Abstract
Shigella spp. are a leading bacterial cause of diarrhea. No widely licensed vaccines are available and there is no generally accepted correlate of protection. We tested a S. sonnei Generalized Modules for Membrane Antigen (GMMA)-based vaccine (1790GAHB) in a phase 2b, placebo-controlled, randomized, controlled human infection model study (NCT03527173) enrolling healthy United States adults aged 18–50 years. We report analyses evaluating immune responses to vaccination, with the aim to identify correlates of risk for shigellosis among assessed immunomarkers. We found that 1790GAHB elicited S. sonnei lipopolysaccharide specific α4β7+ immunoglobulin (Ig) G and IgA secreting B cells which are likely homing to the gut, indicating the ability to induce a mucosal in addition to a systemic response, despite parenteral delivery. We were unable to establish or confirm threshold levels that predict vaccine efficacy facilitating the evaluation of vaccine candidates. However, serum anti-lipopolysaccharide IgG and bactericidal activity were identified as potential correlates of risk for shigellosis.
Subject terms: Vaccines, Drug development
Introduction
Shigella is a major cause of diarrheal disease worldwide, with a broad geographical distribution and affecting all age groups. Mortality due to Shigella remains substantial in children under five years of age in low- and middle-income countries (LMICs)1. Additionally, shigellosis occurring early in childhood is associated with persistent diarrhea, post-infectious sequelae, and linear growth faltering2,3.
There are four Shigella species, two of which are dominant: S. flexneri (with 15 serotypes, 2a being the most prevalent) and S. sonnei. S flexneri is the most common species in LMICs whereas S. sonnei is the leading species in high-income countries1,4,5. Increasing antibiotic resistance for Shigella is a growing concern4,6,7, emphasizing the need for a vaccine to prevent shigellosis. Despite many attempts, no effective vaccine candidate against Shigella is widely available8. Several investigational vaccines based on various technologies are under development, mainly targeting the O Antigen (OAg) moiety of the lipopolysaccharide (LPS)9 which is known to play a key role in naturally-acquired and vaccine-elicited immunity against shigellosis10.
Vaccine development and clinical evaluation are complicated by the lack of an established correlate of protection (CoP) against Shigella. It is widely accepted that the development of anti-LPS serum immunoglobulin (Ig) G antibodies can confer protection against shigellosis10–12. More recently, the role of anti-LPS serum IgG as a CoP has been reinforced: in a re-analysis of serologic and vaccine efficacy (VE) data from a trial of the S. sonnei OAg-Pseudomonas aeruginosa recombinant exoprotein A glycoconjugate vaccine, a threshold of ≥1:1600 titer was associated with reduced risk of shigellosis and predicted VE against S. sonnei shigellosis in young Israeli adults13. Other potential CoPs include functional serum antibodies, mucosal antibodies produced by gut-homing lymphocytes, antibody-secreting cells (ASCs), and antibodies secreted in culture supernatants by lymphocytes isolated from peripheral blood mononuclear cells (PBMC) collected 7–10 days following exposure to Shigella14–21.
The Generalized Modules for Membrane Antigens (GMMA) are outer membrane particles derived from genetically modified Gram-negative bacteria that have been proposed as a platform for OAg delivery22–24. Shigella GMMA are inherently released by bacteria mutated to increase yields and present attenuated endotoxicity through the modification of the lipid A moiety of LPS25,26. We have developed an investigational S. sonnei vaccine (1790GAHB) based on this technology27. 1790GAHB was shown to have an acceptable safety profile28 and induce bactericidal anti-LPS IgG responses in adults from both endemic29,30 and non-endemic settings18,31,32. We previously reported the results of a phase 2b, randomized, controlled human infection model (CHIM) study conducted in adults from the United States (US), in which participants aged 18–50 years received two doses of either 1790GAHB or placebo, 28 days apart and were challenged with S. sonnei strain 53G at day (D) 57 after the first vaccination33. 1790GAHB did not demonstrate clinical efficacy against shigellosis. However, baseline and pre-challenge anti-S. sonnei LPS serum IgG antibody levels and serum bactericidal activity (SBA) were higher in participants who did not develop shigellosis post-challenge, indicating their role in clinical protection33. Here, we further describe the immune responses elicited by 1790GAHB and evaluate the association of various immunological markers with protection from shigellosis, with the aim to identify correlates of risk (CoRs; defined as immunomarkers for which immune responses are associated with protection against clinical outcome)34,35 for shigellosis. We also explored the role of anti-S. sonnei LPS serum IgG as a CoP in the CHIM trial population of US adults. A summary contextualizing the results and potential clinical relevance and impact of the research is provided in the plain language summary (Fig. 1).
Fig. 1. The plain language summary (PLS).

Figure showing the research context, what is new, and the impact of the research.
Results
Demographics
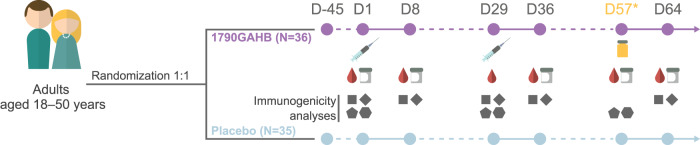
A total of 71 adults were enrolled and vaccinated in the CHIM trial with 36 participants receiving 1790GAHB and 35 receiving placebo (Fig. 2).
Fig. 2. Study scheme.
Study design showing the study population, randomization, and vaccination scheme. All subjects in per protocol analysis received two vaccinations at Day 1 and Day 29, received the challenge agent on day 57, with relevant blood draws for immunogenicity pre and post vaccination. D day, N number of participants. The syringe represents injection with 1790GAHB or placebo. The blood drop and gray container represent blood and stool samples, respectively.
At D57 (28 days after the second vaccination), 33 and 29 participants in the 1790GAHB group and the placebo group, respectively, received a challenge dose consisting of ~1500 colony-forming units of reconstituted lyophilized S. sonnei strain 53G, developed by the Walter Reed Army Institute of Research (Silver Spring, Maryland, US)36. Reasons for elimination from the per-protocol sets were previously reported33; demographic characteristics are summarized in Supplementary Table 1.
Immune responses in the 1790GAHB versus the placebo group
Anti-S. sonnei LPS-specific serum IgG levels and SBA responses were previously reported in detail. Briefly, in the 1790GAHB group, anti-S. sonnei-specific LPS serum IgG geometric mean concentrations increased 2.33- and 5.19-fold at D8 (7 days after first vaccination) and D57 (28 days after second vaccination), respectively, compared with baseline; no increase was observed in the placebo group. At D57, SBA geometric mean titers increased 2.09-fold in the 1790GAHB group and 1.41-fold in the placebo group compared to baseline33.
