Abstract
The connection between cardiovascular illnesses and the gut microbiota has drawn more and more attention in recent years. According to research, there are intricate relationships between dietary elements, gut bacteria, and their metabolites that affect cardiovascular health. In this study, the role of gut microbiota in cardiovascular disorders is examined, with an emphasis on the cardiac consequences brought on by changes in gut microbiota. This essay discusses the gut-heart axis in depth and in detail. It talks about clinical research looking at how soy consumption, probiotic supplements, and dietary changes affected gut microbiota and cardiovascular risk variables. Our goal is to clarify the possible pathways that connect gut microbiota to cardiovascular health and the implications for upcoming treatment approaches. The authors examine the composition, roles, and effects of the gut microbiota on cardiovascular health, including their contributions to hypertension, atherosclerosis, lipid metabolism, and heart failure. Endotoxemia, inflammation, immunological dysfunction, and host lipid metabolism are some of the potential processes investigated for how the gut microbiota affects cardiac outcomes. The research emphasizes the need for larger interventional studies and personalized medicine strategies to completely understand the complexity of the gut-heart axis and its implications for the management of cardiovascular disease. The development of novel treatment strategies and cutting-edge diagnostic technologies in cardiovascular medicine may be facilitated by a better understanding of this axis.
Keywords: cardiac outcomes, cardiovascular diseases, gut microbiota
Introduction
Highlights
The paper highlights the increasing attention given to the relationship between cardiovascular illnesses and the gut microbiota. Research suggests that intricate connections exist between dietary elements, gut bacteria, and their metabolites, which collectively influence cardiovascular health.
The study examines the role of gut microbiota in cardiovascular disorders, with a specific focus on the cardiac consequences resulting from changes in gut microbiota composition. The paper delves into the concept of the ʻgut-heart axisʼ and thoroughly discusses its implications.
The paper presents clinical research findings on the effects of soy consumption, probiotic supplements, and dietary changes on both gut microbiota and cardiovascular risk factors. It explores the potential pathways connecting gut microbiota to cardiovascular health, including influences on hypertension, atherosclerosis, lipid metabolism, heart failure, endotoxemia, inflammation, immunological dysfunction, and host lipid metabolism.
The association between an unhealthy diet and cardiovascular disease (CVD) morbidity has long been recognized as a significant factor. It was initially established based on determinants of metabolic stress and overweight, such as adiposity and visceral fat presence1. However, recent research has highlighted the intricate interactions between dietary components, gut microbiota, and their metabolites, influencing cardiovascular health. Consequently, there is increasing interest in investigating the potential benefits of probiotics in mitigating atherosclerosis and other CVD forms, driven by a growing understanding of the gut microbiota’s role in CVD2,3. While a relationship between coronary heart disease and atherosclerosis has been established, our knowledge about the microbiome composition changes linked to these conditions remains limited4,5.
CVD, encompassing hypertension, atherosclerosis, and heart failure (HF), remains a leading global cause of mortality6. Studies have revealed diverse interactions between the gut microbiota and its metabolic products with the host, influencing the development and occurrence of CVD. Trimethylamine-N-oxide (TMAO), bile acids, and short-chain fatty acids (SCFAs) are among the gut microbiota’s metabolic byproducts associated with CVD7,8. Early studies suggested a potential link between the microbiota and atherosclerosis, as human atherosclerotic plaques contained bacterial DNA. However, it was uncertain whether the DNA originated from live bacteria within the artery wall51. The first studies that provided insight into a potential cause-and-effect relationship between the gut microbiome and CVD focused on TMAO, a metabolite formed after consuming dietary nutrients abundant in a Western diet9.
Rationale for exploring the gut-heart axis and cardiac outcomes
Understanding the gut-heart axis offers new possibilities for preventive and therapeutic interventions in cardiovascular medicine. Researchers and clinicians are exploring the gut microbiota and its metabolites to identify strategies that can reduce cardiovascular risks, improve treatment outcomes, and enhance overall heart health.
