Abstract
The objective of this study was to elaborate Doppler ultrasonographic scan, genetic resistance and serum profile of markers associated with endometritis susceptibility in Egyptian buffalo–cows. The enrolled animals were designed as; twenty five apparently healthy buffalo–cows considered as a control group and twenty five infected buffalo with endometritis. There were significant (p < 0.05) increased of cervical diameter, endometrium thickness, uterine horn diameter, TAMEAN, TAMAX and blood flow through middle uterine artery with significant decrease of PI and RI values in endometritis buffalo–cows. Gene expression levels were considerably higher in endometritis-affected buffaloes than in resistant ones for the genes A2M, ADAMTS20, KCNT2, MAP3K4, MAPK14, FKBP5, FCAMR, TLR2, IRAK3, CCl2, EPHA4, and iNOS. The RXFP1, NDUFS5, TGF-β, SOD3, CAT, and GPX genes were expressed at substantially lower levels in endometritis-affected buffaloes. The PCR-DNA sequence verdicts of healthy and affected buffaloes revealed differences in the SNPs in the amplified DNA bases related to endometritis for the investigated genes. However, MAP3K4 elicited a monomorphic pattern. There was a significant decrease of red blood cells (RBCs) count, Hb and packed cell volume (PCV) with neutrophilia, lymphocytosis and monocytosis in endometritis group compared with healthy ones. The serum levels of Hp, SAA, Cp, IL-6, IL-10, TNF-α, NO and MDA were significantly (P˂0.05) increased, along with reduction of CAT, GPx, SOD and TAC in buffalo–cows with endometritis compared to healthy ones. The variability of Doppler ultrasonographic scan and studied genes alongside alterations in the serum profile of investigated markers could be a reference guide for limiting buffalo endometritis through selective breeding of natural resistant animals.
Keywords: Buffaloes, Endometritis, Transrectal Doppler ultrasound, Gene expression, Single nucleotide polymorphisms, Antioxidants, Immunity
Subject terms: Immunology, Molecular biology, Zoology, Biomarkers, Cardiology, Health care, Risk factors
Although this species is typically raised in tough settings and exhibits limited reproductive and productive potentials, buffaloes are the primary source of high-quality meat and milk in Egypt and some other developing nations1. According to2,3, endometritis is characterized as a local inflammatory condition of the endometrium that results in large economic losses due to the delay in conception, increased artificial inseminations, expense of veterinary services, loss of the calf crop, and higher culling rate. Due to inadequate cleanliness, the shape of vulval lips, vaginal stimulation for milk let down, and wallowing behavior, buffalo–cows have a substantially greater prevalence rate of uterine infection than cows4. Uterine infection is one of the most significant reproductive issues in buffalo–cows, according to5,6.
In order to establish an accurate diagnosis and appropriate therapy, timing and diagnostic modalities are crucial7. Physical examination, ultrasonography, endometrial biopsies, cytology, and uterine culture are all necessary for the diagnosis of endometritis8. Although the development of transrectal ultrasound represented a significant advance in the assessment of uterine diseases9, it is not always simple to determine the precise diagnosis of endometritis. In order to analyze the reproductive system, Doppler ultrasound, a technique that can measure uterine blood flow, has recently been developed10 because endometrial alterations are linked to abnormalities in uterine blood flow and poor uterine vascular perfusion pattern11. The use of color Doppler ultrasonography to examine the correlations between intrauterine fluid buildup and uterine vascular perfusion is still relatively new, despite the growing popularity of Doppler in reproductive diagnostics.
Blood biochemical investigations give plenty of information about an animal's nutritional status, health, and wellbeing, therefore they can be used to assess the health of animals in general12,13. An estimate of the severity of the damage to the body's tissues could be made by observing a divergence of some blood values from their normal ranges14. An imbalance between the oxidant and antioxidant arms causes oxidative stress15. According to16, it has been reported to occur in bovine endometritis and is characterized by an increase in free radicals and nitric oxide. Sperm, ova, and embryogenesis are all affected by oxidative stress17.
A class of soluble proteins known as cytokines has the ability to control how cells and tissues function even at extremely low concentrations. They are created locally in response to stimuli, have a short half-life, and can act in an autocrine, exocrine, or endocrine manner18. Proinflammatory cytokine IL-6 is released by macrophages and T cells, and it is a strong pyrogen that causes the production of acute phase proteins (APP) by binding to receptors in hepatocytes19. An important antagonist of the Th1 type response is the anti-inflammatory cytokine IL-1020. To evaluate the innate immune system's response to infection or inflammation, serum cytokine levels can be evaluated.
The acute phase proteins (APP) are a class of blood proteins mostly made by the liver that are involved in the protection of infected animals from pathological harm, the maintenance of homeostasis, and the restriction of microbial growth in an antibody-independent way. Numerous pathogenic (including viral and non-infectious disorders) and physiological (including diet, age, sex, pregnancy, breastfeeding, and environmental variables) factors have an impact on APPs levels21. Due to the fact that APP levels are correlated with illness severity, they may also be employed as prognostic indicators and as measures of flock health22. According to23, Acute phase reactants can be classified as positive or negative, depending on their serum concentrations during inflammation. Positive acute phase reactants are upregulated, and their concentrations increase during inflammation. Negative acute phase reactants are downregulated, and their concentrations decrease during inflammation. Positive acute phase reactants include procalcitonin, C-reactive protein, ferritin, fibrinogen, hepcidin, and serum amyloid A. Negative acute phase reactants include albumin, prealbumin, transferrin, retinol-binding protein, and antithrombin. Haptoglobin (Hp) is the main APP in ruminants, and serum amyloid A (SAA) is among the positive APPs that see a rise throughout the AP response24.
The advanced molecular genetic techniques could help as adjunct to control the disease by improving animal health25. Several genetic markers, mostly single nucleotide polymorphisms (SNPs), have been successfully identified for disease susceptibility/resistance in livestock26. This suggests that there are variations between host genomes in the degree of susceptibility/resistance to the disease27. It is hypothesized that alterations in the hemodynamics of the uterus tissue and arteries may happen in buffalo cows presenting endometritis in the puerperal. Thus, the use of more than one diagnostic technique may help in the identification of uterine diseases. So far, there is limited information about the metabolic, immunological, antioxidant alterations; SNPs and gene expression associated with buffalo cow's endometritis. Therefore, the aim of the present study was to validate the diagnostic use of Doppler ultrasonography and exploring SNPs, gene expression and serum profile of APPs, immune and antioxidant markers of endometritis in buffalo cows.
Results
Clinical findings
There was a significant (P < 0.05) increase of body temperature, pulse and respiratory rates (39.9 ± 0.03 °C, 67.6 ± 4.3Beats/min and 31 ± 0.5Breaths/min), respectively in endometritis group in relation to control group (38.1 ± 0.2 °C, 48.6 ± 1.8Beats/min and 22 ± 1.1Breaths/min), respectively (Table 1).
Table 1.
Changes in temperature, pulse and respiratory rates, in control (N = 25) and endometritis (N = 25) buffalo cows (mean ± SE).
| Parameters | Control buffalo–cow | Endometritis buffalo–cow | p value |
|---|---|---|---|
| Temperature (°C) | 38.1 ± 0.2 | 39.9 ± 0.03* | 0.001 |
| Pulse rate (Beats/min) | 48.6 ± 1.8 | 67.6 ± 4.3* | 0.01 |
| Respiratory rate (Breaths/min) | 22 ± 1.1 | 31 ± 0.5* | 0.002 |
*Statistically significant when P < 0.05.
Ultrasonographic and Doppler findings
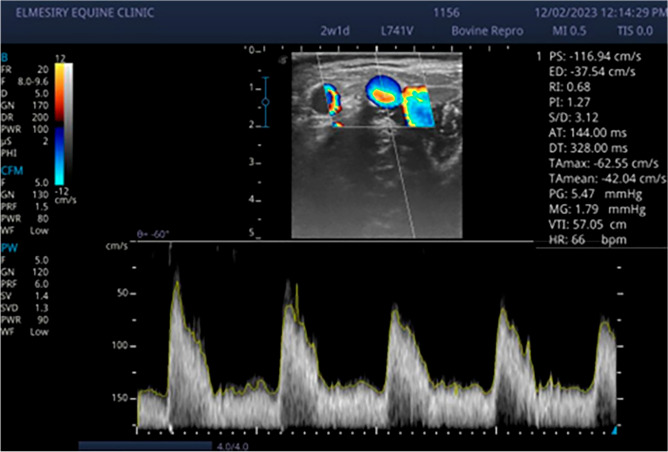
The normal uterus at the time of estrus showed a distinct folding of the endometrium; generally, images of endometritis were characterized by a distended lumen filled to a varying degree with partially echogenic, ‘snowy’ patches and intrauterine heterogeneous content (Fig. 1). There were significant (p < 0.05) increased of cervical diameter, endometrium thickness, and uterine horn diameter between endometritis buffalo–cow and control ones (Table 2). There were significant (p < 0.05) decreased of PI and RI values with significant (p < 0.05) increased of TAMEAN, TAMAX and transverse diameter of middle uterine artery in endometritis buffalo–cows (Table 2 and Fig. 2). Also, significantly higher (p < 0.05) blood flow through middle uterine artery, that was BFV‐TAMAX and BFV‐TAMEAN was evident in endometritis buffalo–cow as compared to normal ones (Table 2).
Figure 1.
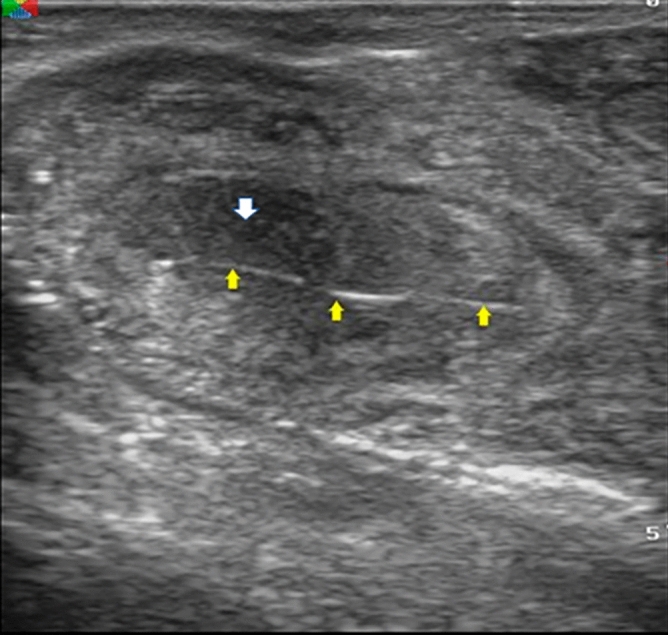
Ultrasonographic images of endometritis in buffalo cow characterized by a distended lumen filled to a varying degree with partially echogenic, ‘snowy’ patches (yellow arrow) and intrauterine heterogeneous content (white arrow).
