Abstract
Nickel–titanium (NiTi) instruments have become the backbone of endodontics due to their exceptional properties, superelasticity, and shape memory. However, challenges such as unexpected breakage, poor cutting efficiency, and corrosion have prompted researchers to explore innovative surface modifications to enhance their performance. This comprehensive review discusses the latest advancements in NiTi metallurgy and their impact on rotary NiTi file systems. Various surface treatment techniques, including ion implantation, cryogenic treatment (CT), thermal nitridation, electropolishing, and physical or chemical vapor deposition, have been investigated to minimize defects, boost surface hardness, and improve cyclic fatigue resistance. Ion implantation has shown promise by increasing wear resistance and cutting efficiency through nitrogen ion incorporation. Thermal nitridation has successfully formed titanium nitride (TiN) coatings, resulting in improved corrosion resistance and cutting efficiency. CT has demonstrated increased cutting efficiency and overall strength by creating a martensite transformation and finer carbide particles. Electropolishing has yielded mixed results, providing smoother surfaces but varying impacts on fatigue resistance. Physical or chemical vapor deposition has proven effective in forming TiN coatings, enhancing hardness and wear resistance. Furthermore, the concept of surface functionalization with silver ions for antibacterial properties has been explored. These advancements present an exciting future for endodontic procedures, offering the potential for enhanced NiTi instruments with improved performance, durability, and patient outcomes.
Keywords: Cryogenic treatments, ion implantation, nickel–titanium alloy, surface modifications, surface treatments, thermal treatment
INTRODUCTION
Nickel–titanium (NiTi) alloy has extensively been utilized as the primary material for producing endodontic instruments. Over time, the demand for NiTi instruments did not drop but, rather, experienced a resurgence. This rebound interest stems from applying contemporary research methods and tools from diverse fields, turning it into an interdisciplinary field of study. In 1988, Walia et al. first introduced NiTi files to the field of endodontics.[1] Initially, Civjan et al.[2] proposed the application of NiTi alloy for crafting rotary and hand files. NiTi rotary files gained popularity due to their ability to shape and debride the canals with greater precision and lesser procedural errors than stainless steel (SS) hand instruments.[3] Although, these files are susceptible to unexpected breakage. Flexural and torsional fractures are two separate fracture modes for NiTi files. Flexural fractures develop due to the files in curved canals experiencing cyclic fatigue. When NiTi files are loaded and unloaded repeatedly during instrumentation, a recurrent phase transition occurs that finally causes torsional fracture once the instrument has passed the point of irreversible plastic deformation.[4]
To enhance their performance, there is a demand for new materials and manufacturing techniques for NiTi rotary instruments. The development of novel rotary endodontic instruments with superior mechanical qualities has been popular in recent years due to the realization that the microstructural changes brought about by thermomechanical treatments can regulate the phase transformations happening in equiatomic NiTi alloys. NiTi instruments have intrinsic flaws that occur during the manufacturing process;[5] hence, efforts have been made to improve their surface properties. Different surface changes have been used to lower or eliminate flaws, boost surface hardness or flexibility, enhance cycle fatigue resistance, and improve cutting effectiveness. Various techniques discussed in this review have been used to give NiTi alloys beneficial properties for the best performance as an endodontic file.
To address material-related concerns with NiTi alloy and the manufacturing of instruments, such as poor cutting efficiency and failure due to fatigue caused by defects, surface changes have been investigated and modified. This review focuses on the latest advancements in NiTi metallurgy and its effect on rotary NiTi files, aiming to pave the way for even more effective and reliable endodontic procedures.
SURFACE CHARACTERISTICS OF NICKEL–TITANIUM ALLOY
The surface of NiTi instruments primarily comprises titanium oxides (TiO2), carbon, and oxygen, with less quantities of nickel oxides (NiO and Ni2O3) and metallic nickel (Ni).[6] The thickness of the oxide layer can vary between 2 and 20 nm, and the surface chemistry and the amount of Ni may differ widely depending on the preparation method.[7] Ni disintegrates more readily as compared to titanium (Ti) due to the less stable nature of its oxide. The superficial layers of NiTi wires exhibit uneven features represented as long island-like formations, suggesting selective dissolution of Ni.[8]
Shabalovskaya[9] observed that the Ti: Ni ratio on the wire surface was 5.5 after mechanical polishing, indicating the presence of five more times Ti on the surface than Ni. However, after boiling or autoclaving the wire in water, the Ti: Ni ratio scaled to 23.4–33.1, and the Ni content was reduced. Similar results were obtained by Hanawa et al.,[6] who found that after immersion in neutral electrolyte solution for 30 days, the Ti: Ni ratio in the polished samples rose from 5.8 to 91. Although the titanium–aluminum–vanadium alloy (Ti6Al4V) in their investigation only had 6% aluminum compared to 50% Ni in NiTi, the surface of the alloy had aluminum levels similar to those in NiTi. However, some chromium and iron were discovered on the exterior of SS, which was devoid of Ni.
