Abstract
Objectives:
This study aimed to analyze the Vaccine Adverse Event Reporting System (VAERS) database and systematically review the literature to provide a comprehensive analysis of reversible cerebral vasoconstriction syndrome (RCVS) and posterior reversible encephalopathy syndrome (PRES) secondary to vaccination.
Methods:
The authors analyzed the VAERS database and conducted a systematic review following PRISMA guidelines. The inclusion criteria for VAERS data were a score of ≥3 on the RCVS2 score and/or radiographic findings consistent with the diagnosis of RCVS or PRES. The systematic review was registered with PROSPERO.
Results:
Our combined data set included 29 cases (9 RCVS and 20 PRES). Most cases were women (72.4%) with a mean age of 50.7 years (SD 19.4 years). Most cases were associated with COVID-19 mRNA vaccines (58.6% Moderna, 20.7% Pfizer). Hypertension (37.9%), hyperlipidemia (13.7%), chronic kidney disease (CKD) (10.3%), and end-stage renal disease (6.8%) were common comorbidities. Furthermore, 20.6% (6/29) of cases were on immunosuppression therapy for various reasons. The mean time to symptom onset was 10.49 days after vaccination (SD 18.60), and the mean duration of hospitalization was 7.42 days (SD 5.94). The symptoms reported the most frequently were headache (41.3%), elevated blood pressure (31.0%), and emesis (17.2%). Typical radiographic findings included T2/FLAIR hyperintensities affecting the parieto-occipital lobes, indicative of vasogenic and/or cytotoxic edema.
Conclusions:
This study provides a comprehensive analysis of postvaccine RCVS and PRES. Both disease states were seen most often in those with pre-existing risk factors such as female sex, age over 50, hypertension, renal disease, and immunosuppression. Vaccines and their associated immune response may cause endothelial dysfunction leading to cerebral vasospasm and loss of cerebral autoregulation. However, further research is required to understand the underlying pathophysiological mechanisms. Despite the associations found, the absolute risk of these syndromes remains extremely low compared to the immense benefits of vaccination.
Keywords: posterior reversible encephalopathy syndrome, PRES, RCVS, reversible cerebral vasoconstriction syndrome, vaccination, vaccine
Introduction
Highlights
The study aimed to provide a comprehensive analysis of reversible cerebral vasoconstriction syndrome (RCVS) and posterior reversible encephalopathy syndrome (PRES) that arise secondary to vaccination through an analysis of Vaccine Adverse Event Reporting System and a systematic review.
A total of 29 cases were analyzed, with most cases (58.6% Moderna, 20.7% Pfizer) associated with COVID-19 mRNA vaccines. Many cases were women, with a mean age of 50.7 years.
Common comorbidities included hypertension, hyperlipidemia, chronic kidney disease, and end-stage renal disease. Additionally, 20.6% of the cases were on immunosuppression therapy. Symptoms frequently reported were headache, elevated blood pressure, and emesis, with radiographic findings often showing T2/FLAIR hyperintensities in the parieto-occipital lobes on MRI.
The risk of postvaccine RCVS and PRES may be higher in those with pre-existing risk factors. Vaccination and its associated inflammation may lead to blood-brain barrier breakdown, endothelial dysfunction leading to loss cerebral autoregulation and vasospasm. However, further research on the exact pathophysiological mechanisms is necessary.
While the study found associations between certain risk factors (female sex, age over 50, hypertension, renal disease, and immunosuppression) and postvaccine RCVS and PRES, the overall risk remains minimal compared to the vast benefits of vaccination.
Vaccines have revolutionized global health, preventing millions of deaths annually by immunizing against various infectious diseases1. However, as with any medical intervention, vaccines can have adverse effects. The Vaccine Adverse Event Reporting System (VAERS) is a national early warning system in the United States that monitors vaccine safety. VAERS provides a platform for healthcare professionals, vaccine manufacturers, and the public to report possible side effects or adverse events (AEs) that may be associated with vaccines2. Despite its limitations, such as under-reporting and the potential for bias, VAERS plays an essential role in postmarketing surveillance of vaccine safety and efficacy2.
Reversible cerebral vasoconstriction syndrome (RCVS) is characterized by recurrent thunderclap headaches and cerebral vasoconstriction that typically resolves over days to weeks3. Posterior reversible encephalopathy syndrome (PRES) (less commonly referred to as reversible posterior leukoencephalopathy syndrome) is a clinical-radiological entity characterized by headaches, seizures, altered consciousness, and visual disturbances associated with typical imaging findings of posterior cerebral white matter edema4. PRES and RCVS are believed to have coinciding pathophysiological mechanisms, including cerebral vasospasm and loss of cerebral autoregulation5. Although rarely reported, thrombotic phenomena, including stroke and vasospastic disorders, have been reported as an adverse reaction to vaccination6,7.
