Abstract
Purpose of Review
The first stages of human life, which include the fetal period, infancy, and early childhood, are the most critical for human growth and development. This is the most vulnerable phase to health challenges due to the immature immune system and rapid development. Mycotoxins such as aflatoxins, ochratoxin A, patulin, fumonisins, zearalenone, and deoxynivalenol are secondary metabolites secreted by various fungal species, primarily Aspergillus, Fusarium, Penicillium, and Alternaria. Aflatoxins are one of the major mycotoxins produced in cereals and cereal-based foods by several species of Aspergillus, mainly Aspergillus flavus. In this context, this review provides a brief overview of the occurrence, exposure, legal regulations, and health effects of aflatoxins (B1, B2, G1, G2, and M1) in cereal-based baby foods and breast milk.
Recent Findings
Human aflatoxin exposure in utero and through breast milk, infant formulas, cereals, and cereal-based foods has been linked to various health consequences, including adverse birth outcomes, impaired growth and development, immune system suppression, and hepatic dysfunction. Recent evidence suggests that especially infants and children are more susceptible to aflatoxins due to their lower body weight, lowered capacity to detoxify harmful substances, more restrictive diet, immature metabolism and elimination, and faster rates of growth and development.
Summary
It is essential for both food safety and infant and child health that aflatoxins in cereal and cereal-based products are precisely detected, detoxified, and managed.
Keywords: Aflatoxins, Breast milk, Cereal-based baby foods, Infant nutrition, Mycotoxin
Introduction
Mycotoxins are toxic metabolites produced by certain fungi belonging to the genus Aspergillus, Penicillium, and Fusarium, causing health problems in humans and animals ranging from allergic responses to death [1]. Mycotoxins cause biochemical, physiological and/or pathological changes in humans, animals, plants, and other microorganisms because of exposure in various ways [2]. In the literature, the most common mycotoxins in food products are aflatoxins (AF), ochratoxin A (OTA), patulin (PAT), fumonisins (FB), zearalenone (ZEN), and deoxynivalenol (DON) [3, 4]. Among the types of mycotoxins, 20 different types of AF were identified. AFB1, AFB2, AFG1, and AFG2 are frequently reported as significant contaminants of food products [5•, 6]. Ochratoxin A is a type of mycotoxin that has carcinogenic, teratogenic, immunotoxic, and neurotoxic effects. Also, it belongs to Group 2B carcinogenic according to the IARC (International Agency for Research on Cancer) [5•, 7]. As a type of mycotoxin, PAT has been identified in various food products, including fruits and vegetables. It is mostly found in apple and apple products, in fruits including pear, apricot, peach, and grapes [8]. According to the IARC, PAT is a group 3 carcinogen, and it has a neurological, gastrointestinal, and immunological adverse effects [9, 10]. Fumonisins are mycotoxins produced by the fungi Fusarium verticillioides and Fusarium proliferatum [11]. Fumonisins have been classified by the IARC as a Group 2B possible human carcinogen [9]. Zearalenone and its derivatives are defined as estrogenic mycotoxins, and they mainly exist in moldy food and crop. The structure of ZEN is like estrogen, so it has estrogen-like effects on various organisms [12]. Deoxynivalenol, produced by Fusarium spp., is a mycotoxin in cereals (wheat, rye, barley, and oats) and cereal-based food products [13].
Consumption of mycotoxins may cause decreased resistance to infectious diseases and impaired immunity. Mycotoxins cause growth retardation by inhibiting protein synthesis, especially in children under the age of 5, and have adverse effects on morbidity and health [14••]. In this context, the aim of the this study was to provide an overview about the aflatoxin occurrence and worldwide exposure of this toxin in human breast milk, infant formula, and cereal-based products for infants and was evaluated regarding the published data during the past decade on aflatoxin prevalence.
Aflatoxins
Aflatoxins are classified by IARC as group 1 carcinogens due to their toxic, carcinogenic, teratogenic, mutagenic, and immunotoxic structure. Aflatoxins are found in cereals and cereal-based products such as grains, bread, breakfast cereals, pasta products, and infant formulas [15]. After immunoaffinity column cleanup and fluorescence detection, high-performance liquid chromatography (HPLC) is commonly used to analyze aflatoxin [16]. Aflatoxins of the B and G groups are named after their blue or green fluorescence under UV light, respectively, whereas aflatoxins of the M group are named after their presence in milk and milk products [17].
In the literature, 20 different types of AF have been identified. Among them AFB1, AFB2, AFG1, AFG2, and AFM1 are frequently reported as the important contaminants of food products [5•]. Dietary exposure to AF in childhood occurs via breast milk and complementary infant foods [2]. A summary of the aflatoxin types, dietary sources, and chemical structures is in Table 1.
Table 1.
| Aflatoxin type | Dietary sources |
|---|---|
| AFB1 |
Plant-based foods Cereal and cereal-based foods |
| AFB2 |
Plant-based foods Cereal and cereal-based foods |
| AFG1 |
Plant-based foods Cereal and cereal-based foods |
| AFG2 |
Plant-based foods Cereal and cereal-based foods |
| AFM1 |
Dairy products Human breast milk |
AFB1
Aflatoxin B1 (AFB1) is the most toxic aflatoxin, being categorized as Group 1 (a human carcinogen), by the IARC and is thought to be the primary cause of human liver cancer [5•]. The liver is thought to be the primary target organ for aflatoxin carcinogenesis. Thus, to reduce aflatoxin exposure, most countries have strict rules for controlling aflatoxin B1 in natural or formulated food products [18].