Table 2.
Transrectal ultrasonographic findings and Blood flow parameters in control (N = 25) and endometritis buffalo–cow (N = 25).
| Parameters | Normal buffalo | Endometritis buffalo | P values |
|---|---|---|---|
| Cervical diameter (cm) | 3 ± 0.01 | 3.6 ± 0.0.04* | 0.001 |
| Uterine horn diameter (cm) | 2.2 ± 0.05 | 2.5 ± 0.01* | 0.006 |
| Endometrium thickness (cm) | 0.65 ± 0.03 | 0.92 ± 0.02* | 0.001 |
| Pulsatility index | 2.5 ± 0.2 | 1.4 ± 0.1* | 0.01 |
| Resistance index | 0.88 ± 0.06 | 0.65 ± 0.02* | 0.02 |
| TAMAX (cm/sec) | 28.7 ± 2.1 | 47 ± 2.1 | 0.004 |
| TAMEAN (cm/sec) | 15.8 ± 1.6 | 29.4 ± 2* | 0.007 |
| Diameter of the MUA (cm) | 0.71 ± 0.03 | 0.89 ± 0.005 | 0.007 |
| Blood flow volume‐ TAMAX (ml/min) | 6.9 ± 1 | 17.4 ± 0.9* | 0.002 |
| Blood flow volume‐ TAMEAN (ml/min) | 3.8 ± 0.5 | 10.9 ± 0.7* | 0.001 |
TAMAX The time average maximum velocity, TAMEAN Time average mean velocity, MUA Middle uterine artery.
*Statistically significant when P < 0.05.
Figure 2.
Doppler sonogram images illustrate waveform of at mid‐estrus buffalo cow; Waveforms diagnosed with clinical endometritis characterized by low RI and PI with significant (p < 0.05) increased of TAMEAN (cm/sec) and TAMAX (cm/sec).
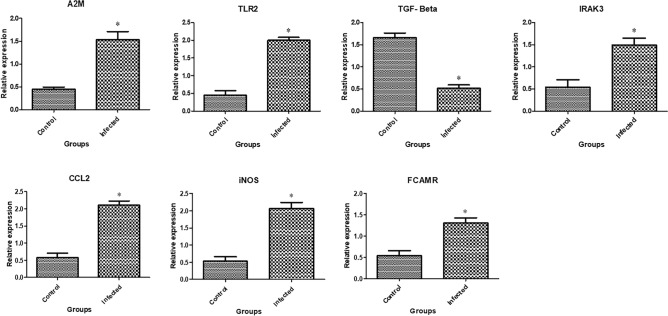
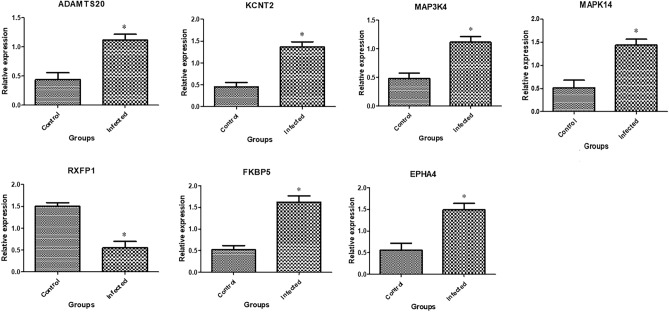
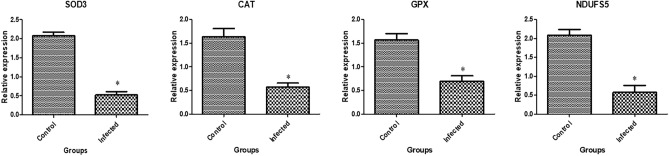
Patterns for transcript levels of immune, metabolic, and antioxidant indicators
In Figs. 3, 4, and 5 the transcript profiles for the assessed immune, metabolic, and antioxidant indicators are displayed. Gene expression levels were considerably higher in endometritis-affected buffaloes than in resistant ones for the genes A2M, TLR2, IRAK3, CCl2, FCAMR, iNOS, ADAMTS20, KCNT2, MAP3K4, MAPK14, FKBP5, and EPHA4. The RXFP1, NDUFS5, TGF-β, SOD3, CAT, and GPX genes were expressed at substantially lower levels in endometritis-affected buffaloes.
Figure 3.
Different immune gene transcript levels between normal and endometritis affected buffaloes. The symbol *denotes significance when p < 0.05.
Figure 4.
Different metabolic gene transcript levels between normal and endometritis affected buffaloes. The symbol *denotes significance when p < 0.05.
Figure 5.
Different antioxidant gene transcript levels between normal and endometritis affected buffaloes. The symbol *denotes significance when p < 0.05.
Genetic polymorphisms of immune, metabolic and antioxidant genes
The PCR-DNA sequence verdicts of healthy and affected buffaloes revealed differences in the SNPs in the amplified DNA bases related to endometritis for the A2M (304-bp), TLR2 (224-bp), TGF-β (420-bp), IRAK3 (310-bp), CCl2 (356-bp), FCAMR (330-bp), iNOS (445-bp), ADAMTS20 (369-bp), KCNT2 (420-bp), MAPK14 (300-bp), FKBP5 (345-bp), RXFP1(360-bp), EPHA4 (420-bp), SOD3 (360-bp), CAT (362-bp), GPX (287-bp), and NDUFS5 (328-bp) genes. However, MAP3K4 (450-bp) elicited a monomorphic pattern. All the discovered SNPs were approved using the DNA sequence differences between immune, metabolic, and antioxidant markers investigated in the researched buffaloes and the reference gene sequences obtained from GenBank. The exonic region changes were present in all of the immune, metabolic, and antioxidant markers under investigation, causing coding DNA sequence alterations in the affected buffaloes compared to healthy ones (Table 3).
Table 3.
Distribution of SNPs, type of mutation in immune and antioxidant genes in healthy and endometritis affected buffaloes.
| Gene | SNPs | Healthy | Mastitis | Total | Type of mutation | Amino acid number and type |
|---|---|---|---|---|---|---|
| A2M | C90T | 0/25 | 14/25 | 14/50 | Synonymous | 30 V |
| A242G | 0/25 | 20/25 | 20/50 | Non-synonymous | 81 H to R | |
| TLR2 | G93A | 8/25 | 0/25 | 8/50 | Synonymous | 31 T |
| TGF-β | T50C | 0/25 | 21/25 | 21/50 | Non-synonymous | 17 I to T |
| T372G | 0/25 | 17/25 | 17/50 | Non-synonymous | 124 D to E | |
| IRAK3 | C73T | 13/25 | 0/25 | 13/50 | Non-synonymous | 25 P to S |
| CCL2 | G177A | 0/25 | 9/25 | 9/50 | Non-synonymous | 59 M to I |
| C222T | 17/25 | 0/25 | 17/50 | Synonymous | 74 P | |
| FCAMR | G178C | 0/25 | 13/25 | 13/50 | Non-synonymous | 60 D to H |
| iNOS | T203C | 0/25 | 14/25 | 14/50 | Non-synonymous | 68 L to P |
| ADAMTS20 | A319C | 19/25 | 0/25 | 19/50 | Non-synonymous | 107 L to I |
| KCNT2 | T50C | 0/25 | 23/25 | 23/50 | Non-synonymous | 17 S to F |
| A226T | 0/25 | 11/25 | 11/50 | Non-synonymous | 76 T to S | |
| MAP3K4 | G46A | 14/25 | 0/25 | 14/50 | Non-synonymous | 16 V to I |
| T159C | 16/25 | 0/25 | 16/50 | Synonymous | 53 T | |
| RXFP1 | T219C | 18/25 | 0/25 | 18/50 | Synonymous | 73 N |
| FKBP5 | A227G | 20/25 | 0/25 | 20/50 | Non-synonymous | 76 N to S |
| EPHA4 | A31G | 9/25 | 0/25 | 9/50 | Non-synonymous | 11 T to A |
| T129G | 12/25 | 0/25 | 12/50 | Non-synonymous | 43 S to R | |
| SOD3 | T117G | 0/25 | 11/25 | 11/50 | Synonymous | 59 G |
| CAT | T175A | 22/25 | 0/25 | 22/50 | Non-synonymous | 59 L to M |
| A340G | 0/25 | 18/25 | 18/50 | Non-synonymous | 114 M to V | |
| GPX | G90T | 0/25 | 13/25 | 13/50 | Synonymous | 30 A |
| NDUFS5 | C179T | 0/25 | 14/25 | 14/50 | Non-synonymous | 60 A to V |
A2M alpha-2-macroglobulin, TLR2 Toll-like receptor 2, TGF-β Transforming growth factor beta, IRAK3 Interleukin 1 receptor associated kinase 3, CCL2 C–C Motif Chemokine Ligand 2, FCAMR Fc alpha and Mu receptor, iNOS Inducible nitric oxide synthase, ADAMTS20 ADAM metallopeptidase with thrombospondin type 1 motif 20, KCNT2 Potassium sodium-activated channel subfamily T member 2, MAP3K4 Mitogen-activated protein kinase kinase kinase 4, MAPK14 Mitogen-activated protein kinase 14, FKBP5 FKBP prolyl isomerase 5, RXFP1 Relaxin family peptide receptor 1, EPHA4 Ephrin type-A receptor 4, SOD3 Superoxide dismutase 3, CAT Catalase, GPX Glutathione peroxidase, and NDUFS5 NADH:ubiquinone oxidoreductase subunit s5. A Alanine, D Aspartic acid, E Glutamic acid, F Phenylalanine, G Glycine, H Histidine, I Isoleucine, L Leucine, M Methionine, N Asparagine, P Proline, R Argnine, S Serine, T Threonine, V Valine.
Hematological and biochemical profile
Hematologically, Red blood cells (RBCs) count, Hb and packed cell volume (PCV) were significantly (P˂0.05) decreased in endometritis group compared with healthy ones; While leukogram revealed enhanced cellular immunity represented by neutrophilia, lymphocytosis and monocytosis (P˂0.05) in endometritis buffalo–cow; however no significant change were observed in values of MCV, MCH and MCHC in both groups (Table 4). Concerning the APPs and cytokines, the serum levels of Hp, SAA, Cp, IL-6, IL-10 and TNF-α were significantly (P˂0.05) increased in buffalo–cows with endometritis compared to healthy ones. With respect to changes in antioxidant/oxidative stress biomarkers, CAT, GPx, NO, SOD and TAC showed a significant decrease associated with a significant increase of MDA in buffalo–cows with endometritis compared to healthy group (Table 5).
