Summary:
Acute compartment syndrome (ACS) is a limb-threatening pathology that necessitates early detection and management. The diagnosis of ACS is often made by physical examination alone; however, supplemental methods such as compartment pressure measurement, infrared spectroscopy, and ultrasound can provide additional information that support decision-making. This practical review aims to incorporate and summarize recent studies to provide evidence-based approaches to compartment syndrome for both resource-rich and -poor settings among several patient populations.
Takeaways
Question: What is the best way to diagnose and treat compartment syndrome in an evidence-based way?
Findings: Compartment syndrome is primarily a clinical diagnosis where mechanism of injury, time since the incident, and physical examination findings are most pertinent. In lower resource areas, Whitesides’ method can convert an arterial line into a pressure gauge for quantitative diagnostics.
Meaning: Compartment syndrome can be effectively treated in areas of varying resource access with a detailed physical examination and low threshold for surgical management.
INTRODUCTION
Necessity of a Practical Review
Acute compartment syndrome (ACS) is a surgical emergency that requires early diagnosis and intervention to prevent severe and possibly irreversible morbidity. The thick facial encasement of muscular compartments allows for ACS to develop when intracompartmental pressures exceed arterial perfusion pressure. This leads to ischemia and edema with the potential for irreversible myonecrosis and nerve damage.1 Traumatic injury comprises ~75% of cases with the lower leg and forearm most frequently involved.1 The incidence of ACS is considerably higher for men compared with women (7.3 per 100,000 versus 0.7 per 100,000) and has been attributed to increased rates of trauma and a higher proportion of muscle-to-overall tissue.2,3
Although devices that directly measure compartment pressure, such as the Stryker needle, can provide objective identification of ACS, clinical diagnosis remains the gold standard. The following practical review aims to clarify current evidence-based practices for recognition and management of ACS. It will additionally provide insight as to diagnosis and treatment in resource-poor areas, notably Whitesides’ method of compartment pressure acquisition, which is explained in the Video. [See Video (online), which shows a demonstration of Whitesides’ method for compartment pressure measurement.]
Video 1. This video shows a demonstration of Whitesides method for compartment pressure measurement.
ACS Pathophysiology and Diagnosis of ACS
Whether through external compression or internal expansion of muscular compartments, ACS develops when compartment pressures exceed perfusion pressure, causing ischemia, myonecrosis, and significant chronic morbidity.1 Volkmann first described myonecrosis and subsequent chronic contracture secondary to prolonged upper extremity ischemia in 1881 without clear identification of treatment options for nearly three decades until Bardenheur first described fasciotomies in 1911.4 Subsequent clinical and basic science studies supported Volkmann’s original theory of ischemic-induced myonecrosis and scar tissue formation with the subsequent chronic flexion contracture deformity being appropriately termed “Volkmann’s contracture.”4,5
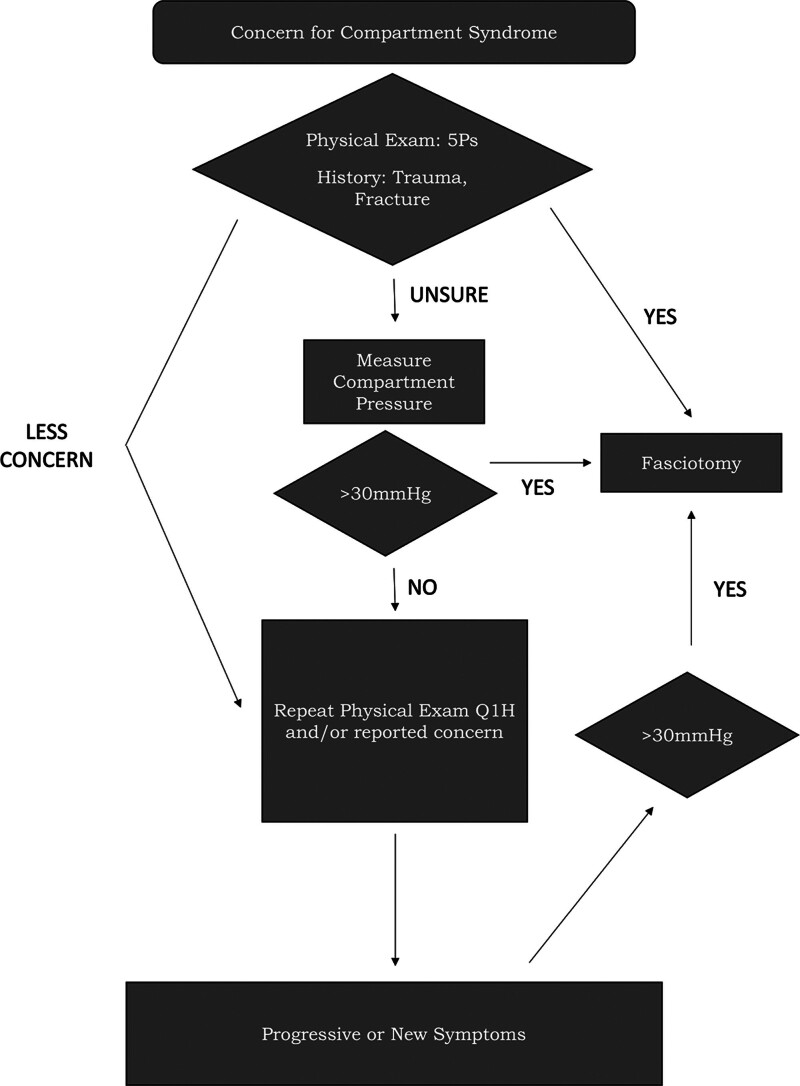
Expeditious diagnosis and subsequent compartment release via fasciotomy are necessary to complete in a timely fashion. Failure to treat ACS within four hours of onset can lead to potentially irreversible muscle damage, and nearly complete myonecrosis within 8 hours.6 An accurate history and physical examination remains the current standard for diagnosing ACS. The classic signs and symptoms of compartment syndrome, the “five Ps,” were first described by Griffiths4 in 1940 and include pain out of proportion on physical examination, pallor, paresthesias, paralysis, and pulselessness. The presence of these signs and symptoms should also be elucidated during the history and physical examination. Sensory disturbances tend to progress from distal to proximal in relation to the point of maximal compression. Muscular pain will be most specific at the most distal/ischemic region of the affected extremity, although this can be confounded by other concomitant traumatic injuries. Of note, pulselessness often is the final symptom to develop and indicates irreversible muscle damage may already be present, warranting immediate release in the operating room.7 History of crush injury, ischemia-reperfusion injury, or fracture should raise suspicion for ACS.8 If a patient is unable to reliably participate in an accurate history and physical examination, or if they are equivocal, objective compartment pressure measurements can be particularly valuable (Fig. 1).
Fig. 1.
Clinical decision algorithm for suspected compartment syndrome.
Quantitative ACS Diagnostic Tests
Stryker Needle
