Abstract
Introduction and importance:
Contralateral subdural effusion (CSDE) is a rare complication secondary to decompressive craniectomy (DC), which can lead to encephalocele and neurologic deterioration. The authors report a case that confirm the existence of unidirectional membrane valve, and cranioplasty is an effective treatment for CSDE.
Case presentation:
The authors reported a case of 43-year-old female was diagnosed with ruptured intracranial aneurysm and treated with interventional embolization. She underwent DC because of postoperative cerebral infarction subsequently. Her conscious state deteriorated accompanied by encephalocele in postoperative 2 week. A craniocerebral computed tomography (CT) confirmed the diagnosis of CSDE with cerebral hernia. A compression bandaging of the skull defect was applicated, whereas, her conscious state progressive deteriorated. She was transferred to the author’s hospital where she underwent burr-hole drainage and clinical symptom has been improved. However, a relapse of CSDE was observed after the removal of drainage tube. Continuous lumbar drainage was employed, and which was ineffective for CSDE in this case. Finally, she underwent cranioplasty, with the help of drainage of subdural effusion, CSDE was completely resolved.
Clinical discussion:
CSDE is occasionally observed in patients after DC. Intracranial pressure (ICP) gradient and unidirectional membrane valve are the possible mechanisms of CSDE. At present, there is no optimal therapy for CSDE. For symptomatic CSDE patients, one or more treatment measures should be applicated.
Conclusion:
Cranioplasty is one of the curative and optimal method to treat symptomatic CSDE patients, early cranioplasty combined with burr-hole drainage should be performed for conservative treatment failed and intractable cases.
Keywords: case report, cerebral spinal fluid, contralateral subdural effusion, cranioplasty, CSF shunt, decompressive craniectomy
Introduction
Highlights
Contralateral subdural effusion is a rare complication after decompressive craniectomy.
We confirm the existence of unidirectional membrane valve is one major reason that causes contralateral subdural effusion.
The burr-hole drainage is a temporary therapeutic measure for contralateral subdural effusion, and continuous lumbar drainage is not effective in treating contralateral subdural effusion.
Early cranioplasty is an effective method for contralateral subdural effusion.
Subdural effusion (SDE) is a common complication secondary to DC. Contralateral subdural effusion (CSDE) is a rare and special type of SDE. CSDE could be divided into the asymptomatic one and the symptomatic one. The previous study mentioned that treatment is not required for CSDE unless the patient is symptomatic1. The symptomatic CSDE has adversely effects of CSDE on neurological function and the prognosis of patients, which needed early aggressive medical intervention. However, the management of symptomatic CSDE represents a clinical challenge, and the literature on managing CSDE is rare and limited to case reports and short series. For the patients with midline shifting of brain tissue and neurologic deterioration, one or more treatment measures should be applicated, such as head-down bed rest (HDBR), compression bandaging, continuous lumbar drainage, burr-hole drainage, Ommaya catheter drainage, cranioplasty, subdural-peritoneal shunt and temporal muscle sticking2. This current case report describes a patient with intractable CSDE that was successfully treated by cranioplasty after other available treatments failure. Should cranioplasty be the preferred opinion for symptomatic patients with CSDE? This case report has been reported in line with the Surgical case report (SCARE) 2023 criteria3.
Case report
A 43-year-old female patient was admitted to the local hospital with sudden intense headache in 26 March 2022, she was diagnosed with ruptured intracranial aneurysm and subarachnoid haemorrhage (SAH). She underwent an interventional embolization with Guglielmi detachable coil (GDC) and endovascular stent in 6 April 2022, and initially followed by a good recovery.
She underwent craniocerebral computed tomography (CT) scan because of the hemiparalysis of left limbs and consciousness disorder, and right cerebral hemisphere infarction with severe midline shift was confirmed. A right decompressive craniotomy was carried out in 18 April 2022, and an improvement in her consciousness disorder was observed during the subsequent 1 week. On 29 April 2022, her conscious state deteriorated again and accompanied by encephalocele. Craniocerebral CT scan confirmed a CSDE with severe midline shifted. Even if a compression bandaging of the right skull defect was applicated, her conscious state progressive deteriorated in the following week.
