Abstract
Cationic Au(I)─NHC (NHC = N-heterocyclic carbene) complexes have become an important class of catalysts for alkyne π-activation reactions in organic synthesis. In particular, these complexes are characterized by high stability of catalytic species engendered by strong σ-donation and metal backbonding. Herein, we report the synthesis and characterization of well-defined [Au(NHC)Cl] complexes featuring recently discovered IPr# family of ligands that hinge upon modular peralkylation of aniline. These ligands have been commercialized in collaboration with MilliporeSigma (IPr#: 915653; Np#: 915912; BIAN-IPr#: 916420). Evaluation of the [Au(NHC)Cl] complexes in a series of Au(I)─NHC-catalyzed π-functionalizations of alkynes, such as hydrocarboxylation, hydroamination and hydration, resulted in the identification of wingtip-flexible [Au(Np#)Cl] as a highly reactive and broadly applicable catalyst with the re-activity outperforming the classical [Au(IPr)Cl] and [Au(IPr*)Cl] complexes. The utility of this catalyst has been demonstrated in the direct late-stage derivatization of complex pharmaceuticals. Structural and computational studies were conducted to determine steric effects, frontier molecular orbitals and bond orders of this class of catalysts. Considering the attractive features of well-defined Au(I)─NHC complexes, we anticipate that this class of bulky and wingtip-flexible Au(I)─NHCs based on the modular peralkylated naphthylamine scaffold will find broad application in π-functionalization of alkynes in various areas of organic synthesis and catalysis.
Graphical Abstract

Introduction
N-heterocyclic carbenes (NHCs) are a powerful class of ancillary ligands to transition metals in organic synthesis and catalysis.1-3 This important class of ligands is characterized by a discrete structural topology featuring steric impact of the N-wingtips towards the metal centre, which permits for a tight protection of the catalytic species.4,5 In terms of electronic properties, strong σ-donation in combination with adjustable π-acceptance and π-donation render NHCs a unique class of ligands in organic synthesis.6 Most notably, NHC ligands favor strong bonding of the metal-ligand bond and accommodate coordination of various metals at different oxidation states.7 The utilization of wingtip-flexible steric effects of NHC ligands has been a major driving force in the development of novel efficient catalysts for activation reactions.1-5
Gold─NHC complexes have emerged as an increasingly important class of catalysts in organic synthesis.8 In particular, cationic Au(I)─NHC complexes have garnered remarkable attention for their superb catalytic reactivity outperforming phosphine-based catalysts.9 In this context, alkyne π-functionalization reactions represent one of the most important classes of processes in academic and industrial settings, enabling the synthesis of a broad range of valuable products from feedstock alkynes.10 The successful implementation of Au(I)─NHC catalysis benefits from a strong σ-donation and metal to ligand backbonding.11 These bonding interactions favor catalyst stability under the reaction conditions required for alkyne activation.12 Another challenge is the tendency of catalysts to form gold nanoparticles, which has been successfully addressed by modifying anionic ligands in Au(I) catalysis.13,14
Steric and structural alteration of NHC ligands have been unexplored in alkyne π-functionalization reactions catalyzed by Au(I)─NHCs.8,9 Notably, the vast majority of studies are currently limited to classical imidazol-2-ylidene ligands, such as ItBu, IMes, IPr and its bulky analogue IPr* under the proviso that less sterically-demanding ItBu and IMes ligands are typically much less reactive than the bulkier IPr and IPr*. Furthermore, effects of non-symmetrical wingtip substitution and steric flexibility of NHC ligands remain unexplored, despite the potential of wingtip modification to improve the efficiency of this class of processes using Au(I)─NHC catalysis.15,16
Recently, our laboratory introduced a new class of IPr# ligands that hinge upon modular peralkylation of aniline.17 The catalysts have been commercialized in collaboration with MilliporeSigma to enable broad access of academic and industrial researchers for reaction screening and optimization (MilliporeSigma: IPr#, 915653; Np#, 915912; BIAN-IPr#, 916420).18 This IPr# imidazol-2-ylidene template exploits bulky and wingtip-flexible NHC ligands to access previously unexplored space of NHC complexes. IPr# ligands have been demonstrated to show high reactivity in Pd and Ni-catalyzed cross-coupling for C─X, C─N, C─O, C─S and C─H bond activation as [Pd(NHC)(cin)Cl] and [Ni(NHC)(η5-Cp)Cl] complexes.17-19
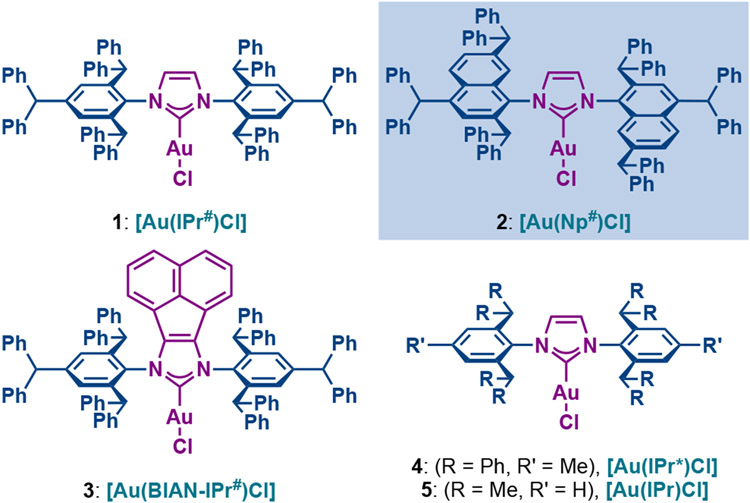
Herein, as a part of our program in ligand design and catalyst,20 we report the synthesis of well-defined [Au(NHC)Cl] complexes from IPr# family of ligands (1–3) and their application in a series of alkyne π-activation reactions, such as hydrocarboxylation,21 hydroamination22 and hydration (Fig. 1).23 Most importantly, we identified [Au(Np#)Cl] (2) as a highly reactive and broadly applicable catalyst for alkyne π-functionalization with the reactivity outperforming the classical [Au(IPr*)Cl] (4) and [Au(IPr)Cl] (5) catalysts. Structural and electronic characterization as well as application to the late-stage functionalization of complex pharmaceuticals is described. Considering the attractive features of well-defined Au(I)─NHC complexes, we anticipate that this class of bulky and wingtip-flexible Au(I)─NHCs based on the modular peralkylated naphthylamine scaffold will find broad application in π-functionalization of alkynes in various areas of organic synthesis and catalysis.