Most studies cite a compartment pressure of 30 mm Hg or greater (with normal compartment pressures being less than 10 mm Hg) or a compartment pressure within 30 mm Hg of the diastolic blood pressure as diagnostic for ACS.9 The Stryker needle is the most widely used compartment pressure measurement device in the United States; however, a modified arterial line can also be used in resource-poor settings and is discussed later in this review [see Video 1 (online)]. To measure compartment pressures with the Stryker needle, the needle is inserted through the skin, subcutaneous tissue, and fascia to enter the involved compartment.10 Studies indicate this is a relatively safe diagnostic procedure with damage to surrounding structures as a rare complication.11
Laboratory Findings
Laboratory measurements that can support diagnosis of ACS include creatine phosphokinase levels, creatinine, and urine myoglobin.12 Elevated creatine phosphokinase is used as a quantitative measure of significant muscle damage/death and can often exceed 1000 international units in cases of ACS myonecrosis. Additionally, the presence of urine myoglobin and rising creatinine associated with myoglobinuric acute kidney injury can also be useful adjuncts in confirming severe muscle damage.13
Near-infrared Spectroscopy
Near-infrared spectroscopy (NIRS) has been proposed as an adjunct to clinical examination and manual measurement of compartment pressure for both diagnosis of ACS and tissue response to treatment. First described by Garr et al14 in the 1990s using porcine models, the use of NIRS for the monitoring of compartment pressures is based on the principle that muscle oxyhemoglobin saturation is directly correlated to perfusion pressure and inversely correlated to compartment pressure.14–16 A follow-up study using critically ill porcine models determined NIRS can be used to diagnose ACS in hypotensive and hypoxic pigs due to significant differences in tissue oxygenation with the concurrent presence of ACS, providing implications for accurate diagnosis in hemodynamically unstable patients.16
Ultrasound
Although not yet often used in the clinical arena, ultrasound has shown promising efficacy in the diagnosis of ACS in experimental models. Multiple measurements have been proposed as diagnostic ultrasonographic criteria for ACS, including compartment fascia flattening pressure (CFFP), the use of intracompartmental pressure to determine relative elasticity (RE) of both unaffected and affected limbs, compartment width (CW), and compartment/fascial displacement.12,17–22
The use of ultrasound in diagnosing ACS has recently been explored in cadaveric models.12,17,21 In 2015, Sellei et al17 described the use of ultrasound to measure compartment displacement wherein they found a significant correlation between the rising compartment pressure and decreased tissue displacement on ultrasound. Marmor et al12 used increasing saline compartment inflation to determine the correlation between anterior CW (measured from interosseous ligament to superficial fascia of anterior compartment) and CFFP (measured as pressure required to flatten superficial fascia of anterior compartment). Using pulse phase–locked loop (PPLL) technique, the authors demonstrated that CW and CFFP were highly correlative to compartment pressures, suggesting there is potential clinical application of PPLL and CW.12 Sellei et al21 later used simulated saline-filled compartments to calculate the RE of compartment pressures, defined as changes in the compartment depth due to a probe pressure of 80 mm Hg correlated to 0 mm Hg, whereby an RE of 100% was represented as compartment depth without compression. It was found that compared with a control group of cadavers without saline-filled compartments, compartments with a pressure of greater than or equal to 30 mm Hg had a significantly decreased RE compared with control compartments overall (12.66% versus 17.06%, P < 0.001).21 In particular, they also demonstrated a significant decrease in anterior tibia compartment RE in patients with elevated compartment pressures compared with their unaffected paired limb (5.14% versus 17.95%, P < 0.0001), citing an RE of less than 10.5% as having a sensitivity of 95.8% and specificity of 87.5% in the diagnosis of ACS.17
Garabekyan et al18 demonstrated similar efficacy of ultrasound in diagnosing ACS by comparing fascial displacement to perfusion pressure. They found that as perfusion pressure ranged from −40 to 80 mm Hg, fascial displacement of infused compartments was significantly greater than in control compartments (P = 0.03), concluding that fascial displacement is greater in compartments with decreased muscle perfusion pressure.18
Lynch et al19 used incremental increases in blood pressure cuff measurements to test the use of ultrasound for estimating intracompartmental pressures by fascial displacement waveforms from arterial pulsations, discovering a sensitivity of 61% and specificity of 94% in diagnosing normal compartment pressures (below 30 mm Hg) in comparison to elevated pressures (>30 mm Hg). Wiemann et al20 further demonstrated that PPLL has the potential for detecting ACS by describing a linear correlation between compartment pressure and PPLL fascial displacement utilizing thigh cuff occlusion (R = 0.8887).
