Abstract
Background:
Racial residential segregation is associated with racial health inequities, but it is unclear if segregation may exacerbate Black-White disparities in cardiovascular disease (CVD) mortality. This study aimed to assess associations between Black-White residential segregation, CVD mortality rates among Non-Hispanic (NH) Black and NH White populations, and Black-White disparities in CVD mortality.
Methods:
This cross-sectional study analyzed Black-White residential segregation as measured by county-level interaction index, of US counties, county-level CVD mortality among NH White and NH black adults aged 25 years and older, and county-level Black-White disparities in CVD mortality in years 2014 to 2017. Age-adjusted, county-level NH Black CVD mortality rates and NH White cardiovascular disease mortality rates, as well as group-level relative risk ratios for Black-White cardiovascular disease mortality were calculated. Sequential generalized linear models adjusted for county-level socioeconomic and neighborhood factors were used to estimate associations between residential segregation and cardiovascular mortality rates among NH Black and NH White populations. Relative risk ratio tests were used to compare Black-White disparities in the most segregated counties to disparities in the least segregated counties.
Results:
We included 1286 counties with ≥5% Black populations in the main analysis. Among adults aged ≥25 years, there were 2,611,560 and 408,429 CVD deaths among NH White and NH Black individuals, respectively. In the unadjusted model, counties in the highest tertile of segregation had 9% higher (95% CI, 1% to 20% higher, P=0.04) rates of NH Black CVD mortality than counties in the lowest tertile of segregation. In the multivariable adjusted model, the most segregated counties had 15% higher (95% CI, 0.5% to 38% higher, P=0.04) rates of NH Black CVD mortality than the least segregated counties. In the most segregated counties, NH Black individuals were 33% more likely to die of CVD than NH White individuals (RR 1.33, 95% CI 1.32 to 1.33, P<0.001).
Conclusions:
Counties with increased Black-White residential segregation have higher rates of NH Black CVD mortality and larger Black-White disparities in CVD mortality. Identifying the causal mechanisms through which racial residential segregation widens disparities in CVD mortality requires further study.
Keywords: Residential Segregation, Social Segregation, Health Inequities, Cross-Sectional Studies, Socioeconomic Factors, Race
Introduction:
Structural racism is the differential access of goods, services, or opportunities of society by race and is exemplified in a range of historic and ongoing racist policies and practices.1–3 Historical government policies such as Jim Crow laws and racial redlining coupled with discriminatory practices within the real estate and banking industries have resulted in and maintained systematic racial segregation of people of color in the United States.4–6 In addition, sustained and systematic neighborhood disinvestment have led to lack of economic opportunities and racialized concentrated poverty, which have continued to reinforce racial segregation in US metropolitan areas.7,8
Prior work has shown that racial residential segregation is strongly associated with Black-White health disparities.9–12 Segregation may influence racial differences in social determinants of health. Early life exposure to racial residential segregation, particularly in the form of school racial segregation and reduced quality of education, have been associated with long-term health outcomes.13,14 Targeted divestment of Black communities has led to concentrated poverty, poor housing quality, increased crime, limited access to healthcare and healthy foods, disproportionate burden of environmental hazards, among other factors, all of which contribute to inequitable health outcomes. Moreover, structurally marginalized racial and ethnic groups living in highly segregated areas tend to face multiple sources of chronic psychological and chemical stressors (e.g., violence, financial stress, air pollution) that may lead to tobacco, alcohol, and drug use, as well as increased risk for obesity, diabetes, and cardiovascular disease (CVD).12,15 In fact, a longstanding body of work has found that neighborhood environments contribute to CVD risk and that racial residential segregation is significantly associated with increased CVD risk among Black Americans.16–19 Previous studies have shown strong associations between high racial residential segregation and increased incidence of hypertension, coronary heart disease, and myocardial infarction among Black Americans.17,20 However, our understanding of the relationship between residential segregation and CVD mortality remains limited.17 In particular, previous studies examining residential segregation and CVD mortality have been limited to small cohorts exclusively in metropolitan areas.21 Further, no prior studies have investigated the extent to which racial residential segregation may be associated with the magnitude of Black-White disparities in CVD mortality.
Racial disparities in CVD mortality are pervasive and continue to widen.22 Understanding the social determinants of Black-White disparities in CVD mortality is crucial to informing interventions aimed at eliminating such disparities. This study seeks to investigate whether increased racial residential segregation is associated with greater Black-White disparities in county-level CVD mortality rates and greater adverse effects on CVD mortality rates among Black Americans compared to White Americans in the United States.
Methods:
This study was considered exempt from review by the University of Pennsylvania Institutional Review Board as all data used are publicly available and routinely collected. No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Data Sources and Measures
County-level mortality data were obtained under agreement from the National Center for Health Statistics (NCHS). These data provide granular information on sex, year of death, race/ethnicity, cause of death, and county of residence, which are extracted from all death certificates filed in the 50 states and the District of Columbia. NCHS data defines causes of death using International Classification of Disease, Tenth Revision (ICD-10) codes. We limited causes of deaths to diseases of the circulatory system (ICD-10 codes I00 to I99), which includes stroke.23 We then tabulated county-level, age-adjusted (to the 2000 US population) CVD mortality rates per 100,000 individuals among Non-Hispanic (NH) Black and NH White adults aged ≥25 years for the period 2014 to 2017.
Data on five types of county-level covariates were collected from the Robert Wood Johnson Foundation County Health Rankings for the year 2017: (1) clinical variables (% of adults with diabetes, % of adults who are obese, % of adults who smoke), (2) demographic variables (% of population that is Hispanic, % of population that is NH Black, % of population that is NH White, % of population that is female, % of population that is aged ≥65 years, % of population not proficient in English), (3) socioeconomic variables (median household income, poverty rate, unemployment rate, income inequality, % of population with high school diploma or equivalent), (4) healthcare access variables (% of population that is uninsured, Health Professional Shortage Area (HPSA) designation), and (5) neighborhood characteristics (food insecurity rate, violent crime rate, homeownership rate). Income inequality was defined as the ratio of household income at the 80th percentile to income at the 20th percentile. Counties were designated metropolitan, micropolitan, or rural based on data from the U.S. Census Bureau.