Fig. 1.
Structures of well-defined and air-stable [Au(NHC)Cl] complexes.
Results and Discussion
Research Design.
For this study, we selected well-defined [Au(NHC)Cl] complexes from IPr# family of ligands (1–3) as well as classical imidazol-2-ylidene based NHC ligands, IPr* (4) and IPr (5) (Fig. 1). The family of IPr# ligands consist of the parent IPr# ligand (1), the flexible Np# ligand (2) and acenaphthene-modified BIAN-IPr# ligand (3). The model IPr* (4) and IPr (5) ligands were selected for comparison of catalytic performance. Previous studies showed that other widely used imidazol-2-ylidene catalysts, such as ItBu and IMes, are significantly less reactive in Au(I)─NHC-catalyzed alkyne π-functionalizations.14
Catalyst Synthesis.
Our studies commenced with the synthesis of well-defined Au(I)─NHC complexes, [Au(IPr#)Cl] (1), [Au(Np#)Cl] (2) and [Au(BIAN-IPr#)Cl] (3) (see SI). Complexes (1–3) were synthesized in 75-90% yields by the method established by Nolan using NHC·HCl and AuCl·Me2S in the presence of K2CO3 in acetone or THF at 70 °C.24,25 Well-defined [Au(IPr*)Cl] (4) and [Au(IPr)Cl] (5) complexes were synthesized by the same method. All complexes were isolated by trituration with cold ether and found to be stable to air and moisture.
Crystallographic Studies.
The model complex [Au(Np#)Cl] (2) was fully characterized by X-ray crystallographic analysis (Fig. 2). The crystals were obtained by slow evaporation of dichloromethane. [Au(Np#)Cl] (2) crystalized as C2-symmetric (rac) anti conformation. Complex (2) features a linear geometry (Fig. 2A). The torsional angle of [Au(Np#)Cl] (2) (C─Au─Cl, 174.31°) is in the range of the previously reported [Au(IPr#)Cl] (1) (C─Au─Cl, 180.00°) and [Au(IPr*)Cl] (4) (C─Au─Cl, 178.35°) complexes.14,17 The metal-ligand bond of [Au(Np#)Cl] (2) (Au─C, 1.983 Å; Au─Cl, 2.282 Å) can be compared with [Au(NHC)Cl] complexes ([Au(IPr#)Cl] (1), Au─C, 1.972 Å; Au─Cl, 2.277 Å and [Au(IPr*)Cl] (4), Au─C, 1.987 Å; Au─Cl, 2.273 Å).14,17 The non-symmetrical Np# ligand dis-plays an anti-conformation of the wingtips with respect to the metal centre (vide infra).
Fig. 2.
(A) X-ray crystal structure of complex [Au(Np#)Cl] (2) (50% ellip-soids). Selected bond lengths [Å] and bond angles [°]: Au1─Cl1, 2.2823(7); Au1─C1, 1.983(2); C1─N1, 1.358(3); C1─N2, 1.353(2); N1─C4, 1.443(2); N2─C54, 1.440(3); Cl1─Au1─C1, 174.31(6); Au1─C1─N1, 125.3(1); Au1─C1─N2, 129.5(1); Au1─C1─N1─C4, 11.4(3); Au1─C1─N2─C54, 19.0(3). (B) Topographical steric map of [Au(Np#)Cl] (2) showing %Vbur per quadrant. Anti C2-symmetric (rac) conformation. Hydrogen atoms have been omitted for clarity. See, SI. CCDC 2260430.
To further evaluate the steric impact of Np# ligand on Au-centre, the percent buried volume (%Vbur) was determined using the method by Cavallo (Fig. 2B).5b The %Vbur of [Au(Np#)Cl] (2) is 46.2% with quadrant distribution of SW, 32.8%; NW, 36.7%; NE, 53.7%; SE, 28.3%. These values can be compared with [Au(IPr#)Cl] (1), (%Vbur = 54.4%; SW, 43.0%; NW, 65.9%; NE, 43.9%; SE, 65.9%) and [Au(IPr*)Cl] (4) (%Vbur = 50.4%; SW, 39.8%; NW, 47.2%; NE, 58.3%; SE, 53.0%).14,17 The topographical map of [Au(Np#)Cl] highlights the non-symmetrical steric impact on the metal centre compared with symmetrical IPr# and IPr* ligands (vide infra).
Catalyst Evaluation.
With access to [Au(NHC)Cl] (1–5) complexes, we next evaluated their catalytic activity in alkyne functionalization reactions. The goal was to explore the catalytic performance of complexes (1–5) in Au(I)─NHC catalysed hydrocarboxylation,21 hydroamina-tion22 and hydration23 reactions of alkynes as modular and broadly useful reactions for C─O and C─N bond functionalization.
Hydrocarboxylation.