Due to the presence of a persistent TiO2 layer, pure Ti and specific Ti alloys are regarded as extremely biocompatible materials.[7] The oxide layer that forms on a Ti implant during implantation expands and takes up minerals and other substances from tissue fluids, resulting in surface remodeling. Hanawa et al.[6] discovered that the calcium phosphate and Ti dioxide layers make up the oxide layer on implants. On an inert oxide layer, calcium phosphate is specifically produced. This layer had a Ca: P ratio that was similar to hydroxyapatite and was denser on pure Ti than Ti alloys (including NiTi). However, the calcium phosphates generated on NiTi or Ti6Al4V were less akin to hydroxyapatite. This is probably because Ni is present on the exterior of NiTi alloy, and aluminum is present on the surface of Ti6Al4V, which may have impacted these results. Similar calcium phosphate layers also exist in SS, although they form more slowly and differently than they do in NiTi.[6,10,11]
SURFACE MODIFICATIONS OF NICKEL–TITANIUM ALLOYS
Numerous strategies have been employed to improve the surface properties of NiTi instruments, aiming to reduce their inherent flaws, enhance surface hardness and flexibility, and improve resistance to cyclic fatigue and cutting efficiency in endodontic procedures.[12] As summarized in Table 1, some of them are:
Table 1.
Studies included in the review and their results obtained
| Surface modification | Author | Year | Method | Result |
|---|---|---|---|---|
| Ion implantation | Gavini et al.[13] | 2010 | Nitrogen ion implantation | Increases the number of CTF |
| Wolle et al.[14] | 2009 | Argon and nitrogen ion implantation | Argon ion implanted file showed double the number of CTF than nitrogen ion implanted file | |
| Rapisarda et al.[15] | 2001 | Nitrogen ion implantation | Increased wear resistance | |
| Conrad et al.[16] Tendys et al.[17] | 1987 1988 | PIII of TiN | Increased wear resistance | |
| Thermal nitridation | Rapisarda et al.[18] | 2000 | TiN coating | Improved cutting ability |
| Lin et al.[19] | 2007 | TiN coating | Greater corrosion resistance when exposed to 5.25% NaOCl | |
| Li et al.[20] | 2006 | TiN coating | Increased cutting efficiency and corrosion resistance | |
| Cryogenic therapy | Kim et al.[21] | 2005 | Cryogenic therapy | Higher microhardness, increased austenitic phase, increased cutting efficiency |
| Vinoth Kumar et al.[22] | 2007 | Deep CT | Improved cutting efficiency, no effect on wear resistance | |
| George et al.[23] | 2011 | Deep CT | Increased cyclic fatigue resistance | |
| Electropolishing | Anderson et al.[24] Tripi et al.[25] da Silva et al.[26] Lopes et al.[27] Condorelli et al.[28] Praisarnti et al.[29] |
2007 2006 2013 2010 | Electropolishing | Increased cyclic fatigue resistance |
| Herold et al.[30] | 2007 | Electropolishing | Does not prevent microfractures | |
| Bui et al.[31] | 2008 | Electropolishing | Electropolished files less resistant to cyclic fatigue | |
| Kaul et al.[32] | 2014 | Electropolishing | Eliminated manufacturing flaws but produced a weak surface highly vulnerable to fresh crack development | |
| Vapor deposition | Schäfer[33] | 2002 | PVD to NiTi K-files | 26.2% increase in cutting efficiency |
| Chi et al.[34] | 2017 | Titanium–zirconium-boron surface layer via PVD | Highly smooth file geometry with greater cyclic fatigue resistance | |
| Bonaccorso et al.[35] | 2008 | PVD and immersion in sodium chloride | Enhanced corrosion resistance, and better pitting resistance | |
| Qaed et al.[36] | 2018 | PVD | Electropolished files performed better than physical vapor-deposited files | |
| Surface functionalization | Cora et al.[37] | 2020 | 2% silver ion dip-coating | Increased efficiency against Enterococcus faecalis without influencing cutting efficiency |
CT: Cryogenic treatment, TiN: Titanium nitride, PIII: Plasma immersion ion implantation, CTF: Cycles to fracture, PVD: Physical vapor deposition, NiTi: Nickel–titanium
-
Ion implantation
-
a)Implantation of nitrogen (N2), argon (Ar), and boron (B) ions
-
b)Plasma immersion ion implantation (PIII).
-
a)
-
Thermal nitridation
-
a)Surface coating with titanium nitride (TiN) layer
-
b)Powder immersion reaction-assisted coating (PIRAC).
-
a)
Cryogenic therapy
Electropolishing
-
Vapor deposition
-
a)Physical vapor deposition (PVD)
- Arc evaporation
- Magnetron sputtering
- Ion plating.
-
b)Chemical vapor deposition.
-
a)
-
Surface functionalization
-
a)Silver ion coating.
-
a)
Ion implantation
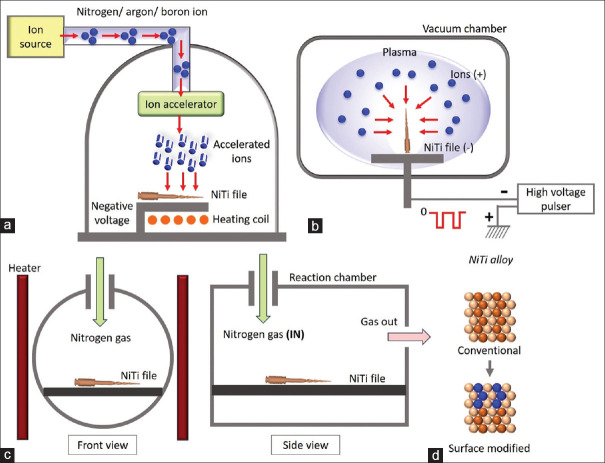
Several efforts have been made to minimize the liberation of Ni from NiTi while preserving the mechanical features of the bulk material. Ion implantation is one such coating technique. As shown in Figure 1a, this involves bombarding gaseous atoms that have been voltage-accelerated into ions such that they get buried beneath the surface of the substrate. The accelerating voltage affects the depth to which they are buried. The end result produces a series of dislocations that increase the material’s durability.[14] Various ions that can be implanted on endodontic files are N2, Ar, and B.