Herring et al22 described an absolute pressure of greater than 100 mbar (~75 mm Hg) or a difference of 50 mbar (~37.5 mm Hg) in the CFFP between affected and unaffected lower extremities as pathological and indicative of ACS, citing an average CFFP of 10.7 ± 10.6 mbar (8.02 ± 7.95 mm Hg) for 10 injured patients without evidence of ACS compared with 157 ± 51.7 mbar (117.76 ± 38.77 mm Hg) for three patients with evidence of ACS (P < 0.02).
MANAGEMENT OF ACS
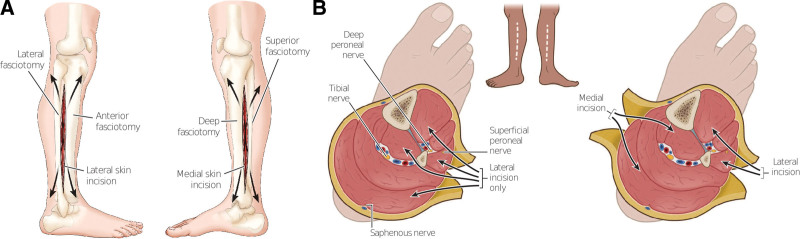
Nonoperative management such as loosening a dressing, replacing a cast, or elevating an extremity can help prevent potential compartment syndrome, but true compartment syndrome requires formal compartment releases (ie, fasciotomies). The surgeon dissects down to the muscular fascia where longitudinal incisions are made to release the fascia, allowing the underlying structures to decompress (Figs. 2A and 3B). The threshold to perform a fasciotomy should remain low, as the morbidity associated with ischemic muscle loss significantly outweighs the reduction in muscle strength and subsequent need for reoperation for closure of wound care.23,24 Immediately following fasciotomy, primary closure may be achieved if limb edema is minimal and allows for adequate tissue reapproximation without significant tension. However, negative pressure therapy can also be placed to limit edema and the need for wound care or skin grafting as it can help prepare the extremity for delayed primary closure.25
Fig. 2.
Lower leg fasciotomy. A, Description of both lateral and medial incisions from lateral point of view. B, Axial cuts depicting the neurovascular bundles that are decompressed through their respective incisions.
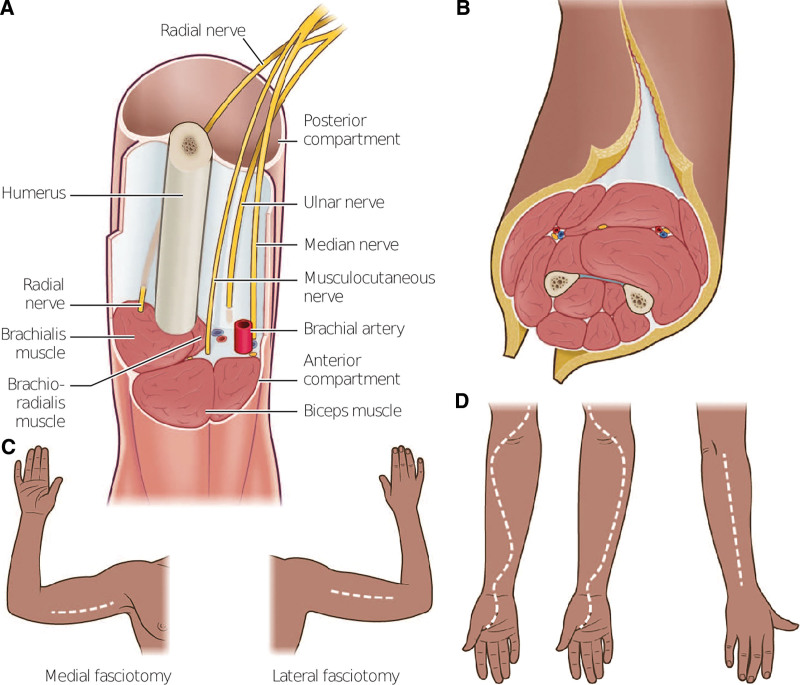
Fig. 3.