The European Union has regulated the strictest maximum levels for aflatoxin B1 (0.10 µg/kg) in infant and baby foods in the Commission Regulation based on the risks associated with mycotoxins in infants [19]. Although aflatoxins are carcinogenic to humans and that the four major aflatoxins may co-occur in infant cereals, no maximum levels for the sum of aflatoxins B1, B2, G1, and G2 have been established.
AFB1-lysine (AFB1-lys) levels, which have a half-life of 2–3 months, are used as a reliable biomarker to measure and evaluate AFB1 exposure in epidemiological studies [20]. In a study conducted by Chen et al., they examined children’s exposures to dietary AFB1-lys and potential impacts on growth in 114 children under 36 months of age in Tanzania. AFB1-lys was detected in serum samples of 72% of the children, with a mean level of 5.1 pg/mg albumin [21]. Liquid chromatography isotope dilution mass spectrometry (LC/MS) was used to determine its concentrations, as described by [22] and [23]. Urine metabolites have a half-life of a few hours or less in humans; the aflatoxin B1-albumin lysine adduct is thought to have the same half-life as albumin itself, about 3 weeks. Because exposure of aflatoxins may be evaluated over months and repeated exposures result in larger AFB1-Lys levels, the utilization of the aflatoxin B1-albumin lysine adduct, measured as aflatoxin B1-lysine (AFB1-lys), is thought to have a higher value as a biomarker [24]. In a prospective cohort study by Lauer et al., the serum concentration of AFB1-lys adduct was used to determine maternal aflatoxin exposure in Uganda. A relationship was found between maternal exposure to aflatoxin during pregnancy and adverse birth outcomes such as low birth weight and smaller head circumference [25]. Gichohi-Wainaina et al. assessed AFB1 exposure in mothers and the risk of stunting, wasting, and underweight in their children under the age of 24 months. The highest AFB1 contamination levels were found in maize grain samples. AFB1 concentration was associated with lower weight for height z scores and weight for age z scores in children [26]. In summary, high levels of AFB1 in mothers and children have been linked to stunting and underweight and low birth weight babies.
Nejad et al. conducted a systematic review and meta-analysis to explore the relationship of AFB1 on infant/children growth parameters such as wasting, underweight, stunting, and weight for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ) z-scores. This is the first meta-analysis to investigate the relationship between AFB1 exposure and child growth parameters. The studies included in the article were conducted in countries such as Zambia, Nepal, Pakistan, Bangladesh, Tanzania, and Ethiopia. AFB1 exposure was found negatively associated with growth z-scores including WHZ and HAZ in infants/children, a possible risk factor for infant/child growth impairment [27].
AFM1
Mammals fed AFB1-contaminated diets excrete 0.3–6.3% of the main 4-hydroxylated metabolite known as aflatoxin M1 (AFM1) in their milk as a modified form of mycotoxin. The International Agency for Research on Cancer (IARC) has classified aflatoxin M1 as 2B (possible human carcinogen) [9]. Aflatoxin M1 has been classified as one of the most common chemical compounds in various dairy products such as milk, cheese, yogurt, butter, and infant formula as well as in animal tissues and human milk [28]. AFM1 is resistant to the high temperatures used in autoclaving and pasteurization processes; thus, it is important to reduce aflatoxin levels during milk and dairy product production, particularly during storage stages [29].
AFM1 can be detected in mother’s milk 12–24 h after consuming contaminated food. It rapidly decreases with time and is no longer detectable after 3 days of not consuming contaminated food [30]. Because AFM1 is secreted in human breast milk, AFM1 exposure in infants and children has been linked to Reye and Kwashiorkor’s syndromes, immunosuppression, dermal irritation, endocrine disruptions, growth retardation, underweight, and infectious diseases. Thus, investigating the presence and level of AFM1 in human breast milk is of particular interest [31].
The Scientific Commission of the European Community regulates a maximum limit of 0.025 µg/kg for AFM1 in infant formulae and follow-on formulae, including infant milk and follow-on milk [19].
In a study conduct by Kabak, it was evaluated that aflatoxin M1 occurs in infant formula, follow-on formula, and toddler formulae marketed in Turkey. Aflatoxin M1 was detected in five of the 62 samples (8%), at levels ranging from 0.016 to 0.022 µg/kg (mean level 0.018 µg/kg) but at levels below the European legislation limit of 0.025 µg/kg. It concluded that the presence of AFM1 in Turkish formulae does not appear to pose severe health danger to children, as none of the samples surpassed the European standard of 0.025 mg kg for AFM1 [32].
Aflatoxin Exposure During the First 1000 Days of Life
Pregnancy, infancy, and early childhood are particularly vulnerable periods to environmental toxins, and any health risks associated with toxicant exposure during these critical periods of life could have long-term consequences [33]. Transplacental transport, breastfeeding, and complementary feeding are important routes of dietary exposure to these contaminants during gestation and early postnatal life [34]. Aflatoxin exposure in utero may contribute to negative pregnancy outcomes, such as impaired fetal growth, premature delivery, and pregnancy losses. In addition, maternal exposure to aflatoxins during pregnancy can result in adverse birth outcomes, such as low birth weight, small-for-gestational-age, preterm birth, and poor growth that lasts into infancy and early childhood [33, 35, 36]. In a systematic review conducted by Alvito et al., they evaluated 17 epidemiological studies and their relationship between adverse pregnancy outcomes and maternal mycotoxin exposure. They found an adverse effect of maternal aflatoxin exposure on fetal growth, a decreased birth weight, and an increased risk of low birth weight among exposed newborn infants [37]. In another prospective cohort study conducted by Tesfamariam et al., the association between chronic aflatoxin exposure during pregnancy and fetal growth trajectories was examined. In this study, aflatoxin was found in 86.6% of maternal blood samples (n, 492), and the aflatoxin-exposed group showed a significantly lower change in fetal weight-for-gestational-age centile change over time than the unexposed group [38].