To measure residential segregation, we used population estimates from the U.S. Census Bureau to calculate county-level values of the Black-White Interaction Index. Massey and Denton’s prior work on measuring residential segregation identified five distinct dimensions of segregation—evenness, exposure, concentration, centralization, and clustering. Exposure is the extent to which minority and majority group members interact with each other in a specific geographic area. Of note, prior work has found that exposure is more significantly correlated with mortality risk than other dimensions of residential segregation, and measures of exposure have been validated as significant predictors of CVD mortality in metropolitan areas.21,24 The Interaction Index measures exposure as the minority-weighted average of the proportion of majority group members in each census tract.25 The tract-level values are then aggregated up to the county level. The Interaction Index ranges from 0 (complete segregation) to 100 (complete integration). Details on the calculation of the Interaction Index are provided in the Supplement. The least segregated counties were defined as those in the lowest tertile of segregation based on the Interaction Index, and the most segregated counties were those in the highest tertile of segregation by Interaction Index. Majority-minority counties with significantly high proportions of NH Black residents (i.e., > 70% NH Black residents) tend to have lower Interaction Index values.
Outcomes
The primary outcome measures were separate county-level, age-adjusted CVD mortality rates (AAMR) for NH Black individuals and NH White individuals. The secondary outcome measure was the relative risk ratio (RR) for Black-White CVD mortality. The RR is calculated as NH Black CVD deaths per 100,000 population divided by NH White CVD deaths per 100,000 population. If RR>1, NH Black individuals are more likely to die of CVD causes than NH White individuals in a given county.
Statistical Analysis
In our main analyses, the analytical cohort was limited to counties with populations that are ≥5% Black, to evaluate residential segregation indices in counties with significant NH Black populations. As of 2017, the overall percentage of NH Black Americans in the US was 12.5%, based on data from the US Census Bureau. The mean county-level percentage of NH Black residents was 8.9%.26 Sensitivity analyses were performed using a cohort of all US counties.
We first summarized county characteristics and CVD mortality rates by tertile of residential segregation defined by interaction indices. In our primary analysis, we assessed the relationship between county-level residential segregation, as defined by Interaction Index, and county-level CVD AAMRs. We fit generalized linear models (GLMs) with negative binomial distribution and log link. Two sets of models were fit, one with the dependent variable as CVD AAMR in NH Black individuals, and one with the dependent variable as CVD AAMR in NH White individuals. County-level covariates were introduced sequentially in this order: metropolitan designation, clinical variables, demographic variables, socioeconomic variables, healthcare access variables, and neighborhood variables. County-level covariates were selected a priori as potential confounders of the association between CVD mortality and residential segregation. All models were adjusted for county population, and results of the GLMs are presented as incident rate ratios (IRR). In addition, variance inflation factors (VIFs) were tabulated for covariates in each model. No VIFs exceeded 5, indicating no significant multicollinearity between covariates. Interaction terms between demographic variables % NH Black population and % NH White population and county-level socioeconomic, healthcare access, and neighborhood characteristics were tested. The interaction terms showed no significant association with NH Black AAMRs or NH White AAMRs and were, thus, excluded from the final models.
In our secondary analysis, we compared group-level RRs for Black-White CVD mortality in the least and most segregated counties by employing a relative risk ratio test.27 We provide 95% CI for all RR and IRR estimates. P-values <0.05 were considered statistically significant. All analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing).
Results:
Of 3138 US counties with complete mortality data, 1286 counties with ≥5% NH Black populations were included in the main analysis. These 1286 counties represent 96% of the total NH Black population in the United States. The median Interaction Index was 55.3 (Range, 0 to 90.7). The lowest tertile of Interaction Index was 0 to 46; the middle tertile was 47 to 64; and the highest tertile was 65 to 91. Counties in the South tended to have the lowest interaction indices (Figure 1). A histogram showing the frequency distribution of Interaction Index values for all counties in the US is shown in Supplemental Figure S2.
Figure 1.