Table 4.
Some hematological parameters of control (N = 25) and endometritis (N = 25) buffalo cows.
| Parameters | Normal buffalo | Endometritis buffalo | P values |
|---|---|---|---|
| RBC (× 1012/L) | 5.2 ± 0.2 | 3.9 ± 0.0.1* | 0.01 |
| Hb (g/dl) | 10.8 ± 0.4 | 9 ± 0.2* | 0.02 |
| PCV% | 32.6 ± 1.7 | 24.3 ± 0.8* | 0.01 |
| MCV (fL) | 50.2 ± 0.9 | 51.6 ± 2.6 | 0.65 |
| MCH (pg) | 17.2 ± 0.14 | 17.3 ± 0.14 | 0.65 |
| MCHC (g/dl) | 34.6 ± 0.7 | 35.2 ± 0.4 | 0.52 |
| WBC(× 109/L) | 6.8 ± 0.4 | 10.6 ± 0.06* | 0.001 |
| Lymphocyte (× 109/L) | 1.6 ± 0.2 | 2.9 ± 0.0.4* | 0.04 |
| Monocyte (× 109/L) | 0.6 ± 0.02 | 0.9 ± 0.08* | 0.02 |
| Neutrophil (× 109/L) | 3.6 ± 0.12 | 7 ± 0.7* | 0.01 |
RBC Erythrocytes count, Hb Hemoglobin, PCV Packed cell volume, MCV mean corpuscular volume, MCH mean corpuscular hemoglobin, MCHC Mean corpuscular hemoglobin concentration, WBC total leukocytes count.
*Statistically significant when P < 0.05.
Table 5.
Serum acute phase proteins, immunological and oxidant/antioxidant values in control (N = 25) and endometritis buffalo–cow (N = 25) (Mean ± SE).
| Parameters | Normal buffalo | Endometritis buffalo | P values |
|---|---|---|---|
| Haptoglobin (ng/ml) | 58.6 ± 0.88 | 87.6 ± 2* | 0.002 |
| Serum amyloid A (mg/l) | 4.9 ± 0.08 | 9.6 ± 0.17* | 0.001 |
| Ceruloplasmin (mg/dl) | 22 ± 3.4 | 46.6 ± 3.2* | 0.007 |
| Interleukin 6 (pg/ml) | 25.6 ± 3.1 | 85.6 ± 6.3* | 0.004 |
| Interleukin 10 (pg/ml) | 27.3 ± 3.9 | 98 ± 8.5* | 0.006 |
| TNF-α (pg/mL) | 42 ± 5.2 | 132 ± 16.7* | 0.02 |
| Catalase (U/l) | 36 ± 0.57 | 16.3 ± 1.4* | 0.002 |
| GPx (U/mL) | 50 ± 2.8 | 20 ± 1.1* | 0.004 |
| Nitric oxide (µmol/L) | 22.2 ± 9.7 | 49.6 ± 2.6* | 0.04 |
| SOD (U/ml) | 27.3 ± 1.4 | 64 ± 2* | 0.001 |
| TAC (mM/L) | 54.3 ± 2.3 | 24.6 ± 2.3* | 0.001 |
| MDA (nmol/mL) | 10.9 ± 3.8 | 30.6 ± 1.2* | 0.02 |
TNF-α Tumor necrosis factor-alpha, GPx Glutathione peroxidase, SOD Super oxide dismutase, TAC Total antioxidant capacity, MDA Malondialdhyde.
*Statistically significant when P < 0.05.
Correlation between ultrasonographic and Doppler findings, gene expression pattern and serum profile of immune, APPs and antioxidant markers
The uterine horn diameter was positively correlated with the mRNA levels of CATand GPXand serum level of Cp (r = 1, P = 0.014, r = 1, P = 0.012 and r = 0.997, P = 0.049) respectively. Uterine artery diameter was positively correlated with the mRNA levels of A2M and FCAMR (r = 1, P = 0.016 and r = 0.998, P = 0.038) respectively and negatively correlated with mRNA levels of MAPK14 (r = − 998, P = 0.035). The endometrium thickness was positively correlated with serum level of Cp and IL10 (r = 1, P = 0.019 and r = 0.999, P = 0.032), respectively and negatively correlated with serum level of SAA (r = − 999, P = 0.023). Pulsatility index (PI) was positively correlated with the mRNA levels of MAP3K4, iNOS and CCl2 (r = 1, P = 0.019, r = 1, P = 0.015 and r = 0.999, P = 0.031) respectively and negatively correlated with mRNA levels of KCNT2 (r = − 998, P = 0.045). Resistance index (RI) was positively correlated with the mRNA levels of MAPK14 (r = 0.998, P = 0.035) and negatively correlated with mRNA levels of A2M and FCAMR (r = − 1, P = 0.016 and r = − 0.998, P = 0.038), respectively. Time average mean velocity (TAMEAN) was negatively correlated with mRNA levels of GPX(r = − 999, P = 0.024) and positively correlated with serum level of Cp (r = 1, P = 0.013).Blood flow volume‐ TAMAX was positively correlated with the mRNA levels of TGF (r = 0.998, P = 0.039). Blood flow volume‐ TAMEAN was negatively correlated with serum level of TNF-α (r = − 997, P = 0.049). The serum level of MDA was positively correlated with mRNA levels of ADAMTS20 and RXFP1 (r = 0.999, P = 0.031 and r = 0.998, P = 0.042), respectively. The serum level of TNF-α was positively correlated with mRNA levels of CAT and GPX (r = 1, P = 0.008 and r = 0.999, P = 0.034), respectively.
Discussion
The objectives of the study were to validate the use Doppler ultrasonographic scan, gene expression and serum profile of metabolic, immune, APPs and antioxidant markers as diagnostic criteria for clinical endometritis in Egyptian Buffalo–Cows. Postpartum uterine infection is one of the most important disorders in bovines6. It causes great economic losses due to its negative effect on reproductive performance as it increases services per conception, the calving to first service interval and the calving to conception interval, reduces the risk of pregnancy, and decreases the conception rate28.
The significant increase of body temperature, pulse and respiratory rates in endometritis group could be attributed to interaction between the host immune system and bacterial endotoxins which trigger the cascade of events that lead to elevated temperature. Our clinical findings were similar to that observed by29 in cows.
The statistical examination of cervical diameter, endometrium thickness, and uterine horn diameter showed that endometritis buffalo–cow levels were significantly higher than normal levels (p > 0.05). Our findings were consistent with those reported by30, but in contrast to findings of31 in dairy cow. The later authors reported non-significant difference of cervical diameter, endometrium thickness, and uterine horn diameter between normal and endometritis animals. During uterine inflammation, where minimum penetration is necessary32, color Doppler method is a valuable tool for visualizing physiological and pathological processes of the reproductive system in cattle33. A greater PI denotes less perfusion to distant tissues, whereas a lower RI implies increased perfusion to the specific organ34. Our results were comparable to those in dairy cows published by35, But way from that study30, found no changes between groups in their spectral Doppler analysis of the uterine arteries. According to36, the bacterial infection in women leads to hyperaemia in pelvic organs, increasing uterine blood flow but causing low PI and RI values in the uterine artery. Clearance of infection leads to increased PI and RI values37. Studies by35, showed a negative correlation between RI and velocity and volume of blood flow. In the same context38, found that, TAMEAN and TAMAX were having an inverse relationship with RI and PI, while35 found non-significantly higher uterine artery diameter.
Through measuring the mRNA levels of immune (A2M, TLR2, TGF-β, IRAK3, CCl2, FCAMR, and iNOS), metabolic (ADAMTS20, KCNT2, MAP3K4, MAPK14, RXFP1, FKBP5, RXFP1, and EPHA4), and antioxidant (SOD3, CAT, GPX, and NDUFS5) genes, we examined the changes in the immune, metabolic, and antioxidant state in postparturient endometritis-affected buffalo cows compared with healthy ones. Gene expression levels were considerably higher in endometritis-affected buffaloes than in resistant ones for the genes A2M, TLR2, IRAK3, CCl2, FCAMR, iNOS, ADAMTS20, KCNT2, MAP3K4, MAPK14, FKBP5, and EPHA4. The RXFP1, NDUFS5, TGF-β, SOD3, CAT, and GPX genes were expressed at substantially lower levels in endometritis-affected buffaloes.
This is the first study to fully analyze the transcript levels of the immune, metabolic, and antioxidant indicators linked to the hazard of buffalo endometritis. Consequently, qualitative and quantitative differences in the investigated genes’ expression precede the development of bovine uterine disease. Greater relative quantities of mRNA for the IL1A, IL6, IL17A, TNF, PGES, and PGHS2 genes were found in primiparous Holstein cows postpartum when compared to healthy cows39. Additionally, C3, C2, LTF, PF4, and TRAPPC13 had unique mRNA expression patterns in the blood and endometrial tissue of dairy cows with subclinical endometritis40. In contrast to control cows, cows with clinical and subclinical endometritis displayed a significant change in the mRNA expression of uterus-associated proinflammatory markers, according to41. In the endometrium of repeat breeding cows with and without subclinical endometritis, there were significantly more transcript levels of tumor necrosis factor and inducible nitric oxide synthase42. Three examined cytokines, including IL-1, IL-1β, and IL-6, were found to have increased gene expression in buffaloes with endometritis compared to healthy animals43. IL10, ATOX1, and GST genes were expressed at substantially lower levels with higher level of genes TLR4, TLR7, TNF-α, NCF4, LITAF, OXSR1, TKT, RPIA, and AMPD1 in endometritis-affected cows as compared with resistant ones44. Buffaloes were significantly more likely to express the inflammatory (IKBKG, LGALS, IL1B, CCL2, RANTES, MASP2, HMGB1, and S-LZ) genes when they had inflammatory reproductive diseases45.
The immune (A2M, TLR2, TGF-β, IRAK3, CCl2, and iNOS), metabolic (ADAMTS20, KCNT2, MAP3K4, MAPK14, RXFP1, FKBP5, RXFP1 FCAMR, and EPHA4), and antioxidant (SOD3, CAT, GPX, and NDUFS5) genes in endometritis-affected and healthy Holstein dairy cows were characterized in this research using a PCR-DNA sequencing technique. The findings show that the SNPs involving both categories vary. It is important to emphasize that the polymorphisms found and made available in this context provide additional data for the evaluated indicators when compared to the corresponding datasets acquired from GenBank. There have been recent studies targeting novel genes specific to livestock endometritis susceptibility using genome-wide association analysis46,47, but up to this point, no studies have examined the link between the SNPs in these genes and endometritis risk. The Bubalus bubalis gene sequences used in our study, which were reported in PubMed, are the first to demonstrate this association.