Figure 1.
Illustration of various surface treatments. (a) Ion implantation, (b) plasma immersion ion implantation, (c) thermal nitridation, (d) surface morphology of NiTi file before and after surface treatment. NiTi: Nickel–titanium
With the implantation of N2 ion, the hardness of endodontic files is decreased, while resistance to wear, cutting efficiency, and cyclic fatigue resistance is increased.[13,15,18,38] Gavini et al.[13] demonstrated that compared to nonimplanted (381 cycles) and annealed files (428 cycles), N2 ion-implanted instruments had a considerably greater number of cycles to fracture (CTF) (510 cycles). Wolle et al.[14] examined the impact produced on file morphology by Ar and N2 ion implantation. The studies demonstrated the potential growth and spread of cracks and their resistance to cycle fatigue. While endodontic files ingrained with N2 performed inferiorly in the fatigue test, merely attaining approximately half the mean CTF, authors found files ingrained with Ar have higher CTF than endodontic files without any alterations. Contrary to findings from Rapisarda et al.,[15] who showed a rise in files’ wear resistance ingrained with N2 ions, this investigation did not discover any significant development or propagation of cracks.
This might result from N2’s higher atomic mass, which restricts the generation of point defects within the crystalline structure. Additionally, N2 forms an exceptionally hard material called TiN when combined with Ti in the file, preventing microfractures.[15] Clinically, a surge in wear resistance may extend the instrument’s life while retaining its accuracy and blade shape after usage and lowering the danger of instrument fracture.
To successfully increase the surface hardness of NiTi alloys, Lee et al. inserted B ions into them using a nonequilibrium technique.[39] N2 was substituted with B because Ti-B has greater mechanical strength than Ti-N. The therapeutic applicability of the findings is constrained using a flatter polycrystalline substrate of NiTi alloy in this investigation rather than an endodontic file.
In the late 1980s, Conrad et al.[16] and Tendys et al.[17] first developed PIII. In this procedure, as shown in Figure 1b, the specimen is placed in a chamber surrounded by plasma ions. A powerful negative pulsating voltage is then used to suck the ions from the plasma, accelerate them, and batter them onto the object’s surface. This method only alters surface features by adding a coating of TiN, which gives items a golden appearance. As a result, wear resistance is increased without sacrificing the material’s natural flexibility or microstructure.[40,41]
Thermal nitridation
Thermal nitridation is another method for producing a hard surface layer that increases wear resistance and surface hardness, as shown in Figure 1c. The sample is heated up thermally in an N2 atmosphere, typically between 200 and 500°C,[18,19] which coats the NiTi files with a layer of TiN, as shown in Figure 1d. Ion implantation and thermal nitridation, two alternative TiN surface treatments, were contrasted by Rapisarda et al.[18] Compared to unaltered files, both procedures demonstrated a higher TiN presence, while ion implantation demonstrated a higher N2-to-Ti ratio. Ion implantation showcased a higher cutting efficiency than thermal nitridation, but both techniques had improved cutting ability compared to no surface treatment.[18]
Shenhar et al.[42] and Huang et al.[43] demonstrated that the presence of a TiN coating significantly enhanced the resistance to corrosion of Ti and Ti alloys when exposed to a corrosive environment. When exposed to 5.25% sodium hypochlorite, Lin et al.[19] showed that including TiN on NiTi endodontic files considerably boosted corrosion resistance. The highest corrosion resistance was achieved at 300°C nitriding temperatures, although following treatment, NiTi’s superelastic characteristics were lost. As a result, nitriding at a temperature of 250°C was advised for usage in clinical settings.[19] Li et al.’s[20] investigation of thermal nitridation at various temperatures revealed that the presence of TiN increased cutting effectiveness and corrosion resistance.
PIRAC is another technique for creating a layer of TiN. Substrates are annealed in enclosed steel foil vessels at less pressure and high temperatures (800°C–1100°C).[44,45] An N2-rich layer with a thin outer layer of TiN and a thicker layer of Ti2Ni is formed on the surface of the sample due to the diffusion of highly reactive monatomic N2.[46] It is noticeable that PIRAC layers have good adherence to the substrate and are comparable to oxide layers on NiTi alloys. Although this method has been applied to biomedical NiTi alloys, it has not been explicitly researched on NiTi endodontic files.
Cryogenic therapy of nickel-titanium alloys
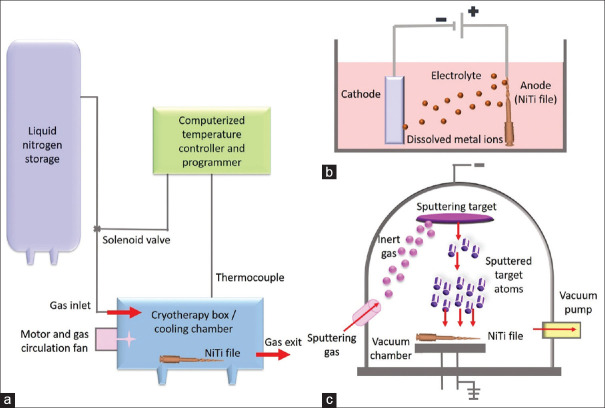
As shown in Figure 2a, cryogenic treatment (CT) is a manufacturing process recommended to enhance the surface hardness and thermal stability of metals.[47] The optimal temperature range for this therapy is typically between − 60°C and − 80°C, which may vary according to the material and specific quenching parameters present.[47] Over the past three decades, reports have shown significant advantages of exposing metals used for industrial purposes to CT.[47,48,49]
Figure 2.