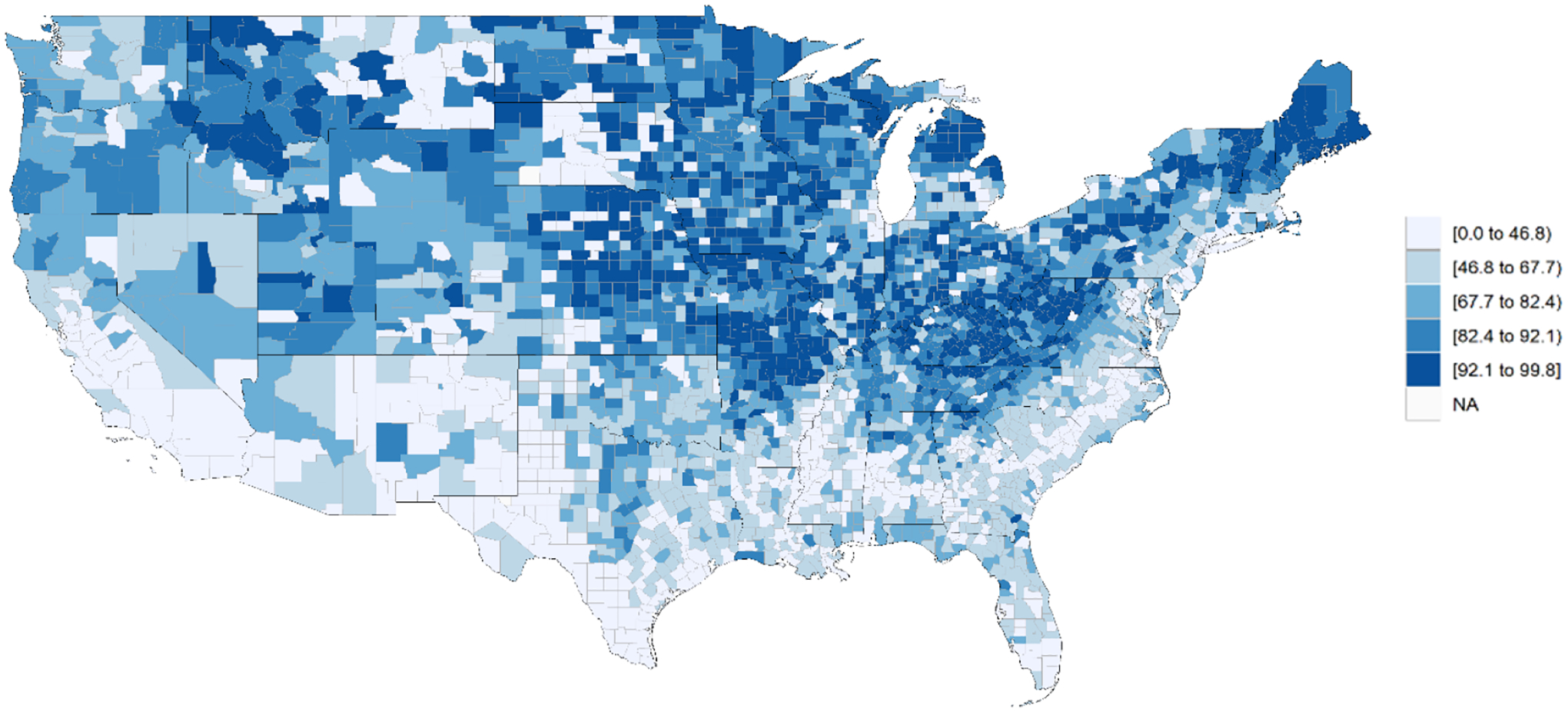
County-Level Black-White Residential Segregation Measured by Interaction Index
Lower numerical values of the interaction index correspond to higher levels of segregation.
Table 1 summarizes county characteristics and CVD mortality rates by tertile of residential segregation defined by interaction indices. The median (IQR) poverty rate for counties in the highest tertile of segregation was 18.9% (14.6% to 24.9%), compared to 14.9% (11.4% to 17.7%) for counties in the lowest tertile of segregation. The most segregated counties had a median (IQR) food insecurity rate of 16.3% (12.6% to 20.3%), and the least segregated counties had a median (IQR) food insecurity rate of 13.3% (11.4% to 14.9%). Additionally, counties in the highest tertile of segregation had a median (IQR) violent crime rate of 389 offenses per 100,00 (230 to 581), whereas counties in the lowest tertile of segregation had a median (IQR) violent crime rate of 209 offenses per 100,000 (141 to 313). Details on county characteristics for all US counties are provided in Supplemental Table S3.
Table 1.
County-level characteristics and CVD AAMRs by Tertile of Black-White Residential Segregation based on Interaction Indices in Counties with ≥5% Black population.
| Overall (n = 1286) | Lowest Tertile of Segregation2 (n = 429) | Middle Tertile of Segregation3 (n = 429) | Highest Tertile of Segregation4 (n = 428) | |
|---|---|---|---|---|
| Metropolitan Designation, n (%) | ||||
| Metropolitan | 683 (53.1%) | 221 (51.6%) | 217 (50.6%) | 245 (57.1%) |
| Micropolitan | 230 (17.9%) | 80 (18.7%) | 73 (17.0%) | 77 (17.9%) |
| Rural | 373 (29.0%) | 127 (29.7%) | 139 (32.4%) | 107 (24.9%) |
| NH White CVD AAMR1 per 100,000, Median (IQR) | 389 (335 to 452) | 385 (334 to 446) | 396 (338 to 460) | 386 (333 to 451) |
| NH Black CVD AAMR per 100,000, Median (IQR) | 475 (394 to 561) | 462 (373 to 558) | 483 (411 to 561) | 480 (399 to 562) |
| Clinical Variables | ||||
| Diabetes Rate (%), Median (IQR) | 12.8 (10.2 to 15.7) | 12.6 (10.4 to 15.0) | 13 (10.2 to 15.7) | 12.6 (10.2 to 16.4) |
| Obesity Rate (%), Median (IQR) | 34.6 (30.0 to 38.2) | 34.3 (30.5 to 37.7) | 35.0 (30.4 to 38.4) | 34.3 (28.9 to 39.3) |
| Smoking Rate (%), Median (IQR) | 18.0 (15.6 to 20.3) | 18.0 (16.0 to 20.4) | 18.2 (15.7 to 20.0) | 17.9 (14.9 to 20.5) |
| Demographic Variables | ||||
| % Population that is Hispanic, Median (IQR) | 5.3 (2.8 to 12.2) | 3.9 (2.5 to 6.3) | 6.0 (3.2 to 12.2) | 7.7 (3.1 to 28.0) |
| % Population that is NH Black, Median (IQR) | 16.6 (8.7 to 32.1) | 8.7 (6.4 to 14.0) | 22 (12.3 to 31.9) | 32.2 (14.4 to 50.9) |
| % Population that is NH White, Median (IQR) | 78.3 (64 to 87.4) | 88.9 (83.7 to 91.5) | 73.5 (66.5 to 83.0) | 60.7 (45.7 to 76.1) |
| % Population that is Female, Median (IQR) | 50.9 (49.7 to 51.6) | 50.8 (49.7 to 51.3) | 50.8 (49.7 to 51.5) | 51.2 (49.7 to 52) |
| % Population Aged 65 and Older, Median (IQR) | 17.2 (14.8 to 19.7) | 17.7 (15.4 to 20.4) | 17.3 (15 to 19.4) | 16.3 (13.9 to 19.1) |
| % Population Not Proficient in English, Median (IQR) | 1.1 (0.4 to 2.5) | 0.7 (0.3 to 1.4) | 1.2 (0.6 to 2.5) | 1.7 (0.7 to 4.9) |
| Socioeconomic Variables | ||||
| Median Household Income, $, Median (IQR) | $46,800 ($39,800 to $56,400) | $48,600 ($43,400 to $58,100) | $45,100 ($39,500 to $54,300) | $45,400 ($36,800 to $55,900) |
| Poverty Rate (%), Median (IQR) | 16.8 (12.8 to 21.2) | 14.9 (11.4 to 17.7) | 17.7 (13.5 to 21.2) | 18.9 (14.6 to 24.9) |
| Unemployment Rate (%), Median (IQR) | 4.6 (4 to 5.6) | 4.4 (3.8 to 5.1) | 4.7 (4 to 5.5) | 5.1 (4.2 to 6.1) |
