Abstract
Dorsal functions as both an activator and repressor of transcription to determine dorsoventral fate in the Drosophila melanogaster embryo. Repression by Dorsal requires the corepressor Groucho (Gro) and is mediated by silencers termed ventral repression regions (VRRs). A VRR in zerknüllt (zen) contains Dorsal binding sites as well as an essential element termed AT2. We have identified and purified an AT2 DNA binding activity in embryos and shown it to consist of cut (ct) and dead ringer (dri) gene products. Studies of loss-of-function mutations in ct and dri demonstrate that both genes are required for the activity of the AT2 site. Dorsal and Dri both bind Gro, acting cooperatively to recruit it to the DNA. Thus, ventral repression may require the formation of a multiprotein complex at the VRR. This complex includes Dorsal, Gro, and additional DNA binding proteins, which appear to convert Dorsal from an activator to a repressor by enabling it to recruit Gro to the template. By showing how binding site context can dramatically alter transcription factor function, these findings help clarify the mechanisms responsible for the regulatory specificity of transcription factors.
Establishment of the dorsoventral axis in a Drosophila melanogaster embryo depends upon the maternal morphogen Dorsal. This transcription factor is localized in a monotonic nuclear concentration gradient in early blastoderm embryos, with ventral nuclei containing the highest and dorsal nuclei containing the lowest concentrations of this protein (38, 40, 44). The dorsoventral fate map of the Drosophila embryo is dictated by the Dorsal nuclear concentration gradient, and mutations that disrupt the gradient also disrupt the fate map. Nuclear localization of Dorsal is dependent upon the activity of 12 maternal gene products, which transduce a signal originating in the ventral perivitelline space to the interior of the embryo, resulting in the release of Dorsal from its cytoplasmic inhibitor, Cactus (for reviews see references 2, 10, and 14). Once Dorsal is free from Cactus, it traverses the nuclear membrane and modifies the transcriptional program of the embryo, generating multiple distinct domains of gene activity along the dorsoventral axis. The ability of Dorsal to subdivide the embryo into multiple domains is critically dependent upon the ability of this factor to function as both an activator and a repressor of transcription. An understanding of what determines whether Dorsal will function as an activator or a repressor of any given target gene is therefore essential to an understanding of pattern formation in the Drosophila embryo.
Dorsal functions as a regulator of a number of cellular determinant-encoding genes. For example, it activates the mesoderm-determining genes twist (twi) and snail (sna) and represses the dorsal ectoderm-determining genes zerknüllt (zen) and decapentaplegic (dpp) (37). Dorsal activates twi through ventral activation regions (VARs) upstream of the core promoter. The only elements within the VARs that are essential for activation are the Dorsal binding sites themselves (19, 21, 34, 46). Dorsal represses zen via a set of Dorsal binding sites in a 5′ regulatory region termed the ventral repression region (VRR). The zen VRR is sufficient to direct ventral repression of a lacZ reporter gene under control of the minimal even skipped (eve) stripe 2 enhancer (MSE) in blastoderm embryos (23, 25). While the Dorsal binding sites in the zen VRR are essential, they are not sufficient for repression. For example, when one of the Dorsal binding sites in zen is removed from the context of the VRR and multimerized upstream of a core promoter driving a lacZ reporter gene, ventral activation of lacZ results (22, 34). Thus, isolated Dorsal binding sites direct activation, while repression apparently requires additional elements in the VRR.
Several approaches have been employed to identify elements other than Dorsal binding sites in the zen VRR that are necessary for ventral repression. A search for conserved elements in the VRR revealed four AT-rich sites termed AT1 through AT4 in addition to three Dorsal binding sites termed dl1 through dl3. In some cases, mutagenesis of these sites resulted in a loss of repression (23, 25). Mutating the AT1 site in the context of a 110-bp region from the VRR containing dl1, dl2, AT1, and AT2 resulted in partial derepression of a linked reporter gene. In contrast, mutating the AT2 site in the context of the same 110-bp region resulted in complete derepression, and the reporter was weakly activated along the entire ventral surface of the embryo. These results demonstrate that the AT1 and AT2 elements are likely binding sites for proteins that convert Dorsal from an activator to a repressor and that the AT2 site plays a dominant role in repression.
Not only are the AT-rich sites important for repression, but proper spacing between the AT-rich and Dorsal sites is also required. A 180-bp region from the zen VRR (which we henceforth refer to as the minimal zen VRR) containing three AT-rich sites, AT1 to AT3, and three Dorsal binding sites, dl1 to dl3, was altered by the insertion of a 5-bp spacer between the AT2 and dl2 sites. This change resulted in the elimination of repression, while the insertion of a 10-bp spacer restored repression (7). This result implies that the correct stereospecific positioning of Dorsal relative to AT2 bound proteins is critical for repression. This, in turn, implies an interaction (direct or indirect) between Dorsal and AT2-bound proteins.
Identifying the repressor proteins that operate from the AT-rich repression elements has proved to be a challenging task. Dorsal switch protein 1 (DSP1) was identified in a yeast screen for cDNAs encoding proteins that convert Dorsal from an activator into a repressor (29). Conversion of Dorsal to a repressor by DSP1 may require a sequence in the zen VRR termed the negative regulatory element (29). The negative regulatory element is distinct from the AT-rich elements and is not known to be a critical component of the VRR. Recent evidence suggests that DSP1 can interfere with all activated transcription in a binding-site-independent manner (26).
The ability of Dorsal- and AT2-bound proteins to repress zen transcription depends critically upon the WD repeat-containing corepressor encoded by groucho (gro) (11). The use of germ line clones to eliminate maternal Gro from the embryo results in nearly complete ventral derepression of both zen and dpp. In addition, a lacZ reporter under control of the minimal zen VRR is ventrally derepressed in embryos derived from gro female germ line clones, indicating that the role of Gro in zen repression is likely to be direct. The idea that Gro plays a direct role in Dorsal-mediated repression is further supported by the finding that Dorsal and Gro bind one another in vitro (11).
