Abstract
Background:
Some studies have found that the application of traditional Chinese medicine in the treatment of lung cancer has achieved satisfying results. Polyphyllin Ⅲ (PP Ⅲ) is a natural steroid saponin from P. polyphylla var. yunnanensis, and its analogs have played a wide role in anticancer research. This study aimed to investigate the effect of PP Ⅲ on the development of lung cancer and its molecular mechanism.
Methods:
A549 and NCI-H1299 cell lines were treated with PP Ⅲ in gradient concentration to detect the IC50 of the cells, and the optimal concentration was selected for subsequent experiments. The effects of PP III treatment on lung cancer were investigated in vitro and in vivo.
Results:
In vitro experiments, it was found that the proliferation, invasion, migration, and colony formation ability of cancer cells were significantly reduced after PP III treatment, while accompanied by a large number of cell apoptosis. Further detection showed that N-cadherin was significantly decreased, E-cadherin was increased, and Snail and Twist were decreased in A549 cells and NCI-H1299 cells, respectively. In addition, GSK-3β expression was increased, while β-catenin expression was reduced with PP III treatment. In the mouse model, it was demonstrated that the volume of transplanted tumors was significantly reduced after PP Ⅲ treatment.
Conclusions:
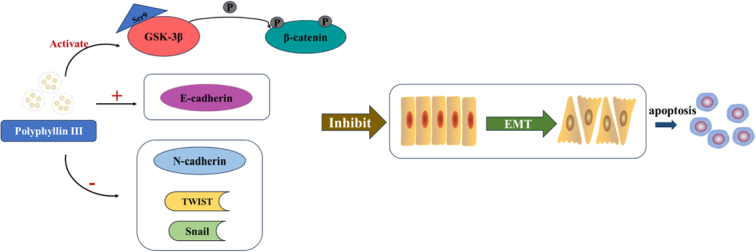
PP Ⅲ has the capacity to inhibit the progression of lung cancer and regulate epithelial-mesenchymal transition through the GSK-3β/β-catenin pathway to suppress the malignant behavior of cancer cells. The application of PP Ⅲ is expected to be an effective method for the treatment of lung cancer.
Keywords: EMT, GSK-3β/β-catenin, lung cancer, Polyphyllin Ⅲ
Introduction
Highlights
-
Polyphyllin Ⅲ regulates epithelial-mesenchymal transition through GSK-3β/β-catenin pathway to suppress malignant behavior of lung cancer cells.
What is known and what is new?
Polyphyllin I, polyphyllin II, polyphyllin VI, and polyphyllin VII have antitumor efficacy.
-
This manuscript investigated that Polyphyllin Ⅲ has the capacity to inhibit the progression of lung cancer.
What is the implication, and what should change now?
The application of PP Ⅲ is expected to be an effective method for the treatment of lung cancer.
Lung cancer, one of the most widespread cancers in the world, has a 5-year survival rate of less than 15%, and the mortality rate is the highest among all types of cancer1. For decades, researchers have tried surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy to slow the progression of lung cancer, but these treatments have generally been associated with terrible side effects and high costs2. Moreover, there are complicated pathogenesis, frequent gene mutation, and incomplete treatment for lung cancer3, and it can indirectly or directly cause patients to gradually reduce sensitivity or even develop tolerance to the above therapies4. As a consequence, the development of valuable treatment for lung cancer patients is critical. Traditional Chinese medicine (TCM) therapy is based on the theory of TCM and has been used for thousands of years. With in-depth research, the potential anticancer effect of natural medicine products has gradually provided new ideas for anticancer drugs, and many natural medicine and their extracts have been approved as powerful antitumor drugs5. Paris polyphylla Smith var. chinensis (Franch.) Hara is a plant in the lily family whose roots have been used in TCM with a long history6. Paris polyphylla var. yunnanensis contains steroids, amino acids, flavonoids, and polysaccharides, and has been used as the main components of nearly 100 kinds of Chinese patent medicines including Yunnan Baiyao and Qingre Jiedu capsules7–9. The most important active ingredient of Paris polyphylla var. yunnanensis is a steroid substance extracted from its rhizome-Polyphyllin (PP). It plays an effective pharmacological role in the treatment of clearing away heat and toxic materials, detumescence and pain relief, and antibacterial, anti-inflammatory, and antitumor10–12. Numerous studies have shown that a variety of chemical components in PP have antitumor efficacy, such as polyphyllin I, polyphyllin II, polyphyllin VI, and polyphyllin VII. These extracts have shown good effects in the treatment of a number of cancers by inducing cancer cell cycle arrest, apoptosis, autophagy, reducing angiogenesis, and increasing anticancer drug sensitivity13–15. PP Ⅱ can induce autophagosome formation in non-small cell lung cancer (NSCLC), increase apoptosis of cancer cells, and eventually inhibit cancer development16. Moreover, PP Ⅱ can activate the JNK pathway and endoplasmic reticulum stress, increase the cytotoxicity of cisplatin, and reduce the drug resistance of NSCLC cells17. Niu et al.15 reported PP Ⅱ regulated epithelial-mesenchymal transition (EMT)-related factors and matrix metalloproteinase to inhibit bladder cancer. The cadherin family, one of the members of the EMT process, is a class of intercellular adhesion promotion molecules, mainly distributed in various epithelial tissues, responsible for homotypic or heterotypic intercellular adhesion, and involved in the metastasis process of tumor cells. E-cadherin regulates various pathways including β-catenin and actin to maintain epithelial cell phenotype and tissue homeostasis, and the loss of E-cadherin is often regarded as critical for EMT development and cell separation18. Genetic mutations or epigenetic changes during the development of malignant tumors can hinder E-cadherin expression and function19. Marina Rosso et al.20 found that the expression level of E-cadherin is closely related to the progression of ovarian cancer, meaning that E-cadherin decreases with the development of ovarian cancer and the dissemination of cancer cells. N-cadherin is responsible for mediating the adhesion between homotypic or allotypic cells, helping cancer cells to colonize the tumor niche after metastasis21. Recent studies have reported that N-cadherin is highly expressed in a variety of malignant tumor tissues, and patients with a higher content of serum N-cadherin have a poorer prognosis, suggesting a certain correlation between N-cadherin and tumor metastasis and invasion. Snail protein, a transcriptional repressor of E-cadherin in EMT, can enhance drug resistance, proliferation ability, and dormancy survival ability of tumor cells22. Snail overexpression enhances the malignant behavior of cancer cells by promoting cells to acquire mesenchymal markers, while at the same time allowing tumor cells to acquire stemness that makes them resistant to treatment23. TWIST1 is a transcription factor that regulates cell migration and tissue remodeling during development and is also one of the transcription factors that inhibit E-cadherin24. Studies have shown that TWIST1 can act as an inducible factor of EMT and participate in the occurrence of cancer. TWIST1 is generally highly expressed in breast cancer tissues, which leads to poor prognosis25. TWIST1 and its downstream signaling activate the AKT pathway and induce EMT and stemness in colorectal cancer26.