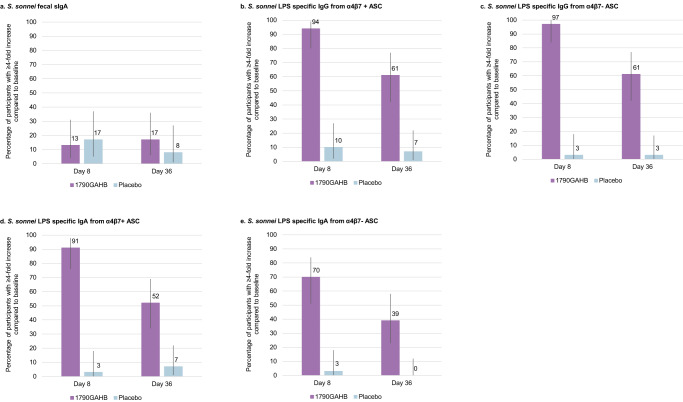
Four (13%) and 5 (17%) participants in the vaccine group and 4 (17%) and 2 (8%) participants in the placebo group showed a ≥4-fold increase in S. sonnei LPS fecal secretory immunoglobulin A (sIgA) titers at D8 and D36 (7 days after second vaccination), respectively (Fig. 3a). However median values did not substantially increase at post-vaccination timepoints for either the 1790GAHB or the placebo group (Supplementary Table 2).
Fig. 3. Percentage of participants with ≥4-fold increase in levels of S. sonnei LPS fecal sIgA and S. sonnei LPS specific IgG/IgA levels from α4β7+ and α4β7− ASCs.
Bar graph showing from (a) to (e), S.sonnei fecal sIgA (a); S.sonnei LPS specific IgG from α4β7+ ASCs (b); S.sonnei LPS specific IgG from α4β7− ASCs (c); S.sonnei LPS specific IgA from α4β7+ ASCs (d); and S.sonnei LPS specific IgA from α4β7− ASCs (e). The 1790GAHB group shows a higher percentage of participants (represented by higher bars) achieving a 4-fold increase or more from baseline for all the parameters, at both Day 8 and Day 36, except for fecal IgA which was similar between recipients of 1790GAHB and placebo at both timepoints. The 95% confidence intervals are represented by the vertical overlay error bars in each case.
The percentage of participants with ≥4-fold increase in IgG/IgA levels from α4β7+/α4β7− ASCs was higher in the 1790GAHB group compared to placebo recipients. Thirty-one (94%) and 20 (61%) participants in the 1790GAHB group versus 3 (10%) and 2 (7%) participants in the placebo group had ≥4-fold increase in IgG levels from α4β7+ ASCs at D8 and D36, respectively (Fig. 3b). Thirty-two (97%) and 20 (61%) participants in the 1790GAHB group versus 1 (3%) and 1 (3%) participant in the placebo group had ≥4-fold increase in IgG levels from α4β7− ASCs at D8 and D36, respectively (Fig. 3c). Thirty (91%) and 17 (52%) 1790GAHB recipients versus 1 (3%) and 2 (7%) placebo recipients for IgA levels from α4β7+ ASCs and 23 (70%) and 13 (39%) 1790GAHB recipients versus 1 (3%) and 0 (0%) placebo recipients for IgA levels from α4β7− ASCs had ≥4-fold increases at D8 and D36, respectively (Fig. 3d, e)
Higher median S. sonnei LPS specific IgG/IgA levels from α4β7+ and α4β7− ASC were observed in the 1790GAHB group than in the placebo group at D8 and D36 (Supplementary Table 2). When stratified by outcome, the percentage of participants with ≥4-fold increases in S. sonnei LPS specific IgG/IgA levels from α4β7+ and α4β7− ASC were overall similar between participants developing or not developing shigellosis post-challenge, as shown by overlapping 95% confidence intervals (CIs) (Supplementary Fig. 1).
SBA against OAg-positive and OAg-negative S. sonnei strains was assessed after LPS-specific antibody depletion. For the OAg-positive S. sonnei strain, no residual bactericidal activity was detected in sera depleted of anti-LPS IgG antibodies compared to non-depleted sera of 1790GAHB vaccinees (previously reported33; Table 1). Depleted sera were bactericidal against the OAg-negative strain and SBA titers tended to be higher in the vaccine than in placebo recipients (Supplementary Table 2).
Table 1.
Serum bactericidal activity against OAg-positive S. sonnei strain for sera depleted and non-depleted of LPS specific antibodies, in participants vaccinated with 1790GAHB (per protocol immunogenicity set)
| Depleted sera | Non-depleted sera33 | |||
|---|---|---|---|---|
| N | GMT (95% CI) | N | GMT (95% CI) | |
| Day 1 | 34 | 16.50 (16.50–16.50) | 32 | 81.95 (57.27–117.26) |
| Day 29 | 34 | 16.50 (16.50–16.50) | 32 | 204.65 (113.56–368.80) |
| Day 57 | 34 | 16.50 (16.50–16.50) | 32 | 171.11 (99.57–294.04) |
The lower limit of quantification (LLOQ) for the assay used to assess S. sonnei bactericidal titers after LPS-specific antibodies depletion was an inhibition concentration 50 (IC50) of 33. Titers below the LLOQ were set to half that limit for the purpose of the analysis.
OAg O antigen, LPS lipopolysaccharide, N number of participants, GMT geometric mean titer, CI confidence interval.
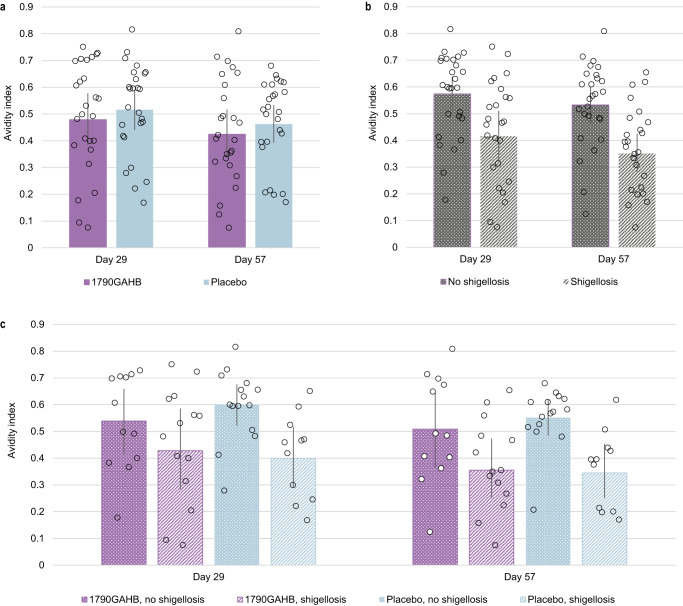
The avidity of anti-S. sonnei LPS antibodies was also evaluated. The avidity index, expressed as the ratio of IgG concentration determined by enzyme-linked immunosorbent assay (ELISA) in the presence or absence of chaotropic agent for the same sample, was similar for participants who received 1790GAHB or placebo, at D29 and D57 (Fig. 4a). Mean antibody avidity at D29 and D57 tended to be higher in participants who did not develop shigellosis post-challenge (Fig. 4b). When participants were grouped both by treatment and challenge outcome, the mean avidity index of participants who did not develop shigellosis was higher (with non-overlapping CIs of the means) compared to that of participants with shigellosis in the placebo group at both D29 and D57; in contrast, only a trend was observed in the 1790GAHB group (Fig. 4c).