First, we evaluated catalytic performance of [Au(NHC)Cl] complexes in hydrocarboxylation of alkynes (Scheme 1).21 This reaction was selected as a model reaction due to the ubiquitous presence of carboxylic acids in medicinal chemistry research and the potential of this platform in late-stage modification of pharmaceuticals. Hydrocarboxylation is an atom economic method to directly access functionalized enol esters as valuable synthetic products and important building blocks for polymers synthesis.26 We conducted studies at 2 mol% catalyst loading (Scheme 1 and 2), which revealed that [Au(Np#)Cl] (2) is a superior catalyst to other catalysts tested. Furthermore, we found that the reactivity of [Au(IPr#)Cl] (1) is comparable to [Au(IPr*)Cl] (4), albeit [Au(IPr*)Cl] (4) was the second most reactive catalyst indicating that excessive steric hindrance inhibits this reaction. Moreover, [Au(IPr)Cl] (5) was the least reactive in the series, while the BIAN-based [Au(BIAN-IPr#)Cl] (3) was less reactive than its imidazol-2-ylidene counterpart, further confirming that a delicate balance of steric is required in this π-functionalization. NaBArF4 was used at twice the amount as the gold catalyst. The reaction at 1:1 ratio gave lower yields. Control experiments in the absence of NaBArF4 and in the absence of gold catalyst gave no conversion. The order of catalytic reactivity in the hydrocarboxylation reaction was established as follows: [Au(Np#)Cl] (2) > [Au(IPr*)Cl] (4) > [Au(IPr#)Cl] (1) > [Au(BIAN-IPr#)Cl] (3) > [Au(IPr)Cl] (1).
Scheme 1.
Hydrocarboxylation of Alkynes using [Au(NHC)Cl] Complexes. Conditions: 1-Octyne (6b) (1.0 equiv), benzoic acid (7a) (1.2 equiv), [Au(NHC)Cl] (1–5) (2.0 mol%), NaBArF4 (4.0 mol%), toluene (0.2 mL), 80 °C, 15 h.
Scheme 2.
Kinetic Profile of Hydrocarboxylation of 1-Octyne with Benzoic Acid using [Au(NHC)Cl]. Conditions: 1-Octyne (6b) (1.0 equiv), benzoic acid (7a) (1.2 equiv), [Au(NHC)Cl] (1–5) (2.0 mol%), NaBArF4 (4.0 mol%), toluene (0.2 mL), 80 °C. [Au(IPr#)Cl] (1); [Au(Np#)Cl] (2); [Au(BIAN-IPr#)Cl] (3); [Au(IPr*)Cl] (4); [Au(IPr)Cl] (5).
Substrate Scope.
Having identified [Au(Np#)Cl] (2) as a highly reactive catalyst for alkyne hydrocarboxylation, the scope was briefly investigated (Scheme 3). First, the substrate scope of hydrocarboxylation with structurally different alkynes was explored. We were pleased to find that [Au(Np#)Cl] (2) catalyst is applicable under the standard reaction conditions at 2.0 mol% catalyst loading across aliphatic terminal alkynes, including long chain (8a–8c), α-cyclic (8e) and α,β-unsaturated-α-cyclic (8f) alkynes. Thus, the catalyst is reactive for hydroelementation of aliphatic terminal alkynes. Pleasingly, this catalyst was also capable of delivering the addition product using internal alkynes (8d) (vide infra). Importantly, the catalyst is compatible with acid sensitive function groups, such as silyl ethers (8c) and α,β-unsaturated alkynes (8f). Next, the performance of [Au(Np#)Cl] (2) in hydrocarboxylation of structurally differentiated carboxylic acids was investigated. Gratifyingly, the catalyst [Au(Np#)Cl] (2) can promote hydrocarboxylation of a wide range of aromatic carboxylic acids, including electronically-neutral (8g), electron rich (8h) and electron-deficient (8i) as well as sterically-hindered (8j) carboxylic acids. Interestingly, the catalyst is compatible with aryl chlorides (8i) under the standard reaction conditions, which allows for further functionalization of the hydrocarboxylation products. Furthermore, aliphatic carboxylic acids (8k–8n) were tolerated under the standard reaction conditions. Challenging benzylic (8l) and α,β-unsaturated (8n) carboxylic acids were also successful substates. It should be noted that in general only the product and starting materials are present after the end of the reaction time. The isomers have not been detected.
Scheme 3.
Substrate Scope of Hydrocarboxylation of Alkynes Catalyzed by [Au(Np#)Cl]. Conditions: Alkyne (6) (1.0 equiv), carboxylic acid (7) (1.2 equiv), [Au(Np#)Cl] (2) (2.0 mol%), NaBArF4 (4.0 mol%), toluene (0.2 mL), 80 °C. Isolated yields.
Late-Stage Functionalization.
Prompted by the broad substrate scope of hydrocarboxylation accommodated by [Au(Np#)Cl] (2), we were intrigued to demonstrate the utility of this catalyst in the direct late-stage functionalization of pharmaceuticals (Scheme 3B). As shown, [Au(Np#)Cl] (2) was effective in promoting hydrocarboxylation of nonsteroidal anti-inflammatory drugs, Indomethacin (8o) and Sulindac (8p) as well as PTC-124 (8q), a novel drug for treatment of genetic disorders, and antihyperuricemic Febuxostat (8r). These experiments further demonstrate excellent functional group tolerance of [Au(Np#)Cl] (2) and its potential in pharmaceutical and medicinal chemistry settings.
Hydroamination.
Encouraged by the high reactivity of [Au(Np#)Cl] (2) in hydrocarboxylation, we next evaluated its catalytic efficiency in hydroamination of alkynes (Scheme 4 and 5).22 Hydroamination of alkynes represents an important class of atom economic C─N addition reactions to access functionalized imines as versatile intermediates in organic synthesis. The catalyst evaluation was performed using hydroamination between of phenylacetylene with aniline at 0.1 mol% catalyst loading (Table 4, entries 1-5). In the series of [Au(NHC)Cl] (1–5) complexes, the naphthyl-based [Au(Np#)Cl] (2) again showed the highest reactivity (entry 2). Similarly, hydroamination of 1-octyne with aniline was used as a model system for aliphatic alkynes and revealed [Au(Np#)Cl] (2) as the most reactive catalyst (see SI). Kinetic studies showed that [Au(Np#)Cl] (2) is a superior catalyst to other Au(I)─NHC complexes tested (Scheme 5). Interestingly, [Au(IPr#)Cl] (1) and [Au(IPr*)Cl] (4) showed comparable performance, while [Au(IPr)Cl] (5) was the least reactive under these conditions. The order of catalytic reactivity of Au(I)─NHC complexes in hydroamination of alkynes is as follows: [Au(Np#)Cl] (2) > [Au(IPr*)Cl] (4) ≈ [Au(IPr#)Cl] (1) > [Au(BIAN-IPr#)Cl] (3) > [Au(IPr)Cl] (5).