Infants and children are the most vulnerable to aflatoxins because of their lower body weight, decreased ability to detoxify hazardous agents, more restricted diet, immature metabolism and elimination, and faster growth and development rates [39, 40]. Furthermore, some nutritional factors influence aflatoxin toxicity. Children who are protein deficient, for example, are more sensitive to aflatoxins [5•]. Aflatoxin exposure is associated with childhood kwashiorkor and marasmus (malnutrition-related childhood disease). Protein deficiency is a major cause of kwashiorkor and marasmus. Children with kwashiorkor or marasmus have higher levels of aflatoxins or their metabolites in their blood and urine. In these malnutrition-related diseases, liver function has reduced, and aflatoxin metabolism has altered. For this reason, children with kwashiorkor or marasmus are more vulnerable to the hazards and toxicity of aflatoxin in their nutrition [41, 42].
Cereal-based baby foods, which are frequently the first solid meal used in infant feeding, gradually replace breast milk during the first several months of life, mainly after 6 months of their birth [43]. Cereals (wheat, corn, oats, rice, barley, malt, soy, and rye), honey, sugar, dried fruits, and cocoa are among the ingredients in these products [44]. Cereal-based foods are sources of energy, starch, fiber, protein, high amounts of vitamins, minerals, and bioactive compounds [45]. It contains indigestible carbohydrates that are central to increasing the intestinal microbiota population. When infants are weaned, a cereal-based feeding increases the fermentation activity of the gut microbiota population [18]. Because these foods have a mild taste and semi-solid texture, they are the good choice for babies who are transitioning from breast milk to solid foods at the start of complementary feeding [46]. Despite all the benefits mentioned, the presence and exposure to aflatoxins because of the consumption of these products is unavoidable because of different grains that are used as ingredients in most baby foods for infants and children, and the likelihood of multiple mycotoxins increases [47]. To protect vulnerable populations, surveillance studies to determine the extent of contamination with the five aflatoxins in foods intended for infants and children are required [48]. The mechanism of aflatoxin and infant nutrition is summarized in Fig. 1. Studies that examine the relationship between aflatoxin exposure and child growth and aflatoxin occurrence of infant foods in different countries are summarized in Tables 2 and 3.
Fig. 1.
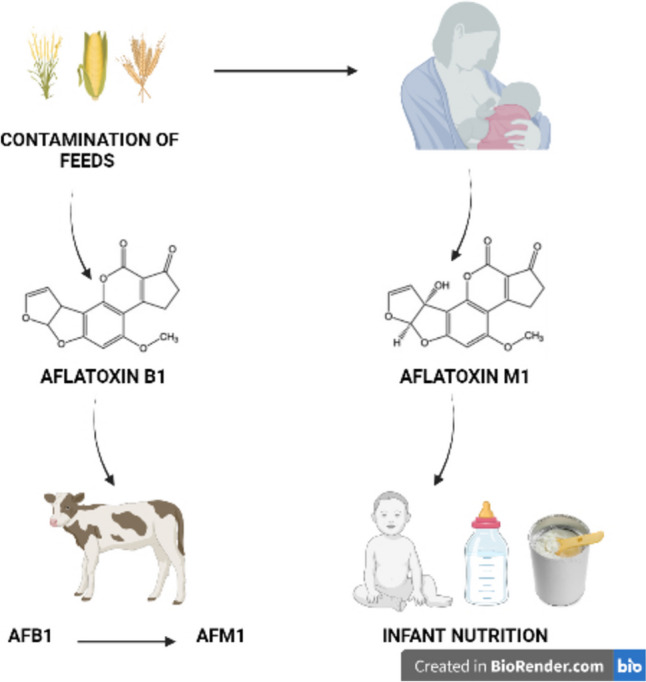
The mechanism of aflatoxin and infant nutrition
Table 2.
Summary of the studies on the association between aflatoxin exposure and child growth parameters
| Country | Study design | Study population | Aflatoxin type | Analysis technique | Detection rate | Outcome | Finding | Reference |
|---|---|---|---|---|---|---|---|---|
| Nepal | Longitudinal birth cohort | 1675 mother and infants (from pregnancy to 2 years of age follow-up) |
AFB1-lysine AFM1 |
HPLC |
1.54 pg/mg 0.04–315.99 ng/L (%94.1) |
LAZ, WAZ, WLZ z-scores, length, knee-heel length and stunting | AFB1-lysine adduct concentrations were significantly associated with changes in LAZ, length, knee-heel length and WAZ z-scores. Serum aflatoxin concentrations were associated with stunting | [49] |
| Zambia | Cross-sectional | 400 mothers with children aged 6–24 months | AFB1-lys | LC–MS/MS | NR | HAZ, WAZ and WHZ z-scores, stunting, underweight, and wasting | AFB1-lys level were found to be significantly associated with child stunting | [24] |
| Tanzania | RCT | Intervention group n = 150, control group n = 150 | Total AFs (AFB1, AFB2, AFG1 and AFG2) | HPLC | NR | WAZ z-score, dietary assessment | Mean concentration of AFs was significantly lower in the intervention than control group. Mean WAZ z-score difference between the groups was 0.57 (p < 0.05) | [50] |
| Kenya | RCT | Intervention group n = 489, control group n = 392 (mother and infants) | AFB1-lys | HPLC |
Intervention group: 2.69 pg/mL Control group: 2.74 pg/ mL |
LAZ z-score, stunting and child serum AFB1-lysine adduct | The intervention significantly reduced endline in serum AFB1-lysine adduct levels but had no effect on endline LAZ or stunting | [51] |
| Nepal | Longitudinal cohort | 85 children (15, 24, and 36 months follow-up) | AFB1-lys | UPLC | 3.62 pg/mg | LAZ, WAZ and WLZ z-scores | The chronic AF exposure was not significantly associated with anthropometric z-scores | [52] |
| Pakistan | Cross-sectional | 150 children aged 3–5 years | AFM1 | ELISA | 0.09 ± 0.32 ng/ml | HAZ, WAZ, and WHZ z-scores | A non-significant correlation was recorded between urinary AFM1 levels and the evaluated anthropometric indices | [53] |
| Tanzania | Longitudinal cohort |
143 infants (1st, 3rd and 5th months follow-up) |
AFM1 (breast milk) |
HPLC | AFM1 levels ranging from 0.01 to 0.55 ng/ml | WAZ, HAZ and WHZ z-scores | A significant association was observed between AFM1 exposure levels and WAZ or HAZ z-scores | [2] |
AF aflatoxin, HAZ height-for-age, HPLC high-performance liquid chromatography, LAZ length-for-age, LC–MS/MS liquid chromatography-mass spectrometry tandem mass spectrometry, NR not reported, UPLC ultra performance liquid chromatography, RCT randomized controlled trial, WAZ weight-for-age, WHZ weight-for-height, WLZ weight-for-length
Table 3.