Fig. 4. S. sonnei LPS specific total IgG avidity at 28 days post-first and second vaccination, in samples grouped by treatment, outcome and both, post-challenge dose.
Bar chart showing the avidity index on the y axes, at Day 29 and Day 57, of participants grouped by a treatment i.e. who received 1790GAHB or placebo; b outcome, i.e. who did not develop, and developed shigellosis, and c combination of both. The bars representing participants who did not develop shigellosis were higher than those for participants with shigellosis, both among 1790GAHB and placebo recipients. Gray points represent individual participant values’ and the 95% confidence intervals are represented by the vertical overlay line in each case. LPS, lipopolysaccharide; IgG immunoglobulin G.
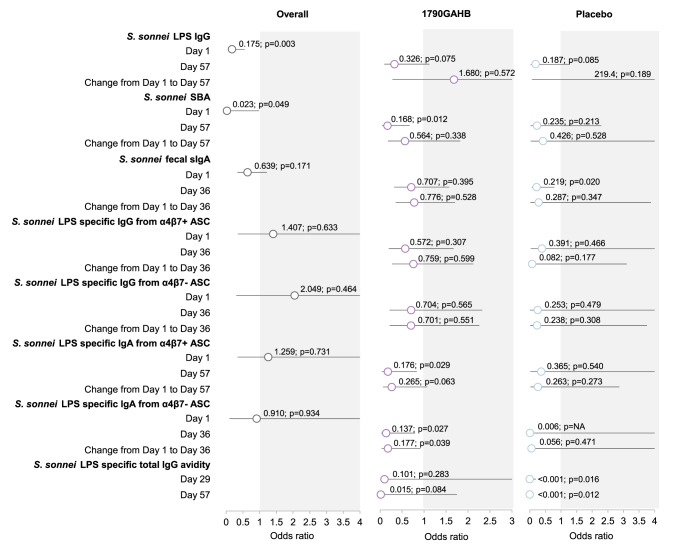
Correlates of risk are identified using the results of logistic regression models as summarized in Fig. 5 and Supplementary Table 3; odds ratio (OR) < 1 indicate that higher values were associated with lower risk of shigellosis. As no VE was observed33, data for the two groups were pooled at D1 (pre-vaccination), to ensure a higher power to detect association between each immunomarker and shigellosis outcome.
Fig. 5. Correlation of risk analysis: association between assessed immunomarkers and risk of shigellosis, by timepoint and group.
Graphical representation of correlate of risks: for each immunomarker, association with risk of developing shigellosis is assessed, by timepoint and by change between timepoints in individual rows; overall and among recipients of 1790GAHB and placebo in the columns. Odds ratios of shigellosis are represented (circles) along with 95% confidence intervals (horizontal bars). Odds ratio values < 1 (white area in the graphs) indicate that higher values of immunomarker are associated with lower risk of shigellosis The p-values show if association between immunomarker and shigellosis is statistically significant (i.e. horizontal bar not crossing the gray area).
Higher S. sonnei LPS-specific IgG levels (OR = 0.175; p = 0.003) at D1 were strongly associated with lower risk of shigellosis, while SBA levels (OR = 0.023; p = 0.049) showed borderline association. None of the other immunomarkers showed association with a lower risk of shigellosis at baseline (Fig. 5).
Increases in S. sonnei LPS-specific IgG levels and SBA from D1 to D57 and higher S. sonnei LPS-specific IgG levels at D57 were not associated with a reduced risk of shigellosis. The OR for shigellosis was <1 for SBA levels against S. sonnei at D57 in the 1790GAHB group (OR = 0.168, p = 0.012).
For higher S. sonnei LPS-specific fecal sIgA levels, the OR was <1 in the placebo group at D36 (OR = 0.219, p = 0.020), but not in the 1790GAHB group (Fig. 5).
In participants vaccinated with 1790GAHB, ORs < 1 were observed for higher S. sonnei LPS-specific IgA levels from α4β7+/α4β7− ASCs at D36 (OR = 0.176, p = 0.029/ OR = 0.137, p = 0.027), as well as their increase from D1 to D36 (OR = 0.265; p = 0.063/ OR = 0.177; p = 0.039). No association was observed for S. sonnei LPS-specific IgG levels from α4β7+/α4β7− ASCs (Fig. 5).
There was a clear association between higher S. sonnei LPS specific IgG avidity and protection in the placebo group at D29 (OR < 0.001; p = 0.0162) and D57 (OR < 0.001; p = 0.0121), but not in the 1790GAHB group (Fig. 5).
Anti-S. sonnei LPS specific serum IgG levels as correlate of protection in US adults
Immunomarker-derived VE (VEIM) was calculated using data from the US adults in the CHIM study, based on the proportion of participants vaccinated with 1790GAHB with S. sonnei LPS serum IgG levels below the protection threshold of 1:1600 proposed by Cohen et al.13. A cut-off of 396 ELISA units (EU)/mL in the GSK Vaccines Institute for Global Health (GVGH) assay was established as the equivalent of this threshold from the Tel Aviv University (TAU) assay. This cut-off did not completely predict protection as 5/27 (18.5%) of participants from the pooled 1790GAHB and placebo groups who developed shigellosis had anti-S. sonnei LPS serum IgG ≥396 EU/mL at the pre-challenge timepoint (D57). The IgG threshold showed a sensitivity (probability of correctly identifying infected participants) of 81.5% and a specificity (probability of correctly identifying protected participants) of 33.3% (Table 2).
Table 2.
Distribution of shigellosis cases and non-cases from the CHIM study population according to S. sonnei LPS serum IgG threshold at 28 days post-second vaccination
| Shigellosis outcome | ||||
|---|---|---|---|---|
| Threshold | Cases | Non-cases | Sensitivity | Specificity |
| <396 EU/mL | 22 | 22 | 81.5% | |
| ≥396 EU/mL | 5 | 11 | 33.3% | |
| Total | 27 | 33 | ||
396 EU/mL in the current GSK Vaccines Institute for Global Health enzyme-linked immunosorbent assay used is equivalent to the 1:1600 titer in the Tel Aviv University assay13.
CHIM controlled human infection model, LPS lipopolysaccharide, IgG immunoglobulin G, EU enzyme-linked immunosorbent assay unit.
Sensitivity was calculated as the number of participants with shigellosis and S. sonnei LPS serum IgG levels below the threshold over the total number of participants with shigellosis. Specificity was defined as the number of participants without shigellosis and S. sonnei LPS serum IgG levels equal to or above the threshold over the total number of participants without shigellosis.