GSK-3β is an evolutionarily conserved serine/threonine kinase. Reduced phosphorylation of GSK-3β activates GSK-3β, thereby promoting the phosphorylation and degradation of β-catenin, reducing the expression of β-catenin in the cytoplasm, and inhibiting the Wnt signaling pathway27. The Wnt/β-catenin pathway is the most important and studied mechanism regulating EMT28, while GSK-3β and β-catenin are pivotal molecules in the Wnt/β-catenin pathway. β-catenin regulates the progression of EMT in various ways, forms a complex with E-cadherin on the cell membrane to participate in intercellular adhesion, and also acts as an important transcription factor involved in regulating and driving the occurrence and development of EMT28. EMT refers to the process in which epithelial cells lose adhesion ability and gain interstitial cell properties, lose polarity and cell junction, and thus gain movement and migration ability, so that cells detach from the primary site and infiltrate into the matrix, and migrate to distant places through the lymphatic channel. EMT is an important approach to lung cancer spread and metastasis29. Chen et al.30 proved that Fangchinoline inhibits NSCLC metastasis by reversing EMT. In addition, the inhibition of EMT promotes NSCLC cell death31.
The combined treatment of TCM and modern medicine has gradually become a promising direction in the clinical treatment of malignant tumors. Most of the existing studies focus on the biological effects of PP Ⅰ, Ⅱ, Ⅶ (G), and D on cancer cells32, but there are no studies on the effects of PP Ⅲ on lung cancer. Moreover, the progression of EMT is closely related to metastasis, invasion, and infiltration of lung cancer. GSK-3β/β-catenin is a key signaling molecule of EMT, so this study aims to explore the impacts of PP Ⅲ on lung cancer by EMT through the GSK-3β/β-catenin signaling pathway.
Methods
Cell culture
Human lung cancer cell lines A549 and NCI-H1299 were cultured in DMEM medium adding fetal bovine serum and double antibody at 37°C and 5% CO2. A549 control group and NCI-H1299 control group were set up: cells were untreated; PP Ⅲ treatment group: A549 cells and NCI-H1299 cells were treated with the corresponding concentration of PP Ⅲ.
The IC50 of PP Ⅲ on A549 and NCI-H1299 was determined by CCK-8
The cells of the logarithmic growth phase were inoculated into a 96-well plate with 2×104 cells per well, and the cells treated with PP Ⅲ concentrations of 0, 2.5, 5, 10, 20, 40, 80, 160 μmol/l were added, respectively. Five repeated experiments were set up in each group, and the cells were incubated at 37°C for 24 h.
20 μl CCK-8 solution was applied to each well away from the light, and the light absorption value of each well was measured under 450 nm wavelength by a microplate reader. The IC50 of PP Ⅲ on A549 cells and NCI-H1299 cells was calculated, which was used as PP Ⅲ concentration in subsequent experiments.
CCK-8 detected cell proliferation
The cells of the logarithmic growth phase were inoculated into a 96-well plate with 2×104 cells per well, cultured for 24, 48, 72, and 96 h at 37°C. 20 μl CCK-8 solution was applied to each well away from the light, and the light absorption value of each well was measured under 450 nm wavelength by a microplate reader, while cell proliferation capacity was calculated.
Colony formation
A549 cells and NCI-H1299 cells in each group were collected, and the cells were inoculated in a 6-well plate culture dish for 14 days. When the cloned cells were visible to the naked eye, the medium was discarded and 1 ml/well crystal violet staining solution was used for dyeing 10–20 min. Then samples were rinsed with distilled water and photographed for counting.
Apoptosis was detected by flow cytometry
Cells were inoculated into the 6-well plate, cultured for 24 h, and treated with PP Ⅲ for 24 h. Samples were collected and stained according to the instructions of the apoptosis detection kit, and flow cytometry was used to detect the apoptosis rate.
Transwell
The invasion ability of the cells was measured using the 3 μm Transwell chamber filter. 50 mg/l Matrigel (BD, USA) was diluted at a ratio of 1:9 and coated on the upper surface of the bottom membrane of the Transwell chamber. About 1×105 cells were inoculated in the upper chamber, and 500 μl DMEM containing 15% fetal bovine serum was applied to the lower chamber and cultured for 24 h. The cells were fixed with formaldehyde when they migrated to the adhesion membrane of the lower chamber, and stained with crystal violet for 15 min. The cells were photographed and counted under a microscope.
Scratch test
8×105 cells were inoculated on a 6-well plate and cultured for 24 h using a 200 μl pipette tip to make scratches, PBS washed away floating cells, and continued culture. The cell migration ability was evaluated at 0, 12, and 24 h.