| Income Inequality Ratio, Median (IQR) | 4.7 (4.3 to 5.2) | 4.5 (4.2 to 4.9) | 4.7 (4.3 to 5.2) | 4.9 (4.5 to 5.6) |
| % Population with High School Diploma and Higher, Median (IQR) | 87.7 (83.3 to 91.8) | 89.7 (86 to 93.5) | 87.3 (82.9 to 91.1) | 85.9 (81.3 to 90.6) |
| Healthcare Access Variables | ||||
| % Population Uninsured, Median (IQR) | 14.9 (10.3 to 19.4) | 13.2 (8.7 to 16.4) | 15.9 (11.2 to 20.3) | 15.5 (11.8 to 20.4) |
| Primary Care HPSA, n (%) | 867 (67.4%) | 286 (66.8%) | 313 (73%) | 268 (62.5%) |
| Neighborhood Characteristics Variables | ||||
| Food Insecurity Rate (%), Median (IQR) | 14.3 (12.0 to 17.2) | 13.3 (11.4 to 14.9) | 14.6 (12.3 to 17.4) | 16.3 (12.6 to 20.3) |
| Violent Crime Rate, Offenses per 100,000 Population, Median (IQR) | 283 (174 to 433) | 209 (141 to 313) | 308 (198 to 425) | 389 (230 to 581) |
| Homeownership Rate (%), Median (IQR) | 70 (64 to 74.8) | 72.4 (68.1 to 76.8) | 70.7 (65.4 to 74.8) | 65.8 (59.1 to 72.2) |
“AAMR” refers to county-level age-adjusted mortality rate.
Counties in the lowest tertile of segregation had interaction indices between 0 and 46.
Counties in the middle tertile of segregation had interaction indices between 47 and 64.
Counties in the highest tertile of segregation had interaction indices between 65 and 91.
Between 2014 and 2017, among adults aged 25 years and older, there were 2,611,560 CVD deaths among NH White individuals and 408,429 CVD deaths among NH Black individuals across all US counties. Figure 2 shows county-level CVD AAMRs among NH White and NH Black individuals during the study period for all US counties. Among counties with ≥5% Black populations, there were 1,726,469 CVD deaths among NH White individuals and 397,636 CVD deaths among NH Black individuals. Overall, median county-level CVD AAMR in the analytical cohort was 389 per 100,000 (IQR, 335 to 452) for NH White individuals and 475 deaths per 100,000 (IQR, 394 to 561) for NH Black individuals. Median CVD AAMR among NH Black individuals was 480 deaths per 100,000 (IQR, 399 to 562) for counties in the highest tertile of segregation.
Figure 2.
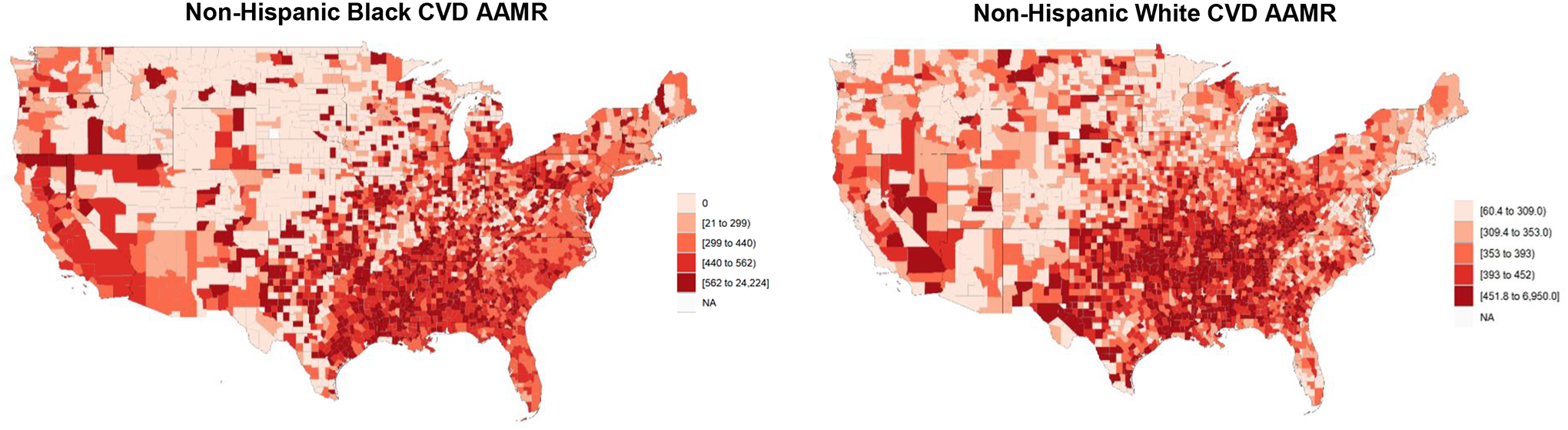
County-Level CVD AAMR in Adults Aged 25 years and Older
Primary Analysis
Results of our primary analysis measuring the associations between county-level Black-White Interaction Index and CVD AAMRs among NH Black and NH White individuals are summarized in Table 2. In the unadjusted model, counties in the highest tertile of segregation had 9% higher (95% CI, 1% to 20% higher, P=0.04) rates of NH Black CVD mortality than counties in the lowest tertile. In the multivariable adjusted model, the most segregated counties had 15% higher (95% CI, 0.5% to 38% higher, P=0.04) rates of NH Black CVD mortality than the least segregated counties. Additionally, counties in the highest tertile of segregation had 8% higher rates (95% CI, 1% to 16%, P<0.001) of NH White CVD mortality than the least segregated counties, but residential segregation was not significantly associated with NH White CVD mortality in the multivariable adjusted model. In the unadjusted sensitivity models of all US counties, the most segregated counties had 42% higher (95% CI, 20% to 69% higher) rates of NH Black CVD mortality and 7% higher (95% CI, 2% to 13% higher) rates of NH White CVD mortality than counties in the lowest tertile of segregation (P<0.001). In the multivariable adjusted models of all US counties, residential segregation was not significantly associated with CVD mortality (Supplemental Table S4).