Illustration of various surface modifications. (a) Cryogenic therapy, (b) electropolishing, (c) physical vapor deposition. NiTi: Nickel–titanium
CT is a relatively newer cooling approach involving immersing the metal in a super-cooled bath of liquid N2 at extremely low temperatures of around − 196°C (−320°F).[47,48] The metal is then allowed to gradually warm to room temperature.[49,50] Compared to traditional cold treatment, CT offers more favorable effects.[51] The advantages include increased cutting efficiency and strength of the alloy.[47,49] Additionally, CT is a cost-effective therapy that influences the whole cross-section of the alloy, unlike surface treatments such as vapor deposition and ion implantation, which only impact the surface.[48]
During CT, two mechanisms can change the metal properties. The first mechanism follows CT and entails a more thorough martensite transition from the austenite phase.[50] The next mechanism is the precipitation of smaller carbide particles inside the crystalline structure.[49] However, there is some debate about which mechanism plays the primary role in these changes.
Regarding NiTi rotary instruments, there have been relatively few studies on CT. Kim et al.[21] assessed the impact of cryogenic therapy on the cutting efficiency, composition, and microhardness of NiTi files. Their results revealed that cryogenically modified files exhibited considerably higher microhardness than controls. The composition of the test and control groups was 56% Ni (by weight), 44% Ti (by weight), and no N, with the bulk of the material in the austenite phase. The deep dry CT greatly improved the cutting effectiveness of NiTi instruments but had no appreciable impact on wear resistance, according to a different study by Vinothkumar et al.[22] A deep CT considerably increased the cyclic fatigue resistance of NiTi rotary files, according to George et al.’s[23] research.
Electropolishing
As shown in Figure 2b, electropolishing is an electrochemical process that removes surface imperfections.[52] A direct current is passed through the solution while the file (anode) is submerged in an electrolytic bath with a cathode that is kept at a specific temperature.[53] As a protective layer, a surface oxide layer is created, which improves corrosion resistance and cyclic fatigue resistance, and there is a reduction in surface residual stress.[5,12,24,54,55] It is debatable if electropolishing actually increases the resistance to corrosion and fatigue life of NiTi instruments. There is general agreement that electropolishing improves the endodontic file’s surface texture, making it smoother.[25,30,32,35,56,57]
Compared to nonelectropolished files, electropolished instruments need a larger potential to form pitting, indicating greater corrosion resistance.[35] While the existence of a corrosion pit was linked to the onset of cracks, other studies revealed that electropolishing did not increase corrosion resistance.[56,57] Various studies by Anderson et al.,[24] Tripi et al.,[25] da Silva et al.,[26] Lopes et al.,[27] Condorelli et al.,[28] and Praisarnti et al.[29] have shown that electropolishing increases cycle fatigue resistance. Larger groove flaws lead to fewer cycles till fracture,[58] whereas surface irregularities would act as places of stress concentration and lead to crack initiation.[24] In contrast, Herold et al.[30] discovered that when compared to regular, untreated ProFiles, electropolishing (EndoSequence) does not prevent microfractures. Bui et al.[31] discovered, on the other hand, that in simulated canals made of plastic blocks, electropolished profiles were considerably less resistant to cycle fatigue than conventional ProFiles. When utilizing electropolished files, greater maximum torque values were needed,[59] suggesting that electropolishing may level and dull the cutting edges, necessitating a larger torque to obtain the same amount of preparation. Examination revealed that the crack lines were occasionally inconsistent with the machined grooves.[60]
While electropolishing eliminated all manufacturing flaws, Kaul et al.[32] discovered a weak surface and highly vulnerable to developing fresh cracks. Altogether, many other investigations indicated that electropolished instruments had not shown higher resilience to cyclic fatigue than any other instrument.[61] Electropolishing had a minimal impact on cutting effectiveness and torsional resistance.[24,26,31] In conclusion, electropolishing gives endodontic files a smoother finish; however, the evidence for the advantages of this surface treatment is inconsistent.
Physical or chemical vapor deposition
Since the late 1980s, medical devices have been coated using the PVD technique to increase wear resistance.[62] PVD has three main types: arc evaporation, magnetron sputtering, and ion plating.[33] PVD produces a dense, homogeneous layer that is highly resistant to corrosion, has enhanced surface hardness, and is biocompatible.[33] The cathodic arc evaporation process is frequently utilized for the best coating to metal adherence. As a result, TiN forms a hard coating over the surface, as shown in Figure 2c.