Western blot
The cells of each group were collected and lysed, while the total protein was extracted. The protein concentration was examined by BCA, and we adjusted the protein concentration of each group. We loaded samples 30 μg/well, and electrophoresis was performed with SDS-PAGE gel. The protein was transferred to the PVDF membrane, sealed with skim milk at 25°C, incubated with primary antibody at 4°C overnight, incubated with HRP-linked secondary antibody, developed and photographed with an ECL kit. Primary antibodies included Snail antibody (ab216347), Twist antibody (ab50887), E-cadherin antibody (ab231303), N-cadherin antibody (ab76011), GSK3β antibody (ab32391), β-catenin antibody (ab32572), Bax antibody (ab32503), Bcl-2 antibody (ab182858), and GAPDH antibody (ab8245), all derived from Abcam.
Immunofluorescent staining
After the fusion growth of cells on the cover glass, 4% polyformaldehyde was applied to cold fix for 20 min, 0.2% Triton X-100 was permeabilized for 10 min, and PBS was used to wash cells for three times. 5% BSA was used to seal the sample at room temperature for half an hour. Antibody against E-cadherin (Santa Cruz) was added and incubated at 4°C overnight, and washed three times with PBS for 10 min each time. The secondary antibody was added and incubated for 1 h away from light, and washed three times with PBS. Alexa Fluor 488 (Molecular Probes, 1:500) and Alexa Fluor 594 goat anti-mouse (Molecular Probes, 1:500) antibodies were added and incubated for 2 h. Samples were washed with PBS three times for 10 min each time. DAPI staining was performed, and 95% glycerin was used to seal the sample. Finally, the confocal microscope was used to observe and photograph.
Nude mouse tumor formation experiment
Thirty-two healthy 4-week-old nude mice were purchased from Beijing Weitonglihua Experimental Animal Technology Co., LTD. NCI-1299 cells were collected by PBS and mixed with the same volume of Matrigel into a concentration of 1×107/ml, then lung cancer cell suspension (100 μl) was injected subcutaneously, and mice were cultured for 15 days. Thirty-two nude mice were randomly divided into a model group (intraperitoneal injection of normal saline) and a PP Ⅲ treatment group (MD, 10 mg/kg· 2 days). Intraperitoneal injection lasted for 28 days. Six nude mice in good growth states were selected from each group, and the tumor volume was measured. The work has been reported in line with the ARRIVE criteria33.
KI67 was detected by immunohistochemistry
The transplanted tumor was fixed with 4% paraformaldehyde at 4°C for 24 h, and then washed with PBS to remove the fixing fluid. 70%, 80%, and 90% ethanol were used to dehydrate one time for 30 min, and 95% and 100% ethanol were treated twice each for 20 min. Xylene and ethanol were mixed 1:1 and used to soak the sample for 2 h. Xylene and paraffin were mixed 1:1 and used to soak the sample for 1 h, and paraffin was used to soak the sample twice for 1 h each time. The sample was sliced with a slicer and baked at 60°C for 2 h. The sample was dewaxed with xylene for 10 min. The sample was treated with 100%, 95%, 80%, and 70% ethanol for 5 min, and distilled water was used to soak the sample for 2 min. The antigen retrieval solution was preheated to 95°C, and the slices were immersed in the repair solution for 15 min and cooled for 40 min. The sample was sealed with TBST for 1 h. PSD95 (cell assay) or NMDA (animal assay) primary antibody was incubated for 1 h. The sample was washed with PBS three times, and HRP-labeled secondary antibody was incubated for 30 min. Then the sample was washed with PBS for three times, and DAB color development was conducted for 7 min. The sample was washed with PBS, hematoxylin was used to redye the sample for 2 min, and the sample was permeabilized with 70, 80, and 95% alcohol for 2 min each, anhydrous ethanol for 4 min, and No. 1 xylene and No. 2xylene for 3 min each. Permount TM Mounting Medium was added to seal the sample, the coverslip was covered, and a microscope was used for photograph.
Statistical analysis
GraphPad version 9.0 was employed to analyze data expressed as mean±SD from the above experiments. Regarding the difference test, we conducted a t-test for 2 groups and ANOVA for more than two groups. The P-value<0.05 was considered statistically significant.