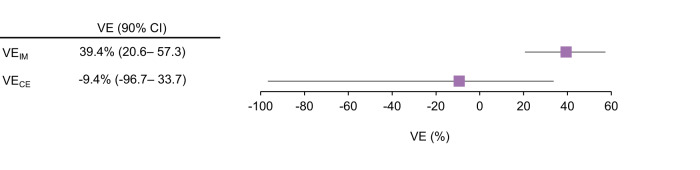
Over the 8-day post-challenge period, VEIM (i.e., VE calculated using the IgG protection threshold) was 39.4% (90% CI 20.6–57.3). VEIM and the VE observed for the primary clinical event (VECE) previously reported33 were not comparable (39.4% versus −9.4%), although 90% CIs overlapped (Fig. 6).
Fig. 6. Forest plot of immunomarker-derived vaccine efficacy (VEIM) and vaccine efficacy against clinical event (shigellosis) (VECE) estimated in the CHIM study population.
Graphical representation of Immunomarker-derived VE (VEIM) calculated using the IgG protection threshold proposed by Cohen et al.13. and vaccine efficacy against observed clinical event (VECE), represented by squares with their corresponding 90% confidence intervals (horizontal lines).
Discussion
During its clinical development, the 1790GAHB candidate vaccine showed an acceptable safety profile and was shown to induce robust anti-S. sonnei LPS serum IgG responses18,30,31. Moreover, serum IgG antibodies were bactericidal and 1790GAHB showed ability to boost the response in individuals pre-immunized or naturally exposed to Shigella29,32. While the vaccine did not show clinical efficacy against shigellosis after challenge with S. sonnei in this CHIM trial33, the study design allowed us to explore the role of several immunomarkers in predicting the risk of shigellosis.
We found that 1790GAHB-induced low levels of S. sonnei LPS-specific fecal sIgA that increased only slightly after the second vaccination, with similar levels observed in the placebo group. Fecal OAg-specific IgA levels have been previously correlated with protection against shigellosis in the case of infection14 or post-immunization with live oral Shigella vaccines37,38, likely as a result of induced mucosal immunity. Thus, at 7 days post-infection with lyophilized S. sonnei 53G in a different CHIM trial in adults, LPS-specific fecal IgA responses were higher in volunteers not progressing to shigellosis than those developing shigellosis after challenge. Moreover, fecal IgA responses continued to increase throughout 14 days post-infection only in volunteers not progressing to shigellosis14. To our knowledge, there is no other assessment of fecal sIgA responses reported after immunization with parenteral Shigella vaccines, as parenteral vaccines are known to induce systemic, rather than mucosal immune responses8. As expected, the magnitude of the fecal sIgA response in our study was lower than that reported after the administration of two live oral S. sonnei vaccine candidates, WRSs2 and WRSs337. In addition to the parenteral administration of the vaccine, other factors could contribute to the low fecal sIgA responses observed after vaccination with 1790GAHB. For instance, antigen-specific fecal sIgA levels were shown to peak between 10 and 14 days and decrease at 28 days post-vaccination with the live oral WRSs2 and WRSs3 vaccines37, therefore the timing of the assessment in our study may not have been optimal to detect an increase in IgA response.
Nevertheless, we found that 1790GAHB was able to induce S. sonnei LPS-specific IgG and IgA responses from ASCs with gut-homing receptors. Both IgG and IgA from α4β7+ ASCs peaked at 7 days post-first vaccination with 1790GAHB, with 94% and 91% of vaccine recipients, respectively, showing ≥4-fold increases from baseline, compared with 10% and 3% in the placebo group. The percentage of participants receiving 1790GAHB with ≥4-fold increase in S. sonnei LPS specific IgG and IgA levels from α4β7+ ASCs remained higher than in the placebo group at 7 days post-second vaccination. This indicates the ability of the S. sonnei GMMA-based vaccine to induce a mucosal response, despite the low fecal sIgA levels observed, as circulating plasmablasts expressing α4β7 integrin are likely homing to the intestinal mucosa. A similar finding was previously reported for another parenteral Shigella vaccine, a bioconjugate composed of the O-polysaccharide of S. flexneri 2a enzymatically linked to the exotoxin A of Pseudomonas aeruginosa (Flexyn2a). Flexyn2a induced α4β7+ IgG and IgA responses that were strongly correlated with each other39. Taken together, our results show that parenteral GMMA-based vaccines have the ability to induce mucosal response in addition to systemic antibody responses.
An increase in α4β7− IgG and IgA levels compared to baseline was observed post-vaccination with 1790GAHB in our study, similarly to findings for Flexyn2a39. We observed no increase in α4β7− IgG/IgA responses post-challenge in the current trial. In contrast, significant increases in LPS specific α4β7− IgG/IgA responses post-infection with lyophilized S. sonnei 53G, in non-immunized adults, were observed in another CHIM trial14.
A key factor in the design of vaccines against infectious diseases is determining the phenotype and magnitude of vaccine-induced immune response that confers protection against infection or at least against a severe clinical outcome. Such immunological markers could then be used in assessing VE in clinical trials without having to conduct trials to measure severe clinical outcomes, thus reducing overall cost and time delays. Approval of a vaccine for marketing by health authorities often relies on demonstrating VE based on an accepted surrogate of protection. The application of surrogates of protection for licensure of vaccine candidates has been extensively used for vaccines against meningitis, pneumococcal, and influenza, to mention a few40. However, establishing a CoP for many infectious diseases can be extremely complex and to date, there is no accepted CoP for shigellosis. Qin et al. define CoPs as immunological measurements that predict a vaccine’s level of protective efficacy based on differences between the vaccinated and unvaccinated groups. In contrast, CoRs are defined as immunomarkers predicting a clinical endpoint in some populations (i.e., in the context of clinical trials, but without being generalized across settings or populations) and irrespective of the intervention35. A CoR is not necessarily a CoP but once identified, it can be validated as a CoP in larger clinical trials, where a vaccine effect is observed. Because we could not demonstrate efficacy against shigellosis following vaccination with 1790GAHB in this CHIM study, a CoP and a threshold predicting VE cannot be identified among the investigated immunomarkers. However, based on the clinical endpoint evaluated (shigellosis), CoRs can be evaluated34. We found that at baseline, higher anti-S. sonnei LPS-specific serum IgG titers were associated with a reduced risk of shigellosis, while SBA titers were borderline associated with a reduced risk. Our findings for LPS-specific serum IgG further confirm the accumulated data on the role of this immunomarker as a CoP or CoR13,41,42. However, there is less evidence in the literature on the use of functional antibody titers as a CoP/CoR for shigellosis. In a previous challenge study, higher baseline SBA titers correlated with resistance to infection post-challenge with S. sonnei 53G, although this correlation was less robust than for other assessed immunomarkers, such as LPS-specific serum IgA response14. Another CHIM study found a strong association between specific SBA titers against S. flexneri 2a and a reduction in disease severity post-challenge43. In a different CHIM study evaluating the efficacy of the Flexyn2a bioconjugate vaccine, vaccinees protected against S. flexneri 2a shigellosis had higher SBA titers than unprotected vaccine recipients; however, in contrast with the findings from the other CHIM trial, this difference was not statistically significant39. Interestingly, in the same study, when assessing correlation with other immunomarkers, bactericidal activity showed the strongest correlation with α4β7+ IgG responses, followed by serum IgG1 responses39. We also previously reported a strong correlation between the 1790GAHB elicited SBA titers and anti-S. sonnei LPS serum IgG levels in adults from both Shigella-endemic29 and non-endemic countries18, indicating the functionality of antibodies induced by vaccination. The magnitude of antibody responses may play a less significant role in protection than their functionality; for instance, findings from a CHIM study suggest that Shigella-specific serum IgG1 may play an important role in protection from shigellosis after parenteral immunization even at relatively low levels elicited post-vaccination39, due to their high efficiency in activating the complement cascade44. Current recommendations for the prioritization of Shigella-specific immunoassays list serum antibody functionality by SBA among first-priority tier analysis45. The use of functional antibodies as an immunomarker predictive of protection is well established for other diseases caused by pathogens invading mucosal surfaces, such as pneumococcal and meningococcal infection19.
