [Google Scholar]
- 10. Sigall Boneh R, Sarbagili Shabat C, Yanai H, et al. Dietary therapy with the Crohn’s disease exclusion diet is a successful strategy for induction of remission in children and adults failing biological therapy. J Crohns Colitis. 2017;11(10):1205-1212. doi: 10.1093/ecco-jcc/jjx071 [DOI] [PubMed] [Google Scholar]
- 11. van Rheenen PF, Aloi M, Assa A, et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. 2020;15(2):171-194. doi: 10.1093/ecco-jcc/jjaa161 [DOI] [PubMed] [Google Scholar]
- 12. Yanai H, Levine A, Hirsch A, et al. The Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-AD): an open-label, pilot, randomised trial. Lancet Gastroenterol Hepatol. 2022;7(1):49-59. doi: 10.1016/S2468-1253(21)00299-5 [DOI] [PubMed] [Google Scholar]
- 13. Malchow H, Steinhardt HJ, Lorenz-Meyer H, et al. Feasibility and effectiveness of a defined-formula diet regimen in treating active Crohn’s disease. European Cooperative Crohn’s Disease Study III. Scand J Gastroenterol. 1990;25(3):235-244. [PubMed] [Google Scholar]
- 14. Wall CL, Gearry RB, Day AS.. Treatment of active Crohn’s disease with exclusive and partial enteral nutrition: a pilot study in adults. Inflamm Intest Dis. 2018;2(4):219-227. doi: 10.1159/000489630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maaser C, Sturm A, Vavricka SR, et al. ; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144-164. doi: 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 16. Cederholm T, Jensen GL, Correia M, et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10(1):207-217. doi: 10.1002/jcsm.12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hashash JG, Elkins J, Lewis JD, Binion DG.. AGA Clinical practice update on diet and nutritional therapies in patients with inflammatory bowel disease: expert review. Gastroenterology. 2024;166(3):521-532. doi: 10.1053/j.gastro.2023.11.303 [DOI] [PubMed] [Google Scholar]
- 18. Castiglione F, Imperatore N, Testa A, et al. Exploring the concept of deep remission in Crohn’s disease: correlation between transmural healing and biomarkers. Therap Adv Gastroenterol. 2022;15:17562848221110643. doi: 10.1177/17562848221110643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma L, Li W, Zhuang N, et al. Comparison of transmural healing and mucosal healing as predictors of positive long-term outcomes in Crohn’s disease. Therap Adv Gastroenterol. 2021;14:17562848211016259. doi: 10.1177/17562848211016259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nardone OM, Calabrese G, Testa A, et al. The impact of intestinal ultrasound on the management of inflammatory bowel disease: from established facts toward new horizons. Front Med (Lausanne). 2022;9:898092. doi: 10.3389/fmed.2022.898092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Novak KL, Nylund K, Maaser C, et al. Expert consensus on optimal acquisition and development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: a reliability and inter-rater variability study on intestinal ultrasonography in Crohn’s disease. J Crohns Colitis. 2021;15(4):609-616. doi: 10.1093/ecco-jcc/jjaa216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danese S, Solitano V, Jairath V, Peyrin-Biroulet L.. The future of drug development for inflammatory bowel disease: the need to ACT (advanced combination treatment). Gut. 2022;71(12):2380-2387. doi: 10.1136/gutjnl-2022-327025 [DOI] [PubMed] [Google Scholar]
- 23. Kedia S, Virmani S, S KV, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut. 2022;71(12):2401-2413. doi: 10.1136/gutjnl-2022-327811 [DOI] [PubMed] [Google Scholar]
- 24. Ruemmele FM, Veres G, Kolho KL, et al. ; European Crohn's and Colitis Organisation. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8(10):1179-1207. doi: 10.1016/j.crohns.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 25. Castiglione F, Imperatore N, Testa A, et al. One-year clinical outcomes with biologics in Crohn’s disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther. 2019;49(8):1026-1039. doi: 10.1111/apt.15190 [DOI] [PubMed] [Google Scholar]
- 26. Sebastian S, Segal JP, Hedin C, et al. ECCO topical review: roadmap to optimal peri-operative care in IBD. J Crohns Colitis. 2023;17(2):153-169. doi: 10.1093/ecco-jcc/jjac129 [DOI] [PubMed] [Google Scholar]
- 27. Nguyen DL, Palmer LB, Nguyen ET, McClave SA, Martindale RG, Bechtold ML.. Specialized enteral nutrition therapy in Crohn’s disease patients on maintenance infliximab therapy: a meta-analysis. Therap Adv Gastroenterol. 2015;8(4):168-175. doi: 10.1177/1756283X15578607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Caro S, Fragkos KC, Keetarut K, et al. Enteral nutrition in adult Crohn’s disease: toward a Paradigm shift. Nutrients. 2019;11(9):2222-2253. doi: 10.3390/nu11092222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wands DIF, Gianolio L, Wilson DC, et al. Nationwide real-world exclusive enteral nutrition practice over time: persistence of use as induction for Pediatric Crohn’s disease and emerging combination strategy with biologics. Inflamm Bowel Dis. 2023:izad167. doi: 10.1093/ibd/izad167 [DOI] [PubMed] [Google Scholar]
- 30. de Sire R, Nardone OM, Testa A, Calabrese G, Caiazzo A, Castiglione F.. Exclusive enteral nutrition in adult Crohn’s disease: an overview of clinical practice and perceived barriers. Clin Exp Gastroenterol. 2021;14:493-501. doi: 10.2147/CEG.S267172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kakkadasam Ramaswamy P; Gold Coast Inflammatory Bowel Diseases Research Group. Exclusive enteral nutrition with oral polymeric diet helps in inducing clinical and biochemical remission in adults with active Crohn’s disease. JPEN J Parenter Enteral Nutr. 2022;46(2):423-432. doi: 10.1002/jpen.2273 [DOI] [PubMed] [Google Scholar]
- 32. Little RD, Swaine A, Reynolds R, et al. Adalimumab drug levels at secondary loss of response do not predict response to dose-intensification in Crohn’s disease: a retrospective, international multicenter study. Inflamm Bowel Dis. 2023:izad248. doi: 10.1093/ibd/izad248 [DOI] [PubMed] [Google Scholar]
- 33. Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. doi: 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 34. Kucharzik T, Wilkens R, D’Agostino MA, et al. ; STARDUST Intestinal Ultrasound study group. Early ultrasound response and progressive transmural remission after treatment with Ustekinumab in Crohn’s disease. Clin Gastroenterol Hepatol. 2023;21(1):153-163.e12. doi: 10.1016/j.cgh.2022.05.055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.