Results
PP Ⅲ treatment inhibited proliferation and promoted apoptosis of lung cancer cells
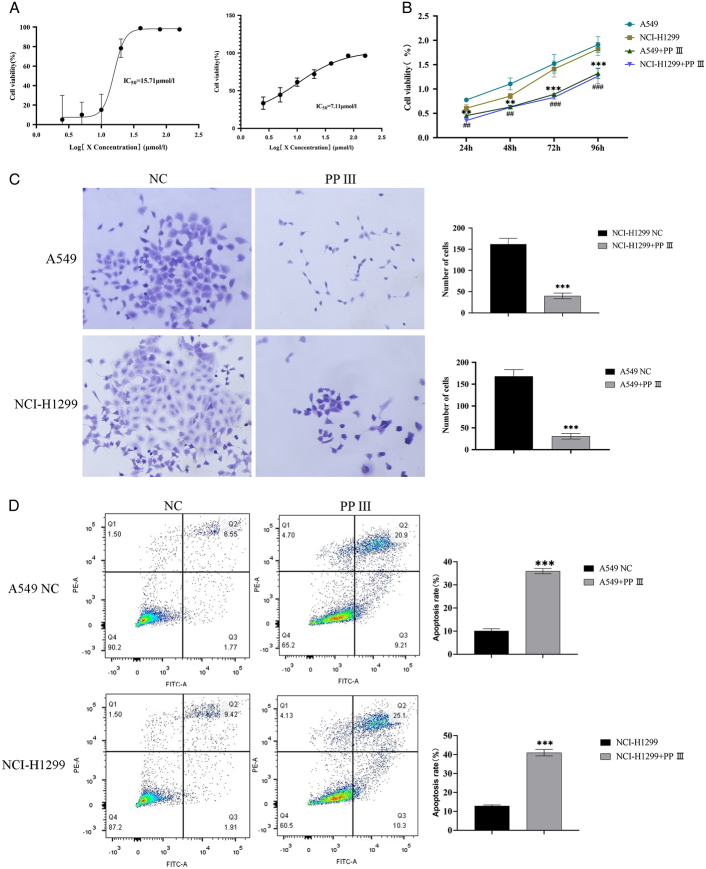
To determine the appropriate PP Ⅲ treatment concentration, we first cultured lung cancer cells A549 and NCI-H1299 treated with PP Ⅲ concentrations of 0, 2.5, 5, 10, 20, 40, 80, and 160 μmol/l for 24 h. The IC50 of A549 and NCI-H1299 in each group was detected by CCK-8 cell activity assay. The IC50 of PP Ⅲ for A549 was 15.71 μmol/l, and that for NCI-H1299 was 7.11 μmol/l (Fig. 1A). We then selected 8 μmol/l PP Ⅲ and 3.5 μmol /l PP Ⅲ to treat A549 cells and NCI-H1299 cells, respectively. A549 and NCI-H1299 cells were treated with PP Ⅲ and continued to be cultured for 72 h. The proliferation and clone formation of A549 and NCI-H1299 cells in each group were detected by CCK-8 and clone formation assay, and the results revealed that the proliferation and clone formation ability of the two cells were significantly reduced after PP Ⅲ treatment (Fig. 1B, C). The cell viability after PP Ⅲ treatment of A549 cells (24 h: P=0.02; 48 h: P=0.03) and NCI-H1299 cells (24 h: P=0.02; 48 h: P=0.04) was lower than before treatment (72 h and 96 h, P all <0.001). The number of A549 cells was lower after PP Ⅲ treatment than before treatment (P<0.001), as well as NCI-H1299 cells (P<0.001). Flow cytometry showed that the apoptosis rates of A549 cells and NCI-1299 cells in control groups were about 9.8±3% and 13±1%, while the apoptosis rates of the two cancer cells treated with PP Ⅲ increased to about 35 and 40% (P all<0.001, Fig. 1D). These results suggested that PP Ⅲ can inhibit the proliferation of lung cancer cells and promote their apoptosis.
Figure 1.
The proliferation and apoptosis of lung cancer cells with PP Ⅲ treatment. A: Detection of IC50 for A549 and NIC-H1299 by CCK-8; B: Detection of proliferation activity of A549 and NIC-H1299 by CCK-8; C: The clone formation of A549 and NIC-H1299; D: The apoptosis rates of A549 and NIC-H1299 with PP Ⅲ treatment.
PP Ⅲ treatment inhibited the migration, invasion, and EMT of lung cancer cells
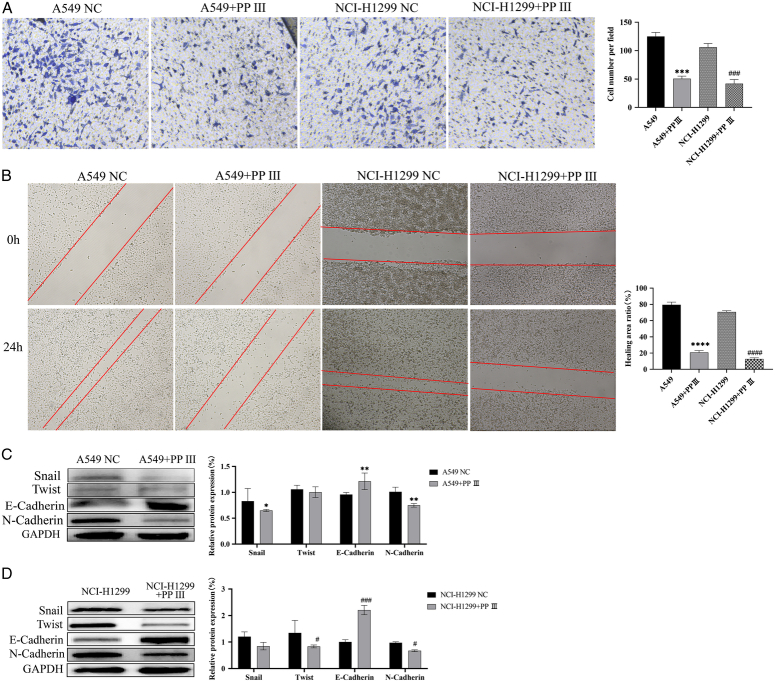
Transwell assay showed a decrease in the number of A549 and NIC-H1299 cells that invaded the lower chamber after PP Ⅲ treatment (P<0.001, Fig. 2A). At the same time, the scratch test also showed that the scratch healing area after PP Ⅲ treatment of the two cancer cells was much smaller than that of the control group (P<0.001, Fig. 2B). Considering that plenty of studies of cancer development have confirmed the presence of EMT in the pathological process related to tumor progression, we tested the expression of EMT-related proteins (E-cadherin, N-cadherin, Snail, and TWIST1) in this study. The expression of E-cadherin in two lung cancer cells was significantly increased after PP Ⅲ treatment, and the effect was more obvious in NCI-H1299 cells (P<0.001) than in A549 cells (P=0.008). The expression of N-cadherin decreased significantly, and the difference was more obvious before and after treatment in the A459 cell line (P=0.002) than in NCI-H1299 cells (P=0.04). Snail protein expression decreased significantly in the A549 cell line (P=0.04), but not significantly in the NCI-H1299 cell line (P=0.06). The expression of TWIST1 protein decreased significantly in the NCI-H1299 cell line (P=0.02), but not in the A549 cell line (P=0.50). More details can be found in Figure 2C, D. According to the results of the Western blot, it can be inferred that the inhibition of malignant behavior of lung cancer cells by PP Ⅲ may occur through the inhibition of EMT of lung cancer cells.