Scheme 4.
Hydrocarboxylation of Alkynes using [Au(NHC)Cl] Complexes. Conditions: Alkyne (6) (1.0 equiv), aniline (9a) (1.2 equiv), [Au(NHC)Cl] (1-5) (0.05 mol%), NaBArF4 (0.1 mol%), 50 °C, 15 h.
Scheme 5.
Kinetic Profile of Hydrocarboxylation of 1-Octyne with Benzoic Acid using [Au(NHC)Cl]. Conditions: Phenylacetylene (6g) (1.0 equiv), aniline (9a) (1.2 equiv), [Au(NHC)Cl] (1–5) (0.05 mol%), NaBArF4 (0.1 mol%), 50 °C. [Au(IPr#)Cl] (1); [Au(Np#)Cl] (2); [Au(BIAN-IPr#)Cl] (3); [Au(IPr*)Cl] (4); [Au(IPr)Cl] (5).
Next, the substrate scope of hydroamination of alkynes using [Au(Np#)Cl] (2) was evaluated (Scheme 6). This catalyst was found to be broadly applicable across a range of alkynes and aromatic amines. The imine products were directly subjected to reduction to facilitate purification. The scope of hydroamination of structurally different alkynes was first investigated. Pleasingly, the catalyst [Au(Np#)Cl] (2) was effective with a variety of aliphatic terminal alkynes (10a–10c), internal aliphatic alkynes (10d) and α-cyclic terminal alkynes (10e). It is worth noting that hydroamination of aromatic terminal alkynes proceeds at 0.05 mol% loading (10f–10g), attesting to the high reactivity of [Au(Np#)Cl]. Next, the performance of [Au(Np#)Cl] was investigated using structurally different aromatic amines. Gratifyingly, we found that [Au(Np#)Cl] (2) is highly efficient in promoting the hydroamination of electronically-neutral (10h), electron-rich (10i) and electron-deficient (10j–10l) as well as sterically-hindered (10m–10n) aromatic amines. Importantly, [Au(Np#)Cl] readily accommodates halogen substitution (Cl, Br) (10k–10l), which allows for further functionalization. Furthermore, polycyclic aromatic amines (10o) are also compatible substrates using [Au(Np#)Cl], delivering amination products which have found applications in agrochemicals and functional materials.
Scheme 6.
Substrate Scope of Hydrocarboxylation of Alkynes Catalyzed by [Au(Np#)Cl].a aConditions: Alkyne (6) (1.0 equiv), amines (9) (1.2 equiv), [Au(Np#)Cl] (2) (1.5 mol%), NaBArF4 (3.0 mol%), 50 °C, 15 h. b[Au(Np#)Cl] (2) (0.05 mol%). Isolated yields.
Hydration.
Furthermore, we tested the performance of [Au(Np#)Cl] as a catalyst in hydration of alkynes (Scheme 7 and 8).23 Au(I)-catalyzed hydration is one of the most important processes for the synthesis of ketones. Au(I)─NHC catalysts (1–5) were evaluated in the hydration of phenylacetylene at 80 °C. AgSbF6 was used following the previous protocol.14c Control reactions were performed in the absence of gold catalyst and resulted in no conversion. [Au(Np#)Cl] (2) performed as the best catalyst. Interestingly, the BIAN-based catalyst [Au(BIAN-IPr#)Cl] (3) was also highly reactive under these conditions. Sterically-hindered catalysts [Au(IPr#)Cl] (1) and [Au(IPr*)Cl] (4) showed comparable reactivity, while [Au(IPr)Cl] (5) was the least reactive. The order of catalytic activity of Au(I)─NHC complexes in hydration of alkynes is as follows: [Au(Np#)Cl] (2) > [Au(BIAN-IPr#)Cl] (3) > [Au(IPr#)Cl] (1) ≈ [Au(IPr*)Cl] (4) > [Au(IPr)Cl] (5).
Scheme 7.
Hydrocarboxylation of Alkynes using [Au(NHC)Cl] Complexes. Conditions: Phenylacetylene (6g) (1.0 equiv), [Au(NHC)Cl] (1–5) (50 ppm), AgSbF6, MeOH (1.0 mL), water (0.2 mL), 80 °C, 15 h.
Scheme 8.
Substrate Scope of Hydrocarboxylation of Alkynes Catalyzed by [Au(Np#)Cl].a aConditions: Alkyne (6) (1.0 equiv), [Au(Np#)Cl] (2) (50 ppm), AgSbF6, MeOH (1.0 mL), H2O (0.2 mL), 80 °C, 15 h. b[Au(Np#)Cl] (2) (0.1 mol%), 1,4-dioxane (1.0 mL), 120 °C. Isolated yields.
The substrate scope of hydration of alkynes using [Au(Np#)Cl] (2) was evaluated (Scheme 5). Pleasingly, we found that this catalyst is highly efficient at very low catalyst loading (50 ppm) for a variety of structurally different alkynes, including aliphatic terminal alkenes (11a–11b), aliphatic internal alkynes (11c), α-cyclic alkynes (11d) and α,β-unsaturated-α-cyclic (11e) alkynes. Furthermore, this catalyst is also capable of promoting hydration of aromatic terminal alkynes, including, electronically-neutral (11f–11g), chloro-functionalized (11h) and even the challenging internal alkynes (11i). Overall, the catalytic activity of [Au(Np#)Cl] supersedes other Au(I)─NHC catalysts reported to date for π-functionalization of alkynes.9,21-23
Limitations.