According to our knowledge, there has not been any prior research on the variation of the immune (A2M, TLR2, TGF-β, IRAK3, CCl2, and iNOS), metabolic (ADAMTS20, KCNT2, MAP3K4, MAPK14, RXFP1, FKBP5, RXFP1 FCAMR, and EPHA4), and antioxidant (SOD3, CAT, GPX, and NDUFS5) markers and how they relate to postparturient endometritis in buffaloes. The candidate gene method, however, was employed to keep track of the soundness of endometritis-affected livestock. For example, endometritis and CXCR1 SNPs have been linked in Holstein dairy cows48. In dairy cattle, uterine infection was linked to lactoferrin (LTF) gene polymorphism49. There has also been evidence linking the beta defensin gene polymorphism and clinical endometritis in dairy cows50. SNPs in the TLR4 and TLR2 genes and endometritis tolerance in buffalo have been elaborated51. Nucleotide sequence variations between healthy and endometritis-affected cows were revealed using PCR-DNA sequencing for immune (TLR4, TLR7, TNF-α, IL10, NCF4, and LITAF), antioxidant (ATOX1, GST, and OXSR1), and erythritol-related (TKT, RPIA, and AMPD1) genes were reported by44. The immunological (IKBKG, LGALS, IL1B, CCL2, RANTES, MASP2, HMGB1, and S-LZ) genes’ nucleotide sequence differences between healthy buffaloes and buffaloes affected by inflammatory reproductive diseases were found by employing PCR-DNA sequencing45.
The alpha-macroglobulin (aM) family of proteins, which includes C3, C4, and C5, also includes alpha-2-macroglobulin (A2M)52. Additionally, it promotes the growth of macrophages and T cells53. Mutations in A2M contributed to mastitis susceptibility in dairy cows54. Innate immune systems, particularly Toll-like receptors and antimicrobial peptides, are vital for the endometrium’s first defense in contradiction of microorganisms55.
Transforming growth factor-beta (TGF-β) is a multifunctional peptide, belonging to a family of cytokines present in many cell types, involved in regulating proliferation, differentiation, adhesion, migration, and immune regulation56. Interleukin 1 receptor associated kinase 3 (IRAK3) is mainly found in monocytes and macrophages, hence it is also known as IRAK-M57. Thus, IRAK3 plays a crucial role in modulating TLR signalling pathways of innate immunity.
C–C Motif Chemokine Ligand 2 (CCL2), which encodes two tiny proteins that bind to the CCR2 receptors present on the surface of monocytes and neutrophils, respectively, acts as a potent chemokine for these cells58. The relationship between single nucleotide polymorphisms in the bovine CCL2 gene and production and health was investigated using Canadian Holstein cattle59. Nitric oxide (NO) plays a vital role in many physiological and pathological processes, and it is synthesized from the amino acid L-arginine by NO synthase (NOS). iNOS is encoded by the NOS2A gene, which is under the transcriptional control of inflammatory mediators produced by immunocompetent cells such as macrophages and neutrophils60.
The role of ADAMTS family proteases in reproductive function and disorders in humans is well known and mutations in ADAMTS20 were associated with endometrial tissue remodeling and inflammation61. A promising candidate gene is the KCNT2 gene on BTA 16 in cattle62 identified KCNT2 as a candidate gene for endometritis within 150 d after calving in first-parity Canadian Holstein cows. Recently63, applied a genome wide association analysis (GWAS) and fine mapping study for disease traits and identified the KCNT2 gene as a main candidate for ketosis in dairy cattle.
Inflammatory diseases may be treated by targeting mitogen-activated protein kinases (MAPKs), which are typically activated in response to inflammatory cytokines and cellular stress64. FKBP5 as a candidate gene interacting with polychlorinated biphenyls, which are increased in human endometritis65. Interestingly, FCAMR may function in immune response to microbes mediated by IgA and IgM66 showed that IgA, IgM, and IgG are the major immunoglobulins for blocking bacterial pathogens from adhering mucosal surface in the uterus. RXFP1 encodes a member of the leucine-rich repeat-containing subgroup of the G protein-coupled 7-transmembrane receptor superfamily. The encoded protein plays a critical role in pregnancy and parturition as a receptor for the protein hormone relaxin.
Erythropoietin-producing hepatocellular receptor A4 (EphA4) gene, as a crucial member of Eph–Ephrin family, encodes a pleiotropic cytokine and EphA4 protein is normally produced by the endometrium67.
Multi-pathogen bacterial infections of the vaginal tract develop in dairy cattle following urination68. A bacterial infection of the endometrium causes the production of chemokines and cytokines, which activates an inflammatory response. Leucocyte recruitment during inflammation has been reported to be interceded by inflammatory cytokines and complement fragments69. Endometritis is also characterized by unchecked extended inflammation linked to tissue damage, which causes the release of molecular forms accompanying injury, further aggravating inflammation and guaranteeing its perseverance47. Afterwards, oxidative stress is brought on by the extreme gathering of ROS70. These modifications are also associated with increased expression of molecules involved in LPS signaling, tissue remodeling, and acute phase response40. The aforementioned reasons could account for the significant amendment in the expression configuration of immune (A2M, TLR2, TGF-β, IRAK3, CCl2, and iNOS), metabolic (ADAMTS20, KCNT2, MAP3K4, MAPK14, RXFP1, FKBP5, RXFP1 FCAMR, and EPHA4), and antioxidant (SOD3, CAT, GPX, and NDUFS5) indicators in endometritis-affected buffaloes. Thus, we assume that an infectious etiology is to blame for the bovine endometritis in the study’s buffaloes. The endometritis-affected buffaloes were exhibiting a substantial inflammatory response, as shown by our real-time PCR data. Gene expression disruption can be used to characterize the common pathological processes, whereas normal gene expression controls the bulk of physiological mechanisms71.
The current study found that buffalo cows with clinical endometritis had normocytic hypochromic anemia, which was demonstrated by a substantial reduction in RBCs, Hb, PCV, neutrophilia, and monocytosis. Buffalo–cows undergo unanticipated nutritional and hormonal changes during the transition phase, which compromises their immune system72. The buffalo–cows are more susceptible to uterine infection as a result of this impaired immune response73. Since the MDA level in these buffalo cows was greater than it was in healthy buffalo cows, it is likely that the large increases in (TLC) and granulocytes were caused by elevated cortisol levels74. In buffalo–cows with endometritis, the TLC, granulocytes, and monocytes continued to rise, suggesting a possible prolonged peripheral inflammatory response in the buffalo–cows with uterine infection74. Bacterial toxins that are circulating in the circulation cause red blood cells to have certain shape abnormalities that cause them to become caught in the spleen network and cause regenerative anemia in animals. The immune system is also stimulated by the presence of germs to create more phagocytic cells, such as neutrophils and monocytes75. Our results disagreed with those published by76 in Egyptian Buffalo–Cows and Murrah buffaloes and77 in crossbreed cows.
APR (acute phase reaction) is one of the many systemic reactions that happen after an infection, damage, or even when the usual physiological balance changes. APR is a series of internal responses to inflammation that are mostly controlled by cytokines, which are produced by macrophages or other inflammatory cells. Production of pro-inflammatory cytokines (mainly IL-1β, TNF-α, and IL-6) at the site of injury, subsequently stimulates the production of APPs from a local site or at the liver. Several writers examined the use of APPs to assess domestic animal health status78. When compared to healthy buffalo–cows, there was a significant rise in the blood levels of Hp, SAA, Cp, IL-6, IL-10, and TNF- in the current study. Our results agreed with those provided by79–81.
In the current investigation, buffalo's cows with endometritis showed disrupted oxidative state, with significantly higher MDA and NO levels and lower activity of CAT, GPx, SOD, and TAC values than in healthy animals. It is well knowledge that inflammatory disorders are linked to heightened oxidative responses and diminished antioxidant defenses82. Reactive oxygen species (ROS), which damage DNA and cell membranes during infection, are produced at high levels in animals with clinical endometritis, as indicated by increased MDA levels83. The inflammation of uterine tissue is associated with increase the serum level of NO resulting in relaxation of smooth muscle and accumulation of inflammatory products in the uterus playing an important role in the increased severity of infection of the uterus. The higher NO level in blood is guessed as an inflammatory reaction of uterine tissue. Elevated values of serum nitric oxide causing loosening of smooth muscle and gathering of inflammatory consequences in the uterus assuming a significant part in the expanded seriousness of infection of the uterus84. The decrease in the concentration of antioxidant enzymes suggests that antioxidant enzymes are faithfully involved in the neutralization and scavenging of free radicals generated during oxidative stress. Reduced levels of antioxidant enzymes are thought to be due to the enzymes' role in converting harmful free radicals into harmless molecules. Our outcomes were like that detailed by76,79,80,85,86.
Conclusion
This study confirmed that non-invasive transrectal Doppler ultrasound can be a useful tool to assess hemodynamic changes during uterine inflammation. Reference values were also provided for further studies on blood flow in endometritis in Egyptian buffaloes. This technique also has great potential in the field of buffalo breeding to determine future fertility and complications during pregnancy. Our findings highlight the significance of SNPs in investigated immune and antioxidant genes as genetic markers and predisposing factors for endometritis resistance/susceptibility. These findings suggest that variability in these genes could be used as proxy biomarkers for such disorder in Egyptian-buffalo cow. The variable expression pattern of immune and antioxidant genes in resistant and non-resistant buffalo–cow to endometritis could be a reference guide and a biomarker that can be used to follow up health status of buffalo–cow. These data open a promising opportunity for limiting endometritis through selective breeding of animals based on genetic markers associated with natural resistance to infection.