In our study, higher IgA (but not IgG) levels from α4β7+ and α4β7− ASCs post-vaccination, as well as their increase from baseline were associated with reduced risk of shigellosis. However, post-first immunization with Flexyn2a, LPS specific α4β7+/− IgG responses were significantly higher in vaccinees protected versus non-protected against shigellosis post-challenge with virulent S. flexneri 2a, whereas no significant differences were observed for α4β7+/− IgA responses39. Differences between the elicited response can be due to the nature of the two parenteral vaccines (GMMA-based, Alhydrogel-adjuvanted 1790GAHB versus the bioconjugate unadjuvanted Flexyn2a). In addition, LPS specific α4β7+ IgG and IgA responses have previously been shown to be significantly increased from baseline in volunteers with shigellosis after challenge with S. sonnei than those without, indicating a predominantly mucosal immune response after oral challenge with the wild-type organism14. In contrast, challenge with S. flexneri 2a is believed to lead to a balanced mucosal/systemic response15. These differences between serotypes with regard to responses mounted to natural infection may also apply to immunization with serotype-specific vaccines, although the underlying mechanism is most likely different. This corroborates previous observations that the definition of a single immune CoP may not be adequate for Shigella, considering the complexities associated with the immune response following infection or vaccination combined with potential differences across diverse serotypes15. However, based on our findings, we suggest that future research may focus on the design of vaccines able to elicit mainly anti-S. sonnei LPS serum IgG responses, but also to induce high SBA titers and robust LPS-specific α4β7+/− IgG responses. The CoRs identified in our study may also be CoPs, but this needs to be validated in additional clinical trials in which VE is observed. Of note, we also observed differences in the OR of shigellosis between the 1790GAHB and placebo groups, which should not arise in the context of the low VECE of 1790GAHB observed in the CHIM trial33. Therefore, associations between immunomarkers and risk of shigellosis identified in our study are difficult to interpret, and might be at least partly due to the lack of correction for multiplicity. In addition, the CoR analyses were exploratory in nature; therefore, the sample size was not driven by statistical hypothesis and p-values were not adjusted by multiple comparisons.
To evaluate whether 1790GAHB elicits antibodies with different specificity able to induce complement-mediated killing, we also tested the SBA of LPS-depleted sera. No residual bactericidal activity against OAg-positive bacteria was detected in sera depleted of anti-LPS antibodies compared to non-depleted sera of 1790GAHB recipients post-vaccination33, indicating that 1790GAHB-induced SBA is mediated by anti-LPS antibodies. However, when tested in SBA against a S. sonnei OAg-negative strain, anti-GMMA protein antibodies were able to kill the bacteria and a slight increase of SBA titers upon vaccination with 1790GAHB was observed versus no change in participants receiving placebo. This is in line with previous preclinical observations that showed anti-OAg antibodies are the main drivers of SBA against OAg-positive Shigella strains, while antibodies not targeting OAg are functional against OAg‑negative bacteria46,47.
The anti-Shigella LPS-specific IgG avidity analysis suggested an association between S. sonnei LPS-specific total IgG avidity and protection from shigellosis. Antibody avidity was similar between 1790GAHB and placebo recipients regardless of the challenge outcome, suggesting that this association is not impacted by vaccination and that this immunomarker may be a suitable CoP. When grouped by both treatment and challenge outcome, the median avidity index for participants who did not develop shigellosis post-challenge with S. sonnei was higher than that for participants developing shigellosis in the placebo group. Only a trend for higher antibody avidity was observed in the vaccine group. However, these results require confirmation in a larger population and should be interpreted with caution due to the relatively small number of samples tested, which also varied across timepoints.
The S. sonnei LPS serum IgG protection threshold of 1:1600 proposed by Cohen et al. in young Israeli adults13 was not validated in US adults in this CHIM study. In our study, the VEIM and VECE point estimates were not comparable (39.4% versus −9.4%), although a slight overlap of the 90% CI was observed. To formally assess the equivalence of VEIM and VECE, a large population and a priori-defined equivalence margins are required and this was not possible with the small sample size of a CHIM study33. Therefore, although our data confirmed the role of anti-S. sonnei LPS serum IgG as a CoR for shigellosis, we could not provide evidence for its role as a CoP, nor that the 1:1600 titer threshold can be used to predict efficacy. There were also other factors that were hindering this comparison. First, the assays used in the two studies were different and required a conversion that may have led to additional uncertainty and variability of the data. Second, VE is known to differ with setting48, and the Cohen et al. study was conducted in field settings, with shigellosis cases being collected 71–155 days post-vaccination13, while we tested the CoP in a CHIM model and collected shigellosis cases during 29–36 days post-vaccination. Finally, the confidence limits around the VEIM estimate did not account for variability around threshold, therefore the real CIs might be larger than those reported.
The results of this CHIM study supported the design of an improved version of the four-component GMMA vaccine, which was formulated with 10-fold higher OAg amount for S. sonnei and three additional serotypes (S. flexneri 1b, 2a and 3a)27. This candidate vaccine is now being tested in adults 18–50 years of age from non-endemic Shigella countries, as a first stage of a phase 1/2 study (NCT05073003) before progressing to investigations in endemic settings.
Overall study limitations have been previously discussed in detail33 and include the low sample size and the fact that there was no assessment of S. sonnei LPS-specific serum IgA or IgG isotyping. In addition to these and the limitations discussed for the CoR analysis, the study had all limitations inherent to a CHIM48.
In conclusion, although these analyses cannot validate a CoP that predicts VE, they indicated immunological measures which are associated with reduced risk of shigellosis, and further confirm the critical role of LPS in protection. We were unable to establish or confirm threshold levels that can predict protection against shigellosis, which would have facilitated the development and evaluation of Shigella vaccine candidates. However, we confirmed serum anti-LPS IgG and identified bactericidal activity as potential CoRs for shigellosis.