In a broader sense, the covered examples include diversity of alkynes (terminal and internal) and carboxylic acids (aliphatic and aromatic). There are several limitations of the present system. Specifically, hydrocarboxylation is not suitable for aromatic alkynes. Hydroamination is not suitable for aliphatic amines. Furthermore, the reactions are differentiated by the reaction temperature. Hydroamination is suitable at 50 °C, and hydrocarboxylation can be performed at 80 °C. This temperature difference originates from the fact that amines are more nucleophilic than carboxylic acids. The hydration is generally suitable at 80 °C, while only one example (diphenylacetylene) required 120 °C because it gave lower yield at 80 °C.
Computational Studies.
To gain insight into the reactivity of [Au(Np#)Cl] (2), steric and electronic properties were evaluated at the B3LYP 6-311++g(d,p)) level. [Au(IPr*)Cl] (4) and [Au(IPr)Cl] (5) complexes were evaluated for comparison. It should be noted that Np# ligand features non-symmetrical wingtips, affording C2-symmetric anti (rac) and Cs-symmetric syn (meso) atropisomers with respect to the metal center. Previous studies showed that anti-Np# free carbene is more stable than meso-Np# (ΔE = 0.56 kcal/mol, B3LYP 6-311++g(d,p)).
First, to eliminate effects from crystal packing, the percent buried volume (%Vbur) was calculated from the optimized structures of [Au(Np#)Cl] (2) (Fig. 3). The calculated %Vbur of anti-Np#, [Au(anti-Np#)Cl] (2), is 38.6% (SW, 35.6%; NW, 40.1%; NE, 42.2%; SE, 36.6%), and %Vbur of syn-Np#, [Au(syn-Np#)Cl] (2), is 39.8% (SW, 50.9%; NW, 31.2%; NE, 42.8%; SE, 34.4%). These values can be compared with the %Vbur of [Au(IPr*)Cl] (4) (%Vbur, 46.5%; SW, 38.8%; NW, 46.5%; NE, 51.6%; SE, 49.2%) and [Au(IPr)Cl] (5) (%Vbur, 40.3%; SW, 40.3%; NW, 40.3%; NE, 40.3%; SE, 40.3%). It should be noted that (1) the total steric impact of Np# is comparatively lower than that of IPr* and IPr, and (2) Np# permits for a non-symmetrical quadrant distribution, indicating significant steric flexibility of the Np# ligand on the Au center.
Fig. 3.
Topographical steric maps of [Au(Np#)Cl]; (A) anti; (B) syn; showing %Vbur per quadrant (B3LYP 6-311++g(d,p)). (C), (D) The corresponding C2-symmetric (rac) anti and Cs-symmetric (meso) syn conformations of [Au(Np#)Cl].
Next, to gain insight into the effect of Np# ligand on metal-ligand bonding properties, the HOMO and LUMO energy levels of [Au(Np#)Cl] (2) were calculated and compared with [Au(IPr*)Cl] (4) and [Au(IPr)Cl] (5) (Fig. 4). It should be noted that orbitals associated with metal-ligand bond are most indicative of catalyst activation and stability. The energy of HOMO, located within Au─Cl bond, for [Au(anti-Np#)Cl] (2) is −6.20 eV and for [Au(syn-Np#)Cl] (2) is −6.31 eV (HOMO-2). These values are in a similar range to HOMO of [Au(IPr*)Cl] (4) (−6.32 eV) and [Au(IPr)Cl] (−6.21 eV). In contrast, the LUMO, located within Au─C(carbene) bond, of [Au(anti-Np#)Cl] (2) is −1.17 eV (LUMO+2) and [Au(syn-Np#)Cl] (2) is −1.17 eV (LUMO+2), which can be compared with [Au(IPr*)Cl] (4) (−1.22 eV) and [Au(IPr)Cl] (5) (−1.04 eV). [Au(anti-Np#)Cl] (2) has the lowest energy gap, while [Au(IPr)Cl] (5) has the largest energy gap.
Fig. 4.
HOMO and LUMO energy levels of [Au(Np#)Cl] (2), [Au(IPr*)Cl] (4) and [Au(IPr)Cl] (5) at (B3LYP 6-311++g(d,p)).
To gain further insight into the relative strength of metal-ligand bond of the [Au(Np#)Cl] (2) complex, NBO analysis was performed (Table 1). The Wiberg bond orders of [Au(anti-Np#)Cl] (2) (Au─C(carbene), 0.6186; Au─Cl, 0.5048) and [Au(syn-Np#)Cl] (2) (Au─C(carbene), 0.6185; Au─Cl, 5042) can be compared with [Au(IPr*)Cl] (4) (Au─C(carbene), 0.6166; Au-Cl, 0.5153) and [Au(IPr)Cl] (5) (Au─C(carbene), 0.6287; Au─Cl, 0.5504). Thus, [Au(IPr)Cl] (5) features the strongest Au─C(carbene) bond, and Au(IPr*)Cl] (4) features the weakest Au─C(carbene) bond.
Table 1.
Bond Orders of [Au(NHC)Cl] Complexes
| entry | complex | Au─C(carbene) | Au─Cl |
|---|---|---|---|
| 1 | 2: [Au(Np#)Cl] (anti) | 0.6186 | 0.5048 |
| 2 | 2: [Au(Np#)Cl] (syn) | 0.6185 | 0.5042 |
| 3 | 4: [Au(IPr*)Cl] | 0.6166 | 0.5153 |
| 4 | 5: [Au(IPr)Cl] | 0.6287 | 0.5504 |
The structural and electronic characterization highlight [Au(Np#)Cl] (2) as a highly promising catalyst for alkyne π-functionalization. Sterically, [Au(Np#)Cl] (2) shows flexible and adjustable steric impact of Np# on the gold center. Electronically, [Au(Np#)Cl] (2) features a strong Au─C(carbene) bond.