Materials and methods
Animals and study design
A total number of 50 Egyptian buffaloes cows with an average of 7–12 years (mean ± SD: 9.42 ± 1.8) and a range of body weight 550–650 kg (mean ± SD: 600 ± 40.82) were used in this study. The experiment was carried out at Siwa Oasis, Egypt which lies between longitudes (Lat: 29° 06″ 29° 24″ N Long: 25° 16″ 26° 12″ E), and located 330 km southwest of the Mediterranean shoreline and at 65 km east of the Libyan borders and in Animal Reproduction Research Institute. Animals were housed in barns, both water and grasses were offered ad lib, with nearly 3 kg/day of commercial concentrate was offered for each buffalo cow. The examined animals were 25 cycling healthy buffalo–cow and 25 buffalo–cow with endometritis. The investigated buffalo–cows were subjected to through clinical examination including recording of temperature, pulse and respiratory rates87. Clinical endometritic was diagnosed as expulsed purulent (> 50% pus) uterine discharge detectable in the vagina more than 21 days after calving or muco-purulent (50% pus–50% mucus) uterine discharge detectable in the vagina after 26 days after calving. Estrus was synchronized through application of Ovisynch protocol as follow: on day zero the buffallo-cow received 100 µg GnRH in form of Gonadroline (Gonavet®,Veyx,Germany), on the 7th day the same buffalo–cows received 500 µg PGF2α in form of Cloprostenol (PGF Veyx Forte®,Veyx,Germany), on the 9th day 100 µg of GnRH (Gonavet®) was injected to the same buffalo–cows. All hormones injections were administrated I.M.
Blood sampling
Ten milliliters of blood was collected from each buffalo–cow via jugular venipuncture at 8 O’clock morning. The collected blood was added to plain tubes (i.e., without anticoagulants) and to others containing EDTA to yield serum or whole blood, respectively. All samples were cooled on crushed ice and were transported immediately to the laboratory for further processing. Tubes containing whole blood were used for CBC and RNA extraction while those in plain tubes were kept overnight at room temperature and centrifuged at 3000 rpm for 15 min. Only clear sera were collected then aliquoted and kept frozen at − 20 °C for subsequent biochemical analyses of energetic and oxidative stress markers.
Ultrasonography and Doppler mode
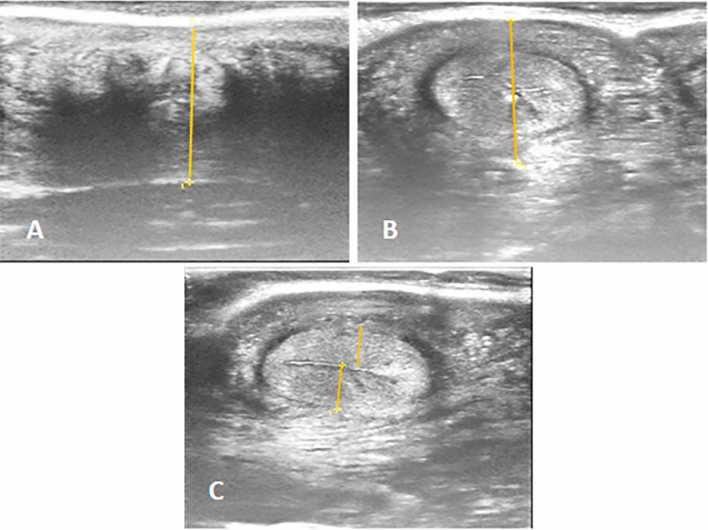
All animals were reproductively assessed by the same operator using ultrasound machine (Sonoscape, E1 Expert, China) provided with high frequency linear transducer: L741, 5–15 MHz to record the diameter of the cervix and uterine horn as well as endometrial thickness (Fig. 6A–C) according to88. The gain, brightness and contrast were set in optimal range for each examination.
Figure 6.
Transrectal ultrasonography, (A): Cervical diameter, (B): Uterine horn diameter, (C): Uterine thickness.
All animals were enrolled for quantitative analysis of blood flow through the middle uterine artery (MUA), a branch of the internal iliac artery that is situated cranial to the external iliac artery and can be found in the mesometrium as a movable arterial vessel and easily seen by the color Doppler technique after the conventional ultrasonographic evaluation35,89. A high frequency linear transducer with a filter of 100 Hz, power of 90%, pulse repetition frequency (PRF) of 6 HZ, and a Doppler angel ranging between 0° and 60° was used to perform the Doppler assessment. The diameter (D; cm) of middle uterine arteries was measured in B-mode, and the mean of three measurements of vessel diameter per examination was calculated from a frozen, two-dimensional, greyscale image were used for statistical analysis. Blood flow indices including pulsatility index (PI), resistance index (RI), TAMEAN (Time average mean velocity) and the time average maximum velocity (TAMAX, cm/s).
Blood flow volume in mL/min was calculated using the equation90:
Total RNA extraction, reverse transcription and quantitative real time PCR
Total RNA was extracted from buffalo blood using Trizol reagent following the manufacturer instructions (RNeasy Mini Ki, Catalogue no.74104). The amount of extracted RNA was quantified and qualified using NanoDrop® ND-1000 Spectrophotometer. The cDNA of each sample was synthesized following the manufacture protocol (Thermo Fisher, Catalog no, EP0441). The gene expression pattern for coding fragments of genes encoding immune (A2M, TLR2, TGF-β, IRAK3, CCl2, FCAMR, and iNOS), metabolic (ADAMTS20, KCNT2, MAP3K4, MAPK14, RXFP1, FKBP5, RXFP1, and EPHA4), and antioxidant (SOD3, CAT, GPX, and NDUFS5) was assessed using quantitative RT-PCR using SYBR Green PCR Master Mix (2 × SensiFastTM SYBR, Bioline, CAT No: Bio-98002). Relative quantification of mRNA level was performed by real-time PCR using SYBR Green PCR Master Mix (Quantitect SYBR green PCR kit, Catalog no, 204141). Primer sequences were designed according to the PubMed published sequence of Bubalus bubalis (Table 6). The housekeeping gene ß. actin was used as a constitutive control for normalization. The reaction mixture was carried out in a total volume of 25 µl consisted of total RNA 3 µl, 4 µl 5 × Trans Amp buffer, 0.25 µl reverse transcriptase, 0.5 µl of each primer, 12.5 µl 2 × Quantitect SYBR green PCR master mix and 8.25 µl RNase free water. The final reaction mixture was placed in a thermal cycler and the following program was carried out: reverse transcription at 50 °C for 30 min, primary denaturation at 94 °C for 10 min followed by 40 cycles of 94 °C for 15 s, annealing temperatures for 1 min as shown in Table 6, and 72 °C for 30 s. At the end of the amplification phase, a melting curve analysis was performed to confirm the specificity of the PCR product. The relative expression of each gene per sample in comparison with ß. actin gene was carried out and calculated according to the 2−ΔΔCt method91.
Table 6.
Oligonucleotide primers sequence, accession number, annealing temperature and PCR product size of immune, metabolic, and antioxidant genes used in real time PCR.
| Gene | Primer | Product length (bp) | Annealing temperature (°C) | Accession number | Source |
|---|---|---|---|---|---|
| A2M | F5′-TGTGCTGTGGTCCGTTTTTACC-3′ | 304 | 58 | KF928932.1 | Current study |
| R5′-CGAGAATATGGGATGGGAGAA-3′ | |||||
| TLR2 | F5′-GCCTCTCATCAGGCTTCTTC-3′ | 224 | 56 | NM_001290891.1 | Current study |
| R5′- GAATCTTCCTCCACTGTGTGA-3′ | |||||
| TGF-β | F5′-CTGTGGTTGCTAATGCTGACG-3′ | 420 | 58 | JQ995536.1 | Current study |
| R5′-CAGCTCTGCCCGAGAGAGCAAC-3′ | |||||
| IRAK3 | F5′-TGGCCGTCGTCGTGCTAGCAG-3′ | 310 | 60 | XM_006066455.3 | Current study |
| R5′-CAATGTGACGAACATCCAGC-3′ | |||||
| CCL2 | F5′-CCAACAGCTTCCACGCTGAA-3′ | 356 | 58 | HQ889748.1 | Current study |
| R5′-TGTGGAGTGAGTGCTCAAGGCT-3′ | |||||
| FCAMR | F5′-GGACCTCGAACTTGCTGCAG-3′ | 330 | 60 | XM_006043810.4 | Current study |
| R5′- CAGCTCCTGGTGGCTGCTGGC-3′ | |||||
| iNOS | F5′-TGGTTCAAGGCATCCTTGAGCG-3′ | 445 | 60 | EF562453.1 | Current study |
| R5′-CGGTACGTGAGCACGGCCACAG-3′ | |||||
| ADAMTS20 | F5′-TATACTTCCGCTACAGGGCACG-3′ | 369 | 56 | XM_006077068.4 | Current study |
| R5′- TCACGTAGTGTTGAAGCCTGGC-3′ | |||||
| KCNT2 | F5′-CTCCATTCGTTGTCTCACGT-3′ | 420 | 58 | XM_044943223.2 | Current study |
| R5′- TAACTGAGATAACCAAGCAGTA-3′ | |||||
| MAP3K4 | F5′-ATCAGACATTGGCTGGCCGGT-3′ | 450 | 60 | XM_055536148.1 | Current study |
| R5′- ACTGGACCGTCGCTCTTAACA-3′ | |||||
| MAPK14 | F5′-TGACAGGCTATGTGGCCACCA-3′ | 300 | 58 | XM_006050391.4 | Current study |
| R5′-CCGGTTCGTCGTCAGGATCGTG-3′ | |||||
| RXFP1 | F5′-TAGTACTGATGAATAACGTCC-3′ | 360 | 58 | XM_044930387.2 | Current study |
| R5′- AAGAGAGATTCATGAGAGGT-3′ | |||||
| FKBP5 | F5′-CAATGACTACTGATGAAGCTG-3′ | 345 | 56 | XM_044930861.2 | Current study |
| R5′- GAGCCATATGCATATTCTGGT-3′ | |||||
| EPHA4 | F5′-ATAGAAGCGGCAGGAGCAGCG-3′ | 221 | 58 | XM_044937879.2 | Current study |
| R5′- CATCCATAATACTCACTTCCT-3′ | |||||
| SOD3 | F5′-CCTGTGACCAGCTGGTGAGGT-3′ | 360 | 56 | XM_006041480.4 | Current study |
| R5′-CCGGGTCGATGGCCGCCGCCTG-3′ | |||||
| CAT | F5′-TGACCACTGGAGGCGGTAATC-3′ | 362 | 58 | XM_044929272.2 | Current study |
| R5′- TCCAACAAGATCCCAATTAC-3′ | |||||
| GPX | F5′- TCTTGTTCTTCAAGTCCGCG -3′ | 287 | 58 | XM_006053253.3 | Current study |
| R5′- AAGCCGAGCACGACCAGGCC-3′ | |||||
| NDUFS5 | F5′-TACGGCAGGCCTCCTAGTCG-3′ | 328 | 58 | XM_006080987.3 | Current study |
| R5′- CGCTGTCTCTTGATGGCATTCA- 3′ | |||||
| ß. actin | F5′- GGAATCCTGCGGTATTCACGA-3′ | 222 | 60 | NM_001290932.1 | Current study |
| R5′- CCGCCAATCCACACAGAGTA -3′ |
A2M alpha-2-macroglobulin, TLR2 Toll-like receptor 2, TGF-β Transforming growth factor beta, IRAK3 Interleukin 1 receptor associated kinase 3, CCL2 C–C Motif Chemokine Ligand 2, FCAMR Fc alpha and Mu receptor, iNOS Inducible nitric oxide synthase, ADAMTS20 ADAM metallopeptidase with thrombospondin type 1 motif 20, KCNT2 Potassium sodium-activated channel subfamily T member 2, MAP3K4 Mitogen-activated protein kinase kinase kinase 4, MAPK14 Mitogen-activated protein kinase 14, FKBP5 FKBP prolyl isomerase 5, RXFP1 Relaxin family peptide receptor 1, EPHA4 Ephrin type-A receptor 4, SOD3 Superoxide dismutase 3, CAT Catalase, GPX Glutathione peroxidase, and NDUFS5 NADH:ubiquinone oxidoreductase subunit s5.