Table 2.
Association between Black-White Interaction Index and CVD AAMR for Sequential Models Controlling for Clinical, Demographic, Socioeconomic, Health Access, and Neighborhood variables in Counties with ≥5% Black population.
| Model Covariates | NH Black CVD AAMR Incident Rate Ratio for Tertile of Highest Segregation, Reference = Tertile of Lowest Segregation (95% CI) | P-Value | NH White CVD AAMR Incident Rate Ratio for Tertile of Highest Segregation, Reference = Tertile of Lowest Segregation (95% CI) | P-Value |
|---|---|---|---|---|
| Residential Segregation | 1.09 (1.01 to 1.20) | 0.04 | 1.08 (1.01 to 1.16) | <0.001 |
| Residential Segregation + Metro/Micro/Rural Designation | 1.09 (1.003 to 1.21) | 0.03 | 1.09 (1.01 to 1.19) | <0.001 |
| Residential Segregation + Metro/Micro/Rural Designation + Clinical Variables | 1.09 (1.01 to 1.23) | 0.04 | 1.09 (1.0 to 1.2) | <0.001 |
| Residential Segregation + Metro/Micro/Rural Designation + Clinical Variables + Demographic Variables | 1.11 (0.95 to 1.3) | 0.12 | 0.98 (0.92 to 1.04) | 0.41 |
| Residential Segregation + Metro/Micro/Rural Designation + Clinical Variables + Demographic Variables + Socioeconomic Variables | 1.10 (0.95 to 1.28) | 0.16 | 0.97 (0.93 to 1.03) | 0.30 |
| Residential Segregation + Metro/Micro/Rural Designation + Clinical Variables + Demographic Variables + Socioeconomic Variables + Healthcare Access Variables | 1.12 (0.96 to 1.31) | 0.09 | 0.98 (0.93 to 1.04) | 0.52 |
| Residential Segregation + Metro/Micro/Rural Designation + Clinical Variables + Demographic Variables + Socioeconomic Variables + Healthcare Access Variables + Neighborhood Characteristics | 1.15 (1.005 to 1.38) | 0.04 | 0.97 (0.92 to 1.03) | 0.32 |
Secondary Analysis
In counties in the lowest tertile of residential segregation defined by Interaction Index, NH Black individuals were 22% more likely to die of CVD than NH White individuals (RR 1.22, 95% CI 1.21 to 1.24). In the most segregated counties, NH Black individuals were 33% more likely to die of CVD than NH White individuals (RR 1.33, 95% CI 1.32 to 1.33). Thus, disparities in Black-White CVD mortality were larger for counties in the highest segregation group compared to those in the lowest segregation group (P<0.001). Similar results were obtained in the sensitivity analysis of all US counties (Table 3).
Table 3.
Relative Risk Ratios of Black-White CVD AAMR for Counties in the Lowest and Highest Groups of Segregation Measured by Interaction Index in Two Analytical Cohorts.
| Analytical Cohort | Lowest Segregation Group Relative Risk Ratio (95% CI) | Highest Segregation Group Relative Risk Ratio (95% CI) | P-Value |
|---|---|---|---|
| Counties with ≥5% Black population | 1.22 (1.21 to 1.24) | 1.33 (1.32 to 1.33) | <0.001 |
| All US Counties | 1.03 (1.0 to 1.06) | 1.32 (1.32 to 1.33) | <0.001 |
Discussion
In this study, we found that after controlling for clinical, socioeconomic, demographic, and neighborhood-level factors, counties with the highest degrees of racial residential segregation defined by Black-White interaction indices had significantly higher NH Black CVD mortality rates than counties with the lowest degrees of segregation. Second, we found that Black-White disparities in CVD mortality from 2014 to 2017 were larger in U.S. counties with high degrees of Black-White residential segregation than in counties with low degrees of segregation. To our knowledge, this is the first nationally comprehensive study to examine and quantify associations between county-level residential segregation and county-level disparities in CVD mortality among NH White and NH Black populations.