While research in the gut-heart axis is still emerging, promising results from preclinical studies and early human research have generated considerable interest in this field. By unraveling the intricate mechanisms linking gut microbiota to cardiac outcomes, we may pave the way for novel therapeutic approaches and personalized interventions for CVDs in the future. However, further research is needed to fully comprehend the complexities of this relationship and translate these findings into effective clinical applications. In this paper, we delve into the gut-heart axis and explore various aspects of it.
Gut microbiota and cardiovascular health
Composition and functions of the gut microbiota
The gut microbiota is a diverse community of microorganisms, comprising bacteria, yeast, and viruses. Bacteria are classified into various hierarchical levels, including phyla, classes, orders, families, genera, and species9. Over 160 species have been identified, with a majority belonging to dominant phyla9. The primary gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with Firmicutes and Bacteroidetes making up around 90% of the gut microbiota10. The Firmicutes phylum encompasses over 200 genera, such as Lactobacillus, Bacillus, Clostridium, Enterococcus, and Ruminicoccus, with the Clostridium genera being particularly abundant10. Bacteroidetes is mainly composed of notable genera like Bacteroides and Prevotella, while the less abundant Actinobacteria phylum is primarily represented by the Bifidobacterium genus10,11. The gut microbiota serves various vital functions within the human body, including protection against pathogens through colonization of mucosal surfaces and the production of antimicrobial substances, which contributes to enhancing the immune system12. Additionally, the gut microbiota plays essential roles in digestion, metabolism, epithelial cell proliferation and differentiation, as well as influencing insulin resistance and secretion13–15. Moreover, the gut microbiota plays a significant role in brain-gut communication, impacting the mental and neurological functions of the host16. Hence, the gut microbiota’s role within the human body is evident and multifaceted17.
Gut microbiota-host interactions and their influence on cardiovascular health
The gut microbiota plays a significant role in food digestion through two main catabolic pathways known as saccharolytic and proteolytic pathways18. In the saccharolytic pathway, the gut microbiota break down sugars and produce the majority of SCFAs. On the other hand, the proteolytic pathway involves protein fermentation, leading to SCFA production along with other co-metabolites such as ammonia, various amines, thiols, phenols, and indoles. Some of these metabolites can be toxic, and their accumulation, primarily cleared by the kidneys, are referred to as microbial uremic toxins19. Besides their role in food digestion, the gut microbiota performs multiple functions and interacts with the host in various ways. It contributes to the formation and regulation of the intestinal mucosal barriers, controls nutrient uptake and metabolism, aids in the maturation of immunological tissues, and prevents the proliferation of pathogenic microorganisms20–24. Under normal conditions, the gut microbiota continues to stimulate the immune system, serving as a rapid and effective defense mechanism against pathogens25. Overall, the microbiota have a fundamental impact on systemic immunity and metabolism, and a healthy gut microbiota is crucial for the overall health of the host3,26.
A small study demonstrated that hypertensive patients had lower microbial community diversity and distinct clustering compared to normotensive individuals27. Both prehypertensive and hypertensive patients exhibited lower gene richness and α-diversity than healthy controls, along with a higher percentage of bacteria from the genus Prevotella28. Recent research has connected the gut microbiota with CVD by reporting cases of bacterial translocation from the gut to the heart29 and detecting gut bacterial DNA in atherosclerotic plaques2. These findings suggest that the intestine may act as a potential reservoir of pathogenic microorganisms, and the gut microbiota may play a role in atherosclerosis development. Metagenomic analysis revealed differences in the gut microbiome between atherosclerotic cardiovascular patients and healthy individuals, with elevated levels of Streptococcus and Enterobacteriaceae species in patients30. Another study, using the terminal restriction fragment length polymorphism method, found altered gut microbiota profiles in patients with coronary artery diseases (CADs), with an increase in Lactobacillales and Clostridium subcluster XIVa, and a reduction in Bacteroides in fecal samples31. Extensive evidence supports the involvement of the gut microbiota in HF development and progression, leading to the proposal of the ʻgut hypothesisʼ. This hypothesis suggests that reduced cardiac output-induced intestinal hypoperfusion and congestion may lead to bowel wall edema and impaired barrier function, resulting in increased bacterial translocation-related inflammatory responses and changes in the gut microbiota that can further exacerbate HF32–34. HF patients with lower intestinal blood flow were found to have higher anti-LPS IgA serum levels, associated with increased growth of bacteria from colonic mucosa biopsies35. A comparison of fecal bacteria in HF patients and healthy individuals revealed higher colonization of pathogenic bacteria, including Campylobacter, Shigella, Salmonella, and Yersinia enterocolitica, in chronic HF patients36. Furthermore, species such as Candida, Campylobacter, and Shigella were positively correlated with HF severity37,38.