Using this method, a thin layer of fine-grained TiN film is formed at low temperatures over the surface of files. This TiN layer can increase wear resistance, surface hardness, and cutting efficiency.[15,18] The surface hardness can reach 2200 VHD when the coating thickness ranges between 1 and 7 microns. Surface imperfections, fissures, and potential residual stresses are eliminated by forming a continuous amorphous coating over the file’s surface, extending the life of the endodontic instrument.[63] When Schafer initially applied PVD to NiTi K-files, he discovered a 26.2% rise in cutting efficiency compared to files without coating.[33] Chi et al. added a unique Ti-zirconium-B (TiZB) surface layer via PVD, resulting in a TiZB film with a highly smooth file geometry and greater cyclic fatigue resistance than untreated files.[34] In PVD instruments, Bonaccorso et al.[35] showed enhanced corrosion resistance. The study discovered that when PVD files were submerged in sodium chloride solution for 1.5 h, they had better pitting resistance than electropolished or nonelectropolished files. Since sodium chloride solutions are not usually employed in endodontic treatments, the findings of this study cannot be easily applied to clinical settings. However, electropolished files outperformed PVD files, according to Qaed et al.[36]
At high temperatures of 300°C, chemical vapor deposition (CVD) also forms a superficial layer made up of TiN.[64,65] Early research proved metal-organic CVD as the elected technique as it can enhance the Ni: Ti ratio on the substrate’s surface by up to twofold.[65] While both CVD and PVD may provide a tough surface layer to NiTi devices, PVD deposits films with clearly defined grains, whereas CVD produces uninterrupted coatings of amorphous materials having weak crystalline structures.[64] Notably, the superficial layers may become exposed as the file’s cutting edges deteriorate,[33] and the fragments may end up trapped within the canal space. Toxic effects may arise from the liberation of these nanoparticles or metal ions.[66]
Surface functionalization of nickel-titanium endodontic files
The objective of surface functionalization is to either block a potentially harmful reaction or to elicit a desired response. To reinforce the NiTi wire, several coatings have been used.[67] Early Teflon and polyethylene coatings have been shown to increase corrosion resistance.[68] Rhodium coatings with low reflectivity and epoxy resin have been used as surface coatings. Compared to uncoated NiTi wires, these coatings can minimize surface roughness.[69] Damage to the surface morphology, however, could be harmful in an endodontic situation because it could cause fractured particles from the surface coating to get dislodged inside the root canal space or move into the periapical area, potentially causing a foreign body response.[70] Most endodontic studies on surface modifications have mainly concentrated on the geometry and shape created by these files, such as conicity, taper, and centering ability, as well as their mechanical features, such as metallurgy, flexibility, cyclic fatigue, torsional fatigue, cutting efficiencies, and so on.
A single study has probed into the idea of giving endodontic instruments a new use beyond their current capacity of shaping the root canal and clearing away the debris. Cora et al.[37] coated the surface of NiTi rotary files with silver ions and examined their antibacterial properties. They used the dip-coating process at 25 or 50 mm/min for coating the ProTaper Universal NiTi files with a silane-based, silver-complex solution (2% silver ion coating).[37] Enterococcus faecalis was used as a test subject for its antibacterial activity. Cultures were taken from the sample and incubated; then, the bacterial colony count was recorded. The debris lost in clear resin blocks after creating an artificial root canal was used to compare the cutting effectiveness of coated and uncoated files.[37] Additionally, they examined the files using scanning electron microscopy and observed that the silver ion surface coating did not influence the cutting efficacy of rotary NiTi endodontic file, while it was effective against E. faecalis. This encapsulates the conceptual framework and potential for functionalizing NiTi rotary files in the future.
CONCLUSION
NiTi instruments have seen a resurgence in endodontics, driven by cutting-edge research and interdisciplinary approaches. Exciting surface modifications, such as N2 ion implantation for wear and fatigue resistance and PIII for a durable TiN layer, are elevating performance. Thermal nitridation creates a corrosion-resistant TiN coating, while CT at super-cooled temps enhances strength. Electropolishing offers smoother surfaces, but the impact on fatigue varies. Physical or chemical vapor deposition provides tough TiN coatings. Surface functionalization with silver ions shows potential for antibacterial properties. These innovations promise a bright future for enhanced endodontic procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Walia H, Brantley WA, Gerstein H. An initial investigation of the bending and torsional properties of Nitinol root canal files. J Endod. 1988;14:346–51. doi: 10.1016/s0099-2399(88)80196-1. [DOI] [PubMed] [Google Scholar]
- 2.Civjan S, Huget EF, DeSimon LB. Potential applications of certain nickel-titanium (nitinol) alloys. J Dent Res. 1975;54:89–96. doi: 10.1177/00220345750540014301. [DOI] [PubMed] [Google Scholar]