Conclusions
In summary, we have identified wingtip-flexible [Au(Np#)Cl] as a highly reactive and broadly applicable Au(I)─NHC catalyst for alkyne π-functionalization with the reactivity outperforming classical [Au(IPr)Cl] and [Au(IPr*)Cl] complexes. The synthetic utility of [Au(Np#)Cl] was highlighted in the direct late-stage derivatization of complex pharmaceuticals. The broad reactivity was demonstrated in hydrocarboxylation, hydroamination and hydration of alkynes, three classes of modular processes that represent atom-economic C─O and C─N functionalizations in organic synthesis. Another notable finding of this study is that although [Au(IPr)Cl] is by far the most common precatalyst used π-activation reactions to date, more sterically-demanding catalysts showed improved reactivity in each of the reactions studied. [Au(Np#)Cl] catalyst is well-defined, air- and bench-stable, and the NHC scaffold is based on the modular peralkylation approach, enabling for the synthesis of analogues. This class of bulky and flexible catalysts (MilliporeSigma: IPr#, 915653; Np#, 915912; BIAN-IPr#, 916420) represents an attractive entry for wide application in organic synthesis and catalysis.
Supplementary Material
Acknowledgements
We gratefully acknowledge Rutgers University, the NIH (R35GM133326), the NSF (CAREER CHE-1650766) and the ACS PRF (DNI-55549) for generous financial support. Additional support was provided by the Rutgers Graduate School in the form of Dean’s Dissertation Fellowship. Supplement funding for this project was provided by the Rutgers University – Newark Chancellor’s Research Office. We thank the Wroclaw Center for Networking and Supercomputing (grant number WCSS159).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details and computational data. See DOI: 10.1039/x0xx00000x
Conflicts of interest
The authors declare the following competing financial interest(s): Rutgers University has filed patent(s) on ligands and precatalysts described in this manuscript (US 63/155,492, Mar 2, 2021).
Notes and references
- 1.(a) Hopkinson MN, Richter C, Schedler M and Glorius F, Nature, 2014, 510, 485–496; [DOI] [PubMed] [Google Scholar]; (b) N-Heterocyclic Carbenes: Effective Tools for Or-ganometallic Synthesis, ed. Nolan SP, Wiley, Weinheim, 2014; [Google Scholar]; (c) N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, ed. Diez-Gonzalez S, RSC, Cambridge, 2016; [Google Scholar]; (d) Science of Synthesis: N-Heterocyclic Carbenes in Catalytic Organic Synthesis, ed. Nolan SP and Cazin CSJ, Thieme, Stuttgart, 2017; [Google Scholar]; (e) Huynh HV, The Organometallic Chemistry of N-Heterocyclic Carbenes, Wiley, Hoboken, 2017; [Google Scholar]; (f) Hopkinson MN and Glorius F, An overview of NHCs, Wiley-VCH, 2018. [Google Scholar]
- 2.(a) Lee KM, Lee CK and Lin IJB, Angew. Chem. Int. Ed, 1997, 36, 1850–1852; [Google Scholar]; (b) Boydston AJ, Wil-liams KA and Bielawski CW, J. Am. Chem. Soc, 2005, 127, 12496–12497; [DOI] [PubMed] [Google Scholar]; (c) Hickey JL, Ruhayel RA, Barnard PJ, Baker MV, Berners-Price SJ and Filipovska J. Am. Chem. Soc, 2008, 130, 12570–12571; [DOI] [PubMed] [Google Scholar]; (d) Hindi KM, Panzner MJ, Tessier CA, Cannon CL and Youngs WJ, Chem. Rev, 2009, 109, 3859–3884; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Mercs L and Al-brecht M, Chem. Soc. Rev, 2010, 39, 1903–1912; [DOI] [PubMed] [Google Scholar]; (f) Oisaki K, Li Q, Furukawa H, Czaja AU, and Yaghi OMA, J. Am. Chem.Soc, 2010, 132, 9262–9264; [DOI] [PubMed] [Google Scholar]; (g) Ranganath KVS, Kloesges J, Schafer AH and Glorius F, Angew. Chem. Int. Ed, 2010, 49, 7786–7789; [DOI] [PubMed] [Google Scholar]; (h) Lara P, Rivada-Wheelaghan O, Conejero S, Poteau R, Phillippot K and Chaudret B, Angew. Chem. Int. Ed, 2011, 50, 12080–12084; [DOI] [PubMed] [Google Scholar]; (i) Zhukhovitskiy AV, Mavros MG, Voorhis TV and Johnson JA, J. Am. Chem. Soc, 2013, 135, 7418–7421; [DOI] [PubMed] [Google Scholar]; (j) Visbal R and Gimeno MC, Chem. Soc. Rev, 2014, 43, 3551–3574. [DOI] [PubMed] [Google Scholar]