Clinical trials
In the study conducted by Malik et al., the researchers investigated the impact of supplementing patients with stable CAD with Lactobacillus plantarum 299v probiotics. The 6-week intervention resulted in improved endothelial function, but other vascular parameters remained unchanged. Probiotic supplementation also led to reduced levels of inflammatory cytokines and plasma leptin, and specific changes were observed in the gut microbiome composition, with an enrichment of Lactobacillus and Bacillus species. However, there was no significant change in the plasma TMAO concentration. The findings suggest that Lactobacillus plantarum 299v supplementation may have a positive effect on vascular function and certain plasma biomarkers in patients with CAD. Nevertheless, further research is needed to gain a comprehensive understanding of its impact on cardiovascular health39.
In a randomized, controlled crossover trial, Wang et al. investigated the effects of different dietary interventions on gut microbiota composition and cardiovascular risk factors. The participants followed either a healthy lacto-ovo vegetarian diet (VD) or the same diet supplemented with cooked unprocessed or processed lean red meat for three weeks each, with washout periods in between. While specific changes were observed in certain gut microbiota genera and operational taxonomic units in response to the diets, the overall gut microbiota structure remained unaffected. The healthy dietary pattern led to improvements in blood lipid profiles, resulting in reduced total cholesterol and low-density lipoprotein cholesterol levels, regardless of red meat consumption. Additionally, certain gut bacteria were found to be associated with cholesterol levels. The study emphasizes the potential advantages of adopting a healthy dietary pattern for gut microbiota and cardiovascular health, with individual responses varying among participants40.
Shah et al. conducted a research study to investigate the links between dietary soy intake, gut microbiota, and metabolites in healthy individuals. The study identified two distinct gut enterotypes (Enterotype 1 and Enterotype 2) based on the composition of the gut microbiome. The association between soy intake and plasma and stool metabolites varied significantly between these enterotypes. Moreover, specific gut microbiota taxa were found to be connected to blood pressure, with Prevotella associated with increased blood pressure and Dialister linked to decreased blood pressure. Notably, the presence of Prevotella without the protective co-occurrence of Dialister was associated with higher blood pressure and unfavorable cardiometabolic risk markers. The research suggests that the gut microbiome plays a crucial role in influencing the effects of soy intake on metabolites and blood pressure, independent of dietary sodium intake, highlighting the intricate interactions between diet, gut microbiota, and cardiovascular health. More research is needed to fully comprehend the underlying mechanisms and implications for human health41.
Djekic et al. conducted a clinical trial to compare the effects of a VD and a meat-based diet (MD) on patients with ischemic heart disease (IHD) receiving optimal medical therapy. The VD group exhibited lower levels of plasma oxidized LDL-C, total cholesterol, LDL-C, body weight, and BMI compared to the MD group. Although the overall composition of the gut microbiota remained similar between the diets, specific microbial genera differed. The VD induced changes in plasma metabolites, including acylcarnitine metabolites and phospholipids. Plasma concentrations of TMAO and l-carnitine were reduced after VD, while choline levels increased. Individual responses varied, with some participants experiencing greater benefits from VD, resulting in reduced oxidized LDL-C and BMI. The study suggests that VD may have favorable effects on cardiovascular risk markers, possibly attributed to alterations in gut microbiota and plasma metabolites, in patients with IHD42.