Isolated postvaccine RCVS and PRES cases have been reported; however, no comprehensive analysis of these events has been conducted concerning vaccine administration. This study aims to provide an analysis of RCVS and PRES after vaccination, as reported in the VAERS database as well as a systematic review of the literature. We aim to contribute to our understanding of these rare postvaccine neurological syndromes, including compounding risk factors and outcomes, to guide clinicians in managing these potential AEs.
Methods
VAERS database analysis
The VAERS database (https://vaers.hhs.gov/) is a national early warning system that monitors the safety of vaccines in the United States and was searched to identify cases of PRES and RCVS after vaccination2. The VAERS database was accessed on 1 August 2023, and no constraints were placed on the reporting year. The search strategy was constructed using the key indexing terms ‘reversible cerebral vasoconstriction syndrome’ and ‘posterior reversible encephalopathy syndrome’. VAERS ID was identified for all cases and extracted in a txt file. Each case was assessed by two reviewers. Cases of RCVS were included in the results if they had a score of ≥3 on the validated RCVS2 score for reversible cerebral vasoconstriction syndrome or if there were radiographic findings consistent with the diagnosis. The RCVS2 score is a clinical prediction rule used to diagnose RCVS. The RCVS2 score is calculated based on several clinical and radiological factors to differentiate RCVS from other conditions like aneurysmal subarachnoid hemorrhage, primary angiitis of the central nervous system, and other causes of thunderclap headache or reversible cerebral vasoconstriction. The score typically incorporates elements such as: (1) internal carotid artery involvement (not affected 0; affected −5), (2) sex (male 0; female +1), (3) vasoconstrictive trigger identified (no 0; yes +3), (4) subarachnoid hemorrhage present on imaging (absent 0; present +1) (5) recurrent or single thunderclap headache (absent 0; present +5). Scores 3–4 had 86% specificity and 10% sensitivity for the diagnosis of RCVS8. This study was completed according to the Strengthening the Reporting of Cohort Studies in Surgery (STROCSS) criteria9. The research protocol was prospectively registered on the Center for Open Science (OSF) registry.
Systematic review and search strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) and assessing the methodological quality of systematic reviews (AMSTAR) guidelines10,11. The study was registered with the International Prospective Register of Systematic Reviews (PROSPERO). A comprehensive literature search of PubMed/MEDLINE, ScienceDirect, Hinari, and Scopus databases was performed to identify vaccination-related RCVS and PRES. The literature search was conducted on 1 August 2023, and no constraints were placed on the reporting year. The search strategy was built around combinations of key terms: (vaccine OR vaccination) AND (Reversible Cerebral Vasoconstriction Syndrome OR RCVS OR Posterior Reversible Encephalopathy Syndrome OR PRES). The specific search string utilized is identified in Table 1. The search was not restricted by age, sex, type of vaccine, or time frame. Indexing tools such as MeSH terms were used in PubMed/MEDLINE. A gray literature search was conducted by reviewing the first 100 results obtained from Google Scholar. Both forward and backward citations were utilized to include relevant articles.
Table 1.
Search term utilized for each respective database in systematic review.
| Database | Search string |
|---|---|
| PubMed/Medline | (vaccine OR vaccination) AND (“Reversible Cerebral Vasoconstriction Syndrome”[MeSH/Tiab] OR “RCVS” OR “Posterior Reversible Encephalopathy Syndrome”[MeSH/Tiab] OR “PRES”) |
| Scopus | vaccine OR vaccination AND “reversible cerebral vasoconstriction syndrome” OR RCVS OR “posterior reversible encephalopathy syndrome” OR PRES |
| ScienceDirect | (vaccine OR vaccination) AND (Reversible Cerebral Vasoconstriction Syndrome OR Posterior Reversible Encephalopathy Syndrome) |
| Hinari | vaccine OR vaccination AND Reversible Cerebral Vasoconstriction Syndrome OR Posterior Reversible Encephalopathy Syndrome |
Study selection and data extraction
The exported search results were uploaded to Microsoft EndNote X9 and duplicate records were removed. Screening of titles and abstracts was performed independently by two authors using the Rayyan QCRI web application, a systematic review tool that allows for blind screening and collaboration12. Discrepancies between reviewers were resolved through discussion, and if necessary, a third reviewer was consulted. Full-text articles identified as potentially eligible were obtained and evaluated for inclusion based on the following criteria: (1) case reports/series and observational studies of patients who developed RCVS or PRES after vaccination, (2) reports available in English. Exclusion criteria included (1) studies not reporting original patient data (i.e. reviews, editorials, commentaries, and letters), (2) reports where RCVS or PRES could not definitively be attributed to vaccination or where another causative factor was present, (3) records published in nonpeer-reviewed journals. Data extraction was performed by two independent reviewers and consistency was cross-checked. The extracted data included the type of vaccine, the time to onset of RCVS or PRES symptoms after vaccination, patient comorbidities demographic information, radiographic findings, clinical manifestations, hospital stay, time to symptom onset, imaging findings, treatment, and outcomes.