- 3.(a) Hermann WA, Angew. Chem. Int. Ed, 2002, 41, 1290–1309; [DOI] [PubMed] [Google Scholar]; (b) Glorius F, Top. Organomet. Chem, 2007, 21, 1–231; [Google Scholar]; (c) Kantchev EAB, O’Brien CJO and Organ MG, Angew. Chem. Int. Ed, 2007, 46, 2768–2813; [DOI] [PubMed] [Google Scholar]; (d) Würtz S and Glorius F, Acc. Chem. Res, 2008, 41, 1523–1533; [DOI] [PubMed] [Google Scholar]; (e) Diez-Gonzalez S, Marion N and Nolan SP, Chem. Rev, 2009, 109, 3612–3676; [DOI] [PubMed] [Google Scholar]; (f) Fortman GC and Nolan SP, Chem. Soc. Rev, 2011, 40, 5151–5169; [DOI] [PubMed] [Google Scholar]; (g) N-Heterocyclic Carbenes in Transition Metal Catalysis, ed. Cazin CSJ, Springer, New York, 2011; [Google Scholar]; (h) Peris E, Chem. Rev, 2018, 118, 9988–10031; [DOI] [PubMed] [Google Scholar]; (i) Sipos G and Dorta R, Coord. Chem. Rev, 2018, 375, 13–68; [Google Scholar]; (j) Iglesias M and Oro LA, Chem. Soc. Rev, 2018, 47, 2772–2808; [DOI] [PubMed] [Google Scholar]; (k) Zhao Q, Meng G, Nolan SP and Szostak M, Chem. Rev, 2020, 120, 1981–2048; [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Chen C, Liu FS and Szostak M, Chem. Eur. J; 2021, 27, 4478–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahra F, Nelson DJ and Nolan SP, Trends Chem, 2020, 2, 1096–1113. [Google Scholar]
- 5.(a) Clavier H and Nolan SP, Chem. Commun, 2010, 46, 841–861; [DOI] [PubMed] [Google Scholar]; (b) Falivene L, Cao Z, Petta A, Serra L, Poater A, Oliva R, Scarano V and Cavallo L, Nat. Chem, 2019, 11, 872–879. [DOI] [PubMed] [Google Scholar]
- 6.(a) Diez-Gonzalez S and Nolan SP, Coord. Chem. Rev, 2007, 251, 874–883; [Google Scholar]; (b) Jacobsen H, Correa A, Poater A, Costabile C and Cavallo L, Coord. Chem. Rev; 2009, 253, 687–703; [Google Scholar]; (c) Dröge T and Glorius F, Angew. Chem. Int. Ed, 2010, 49, 6940–6952; [DOI] [PubMed] [Google Scholar]; (d) Nelson DJ and Nolan SP, Chem. Soc. Rev, 2013, 42, 6723–6753. [DOI] [PubMed] [Google Scholar]
- 7.(a) Melaimi M, Soleihavoup M and Bertrand G, Angew. Chem. Int. Ed, 2010, 49, 8810–8849; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Martin D, Melaimi M, Soleilhavoup M and Bertrand G, Organometallics, 2011, 30, 5304–5313; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Soleilhavoup M and Bertrand G, Acc. Chem. Res, 2015, 48, 256–266; [DOI] [PubMed] [Google Scholar]; (d) Paul USD and Radius U, Eur. J. Inorg. Chem, 2017, 3362–3375; [Google Scholar]; (e) Cheng J, Wang L and Deng L, Chem. Rev, 2018, 118, 9930–9987. [DOI] [PubMed] [Google Scholar]
- 8.(a) Gatineau D, Goddard J-P, Mouries̀-Mansuy V and Fensterbank L, Isr. J. Chem, 2013, 53, 892–900; [Google Scholar]; (b) Nolan SP, Acc. Chem. Res, 2011, 44, 91–100; [DOI] [PubMed] [Google Scholar]; (c) Marion N and Nolan SP, Chem. Soc. Rev, 2008, 37, 1776–1782; [DOI] [PubMed] [Google Scholar]; (d) Wurm T, Mohamed A and Hashmi ASK, NHC-Au(I) Complexes: Synthesis, Activation, and Application. In N-Heterocyclic Carbenes, ed. Nolan SP, John Wiley & Sons, Inc., 2014. [Google Scholar]
- 9.Collado A, Nelson DJ and Nolan SP, Chem. Rev, 2021, 121, 8559–8612. [DOI] [PubMed] [Google Scholar]
- 10.(a) Teles JH, Brode S and Chabanas M, Angew. Chem. Int. Ed, 1998, 37, 1415–1418; [DOI] [PubMed] [Google Scholar]; (b) Campeau D, Rayo DFL, Mansour A, Muratov K and Gagosz F, Chem. Rev, 2021, 121, 8756–8867; [DOI] [PubMed] [Google Scholar]; (c) Dorel R and Echavarren AM, Chem. Rev, 2015, 115, 9028–9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Marion N and Nolan SP, Chem. Soc. Rev, 2008, 37, 1776–7782; [DOI] [PubMed] [Google Scholar]; (b) Lin IJB and Vasam CS, Can. J. Chem, 2005, 83, 812–825. [Google Scholar]
- 12.Duan H, Sengupta S, Petersen JL, Akhmedov NG and Shi X, J. Am. Chem. Soc, 2009, 131, 12100–12102. [DOI] [PubMed] [Google Scholar]
- 13.Zhang G, Huang X, Li G and Zhang L, J. Am. Chem. Soc, 2008, 130, 1814–1815. [DOI] [PubMed] [Google Scholar]
- 14.(a) Gómez-Suárez A, Ramón RS, Songis O, Slawin AMZ, Cazin CSJ and Nolan SP, Organometallics, 2011, 30, 5463–5470; [Google Scholar]; (b) Nahra F, Patrick SR, Bello D, Brill M, Obled A, Cordes DB, Slawin AMZ, O’Hagan D and Nolan SP, ChemCatChem, 2015, 7, 240–244; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Marion N, Ramón RS and Nolan SP, J. Am. Chem. Soc; 2009, 131, 448–449. [DOI] [PubMed] [Google Scholar]
- 15.For selected recent studies on Au(I)─NHC catalysis, see: (a) Chintawar CC, Yadav AK, Kumar A, Sancheti SP and Patil NT, Chem. Rev, 2021, 121, 8478–8558; [DOI] [PubMed] [Google Scholar]; (b) Pflästerer D, Hashmi ASK, Chem. Soc. Rev, 2016, 45, 1331–1367; [DOI] [PubMed] [Google Scholar]; (c) Hashmi ASK, Chem. Rev, 2021, 121, 8309–8310; [DOI] [PubMed] [Google Scholar]; (d) Kumar A and Patil NT, ACS Sust. Chem. Eng 2022, 10, 6900–6918; [Google Scholar]; (e) Tang Y, Benaissa I, Huynh M, Vendier L, Lugan N, Bastin S, Belmont P, César V, Michelet V, Angew. Chem. Int. Ed, 2019, 58, 7977–7981; [DOI] [PubMed] [Google Scholar]; (f) Laher R, Marin C and Michelet V, Org. Lett, 2020, 22, 4058–4062; [DOI] [PubMed] [Google Scholar]; (g) Teixeira P, Bastin S and César V, Isr. J. Chem, 2023, 68, e202200051; [Google Scholar]; (h) Pedrazzani R, Pintus A, De Ventura R, Marchini M, Ceroni P, Silva López C, Monari M and Bandini M, ACS Org. Inorg. Au, 2022, 2, 229–235; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Pedrazzani R, Pinosa E, Bertuzzi G, Monari M, Lauzon S, Ollevier T and Bandini M, Chem. Commun, 2022, 58, 8698–8701. [DOI] [PubMed] [Google Scholar]