Occurrence of aflatoxins in infant foods in different countries
| Type of aflatoxin | Dietary source | Country | Method of detection | Sample size | Positive samples (%) |
Mean concentration of AF |
Reference |
|---|---|---|---|---|---|---|---|
| AFB1 | Cereal-based baby foods | Lebanese | ELISA | 42 | ND | ND | [54] |
| AFB1 | Cereal-based baby foods | Iran | HPLC | 48 | 33 (68.7) | 2.6 ± 4.0 µg/kg | [55] |
| AFB1 | Cereal-based baby foods | Kosovo | ELISA and LC-MS/MS | 103 | 64 (62.1) | Ranged from 0.008 to 0.116 μg/kg | [56] |
| AFM1 | Baby milk | 40 | 6 (15) | Ranged from 0.008 to 0.123 μg/kg | |||
| AFB1 | Cereal-based baby foods | Turkey | LC-MS/MS | 85 | 11 (12.9) | 0.03 ± 0.01 (Mean ± SD) | [57] |
| AFB2 | 3 (3.5) | 0.05 ± 0.01 (Mean ± SD) | |||||
| AFG1 | ND | ND | |||||
| AFG2 | ND | ND | |||||
| AFB1 | Cereal-based baby foods | Spain | HPLC | 60 | 11 (18.3) | 0.03 ± 0.05 (Mean ± SD) | [48] |
| AFB2 | 1 (1.7) | 0.01 ± 0.02 (Mean ± SD) | |||||
| AFG1 | 6 (10) | 0.02 ± 0.04 (Mean ± SD) | |||||
| AFG2 | 1 (1.7) | 0.01 ± 0.01 (Mean ± SD) | |||||
| AFM1 | Baby formula | Lebanese | ELISA | 84 | 8 (9.5) | 5.72 ± 0.014 ng/L | [54] |
| AFM1 | Breast milk | Mexico | ELISA | 123 | 123 (100) | 17.04 ng/L | [31] |
| AFM1 | Breast milk | Turkey | HPLC | 100 | 53 (53) | 6.36 ng/L | [58] |
| AFM1 |
Breast milk (BM) and infant powdered milk (IPM) |
Brazil | HPLC |
94 (BM) 16 (IPM) |
5 (5.3) 7 (43.8) |
0.018 ± 0.005 (BM) 0.024 ± 0.01 (IPM) |
[59] |
| AFM1 | Breast milk | Iran | ELISA | 85 | 85 (100) | 5.91 ng/L | [60] |
| AFM1 | Baby formula | Turkey | HPLC | 62 | 5 (8) | 0.018 μg/kg | [32] |
AF aflatoxin, ND not determined, BM breast milk, IPM infant powdered milk
In Vitro Bioaccessibility of Aflatoxins
Bioaccessibility, refers to the portion of a food contaminant that is released into the gastrointestinal tract, whereas bioavailability refers to the portion of an ingested food contaminant that enters the systemic circulation and can negatively impact health [61]. In vivo bioavailability studies are more difficult in terms of time, ethics, and cost than in vitro methods [61]. The bioavailability of nutrients is generally studied using in vitro systems based on the compartments of the gastrointestinal tract [62]. In vitro bioavailability analyses were performed in simulated mouth, stomach, and small intestine models [63]. The food product, level of contamination, and method of contamination all affect mycotoxin bioaccessibility. By simulating the digestion in vitro, mycotoxins’ bioaccessibility and bioavailability are assessed [64, 65]. Many studies have used different in vitro digestion models to determine the bioaccessibility or absorption of mycotoxins, avoiding the use of more complex cell cultures and the use of animals in in vivo experiments [66].
Legal Regulations
The World Health Organization (WHO) has identified AFs as a global food safety concern [67]. Because of their toxic, carcinogenic, mutagenic, teratogenic, and immunotoxic properties, AFs were classified as group 1 carcinogens by the IARC [9]. Countries have implemented strict regulations to prevent AF contamination in food and feed due to serious health complications in humans and animals. The maximum permissible limit for total AFs according to the European Commission Regulation is present in Table 4 [19].
Table 4.