Fasciotomies of the arm and forearm. A, Upper arm fasciotomy requires decompression of the main neurovascular bundle and brachial plexus. B, Upper arm fasciotomy that displays the axial view of medial and lateral fasciotomies. C, Display of rough placement for these incision lines. D, Forearm fasciotomy with S-shaped incision to include the volar hand and carpal tunnel release.
In 2000, Fitzgerald et al26 described the long-term outcomes of ACS and noted that 77% of their patient cohort had persistent altered limb sensation and pain within the limits of the fasciotomy wounds, which was notably more common in patients who required skin grafting for wound closure. This can be mitigated with proper rehabilitation programs that include protection, resting, ice, compression, and elevation management the first 3 weeks with progressive return to full range of motion and strengthening exercises until 12 weeks.27
Burn and Electrical Injuries
ACS is a rare sequelae of thermal burn injuries that can be caused by circumferential extracompartmental eschar formation, which can act as a tourniquet. After prolonged ischemia, the release of a circumferential eschar can lead to ischemia-reperfusion injury, and subsequent intracompartmental edema and ACS.28
Electrical injuries have a particularly high incidence of ACS compared with thermal burns. These injuries account for 3%–5% of admissions to burn units and usually involve the upper extremity.29–31 The pathophysiology of electrical injury-induced ACS is based on Ohm’s law, which states that the greater the resistance of a medium, the higher the voltage and the lower the current will be present. As bone has the highest density of any tissue, a large amount of thermal heat is generated by its resistance to electricity and radiates to adjacent muscle bellies (such as pronator quadratus), causing deep severe burns and edema which elevates compartment pressures and predisposes to ACS.30
Piccolo et al32 discussed an algorithm for fasciotomies in electrical burn patients based on pulse oximetry (infrared spectroscopy) suggesting that in the event of an electrical injury, less than 90% on index digit pulse oximetry, regardless of whether or not a Doppler signal was present, was an indication for fasciotomy. Interestingly, pulse oximetry was valued more in the algorithm for both escharotomies and fasciotomies than the loss of the peripheral pulse. Given that the tissue becomes poorly perfused/ischemic well before the pulse is lost, the clinician should never wait for the loss of pulses before acting on an expected compartment syndrome.32 However, there is a general lack of consensus in surgical literature as to how NIRS corresponds to the critical pressure threshold for 30 mm Hg for ACS.33,34
Pediatric ACS
Given their rapidly changing physiology, pediatric populations present with a unique set of risk factors for the development of ACS.35 In particular, they have been cited as having a relatively higher risk for the development of leg ACS in the setting of long-bone fractures.36 In the setting of neonatal care (<28 days of age), forearm compartment syndrome is the most frequently described.37 Early detection of associated skin changes, such as ecchymosis, bullae, or distal gangrene, should prompt emergent surgical intervention within hours of presentation to prevent contracture and functional limitations.38,39
It should be noted that current compartment measurement thresholds of greater than 30 mm Hg are still used in this population.40 Pediatric patients may face long-term consequences from fasciotomies due to scar formation and risk of contracture with growth-related changes.41 This is especially true in neonates.37 Current research aims at preventing and limiting scar formation, notably in postoperative wound and skin graft care.
DETECTION, INCIDENCE, AND TREATMENT OF ACS IN LOW-RESOURCE ENVIRONMENTS
Given the high degree of functional morbidity associated with untreated or delayed detection of ACS, it is equally important for surgeons in resource-poor environments/developing nations to be able to accurately and efficiently diagnose this condition. The delayed treatment of ACS in developing nations can predispose patients to both acute ischemia and gangrene, and chronic sequelae not as commonly seen in developed countries, such as the development of Volkmann’s ischemic contractures.5 Although there is a deficit of epidemiological studies which detail incidence of compartment syndrome in developing nations on a global scale, Nwadinigwe et al42 reported an incidence of ACS in 7.1% and frank gangrene in 3.1% of pediatric patients presenting to their medical center in Nigeria. Additionally, Saikia et al43 note an incidence of ACS among 2.67% of patients presenting with closed lower extremity fractures to their medical center in India.
As the consequences of inefficiently diagnosing ACS are significant, there is a clear need for accessible and reliable modalities which allow physicians in resource-poor environments to diagnose ACS. A prompt physical examination paying attention to certain pathognomonic findings (previously described six “Ps”) is often the best diagnostic tool, but when physical examination findings are equivocal or impossible to ascertain (eg, due to obtunded/sedated state), compartment pressures need to be measured. As many hospitals in developing countries may not have access to a readily quantifiable compartment pressure, as through the Stryker needle, clinicians may need to improvise. Recognizing this need, Whitesides et al6 described a modification of an arterial line pressure gauge in 1975, which uses a modified arterial line which is inserted into the affected compartment and measures compartment pressure. Whitesides’ method uses a needle that is equalized outside of the compartment to atmospheric pressure and is allowed to equilibrate to gravity. The movement of saline into the column will standardize the sphygmomanometer to then measure the arterial line. Video 1 demonstrates Whitesides’ method.