To identify potential AT2 binding repressors, we have purified proteins from Drosophila embryonic extracts that specifically bind to this element. The purified proteins were found to be the products of cut (ct) and dead ringer (dri). We present genetic evidence demonstrating that these proteins may each play a role in the Dorsal-mediated repression of zen. We show additionally that, like Dorsal, Dri binds Gro in vitro and that Dri and Dorsal can cooperatively recruit Gro to DNA.
MATERIALS AND METHODS
Purification of ZREB.
Nuclear extracts were prepared from 0- to 12-h embryos (43), and the resulting ammonium sulfate pellet was dialyzed against HEMG buffer (25 mM HEPES [pH 7.6], 0.1 mM EDTA, 2.5 mM MgCl2, 10% [vol/vol] glycerol, 1 mM dithiothreitol [DTT], 1 mM sodium metabisulfite, 0.2 mM phenylmethylsulfonyl fluoride [PMSF]) containing 50 mM KCl. Nuclear extracts prepared from approximately 300 g of embryos were centrifuged at 9,000 rpm for 6 min in a GSA rotor, and the supernatant was applied to a 90-ml SP-Sepharose (Pharmacia) column preequilibrated in HEMG containing 50 mM KCl. After loading of the nuclear extract, the column was washed extensively with HEMG buffer containing 50 mM KCl and then stepwise eluted. Sodium metabisulfite and PMSF were omitted from the HEMG buffer from the SP-Sepharose wash step on. zen repression element binding activity (ZREB) was eluted from the column in the first elution step with HEMG containing 0.15 M KCl. Protein fractions from the 0.15 M KCl step were diluted to 50 mM KCl with HEMG buffer without KCl and applied to a 35-ml DEAE Sephacel column. After loading, the DEAE column was extensively washed with HEMG containing 50 mM KCl and stepwise eluted with HEMG containing 0.15 and 0.25 M KCl. Fractions containing DNase I footprinting activity were subjected to DNA affinity chromatography. The columns were prepared as described by Kadonaga and Tjian (24). The DNA sequences of the complementary oligodeoxynucleotides coupled to the wild-type AT2 DNA affinity column used in the purification were 5′-AGGATCGAATATTGATTGG-3′ and 5′-GATCCTCCAATCAATATTC-3′. The DNA sequences of the complementary oligodeoxynucleotides coupled to the mutant AT2 DNA affinity column were 5′-AGGATCGCCTATATGAACGAAGCGGTCGTGGGTTTCTCCCAGTTA-3′ and 5′-GATCCTTAACTGGGAGAAACCCACGACCGCTTCGTTCATATAGGC-3′. The KCl concentration of the fractions containing ZREB activity from the DEAE column was diluted to 0.2 M KCl, and Nonidet P-40 (NP-40) was added to a 0.025% (vol/vol) final concentration. This mixture was then split into four equal parts, and each portion was loaded onto a 1-ml mutant AT2 DNA affinity column. The flowthrough from the mutant columns was pooled, and poly(dI-dC) and poly(dA-dT) were added at a concentration of 20 μg/ml for each competitor DNA. The mixture was incubated for 10 min on ice and loaded onto a 0.5-ml wild-type AT2 DNA affinity column. The wild-type column was then washed extensively with HEMG containing 0.2 M KCl and 0.025% NP-40. The ZREB activity was eluted from the wild-type column with HEMG containing 1.0 M KCl and 0.025% NP-40.
Expression and purification of recombinant transcription factors.
The expression and purification of Dorsal and M2 FLAG-tagged Gro were performed as described previously (11, 41). To produce six-histidine-tagged Dri (6His-Dri), an ApaLI to EcoRI fragment of Dri cDNA was cloned into the blunted StuI site of pAcSG-His-NT-B baculovirus transfer vector (Pharmingen). The recombinant baculovirus expressing 6His-Dri was generated and selected according to the instructions provided by Pharmingen. Nuclear extract was prepared from Sf9 cells infected with the recombinant 6His-Dri virus, and 6His-Dri was purified from the extract with nickel-nitrilotriacetic acid-agarose (Qiagen) according to the directions provided by the manufacturer.
DNA binding and protein-protein interaction assays.
DNase I footprinting assays were performed as described previously (33). Electrophoretic mobility shift assays were performed as previously described (34). UV cross-linking was performed as follows. A double-stranded oligonucleotide containing the wild-type AT2 site was synthesized, with 5-iodouracil substituted for each thymine (iodouracil phosphoramidite was obtained from Glen Research, Inc.). The oligonucleotide was end labeled with 32P. Protein fractions were mixed with approximately 1.33 ng of probe for 10 min on ice in 10 mM HEPES (pH 7.6), 150 mM KCl, 1 mM DTT, 2.5 mM MgCl2, 10% glycerol, and 0.1 mg of bovine serum albumin per ml. After incubating 10 min on ice, the mixture was transferred into 24-well tissue culture plates and pulsed twice at 7,500 J on ice with a Stratalinker UV-cross-linker from Stratagene.
Coimmunoprecipitation assays to analyze the protein-protein interactions between Dorsal, Dri, and M2-Gro in vitro were performed as follows. Equal volumes (20 μl) of anti-M2 affinity resin (with or without 1 μg of purified immobilized M2-Gro) were incubated with 250 ng of purified Dorsal, 400 ng of purified 6His-Dri alone, or both in a total volume of 200 μl of interaction buffer (25 mM HEPES [pH 7.9], 1.5 mM MgCl2, 100 mM NaCl, 10% glycerol, 2 mM DTT, 0.5% NP-40, 1 mM PMSF, 0.5 mg of BSA per ml). The beads were extensively washed with the interaction buffer, and bound proteins were eluted by boiling in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and resolved by SDS-PAGE. Bound proteins were visualized by probing immunoblots with the appropriate antibodies.