Prior studies have found significant associations between incident CVD risk and residential segregation across the lifespan.14,17,20,28 Building on this work, our findings show that these associations extend to CVD mortality. Black-White residential segregation is differentially associated with NH White and NH Black CVD mortality and exacerbates Black-White disparities in CVD mortality. Further, our results show that residential segregation is independently associated with higher CVD mortality rates among NH Black individuals, even after controlling for a wide array of socioeconomic and neighborhood factors.
When we included counties with extremely small NH Black populations, we continued to observe an association between residential segregation and higher CVD mortality rates among NH Black individuals. The association between segregation and CVD mortality was completely attenuated when clinical and demographic variables were added to the model, and no significant association between segregation and CVD mortality was observed when socioeconomic factors, healthcare access variables, and neighborhood characteristics were added to the model. Further work must be done to better understand the mechanisms in which neighborhood factors and segregation jointly influence health outcomes in rural, predominantly White communities. Nonetheless, when including these counties with fewer than 5% NH Black populations, the relative risk ratios of Black-White CVD mortality in the lowest and highest tertile of segregation showed significant increases with increased residential segregation.
While this paper does not evaluate the causal pathways through which residential segregation affects CVD mortality, it is helpful to consider the findings of this study within the larger historical context of residential segregation and structural racism. Racialized residential segregation is reflective of a long and continued history of racist policies and practices that perpetuate health inequities among Black Americans. For example, segregation represents a critical link between reduced access to health services, limited access to healthy foods, and increased exposure to water and air pollution and other environmental hazards in Black communities and poorer health outcomes among Black Americans.15,29–33 Segregated neighborhoods also tend to have increased frequency of psychological stressors (e.g., concerns for safety, over policing, violent crime), which have been shown to decrease healthy behaviors, lead to delays in seeking care and filling prescriptions, and are associated with worse community cardiovascular health.34–36 Moreover, prior work has found that neighborhood deprivation is associated with markers of biological adversity, which may influence CVD risk. For example, neighborhood social deprivation and individual-level socioeconomic status has been found to be associated with a number of pathophysiological features modulated by chronic stress (e.g., cortisol levels, telomere length, monocyte CCR2 levels) that have been implicated in the etiology certain cardiovascular diseases.37–43 These effects are compounded by economic distress as well as neighborhood conditions marked by deterioration in highly segregated areas (e.g., crumbling housing stock, blighted vacant land, and limited greenspace), which have been associated with poor cardiovascular outcomes.16,44–46
Policies aimed at addressing the greater health consequences of residential segregation and structural racism are necessary. Some long-term success has been found in housing voucher programs that assist residents of highly segregated areas with relocating to better quality neighborhoods. Moreover, removal of exclusionary land use restrictions can reduce metropolitan area-level segregation by giving lower-income households greater access to housing in higher-resourced areas, which has the potential to lessen health disparities arising from neighborhood deprivation.47 Some research has also found that improving features of the built environment in highly segregated neighborhoods may be one pathway to reducing CVD health disparities in minority populations, though evidence for the efficacy of such interventions remains limited.19,48–51 Further work is needed to design interventions that can address the specific causal relationships between racial residential segregation and Black-White disparities in CVD mortality.
Limitations
This study has several limitations. First, as the study was cross-sectional and observational, causality cannot be inferred. Moreover, as all data were aggregated to the county level, no inferences at the individual level could be made. Second, this study is limited to studying only NH Black and NH White populations. Further work must be done to understand the associations between residential segregation and disparities in CVD mortality between other racial and ethnic groups. Third, we did not account for other forms of segregation, such as residential clustering of rich and poor households, that may influence CVD mortality risk. However, all county-level models were adjusted for demographic, socioeconomic, health access, and neighborhood characteristics to identify the independent effects of residential segregation on mortality rates. Fourth, interaction indices were calculated at the county area level, which impedes a more granular understanding of the heterogeneous effects of residential segregation at the block level. Fifth, there are limitations to the county-level data available from the Robert Wood Johnson Foundation County Health Rankings that preclude a nuanced analysis of the association between neighborhood characteristics and CVD AAMR. For instance, crime rates may be underreported in communities subject to police violence, and rates of food insecurity do not take into account the quality of food available within a neighborhood. Similarly, there exist a number of other clinical variables that are associated with CVD mortality rates (e.g., rates of atrial fibrillation, heart failure, etc.) but were not available in the Robert Wood Johnson Foundation County Health Rankings data. Moreover, due to data limitations, this study did not account for all county-level factors that may be associated with CVD mortality (e.g., environmental pollution, neighborhood investment). Further work is needed to elucidate the interactions between various sociodemographic and environmental variables that may influence CVD mortality risk. Lastly, this study may be biased by ecological fallacy, given that all analyses were conducted using county-level variables. However, one potential advantage of such variables is that they offer a wide range of socioeconomic, neighborhood, demographic, and healthcare access information for every county in the nation, whereas individual-level data are often limited by small cohort sizes and/or geographic limitations. We further underscore that teasing apart specific effects of residential segregation remains challenging even when relying on individual-level data because residential segregation is known to influence health disparities at multiple levels beyond the level of the individual.15,52 Certainly, further work, potentially employing multilevel and/or hierarchical analyses, is warranted to identify specific associations between racial residential segregation and health disparities at the individual patient level.
Conclusion
This study assessed associations between county-level Black-White residential segregation and Black-White disparities in county-level CVD mortality rates in the United States. Increased county-level residential segregation was associated with increased NH Black CVD mortality rates. Counties with higher levels of residential segregation also had larger Black-White disparities in CVD mortality. Identifying the causal mechanisms through which residential segregation widens racial disparities in cardiovascular health and designing effective interventions to abate those inequities is essential.