- 3.Prasad PS, Sam JE, Kumar A, Kannan The effect of 5% sodium hypochlorite, 17% EDTA and triphala on two different rotary Ni-Ti instruments: An AFM and EDS analysis. J Conserv Dent. 2014;17:462–6. doi: 10.4103/0972-0707.139842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair AS, Tilakchand M, Naik BD. The effect of multiple autoclave cycles on the surface of rotary nickel-titanium endodontic files: An in vitro atomic force microscopy investigation. J Conserv Dent. 2015;18:218–22. doi: 10.4103/0972-0707.157256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhn G, Tavernier B, Jordan L. Influence of structure on nickel-titanium endodontic instruments failure. J Endod. 2001;27:516–20. doi: 10.1097/00004770-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Hanawa T, Hiromoto S, Asami K, Okuno O, Asaoka K. Surface oxide films on titanium alloys regenerated in Hanks'solution. Mater Trans. 2002;43:3000–4. [Google Scholar]
- 7.Vadiraj A, Kamaraj M. Effect of surface treatments on fretting fatigue damage of biomedical titanium alloys. Tribol Int. 2007;40:82–8. [Google Scholar]
- 8.Oshida Y, Sachdeva RC, Miyazaki S. Microanalytical characterization and surface modification of TiNi orthodontic archwires. Biomed Mater Eng. 1992;2:51–69. [PubMed] [Google Scholar]
- 9.Shabalovskaya SA. On the nature of the biocompatibility and on medical applications of NiTi shape memory and superelastic alloys. Biomed Mater Eng. 1996;6:267–89. [PubMed] [Google Scholar]
- 10.Garg S, Mahajan P, Thaman D, Monga P. Comparison of dentinal damage induced by different nickel-titanium rotary instruments during canal preparation: An in vitro study. J Conserv Dent. 2015;18:302–5. doi: 10.4103/0972-0707.159730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanawa T, Asami K, Asaoka K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J Biomed Mater Res. 1998;40:530–8. doi: 10.1002/(sici)1097-4636(19980615)40:4<530::aid-jbm3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Gutmann JL, Gao Y. Alteration in the inherent metallic and surface properties of nickel-titanium root canal instruments to enhance performance, durability and safety: A focused review. Int Endod J. 2012;45:113–28. doi: 10.1111/j.1365-2591.2011.01957.x. [DOI] [PubMed] [Google Scholar]
- 13.Gavini G, Pessoa OF, Barletta FB, Vasconcellos MA, Caldeira CL. Cyclic fatigue resistance of rotary nickel-titanium instruments submitted to nitrogen ion implantation. J Endod. 2010;36:1183–6. doi: 10.1016/j.joen.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Wolle CF, Vasconcellos MA, Hinrichs R, Becker AN, Barletta FB. The effect of argon and nitrogen ion implantation on nickel-titanium rotary instruments. J Endod. 2009;35:1558–62. doi: 10.1016/j.joen.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Rapisarda E, Bonaccorso A, Tripi TR, Condorelli GG, Torrisi L. Wear of nickel-titanium endodontic instruments evaluated by scanning electron microscopy: Effect of ion implantation. J Endod. 2001;27:588–92. doi: 10.1097/00004770-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Conrad JR, Radtke JL, Dodd RA, Worzala FJ, Tran NC. Plasma source ion-implantation technique for surface modification of materials. J Appl Phys. 1987;62:4591–6. [Google Scholar]
- 17.Tendys J, Donnelly IJ, Kenny MJ, Pollock JT. Plasma immersion ion implantation using plasmas generated by radio frequency techniques. Appl Phys Lett. 1988;53:2143–5. [Google Scholar]
- 18.Rapisarda E, Bonaccorso A, Tripi TR, Fragalk I, Condorelli GG. The effect of surface treatments of nickel-titanium files on wear and cutting efficiency. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:363–8. doi: 10.1016/s1079-2104(00)70103-x. [DOI] [PubMed] [Google Scholar]
- 19.Lin MC, Liu JF, Li UM, Lin CP, Tsai WF, Ai CF, et al. Thermal nitriding treatment increases the corrosion resistance of Ni-Ti file. Iran Endod J. 2007;9:235–40. [Google Scholar]
- 20.Li UM, Chiang YC, Chang WH, Lu CM, Chen YC, Lai TM, et al. Study of the effects of thermal nitriding surface modification of nickel titanium rotary instruments on the wear resistance and cutting efficiency. J Dent Sci. 2006;1:53–8. [Google Scholar]
- 21.Kim JW, Griggs JA, Regan JD, Ellis RA, Cai Z. Effect of cryogenic treatment on nickel-titanium endodontic instruments. Int Endod J. 2005;38:364–71. doi: 10.1111/j.1365-2591.2005.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinothkumar TS, Miglani R, Lakshminarayananan L. Influence of deep dry cryogenic treatment on cutting efficiency and wear resistance of nickel-titanium rotary endodontic instruments. J Endod. 2007;33:1355–8. doi: 10.1016/j.joen.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 23.George GK, Sanjeev K, Sekar M. An in vitro evaluation of the effect of deep dry cryotreatment on the cutting efficiency of three rotary nickel titanium instruments. J Conserv Dent. 2011;14:169–72. doi: 10.4103/0972-0707.82627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson ME, Price JW, Parashos P. Fracture resistance of electropolished rotary nickel-titanium endodontic instruments. J Endod. 2007;33:1212–6. doi: 10.1016/j.joen.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Tripi TR, Bonaccorso A, Condorelli GG. Cyclic fatigue of different nickel-titanium endodontic rotary instruments. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e106–14. doi: 10.1016/j.tripleo.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 26.da Silva MA, Ponciano Gomes JA, Ormiga F. Influence of electrochemical polishing on the mechanical behaviour of nickel-titanium rotary files. Aust Endod J. 2013;39:73–7. doi: 10.1111/j.1747-4477.2011.00308.x. [DOI] [PubMed] [Google Scholar]