DNA-affinity chromatography assays to examine interactions between Dorsal, Dri, and Gro were performed as follows. Twenty microliters of DNA affinity resin (containing about 1 μg of DNA) was incubated with 4 μg of purified Dorsal, 4 μg of 6His-Dri, or both. The resin was washed three times with the interaction buffer and then incubated with 20 μl of in vitro-translated and [35S]Met-labeled Gro in a total volume of 200 μl of interaction buffer at room temperature for 1 h. After the beads were washed four times with the interaction buffer, the proteins retained on the beads were eluted by boiling in SDS-PAGE sample buffer and resolved by SDS-PAGE. The 35S-Gro was visualized and quantitated with a phosphorimager. Dorsal and Dri were visualized by immunoblotting.
ct and dri alleles.
Experiments examining the role of ct in ventral repression employed ctC145, an X-ray-induced probable null allele (20). Experiments examining the role of dri in ventral repression employed dri2, an ethyl methanesulfonate-induced strong hypomorphic allele (40a).
P-element-mediated transformation and in situ hybridization.
P-element mediated transformation (39) and in situ hybridization to whole mount embryos with digoxigenin-labeled probes (45) were performed as described previously. The stained embryos were examined and photographed with Normarski optics. Embryo staging was as described by Campos-Ortega and Hartenstein (8).
RESULTS
Purification of an AT2 binding activity.
To elucidate the mechanism of Dorsal-mediated transcriptional repression, a nuclear extract prepared from 0- to 4-h old embryos was assayed for proteins that could specifically bind the zen VRR. The extract was found to contain proteins capable of protecting the AT1, AT2, and AT3 sites as well as the dl1, dl2, and dl3 sites from DNase I digestion (data not shown). Since the AT2 element is the only one of the AT-rich elements that is essential for zen VRR activity (23), chromatographic purification of the AT2 binding activity was initiated with DNase I footprinting as a binding assay. This activity was termed ZREB.
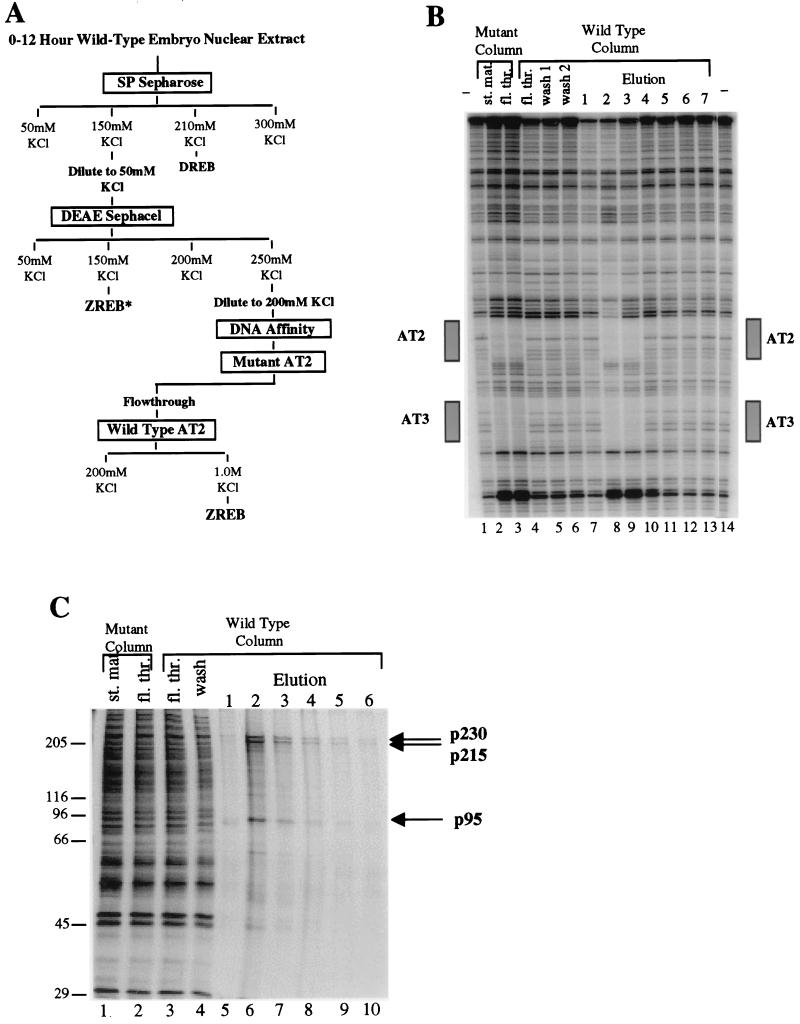
ZREB was purified to homogeneity from nuclear extracts of 0- to 12-h-old embryos (Fig. 1A). The activity was partially purified by cation-exchange (SP Sepharose) and then anion-exchange (DEAE Sephacel) chromatography. DEAE Sephacel chromatography yielded two separate peaks of AT2 binding activity, one eluted at 150 mM KCl and the other at 250 mM KCl. Subsequent analysis showed that the activity in the 150 mM KCl eluate (ZREB*) was due to a degraded form of one of the polypeptides present in the 250-mM KCl peak (see below). The material in the 250 mM KCl eluate was subjected to two rounds of DNA affinity chromatography. The first DNA affinity column contained a mutant AT2 site, and thus the AT2 binding activity flowed through the column. The flowthrough from the first affinity column was then applied to a wild-type AT2 column. The activity bound to the column and eluted at high salt concentrations. ZREB binds to the AT2 element in the zen VRR as well as to the AT3 element (Fig. 1B). A comparison of the two sites reveals an identical 7-bp sequence (TATTGAT) in the center of both the AT2- and AT3-protected sequences.
FIG. 1.