Maximum limits of AFB1 and AFM1 in cereal-based products and baby formulas according to the European Commission
| Legal regulations | Infant products | Type of aflatoxin | Maximum limits |
|---|---|---|---|
|
European Commission 1881/2006/EC |
Cereal-based and infant foods | AFB1 | 0.1 µg/kg |
|
Infant formula and follow-on formula |
AFM1 | 0.025 µg/kg |
Conclusions and Future Perspectives
Humans can be exposed to AFs during the early stages of life, including in utero exposure, breast milk, infant formula milk, and cereal-based infant foods used up to the age of 2. Infants and children are among the vulnerable groups in the population in terms of their physiology and nutrition. The presence of AFs in infant nutrition is of high importance. Aflatoxin contamination is inevitable due to the presence of many components such as cereals (wheat, corn, oats, rice, barley, malt, soy, and rye, milk powder, and fruits in baby products). In the studies examined recently, it is seen that the presence of aflatoxins is quite common in cereal-based baby products, milk, and dairy products. The presence of AFB1 in cereal-based baby products and AFM1 in milk and dairy products can cause negative health consequences for infant and child health. Due to their thermostable nature, most mycotoxins are resistant to food processing techniques. In this context, by periodically testing baby foods for aflatoxins, safe food will be provided for infant and child nutrition.
Breast milk and continuity of breastfeeding are very important for infant nutrition. The American Academy of Pediatrics (AAP) and WHO recommend exclusive breastfeeding for approximately 6 months after birth and continued breastfeeding with complementary foods for at least 2 years. Since the mother’s nutrition will affect the composition of breast milk, it is important for public health and mycotoxin contamination to inform mothers about the consumption of foods containing mycotoxins, especially during pregnancy and breastfeeding, and to carry out the necessary legal regulations and inspections on food safety.
There are differences between countries regarding the presence of mycotoxins in breast milk, formula milk, and/or cereal-based baby foods. Epidemiological studies in the literature examining the effects of mycotoxin contamination on growth parameters in infants and children were generally conducted in countries such as Gambia, Nigeria, Kenya, Pakistan, Tanzania, Ethiopia, and Nepal. In countries with low income, ensuring food safety and carrying out legal regulations to prevent mycotoxin contamination are important to protect the health of mothers, infants, and children.
In this context, to prevent AF contamination in cereal-based baby foods, milk, and dairy products the limit of legal regulations should not be exceeded, and AF levels should be strictly monitored in high-risk areas and in commercially sold products.
Author Contribution
YA: Designed research and wrote paper (conceptualization, writing, review, and editing); GA: Designed research (conceptualization and supervision). All authors have read and approved the final manuscript.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Pereira C. SC Cunha, JO Fernandes. Mycotoxins of concern in children and infant cereal food at European level: incidence and bioaccessibility. Toxins. 2022;14(7):488. doi: 10.3390/toxins14070488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magoha H, Kimanya M, Meulenaer BD, Roberfroid D, Lachat C, Kolsteren P. Association between aflatoxin M1 exposure through breast milk and growth impairment in infants from Northern Tanzania. World Mycotoxin J. 2014;7(3):277–284. doi: 10.3920/WMJ2014.1705. [DOI] [Google Scholar]
- 3.Abrunhosa L, Morales H, Soares C, Calado T, Vila-Chã AS, Pereira M, et al. A review of mycotoxins in food and feed products in Portugal and estimation of probable daily intakes. Crit Rev Food Sci Nutr. 2016;56(2):249–265. doi: 10.1080/10408398.2012.720619. [DOI] [PubMed] [Google Scholar]
- 4.Oteiza JM, Khaneghah A, Campagnollo FB, Granato D, Mahmoudi M, Sant’Ana A, et al. Influence of production on the presence of patulin and ochratoxin A in fruit juices and wines of Argentina. Lebensm Wiss Technol. 2017;80:200–207. doi: 10.1016/J.LWT.2017.02.025. [DOI] [Google Scholar]
- 5.Coppa C, Khaneghah Mousavi A, Alvito PC, Assunção R. The occurrence of mycotoxins in breast milk, fruit products and cereal-based infant formula: a review. Trends Food Sci Technol. 2019;92:81–93. doi: 10.1016/j.tifs.2019.08.014. [DOI] [Google Scholar]
- 6.Mousavi Khaneghah A, Eş İ, Raeisi S, Fakhri Y. Aflatoxins in cereals: state of the art. J Food Saf. 2018;38(6):12532. doi: 10.1111/jfs.12532. [DOI] [Google Scholar]
- 7.Ostry V, Malir F, Toman J, Grosse Y. Mycotoxins as human carcinogens—the IARC Monographs classification. Mycotoxin Res. 2017;33(1):65–73. doi: 10.1007/s12550-016-0265-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhong L, Carere J, Lu Z, Lu F, Zhou T. Patulin in apples and apple-based food products: the burdens and the mitigation strategies. Toxins. 2018;10(11):475. doi: 10.3390/toxins10110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. International Agency for Research on Cancer. Agents classified by the IARC Monographs. 2023. https://monographs.iarc.who.int/agents-classified-by-the-iarc/. Accessed 10 May 2023.