Data characterization and analysis
Descriptive statistics were used to analyze the extracted data. Data were presented as mean±SD and as frequencies and percentages for categorical variables. All statistical analyzes were performed using Python v3.8 with Pandas v1.1.3 and NumPy v1.18.5 libraries for data management and manipulation. Data visualization was completed using the Python library Matplotlib v3.3.2.
Quality and risk of bias assessment
The quality of the included records within the systematic review was evaluated using the Joanna Briggs Institute (JBI) critical appraisal tool for case reports. The tool consists of eight items addressing different aspects of the methodological quality of case reports, including precise patient demographics, accurate diagnosis, objective measurements of intervention outcomes, and follow-up information. The quality and risk of bias assessment were conducted by two authors; a third author was consulted for any discrepancy between reviewers.
Results
VAERS database results
A total of 11 RCVS and 26 PRES diagnoses were registered in the VAERS database. Our analysis yielded a total of 7 RCVS and 18 PRES events based on manual review using the inclusion criteria. Thus, our VAERS data set is made up of 25 cases. Most of the reports were from women (72%), averaging 52.42±18.42 years (range 18–93). In terms of regional distribution, Ohio was the most reported region, with four instances, followed by Florida and Illinois, each with three cases. The onset of symptoms after vaccination was on average, 11.42 days (SD 19.71, range 1–90 days). For those hospitalized, the average duration of hospitalization was 7.42 days (SD 5.94, range 1–25 days). The most reported comorbidities were hypertension (10/25; 40%) and hyperlipidemia (4/25; 16%). Chronic kidney disease (CKD) was observed in 3/25 (12%) cases, and end-stage renal disease (ESRD) in 2/25 (8%) cases. Additionally, 6/10 (24%) individuals were on immunosuppression therapy, including pembrolizumab for metastatic breast cancer, tacrolimus and mycophenolate after a kidney transplant, and voclosporin for lupus nephritis. The symptoms reported the most frequently included headache (10/25; 40%), elevated blood pressure (9/25; 36%), and emesis (5/25; 20%). Regarding the types of vaccine-associated with these events, most were related to the COVID-19 mRNA (Moderna) vaccine, accounting for 15/25 (60%) cases. The COVID-19 mRNA vaccine (Pfizer) was reported in 6/25 (24%) instances, while other vaccines (i.e. pneumococcal, influenza, COVID-19 [Janssen]) comprised the rest. Management was often symptomatic for related symptoms, including control of blood pressure and levetiracetam for managing seizures. Outcomes were only reported in 7/25 (28%) cases and were favorable. Table 2 provides complete clinical details for each case alongside the respective VAERS ID.
Table 2.
VAERS analysis for cases of RCVS and PRES after vaccination.
| VAERS ID | Diagnosis | Age | Sex | Region | Year Reported | Comorbidities | Symptoms | Hospitalization | Vaccine Type | Time to onset | Neurodiagnostic Testing | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1272049-1 | RCVS | 41 | M | Florida | 2021 | – | Dizziness, nausea, emesis, weakness, speech impairment | No | COVID-19 mRNA (Pfizer) | 4 days | MRI unspecified findings of a CVA | – | – |
| 1040300-1 | RCVS | 41 | M | Connecticut | 2021 | Hypertension, caffeine consumption (60 oz daily) | Thunderclap headache | 10 days | COVID-19 mRNA (Moderna) | 5 days | CTH showing SAH. DSA: Evidence of the bilateral PCAs, SCA, and L PICA narrowing. | Nimodipine, GABApentin, and 1 week course of levetiracetam. | – |
| 1071969-1 | RCVS | 51 | M | Wisconsin | 2021 | Postcoital headaches | Headache, nausea, emesis | 4 days | COVID-19 mRNA (Moderna) | 4 days | CTH: subarachnoid hemorrhage | – | – |
| 1354795-1 | RCVS | 75 | F | Ohio | 2021 | Hypertension | Severe headache | No | COVID-19 mRNA (Moderna) | 1 day | DSA: near-complete occlusion of L MCA | – | Unspecified improvement. |