Purification of ZREB. (A) Flow chart of the ZREB purification scheme. Columns were eluted in a stepwise fashion with the indicated concentrations of KCl. DREB is the decapentaplegic repression element binding protein, which is equivalent to NTF-1 (18). Column fractions were assayed for AT2 site-specific DNase I footprinting activity. ZREB activity was purified to near homogeneity from the DEAE Sephacel 0.25 M KCl step by multiple rounds of DNA affinity chromatography. Another peak of AT2 binding activity (termed ZREB*) was detected in the DEAE Sephacel 0.15 M KCl step. This was later found to represent a degraded form of ZREB. (B) DNase I footprinting of material from the last two steps in the purification of ZREB. Lanes 1 and 14, no protein controls; lanes 2 and 3, 25 μl of starting material and flowthrough from mutant AT2 site affinity column. The flowthrough from the mutant AT2 site column was applied to the wild-type AT2 column. Footprints of fractions from this column are shown in lanes 4 to 13. Lane 4, 25 μl of flowthrough; lanes 5 and 6, 12.5 μl of serial 0.25 M KCl washes; lanes 7 to 13, 2.5 μl of serial 1 M KCl washes. The boxes beside the gel designate the location of the AT2 and AT3 elements in the zen VRR probe. (C) Silver-stained SDS-PAGE gel of the affinity column fractions. Lanes 1 and 2 contain the mutant affinity column starting (st.) and flowthrough (fl. thr.) fractions (2.5 μl). Lanes 3 and 4, wild-type affinity column flowthrough and wash fractions; lanes 5 to 10, column fractions eluted with 1.0 M KCl (12 μl). Molecular size standards (in kilodaltons) are indicated to the left. The positions of the three major polypeptide species (p230, p215, and p95) coeluting with the footprinting activity are indicated by arrows.
To assess the purity of ZREB, fractions from the final DNA affinity column were subjected to SDS-PAGE; the protein bands were visualized by silver staining (Fig. 1C). The column fractions with peak activity contained three major protein species with apparent molecular sizes of approximately 230, 215, and 95 kDa, which were termed p230, p215, and p95, respectively. The elution profiles of the three polypeptides were identical with one another and correlated with the elution profile of the DNase I footprinting activity (Fig. 1B).
ZREB binding specificity correlates with repression in vivo.
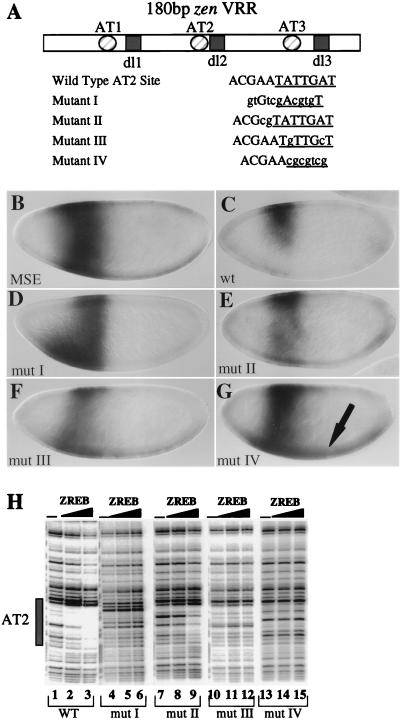
The binding specificity of purified ZREB was tested with mutations in the AT2 element that disrupt repression in vivo. The goal was to determine if there was a correlation between the in vitro binding specificity of this preparation and the sequence requirements for repression in vivo. These experiments employed the minimal zen VRR (Fig. 2A). Four different mutant versions of the VRR contained different alterations in the AT2 element. Mutation I was shown previously to eliminate repression by the zen VRR when it was placed upstream of the MSE, driving lacZ expression (23). Mutation II is a double mutation of two residues immediately adjacent to the TATTGAT element that is conserved between AT2 and AT3. Mutation III is a double-point mutation within the conserved TATTGAT element. Mutation IV is a 7-bp substitution of the entire TATTGAT element.
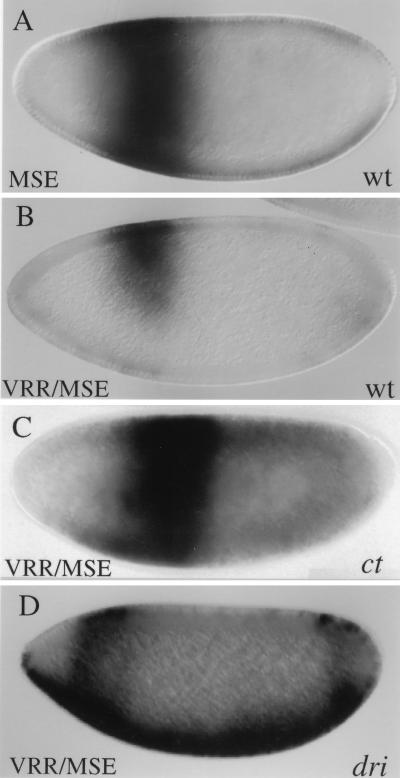
FIG. 2.
Correlation between ZREB binding and repression in vivo. (A) Diagram of the minimal zen VRR and sequences of the wild-type and mutant AT2 elements. Shown are the three AT-rich sites (circles) and the three Dorsal sites (squares). The AT2 site was mutated; lowercase letters indicate the bases that were mutated. The 7-bp core sequence found in both AT2 and AT3 is underlined in each sequence. Two copies of the wild type and of each mutant zen VRR were placed upstream of the MSE driving lacZ expression. The resulting lacZ expression patterns are shown in panels B through G. (B through G) Whole mount in situ hybridizations with an antisense lacZ riboprobe. All embryos are stage 4, nuclear cycle 13, and are oriented with the anterior on the left and the dorsal side up. (B) Embryo containing the MSE driving lacZ expression. (C) Embryo containing two copies of the wild-type (wt) minimal zen VRR upstream of the MSE driving lacZ. (D through G) Embryos containing the minimal zen VRR AT2 mutants I to IV upstream of the MSE driving lacZ expression. The arrow in G points to the ventral expression resulting from mutation IV. (H) DNase I footprinting of the AT2 site with partially purified ZREB. Lanes 1 to 3, wild-type (wt) probe; lanes 4 to 6, mutant (mut) I; lanes 7 to 9, mutant II; lanes 10 to 12, mutant III; and lanes 13 to 15, mutant IV; lanes 1, 4, 7, 10, and 13, DNA alone; lanes 2, 5, 8, 11, and 14, approximately 3 ng of ZREB; lanes 3, 6, 9, 12, and 15, approximately 7.5 ng of ZREB. The box designates the position of the AT2 site.