- 10.Pal S. Toxicological effects of patulin mycotoxin on the mammalian system: an overview. Toxicol Res. 2017;6(6):764–771. doi: 10.1039/c7tx00138j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Riley RT, Wu F. Dietary fumonisin and growth impairment in children and animals: a review. Compr Rev Food Sci Food Saf. 2018;17(6):1448–1464. doi: 10.1111/1541-4337.12392. [DOI] [PubMed] [Google Scholar]
- 12.Han X, Huangfu H, Xu T, Xu W, Asakiya C, Huang K, et al. Research progress of safety of zearalenone: a review. Toxins. 2022;14(6):386. doi: 10.3390/toxins14060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji X, Xiao Y, Lyu W, Li M, Wang W, Tang B, et al. Probabilistic risk assessment of combined exposure to deoxynivalenol and emerging alternaria toxins in cereal-based food products for infants and young children in China. Toxins. 2022;14(8):509. doi: 10.3390/toxins14080509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P, Gupta A, Kumar Mahato D, Pandhi S, Kumar Pandey A, Kargwal A, et al. Aflatoxins in cereals and cereal-based products: occurrence, toxicity, impact on human health, and their detoxification and management strategies. Toxins. 2022;14(10):687. doi: 10.3390/toxins14100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaneghah AM, Fakhri Y, Raeisi S, Armoon B, Sant'Ana AS. Prevalence and concentration of ochratoxin A, zearalenone, deoxynivalenol and total aflatoxin in cereal-based products: a systematic review and meta-analysis. Food Chem Toxicol. 2018;118:830–848. doi: 10.1016/j.fct.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Alvito PC, Sizoo EA, Almeida CMM, van Egmond HP. Occurrence of aflatoxins and ochratoxin A in baby foods in Portugal. Food Anal Methods. 2010;3(1):22–30. doi: 10.1007/s12161-008-9064-x. [DOI] [Google Scholar]
- 17.Gong YY, Watson S, Routledge MN. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 2016;4(1):14–27. doi: 10.14252/foodsafetyfscj.2015026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashiry M, Javanmardi F, Sadeghi E, Shokri S, Hossieni H, Oliveira CAF, et al. The prevalence of aflatoxins in commercial baby food products: a global systematic review, meta-analysis, and risk assessment study. Trends Food Sci Technol. 2021;114:100–115. doi: 10.1016/j.tifs.2021.05.014. [DOI] [Google Scholar]
- 19.Commission regulation (EC), No 1881, 2006 of 19, 2006 Setting maximum levels for certain contaminants in foodstuffs. Official J Eur Union. 2006;364(5):24. [Google Scholar]
- 20.Cao W, Yu P, Yang K, Cao D. Aflatoxin B1: metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol Mech Methods. 2022;32(6):395–419. doi: 10.1080/15376516.2021.2021339. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Mitchell NJ, Gratz J, Houpt ER, Gong Y, Egner PA, et al. Exposure to aflatoxin and fumonisin in children at risk for growth impairment in rural Tanzania. Environ Int. 2018;115:29–37. doi: 10.1016/j.envint.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCoy LF, Scholl PF, Sutcliffe AE, Kieszak SM, Powers CD, Rogers HS, et al. Human aflatoxin albumin adducts quantitatively compared by ELISA, HPLC with fluorescence detection, and HPLC with isotope dilution mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1653–1657. doi: 10.1158/1055-9965.EPI-07-2780. [DOI] [PubMed] [Google Scholar]
- 23.Groopman JD, Egner PA, Schulze KJ, Wu LSF, Merrill R, Mehra S, et al. Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B1-lysine albumin biomarkers. Food Chem Toxicol. 2014;74:184–189. doi: 10.1016/j.fct.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alamu EO, Gondwe T, Akello J, Maziya-Dixon B, Mukanga M. Relationship between serum aflatoxin concentrations and the nutritional status of children aged 6–24 months from Zambia. Int J Food Sci Nutr. 2020;71(5):593–603. doi: 10.1080/09637486.2019.1689547. [DOI] [PubMed] [Google Scholar]
- 25.Lauer JM, Duggan CP, Ausman LM, Griffiths JK, Webb P, Wang JS, et al. Maternal aflatoxin exposure during pregnancy and adverse birth outcomes in Uganda. Matern Child Nutr. 2019;15(2):e12701. doi: 10.1111/mcn.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gichohi-Wainaina WN, Kimanya M, Muzanila YC, Kumwenda NC, Msere H, Rashidi M, et al. Aflatoxin contamination, exposure among rural smallholder farming Tanzanian mothers and associations with growth among their children. Toxins (Basel) 2023;15(4):257. doi: 10.3390/toxins15040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghorbani Nejad B, Mostafaei Z, Balouchi Rezaabad A, Mehravar F, Zarei M, Dehghani A, et al. A systematic review with meta-analysis of the relation of aflatoxin B1 to growth impairment in infants/children. BMC Pediatr. 2023;23(1):1–15. doi: 10.1186/s12887-023-04275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahmani J, Solmaz A, Miri A, Fakhri Y, Riahi SM, Keramati H, et al. The prevalence of aflatoxin M1 in milk of Middle East region: a systematic review, meta-analysis and probabilistic health risk assessment. Food Chem Toxicol. 2018;118:653–666. doi: 10.1016/j.fct.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Fakhri Y, Rahmani J, Oliveira CAF, Tuanny Franco L, Humberto Corassin C, Saba S, et al. Aflatoxin M1 in human breast milk: a global systematic review, meta-analysis, and risk assessment study (Monte Carlo simulation) Trends Food Sci Technol. 2019;88(12):333–342. doi: 10.1016/j.tifs.2019.03.013. [DOI] [Google Scholar]