On 6 May 2022, the patient was transferred to the author’s hospital. Craniocerebral CT scan confirmed a CSDE with the midline shifted more than 1 cm (Fig. 1). Her condition recovered after an immediate burr-hole drainage was employed; however, her condition deteriorated again after the drainage tube was removed (Fig. 1). A second burr-hole drainage and continuous lumbar drainage of cerebrospinal fluid (CSF) was applied. The patient’s condition only improved dramatically during the drainage tube of effusion chamber was maintained open; however, continuous lumbar drainage had no effect on her condition (Fig. 2). A three-dimensional designed titanium plate suitable for her cranial defect was ordered, and she underwent cranioplasty with this titanium plate. The patient’s consciousness improved progressively after cranioplasty, and CT scan indicated that CSDE decreased significantly (Fig. 3A). The drainage tubes of effusion chamber and lumbar cistern were removed, and the following CT scan indicated that CSDE disappeared completely (Fig. 3B).
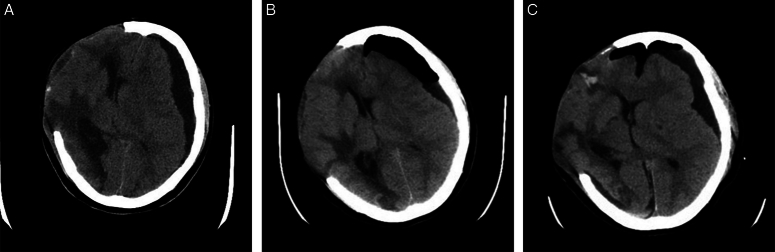
Figure 1.
Computed tomography (CT) images of a 43-year-old female patient diagnosed with contralateral subdural effusion (CSDE). (A) CT scan showing a CSDE and a severe midline shift with encephalocele after the patient was admitted to hospital. (B) CT scan showing the reduction of subdural effusion after burr-hole drainage. (C) CT scan showing the recurrence of CSDE after the drainage tube was removed.
Figure 2.
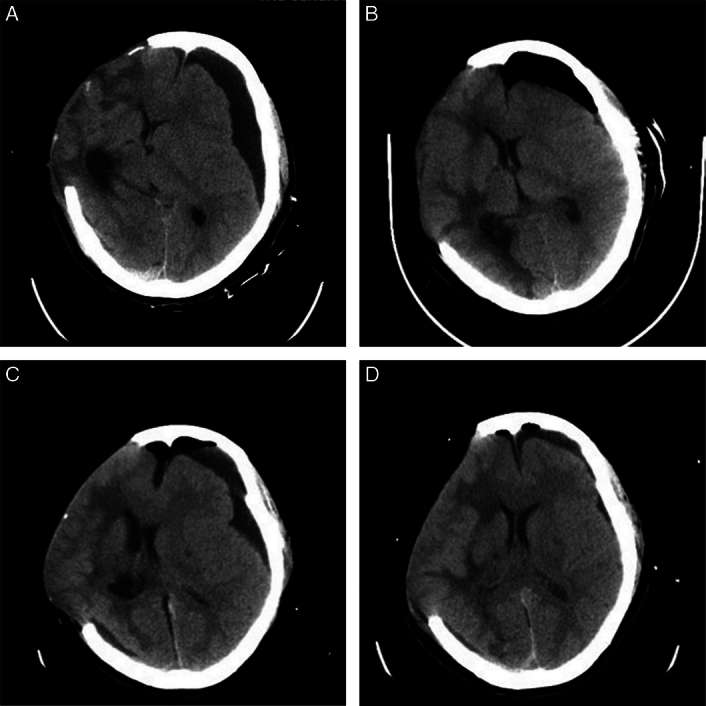
(A) Computed tomography (CT) scan showing the recurrence of contralateral subdural effusion (CSDE) after the drainage tube was removed. (B) CT scan showing the reduction of subdural effusion after a second burr-hole drainage and continuous lumbar drainage of cerebrospinal fluid. (C) CT scan showing the recurrence of CSDE after the drainage tube of lumbar cistern was kept open, and the drainage tube of effusion chamber was kept closed. (D) CT scan showing the significantly reduction of subdural effusion after the drainage tube of effusion chamber was kept open, and the drainage tube of lumbar cistern was kept closed.