Saikia et al43 further validate Whitesides’ technique to measure anterior compartment syndrome in the lower leg with the use of inexpensive and commonly available supplies in their Indian medical center, including a mercury manometer, plastic intravenous extension tubes, 18-gauge needles, a 20-mL syringe, a three-way stopcock, and bacteriostatic normal saline. Although several studies have supported Whitesides’ method and recommended it as a safe alternative to the commercialized version, others have found discrepancies in the accuracy of Whitesides’ method.13,43,44 For example, Uliasz et al44 demonstrated similar accuracy of the Stryker needle when compared with Whitesides’ method and a pressurized, intravenous pump. Interestingly, Whitesides’ method lacked precision compared with either of the former modalities.44 Of note, Whitesides’ method used in this article differed from modern versions by not equalizing with a hanging IV bag and could account for this difference.44 This is further supported by Collinge and Kuper45 when comparing a solid-state transducer intracompartmental catheter, an electronic transducer catheter, and modern Whitesides’ method with an 18-gauge needle (interdevice reliability 0.83). Although there is certain evidence against the use of Whitesides’ method, the majority of the literature indicates it can be a useful adjunct for physicians in resource-poor environments.
After diagnosis, the need for urgent surgical intervention should not be understated. However, this may be determined by discrepancies in access to care. Patients in low-resource environments may face additional barriers, such as transportation and access to trained medical practitioners. These may further complicate and prolong detection and definitive treatment, unfortunately potentially leading to devastating consequences. Having a high index of suspicion is paramount.
PROPHYLACTIC FOREARM FASCIOTOMIES
Crush Injury
The pathophysiology of crush injury entails localized traumatic compression injury which can be associated with muscular, neurovascular, and osseous damage. After significant crush injury, the inflammatory cascade that results from rhabdomyolysis can cause significant edema and raise intracompartmental pressures.13 Previous studies have indicated that crush injury patients may have an earlier onset of irreversible myonecrosis compared with other ACS etiologies (6 versus 8 hours).46,47 Therefore, similar to other etiologies of ACS, emergent fascial release is recommended for crush injury patients with a high clinical suspicion to minimize morbidity.48
Vascular/Reperfusion Injury
Prophylactic fasciotomy in the setting of vascular injury and limb ischemia has remained a topic of debate in recent literature. The pathophysiology of ACS in the setting of arterial ischemia can be attributed to both initial ischemic insult and reperfusion injury following limb revascularization. As tissue is exposed to prolonged ischemia, anaerobic metabolism ensues which leads to gradual tissue degradation and with time, irreversible neuromuscular damage, inability for reperfusion, and permanent tissue loss.49,50 Among limbs that are able to be reperfused, the introduction of additional blood into the compartment can lead to production of oxygen free radicals which further promotes intimal vascular injury, platelet aggregation, and thrombosis, thereby reintroducing ischemia and anaerobic metabolism.51
As the pathophysiology of vascular ischemia allows for the development for ACS in both the acute and delayed settings, many have adopted the use of prophylactic fasciotomies (as compared with therapeutic fasciotomies after the development of ACS) at time of revascularization.52 Wesslén and Wahlgren51 describe a statistically lower incidence of postoperative neuromuscular sequelae among patients who undergo prophylactic four-compartment, double-incision lower extremity fasciotomy as compared with therapeutic fasciotomy following elective lower extremity revascularization procedures (18% versus 42%, P < 0.05). As the degree of ischemia and total ischemia time are direct contributors to the development of ACS, many studies have delineated the importance of early versus late fasciotomies particularly in the treatment of those with traumatic acute limb ischemia.53,54 In their multivariate adjusted analysis, Farber et al54 describe a four-fold lower risk of amputation for traumatic acute limb ischemia patients who underwent early (<8 hours) as compared with late (>18 hours) lower extremity fasciotomy which persisted among subgroup analysis defined by type of vessel injured, mechanism of injury, procedure performed, and presence of associated venous injury or fracture. Additionally, they demonstrate a 23% lower hospital length of stay for early- as compared with late-fasciotomy patients.54 Similarly, Rothenberg et al53 report a significantly lower rate of amputation within 30 days of presentation among acute limb ischemia patients who underwent early versus late fasciotomy (5.9% versus 50%, P = 0.002), further supporting the notion that prophylactic fasciotomy at the time of revascularization or early fasciotomy from the time of presentation (6–8 hours may decrease the incidence of ACS, its corresponding neuromuscular sequelae, and overall risk of amputation in the affected extremity.