- 16.For a recent review, see: Gao P and Szostak M, Coord. Chem. Rev., 2023, 485, 215110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Q, Meng G, Li G, Flach C, Mendelsohn R, Lalancette R, Szostak R and Szostak M, Chem. Sci, 2021, 12, 10583–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.For the availability of IPr# NHC ligands, see: www.sigmaaldrich.com/catalog/product/aldrich/915653 (accessed on May 24, 2023). [Google Scholar]
- 19.Rahman MM, Zhao Q, Meng G, Szostak R and Szostak M, Organometallics, 2022, 41, 2597–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Rahman MM, Meng G, Bisz E, Dziuk B, Lalancette R, Szostak R and Szostak M, Chem. Sci, 2023, 14, 5141–5147; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gao P, Xu J, Zhou T, Liu Y, Bisz E, Dziuk B, Lalancette R, Szostak R, Zhang D and Szostak M, Angew. Chem. Int. Ed, 2023, 62, e202218427; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang J, Li X, Li T, Zhang G, Wan K, Ma Y, Fang R. Szostak R and Szostak M, ACS Catal., 2022, 12, 15323–15333; [Google Scholar]; (d) Zhang J, Wang Y, Zhang Y, Liu T, Fang S, Wang R, Ma Y, Fang R, Szostak R, and Szostak M, Organometallics, 2022, 41, 1115–1124; [Google Scholar]; (e) Xia Q, Shi S, Gao P, Lalancette R, Szostak R and Szostak M, J. Org. Chem, 2021, 86, 15648–15657; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Lei P, Wang Y, Zhang C, Hu Y, Feng J, Ma Z, Liu X, Szostak R and Szostak, Org. Lett, 2022, 24, 6310–6315; [DOI] [PubMed] [Google Scholar]; (g) Zhou T, Ma S, Nahra F, Obled AMC, Poater A, Cavallo L, Cazin CSJ, Nolan SP and Szostak M, iScience, 2020, 23, 101377; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Yang S; Zhou T, Poater A, Cavallo L, Nolan SP and Szos-tak M, Catl. Sci. Technol, 2021, 11, 3189–3197; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yang S, Yu X and Szostak M, ACS Catal, 2023, 13, 1848–1855; [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Zhang J, Li T, Li X, Lv A, Li X, Wang Z, Wang R, Ma Y, Fang R, Szostak R and Szostak M, Commun Chem, 2022, 5, 60–70; [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Rahman MM, Zhang J, Zhao Q, Feliciano J, Bisz E, Dziuk B, Lalancette R, Szostak R and Szostak M, Organometallics, 2022, 41, 2281–2290; [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Zhang J, Rahman MM, Zhao Q, Feliciano J, Bisz E, Dziuk B, Lalancette R, Szostak R and Szostak M, Organometallics, 2022, 41, 1806–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Rotem M and Shvo Y, Organometallics, 1983, 2, 1689–1691; [Google Scholar]; (b) Goossen LJ, Paetzold J and Koley D, Chem. Commun, 2003, 706–707; [PubMed] [Google Scholar]; (c) Chary BC ad Kim S, J. Org. Chem, 2010, 75, 7928–7931; [DOI] [PubMed] [Google Scholar]; (d) Dupuy S, Gasperini D and Nolan SP, ACS Catal, 2015, 5, 6918–6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Müller TE and Beller M, Chem. Rev, 1998, 98, 675–704; [DOI] [PubMed] [Google Scholar]; (b) Alonso F, Beletskaya IP and Yus M, Chem. Rev, 2004, 104, 3079–3160; [DOI] [PubMed] [Google Scholar]; (c) Severin R and Doye S, Chem. Soc. Rev, 2007, 36, 1407–1420. [DOI] [PubMed] [Google Scholar]; (d) Müller TE, Hultzsch KC, Yus M, Foubelo F and Tada M, Chem. Rev, 2008, 108, 3795–3892; [DOI] [PubMed] [Google Scholar]; (e) Doye S, Synlett, 2004, 1653–1672; [Google Scholar]; (f) Lee AV and Schafer LL, Eur. J. Inorg. Chem, 2007, 2243–2255; [Google Scholar]; (g) Odom AL, Dalton Trans, 2005, 225–233; [DOI] [PubMed] [Google Scholar]; (h) Zeng X, Soleilhavoup M and Bertrand G, Org. Lett, 2009, 11, 3166–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Modern Carbonyl Chemistry, ed. Otera J, Wiley-VCH, Weinheim, Germany, 2000; [Google Scholar]; (b) Mizushima E, Sato K, Hayashi T and Tanaka M, Angew. Chem. Int. Ed, 2002, 41, 4563–4565. [DOI] [PubMed] [Google Scholar]
- 24.de-Frémont P, Scott NM, Stevens ED and Nolan SP, Organometallics, 2005, 24, 2411–2418. [Google Scholar]
- 25.Tzouras NV, Nahra F, Falivene L, Cavallo L, Saab M, Hecke KV, Collado A, Collett CJ, Smith AD, Cazin CSJ and Nolan SP, Chem. Eur. J, 2020, 26, 4515–4519. [DOI] [PubMed] [Google Scholar]
- 26.(a) Yu D-G, Li B-J and Shi Z-J, Acc. Chem. Res, 2010, 43, 1486–1495; [DOI] [PubMed] [Google Scholar]; (b) Rosen BM, Quasdorf KW, Wilson DS, Zhang N, Resmerita A-M, Garg NK and Percec V, Chem. Rev, 2011, 111, 1346–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.