As shown previously (23), the wild-type minimal zen VRR can ventrally repress the MSE/lacZ reporter gene (compare Fig. 2B and 2C). Mutations I, III, and IV completely abolish ventral repression (Fig. 2D, F, and G). In addition, mutation IV and, to a lesser extent, mutation III result in weak expression across the ventral side of the embryo, most likely from Dorsal-mediated activation following a complete loss of repression (arrow in Fig. 2G). Mutation II showed weak derepression, but there was clearly asymmetry in the lacZ stripe, with more staining on the dorsal side than on the ventral side of the embryo (Fig. 2E).
The binding of ZREB to the various mutant forms of the AT2 site correlated with the levels of repression observed in vivo. ZREB failed to bind to mutations I, III, and IV (compare lanes 1 to 3 with lanes 4 to 6 and 10 to 15 in Fig. 2H), the same mutations that result in complete loss of repression in the embryo (Fig. 2D, F, and G). Furthermore, reduced but still detectable binding of ZREB to mutation II correlated with weak derepression in vivo (Fig. 2E and H, lanes 7 to 9). These experiments suggest that the loss of ZREB binding to the zen VRR through mutations in the AT2 repression element results in loss of repression. The mutagenesis data (Fig. 2 and additional mutants; data not shown) suggest that the conserved seven-nucleotide sequence, TATTGAT, found in both AT2 and AT3 represents the critical core of the sequence. Mutations inside this 7-bp sequence have dramatic effects on DNA binding and repression, while mutations outside the core element have smaller effects.
ZREB consists of ct and dri gene products.
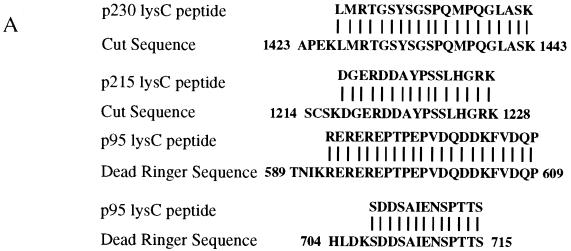
To identify the polypeptides in the ZREB preparation, the three major species in the affinity-purified material were separated by preparative SDS-PAGE and digested with Lys-C endoprotease. Lys-C peptides were fractionated via reverse-phase high-pressure liquid chromatography and sequenced by automated Edman degradation. Amino acid sequences from p230, p215, and p95 were compared to a variety of databases with the BLAST algorithm. Perfect matches to the p230 and p215 Lys-C peptides were found in the homeodomain-containing Cut (Ct) protein (Fig. 3A, top two sequences). Perfect matches to the p95 Lys-C peptides were found in the Dri protein (Fig. 3A, bottom two sequences). As expected for bona fide Lys-C-generated peptides, all the sequenced peptides were preceded by lysine residues in the Ct or Dri sequences.
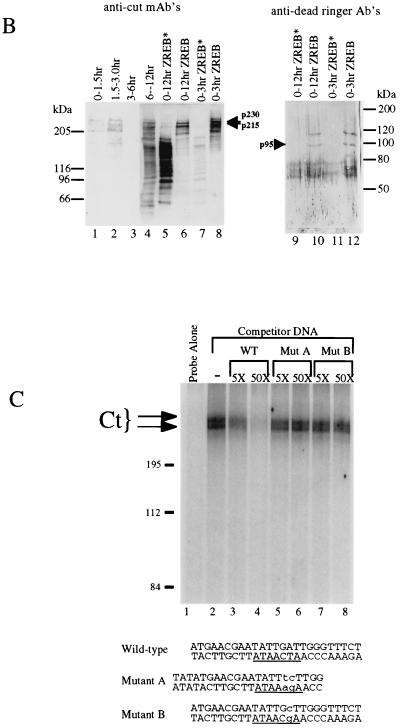
FIG. 3.