Gut microbiota and specific CVDs
Gut microbiota in hypertension and blood pressure regulation
The gut microbiota, with Firmicutes and Bacteroidetes as dominant phyla, plays a vital role in regulating various aspects of cardiovascular health43. It can adapt to lifestyle modifications, including diet and exercise, and influences about 10% of the host’s transcriptome, impacting immunity, cell proliferation, and metabolism44. The gut microbiota’s role in CVD, particularly arteriosclerosis and hypertension, has been studied extensively. Elevated levels of TMAO and toxic metabolites like p-cresol and indoxyl sulfate, derived from the fermentation of protein by gut microbes, are associated with CVD45. SCFA, produced by the gut microbiota, influence blood pressure through the activation of specific receptors, GPR41, GPR43, and Olfr7846. Chronic low-grade inflammation and alterations in microbial gene richness are linked to hypertension47. Probiotic consumption has been shown to modestly decrease blood pressure in humans48. The impact of specific gut microbial species on blood pressure regulation is complex and influenced by genetic factors49. Further research is needed to better understand the specific role of different gut microbial species in cardiovascular health.
Gut-heart axis in atherosclerosis and lipid metabolism
Recent studies have revealed the presence of bacterial DNA in atherosclerotic plaques, suggesting a potential role of the gut microbiota in the development of CVD50. Patients with atherosclerosis have shown distinct differences in gut microbiota compared to those without51. Some studies found that patients with coronary heart disease or high IMT values, a marker of subclinical atherosclerosis, had a greater Firmicutes/Bacteroidetes ratio, while others reported enrichment of the phyla Escherichia in patients with subclinical carotid atherosclerosis and CAD52. Atherosclerotic plaques exhibit dominance of the phylum Proteobacteria and also contain the gut-dominant phylum Firmicutes. However, the role of the gut microbiota in atherosclerosis development remains inconclusive. Some studies suggest significant differences in gut microbiota between patients with stable and unstable plaques, while others found no major distinctions53. Bacterial DNA in plaques may trigger macrophages and activate the innate immune system through Toll-like receptor 2 (TLR2) and TLR4, which could be linked to plaque stability54.
Gut microbiota modulation and its impact on HF
The ʻgut hypothesisʼ in HF proposes a significant connection between the gut microbiota, its metabolites, and HF pathogenesis33. Evidence suggests that bacterial translocation in HF results from various mechanisms leading to structural and functional changes in the gastrointestinal tract, including splanchnic congestion and alterations in the host’s immune defense system55. The gut microbiota, as essential components of the intestinal micro-ecosystem, play a crucial role in HF, influencing inflammation and intestinal permeability. Studies have shown increased pathogenic bacteria and yeasts in stable chronic HF patients, with levels correlating with HF severity37. The presence of the Escherichia/Shigella genus is also increased during the decompensated phase of HF compared to the compensated phase56. This pathogen overgrowth raises the risk of gastrointestinal infections in HF patients, leading to a worse in-hospital prognosis, especially when combined with antibiotic treatment57.