- 27.Lopes HP, Elias CN, Vieira VT, Moreira EJ, Marques RV, de Oliveira JC, et al. Effects of electropolishing surface treatment on the cyclic fatigue resistance of BioRace nickel-titanium rotary instruments. J Endod. 2010;36:1653–7. doi: 10.1016/j.joen.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Condorelli GG, Bonaccorso A, Smecca E, Schäfer E, Cantatore G, Tripi TR. Improvement of the fatigue resistance of NiTi endodontic files by surface and bulk modifications. Int Endod J. 2010;43:866–73. doi: 10.1111/j.1365-2591.2010.01759.x. [DOI] [PubMed] [Google Scholar]
- 29.Praisarnti C, Chang JW, Cheung GS. Electropolishing enhances the resistance of nickel-titanium rotary files to corrosion-fatigue failure in hypochlorite. J Endod. 2010;36:1354–7. doi: 10.1016/j.joen.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Herold KS, Johnson BR, Wenckus CS. A scanning electron microscopy evaluation of microfractures, deformation and separation in EndoSequence and profile nickel-titanium rotary files using an extracted molar tooth model. J Endod. 2007;33:712–4. doi: 10.1016/j.joen.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Bui TB, Mitchell JC, Baumgartner JC. Effect of electropolishing ProFile nickel-titanium rotary instruments on cyclic fatigue resistance, torsional resistance, and cutting efficiency. J Endod. 2008;34:190–3. doi: 10.1016/j.joen.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Kaul R, Farooq R, Kaul V, Khateeb SU, Purra AR, Mahajan R. Comparative evaluation of physical surface changes and incidence of separation in rotary nickel-titanium instruments: An in vitro SEM study. Iran Endod J. 2014;9:204–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Schäfer E. Effect of physical vapor deposition on cutting efficiency of nickel-titanium files. J Endod. 2002;28:800–2. doi: 10.1097/00004770-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Chi CW, Deng YL, Lee JW, Lin CP. Fracture resistance of dental nickel-titanium rotary instruments with novel surface treatment: Thin film metallic glass coating. J Formos Med Assoc. 2017;116:373–9. doi: 10.1016/j.jfma.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Bonaccorso A, Tripi TR, Rondelli G, Condorelli GG, Cantatore G, Schäfer E. Pitting corrosion resistance of nickel-titanium rotary instruments with different surface treatments in seventeen percent ethylenediaminetetraacetic acid and sodium chloride solutions. J Endod. 2008;34:208–11. doi: 10.1016/j.joen.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Qaed NA, Mourshed BD, Al-Shamiri HM, Alaizari N, Alhamdah SS. The effect of surface topographical changes of two different surface treatments rotary instrument. J Clin Exp Dent. 2018;10:e49–53. doi: 10.4317/jced.54472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cora S, Er K, Taşdemir T, Kiraz N, Becer B, Felek R, et al. Effect of silver ion surface coating on antimicrobial and cutting efficiencies of nickel-titanium rotary files. Meandros Med Dent J. 2020;21:26–33. [Google Scholar]
- 38.Alves-Claro AP, Claro FA, Uzumaki ET. Wear resistance of nickel-titanium endodontic files after surface treatment. J Mater Sci Mater Med. 2008;19:3273–7. doi: 10.1007/s10856-008-3439-9. [DOI] [PubMed] [Google Scholar]
- 39.Lee DH, Park B, Saxena A, Serene TP. Enhanced surface hardness by boron implantation in nitinol alloy. J Endod. 1996;22:543–6. doi: 10.1016/S0099-2399(96)80015-X. [DOI] [PubMed] [Google Scholar]
- 40.Li UM, Iijima M, Endo K, Brantley WA, Alapati SB, Lin CP. Application of plasma immersion ion implantation for surface modification of nickel-titanium rotary instruments. Dent Mater J. 2007;26:467–73. doi: 10.4012/dmj.26.467. [DOI] [PubMed] [Google Scholar]
- 41.Alves FR, Ribeiro TO, Moreno JO, Lopes HP. Comparison of the efficacy of nickel-titanium rotary systems with or without the retreatment instruments in the removal of gutta-percha in the apical third. BMC Oral Health. 2014;14:102. doi: 10.1186/1472-6831-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shenhar A, Gotman I, Radin S, Ducheyne P. Microstructure and fretting behavior of hard TiN-based coatings on surgical titanium alloys. Ceram Int. 2000;26:709–13. [Google Scholar]
- 43.Huang HH, Hsu CH, Pan SJ, He JL, Chen CC, Lee TL. Corrosion and cell adhesion behavior of TiN-coated and ion-nitrided titanium for dental applications. Appl Surf Sci. 2005;244:252–6. [Google Scholar]
- 44.Shenhar A, Gotman I, Gutmanas EY, Ducheyne P. Surface modification of titanium alloy orthopaedic implants via novel powder immersion reaction assisted coating nitriding method. Mater Sci Eng A. 1999;268:40–6. [Google Scholar]
- 45.Starosvetsky D, Gotman I. Corrosion behavior of titanium nitride coated Ni-Ti shape memory surgical alloy. Biomaterials. 2001;22:1853–9. doi: 10.1016/s0142-9612(00)00368-9. [DOI] [PubMed] [Google Scholar]
- 46.Mohammadi Z, Soltani MK, Shalavi S, Asgary S. A review of the various surface treatments of NiTi instruments. Iran Endod J. 2014;9:235–40. [PMC free article] [PubMed] [Google Scholar]