Identification of ZREB. (A) Approximately 10 pmol of p230, p215, and p95 were digested with endoproteinase Lys-C (13). Peptide fragments were purified by high-pressure liquid chromatography and sequenced by automated Edman degradation with a Perkin-Elmer/Applied Biosystems Division device (model 494), and the amino acid sequences were subjected to a BLAST sequence search. Results of the search are shown for each peptide. Both p230 and p215 peptides were exact matches to the homeodomain-containing protein Cut. Two separate peptides were sequenced from p95, and both were identical to Dri. One of the p95 peptides contains an internal lysine residue, clearly resulting from incomplete Lys-C digestion, since another p95 peptide was sequenced that was identical to this peptide, except that it terminated after the lysine residue (data not shown). (B) Western blots of crude embryo extracts and of purified ZREB and ZREB* were probed with anti-Ct (lanes 1 to 8) or anti-Dri (lanes 9 to 12) monoclonal antibodies (mAb’s) or antibodies (Ab’s). Lane 1, nuclear extracts of 0- to 1.5-h embryos; lane 2, nuclear extracts of 1.5- to 3-h embryos; lane 3, nuclear extracts of 3- to 6-h embryos; lane 4, nuclear extracts of 6- to 12-h embryos; lanes 5 and 9, ZREB* prepared from 0- to 12-h embryos; lanes 6 and 10, ZREB prepared from 0- to 12-h embryos; lanes 7 and 11, ZREB* prepared from 0- to 3-h embryos; lanes 8 and 12, ZREB prepared from 0- to 3-h embryos. In the anti-Dri immunoblot, the band above p95 with an apparent molecular size of about 115 kDa corresponds to a minor species seen in many ZREB preps and may represent full-length Dri, while p95 probably represents a truncated form of Dri. The series of bands extending downward from the 80-kDa marker represent a keratin contaminant in the ZREB and ZREB* preps against which the anti-Dri antibodies avidly react. (C) DNA affinity-purified ZREB was cross-linked to an iodinated DNA probe. The sequence of the probe is the same as that of the wild-type competitor, except that thymine residues were replaced with 5-iodouracil. All fractions were exposed to UV treatment. Lane 1, probe alone without protein; lanes 2 to 8, probe and 5 μl of affinity-purified ZREB; lanes 3 and 4, 5- and 50-fold excesses of cold wild-type (WT) competitor, respectively; lanes 5 and 6, 5- and 50-fold excesses of cold mutant competitor A, respectively; lanes 7 and 8, 5- and 50-fold excesses of cold mutant competitor B, respectively. Sequences of the wild-type and mutant competitors are indicated below the gel. Lowercase letters indicate mutations. (D) Recombinant Dri was assayed for binding to the AT2 site by electrophoretic mobility shift assays. The sequence of one strand of the radiolabeled double-stranded probe is TATGAACGAATATTGATTGGGA (the 7-bp core of the AT2 site is underlined). Lane 1, radiolabeled probe alone (100 fmol); lanes 2 to 17, recombinant Dri (250 ng) and radiolabeled probe (100 fmol). In addition to the radiolabeled probe and Dri, the binding reactions included 5-fold (lanes 3, 7, 11, and 15), 50-fold (lanes 4, 8, 12, and 16), or 250-fold (lanes 5, 9, 13, and 17) molar excess of the indicated cold competitors. The AT2-WT competitor is identical in sequence to the labeled probe. The AT2-mII, AT2-mIII, and AT2-mIV competitors have the same mutations as mutants II, III, and IV, respectively, in Fig. 2.
Immunoblots of the crude extracts and of purified ZREB were probed with anti-Ct and anti-Dri antibodies, confirming that p230 and p215 are Ct and that p95 is Dri (Fig. 3B). The Ct antibodies clearly detect p230 and p215 in extracts of 0- to 1.5-h embryos (lane 1), indicating that the gene is probably maternally expressed. The protein is also present in extracts of 1.5- to 3-h embryos (lane 2), disappears from extracts of 3- to 6-h embryos (lane 3), and then reappears in later embryos (lane 4). In ZREB preparations from either 0- to 12-h embryos (lane 6) or 0- to 3-h embryos (lane 8), the Ct antibody primarily detects p215 and p230. However, in ZREB* preparations, p215 and p230 are largely replaced with a ladder of lower-molecular-weight bands, presumably representing degradation products of Ct (lanes 5 and 7). The Dri antibodies are not sufficiently sensitive to detect Dri in crude extracts (data not shown) but clearly bind to two bands in ZREB (but not ZREB*) preparations from 0- to 12-h embryos and 0- to 3-h embryos (lanes 9 to 12). One of these bands corresponds to p95, while the other has an apparent molecular size of about 115 kDa and corresponds to a minor species seen in silver-stained gels of some of our ZREB preparations. Although we have not sequenced this minor species, it comigrates with recombinant Dri (see Fig. 6A; data not shown). We therefore suspect that it represents full-length Dri and that p95 represents a truncated form of Dri. Apparently, the Dri antibody binds more avidly to this putative full-length species than to p95, explaining why the relative intensities of the bands in the immunoblot do not correspond to their relative intensities in the silver-stained gel.
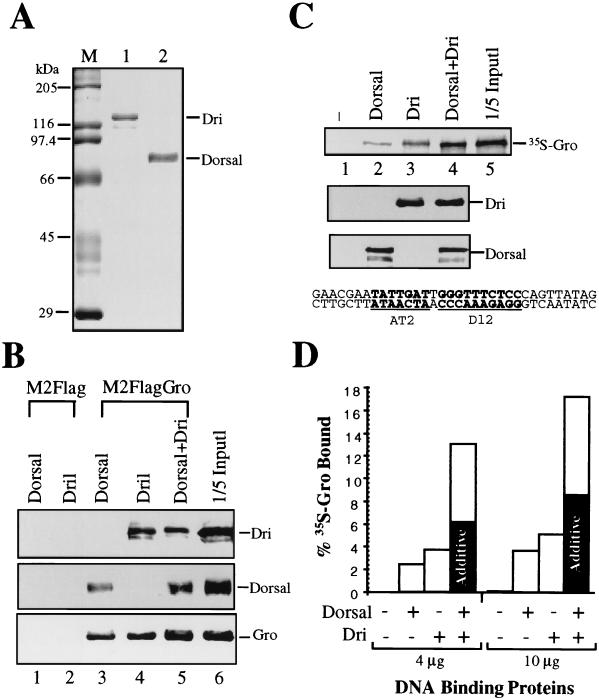
FIG. 6.