Hand Compartment Syndrome
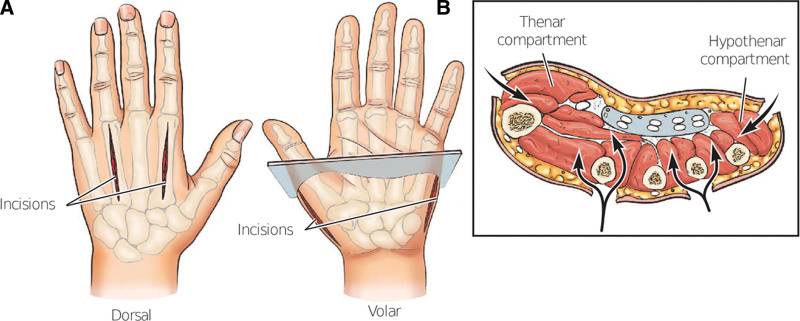
In contrast to the traditional cutoff of 30 mm Hg threshold for diagnosis of compartment syndrome in other regions of the upper extremity, compartment syndrome in one or multiple of the 10 compartments of the hand is defined as pressures greater than 15 mm Hg per compartment.55 Isolated hand compartment is exceedingly rare outside of traumatic etiologies and cardiovascular instrumentation, wherein case reports are limited to systemic diagnoses such as bleeding or sclerosing conditions.56,57 An argument can be made for prophylactic release of the hand compartments when the forearm is affected, especially when the hand has visible deformation of the dorsal aspect or a traumatic etiology. Various compartment release approaches are included in Figure 4. A carpal tunnel release may also be necessary considering neurological involvement, which occurs 21% of the time.58 Determination of which compartments to release can be done by clinical examination; with presence of taut overlying skin, pain within the median nerve distribution, and hand held in the intrinsic-minus position being the most specific.55
Fig. 4.
Concern for hand compartment syndrome requires more specific release beyond the S-shaped incision of the forearm. A, Depicts the volar and dorsal incisions, with a total of four incisions. B, Axial viewpoint to display decompression of the thenar, hypothenar, and digital compartments.
ADDITIONAL CONSIDERATIONS
Medicolegal Considerations
DePasse et al59 reviewed the VerdictSearch database (which contains >180,000 cases) in 2017 and described results from the US litigation surrounding compartment syndrome claims from 1988 to 2015. In 139 of these cases, more than 50% ruled in favor of the defendant or resulted in a settlement, demonstrating the difficulty to defend inaction by the surgical team if there is concern for compartment syndrome.59
CONCLUSIONS
ACS is a true surgical emergency that can threaten limb viability and predispose to significant chronic functional morbidity. Despite recent advances in technology and useful adjunct tools, clinical diagnosis remains the gold standard in both resource-rich and -poor environments. One possible exception is critically ill or unreliable physical examination patients in which recent technology may be particularly useful. Regardless of the etiology of ACS, expeditious diagnosis and a low threshold for emergent fasciotomy remains as the standard of care.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Elliott KGB, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85:625–632. [PubMed] [Google Scholar]
- 2.Torlincasi AM, Lopez RA, Waseem M. Acute compartment syndrome. In: StatPearls. Bethesda, MD: StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 3.McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome. Who is at risk? J Bone Joint Surg Br. 2000;82:200–203. [PubMed] [Google Scholar]
- 4.Griffiths DL. Volkmann’s ischaemic contracture. BJS Br J Surg. 1940;28:239–260. [Google Scholar]
- 5.Cugola L, Fasolo G. Clinical and demographic profile Volkmann’s ischemic contracture presenting in Tigray (Ethiopia). Acta Biomed 2022;92(S3):e2021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitesides TE, Haney TC, Morimoto K, et al. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop. 1975;43–51. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt AH, Bosse MJ, Frey KP, et al. ; METRC. Predicting acute compartment syndrome (PACS): the role of continuous monitoring. J Orthop Trauma. 2017;31:S40–S47. [DOI] [PubMed] [Google Scholar]
- 8.Bouklouch Y, Schmidt AH, Obremskey WT, et al. Big data insights into predictors of acute compartment syndrome. Injury. 2022;53:2557–2561. [DOI] [PubMed] [Google Scholar]
- 9.Leversedge FJ, Moore TJ, Peterson BC, et al. Compartment syndrome of the upper extremity. J Hand Surg. 2011;36:544–559; quiz 560. [DOI] [PubMed] [Google Scholar]
- 10.Wilsey B. Direct measurement of compartment pressure using a Stryker device. 2018. Available at https://pdf.medicalexpo.com/pdf/stryker/intra-compartmental-pressure-monitor/70192-169461-_2.html. Accessed June 20, 2023.