DNA sequencing and polymorphism detection
Before DNA sequencing, removing primer dimmers, nonspecific bands and other impurities was done. As described by92, purification of real time PCR products with expected size (target bands) was carried out using PCR purification kit following the manufacturer procedures (Jena Bioscience # pp-201 × s/Germany). Quantification of PCR product was carried out using Nanodrop (Uv–Vis spectrophotometer Q5000/USA) in order to yield high products and to ensure enough concentrations and purity of the PCR products93. To detect SNPs in genes investigated in control, and endometritis affected buffaloes, PCR products with target band were sent for DNA sequencing in forward and reverse directions using ABI 3730XL DNA sequencer (Applied Biosystem, USA), depending on enzymatic chain terminator technique developed by94.
Analysis of DNA sequencing data was carried out by chromas 1.45 and blast 2.0 software95. Differences were classified as single-nucleotide polymorphisms (SNPs) between PCR products of investigated genes and reference sequences available in GenBank. Based on data alignment of DNA sequencing, variation of amino acid sequence of the investigated genes between enrolled buffaloes was performed using the MEGA6 software package96.
Biochemical analysis
The following commercial kits were used according to the standard protocol of the suppliers to quantify each of: serum amyliod A (SAA) using IBL International Crop (canda)® ELISA kits, haptoglobin (Hp) by Eagle Biosciences (Columbia) ELISA kits, caeruloplasmin (Cp) levels by Arbor Assays DetectX ® (USA)® kits. For malondialdehyde (MDA) (Biodiagnostic Egypt, CAT No: MD2529), catalase (CAT) (Biodiagnostic Egypt, CAT No: CA252417); glutathione peroxidase (GPx) (Biodiagnostic Egypt, CAT No: GR 2511), total antioxidant capacity (TAC) (Biodiagnostic Egypt, CAT No: TA25 13), nitric oxide (NO) (Biodiagnostic Egypt, CAT .No.NO2533), super oxide dismutase (SOD) (Biodiagnostic Egypt, CAT No: SD 25 20), IL 6 (BOSTER BIOLOGICAL TECHNOLOGY, CAT No: EK0412) and TNF-α ELISA Kit (AVIVA SYSTEM BIOLOGY); IL-10 (ELISA kits of Ray Biotech Company®).
Statistical analysis
Statistical analyses were carried out using a statistical software program (SPSS, ver.20, Inc., Chicago, USA). Descriptive statistics were performed for all parameters. Student’s t-test was used analyze the data. Results were considered statistically significant at P ˂ 0.05.
Ethical approval and informed consent
The experimental procedures were approved by the Experimental Animal Care Committee of Desert Research Center, Egypt (Approval No. 2022–0180), and all protocols were carried out in accordance with guidelines and regulations of the Universal Directive on the Protection of Animals Used for Scientific Purposes. All protocols follow the ARRIVE guidelines for reporting animal research (https://arriveguidelines.org).
Acknowledgements
The authors acknowledge the staff members of Animal Health and Poultry Department, Desert Research Center, Egypt and the staff members in Mariut Research Station, Alexandria, Egypt.
Abbreviations
- MUA
Middle uterine artey
- PI
Pulsatility index
- RI
Resistance index
- TAMAX
The time average maximum velocity
- TAMEAN
Time average mean velocity
- RBC
Erythrocytes count
- Hb
Hemoglobin
- PCV
Packed cell volume
- MCV
Mean corpuscular volume
- MCH
Mean corpuscular hemoglobin
- MCHC
Mean corpuscular hemoglobin concentration
- WBC
Total leukocytes count
- TNF-α
Tumor necrosis factor-alpha
- GPx
Glutathione peroxidase
- SOD
Super oxide dismutase
- TAC
Total antioxidant capacity
- MDA
Malondialdhyde.
- A2M
Alpha-2-macroglobulin
- TLR2
Toll-like receptor 2
- TGF-β
Transforming growth factor beta
- IRAK3
Interleukin 1 receptor associated kinase 3
- CCL2
C–C motif chemokine ligand 2
- FCAMR
Fc alpha and Mu receptor
- iNOS
Inducible nitric oxide synthase
- ADAMTS20
ADAM metallopeptidase with thrombospondin type 1 motif 20
- KCNT2
Potassium sodium-activated channel subfamily T member 2
- MAP3K4
Mitogen-activated protein kinase kinase kinase 4
- MAPK14
Mitogen-activated protein kinase 14
- FKBP5
FKBP prolyl isomerase 5
- RXFP1
Relaxin family peptide receptor 1
- EPHA4
Ephrin type-A receptor 4
- SOD3
Superoxide dismutase 3
- CAT
Catalase
- GPX
Glutathione peroxidase
- NDUFS5
NADH:ubiquinone oxidoreductase subunit s5
- A
Alanine
- D
Aspartic acid
- E
Glutamic acid
- F
Phenylalanine
- G
Glycine
- H
Histidine
- I
Isoleucine
- L
Leucine
- M
Methionine
- N
Asparagine
- P
Proline
- R
Argnine
- S
Serine
- T
Threonine
- V
Valine
Author contributions
A.El.-S. and conceived, designed the experiment, performed biochemical analysis and wrote the manuscript. M.R. collected blood samples, and analyzed data. A.A.performed real-time PCR and contributed to writing the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perera B. Reproductive cycles of buffalo. Anim. Reprod. Sci. 2011;124:194–199. doi: 10.1016/j.anireprosci.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Pleticha S, Drillich M, Heuwieser W. Evaluation of the Metricheck device and the gloved hand for the diagnosis of clinical endometritis in dairy cows. J. Dairy Sci. 2009;92:5429–5435. doi: 10.3168/jds.2009-2117. [DOI] [PubMed] [Google Scholar]
- 3.Tsousis G, Sharifi AR, Hoedemaker M. Increased risk of conception failure in German Holstein Friesian cows with chronic endometritis. Reprod. Domest. Anim. 2010;45:1114–1117. doi: 10.1111/j.1439-0531.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- 4.Moghaddam, A. & M. Mamoei. A survey on some of the reproductive and productive traits of the buffalo in Iran. 23rd World Buiatrics 1910 (2004).
- 5.Melendez P, McHale J, Bartolome J, Archbald L, Donovan G. Uterine involution and fertility of Holstein cows subsequent to early postpartum PGF2α treatment for acute puerperal metritis. J. Dairy Sci. 2004;87:3238–3246. doi: 10.3168/jds.S0022-0302(04)73460-8. [DOI] [PubMed] [Google Scholar]
- 6.Földi J, Kulcsar M, Pecsi A, Huyghe B, De Sa C, Lohuis J, Cox P, Huszenicza G. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 2006;96:265–281. doi: 10.1016/j.anireprosci.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Rua MAS, Quirino CR, Ribeiro RB, Carvalho ECQ, Bernadino MdLA, Junior AB, Cipagalta LF, Barreto MAP. Diagnostic methods to detect uterus illnesses in mares. Theriogenology. 2018;114:285–292. doi: 10.1016/j.theriogenology.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Pisello L, Rampacci E, Stefanetti V, Beccati F, Hyatt DR, Coletti M, Passamonti F. Temporal efficacy of antimicrobials against aerobic bacteria isolated from equine endometritis: An Italian retrospective analysis (2010–2017) Vet. Rec. 2019;185:598–598. doi: 10.1136/vr.105413. [DOI] [PubMed] [Google Scholar]
- 9.Squires E, McKinnon A, Shideler R. Use of ultrasonography in reproductive management of mares. Theriogenology. 1988;29:55–70. doi: 10.1016/0093-691X(88)90031-3. [DOI] [Google Scholar]
- 10.Abdelnaby E, El-Maaty AA. Luteal blood flow and growth in correlation to circulating angiogenic hormones after spontaneous ovulation in mares. Bulg. J. Vet. Med. 2017;20:97–107. doi: 10.15547/bjvm.998. [DOI] [Google Scholar]
- 11.Ferreira J, Canesin HdS, Ignácio FS, Rocha NS, Pinto CR, Meira Cd. Effect of age and endometrial degenerative changes on uterine blood flow during early gestation in mares. Theriogenology. 2015;84:1123–1130. doi: 10.1016/j.theriogenology.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Faye B, Bengoumi M. Camel Clinical Biochemistry and Hematology. Springer; 2018. [Google Scholar]
- 13.Faraz A, Younas M, Waheed A, Yaqoob M, Ishaq K. Growth performance and hair mineral status of Marecha (Camelus dromedarius) calves reared under different management systems. Pak. J. Zool. 2019;51:503. doi: 10.17582/journal.pjz/2019.51.2.503.509. [DOI] [Google Scholar]
- 14.Abebe W, Getinet A, Mekonnen H. Study on live weight, carcass weight and dressing percentage of Issa camels in Ethiopia. Revue de médecine vétérinaire. 2002;153:713–716. [Google Scholar]
- 15.Celi P. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 2011;33:233–240. doi: 10.3109/08923973.2010.514917. [DOI] [PubMed] [Google Scholar]
- 16.Kaya S, Öğün M, Özen H, Kuru M, Şahin L, Kükürt A, Kaçar C. The impact of endometritis on specific oxidative stress parameters in cows. J. Hell. Vet. Med. Soc. 2017;68:231–236. doi: 10.12681/jhvms.15610. [DOI] [Google Scholar]
- 17.Zhong R-Z, Zhou D-W. Oxidative stress and role of natural plant derived antioxidants in animal reproduction. J. Integr. Agric. 2013;12:1826–1838. doi: 10.1016/S2095-3119(13)60412-8. [DOI] [Google Scholar]
- 18.Khalid A, Na Y, Jinyou Z, Khudhair N, Guixue Z. Responses of chicken Sertoli cells and fibroblasts after transfection with plasmids pEGFP-N3-HNP-1. Pak. Vet. J. 2015;35:504–509. [Google Scholar]
- 19.Petersen H, Nielsen J, Heegaard PMH. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- 20.Balaram P, Kien PK, Ismail A. Toll-like receptors and cytokines in immune responses to persistent mycobacterial and Salmonella infections. Int. J. Med. Microbiol. 2009;299:177–185. doi: 10.1016/j.ijmm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Nazifi S, Esmailnezhad Z, Haghkhah M, Ghadirian S, Mirzaei A. Acute phase response in lame cattle with interdigital dermatitis. World J. Microbiol. Biotechnol. 2012;28:1791–1796. doi: 10.1007/s11274-011-0995-9. [DOI] [PubMed] [Google Scholar]