| 2309661-1 | RCVS | 69 | F | Illinois | 2022 | Hyperlipidemia, depression | Thunderclap headache, disorientation, emesis, speech impairment | No | COVID-19 mRNA (Moderna) | 8 days | – | Intramuscular Toradol and promethazine. | – |
| 2367879-1 | RCVS | 56 | F | Florida | 2022 | CVST, IIH, Lyme meningitis, hypertension, hypothyroidism | Thunderclap headache | No | COVID-19 mRNA (Pfizer) | 36 days | MRV: Moderate to severe narrowing of the L transverse sinus (chronic) | Acetazolamide | Improved severity of chronic headaches |
| 2596001-1 | RCVS | 47 | F | Texas | 2023 | Depression (on SSRIs) | Thunderclap headache | 3 days | COVID-19 mRNA (Moderna) | 48 days | MRI and CTH unspecified results | Verapamil | Improvement in headache |
| 0510187-1 | PRES | 23 | F | Illinois | 2013 | ESRD, hypertension | Blurry vision and seizures | Yes | Influenza (quadrivalent) | 1 day | – | – | – |
| 0742509-1 | PRES | – | F | – | 2018 | – | Cortical blindness, hallucinations, weakness. | Yes | Influenza, quadrivalent. | – | T2/FLAIR hyperintensities involving the parieto-occipital lobes | – | Improvement |
| 0933025-1 | PRES | 43 | F | Arizona | 2021 | Hyperthyroidism | Thunderclap headache | 1 day | COVID-19 mRNA (Moderna) | 7 days | MRI/CT: Occipital lobe lesions | Decadron | Improvement in symptoms at 10 days |
| 1060313-1 | PRES | 73 | F | Wyoming | 2021 | Hypertension | Acute altered mental status, elevated BP (SBP >240 mmHg) | 2 days | COVID-19 mRNA (Moderna) | <24 h | MRI: Punctate lesions in the parietal and frontal lobes. | Blood pressure management | |
| 1069614-1 | PRES | 59 | F | West Virginia | 2021 | Metastatic ovarian breast cancer on chemotherapy (pembrolizumab) | Altered mental status, elevated blood pressure | 8 days | COVID-19 mRNA (Moderna) | 2 days | MRI: Vasogenic edema affecting the parietal, temporal, occipital, and cerebellar hemispheres. | Blood pressure management (amlodipine), and levetiracetam. | – |
| 1122987-1 | PRES | 18 | M | – | 2021 | CKD V, Chronic use of tacrolimus due to failed kidney transplant | Generalized tonic-clonic seizures. | 7 days | COVID-19 (Janssen) | 1 day | MRI: Asymmetric scattered regions of predominantly right-sided subcortical signal abnormality of a posterior predominance. EEG: Bisynchronous rhythmic slow waves with wide distribution (GRDAs) | Levetiracetam | – |
| 1176079-1 | PRES | 93 | M | Virginia | 2021 | CKD IV, hypertension | Elevated blood pressure (SBP >220 mmHg), seizures, altered mental status | 6 days | COVID-19 mRNA (Moderna) | 1 day | MRI: T2/FLAIR hyperintensities of the occipital lobes. EEG: Electrographic seizures (unspecified) | Levetiracetam | – |
| 1290132-1 | PRES | 64 | F | Ohio | 2021 | Necrotizing pancreatitis | Altered mental status, acute hypoxic and hypercapnic respiratory failure, splenic vein thrombosis | 25 days | COVID-19 mRNA (Pfizer) | 11 days | MRI: PRES (unspecified) | – | – |
| 1330290-1 | PRES | 33 | F | Ohio | 2021 | Kidney transplant on tacrolimus | Encephalopathy, tachycardia, elevated blood pressure | 8 days | COVID-19 (Janssen) | 6 days | MRI: PRES (unspecified) | – | – |
| 1396565-1 | PRES | 66 | F | Ohio | 2021 | Scleroderma (mycophenolate mofetil 1000 mg twice daily) | Comatose | 3 days | COVID-19 mRNA (Moderna) | 11 days | – | – | – |
| 1430482-1 | PRES | 53 | F | Texas | 2021 | Obesity, macrocytic anemia, CKD, stroke, pulmonary embolus | Headache, nausea, emesis, elevated blood pressure (SBP >250 mmHg) | 3 days | COVID-19 mRNA (Moderna) | 3 days | MRI: T2/FLAIR hyperintensities involving the bilateral frontal, parietal, and occipital lobes. | – | – |
| 1501696-1 | Severe pre-eclampsia + PRES | 33 | F | Delaware | 2021 | Infertility | Severe hypertension, proteinuria, cortical blindness, hallucinations | 8 days | COVID-19 mRNA (Pfizer) | 10 days | – | – | – |
| 1757268-1 | PRES | 44 | F | North Carolina | 2021 | Substance use disorder, hypertension, type 2 diabetes mellitus, hyperlipidemia, vitamin D deficiency | Elevated blood pressure (SBP >220 mmHg), seizures, altered mental status | 10 days | COVID-19 mRNA (Moderna) | 4 days | MRI: Vasogenic edema of the occipital lobes | Amlodipine 10 mg, lisinopril 10 mg, hydrochlorothiazide 12.5 mg and carvedilol 3.125 mg twice daily | – |