Figure 2.
PP Ⅲ inhibited the malignant activity of A549 and NIC-H1299 cells. A: PP Ⅲ inhibited the invasion of lung cancer cells; B: The migration ability of lung cancer cells decreased; C-D: Related proteins expression of A549 and NIC-H1299 cells. (compared with A549: *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; compared with NIC-H1299: # P<0.05, ## P<0.01, ### P<0.001, #### P<0.0001).
PP Ⅲ inhibited EMT of lung cancer cells through the GSK-3β/β-catenin signaling pathway
Since GSK-3β/β-catenin is a key signaling pathway promoting EMT, the expression of GSK-3β and β-catenin were observed by Western blot after PP Ⅲ treatment. Studies have shown that Bax induces apoptosis34,35. Moreover, the upregulation of Bcl-2 promotes cell survival36. As a result, the increase of Bax expression was statistically significant, while the Bcl-2 expression decreased, which promoted the apoptosis of cancer cells. In addition, the increased expression of GSK-3β was statistically significant, and the expression of β-catenin was significantly decreased, suggesting that PP Ⅲ treatment inhibited the occurrence of EMT in cancer cells. The results in A549 cells (P all<0.001, Fig. 3A) were the same as NCI-H1299 cells (P all<0.001, Fig. 3B). The expression of EMT-related protein E-cadherin was detected by immunofluorescence (Fig. 3C). After treatment with PP Ⅲ+GSK-3β inhibitors, the expression of E-cadherin decreased in both A549 and NCI-H1299 cells. These results suggested that PP Ⅲ mediated EMT in lung cancer cells through the GSK-3β/β-catenin pathway.
Figure 3.
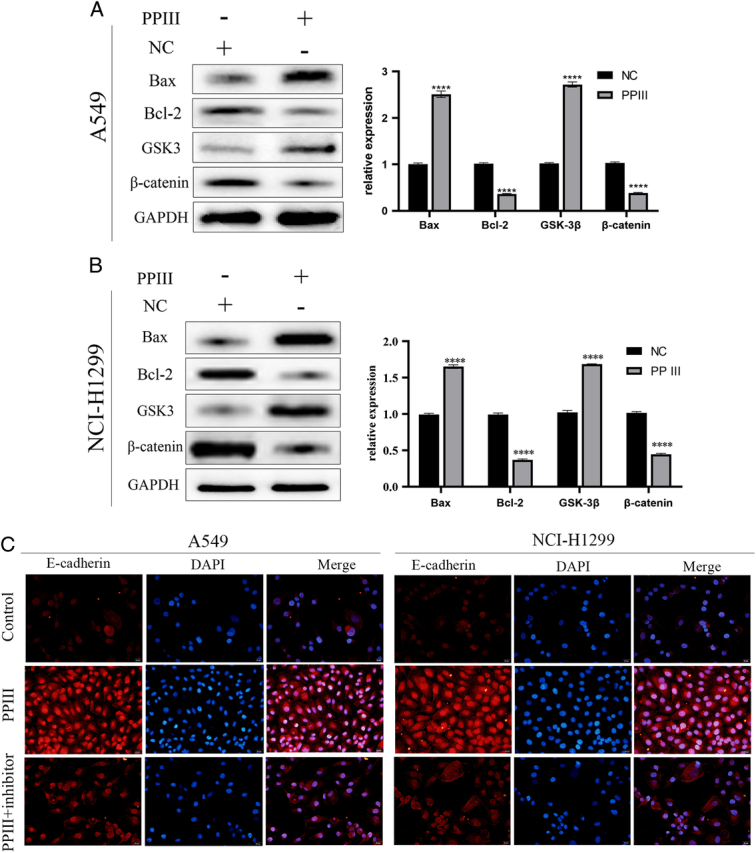
PP Ⅲ inhibited EMT of lung cancer cells through GSKβ/βcatenin pathway. A-B: The expressions of GSKβ, βcatenin, Bax and Bcl-2 in A549 and NCI-H1299 cells; C: E-Cadherin was tested by immunofluorescence assay. (compared with control group: *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
PP Ⅲ inhibited EMT of lung cancer cells through GSK-3β/β-catenin pathway on animal model
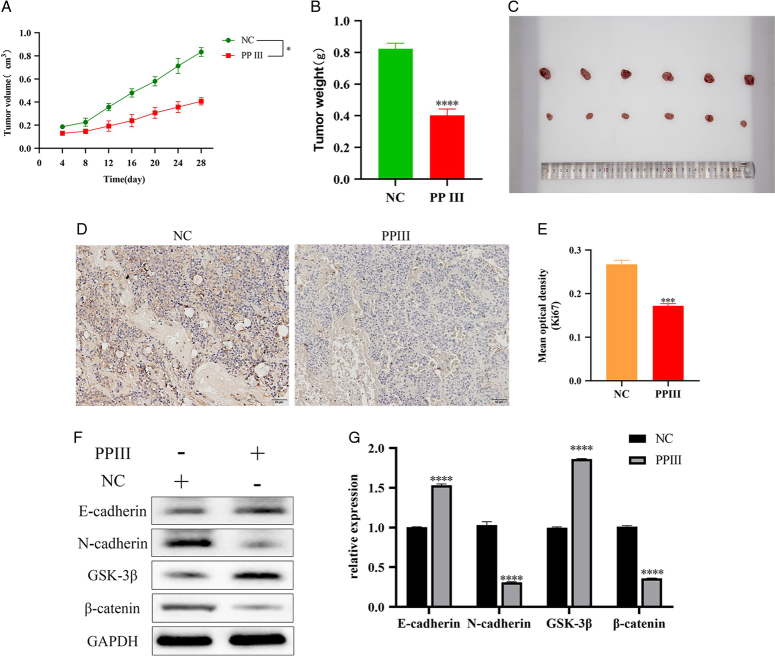
The tumor-bearing mice received intraperitoneal injections of normal saline or PP Ⅲ treatment (10 mg/kg) for 15 days. As shown in Figure 4A–C, the tumor volume (P=0.04) and weight (P<0.001) of nude mice were significantly reduced after PP Ⅲ treatment. Immunohistochemical results showed that the expression of tumor marker Ki67 was significantly decreased after PP Ⅲ treatment (P<0.001, Fig. 4D, E). Western blot results indicated that the expression levels of E-cadherin and GSK-3β increased significantly after treatment with PP Ⅲ (P all<0.001). Moreover, the expression levels of N-cadherin and β-catenin decreased significantly (P all<0.001). More details can be found in Figure 4F, G. The results of the animal experiments were consistent with the experimental results at the cell level. These results all indicated that PP III is effective in the treatment of lung cancer by influencing the EMT of lung cancer cells.