Potential mechanisms underlying gut microbiota-cardiac connections
The gut microbiota’s function is closely linked to the risk of CVDs. Gut microbiota dysbiosis can lead to impaired mucosal barrier, overactivated inflammation, and immune dysfunction, which are crucial steps in CVD development. Gram-negative bacteria, particularly LPS, play a key role in endotoxemia and impaired intestinal mucosal barrier function58. Metabolic endotoxemia, is often associated with the negative effects of gut microbiota-derived LPSs on inflammation, dysglycemia, and metabolic dysfunction. Different types of LPS from various bacteria influence gut barrier function, inflammation, hormones, and blood glucose levels. The interaction between LPS and other bacterial components further affects host metabolism. Interestingly, certain types of LPS can promote a metabolically beneficial endotoxemia, countering the detrimental effects of others. This suggests that the metabolic outcome is influenced by the synergy between different LPS types and other bacterial components58. LPS has been implicated in the development of cardiometabolic diseases59. Studies have shown that a high-fat diet (HFD) reduces levels of beneficial gram-positive Bifidobacteria and increases LPS-containing gut microbiota, contributing to obesity, a major CVD risk factor60. The gut microbiota is described as a key player in regulating energy balance. Changes in its composition due to environmental factors can disrupt this balance, potentially contributing to obesity. Alterations in the gut microbiota can impact the production and release of fat molecules, influencing energy storage. The gut microbiota aids in breaking down dietary polysaccharides, leading to increased fat production in the liver. It also influences the expression of genes related to fat cell accumulation. The gut microbiota can affect the expression of genes involved in fat storage and metabolism. Changes in the structure of the gut microbiota can lead to reduced adenosine monophosphate–activated protein kinase (AMPK) activity, potentially contributing to fat accumulation60. Continuous subcutaneous LPS infusion mimics the effects of a HFD on glucose metabolism and weight gain61. Overall, gut dysbiosis and altered metabolites disrupt nutrient metabolism, promote insulin resistance, and increase adipose tissue storage, heightening the risk of cardiovascular risk factors such as obesity and diabetes.
CVD patients, especially those with HF or hypertension, often show dysfunctional intestinal barriers and increased levels of the systemic microbial component LPS, leading to inflammation62. In HF patients, the intestinal barrier, maintained by factors like tight junctions, mucus, and immunity, is often compromised. This leads to bowel wall swelling and impaired function, known as ʻleaky gut’. This allows bacterial products to enter the bloodstream, causing inflammation. Studies link intestinal changes in HF patients to elevated proinflammatory cytokines, worse symptoms, and outcomes62. Factors like fluid overload, sympathetic activation, and low cardiac output worsen bowel edema and reduce mucosal blood flow, increasing permeability and bacterial biofilm formation. A compromised gut barrier lets lipopolysaccharides (LPS) from Gram-negative bacteria enter the bloodstream, activating immune receptors (TLRs) and releasing proinflammatory cytokines, promoting inflammation in the host62. Long-term consumption of a HFD induces microbial dysbiosis, with an increased proportion of Gram-negative bacteria like Alistipes and Bacteroides, along with higher levels of genes involved in LPS biosynthesis63. Dietary fats can impair the intestinal barrier by activating the secretion of proinflammatory cytokines (e.g. TNF-α, IFNγ, and IL-1β)64. Gut microbes also play a role in CVD through host lipid metabolism. Studies suggest that the gut microbiota is implicated in lipid metabolism disorders like dyslipidemia or hyperlipidemia, which are major CVD risk factors51. TMAO (trimethylamine-N-oxide), a gut microbial cometabolite derived from dietary nutrients, has been linked to CVD risk65. Diets high in choline or carnitine can increase circulating TMAO levels, leading to enhanced macrophage foam cell formation and aortic atherosclerotic plaque development, directly connecting diet-microbiota-related TMAO to CVD progression66.
Results
Table 1.
Table 1.
The results of this paper are summarized in this table
| Rationale for exploring the gut-heart axis | Understanding the gut-heart axis offers new possibilities for preventive and therapeutic interventions in cardiovascular medicine |
| Composition and functions of gut microbiota | The gut microbiota is a diverse community of microorganisms, including bacteria, yeast, and viruses. It plays vital roles in various bodily functions |
| Gut microbiota-host interactions and health | The gut microbiota influences digestion, metabolism, immunity, and brain-gut communication. Disruptions in gut microbiota can impact health outcomes |
| Clinical trials | Various clinical trials have explored the effects of interventions on gut microbiota and cardiovascular health |
| Gut microbiota in specific cardiovascular diseases | The gut microbiota has been studied in relation to hypertension, atherosclerosis, lipid metabolism, and heart failure |
| Potential mechanisms underlying connections | Gut microbiota dysbiosis can lead to impaired mucosal barrier, inflammation, and immune dysfunction, contributing to CVD development |
Challenges and future directions
The limitations of the study pertain mainly to the small sample size of the studies which have been evaluated. The complex nature of gut microbiota may hinder a complete grasp of the gut-heart axis and its implications for cardiovascular outcomes, as many studies rely on observational data, making it difficult to establish definitive causality between gut microbiota and CVDs.