Figure 3.
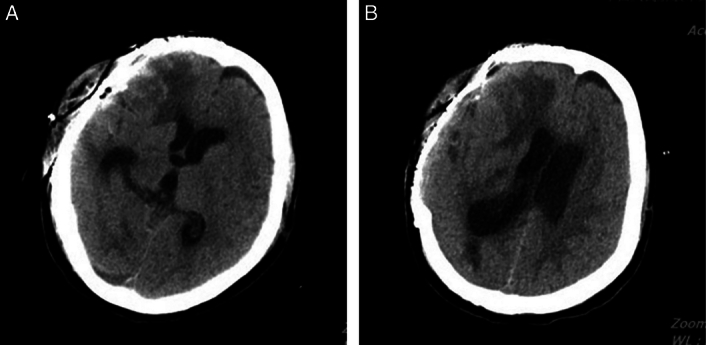
(A) Computed tomography (CT) scan showing the significantly reduction of subdural effusion after the cranioplasty. (B) CT scan showing the completely cured of contralateral subdural effusion after the drainage tubes of effusion chamber and lumbar cistern were removed.
CT scan indicated that no recurrence of CSDE was found in 17 June 2022. On 21 June 2022, the patient was discharged from hospital. Her incision has been healing well, right limb revealed Grade 4 power and left limb showed Grade 1 power. Unfortunately, this case lost follow-up.
Discussion
CSDE is occasionally observed in patients after DC. The most acceptable theories about CSDE mechanism include the rapid decrease in ICP and the outward herniation of brain tissue during the DC procedure generates a pressure gradient between the bilateral hemispheres4,5. In addition, the shear stress caused by trauma or surgical trauma results in the tearing of arachnoid membrane, which creates a unidirectional membrane valve. Based on above theories, the enlargement contralateral subdural space creates a negative pressure zone and only allows CSF flow into the contralateral subdural space through unidirectional membrane valve.
The recent statistics presented that 41.7% of the symptomatic patients underwent burr-hole drainage could control CSDE; however, the recurrence rate was as high as 76%6. In our case, the patient received burr-hole drainage first and achieved a transient improvement of clinical symptoms, however the symptoms deteriorated again after the drainage tube removed. Undoubtedly, burr-hole drainage addresses none of the pressure gradient or unidirectional membrane valve, therefore, burr-hole drainage is only a temporary measure. The statistics of Lin showed that 25% of the symptomatic patients underwent cranioplasty, while the recurrence rate is only 13.3%6. Other studies also confirmed that the cure rate of cranioplasty alone for CSDE as high as 87.5%5,7–9. In our case, CSDE completely eliminated after the patient received cranioplasty. Conceivably, cranioplasty solves the problem of pressure gradient at least, the abatement or disappearance of pressure gradient is beneficial for decreasing the subdural effusion. The above results suggest that cranioplasty is one of the optimal methods to treat CSDE. A few published study and reviews showed that cranioplasty, particularly early cranioplasty, is effective for the treatment of CSDE2,6,10. Previous study showed that 18.3% of symptomatic patients need both cranioplasty and subdural-peritoneal shunt to resolve CSDE completely6. In this case, we found that continuous lumbar drainage is ineffective for CSDE. We suspect that the unidirectional membrane valve only permits CSF to flow into subdural space, cannot backflow into subarachnoid space. Although postoperative hydrocephalus was not observed in this case, it should be noted that some patients may develop hydrocephalus after cranioplasty. CSDE, whether is a special type of hydrocephalus? Which need further research. It has been suggested that CSF shunt, such as subdural-peritoneal shunt or ventriculoperitoneal shunt, is needed for this situation10,11.
The study didn’t follow this case long-term due to the patient was lost to follow-up, so it’s unclear the long-term effect of cranioplasty on CDSE. As there is only one case in our reports, more cases are required to support our conclusion in the future.