- 11.Vogels S, Ritchie ED, Bakker EWP, et al. Measuring intracompartmental pressures for the chronic exertional compartment syndrome: challenging commercially available devices and their respective accuracy. J Biomech. 2022;135:111026. [DOI] [PubMed] [Google Scholar]
- 12.Marmor M, Charlu J, Knox R, et al. Use of standard musculoskeletal ultrasound to determine the need for fasciotomy in an elevated muscle compartment pressure cadaver leg model. Injury. 2019;50:627–632. [DOI] [PubMed] [Google Scholar]
- 13.Stanley M, Chippa V, Aeddula NR, et al. Rhabdomyolysis. In: StatPearls. Bethesda, MD: StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 14.Garr JL, Gentilello LM, Cole PA, et al. Monitoring for compartmental syndrome using near-infrared spectroscopy: a noninvasive, continuous, transcutaneous monitoring technique. J Trauma. 1999;46:613–616; discussion 617–618. [DOI] [PubMed] [Google Scholar]
- 15.Arbabi S, Brundage SI, Gentilello LM. Near-infrared spectroscopy: a potential method for continuous, transcutaneous monitoring for compartmental syndrome in critically injured patients. J Trauma. 1999;47:829–833. [DOI] [PubMed] [Google Scholar]
- 16.Shuler MS, Reisman WM, Kinsey TL, et al. Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am. 2010;92:863–870. [DOI] [PubMed] [Google Scholar]
- 17.Sellei RM, Hingmann SJ, Weber C, et al. Assessment of elevated compartment pressures by pressure-related ultrasound: a cadaveric model. Eur J Trauma Emerg Surg. 2015;41:639–645. [DOI] [PubMed] [Google Scholar]
- 18.Garabekyan T, Murphey GC, Macias BR, et al. New noninvasive ultrasound technique for monitoring perfusion pressure in a porcine model of acute compartment syndrome. J Orthop Trauma. 2009;23:186–193; discussion 193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch JE, Lynch JK, Cole SL, et al. Noninvasive monitoring of elevated intramuscular pressure in a model compartment syndrome via quantitative fascial motion. J Orthopaed Res.2009;27:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiemann JM, Ueno T, Leek BT, et al. Noninvasive measurements of intramuscular pressure using pulsed phase-locked loop ultrasound for detecting compartment syndromes: a preliminary report. J Orthop Trauma. 2006;20:458–463. [DOI] [PubMed] [Google Scholar]
- 21.Sellei RM, Wollnitz J, Reinhardt N, et al. Non-invasive measurement of muscle compartment elasticity in lower limbs to determine acute compartment syndrome: clinical results with pressure related ultrasound. Injury. 2020;51:301–306. [DOI] [PubMed] [Google Scholar]
- 22.Herring MJ, Donohoe E, Marmor MT. A novel non-invasive method for the detection of elevated intra-compartmental pressures of the leg. J Vis Exp JoVE. 2019;(147). [DOI] [PubMed] [Google Scholar]
- 23.Han F, Daruwalla ZJ, Shen L, et al. A prospective study of surgical outcomes and quality of life in severe foot trauma and associated compartment syndrome after fasciotomy. J Foot Ankle Surg. 2015;54:417–423. [DOI] [PubMed] [Google Scholar]
- 24.Janakiram NB, Motherwell JM, Goldman SM, et al. Efficacy of non-surgical interventions for promoting improved functional outcomes following acute compartment syndrome: a systematic review. PLoS One. 2022;17:e0274132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dekker PK, Chang BL, Fleury CM, et al. Retention sutures and negative pressure wound therapy for delayed primary closure of fasciotomy wounds. Plast Reconstr Surg Glob Open. 2021;9:e3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald AM, Gaston P, Wilson Y, et al. Long-term sequelae of fasciotomy wounds. Br J Plast Surg. 2000;53:690–693. [DOI] [PubMed] [Google Scholar]
- 27.Altan L. Postoperative rehabilitation of compartment syndrome following fasciotomy. Turk J Phys Med Rehabil. 2023;69:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer S, Haug V, Diehm Y, et al. Feasibility and safety of enzymatic debridement for the prevention of operative escharotomy in circumferential deep burns of the distal upper extremity. Surgery. 2019;165:1100–1105. [DOI] [PubMed] [Google Scholar]
- 29.Shen YM, Ma CX, Qin FJ, et al. Wound repair and functional reconstruction of high-voltage electrical burns in wrists. Zhonghua Shao Shang Za Zhi. 2017;33:738–743. [DOI] [PubMed] [Google Scholar]
- 30.Friedstat J, Brown DA, Levi B. Chemical, electrical, and radiation injuries. Clin Plast Surg. 2017;44:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DH, Desai MJ, Gauger EM. Electrical injuries of the hand and upper extremity. J Am Acad Orthop Surg. 2019;27:e1–e8. [DOI] [PubMed] [Google Scholar]
- 32.Piccolo NS, Piccolo MS, Piccolo PDP, et al. Escharotomies, fasciotomies and carpal tunnel release in burn patients—review of the literature and presentation of an algorithm for surgical decision making. Handchir Mikrochir Plast Chir. 2007;39:161–167. [DOI] [PubMed] [Google Scholar]
- 33.Cathcart CC, Shuler MS, Freedman BA, et al. Correlation of near-infrared spectroscopy and direct pressure monitoring in an acute porcine compartmental syndrome model. J Orthop Trauma. 2014;28:365–369. [DOI] [PubMed] [Google Scholar]