| 2043972-1 | PRES | 48 | M | Wisconsin | 2022 | ESRD, immunosuppression (tacrolimus, mycophenolate), hypertension | Flu-like symptoms, shortness of breath, hyperpyrexia (102.5F), generalized tonic-clonic seizure | 6 days | COVID-19 mRNA (Moderna) | 1 day | MRI: Unspecified. | – | Oxygen-dependent with significant residual symptoms. |
| 2211495-1 | PRES | 33 | M | West Virginia | 2022 | Hypertension | shakiness, lightheadedness, generalized weakness, difficulty breathing, possible seizures, and headache | 4 days | COVID-19 mRNA (Pfizer) | 10 days | CTH: Small focal areas of low attenuation involving the bilateral occipital lobes, greater than left. R frontal lobe SAH. EEG: No epileptiform discharges. | – | – |
| 2237513-1 | PRES | 50 | F | Illinois | 2022 | Lupus nephritis (on voclosporin) | Hyperpyrexia (101.8F), emesis, headache, blurred vision, elevated blood pressure (SBP >200 mmHg) | 7 days | COVID-19 mRNA (Moderna) | ~90 days | CT/MRI: PRES (unspecified) | – | – |
| 2315645-1 | PRES | 80 | F | Florida | 2022 | Hyperlipidemia, coronary artery disease, dementia | Aphasia | 5 days | COVID-19 mRNA (Moderna) | 1 day | MRI: PRES (unspecified) | – | – |
| 2527853-1 | PRES | 65 | F | Puerto Rico | 2022 | Hyperlipidemia | Headache, paresthesia, weakness, elevated blood pressure | 21 days | COVID-19 mRNA (Pfizer) + pneumococcal (Prevnar 13) | 8 days | PRES (unspecified) | – | Improvement in symptoms at 3 weeks. |
CKD, Chronic kidney disease; COVID-19, Coronavirus disease 2019; CTH, Computerized tomography head; CVA, Cerebrovascular accident; CVST, Cerebral venous sinus thrombosis; DSA, Digital subtraction angiography; EEG, Electroencephalogram; ESRD, End-stage renal disease; GRDA, Generalized rhythmic delta activity; MCA, Middle cerebral artery; MRA, Magnetic resonance angiography; PCA, Posterior cerebral artery; PICA, Posterior inferior cerebellar artery; PRES, Posterior reversible encephalopathy syndrome; RCVS, Reversible cerebral vasoconstriction syndrome; SAH, Subarachnoid hemorrhage; SCA, Superior cerebellar artery; SSRI, Selective serotonin reuptake inhibitor.
Systematic review results
A total of 1094 records were identified from four electronic databases in this systematic review. A total of 860 records procured through the search strategy were excluded at the initial abstract and title screening stage. The reason for the removal was due to irrelevant data to the study protocol. A total of 4 cases were included in the results13–16. A PRISMA flow diagram is provided in Figure 1. Two cases of RCVS and PRES were identified. The average was 40.5 years, and 3/4 cases were reported in women. Two instances of RCVS were reported after administering the mRNA COVID-19 vaccine. A case of PRES was reported secondary to the measles vaccine and another from the mRNA COVID-19 vaccine. 3/4 cases had a symptom onset within 24 h after receiving their vaccine, whereas case no. 4 reported RCVS symptoms, including blurry vision and a focal headache 18 days after vaccination. Common risk factors included a history of RCVS, recreational drug use, alcohol and smoking, and hypertension. Common findings reported in people with PRES include T2/FLAIR hyperintensities on MRI indicative of vasogenic edema. The radiographic findings in RCVS also included vasogenic edema within the posterior circulation and focal stenosis of the posterior cerebral artery (P1 segment). Additionally, case no. 3 had a history of RCVS reported two years before his current presentation and had radiographic findings of basilar stenosis with a ‘beads-on-a-string’ appearance. Treatment included levetiracetam in two cases. Case no. 2 was also treated with three days of pulse-dose steroids. The management of RCVS had a short course of losartan in anticipation of a subsequent dose of the COVID-19 mRNA vaccine and oral nimodipine for cerebral vasospasm. 75% of cases showed favorable outcomes with resolution of symptoms; however, case no. 1 continued to exhibit cognitive deficits at 18 months of follow-up. All included cases are shown in Table 3.
Figure 1.