Figure 4.
PP Ⅲ inhibited the growth of transplanted tumors in a mouse model. A-B: Growth of transplanted tumor volume and weight in mice; C: Pictures of A549 cell transplantation tumor in each group; D-E: Immunohistochemical staining detection of transplanted tumor markers Ki67; F-G: Expression levels of E-cadherin, N-cadherin, GSK-3β and β-catenin. (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Discussion
There are high incidences and fatality rates of lung cancer around the world, which brings adverse consequences for a lot of patients. Most patients diagnosed with lung cancer already have extensive metastasis of cancer cells, and lose the best opportunity for surgery. Common causes of lung cancer include active and passive smoking, air pollution, oil fumes, occupational hazards, a history of lung disease, and nutritional status37. The emerging treatment strategies such as surgery, radiotherapy, chemotherapy, targeted therapy, and conservative therapy have improved the situation of lung cancer to some extent, but there are still problems such as high cost, great side effects, and incomplete treatment.
The prevention and treatment of diseases in TCM are based on the principles of syndrome differentiation, equilibrium between Yin and Yang, and organs and meridians, combined with modern and contemporary progressive medical technology. TCM believes that the pathogenesis of lung cancer is probably summed up as the accumulation of lung cancer precipitating factors and injuries related to treatment, which are transformed into Qi deficiency, Yin deficiency, Yang deficiency, phlegm, stasis, and fire, and lead to body resistance weakened while pathogenic factors prevailing, and systemic deficiency symptoms occur in the lung38. He et al. learned that the vulnerable lung is severely damaged, and Yang is excessive and leads to dryness-heat in patients with lung cancer after numerous surgeries, chemoradiation, blood loss, and respiratory disorders39. The treatment of lung cancer needs invigorating Qi, nourishing Yin, warming Yang and Fuzheng, clearing heat and detoxification, blood activating and stasis removal, etc. Some TCM materials such as Baizhu, Astragalus membranaceus, Shiitake mushroom, and Andrographis paniculata have a targeted inhibition effect on lung cancer38. Jiang et al.40 used invigorating Qi and nourishing Yin, clearing heat and detoxification, removing phlegm and blood stasis to treat cases of EGFR-TKIs drug-resistant lung cancer, treating both symptoms and root causes, and effectively alleviating patients’ conditions. Zhao et al.41 used TCM prescription combined with docetaxel for the targeted treatment of NSCLC and found that the combined therapy could reduce the level of serum tumor markers, with an effective rate of over 65%, which was significantly better than the use of docetaxel alone.
P. polyphylla var. yunnanensis is a kind of traditional natural Chinese medicine used for clearing heat and detoxifying for thousands of years, and its antitumor potential makes it a new research object in the treatment of tumors in recent years. It has been found that P. polyphylla var. yunnanensis and its extracts act on ROS/NF-κB/NLRP3/GSDMD, PI3K/Akt/mTOR, TWIST1/VE-cadherin and JNK signaling pathways to inhibit the progression of cancer cells, promote apoptosis and autophagy and cell cycle arrest16,42,43. In this study, we explored the effects of PP Ⅲ on lung cancer cells A549 and NCI-H1299. It has been proved that PP Ⅲ could inhibit the proliferation and promote apoptosis of lung cancer cells. At the same time, the invasion and migration ability of lung cancer cells was inhibited by PP Ⅲ treatment. These results confirmed that PP III has the potential to alleviate lung cancer.
In addition, the EMT that tumor cells rely on for metastasis may also be a cause of increased tumor drug resistance. According to the relationship between EMT and cancer metastasis, we interpreted that PP Ⅲ could affect the EMT of lung cancer cells. After PP Ⅲ treatment in lung cancer cells, the detection results of EMT-related marker proteins were consistent with expectations. The expression of tumor suppressor E-cadherin in lung cancer cells was significantly increased in the PP Ⅲ treatment group, and the expression of tumor invasion-promoting factor N-cadherin was significantly decreased. Meanwhile, the expressions of cancer-promoting proteins Snail and TWIST were also decreased, but the reduction of TWIST expression was only significant in the NCI-H1299 cell line. Our results were consistent with most studies, which indicated that down-regulation of E-cadherin expression marks the occurrence of EMT. In our study, the results illustrated that the tumor volume of mice with lung cancer decreased significantly after treatment. However, a small number of researchers believe that there is no causal or necessary relationship between E-cadherin and EMT44, and there are even conflicting results. For example, the evidence.18 showed that low expression of E-cadherin in canine breast cancer is a sign of a good prognosis. The result contradicts a large number of other results, which suggest low expression of E-cadherin is associated with poor prognosis in cancer45–47, hence research in this area should be carried out more comprehensively.
However, the clinical application of PP Ⅲ in the treatment of lung cancer still needs to be verified by clinical trials, and there are few studies on the toxic and side effects of PP Ⅲ in the antitumor effect. Therefore, how to safely use PP Ⅲ in the treatment of lung cancer will be the focus of future research.