Dri and Dorsal physically interact with Gro and cooperatively recruit Gro to DNA. (A) A Coomassie blue-stained gel showing the recombinant Dri (lane 1) and Dorsal (lane 2) used in the experiments presented in panels B and C. M, molecular size markers. (B) Coimmunoprecipitation of baculovirus-expressed Dorsal and Dri with FLAG-tagged Gro using monoclonal M2 antibodies against the FLAG epitope. M2-FLAG attached to agarose was unable to bind Dorsal (lane 1) or Dri (lane 2). When Gro was coupled to the M2-agarose resin via the FLAG epitope, both Dorsal and Dri were retained on the resin (lanes 3 to 5). Lane 6 contains 1/15 of the input protein used in the coimmunoprecipitation experiments shown in the other lanes. Dorsal, Dri, and epitope-tagged Gro were detected by immunoblotting with anti-Dorsal polyclonal, anti-Dri polyclonal, and M2 monoclonal antibodies, respectively. (C) Dorsal and Dri cooperatively recruit Gro to DNA. A DNA fragment containing Dorsal (dl2) and Dri (AT2) binding sites was coupled to agarose resin. The sequence of the DNA fragment was derived from the zen VRR and is shown at the bottom. No Dorsal or Dri (lane 1), 4 μg of Dorsal (lane 2), 4 μg of Dri (lane 3), or 4 μg of both Dorsal and Dri (lane 4) was added to the resin, followed by 35S-labeled Gro. This amount of Dorsal and Dri represents an approximately twofold molar excess over the concentration of sites on the column, and as a result, about 50% of the input Dorsal and Dri was retained on the column. The addition of more Dorsal and Dri did not significantly increase the amount of protein retained on the column (Fig. 6D and data not shown), confirming that the column is saturated. After extensive washing, bound proteins were eluted from the resin with SDS-PAGE sample buffer and fractionated by SDS-PAGE. One-fifth of the input protein used for lanes 1 to 4 is shown in lane 5. (Upper panel) Autoradiogram showing 35S-labeled Gro. (Middle panel) Anti-Dorsal immunoblot. (Lower panel) Anti-Dri immunoblot. (D) Quantification of the Gro recruitment assay. The left half of the bar graph shows the quantification of the experiment shown in panel C. The right half of the bar graph shows the quantification of a similar experiment with 10 μg rather than 4 μg of Dorsal and Dri. The shaded portions of the two bars showing the amount of Gro retained in the presence of both Dorsal and Dri indicate the amount of Gro that would have been retained if the manner in which Dorsal and Dri were functioning were merely additive.
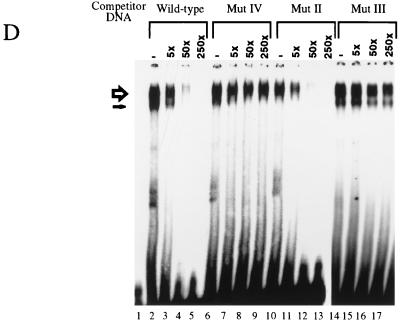
UV cross-linking assays were employed in an effort to verify that the Ct and/or Dri gene products in the ZREB preparation were truly responsible for the AT2 binding activity and to determine if any of the polypeptides in the ZREB complex made intimate contact with the major groove of the DNA. A radioactive AT2 site-containing probe, modified by substitution of 5-iodouracil for thymine, was mixed with affinity-purified ZREB and exposed to UV light to covalently cross-link the DNA to proteins in contact with the iodine atoms in the major groove. The mixture was subjected to SDS-PAGE and autoradiography (Fig. 3C). Both p230 and p215, but not p95, became covalently attached to the DNA (Fig. 3C, lane 2). The binding was specific, since cross-linking was prevented by cold noniodinated wild-type competitor but not by competitors containing double- or single-point mutations in the AT2 site (compare lanes 3 to 4 with lanes 5 to 8). These results together with the DNase I protection assays demonstrate that Ct binds directly and specifically to the major groove of the AT2 site.
It is not surprising that we failed to detect UV-induced covalent attachment of Dri to the iodinated probe, since, by analogy to its mammalian homolog Bright, Dri is expected to bind DNA via the minor groove (16). To determine if Dri bound the AT2 site specifically, we expressed and purified recombinant Dri with a baculovirus expression vector. Electrophoretic mobility shift assays demonstrated the formation of a complex between Dri and AT2 (Fig. 3D, lanes 2, 6, 10, and 14). The formation of this complex is due to sequence-specific DNA binding as evidenced by competition assays. Cold oligonucleotides containing either a 7-bp (lanes 7 to 9) or a 2-bp (lanes 15 to 17) mutation in the 7-bp core AT2 recognition element compete much more poorly for Dri than does a cold oligonucleotide containing the wild-type site (lanes 3 to 5). A 2-bp mutation outside the core AT2 element has no effect on binding (lanes 10 to 12). The effect of this mutation outside the core element on recombinant Dri binding is less than the effect of the same mutation on binding of ZREB (Fig. 2H), suggesting that while both Ct and Dri primarily recognize the 7-bp core, there are probably slight differences between the binding specificity of the two proteins.
The above-described gel shift experiments demonstrate that Dri can specifically bind the AT2 site in the absence of Ct. Due to difficulties producing recombinant Ct, we have not been able to carry out similar experiments to demonstrate Ct binding in the absence of Dri. However, immunoblot analysis of the ZREB* activity that eluted from the DEAE Sephacel column at 150 mM KCl demonstrates that ZREB* contains Ct in a partially degraded form but lacks Dri (Fig. 3B). ZREB* gives DNase I footprints over the AT2 and AT3 sites in the zen VRR that are essentially identical to those obtained with ZREB. Thus, both Dri and Ct can bind the AT2 site independently of one another.
Genetic analysis of the role of ct and dri in zen regulation.
To determine if ct and dri are required for the activity of the AT2 site in vivo, we tested the effects of mutations in these genes on the activity of the lacZ transgene under control of the MSE and the minimal zen VRR (Fig. 4). For both ct and dri, we generated germ line clones to test the effects of eliminating maternally contributed gene products, and, in addition, we examined the effects of eliminating zygotically produced gene products. A null mutation in ct (which is an X-linked gene) resulted in strong ventral derepression of the transgene (Fig. 4C). This ventral derepression was observed in about one-half the embryos derived from a cross between females containing ct germ line clones and hemizygous males. It was never observed in a cross between heterozygous females and hemizygous males, suggesting that derepression requires simultaneous elimination of both maternal and zygotic Ct.