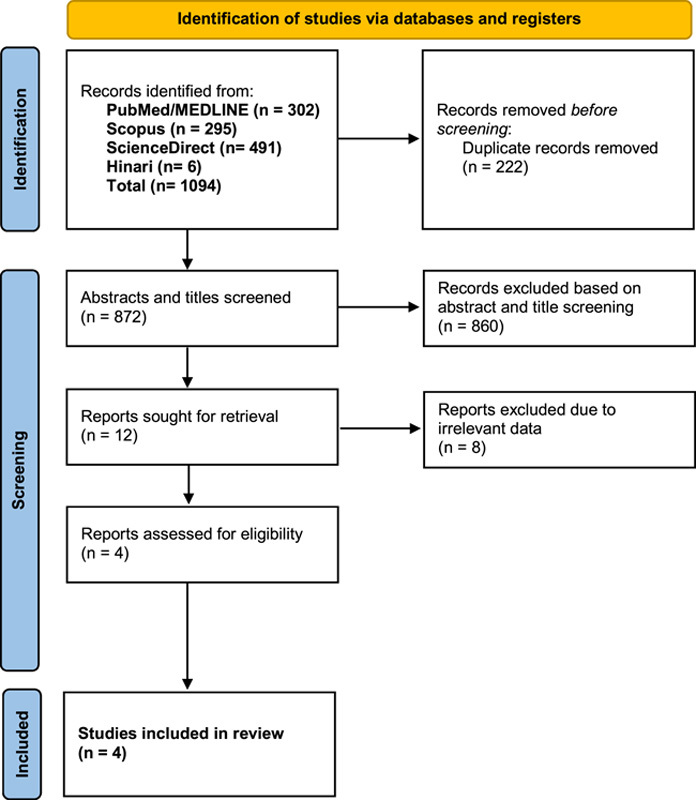
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
Table 3.
Results of the systematic review of cases of PRES and RCVS after vaccination.
| Case no. | Diagnosis | Age | Sex | Risk factors | Symptoms | Vaccine type | Time to onset | Imaging findings | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PRES | 76 | F | Hypertension, alcohol use disorder, shingles | Confusion, blurry vision, unsteady intermittent gait | COVID-19 mRNA (Moderna) | <24 h | T2/FLAIR hyperintensities in the parieto-occipital lobes | levetiracetam 750 mg twice daily | Residual cognitive decline 18 months later |
| 2 | PRES | 18 | F | None | Diffuse pain, pruritus, weakness of the hand, dysthesia | Measles | 8 h | T2/FLAIR hyperintensities in the occipital lobes | 1000 mg steroids for 3 days. MRA showed vasoconstriction of the posterior cerebral arteries | Improvement in T2/FLAIR hyperintensities and posterior circulation vasoconstriction after 3 days of treatment |
| 3 | RCVS | 30 | M | History of RCVS, Bipolar disorder | Thunderclap headache | COVID-19 mRNA (Pfizer) | 12 h | Normal CT head | Losartan 50 mg for 2 weeks | No symptoms at 2-week follow-up |
| 4 | RCVS | 38 | F | Migraine without aura, 10-pack smoking history | Sudden-onset blurred vision bilaterally followed by a focal headache over the right occipital projection | COVID-19 mRNA (Moderna) | 18 days | MRI showed an ischemic stroke on the territory of the right posterior cerebral artery. MRA shows discontinuation of the right P1 segment | Levetiracetam (1000 mg/d) and nimodipine (90 mg/d) for 5 weeks | Normal flow in both PCAs was documented on CTA after 7 days of nimodipine, suggesting vasospasm |
COVID-19, Coronavirus disease 2019; MRA, Magnetic resonance angiography; PRES, Posterior reversible encephalopathy syndrome; RCVS, Reversible cerebral vasoconstriction syndrome.
Quality and risk of bias assessment
The Joanna Briggs Institute critical appraisal tool for case reports was used to assess the risk of bias in reported cases within the systematic review. Overall, a low risk of bias was observed in the four cases. Table 4 provides a checklist for all included records13–16.
Table 4.
Joanna Briggs Institute critical appraisal and risk of bias results for included records.
Combined analysis
Our combined data set included a total of 29 patients [9 (31.0%) RCVS and 20 (69%) PRES], with the majority being female (n=21, 72.4%) compared to males (n=8, 27.6%). The age of the patients ranged from 18 to 93 years, with a mean age of 50.7 years (SD 19.4 years; range 18–93). Geographically, the regions that were the most frequently reported were Florida, Ohio, and Illinois. Other areas represented in the data included Wisconsin, Texas, West Virginia, Connecticut, Arizona, Wyoming, Virginia, Delaware, North Carolina, and Puerto Rico. Among the patients, common comorbidities included hypertension (n=11, 37.9%), and hyperlipidemia (n=4, 13.7%). CKD was observed in 3/3 (10.3%) cases, and end-stage renal disease (ESRD) in 2/3 (6.8%) cases. Furthermore, 6/29 (20.6%) individuals were on immunosuppressive therapy for various reasons, including kidney transplants, breast cancer, and lupus nephritis. The most common CTH finding was hypodensities within the posterior occipital regions of the brain. And MRI often revealed T2/FLAIR hyperintensities consistent with vasogenic edema within the parietal and occipital lobes.