Conclusions
In conclusion, we found that PP Ⅲ has the capacity for anti-lung cancer, and PP Ⅲ inhibits the invasion and migration of lung cancer cells by regulating EMT function via GSK-3β/β-catenin pathway. Therefore, PP Ⅲ is expected to be applied in the treatment of lung cancer.
Ethical statement
The study, which included animal experiments, was approved by the Animal Experiment Ethics Committee of Kunming Medical University (No. kmmu20230373).
Consent
The present study followed international, national and institutional guidelines for humane animal treatment and complied with relevant legislation. The study did not involve patients or volunteers.
Sources of funding
This study was supported by the Regulation of adenylate cyclase in macrophages with chronic obstructive pulmonary disease and its molecular mechanism (#2019J1233) and Effect and mechanism of Polyphyllin on PI3K regulation of autophagy microenvironment in lung cancer (#202101AY070001-089).
Author contribution
Q.L.: conception and design; Z.L.: administrative support; J.Y.: collection and assembly of data; Q.L.: data analysis and interpretation. All authors contributed in provision of study materials or patients, manuscript writing, and final approval of manuscript.
Conflicts of interest disclosure
The authors declare that there is no conflict of interest.
Research registration unique identifying number (UIN)
Name of the registry: not applicable.
Unique identifying number or registration ID: not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): not applicable.
Guarantor
Correspondence: *Zhuang Luo, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Kunming Medical University, No. 295 Xichang Road, Xishan District, Kunming 650032, People’s Republic of China. e-mail: skyny4511@126.com, Tel: 13888671493; Jiao Yang, e-mail: yangjiaokmu@163.com
Data availability statement
The data in this study are available with permission from the corresponding author.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
This study was supported by the Regulation of adenylate cyclase in macrophages with chronic obstructive pulmonary disease and its molecular mechanism (#2019J1233) and Effect and mechanism of Polyphyllin on PI3K regulation of autophagy microenvironment in lung cancer (#202101AY070001-089).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 15 January 2024
Contributor Information
Qian Liu, Email: 309679740@qq.com.
Zhuang Luo, Email: luozhuangyn@outlook.com.
Jiao Yang, Email: yangjiaokmu@163.com.
References
- 1.Suwinski R, Giglok M, Galwas-Kliber K, et al. Blood serum proteins as biomarkers for prediction of survival, locoregional control and distant metastasis rate in radiotherapy and radio-chemotherapy for non-small cell lung cancer. BMC Cancer 2019;19:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonna S, Subramaniam DS. Molecular diagnostics and targeted therapies in non-small cell lung cancer (NSCLC): an update. Discov Med 2019;27:167–170. [PubMed] [Google Scholar]
- 3.Kumar M, Sarkar A. Current therapeutic strategies and challenges in nsclc treatment: a comprehensive review. Exp Oncol 2022;44:7–16. [DOI] [PubMed] [Google Scholar]
- 4.Shen M, Lu C, Gao J. Prognostic influence of PD-1/PD-L1 suppressors in combination with chemotherapeutic agents for non-small cell pulmonary carcinoma: system review and meta-analysis. Front Oncol 2023;13:1137913. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Chen H, Zheng M, Zhang W, et al. Research status of mouse models for Non-Small-Cell Lung Cancer (NSCLC) and antitumor therapy of Traditional Chinese Medicine (TCM) in Mouse Models. Evid Based Complement Alternat Med 2022;2022:6404853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan PC, Li CJ, Zhang Z, et al. Neobacillus paridis sp. nov., an endophyte of Paris polyphylla Smith var. yunnanensis. Arch Microbiol 2022;204:129. [DOI] [PubMed] [Google Scholar]
- 7.Qin XJ, Ni W, Chen CX, et al. Seeing the light: shifting from wild rhizomes to extraction of active ingredients from above-ground parts of Paris polyphylla var. yunnanensis. J Ethnopharmacol 2018;224:134–139. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YY, Jin L, Wu YY, et al. [Chemical constituents of steroidal saponins in rhizome of Paris polyphylla var. yunnanensis cultured in vitro]. China J Chinese Materia Medica 2021;46:4936–4944. [DOI] [PubMed] [Google Scholar]
- 9.Guan LJ, Ding LS, Li YM, et al. A new homo-aro-cholestane glycoside from the rhizome of Paris polyphylla var. chinensis. J Asian Nat Prod Res 2021;23:1107–1114. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Zhou X, Zhao Y, et al. Polyphyllin I ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages through the NF-κB pathway. Front Immunol 2018;9:2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thapa CB, Paudel MR, Bhattarai HD, et al. Bioactive secondary metabolites in Paris polyphylla Sm. and their biological activities: a review. Heliyon 2022;8:e08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao L, Qiuhong L, Fuqiang Y, et al. Plant growth-promoting activities of bacterial endophytes isolated from the medicinal plant Pairs polyphylla var. yunnanensis. World J Microbiol Biotechnol 2021;38:15. [DOI] [PubMed] [Google Scholar]
- 13.Su F, Ye L, Zhou Z, et al. Study of chemical compositions and anticancer effects of Paris polyphylla var. Chinensis leaves. Molecules (Basel, Switzerland) 2022;27:2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J, Yu S, Guo C, et al. Polyphyllin I induces autophagy and cell cycle arrest via inhibiting PDK1/Akt/mTOR signal and downregulating cyclin B1 in human gastric carcinoma HGC-27 cells. Biomed Pharmacother 2019;117:109189. [DOI] [PubMed] [Google Scholar]
- 15.Niu W, Xu L, Li J, et al. Polyphyllin II inhibits human bladder cancer migration and invasion by regulating EMT-associated factors and MMPs. Oncol Lett 2020;20:2928–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao Y, Xin M, Xu J, et al. Polyphyllin II induced apoptosis of NSCLC cells by inhibiting autophagy through the mTOR pathway. Pharmaceutical Biol 2022;60:1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Man S, Lv P, Cui J, et al. Paris saponin II-induced paraptosis-associated cell death increased the sensitivity of cisplatin. Toxicol Appl Pharmacol 2020;406:115206. [DOI] [PubMed] [Google Scholar]