Methods
Study design and objectives
We conducted a single-center, observer-blind, randomized, placebo-controlled, phase 2b CHIM study between August 2018 and November 2019 at the Cincinnati Children’s Hospital Medical Center (CCHMC), Ohio, USA. The study design was previously described in detail33. Briefly, participants aged 18–50 years were randomized (1:1) to receive two doses of either 1790GAHB (0.5 mL dose containing 1.5/25 μg of OAg/protein) or placebo (0.5 mL of Alhydrogel in tris-buffered saline [0.7 mg Al3+/mL]), 28 days apart and were challenged on D57 with S. sonnei strain 53G.
The study was conducted in accordance with all applicable regulatory requirements, the Good Clinical Practice guidelines, and the Declaration of Helsinki. The protocol and study-related documents were approved by the CCHMC institutional review board, and all participants provided written informed consent. The trial is registered on ClinicalTrials.gov (NCT03527173), and the full study protocol is available at www.gsk-studyregister.com (study ID 205626).
The results of the primary and secondary objectives have previously been reported, including serum anti-S. sonnei LPS IgG levels and SBA titers33. Here, we present the results of tertiary and exploratory objectives that evaluated S. sonnei specific sIgA in stool, as well as S. sonnei LPS IgA/IgG specific α4β7+/α4β7− ASC plasmablast response pre- and post-vaccination, as indicated in Fig. 2. We also assessed the association with shigellosis for all evaluated immunomarkers, including those previously reported33, and investigated their role as CoRs. Furthermore, in post-hoc analyses, we evaluated SBA against OAg-positive and OAg-negative Shigella strains after anti-LPS antibody depletion and anti-S. sonnei LPS-specific total IgG avidity (Fig. 2). In addition, we explored the role of the CoP proposed by Cohen et al. in young Israeli adults13 in the population of US adults from the CHIM trial33.
Blood samples for antibody response and evaluation of SBA (~20 mL) and for PBMC isolation (α4β7+/α4β7− plasmablasts response) (~50 mL) and stool samples for the detection of fecal sIgA were collected as indicated in Fig. 2.
S. sonnei LPS fecal sIgA were determined by an ELISA at CCHMC. Stool (2.5–3.0 g) was mixed well with extraction buffer and then centrifuged (20,000 revolutions/minute) for 30 min at 4 °C. The supernatant was collected and transferred into pre-labeled cryovials. Antigen-specific LPS sIgA and total IgA were determined by ELISA. Total IgA were expressed as mg/mL and the LPS-specific sIgA as titer. Results were reported as LPS activity (titers/mg/mL), defined as LPS sIgA divided by total IgA. The lower limit of quantification (LLOQ) for the LPS sIgA was 10. The assay was performed CCHMC Laboratories for Specialized Clinical Studies (Cincinnati, Ohio, US).
S. sonnei LPS-specific IgA/IgG from α4β7+/α4β7− ASCs were assessed from PBMC. Cells were separated into α4β7+ and α4β7− populations. Both cell populations were cultured in vitro to collect antibodies in lymphocyte supernatant (ALS). The ALS samples were assayed using ELISA as previously described14 to determine S. sonnei LPS-specific endpoint titers (expressed as titer/5*106 cells). The assay was performed at the Walter Reed Army Institute of Research (Silver Spring, Maryland, US).
S. sonnei bactericidal titers after LPS specific antibodies depletion were determined using a luminescent serum bactericidal assay33,49, with LLOQs of 33 and 10 of inhibition concentration (IC)50 for the S. sonnei OAg-positive and OAg-negative strains, respectively. Prior to the assay, LPS antibody depletion was performed by overnight incubation with homologous competitor S. sonnei LPS (at a concentration of 50 µg/mL) and confirmed by ELISA using S. sonnei LPS as plate coating antigen (at a concentration of 0.5 µg/mL in phosphate buffer saline [PBS]). To prove a minimal reduction of anti-GMMA protein antibodies, we also performed ELISA using S. sonnei OAg-negative GMMA as plate coating antigen (at a concentration of 1 µg/mL in PBS) of samples pre- and post-LPS antibodies depletion. Effective depletion was confirmed with a reduction of EU/mL on LPS coating >80% and <30% on GMMA OAg-negative coating. These assays were performed at GVGH.
The anti-S. sonnei LPS-specific total IgG avidity was determined by ELISA33 in the presence of chaotropic agent. The avidity index was expressed as the ratio of IgG concentration (EU/mL) after treatment with 0.5 M ammonium thiocyanate, compared to the value for the same sample without chaotropic treatment. The LLOQ was 9.9 EU/mL. The assay was performed at GVGH.
Statistical analysis
All statistical analyses were carried out using Statistical Analysis Systems 9.4.
Sample size considerations have previously been described33. All immunogenicity analyses (except anti-S. sonnei LPS specific total IgG avidity index) used descriptive statistics and were conducted in the per-protocol set for immunogenicity comprising all participants with available data in the full analysis set who correctly received the vaccine/placebo, had no major protocol deviation, and had immunogenicity data at the relevant timepoint. Anti-S. sonnei LPS-specific total IgG avidity index was assessed in a subset of 26 participants in each of the 1790GAHB and the placebo groups.
For Analysis of immunogenicity, for each group, median, median difference, and percentage of participants with ≥4-fold increase post-vaccination in S. sonnei LPS specific fecal sIgA activity compared to baseline and pre-challenge were calculated with two-sided 95% CIs. LPS-specific sIgA activity corresponding to participants with titers below the LLOQ was set to zero. For analysis purposes, to perform log10 transformation, zero values were recoded to the smallest non-zero value in the dataset, divided by 10 before log10 transformation.
Median, median difference, and the number and percentage of participants with a ≥4-fold increase post-vaccination in antibody titers against S. sonnei LPS specific IgA/IgG from α4β7+ and α4β7− ASC plasmablasts were calculated with 95% CIs.
For the evaluation of S. sonnei bactericidal titers after LPS antibody depletion, geometric mean titers, geometric mean ratios, and the percentage of participants with a ≥4-fold increase post-vaccination compared to baseline (or a 4-fold increase compared to LLOQ, if the participant had SBA titers <LLOQ) were calculated with 2-sided 95% CIs. Titers below the LLOQ were set to half that limit for the purpose of the analysis.
Mean anti-S. sonnei LPS-specific total IgG avidity indices with 95% CIs were computed. For analysis purposes, when serum concentration after chaotropic treatment was below the LLOQ, its value was replaced by half of the LLOQ (4.95) and then the avidity index was calculated; values > 1 were replaced by 1.
Analyses of correlates of risk for shigellosis were conducted in the per-protocol set for efficacy33, using univariate logistic regression with shigellosis as dependent variable (present versus absent) and the immunomarker as continuous independent variable after log10 transformation. ORs were calculated with Wald 95% CIs and p-values; the Firth method50 was used for CIs in case of quasi-complete separation of data. The ORs quantify the ratio between odds of shigellosis for a 10-fold increase in immunomarker (original non-transformed value) between different participants at the same timepoint or between baseline and post-vaccination timepoint within the same participant; ORs <1 indicate that higher values are associated with lower risk of shigellosis. Immunomarkers with p < 0.05 from the logistic regression using baseline pooled data were considered CoRs. P-values from all the other remaining logistic regressions were calculated to further explore the association between immunomarkers and shigellosis. Due to the descriptive nature of all analyses, p-values and CIs were not adjusted for multiplicity.