The most frequently reported symptom was headache (n=12, 41.3%), followed by elevated blood pressure (n=9, 31.0%) and emesis (n=5, 17.2%). Note that patients often presented multiple symptoms. The onset of symptoms after vaccination was ~10.49 days (SD=18.60). The mean duration of hospitalization among patients was ~7.42 days, although this varied widely between individuals, with a SD of 5.94 days. COVID-19 mRNA vaccines from Moderna (n=17, 58.6%) and Pfizer (n=6, 20.7%) were the most frequent in our data set. Other vaccines included the quadrivalent influenza vaccine (n=2, 6.8%), the Janssen COVID-19 vaccine from Janssen (n=2, 6.8%), a combination of the Pfizer COVID-19 mRNA vaccine and the pneumococcal vaccine Prevnar 13 (n=1, 3.4%), and the measles vaccine (n=1, 3.4%). Figure 2 provides a visual representation of the vaccines involved. Management was often symptomatic for related symptoms, including control of blood pressure and levetiracetam for managing seizures. Only 10/29 (34.5%) cases reported results, 8/10 (80%) of which were favorable.
Figure 2.
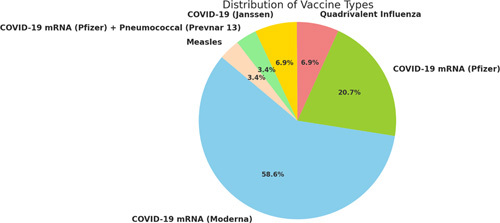
Graphical breakdown of the 29 vaccine related cases of RCVS and PRES.
Discussion
Our analysis of the VAERS database and systematic review represents the most comprehensive evaluation of RCVS and PRES after vaccination. We included 29 cases (9 RCVS and 20 PRES), most of which were reported in women (72.4%), and those with pre-existing risk factors, including hypertension, renal disease, and immunosuppressive therapy. The COVID-19 mRNA vaccines were most often implicated. Understanding the pathophysiology of RCVS and PRES is essential in understanding how vaccines might contribute to their development. RCVS and PRES are understood as disorders of cerebral autoregulation and vasospasm. In RCVS, there is a transient alteration in the tone of the cerebral arteries, leading to vasoconstriction that can fluctuate and eventually reverses completely3. PRES is believed to result from the failure of the cerebral autoregulation mechanism in response to acute changes in blood pressure, resulting in hyperperfusion and endothelial dysfunction, leading to vasogenic edema17. The endothelium of cerebral vessels produces several vasoactive substances including nitric oxide and eicosanoids, which help regulate cerebral blood flow18. Vaccines, particularly COVID-19 mRNA vaccines, have been proposed to induce a robust immune response that could lead to systemic inflammation and endothelial dysfunction19. Thus, disrupting cerebral autoregulation and promoting vasospasm, leading to conditions such as RCVS and PRES. Furthermore, the SARS-CoV-2 spike protein has been suggested to injure endothelial cells directly, and the spike protein produced in response to mRNA vaccines could have similar effects20,21.
In our study, the mean age of the patients was 50.7 years, and common comorbidities included hypertension, hyperlipidemia, immunosuppression, and CKD. These findings are consistent with known risk factors for RCVS and PRES. The typical imaging finding in RCVS includes focal stenosis or narrowing of the cerebrovasculature, as seen on CTA, and digital subtraction angiography. The radiographic findings of PRES also include vasogenic edema on magnetic resonance imaging, as this was our cohort’s most prevalent radiographic finding22,23. Careful monitoring and management of these conditions in the postvaccination period may be needed, especially in patients with compounding risk factors as seen in our cohort. Our study’s clinical presentations of RCVS and PRES were typical, with the most frequently reported symptoms being headache, elevated blood pressure, and emesis. The onset of symptoms after vaccination was ~10.49 days on average, indicating a potential window for early intervention and treatment. The management strategies in our study were largely symptomatic, focusing on controlling blood pressure and managing seizures. About a third of the cases reported favorable outcomes, which may reflect the nature of these conditions and the challenges associated with their management.
Our study has several limitations, including possible under-reporting and reporting bias in the VAERS database. A ʻstimulatedʼ reporting bias may have occurred due to the COVID-19 pandemic. Thus, many cases of non-mRNA vaccine-related AEs prior to the COVID-19 pandemic may have been underreported. As an observational study, we cannot establish a causal relationship between vaccination and RCVS/PRES. Despite these limitations, our study provides important information on these rare postvaccine neurological syndromes, helping inform clinicians and guide future research. The study also underscores the importance of ongoing surveillance and more research to understand the potential relationship between vaccination and the development of these rare neurological syndromes. Future studies are needed to validate our findings in larger cohorts and to elucidate the underlying pathophysiological mechanisms. Such studies can evaluate mRNA vaccines and causality with vasospasm and blood-brain barrier dysfunction. Guiding the development of strategies for preventing, detecting, and managing these potential postvaccine AEs. Despite the associations identified, it is crucial to emphasize that the absolute risk of developing these syndromes after vaccination remains extremely low. The findings of this study should not discourage the public from getting vaccinated but should serve to inform healthcare professionals for better recognition, reporting, and management of these rare events.