Conclusion
In conclusion, CSDE is a rare complication secondary to DC. The restoration of normal skull structure early is benefit to improve cerebral hemodynamics and promote neurological functional recovery. The cranioplasty is a safe and efficient method to CSDE patients, the treatment success rate has reached more than 80%, it can be preferred method to inefficacious patients treated with conservative treatment. For CSDE patients with hydrocephalus after cranioplasty, CSF shunt should be required.
Ethical approval
None. This is a case report involves no experiments. Therefore, it did not require ethical approval from ethics committee.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal on request.
Source of funding
This research was support by Research Project of author’s hospital (2021-XZYG-C37 and 2021-XZYG-A13) and the Spark Talent program of author’s hospital.
Author contribution
Q.O. contributed to writing the case information and discussion. Y.Y. diagnosed the case, collected the data, and preserved the pictures. J.C. contributed to the process of original draft preparation and introduction. B.S. Revised it critically for important intellectual content, contributed to review and editing. Y.M. edited the rough draft into the final manuscript.
Conflicts of interest disclosure
The authors declare that there are no conflicts of interest.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Qing Ouyang.
Data availability statement
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article
Published online 5 February 2024
Contributor Information
Qing Ouyang, Email: oyq911@126.com.
Yongxiang Yang, Email: yangyongxiang2014@163.com.
Jingmin Cheng, Email: sjwkuser@163.com.
Bing Sun, Email: sunbing712@163.com.
Yuan Ma, Email: Tianfu_47@163.com.
References
- 1.Wang HK, Lu K, Liang CL, et al. Contralateral subdural effusion related to decompressive craniectomy performed in patients with severe traumatic brain injury. Injury 2012;43:594–597. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Chen F, Wen L, et al. Cranioplasty as the treatment for contralateral subdural effusion secondary to decompressive craniectomy: a case report and review of the relevant literature. J Int Med Res 2020;48:300060520966890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohrabi C, Mathew G, Maria N, et al. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg 2023, 109:1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang XF, Wen L, Li G, et al. Contralateral subdural effusion secondary to decompressive craniectomy performed in patients with severe traumatic brain injury: incidence, clinical presentations, treatment and outcome. Med Princ Pract 2009;18:16–20. [DOI] [PubMed] [Google Scholar]
- 5.Salunke P, Garg R, Kapoor A, et al. Symptomatic contralateral subdural hygromas after decompressive craniectomy: plausible causes and management protocols. J Neurosurg 2015;122:602–609. [DOI] [PubMed] [Google Scholar]
- 6.Ling H, Yang L, Huang Z, et al. Contralateral subdural effusion after decompressive craniectomy: what is the optimal treatment? Clin Neurol Neurosurg 2021;210:106950. [DOI] [PubMed] [Google Scholar]
- 7.Su TM, Lee TH, Huang YH, et al. Contralateral subdural effusion after decompressive craniectomy in patients with severe traumatic brain injury: clinical features and outcome. J Trauma 2011;71:833–837. [DOI] [PubMed] [Google Scholar]
- 8.Wan Y, Shi L, Wang Z, et al. Effective treatment via early cranioplasty for intractable contralateral subdural effusion after standard decompressive craniectomy in patients with severe traumatic brain injury. Clin Neurol Neurosurg 2016;149:87–93. [DOI] [PubMed] [Google Scholar]
- 9.Kilincer C, Hamamcioglu MK. Contralateral subdural effusion secondary to decompressive craniectomy: differences in patients with large hemispheric infarctions and traumatic brain injury. Med Princ Pract 2010;19:499; author reply 500. [DOI] [PubMed] [Google Scholar]
- 10.Zhou W, Wang Z, Zhu H, et al. Effects of cranioplasty on contralateral subdural effusion after decompressive craniectomy: a literature review. World Neurosurg 2022;165:147–153. [DOI] [PubMed] [Google Scholar]
- 11.Wu R, Ye Y, Ma T, et al. Management of subdural effusion and hydrocephalus following decompressive craniectomy for posttraumatic cerebral infarction in a patient with traumatic brain injury: a case report. BMC Surg 2019;19:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.