Moreover, the gut microbiome is hyper-specific, influenced by various lifestyle, genetic, and diet factors. While valuable associations between gut microbiota and cardiovascular health have been found in existing studies, most of them focus on correlations rather than causation. Therefore, further interventional studies are needed to unveil the relationship between gut microbiota and CVDs. To address these challenges, translational research becomes crucial as it can bridge the gap between basic scientific discoveries and clinical applications. A deeper understanding of the gut-heart axis can lead to innovative diagnostic tools and novel therapeutic approaches for managing CVDs.
Furthermore, interventions such as probiotics, prebiotics, or fecal microbiota transplantation could be explored to deliberately modulate the gut microbiota and potentially improve cardiovascular outcomes. As the gut microbiota varies from person to person, personalized medicine approaches hold promise in tailoring specific interventions for more effective CVD management. By combining these research approaches, we can gain a better understanding of the complex gut-heart axis and its significance in cardiovascular health. Which may lead to more targeted and personalized strategies for preventing and managing CVDs.
Conclusion
By understanding the mechanisms through which the gut microbiota influences cardiovascular health, researchers aim to identify strategies that can mitigate cardiovascular risks, enhance treatment outcomes, and improve overall heart health. Research into the gut-heart axis has shown promising findings, sparking significant interest in this field. These findings suggest that understanding the complex relationship between gut microbiota and cardiac outcomes may lead to therapeutic approaches and personalized interventions for CVDs in the future. However, it is essential to recognize that further research is needed to fully grasp the complexities of this relationship (Fig. 1).
Figure 1.
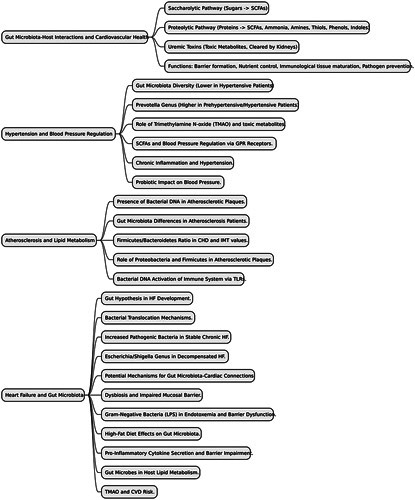
Provides a schematic overview of the mechanisms in which gut microbiota and cardiovascular issues interact.
Ethical approval
Ethics approval was not required for this Review Article.
Consent
Informed consent was not required for this Review Article.
Sources of funding
Not applicable.
Authors’ contributions
S.R. and A.I.S.: designed the study and conceived the idea; M.S.A., A.A., B.A., and T.W.: wrote the first draft; R.A., K.M., and V.K.: wrote the second draft; U.T. and S.K.: worked on the revisions.
Conflicts of interest disclosure
No conflicts of interest declared.
Research registration unique identifying number (UIN)
It is not a human study.
Guarantor
Koushik Majumder.
Data availability statement
Not applicable.
Provenance and peer review
Not commissioned, externally peer reviewed.
Acknowledgements
None.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 18 October 2023
Contributor Information
Safia Rashid, Email: safia.rashid68@gmail.com.
Abdulmaleek Idanesimhe Sado, Email: Sadoabdulmalik@gmail.com.
Muhammad Sohaib Afzal, Email: sohaibafzal237@gmail.com.
Amna Ahmed, Email: aamna.ahmed92@gmail.com.
Bsher Almaalouli, Email: bsher.almaalouli@gmail.com.
Tallha Waheed, Email: allhawaheed@gmail.com.
Rabia Abid, Email: rabx15@gmail.com.