- 47.Vinothkumar TS, Kandaswamy D, Prabhakaran G, Rajadurai A. Microstructure of cryogenically treated martensitic shape memory nickel-titanium alloy. J Conserv Dent. 2015;18:292–6. doi: 10.4103/0972-0707.159727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohan Lal D, Renganarayanan S, Kalanidhi A. Cryogenic treatment to augment wear resistance of tool and die steels. Cryogenics. 2001;41:149–55. [Google Scholar]
- 49.Huang J, Zhu Y, Liao X, Beyerlein I, Bourke M, Mitchell T. Microstructure of cryogenic treated M2 tool steel. Mater Sci Eng A. 2003;339:241–4. [Google Scholar]
- 50.Barron R. Cryogenic treatment of metals to improve wear resistance. Cryogenics. 1982;22:409–13. [Google Scholar]
- 51.Moore K, Collins D. Cryogenic treatment of three heat-treated tool steels. Key Eng Mater. 1993;86:47–54. [Google Scholar]
- 52.Shabalovskaya S, Anderegg J, Van Humbeeck J. Critical overview of nitinol surfaces and their modifications for medical applications. Acta Biomater. 2008;4:447–67. doi: 10.1016/j.actbio.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Ounsi HF, Nassif W, Grandini S, Salameh Z, Neelakantan P, Anil S. Evolution of nickel-titanium alloys in endodontics. J Contemp Dent Pract. 2017;18:1090–6. doi: 10.5005/jp-journals-10024-2181. [DOI] [PubMed] [Google Scholar]
- 54.Bonaccorso A, Schäfer E, Condorelli GG, Cantatore G, Tripi TR. Chemical analysis of nickel-titanium rotary instruments with and without electropolishing after cleaning procedures with sodium hypochlorite. J Endod. 2008;34:1391–5. doi: 10.1016/j.joen.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Thierry B, Tabrizian M, Trepanier C, Savadogo O, Yahia L. Effect of surface treatment and sterilization processes on the corrosion behavior of NiTi shape memory alloy. J Biomed Mater Res. 2000;51:685–93. doi: 10.1002/1097-4636(20000915)51:4<685::aid-jbm17>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 56.Cheung GS, Shen Y, Darvell BW. Does electropolishing improve the low-cycle fatigue behavior of a nickel-titanium rotary instrument in hypochlorite? J Endod. 2007;33:1217–21. doi: 10.1016/j.joen.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 57.Peters OA, Roehlike JO, Baumann MA. Effect of immersion in sodium hypochlorite on torque and fatigue resistance of nickel-titanium instruments. J Endod. 2007;33:589–93. doi: 10.1016/j.joen.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Lopes HP, Elias CN, Vieira MV, Vieira VT, de Souza LC, Dos Santos AL. Influence of surface roughness on the fatigue life of nickel-titanium rotary endodontic instruments. J Endod. 2016;42:965–8. doi: 10.1016/j.joen.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Boessler C, Paque F, Peters OA. The effect of electropolishing on torque and force during simulated root canal preparation with ProTaper shaping files. J Endod. 2009;35:102–6. doi: 10.1016/j.joen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Kim HC, Yum J, Hur B, Cheung GS. Cyclic fatigue and fracture characteristics of ground and twisted nickel-titanium rotary files. J Endod. 2010;36:147–52. doi: 10.1016/j.joen.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 61.Oh SR, Chang SW, Lee Y, Gu Y, Son WJ, Lee W, et al. Acomparison of nickel-titanium rotary instruments manufactured using different methods and cross-sectional areas: Ability to resist cyclic fatigue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:622–8. doi: 10.1016/j.tripleo.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 62.Chopra D, Jayasree A, Guo T, Gulati K, Ivanovski S. Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioact Mater. 2022;13:161–78. doi: 10.1016/j.bioactmat.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alapati SB, Brantley WA, Svec TA, Powers JM, Mitchell JC. Scanning electron microscope observations of new and used nickel-titanium rotary files. J Endod. 2003;29:667–9. doi: 10.1097/00004770-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 64.Tripi TR, Bonaccorso A, Condorelli GG. Fabrication of hard coatings on NiTi instruments. J Endod. 2003;29:132–4. doi: 10.1097/00004770-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Tripi TR, Bonaccorso A, Rapisarda E, Tripi V, Condorelli GG, Marino R, et al. Depositions of nitrogen on NiTi instruments. J Endod. 2002;28:497–500. doi: 10.1097/00004770-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Gulati K, Scimeca JC, Ivanovski S, Verron E. Double-edged sword: Therapeutic efficacy versus toxicity evaluations of doped titanium implants. Drug Discov Today. 2021;26:2734–42. doi: 10.1016/j.drudis.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Bącela J, Łabowska MB, Detyna J, Zięty A, Michalak I. Functional coatings for orthodontic archwires-a review. Materials (Basel) 2020;13:3257. doi: 10.3390/ma13153257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brantley WA. Evolution, clinical applications, and prospects of nickel-titanium alloys for orthodontic purposes. J World Fed Orthod. 2020;9:S19–26. doi: 10.1016/j.ejwf.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Iijima M, Muguruma T, Brantley W, Choe HC, Nakagaki S, Alapati SB, et al. Effect of coating on properties of esthetic orthodontic nickel-titanium wires. Angle Orthod. 2012;82:319–25. doi: 10.2319/021511-112.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nair PN. On the causes of persistent apical periodontitis: A review. Int Endod J. 2006;39:249–81. doi: 10.1111/j.1365-2591.2006.01099.x. [DOI] [PubMed] [Google Scholar]