Supplementary Material
Disclosures:
Kriyana P Reddy has no disclosures. Ashwin S Nathan has received research funds and speaker fees from Abiomed and research funds from Biosense Webster. Jay Giri has served on advisory boards and received research funds to the institution from Abiomed, Astra Zeneca, Boston Scientific, Inari Medical, and Abbott Vascular and he has equity in Endovascular Engineering. Alexander C Fanaroff has no disclosures. Sameed Ahmed M. Khatana receives grants from the National Heart, Lung, and Blood Institute (5K23HL153772-02) and the American Heart Association (20CDA35320251). Lauren A. Eberly has no disclosures. Howard M Julien has no disclosures. Peter W. Groeneveld has no disclosures.
Abbreviations:
- CVD
Cardiovascular Disease
- NH
Non-Hispanic
- NCHS
National Center for Health Statistics
- HPSA
Health Professional Shortage Area
- AAMR
age-adjusted mortality rate
- RR
relative risk
- GLM
generalized linear model
- IRR
incident rate ratio
References
- 1.Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. Aug 2000;90(8):1212–5. doi: 10.2105/ajph.90.8.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kushner JA. Apartheid in America: An Historical and Legal Analysis of Contemporary Racial Segregation in the United States. Harvard Law Journal. 1979;22(547):559–560. [Google Scholar]
- 3.Krieger N, Van Wye G, Huynh M, et al. Structural Racism, Historical Redlining, and Risk of Preterm Birth in New York City, 2013–2017. Am J Public Health. Jul 2020;110(7):1046–1053. doi: 10.2105/AJPH.2020.305656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reina VJ, Pritchett WE, Wachter SM, eds. Perspectives on fair housing. University of Pennsylvania Press; 2020. [Google Scholar]
- 5.Rights UCoC. Understanding Fair Housing US Government Printing Office; 1973. [Google Scholar]
- 6.Turner MA, Santos R, Levy DK, Wissoker D, Aranda C, Pittingolo R. Housing Discrimination against Racial and Ethnic Minorities 2012. US Departrment of Housing and Urban Development, Office of Policy Development and Research; 2013. [Google Scholar]
- 7.Cashin S The Failures of Integration: How Race and Class Undermine America’s Dream.. Public Affairs; 2004.
- 8.Charles CZ. The Dynamics of Residential Segregation. Annual Review of Sociology. 2003;29:167–207. [Google Scholar]
- 9.Hayanga AJ, Zeliadt SB, Backhus LM. Residential segregation and lung cancer mortality in the United States. JAMA Surg. Jan 2013;148(1):37–42. doi: 10.1001/jamasurgery.2013.408 [DOI] [PubMed] [Google Scholar]
- 10.Jackson SA, Anderson RT, Johnson NJ, Sorlie PD. The relation of residential segregation to all-cause mortality: a study in black and white. Am J Public Health. Apr 2000;90(4):615–7. doi: 10.2105/ajph.90.4.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popescu I, Duffy E, Mendelsohn J, Escarce JJ. Racial residential segregation, socioeconomic disparities, and the White-Black survival gap. PLoS One. 2018;13(2):e0193222. doi: 10.1371/journal.pone.0193222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams DR, Lawrence JA, Davis BA. Racism and Health: Evidence and Needed Research. Annu Rev Public Health. Apr 1 2019;40:105–125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert KL, Ransome Y, Dean LT, DeCaille J, Kawachi I. Social Capital, Black Social Mobility, and Health Disparities. Annu Rev Public Health. Apr 5 2022;43:173–191. doi: 10.1146/annurev-publhealth-052020-112623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MH, Schwartz GL, White JS, et al. School racial segregation and long-term cardiovascular health among Black adults in the US: A quasi-experimental study. PLoS Med. Jun 2022;19(6):e1004031. doi: 10.1371/journal.pmed.1004031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. Sep-Oct 2001;116(5):404–16. doi: 10.1093/phr/116.5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. Jul 12 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205 [DOI] [PubMed] [Google Scholar]
- 17.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Circulation. Jan 13 2015;131(2):141–8. doi: 10.1161/CIRCULATIONAHA.114.011345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unger E, Diez-Roux AV, Lloyd-Jones DM, et al. Association of neighborhood characteristics with cardiovascular health in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. Jul 2014;7(4):524–31. doi: 10.1161/CIRCOUTCOMES.113.000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leal C, Chaix B. The influence of geographic life environments on cardiometabolic risk factors: a systematic review, a methodological assessment and a research agenda. Obes Rev. Mar 2011;12(3):217–30. doi: 10.1111/j.1467-789X.2010.00726.x [DOI] [PubMed] [Google Scholar]
- 20.Gao X, Kershaw KN, Barber S, et al. Associations Between Residential Segregation and Incident Hypertension: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. Feb 2022;11(3):e023084. doi: 10.1161/JAHA.121.023084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greer S, Kramer MR, Cook-Smith JN, Casper ML. Metropolitan racial residential segregation and cardiovascular mortality: exploring pathways. J Urban Health. Jun 2014;91(3):499–509. doi: 10.1007/s11524-013-9834-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh GK, Siahpush M, Azuine RE, Williams SD. Widening Socioeconomic and Racial Disparities in Cardiovascular Disease Mortality in the United States, 1969–2013. Int J MCH AIDS. 2015;3(2):106–18. [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. Feb 22 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 24.Yang TC, Matthews SA. Death by Segregation: Does the Dimension of Racial Segregation Matter? PLoS One. 2015;10(9):e0138489. doi: 10.1371/journal.pone.0138489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massey DS, Denton NA. The Dimensions of Residential Segregation. Social Forces. 1988;67(2):281–315. [Google Scholar]
- 26.Prevention CfDCa. Single-Race Population Estimates. Accessed June 4, 2023,