Koushik Majumder, Email: majumder382@gmail.com.
Vikash Kumar, Email: Kumarvikashmd@gmail.com.
Usha Tejwaney, Email: Utejwaney@gmail.com.
Sarwan Kumar, Email: skumar2@med.wayne.edu.
References
- 1.Attaye I, Pinto-Sietsma S, Herrema H, et al. A crucial role for diet in the relationship between gut microbiota and cardiometabolic disease. Annu Rev Med 2020;71:149–161. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol 2017;14:79–87. [DOI] [PubMed] [Google Scholar]
- 3.Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res 2017;120:1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang WHW, Bäckhed F, Landmesser U, et al. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2089–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra SA, Narula J, Bhagat N, et al. Pathophysiology and implications of the immune-microbiome relationship in cardiovascular disease. Trends Cardiovasc Med 2020;31:473–484. [Google Scholar]
- 6.Murray CJL, Barber RM, Foreman KJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. The Lancet 2015;386:2145–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Rodriguez E, Egea-Zorrilla A, Plaza-Díaz J, et al. The gut microbiota and its implication in the development of atherosclerosis and related cardiovascular diseases. Nutrients 2020;12:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koren O, Spor A, Felin J, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A 2021;108(suppl 1):4592–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laterza L, Rizzatti G, Gaetani E, et al. The gut microbiota and immune system relationship in human graft-versus-host disease. Mediterr J Hematol Infect Dis 2016;8:e2016025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature 2011;473:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mills S, Stanton C, Lane J, et al. Precision nutrition and the microbiome, Part I current state of the science. Nutrients 2019;11:923–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018;555:210–215. [DOI] [PubMed] [Google Scholar]
- 14.Wiley N, Dinan T, Ross R, et al. The microbiota-gut-brain axis as a key regulator of neural function and the stress response: implications for human and animal health. J Anim Sci 2017;95:3225–3246. [DOI] [PubMed] [Google Scholar]
- 15.Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015;17:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Science Advances 2019;5eaau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek 2020;113:567–588. [DOI] [PubMed] [Google Scholar]
- 18.Sekirov I, Russell SL, Antunes LC, et al. Gut microbiota in health and disease. Physiol Rev 2010. Oct;90:859–904. [DOI] [PubMed] [Google Scholar]
- 19.Nallu A, Sharma S, Ramezani A, et al. Gut microbiome in chronic kidney disease: challenges and opportunities. Transl Res 2017;179:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science 2005;307:1915–1920. [DOI] [PubMed] [Google Scholar]
- 21.Savage DC. Associations of indigenous microorganisms with gastrointestinal mucosal epithelia. Am J Clin Nutr 1970;23:1495–1501. [DOI] [PubMed] [Google Scholar]
- 22.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004;101:15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005;122:107–118. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Wang B, Zhang M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci USA 2008;105:2117–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004;118:229–241. [DOI] [PubMed] [Google Scholar]
- 26.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology 2009;136:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension 2015;65:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitra S, Drautz‐Moses DI, Alhede M, et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 2015;3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 2017;8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emoto T, Yamashita T, Kobayashi T, et al. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels 2017;32:39–46. [DOI] [PubMed] [Google Scholar]
- 32.Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 2007;50:1561–1569. [DOI] [PubMed] [Google Scholar]
- 33.Nagatomo Y, Tang WH. Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail 2015;21:973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Organ CL, Otsuka H, Bhushan S, et al. Choline diet and its gut microbe‐derived metabolite, trimethylamine N‐oxide, exacerbate pressure overload‐induced heart failure. Circ Heart Fail 2016;9:e002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandek A, Swidsinski A, Schroedl W, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol 2014;64:1092–1102. [DOI] [PubMed] [Google Scholar]