- 34.Walters TJ, Kottke MA, Hargens AR, et al. Noninvasive diagnostics for extremity compartment syndrome following traumatic injury: a state-of-the-art review. J Trauma Acute Care Surg. 2019;87(1S Suppl 1):S59–S66. [DOI] [PubMed] [Google Scholar]
- 35.Lin JS, Samora JB. Pediatric acute compartment syndrome: a systematic review and meta-analysis. J Pediatr Orthoped B. 2020;29:90–96. [DOI] [PubMed] [Google Scholar]
- 36.Martus JE, Preston RK, Schoenecker JG, et al. Complications and outcomes of diaphyseal forearm fracture intramedullary nailing: a comparison of pediatric and adolescent age groups. J Pediatr Orthop. 2013;33:598–607. [DOI] [PubMed] [Google Scholar]
- 37.Ragland R, Moukoko D, Ezaki M, et al. Forearm compartment syndrome in the newborn: report of 24 cases. J Hand Surg. 2005;30:997–1003. [DOI] [PubMed] [Google Scholar]
- 38.Neri I, Magnano M, Pini A, et al. Congenital Volkmann syndrome and aplasia cutis of the forearm: a challenging differential diagnosis. JAMA Dermatol. 2014;150:978–980. [DOI] [PubMed] [Google Scholar]
- 39.Caouette-Laberge L, Bortoluzzi P, Egerszegi EP, et al. Neonatal Volkmann’s ischemic contracture of the forearm: a report of five cases. Plast Reconstr Surg. 1992;90:621–628. [DOI] [PubMed] [Google Scholar]
- 40.Tharakan SJ, Subotic U, Kalisch M, et al. Compartment pressures in children with normal and fractured forearms: a preliminary report. J Pediatr Orthop. 2016;36:410–415. [DOI] [PubMed] [Google Scholar]
- 41.Shirley ED, Mai V, Neal KM, et al. Wound closure expectations after fasciotomy for paediatric compartment syndrome. J Child Orthop. 2018;12:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nwadinigwe CU, Ihezie CO, Iyidiobi EC. Fractures in children. Nigerian J Med. 2006;15:81–84. [DOI] [PubMed] [Google Scholar]
- 43.Saikia KC, Bhattacharya TD, Agarwala V. Anterior compartment pressure measurement in closed fractures of leg. Indian J Orthop 2008;42:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uliasz A, Ishida JT, Fleming JK, et al. Comparing the methods of measuring compartment pressures in acute compartment syndrome. Am J Emerg Med. 2003;21:143–145. [DOI] [PubMed] [Google Scholar]
- 45.Collinge C, Kuper M. Comparison of three methods for measuring intracompartmental pressure in injured limbs of trauma patients. J Orthop Trauma. 2010;24:364–368. [DOI] [PubMed] [Google Scholar]
- 46.Heppenstall RB, Scott R, Sapega A, et al. A comparative study of the tolerance of skeletal muscle to ischemia. Tourniquet application compared with acute compartment syndrome. J Bone Joint Surg Am. 1986;68:820–828. [PubMed] [Google Scholar]
- 47.Bodansky D, Doorgakant A, Alsousou J, et al. Acute compartment syndrome: do guidelines for diagnosis and management make a difference? Injury. 2018;49:1699–1702. [DOI] [PubMed] [Google Scholar]
- 48.Coccolini F, Improta M, Picetti E, et al. Timing of surgical intervention for compartment syndrome in different body region: systematic review of the literature. World J Em Surg. 2020;15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gourgiotis S, Villias C, Germanos S, et al. Acute limb compartment syndrome: a review. J Surg Educ. 2007;64:178–186. [DOI] [PubMed] [Google Scholar]
- 50.Fukuda I, Chiyoya M, Taniguchi S, et al. Acute limb ischemia: contemporary approach. Gen Thorac Cardiovasc Surg. 2015;63:540–548. [DOI] [PubMed] [Google Scholar]
- 51.Wesslén C, Wahlgren CM. Contemporary management and outcome after lower extremity fasciotomy in non-trauma-related vascular surgery. Vasc Endovascular Surg. 2018;52:493–497. [DOI] [PubMed] [Google Scholar]
- 52.Chung J, Modrall J. Compartment syndrome. In: Rutherford’s Vascular Surgery. 8th ed. Minneapolis, MN: Clinical Tree; 2016:2544–2554. [Google Scholar]
- 53.Rothenberg KA, George EL, Trickey AW, et al. Delayed fasciotomy is associated with higher risk of major amputation in patients with acute limb ischemia. Ann Vasc Surg. 2019;59:195–201. [DOI] [PubMed] [Google Scholar]
- 54.Farber A, Tan TW, Hamburg NM, et al. Early fasciotomy in patients with extremity vascular injury is associated with decreased risk of adverse limb outcomes: a review of the national trauma data bank. Injury. 2012;43:1486–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Codding JL, Vosbikian MM, Ilyas AM. Acute compartment syndrome of the hand. J Hand Surg. 2015;40:1213–1216; quiz 1216. [DOI] [PubMed] [Google Scholar]
- 56.Gallagher E, Ruiter T. Spontaneous arterial hemorrhage of the hand resulting in compartment syndrome. Eplasty. 2015;15:ic44. [PMC free article] [PubMed] [Google Scholar]
- 57.Tanagho A, Hatab S, Youssef S, et al. Spontaneous compartment syndrome of the hand in systemic sclerosis. Orthopedics. 2015;38:e849–e851. [DOI] [PubMed] [Google Scholar]
- 58.Oak NR, Abrams RA. Compartment syndrome of the hand. Orthop Clin North Am. 2016;47:609–616. [DOI] [PubMed] [Google Scholar]
- 59.DePasse JM, Sargent R, Fantry AJ, et al. Assessment of malpractice claims associated with acute compartment syndrome. J Am Acad Orthop Surg. 2017;25:e109–e113. [DOI] [PubMed] [Google Scholar]