- 22.Miglio A, Moscati L, Scoccia E, Maresca C, Antognoni MT, Felici A. Reference values for serum amyloid A, haptoglobin, lysozyme, zinc and iron in healthy lactating Lacaune sheep. Acta Veterinaria Scandinavica. 2018;60:1–4. doi: 10.1186/s13028-018-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohebbi-Fani M, Ansari-Lari M, Nazifi S, Abbasi F, Shabbooei Z. Oxidative status and acute phase response in post-transition early-and mid-lactation Holstein cows and their correlations with some performance records. İstanbul Üniversitesi Veteriner Fakültesi Dergisi. 2016;42:65–73. doi: 10.16988/iuvfd.2016.55237. [DOI] [Google Scholar]
- 24.Ceciliani F, Giordano A, Spagnolo V. The systemic reaction during inflammation: The acute-phase proteins. Protein Pept. Lett. 2002;9:211–223. doi: 10.2174/0929866023408779. [DOI] [PubMed] [Google Scholar]
- 25.Berry DP, Bermingham ML, Good M, More SJ. Genetics of animal health and disease in cattle. Irish Vet. J. 2011;64:1–10. doi: 10.1186/2046-0481-64-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallam AM, Reyer H, Wimmers K, Bertolini F, Aboul-Naga A, Braz CU, Rabee AE. Genome-wide landscape of runs of homozygosity and differentiation across Egyptian goat breeds. BMC Genom. 2023;24:573. doi: 10.1186/s12864-023-09679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams LG, Schutta CJ. Natural resistance against brucellosis: A review. Open Vet. Sci. J. 2010;4:61–71. doi: 10.2174/1874318801004010061. [DOI] [Google Scholar]
- 28.Bollwein H, Lüttgenau J, Herzog K. Bovine luteal blood flow: basic mechanism and clinical relevance. Reprod. Fertil. Dev. 2012;25:71–79. doi: 10.1071/RD12278. [DOI] [PubMed] [Google Scholar]
- 29.Perumal P, Chaurasia D, De A, Bhattacharya D, Sunder J, Bhowmick S, Kundu A, Mishra P. Effect of clinical endometritis on physiological, hematological, biochemical and endocrinological profiles in crossbred cows under tropical island ecosystem. Indian J. Anim. Sci. 2020;90:1296–1299. doi: 10.56093/ijans.v90i9.109493. [DOI] [Google Scholar]
- 30.BL, M.R., E. de Souza Meira Júnior, A. Reyes, E. Marques, A. de Castro Nassar & L. Gregory. Assessment of the bovine uterus with endometritis using Doppler ultrasound (2019).
- 31.Nithish HNN, Jayakumar C, Simon S, Abhilash R, Mathew JJ. Endometrial cytology and transrectal ultrasonography in diagnosis of subclinical endometritis in postpartum crossbred cattle. J. Vet. Anim. Sci. 2022 doi: 10.51966/jvas.2022.53.4.558-564. [DOI] [Google Scholar]
- 32.Barr, F. OJ Ginther (Ed.), Ultrasonic Imaging and Animal Reproduction: Color-Doppler Ultrasonography-Book 4, Equiservices Publishing, Cross plains 2007, ISBN: 0964007282, 258pp;£ 60.00 (hard). WB Saunders, (2008).
- 33.Sharma A, Mahajan M, Singh M, Kumar P. Ultrasonographic investigation of monochorionic diamniotic monozygotic twins in a cow. Vet. Sci. Res. Rev. 2018;4:62–71. [Google Scholar]
- 34.Varughese E, Brar P, Dhindsa S. Uterine blood flow during various stages of pregnancy in dairy buffaloes using transrectal Doppler ultrasonography. Anim. Reprod. Sci. 2013;140:34–39. doi: 10.1016/j.anireprosci.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Sharma A, Singh M, Abrol A, Soni T. Doppler sonography of uterine blood flow at mid-oestrus during different degree of clinical endometritis in dairy cows. Reprod. Domest. Anim. 2019;54:1274–1278. doi: 10.1111/rda.13512. [DOI] [PubMed] [Google Scholar]
- 36.Tinkanen H, Kujansuu E. Doppler ultrasound studies in pelvic inflammatory disease. Gynecol. Obstet. Invest. 1992;34:240–242. doi: 10.1159/000292770. [DOI] [PubMed] [Google Scholar]
- 37.Honnens A, Niemann H, Paul V, Meyer H, Bollwein H. Doppler sonography of the uterine arteries during a superovulatory regime in cattle: Uterine blood flow in superovulated cattle. Theriogenology. 2008;70:859–867. doi: 10.1016/j.theriogenology.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 38.Bollwein H, Baumgartner U, Stolla R. Transrectal Doppler sonography of uterine blood flow in cows during pregnancy. Theriogenology. 2002;57:2053–2061. doi: 10.1016/S0093-691X(02)00706-9. [DOI] [PubMed] [Google Scholar]
- 39.Johnson H, Torres CG, Carvallo F, Duchens M, Peralta OA. Endometrial expression of selected transcripts in postpartum of primiparous Holstein cows with clinical and subclinical endometritis. Anim. Reprod. Sci. 2015;156:34–39. doi: 10.1016/j.anireprosci.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Raliou M, Dembélé D, Düvel A, Bolifraud P, Aubert J, Mary-Huard T, Rocha D, Piumi F, Mockly S, Heppelmann M. Subclinical endometritis in dairy cattle is associated with distinct mRNA expression patterns in blood and endometrium. PLoS One. 2019;14:e0220244. doi: 10.1371/journal.pone.0220244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pothmann H, Flick P, Tichy A, Gabler C, Drillich M. Messenger RNA expression of selected factors at different sites of the bovine endometrium associated with uterine health. Front. Vet. Sci. 2021;8:649758. doi: 10.3389/fvets.2021.649758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janowski T, Baranski W, Lukasik K, Skarzynski D, Rudowska M, Zdunczyk S. Prevalence of subclinical endometritis in repeat breeding cows and mRNA expression of tumor necrosis factor α and inducible nitric oxide synthase in the endometrium of repeat breeding cows with and without subclinical endometritis. Polish J. Vet. Sci. 2013;16:693–699. doi: 10.2478/pjvs-2013-0098. [DOI] [PubMed] [Google Scholar]
- 43.Taha DA, Mahfouz ER, Bibars MA, Hassan NA, Othman OE. Cytokine genes expression in uteri of Bubalus bubalis associated with endometritis infection. Jordan J. Biol. Sci. 2021;14:245–251. doi: 10.54319/jjbs/140207. [DOI] [Google Scholar]
- 44.Al-Sharif M, Abdo M, Shabrawy OE, El-Naga EMA, Fericean L, Banatean-Dunea I, Ateya A. Investigating polymorphisms and expression profile of immune, antioxidant, and erythritol-related genes for limiting postparturient endometritis in holstein cattle. Vet. Sci. 2023;10:370. doi: 10.3390/vetsci10060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ateya A, Safhi FA, El-Emam H, Al-Ghadi MQ, Abdo M, Fericean L, Olga R, Mihaela O, Hizam MM, Mamdouh M. DNA polymorphisms and MRNA levels of immune biomarkers as candidates for inflammatory postpartum disorders susceptibility in Italian buffaloes. Vet. Sci. 2023;10:573. doi: 10.3390/vetsci10090573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.May K, Sames L, Scheper C, König S. Genomic loci and genetic parameters for uterine diseases in first-parity Holstein cows and associations with milk production and fertility. J. Dairy Sci. 2022;105:509–524. doi: 10.3168/jds.2021-20685. [DOI] [PubMed] [Google Scholar]
- 47.Pereira G, Guo Y, Silva E, Silva MF, Bevilacqua C, Charpigny G, Lopes-da-Costa L, Humblot P. Subclinical endometritis differentially affects the transcriptomic profiles of endometrial glandular, luminal, and stromal cells of postpartum dairy cows. J. Dairy Sci. 2022;105:6125–6143. doi: 10.3168/jds.2022-21811. [DOI] [PubMed] [Google Scholar]
- 48.Asadpour R, Feyzi L, Jafari-Joozani R, Hamali H, Hajibemani A, Firouzamandi M. The relationship between polymorphism of the CXCR1 gene and the risk of endometritis in Holstein dairy cows. Veterinarski Arhiv. 2020;90:557–563. doi: 10.24099/vet.arhiv.0807. [DOI] [Google Scholar]
- 49.Hajibemani, A., H. Sharifiyazdi, A. Mirzaei & Ghasrodashti, A.R.. Characterization of single nucleotide polymorphism in the 5'-untranslated region (5'-UTR) of Lactoferrin gene and its association with reproductive parameters and uterine infection in dairy cattle. In Veterinary Research Forum. 2012. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran [PMC free article] [PubMed]
- 50.Goroohi Z, Sharifiyazdi H, Mirzaei A. Association between beta defensin gene polymorphism and clinical endometritis in dairy cows. Comp. Clin. Pathol. 2019;28:377–382. doi: 10.1007/s00580-019-02890-6. [DOI] [Google Scholar]
- 51.Mossallam AAA, Osman NM, Othman OE, Mahfouz ER. Polymorphism evaluation of TLR2 gene associated with endometritis infection in buffalo reared in Egypt. Biotechnol. J. Int. 2022;26:45–55. doi: 10.9734/bji/2022/v26i5661. [DOI] [Google Scholar]
- 52.Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, Kafatos FC. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito. Anopheles Gamb. Cell. 2001;104:709–718. doi: 10.1016/s0092-8674(01)00267-7. [DOI] [PubMed] [Google Scholar]
- 53.Bernabucci U, Ronchi B, Lacetera N, Nardone A. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J. Dairy Sci. 2002;85:2173–2179. doi: 10.3168/jds.S0022-0302(02)74296-3. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Huang J, Feng M, Ju Z, Wang C, Yang G, Yuan J, Zhong J. Regulatory mutations in the a 2M gene are involved in the mastitis susceptibility in dairy cows. Anim. Genet. 2014;45:28–37. doi: 10.1111/age.12099. [DOI] [PubMed] [Google Scholar]