- 27.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. Jan 25 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao Q, Heiss G, Kucharska-Newton A, Bey G, Love SM, Whitsel EA. Life-Course Neighborhood Socioeconomic Status and Cardiovascular Events in Black and White Adults in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. Jul 23 2022;191(8):1470–1484. doi: 10.1093/aje/kwac070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soliman MR, Derosa CT, Mielke HW, Bota K. Hazardous wastes, hazardous materials and environmental health inequity. Toxicol Ind Health. Sep-Oct 1993;9(5):901–12. doi: 10.1177/074823379300900511 [DOI] [PubMed] [Google Scholar]
- 30.Hammer PJ. The Flint Water Crisis, the Karegnondi Water Authority and Strategic-Structural Racism. Critical Sociology. 2017;45(1):103–119. [Google Scholar]
- 31.Benmarhnia T, Huang J, Basu R, Wu J, Bruckner TA. Decomposition Analysis of Black-White Disparities in Birth Outcomes: The Relative Contribution of Air Pollution and Social Factors in California. Environ Health Perspect. Oct 4 2017;125(10):107003. doi: 10.1289/EHP490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. Dec 2004;112(17):1645–53. doi: 10.1289/ehp.7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payne-Sturges DC, Gee GC, Cory-Slechta DA. Confronting Racism in Environmental Health Sciences: Moving the Science Forward for Eliminating Racial Inequities. Environ Health Perspect. May 2021;129(5):55002. doi: 10.1289/EHP8186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billimek J, Sorkin DH. Self-reported neighborhood safety and nonadherence to treatment regimens among patients with type 2 diabetes. J Gen Intern Med. Mar 2012;27(3):292–6. doi: 10.1007/s11606-011-1882-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piro FN, Noss O, Claussen B. Physical activity among elderly people in a city population: the influence of neighbourhood level violence and self perceived safety. J Epidemiol Community Health. Jul 2006;60(7):626–32. doi: 10.1136/jech.2005.042697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eberly LA, Julien H, South EC, et al. Association Between Community-Level Violent Crime and Cardiovascular Mortality in Chicago: A Longitudinal Analysis. J Am Heart Assoc. Jul 19 2022;11(14):e025168. doi: 10.1161/JAHA.122.025168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell-Wiley TM, Baumer Y, Baah FO, et al. Social Determinants of Cardiovascular Disease. Circ Res. Mar 4 2022;130(5):782–799. doi: 10.1161/CIRCRESAHA.121.319811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baez AS, Ortiz-Whittingham LR, Tarfa H, et al. Social determinants of health, health disparities, and adiposity. Prog Cardiovasc Dis. May 11 2023;doi: 10.1016/j.pcad.2023.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumer Y, Pita MA, Turner BS, et al. Neighborhood socioeconomic deprivation and individual-level socioeconomic status are associated with dopamine-mediated changes to monocyte subset CCR2 expression via a cAMP-dependent pathway. Brain Behav Immun Health. Jul 2023;30:100640. doi: 10.1016/j.bbih.2023.100640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumer Y, Pita MA, Baez AS, et al. By what molecular mechanisms do social determinants impact cardiometabolic risk? Clin Sci (Lond). Mar 31 2023;137(6):469–494. doi: 10.1042/CS20220304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noels H, Weber C, Koenen RR. Chemokines as Therapeutic Targets in Cardiovascular Disease. Arterioscler Thromb Vasc Biol. Apr 2019;39(4):583–592. doi: 10.1161/ATVBAHA.118.312037 [DOI] [PubMed] [Google Scholar]
- 42.Yeh JK, Wang CY. Telomeres and Telomerase in Cardiovascular Diseases. Genes (Basel). Sep 1 2016;7(9)doi: 10.3390/genes7090058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. Jan 2002;52(1):1–23. doi: 10.1016/s0022-3999(01)00302-6 [DOI] [PubMed] [Google Scholar]
- 44.Sundquist K, Theobald H, Yang M, Li X, Johansson SE, Sundquist J. Neighborhood violent crime and unemployment increase the risk of coronary heart disease: a multilevel study in an urban setting. Soc Sci Med. Apr 2006;62(8):2061–71. doi: 10.1016/j.socscimed.2005.08.051 [DOI] [PubMed] [Google Scholar]
- 45.Diez Roux AV, Kershaw KN, Lisabeth L. Neighborhoods and cardiovascular risk: beyond individual-level risk factors. Curr Cardiovasc Risk Rep. 2008;2:175–180. [Google Scholar]
- 46.Khatana SAM, Venkataramani AS, Nathan AS, et al. Association Between County-Level Change in Economic Prosperity and Change in Cardiovascular Mortality Among Middle-aged US Adults. JAMA. Feb 2 2021;325(5):445–453. doi: 10.1001/jama.2020.26141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steil J, Lens M. Public Policies to Address Residential Segregation And Improve Health. Health Affairs. 2023; [Google Scholar]
- 48.Kershaw KN, Albrecht SS. Racial/ethnic residential segregation and cardiovascular disease risk. Curr Cardiovasc Risk Rep. Mar 2015;9(3)doi: 10.1007/s12170-015-0436-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cummins S, Flint E, Matthews SA. New neighborhood grocery store increased awareness of food access but did not alter dietary habits or obesity. Health Aff (Millwood). Feb 2014;33(2):283–91. doi: 10.1377/hlthaff.2013.0512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mujahid MS, Diez Roux AV, Morenoff JD, et al. Neighborhood characteristics and hypertension. Epidemiology. Jul 2008;19(4):590–8. doi: 10.1097/EDE.0b013e3181772cb2 [DOI] [PubMed] [Google Scholar]
- 51.Mujahid MS, Diez Roux AV, Shen M, et al. Relation between neighborhood environments and obesity in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. Jun 1 2008;167(11):1349–57. doi: 10.1093/aje/kwn047 [DOI] [PubMed] [Google Scholar]
- 52.Diez-Roux AV. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. Am J Public Health. Feb 1998;88(2):216–22. doi: 10.2105/ajph.88.2.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