- 18.Kaszak I, Witkowska-Piłaszewicz O, Niewiadomska Z, et al. Role of cadherins in cancer-a review. Int J Mol Sci 2020;21:7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gloushankova NA, Zhitnyak IY, Rubtsova SN. Role of epithelial-mesenchymal transition in tumor progression. Biochemistry 2018;83:1469–1476. [DOI] [PubMed] [Google Scholar]
- 20.Rosso M, Majem B, Devis L, et al. E-cadherin: a determinant molecule associated with ovarian cancer progression, dissemination and aggressiveness. PLoS One 2017;12:e0184439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piprek RP, Kloc M, Mizia P, et al. The central role of cadherins in gonad development, reproduction, and fertility. Int J Mol Sci 2020;21:8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin Q, Li X, Liang X, et al. Targeting the EMT transcription factor Snail overcomes resistance to osimertinib in EGFR-mutant non-small cell lung cancer. Thorac Cancer 2021;12:1708–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Shi J, Chai K, et al. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets 2013;13:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui J, Ren P, Li Y, et al. ESRP1 as a prognostic factor of non-small-cell lung cancer is related to the EMT transcription factor of Twist. Thorac Cancer 2021;12:2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Hu B, Qin L, et al. SRC-1 and Twist1 expression positively correlates with a poor prognosis in human breast cancer. Int J Biol Sci 2014;10:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh BY, Kim SY, Lee YS, et al. Twist1-induced epithelial-mesenchymal transition according to microsatellite instability status in colon cancer cells. Oncotarget 2016;7:57066–57076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan WX, Wang XX, Zheng DH, et al. Muscone promotes the adipogenic differentiation of human gingival mesenchymal stem cells by inhibiting the Wnt/β-Catenin signaling pathway. Drug Des Dev Ther 2019;13:3291–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jian H, Shen X, Liu I, et al. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev 2006;20:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13:97–110. [DOI] [PubMed] [Google Scholar]
- 30.Chen B, Song Y, Zhan Y, et al. Fangchinoline inhibits non-small cell lung cancer metastasis by reversing epithelial-mesenchymal transition and suppressing the cytosolic ROS-related Akt-mTOR signaling pathway. Cancer Lett 2022;543:215783. [DOI] [PubMed] [Google Scholar]
- 31.Hong W, Xue M, Jiang J, et al. Circular RNA circ-CPA4/ let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug resistance and immune evasion in non-small cell lung cancer (NSCLC). J Experiment Clin Cancer Res 2020;39:149. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Liu Z, Sun Z, Zhang D, et al. Paris polyphylla ethanol extract induces G2/M arrest and suppresses migration and invasion in bladder cancer. Translational Cancer Res 2020;9:5994–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen K, WuWong DJ, Wong S, et al. Pharmacological inhibition of Bax-induced cell death: Bax-inhibiting peptides and small compounds inhibiting Bax. Experiment Biol Med 2019;244:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spitz AZ, Gavathiotis E. Physiological and pharmacological modulation of BAX. Trends Pharmacol Sci 2022;43:206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin L, Chen Y, Cheng D, et al. YAP inhibits autophagy and promotes progression of colorectal cancer via upregulating Bcl-2 expression. Cell Death Dis 2021;12:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med Mar 2020;41:1–24. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Feiyue Z, Gaofeng L. Traditional Chinese medicine and lung cancer--From theory to practice. Biomed Pharmacother 2021;137:111381. [DOI] [PubMed] [Google Scholar]
- 39.Su XL, Wang JW, Che H, et al. Clinical application and mechanism of traditional Chinese medicine in treatment of lung cancer. Chin Med J 2020;133:2987–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang R, Mo C, Man T, et al. An analysis on the experience of Professor Rong Yuanming, a famous Chinese medicine doctor, in treating EGFR-TKIs resistance of lung cancer with nourishing Qi and Yin method. LISHIZHEN Med Mater Med Res 2020;31:2265–2268. [Google Scholar]
- 41.Zhao Y. Effect of Yiqi Yangyin Xiaoai Decoction combined with docetaxel on non-small cell lung cancer after molecular targeted therapy failure and its influence on serum tumor markers. Heilongjiang J Tradit Chin Med 2019;48:81–82. [Google Scholar]
- 42.Yuan YL, Jiang N, Li ZY, et al. Polyphyllin VI induces apoptosis and autophagy in human osteosarcoma cells by modulation of ROS/JNK activation. Drug Des Dev Ther 2019;13:3091–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang D, Liu S, Liu Z, et al. Polyphyllin I induces cell cycle arrest in prostate cancer cells via the upregulation of IL6 and P21 expression. Medicine 2019;98:e17743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brabletz S, Schuhwerk H, Brabletz T, et al. Dynamic EMT: a multi-tool for tumor progression. EMBO J 2021;40:e108647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang K, Jiang L, Sun Y, et al. Effect of E-cadherin on prognosis of colorectal cancer: a meta-analysis update. Mol Diagn Ther 2022;26:397–409. [DOI] [PubMed] [Google Scholar]
- 46.Wong SHM, Fang CM, Chuah LH, et al. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol 2018;121:11–22. [DOI] [PubMed] [Google Scholar]
- 47.Luo SL, Xie YG, Li Z, et al. E-cadherin expression and prognosis of oral cancer: a meta-analysis. Tumour Biol 2014;35:5533–5537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study are available with permission from the corresponding author.