- 55.Botos I, Segal DM, Davies DR. The structural biology of Toll-like receptors. Structure. 2011;19:447–459. doi: 10.1016/j.str.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xue VW, Chung JY-F, Córdoba CAG, Cheung AH-K, Kang W, Lam EW-F, Leung K-T, To K-F, Lan H-Y, Tang PM-K. Transforming growth factor-β: a multifunctional regulator of cancer immunity. Cancers. 2020;12:3099. doi: 10.3390/cancers12113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi K, Hernandez LD, Galán JE, Janeway CA, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/S0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 58.Proudfoot AE. Chemokine receptors: Multifaceted therapeutic targets. Nat. Rev. Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leyva-Baca I, Schenkel F, Sharma B, Jansen G, Karrow N. Identification of single nucleotide polymorphisms in the bovine CCL2, IL8, CCR2 and IL8RA genes and their association with health and production in Canadian Holsteins. Anim. Genet. 2007;38:198–202. doi: 10.1111/j.1365-2052.2007.01588.x. [DOI] [PubMed] [Google Scholar]
- 60.Kendall HK, Marshall RI, Bartold PM. Nitric oxide and tissue destruction. Oral Dis. 2001;7(1):2–10. doi: 10.1034/j.1601-0825.2001.70102.x. [DOI] [PubMed] [Google Scholar]
- 61.Kim Y-S, Hwang J-Y, Kim T-H, Lee E-G, Lee H-H. Genome-wide association study of recurrent endometriosis related with ovarian cancer. Clin. Exp. Obstet. Gynecol. 2019;46:553–559. doi: 10.12891/ceog4600.2019. [DOI] [Google Scholar]
- 62.Guarini A, Lourenco D, Brito L, Sargolzaei M, Baes CF, Miglior F, Misztal I, Schenkel F. Genetics and genomics of reproductive disorders in Canadian Holstein cattle. J. Dairy Sci. 2019;102:1341–1353. doi: 10.3168/jds.2018-15038. [DOI] [PubMed] [Google Scholar]
- 63.Freebern E, Santos DJA, Fang L, Jiang J, Parker KL, Gaddis GE, Liu PM, VanRaden CM, Cole JB, Ma Li. GWAS and fine-mapping of livability and six disease traits in Holstein cattle. BMC Genom. 2020 doi: 10.1186/s12864-020-6461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearson G, Robinson F, Beers Gibson T, Xu B-E, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 65.Roy D, Morgan M, Yoo C, Deoraj A, Roy S, Yadav VK, Garoub M, Assaggaf H, Doke M. Integrated bioinformatics, environmental epidemiologic and genomic approaches to identify environmental and molecular links between endometriosis and breast cancer. Int. J. Mol. Sci. 2015;16:25285–25322. doi: 10.3390/ijms161025285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh J, Murray R, Mshelia G, Woldehiwet Z. The immune status of the bovine uterus during the peripartum period. Vet. J. 2008;175:301–309. doi: 10.1016/j.tvjl.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Fujii H, Tatsumi K, Kosaka K, Yoshioka S, Fujiwara H, Fujii S. Eph–ephrin A system regulates murine blastocyst attachment and spreading. Dev. Dyn.: Off. Pub. Am. Assoc. Anat. 2006;235:3250–3258. doi: 10.1002/dvdy.20977. [DOI] [PubMed] [Google Scholar]
- 68.Sheldon IM, Williams EJ, Miller AN, Nash DM, Herath S. Uterine diseases in cattle after parturition. Vet. J. 2008;176:115–121. doi: 10.1016/j.tvjl.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018;49:397–412. doi: 10.1016/j.immuni.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pisani LF, Lecchi C, Invernizzi G, Sartorelli P, Savoini G, Ceciliani F. In vitro modulatory effect of ω-3 polyunsaturated fatty acid (EPA and DHA) on phagocytosis and ROS production of goat neutrophils. Vet. Immunol. Immunopathol. 2009;131:79–85. doi: 10.1016/j.vetimm.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 71.Shi H, Huang X, Yan Z, Yang Q, Wang P, Li S, Sun W, Gun S. Effect of Clostridium perfringens type C on TLR4/MyD88/NF-κB signaling pathway in piglet small intestines. Microb. Pathog. 2019;135:103567. doi: 10.1016/j.micpath.2019.103567. [DOI] [PubMed] [Google Scholar]
- 72.Sordillo L. Nutritional strategies to optimize dairy cattle immunity. J. Dairy Sci. 2016;99:4967–4982. doi: 10.3168/jds.2015-10354. [DOI] [PubMed] [Google Scholar]
- 73.Senosy W, Izaike Y, Osawa T. Influences of metabolic traits on subclinical endometritis at different intervals postpartum in high milking cows. Reprod. Domest.Anim. 2012;47:666–674. doi: 10.1111/j.1439-0531.2011.01941.x. [DOI] [PubMed] [Google Scholar]
- 74.Islam, R. Studies on immune–endocrine profile of peripartum cows in relation to postpartum reproductive health. Phd IVRI, Izatnagar, UP Bareilly (2012).
- 75.Bernard FF, Zinkl JG, Jain NC, Schalm OW. Schalm's Veterinary Hematology. 5. Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 76.Biswal P, Lathwal S, Baithalu RK, Nag P, Kumar S. Total antioxidant capacity, neutrophil profile, in vitro phagocytic activity, myeloperoxidase (MPO) activity and IL-8 status in uterine infected Murrah buffaloes during peripartum period. Indian J. Anim. Sci. 2022;92:32–37. doi: 10.56093/ijans.v92i1.120914. [DOI] [Google Scholar]
- 77.Patra MK, Kumar H, Nandi S. Neutrophil functions and cytokines expression profile in buffaloes with impending postpartum reproductive disorders. Asian-Australas. J. Anim. Sci. 2013;26:1406. doi: 10.5713/ajas.2012.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ceciliani F, Ceron J, Eckersall P, Sauerwein H. Acute phase proteins in ruminants. J. Proteom. 2012;75:4207–4231. doi: 10.1016/j.jprot.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 79.Niroomandi E, Maleki S, Abdollahpour G, Zakian A, Ahmadvand H. The effect of natural infection with different Leptospira interrogans serovars on oxidative stress biomarkers and acute-phase responses in horses and cattle. Vet. Clin. Pathol. 2022;51:84–92. doi: 10.1111/vcp.13042. [DOI] [PubMed] [Google Scholar]
- 80.Picco, A. M., Cuenca, R., Serrano, E., Tvarijonaviciute, A., Cerón, J., Marga, G., Ahmed, A. & Pastor, J. Acute phase proteins and total antioxidant capacity in free-roaming cats infected by pathogenic leptospires (2020). [DOI] [PMC free article] [PubMed]
- 81.Manimaran A, Kumaresan A, Jeyakumar S, Mohanty T, Sejian V, Kumar N, Sreela L, Prakash MA, Mooventhan P, Anantharaj A. Potential of acute phase proteins as predictor of postpartum uterine infections during transition period and its regulatory mechanism in dairy cattle. Vet. World. 2016;9:91. doi: 10.14202/vetworld.2016.91-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmed W. Overview on some factors negatively affecting ovarian activity in large farm animals. Glob. Vet. 2007;1:53–66. [Google Scholar]
- 83.Yaralioglu-Gurgoze S, Cetin H, Cen O, Yilmaz S, Atli MO. Changes in malondialdehyde concentrations and glutathione peroxidase activity in purebred Arabian mares with endometritis. Vet. J. 2005;170:135–137. doi: 10.1016/j.tvjl.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Oral H, Öğün M, Kuru M, Kaya S. Evaluation of certain oxidative stress parameters in heifers that were administered short term PRID. Kafkas Üniversitesi Veteriner Fakültesi Dergisi. 2015;21:569–573. [Google Scholar]
- 85.Abdelnaby EA, Emam IA, Salem NY, Ramadan ES, Khattab MS, Farghali HA, Abd El Kader NA. Uterine hemodynamic patterns, oxidative stress, and chromoendoscopy in mares with endometritis. Theriogenology. 2020;158:112–120. doi: 10.1016/j.theriogenology.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 86.El-Deeb W, Abdelghani MA, Alhaider A, Fayez M. Oxidative stress, ceruloplasmin and neopterin biomarkers in dromedary camels with clinical endometritis. Anim. Reprod. 2022 doi: 10.1590/1984-3143-AR2022-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Radostits OM, Gay C, Hinchcliff KW, Constable PD. Veterinary Medicine E-Book: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. Elsevier Health Sciences; 2006. [Google Scholar]
- 88.Meira Jr EBS, Henriques LCS, Sá LRMD, Gregory L. Comparison of ultrasonography and histopathology for the diagnosis of endometritis in Holstein-Friesian cows. J. Dairy Sci. 2012;95:6969–6973. doi: 10.3168/jds.2011-4950. [DOI] [PubMed] [Google Scholar]
- 89.Elmetwally MA, Elshopakey GE, Eldomany W, Eldesouky A, Samy A, Lenis YY, Chen DB. Uterine, vaginal and placental blood flows increase with dynamic changes in serum metabolic parameters and oxidative stress across gestation in buffaloes. Reprod. Domest. Anim. 2021;56:142–152. doi: 10.1111/rda.13858. [DOI] [PubMed] [Google Scholar]
- 90.Bollwein H, Meyer H, Maierl J, Weber F, Baumgartner U, Stolla R. Transrectal Doppler sonography of uterine blood flow in cows during the estrous cycle. Theriogenology. 2000;53:1541–1552. doi: 10.1016/S0093-691X(00)00296-X. [DOI] [PubMed] [Google Scholar]
- 91.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45–e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boom R, Sol C, Salimans M, Jansen C, Wertheim-van Dillen P, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boesenberg-Smith KA, Pessarakli MM, Wolk DM. Assessment of DNA yield and purity: An overlooked detail of PCR troubleshooting. Clin. Microbiol. Newslett. 2012;34:1–6. doi: 10.1016/j.clinmicnews.2011.12.002. [DOI] [Google Scholar]
- 94.Sanger, F., Nicklen, S. & Coulson, A. R. DNA sequencing with chain-terminating inhibitors. In Proceedings of the National Academy of Sciences. 74, 5463–5467 (1977). [DOI] [PMC free article] [PubMed]
- 95.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 96.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.