- 30.Battacone G, Nudda A, Palomba M, Mazzette A, Pulina G. The transfer of aflatoxin M1 in milk of ewes fed diet naturally contaminated by aflatoxins and effect of inclusion of dried yeast culture in the diet. J Dairy Sci. 2009;92(10):4997–5004. doi: 10.3168/jds.2008-1684. [DOI] [PubMed] [Google Scholar]
- 31.Salas R, Acosta N, de Jesús GA, Tijerina A, Dávila R, Jiménez-Salas Z, et al. Levels of aflatoxin M1 in breast milk of lactating mothers in Monterrey, Mexico: exposure and health risk assessment of newborns. Toxins (Basel) 2022;14(3):194. doi: 10.3390/toxins14030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabak B. Aflatoxin M1 and ochratoxin A in baby formulae in Turkey: occurrence and safety evaluation. Food Control. 2012;26(1):182–187. doi: 10.1016/j.foodcont.2012.01.032. [DOI] [Google Scholar]
- 33.Ismail A, Naeem I, Gong YY, Routledge MN, Akhtar S, Riaz M, et al. Early life exposure to dietary aflatoxins, health impact and control perspectives: a review. Trends Food Sci Technol. 2021;112:212–224. doi: 10.1016/j.tifs.2021.04.002. [DOI] [Google Scholar]
- 34.Papadopoulou E, Småstuen Haug L, Kaur Sakhi A, Andrusaityte S, Basagaña X, Brantsaeter AL, et al. Diet as a source of exposure to environmental contaminants for pregnant women and children from six European countries. Environ Health Perspect. 2019;127(10):107005. doi: 10.1289/EHP5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews-Trevino JY, Webb P, Shively G, Rogers BL, Baral K, Davis D, et al. Relatively low maternal aflatoxin exposure is associated with small-for-gestational-age but not with other birth outcomes in a prospective birth cohort study of Nepalese infants. J Nutr. 2019;149(10):1818–1825. doi: 10.1093/jn/nxz122. [DOI] [PubMed] [Google Scholar]
- 36.Smith LE, Prendergast AJ, Turner PC, Humphrey JH, Stoltzfus RJ. Aflatoxin exposure during pregnancy, maternal anemia, and adverse birth outcomes. Am J Trop Med Hyg. 2017;96(4):770–776. doi: 10.4269/ajtmh.16-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvito P, Pereira-da-Silva L. Mycotoxin exposure during the first 1000 days of life and its impact on children’s health: a clinical overview. Toxins (Basel) 2022;14(3):189. doi: 10.3390/toxins14030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tesfamariam K, Gebreyesus SH, Lachat C, Hanley-Cook GT, Roro M, Mengistu YG, et al. Chronic aflatoxin exposure during pregnancy is associated with lower fetal growth trajectories: a prospective cohort from the Butajira Nutrition, Mental Health, and Pregnancy (BUNMAP) Study in rural Ethiopia. Am J Clin Nutr. 2022;116(6):1634–1641. doi: 10.1093/ajcn/nqac280. [DOI] [PubMed] [Google Scholar]
- 39.Sherif SO, Salama EE, Abdel-Wahhab MA. Mycotoxins and child health: The need for health risk assessment. Int J Hyg Environ Health. 2009;212(4):347–368. doi: 10.1016/j.ijheh.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Mir SA, Dar MN, Shah MA, Sofi SA, Hamdani AM, Oliveira CAF, et al. Application of new technologies in decontamination of mycotoxins in cereal grains: challenges, and perspectives. Food Chem Toxicol. 2021;148:111976. doi: 10.1016/j.fct.2021.111976. [DOI] [PubMed] [Google Scholar]
- 41.Michael H, Amimo JO, Rajashekara G, Saif LJ, Vlasova AN. Mechanisms of kwashiorkor-associated immune suppression: insights from human, mouse, and pig studies. Front Immunol. 2022;13:826268. doi: 10.3389/fimmu.2022.826268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dabuo B, Wesome Avogo E, Owusu Koomson G, Akantibila M, Ayendo Gbati D. Aflatoxins: toxicity, occurrences and chronic exposure. In: Assaf JC, editor. Aflatoxins-occurrence, detection and novel detoxification strategies. IntechOpen. 2022;-:1–14. doi: 10.5772/intechopen.105723. [DOI] [Google Scholar]
- 43.Al-Taher F, Cappozzo J, Zweigenbaum J, Lee HJ, Jackson L, Ryu D. Detection and quantitation of mycotoxins in infant cereals in the US market by LC-MS/MS using a stable isotope dilution assay. Food Control. 2017;72:27–35. doi: 10.1016/j.foodcont.2016.07.027. [DOI] [Google Scholar]
- 44.Khodaei D, Javanmardi F, Khaneghah AM. The global overview of the occurrence of mycotoxins in cereals: a three-year survey. Curr Opin Food Sci. 2021;39:36–42. doi: 10.1016/j.cofs.2020.12.012. [DOI] [Google Scholar]
- 45.Agostoni C, Decsi T, Fewtrell M, Goulet O, Kolacek S, Koletzko B, et al. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2008;46(1):99–110. doi: 10.1097/01.mpg.0000304464.60788.bd. [DOI] [PubMed] [Google Scholar]
- 46.Sakashita R, Inoue N, Tatsuki T. Selection of reference foods for a scale of standards for use in assessing the transitional process from milk to solid food in infants and pre-school children. Eur J Clin Nutr. 2003;57(7):803–809. doi: 10.1038/sj.ejcn.1601612. [DOI] [PubMed] [Google Scholar]
- 47.Mallmann CA, Tyska D, Almeida CAA, Oliveira MS, Gressler LT. Mycotoxicological monitoring of breakfast and infant cereals marketed in Brazil. Int J Food Microbiol. 2020;331:108628. doi: 10.1016/j.ijfoodmicro.2020.108628. [DOI] [PubMed] [Google Scholar]