Conclusions
We identified 29 cases of RCVS and PRES after vaccination, most of which were associated with COVID-19 mRNA vaccines. Although these events are rare, our study highlights the importance of maintaining vigilance for these potential neurological syndromes in the postvaccination period, particularly in patients with pre-existing risk factors such as female sex, age over 50, underlying hypertension, immunosuppressant usage, and renal disease. Our findings suggest that the immune response triggered by vaccines, particularly mRNA-based COVID-19 vaccines, could theoretically disrupt cerebral autoregulation and promote vasospasm, leading to conditions such as RCVS and PRES. However, establishing a causal relationship will require further investigation.
Ethical approval
The Vaccine Adverse Event Reporting System (VAERS) is under the jurisdiction and ethical approval by the U.S. Department of Health and Human Services (HHS). VAERS is co-managed by the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA). Ethical approval was not required for this research because no patient records were accessed.
Consent
The VAERS complies with all United States Government security standards and protections concerning health information. Use of the VAERS database does not require approval by an institutional review board or informed consent.
Sources of funding
No internal or external funding was received for this manuscript.
Author contribution
B.S.S.: conceptualization, data curation, formal analysis, methodology, project administration, resources, software, validation, visualization, writing – original draft, writing – review and editing; T.F.: writing – original draft; V.K. and M.A.G.D.: conceptualization, writing – original draft, writing – review and editing.
Conflicts of interest disclosure
On behalf of all authors the corresponding author states that there are no conflicts of interest.
Research registration unique identifying number (UIN)
Open Science Framework https://doi.org/10.17605/OSF.IO/KPMDE.
Guarantor
Bahadar S. Srichawla.
Data availability statement
Data is available upon reasonable request from the Editor-In-Chief.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 18 October 2023
Contributor Information
Bahadar S. Srichawla, Email: bahadar.srichawla@umassmemorial.org.
Ton Fang, Email: ton.fang@umassmemorial.org.
Vincent Kipkorir, Email: vincentkipkorir42358@gmail.com.
Maria A. Garcia-Dominguez, Email: maria.garcia-dominguez@umassmemorial.org.
References
- 1.Canoui E, Launay O. History and principles of vaccination. Rev Mal Respir 2019;36:74–81. [DOI] [PubMed] [Google Scholar]
- 2.Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015;33:4398–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol 2012;11:906–917. [DOI] [PubMed] [Google Scholar]
- 4.Triplett JD, Kutlubaev MA, Kermode AG, et al. Posterior reversible encephalopathy syndrome (PRES): diagnosis and management. Pract Neurol 2022;22:183–189. [DOI] [PubMed] [Google Scholar]
- 5.Jeanneret V, Jillella DV, Rangaraju S, et al. PRES and RCVS: two distinct entities or a spectrum of the same disease? J Stroke Cerebrovasc Dis 2022;31:106472. [DOI] [PubMed] [Google Scholar]
- 6.Kleebayoon A, Wiwanitkit V. Vasospastic angina following COVID-19 vaccine-related myocarditis: comment. Cardiol Young 2023;33:672. [DOI] [PubMed] [Google Scholar]
- 7.Jabagi MJ, Botton J, Bertrand M, et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA 2022;327:80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha EA, Topcuoglu MA, Silva GS, et al. RCVS(2) score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology 2019;92:e639–e47. [DOI] [PubMed] [Google Scholar]
- 9.Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finsterer J. First reported case of reversible cerebral vasoconstriction syndrome after a SARS-CoV-2 vaccine. Cureus 2021;13:e19987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamano T, Takeda T, Morita H, et al. Posterior reversible encephalopathy syndrome following measles vaccination. J Neurol Sci 2010;298:124–126. [DOI] [PubMed] [Google Scholar]
- 15.Lund AM, Al-Karagholi MA. COVID-19 vaccination might induce reversible cerebral vasoconstriction syndrome attacks: a case report. Vaccines (Basel) 2022;10:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCullough J, Ahmad M, Tam I, et al. Posterior reversible encephalopathy syndrome onset within 24 hours following moderna mRNA booster COVID-19 vaccination: vaccine adverse event vs. hypertension? Cureus 2022;14:e24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015;14:914–25. [DOI] [PubMed] [Google Scholar]
- 18.Peterson EC, Wang Z, Britz G. Regulation of cerebral blood flow. Int J Vasc Med 2011;2011:823525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terentes-Printzios D, Gardikioti V, Solomou E, et al. The effect of an mRNA vaccine against COVID-19 on endothelial function and arterial stiffness. Hypertens Res 2022;45:846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trougakos IP, Terpos E, Alexopoulos H, et al. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med 2022;28:542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhal AB. Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome as syndromes of cerebrovascular dysregulation. Continuum (Minneap Minn) 2021;27:1301–20. [DOI] [PubMed] [Google Scholar]
- 23.Shankar J, Banfield J. Posterior reversible encephalopathy syndrome: a review. Can Assoc Radiol J 2017;68:147–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon reasonable request from the Editor-In-Chief.