Exploration of correlate of protection against Shigella was done by calculating VEIM on the per-protocol efficacy set from the US adults in the CHIM study using a model proposed by Siber et al.51 as:
In the above formula, the titer of 1:1600 measured using the TAU assay was used as the protection threshold, as identified by Cohen et al.13. To allow comparability between VEIM and VECE, the CIs were calculated with the same method, i.e., 90% 2-sided exact unconditional CIs with the Miettinen–Nurminen method52. This method is expected to underestimate variance of VEIM as it makes the strong assumption that the threshold has no variability, however it was not possible to use the bootstrap method, as we had no access to original data.
In a commutability study, the 1:1600 titer threshold in the TAU assay was shown to correspond to 396 EU/mL in the in-house ELISA currently used by the GVGH. These conversions were performed to allow between-study comparisons. As in the current GVGH ELISA assay the standard serum has been recalibrated with respect to the one used in the CHIM33, ELISA measurements obtained in the original CHIM study were multiplied by 0.45 to correspond to the in-house ELISA currently used by the GVGH.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors thank the study participants and site staff involved in the study, as well as the CCHMC study team and the Shigella project team at GVGH. The authors would like to acknowledge Brielle Barnard (WRAIR) for significant involvement in the collection of α4β7 data, Monica McNeil and Michelle Dickey (CCHMC) for overseeing laboratory and clinical operations, respectively, and Kishor Mariyala, Sateesh Aravapalli, and Rob Mulder (GSK) for providing statistical programming activities. The authors would also like to thank Akkodis Belgium c/o GSK for medical writing and design support (Petronela M. Petrar) and manuscript coordination. This study was funded by the Bill and Melinda Gates Foundation (BMFG) and GlaxoSmithKline Biologicals SA (study sponsor). GlaxoSmithKline Biologicals SA also covered all costs associated with the development of this manuscript.
Author contributions
V.C., O.R., K.A.C., U.N.N., E.S., P.F., A.A., E.M., R.W.F., L.B.M., R.W.K., A.P., and F.Mi. were involved in the study design and data acquisition, analysis, and interpretation. A.K.A. and A.C. were involved in data analysis and interpretation. F.Ma. was involved in data acquisition, analysis, and interpretation. F.N. was involved in the study design and data acquisition and analysis. V.C., O.R., U.N.N., A.C., and F.Mi. contributed to the writing of the first draft. All authors revised and edited the first and following drafts. All authors reviewed the final version of the article and agreed to its contents.
Data availability
Anonymized individual participant data and study documents can be provided upon request from www.clinicalstudydatarequest.com.
Competing interests
V.C., O.R., F.Ma., U.N., E.S., A.C., P.F., A.A., A.K.A., E.M., F.N., L.B.M., A.P., and F.Mi. are or were GSK employees when the study was designed, initiated, and/or conducted. V.C., O.R., A.K.A., F.N., and F.Mi. hold shares in GSK. L.B.M. reports paid travel costs by BMGF to attend Shigella meetings in January 2020. P.F. reports grants/contracts to GSK from BMGF. L.B.M. and F.Mi. report funding to GSK from BMGF for vaccine manufacturing and testing and patent issued on the 4-component Shigella GMMA formulation. K.A.C., R.W.F., and R.W.K. have no financial relationships to disclose. The authors declare no other financial and non-financial relationships and activities or conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-024-00822-2.
References
- 1.Khalil IA, et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990-2016. Lancet Infect. Dis. 2018;18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby TE, et al. Consequences of Shigella infection in young children: a systematic review. Int. J. Infect. Dis. 2023;129:78–95. doi: 10.1016/j.ijid.2023.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasrin D, et al. Pathogens associated with linear growth faltering in children with diarrhea and impact of antibiotic treatment: The Global Enteric Multicenter Study. J. Infect. Dis. 2021;224:S848–S855. doi: 10.1093/infdis/jiab434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shad AA, Shad WA. Shigella sonnei: virulence and antibiotic resistance. Arch. Microbiol. 2021;203:45–58. doi: 10.1007/s00203-020-02034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson M, Sansonetti PJ, Marteyn BS. Shigella diversity and changing landscape: insights for the twenty-first century. Front. Cell. Infect. Microbiol. 2016;6:45. doi: 10.3389/fcimb.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranjbar R, Farahani A. Shigella: antibiotic-resistance mechanisms and new horizons for treatment. Infect. Drug Resist. 2019;12:3137–3167. doi: 10.2147/IDR.S219755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shakoor S, Platts-Mills JA, Hasan R. Antibiotic-resistant enteric infections. Infect. Dis. Clin. North Am. 2019;33:1105–1123. doi: 10.1016/j.idc.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 8.MacLennan CA, Grow S, Ma LF, Steele AD. The Shigella vaccines pipeline. Vaccines. 2022;10:1376. doi: 10.3390/vaccines10091376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raso MM, Arato V, Gasperini G, Micoli F. Toward a Shigella vaccine: opportunities and challenges to fight an antimicrobial-resistant pathogen. Int. J. Mol. Sci. 2023;24:4649. doi: 10.3390/ijms24054649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen D, et al. Serum IgG antibodies to Shigella lipopolysaccharide antigens - a correlate of protection against shigellosis. Hum. Vaccin. Immunother. 2019;15:1401–1408. doi: 10.1080/21645515.2019.1606971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black RE, et al. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J. Infect. Dis. 1987;155:1260–1265. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- 12.Cohen D, et al. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J. Infect. Dis. 1988;157:1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 13.Cohen D, et al. Threshold protective levels of serum IgG to Shigella lipopolysaccharide: re-analysis of Shigella vaccine trials data. Clin. Microbiol. Infect. 2023;29:366–371. doi: 10.1016/j.cmi.2022.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarkson KA, et al. Immune response characterization after controlled infection with lyophilized Shigella sonnei 53G. mSphere. 2020;5:e00988–00919. doi: 10.1128/mSphere.00988-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarkson KA, et al. Shigella-specific immune profiles induced after parenteral immunization or oral challenge with either Shigella flexneri 2a or Shigella sonnei. mSphere. 2021;6:e0012221. doi: 10.1128/mSphere.00122-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feller AJ, et al. Comparative evaluation of the antibody in lymphocyte supernatant (ALS) and enzyme-linked immunospot (ELISPOT) assays for measuring mucosal immune responses to Shigella antigens. Vaccine. 2011;29:8487–8489. doi: 10.1016/j.vaccine.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotloff KL, et al. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine. 1995;13:1488–1494. doi: 10.1016/0264-410X(95)00102-7. [DOI] [PubMed] [Google Scholar]