- 48.Herrera M, Bervis N, Carramiñana JJ, Juan T, Herrera A, Ariño A, et al. Occurrence and exposure assessment of aflatoxins JJ, Juan T, Herrera A, Ariño A, et al. Occurrence and exposure assessment of aflatoxins and deoxynivalenol in cereal-based baby foods for infants. Toxins (Basel). 2019;11(3):150. doi: 10.3390/toxins11030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrews-Trevino JY, Webb P, Shively G, Kablan A, Baral K, Davis D, et al. Aflatoxin exposure and child nutrition: measuring anthropometric and long-bone growth over time in Nepal. Am J Clin Nutr. 2021;113(4):874–883. doi: 10.1093/ajcn/nqaa397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamala A, Kimanya M, De Meulenaer B, Kolsteren P, Jacxsens L, Haesaert G, et al. Post-harvest interventions decrease aflatoxin and fumonisin contamination in maize and subsequent dietary exposure in Tanzanian infants: a cluster randomised-controlled trial. World Mycotoxin J. 2018;11(3):447–458. doi: 10.3920/WMJ2017.2234. [DOI] [Google Scholar]
- 51.Hoffmann V, Jones K, Leroy JL. The impact of reducing dietary aflatoxin exposure on child linear growth: a cluster randomised controlled trial in Kenya. BMJ Glob Health. 2018;3(6):e000983. doi: 10.3920/wmj2017.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell NJ, Hsu HH, Chandyo RK, Shrestha B, Bodhidatt L, Tu YK, et al. Aflatoxin exposure during the first 36 months of life was not associated with impaired growth in Nepalese children: an extension of the MAL-ED study. PLoS One. 2017;12(2):e0172124. doi: 10.1371/journal.pone.0172124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nasir U, Naeem I, Asif M, Ismail A, Gong YY, Routledge MN, et al. Assessment of aflatoxins exposure through urinary biomarker approach and the evaluation of the impacts of aflatoxins exposure on the selected health parameters of the children of Multan city of Pakistan. Food Control. 2021;123:107863. doi: 10.1016/j.foodcont.2021.107863. [DOI] [Google Scholar]
- 54.Daou R, Hoteit M, Bookari K, Al-Khalaf M, Nahle S, Al-Jawaldeh A, et al. Aflatoxin B1 occurrence in children under the age of five’s food products and aflatoxin M1 exposure assessment and risk characterization of Arab infants through consumption of infant powdered formula: a Lebanese experience. Toxins. 2022;14(5):290. doi: 10.3390/toxins14050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mottaghianpour E, Nazari F, Mehrasbi MR, Hosseini MJ. Occurrence of aflatoxin B1 in baby foods marketed in Iran. J Sci Food Agric. 2017;97(9):2690–2694. doi: 10.1002/jsfa.8092. [DOI] [PubMed] [Google Scholar]
- 56.Muharremi H, Raka L, Spahiu J, Tershnjaku I, Topi D. Investigation of aflatoxin M1 in baby milk and aflatoxin B1 in infant cereals marketed in Kosovo. J Food Process Preserv. 2022;46(6):e16285. doi: 10.1111/jfpp.16285. [DOI] [Google Scholar]
- 57.Er Demirhan B, Demirhan B. The investigation of mycotoxins and Enterobacteriaceae of cereal-based baby foods marketed in Turkey. Foods. 2021;10(12):3040. doi: 10.3390/foods10123040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karayağiz Muslu G, Özdemir M. Occurrence of and factors associated with the presence of aflatoxin M1 in breast milk of mothers in Fethiye. Turkey Biol Res Nurs. 2020;22(3):362–368. doi: 10.1177/1099800420919900. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa AT, Takabayashi-Yamashita CR, Sataque Ono EY, Bagatin AK, Rigobello FF, Kawamura O, et al. Exposure assessment of infants to aflatoxin M1 through consumption of breast milk and infant powdered milk in Brazil. Toxins. 2016;8(9):246. doi: 10.3390/toxins8090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maleki F, Abdi S, Davodian E, Haghani K, Bakhtiyaric S. Exposure of infants to aflatoxin M1 from mother's breast milk in Ilam, Western Iran. Osong Public Health Res Perspect. 2015;6(5):283–287. doi: 10.1016/j.phrp.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernández-García E, Carvajal-Lérida I, Pérez-Gálvez A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr Res. 2009;29(11):751–760. doi: 10.1016/j.nutres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 62.Sopade PA, Gidley MJ. A rapid in-vitro digestibility assay based on glucometry for investigating kinetics of starch digestion. Starch. 2009;61(5):245–255. doi: 10.1002/star.200800102. [DOI] [Google Scholar]
- 63.Ménard O, Cattenoz T, Guillemin H, Souchon I, Deglaire A, Dupont D, et al. Validation of a new in vitro dynamic system to simulate infant digestion. Food Chem. 2014;145:1039–1045. doi: 10.1016/j.foodchem.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 64.Raiola A, Tenore GC, Manyes L, Meca G, Ritieni A. Risk analysis of main mycotoxins occurring in food for children: an overview. Food Chem Toxicol. 2015;84:169–180. doi: 10.1016/j.fct.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 65.Versantvoort CH, Oomen AO, Van de Kamp E, Rompelberg CJM, Sips AJAM. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem Toxicol. 2005;43(1):31–40. doi: 10.1016/j.fct.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 66.González-Arias CA, Marin S, Sanchis V, Ramos AJ. Mycotoxin bioaccessibility/absorption assessment using in vitro digestion models: a review. World Mycotoxin J. 2013;6(2):167–184. doi: 10.3920/WMJ2012.1521. [DOI] [Google Scholar]
- 67.World Health Organization (WHO). Estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007–2015. https://apps.who.int/iris/bitstream/handle/10665/199350/9789241565165_eng.pdf?sequence=1&isAllowed=y. Accessed 16 June 2023.