- 36.Lim GB. Heart failure: gut flora–pathogenic role in chronic heart failure. Nat Rev Cardiol 2016;13:61. [DOI] [PubMed] [Google Scholar]
- 37.Pasini E, Aquilani R, Testa C, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail 2016;4:220–227. [DOI] [PubMed] [Google Scholar]
- 38.Xu H, Wang X, Feng W, et al. The gut microbiota and its interactions with cardiovascular disease. Microb Biotechnol 2020;13:637–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik M, Suboc TM, Tyagi S, et al. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ Res 2018;123:1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Lindemann SR, Cross TL, et al. Effects of adding lean red meat to a U.S.-style healthy vegetarian dietary pattern on gut microbiota and cardiovascular risk factors in young adults: a crossover randomized controlled trial. J Nutr 2023;153:1439–1452. [DOI] [PubMed] [Google Scholar]
- 41.Shah RD, Tang ZZ, Chen G, et al. Soy food intake associates with changes in the metabolome and reduced blood pressure in a gut microbiota dependent manner. Nutr Metab Cardiovasc Dis 2020;30:1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djekic D, Shi L, Brolin H, et al. Effects of a vegetarian diet on cardiometabolic risk factors, gut microbiota, and plasma metabolome in subjects with ischemic heart disease: a randomized, crossover study. J Am Heart Assoc 2020;9:e016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sommer F, Nookaew I, Sommer N, et al. Site-specific programming of the host epithelial transcriptome by the gut microbiota. Genome Biol 2015;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 2014;25:657–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 2014;5:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013;500:585–858. [DOI] [PubMed] [Google Scholar]
- 48.Seppo L, Jauhiainen T, Poussa T, et al. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr 2003;77:326–330. [DOI] [PubMed] [Google Scholar]
- 49.Mell B, Jala VR, Mathew AV, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat model. Physiol Genomics 2015;00136:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ott SJ, El Mokhtari NE, Musfeldt M, et al. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation 2006;113:929–937. [DOI] [PubMed] [Google Scholar]
- 51.Koren O, Spor A, Felin J, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA 2011;108(Suppl 1):4592–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szabo H, Hernyes A, Piroska M, et al. Association between gut microbial diversity and carotid intima-media thickness. Medicina 2021;57:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindskog Jonsson A, Hållenius FF, Akrami R, et al. Bacterial profile in human atherosclerotic plaques. Atherosclerosis 2017;263:177–183. [DOI] [PubMed] [Google Scholar]
- 54.van den Munckhof ICL, Kurilshikov A, Ter Horst R, et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes Rev 2018;19:1719–1734. [DOI] [PubMed] [Google Scholar]
- 55.Mu F, Tang M, Guan Y, et al. Knowledge mapping of the links between the gut microbiota and heart failure: a scientometric investigation (2006–2021). Front Cardiovasc Med 2022;9:882660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayashi T, Yamashita T, Watanabe H, et al. Gut microbiome and plasma microbiome-related metabolites in patients with decompensated and compensated heart failure. Circ J 2018;83:182–192. [DOI] [PubMed] [Google Scholar]
- 57.Mamic P, Heidenreich PA, Hedlin H, et al. Hospitalized patients with heart failure and common bacterial infections: a nationwide analysis of concomitant clostridium difficile infection rates and in-hospital mortality. J Card Fail 2016;22:891–900. [DOI] [PubMed] [Google Scholar]
- 58.Anhê FF, Barra NG, Cavallari JF, et al. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep 2021;36:109691. [DOI] [PubMed] [Google Scholar]
- 59.Zhi C, Huang J, Wang J, et al. Connection between gut microbiome and the development of obesity. Eur J Clin Microbiol Infect Dis 2019;38:1987–1998. [DOI] [PubMed] [Google Scholar]
- 60.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol 2009;9:692–703. [DOI] [PubMed] [Google Scholar]
- 61.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57:1470–1481. [DOI] [PubMed] [Google Scholar]
- 62.Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res 2020;127:553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wan Y, Wang F, Yuan J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 2019;68:1417–1429. [DOI] [PubMed] [Google Scholar]
- 64.Yang Y, Zhong Z, Wang B, et al. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology 2019;44:2054–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