- 18.Micoli F, et al. Antibodies elicited by the Shigella sonnei GMMA vaccine in adults trigger complement-mediated serum bactericidal activity: results from a phase 1 dose escalation trial followed by a booster extension. Front. Immunol. 2021;12:671325. doi: 10.3389/fimmu.2021.671325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ndungo E, Pasetti MF. Functional antibodies as immunological endpoints to evaluate protective immunity against Shigella. Hum. Vaccin. Immunother. 2020;16:197–205. doi: 10.1080/21645515.2019.1640427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarker P, et al. Functional antibodies and innate immune responses to WRSS1, a live oral Shigella sonnei vaccine candidate, in Bangladeshi adults and children. J. Infect. Dis. 2021;224:S829–S839. doi: 10.1093/infdis/jiab395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha A, et al. Circulating gut-homing (α4β7+) plasmablast responses against Shigella surface protein antigens among hospitalized patients with diarrhea. Clin. Vaccine Immunol. 2016;23:610–617. doi: 10.1128/CVI.00205-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancini F, et al. GMMA-based vaccines: the known and the unknown. Front. Immunol. 2021;12:715393. doi: 10.3389/fimmu.2021.715393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccioli D, Bartolini E, Micoli F. GMMA as a ‘plug and play’ technology to tackle infectious disease to improve global health: context and perspectives for the future. Expert Rev. Vaccines. 2022;21:163–172. doi: 10.1080/14760584.2022.2009803. [DOI] [PubMed] [Google Scholar]
- 24.Micoli F, MacLennan CA. Outer membrane vesicle vaccines. Semin. Immunol. 2020;50:101433. doi: 10.1016/j.smim.2020.101433. [DOI] [PubMed] [Google Scholar]
- 25.Mancini F, Rossi O, Necchi F, Micoli F. OMV vaccines and the role of TLR agonists in immune response. Int. J. Mol. Sci. 2020;21:4416. doi: 10.3390/ijms21124416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi O, et al. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: relative activation of TLR4 and TLR2 pathways in different mutants. J. Biol. Chem. 2014;289:24922–24935. doi: 10.1074/jbc.M114.566570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Micoli F, Nakakana UN, Berlanda Scorza F. Towards a four-component GMMA-based vaccine against Shigella. Vaccines. 2022;10:328. doi: 10.3390/vaccines10020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Ryck I, et al. GMMA technology for the development of safe vaccines: meta-analysis of individual patient data to assess the safety profile of Shigella sonnei 1790GAHB vaccine in healthy adults, with special focus on neutropenia. Infect. Dis. Ther. 2022;11:757–770. doi: 10.1007/s40121-022-00596-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapulu MC, et al. Complement-mediated serum bactericidal activity of antibodies elicited by the Shigella sonnei GMMA vaccine in adults from a shigellosis-endemic country: Exploratory analysis of a Phase 2a randomized study. Front. Immunol. 2022;13:971866. doi: 10.3389/fimmu.2022.971866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obiero CW, et al. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front. Immunol. 2017;8:1884. doi: 10.3389/fimmu.2017.01884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Launay O, et al. Safety profile and immunologic responses of a novel vaccine against Shigella sonnei administered intramuscularly, intradermally and intranasally: results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. EBioMedicine. 2017;22:164–172. doi: 10.1016/j.ebiom.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Launay O, et al. Booster vaccination with Gvgh Shigella sonnei 1790GAHB GMMA vaccine compared to single vaccination in unvaccinated healthy European Adults: results from a phase 1 clinical trial. Front. Immunol. 2019;10:335. doi: 10.3389/fimmu.2019.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frenck RW, Jr, et al. Efficacy, safety, and immunogenicity of the Shigella sonnei 1790GAHB GMMA candidate vaccine: results from a phase 2b randomized, placebo-controlled challenge study in adults. EClinicalMedicine. 2021;39:101076. doi: 10.1016/j.eclinm.2021.101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 35.Qin L, et al. A framework for assessing immunological correlates of protection in vaccine trials. J. Infect. Dis. 2007;196:1304–1312. doi: 10.1086/522428. [DOI] [PubMed] [Google Scholar]
- 36.Frenck RW, et al. Establishment of a controlled human infection model with a lyophilized strain of Shigella sonnei 53G. mSphere. 2020;5:e00416–e00420. doi: 10.1128/mSphere.00416-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frenck RW, et al. A phase I trial to evaluate the safety and immunogenicity of WRSs2 and WRSs3; two live oral candidate vaccines against Shigella sonnei. Vaccine. 2018;36:4880–4889. doi: 10.1016/j.vaccine.2018.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raqib R, et al. A phase I trial of WRSS1, a Shigella sonnei live oral vaccine in Bangladeshi adults and children. Hum. Vaccin. Immunother. 2019;15:1326–1337. doi: 10.1080/21645515.2019.1575165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarkson KA, et al. Immune response characterization in a human challenge study with a Shigella flexneri 2a bioconjugate vaccine. EBioMedicine. 2021;66:103308. doi: 10.1016/j.ebiom.2021.103308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plotkin SA. Recent updates on correlates of vaccine-induced protection. Front. Immunol. 2023;13:1081107. doi: 10.3389/fimmu.2022.1081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen D, et al. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: a phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis. 2021;21:546–558. doi: 10.1016/S1473-3099(20)30488-6. [DOI] [PubMed] [Google Scholar]
- 42.Cohen D, et al. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J. Clin. Microbiol. 1991;29:386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimanovich AA, et al. Functional and antigen-specific serum antibody levels as correlates of protection against shigellosis in a controlled human challenge study. Clin. Vaccine Immunol. 2017;24:e00412–e00416. doi: 10.1128/CVI.00412-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burton DR, Woof JM. Human antibody effector function. Adv. Immunol. 1992;51:1–84. doi: 10.1016/S0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- 45.Kaminski RW, et al. Consensus report on Shigella controlled human infection model: immunological assays. Clin. Infect. Dis. 2019;69:S596–S601. doi: 10.1093/cid/ciz909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mancini F, et al. Exploring the role of GMMA components in the immunogenicity of a 4-valent vaccine against Shigella. Int. J. Mol. Sci. 2023;24:2742. doi: 10.3390/ijms24032742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancini F, et al. Dissecting the contribution of O-antigen and proteins to the immunogenicity of Shigella sonnei generalized modules for membrane antigens (GMMA) Sci. Rep. 2021;11:906. doi: 10.1038/s41598-020-80421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giersing BK, et al. How can controlled human infection models accelerate clinical development and policy pathways for vaccines against Shigella? Vaccine. 2019;37:4778–4783. doi: 10.1016/j.vaccine.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 49.Rossi O, et al. Intra-laboratory evaluation of luminescence based high-throughput serum bactericidal assay (L-SBA) to determine bactericidal activity of human sera against Shigella. High Throughput. 2020;9:14. doi: 10.3390/ht9020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. doi: 10.1093/biomet/80.1.27. [DOI] [Google Scholar]
- 51.Siber GR, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007;25:3816–3826. doi: 10.1016/j.vaccine.2007.01.119. [DOI] [PubMed] [Google Scholar]
- 52.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat. Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual participant data and study documents can be provided upon request from www.clinicalstudydatarequest.com.