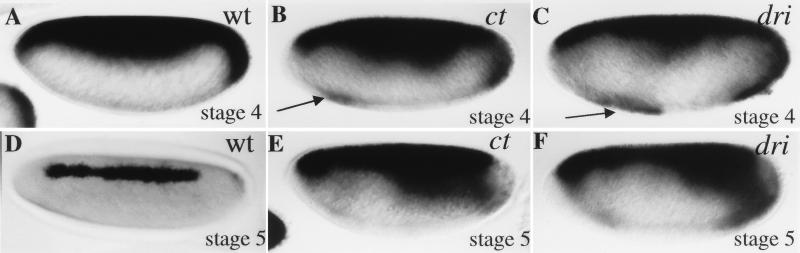
FIG. 4.
Analysis of zen VRR function in ct and dri mutant backgrounds. (A through D) In situ hybridizations of whole mount embryos with an antisense lacZ probe. All embryos are oriented with the anterior to the left and the dorsal side up, and all are at nuclear cycle 13. (A) Expression pattern of the MSE/lacZ transgene in wild-type (wt) embryos. (B) Expression of the VRR/MSE/lacZ transgene in wild-type (wt) embryos. Stage 4 embryos uniformly exhibited ventral repression of the lacZ stripe. (C) Expression of the VRR/MSE/lacZ transgene in a ct mutant background. Females carrying ctC145 germ line clones were crossed with males homozygous for the lacZ transgene and hemizygous for wild-type ct (an X-linked gene). This phenotype was observed in approximately half (8 of 18) of the resulting nuclear cycle 13 embryos, as expected for a phenotype that is due to the simultaneous elimination of both maternal and zygotic gene products. It was never observed in stage 4 embryos resulting from a cross between heterozygous females and hemizygous males. (D) Expression of the VRR/MSE/lacZ transgene in a dri mutant background. The dri2 mutation was recombined onto a second chromosome carrying the lacZ transgene. This phenotype was observed in approximately one-third (11 of 28) of the stage 4 lacZ-expressing embryos produced by this balanced stock, as expected for a recessive zygotic effect.
A strong hypomorphic mutation in dri (which is an autosomal gene) also resulted in strong derepression (Fig. 4D). In contrast to the results observed with ct, this effect is strictly zygotic. It was observed in a cross between heterozygous dri males and females but not in a cross between females carrying dri germ line clones and wild-type males. Most strikingly, in the absence of zygotic Dri, the zen VRR directs strong ventral expression in the blastoderm embryo, reminiscent of the results observed when the AT2 element was mutagenized (e.g., Fig. 2G). These results strongly suggest that, in the context of the minimal zen VRR, Dri plays an essential role in converting Dorsal from an activator into a repressor. We also note that the dri mutation results in a significant weakening of the transverse eve stripe as well as a shift in the position of the stripe toward the anterior pole of the embryo, presumably due to a role for Dri in anteroposterior pattern formation (40a).
Despite the strong effects of the ct and dri mutations on the activity of the minimal zen VRR, both genes make only minor contributions to the ventral repression of the endogenous zen gene in the stage 4 embryo. In the absence of both zygotic and maternal Ct (Fig. 5B) or in the absence of zygotic Dri (Fig. 5C), zen expression in the stage 4 embryo is still largely restricted to the dorsal 40 to 50% of the embryo, although weak ventral patches of zen expression were observed with high frequency (Fig. 5B and C). Such patches were never observed in wild-type embryos stained in parallel with these embryos. The contrast between the strong effect observed for the minimal VRR and the weak effect observed for the endogenous zen gene suggests redundancy in the zen locus. In other words, there may be additional unidentified ventral repression regions in the zen locus that function in a Ct- and Dri-independent manner (see Discussion).
FIG. 5.
Analysis of zen expression pattern in ct and dri mutant backgrounds. (A through F) Whole mount in situ hybridizations with zen cDNA probes. For all embryos, sagittal views are shown, with anterior to the left and dorsal side up, except for that shown in panel D, which is a dorsolateral surface view. (A) A wild-type nuclear cycle 13 embryo. Complete ventral repression is uniformly observed in these embryos. (B) A nuclear cycle 13 embryo derived from a cross between females bearing ctC145 germ line clones and wild-type (hemizygous) males. A ventral patch of expression (arrow) was observed in slightly less than half (25 of 56) of nuclear cycle 13 embryos. Such patches were not observed in embryos resulting from a cross between heterozygous females and hemizygous males. (C) A nuclear cycle 13 embryo derived from a cross between dri2 heterozygous females and males. A ventral patch of expression (arrow) was observed in slightly less than a quarter (8 of 43) of nuclear cycle 13 embryos. (D) A wild-type late stage 5 embryo. By this stage, the expression pattern is refined to a stripe three to five cells wide in 100% of the embryos. (E) A late-stage 5 embryo derived from a cross between females bearing ctC145 germ line clones and wild-type (hemizygous) males. The lack of zen refinement was observed in somewhat less than half (32 of 78) of stage 5 embryos. (F) A late-stage 5 embryo derived from a cross between dri2 heterozygous females and males. The lack of zen refinement was observed in about one-quarter (11 of 41) of stage 5 embryos.
Although neither Ct nor Dri is essential for ventral repression of the endogenous zen gene in the stage 4 embryo, both factors appear to play essential roles in the refinement of the zen pattern that normally occurs in stage 5 embryos. Normally, zen expression refines during cellularization to a stripe approximately three to five cells in width (Fig. 5D). However, in the absence of both maternal and zygotic Ct or in the absence of zygotic Dri, a severe refinement defect was observed (Fig. 5E and F). zen expression in the stage 5 embryo is believed to depend, at least in part, upon dpp (37). We therefore examined these embryos for the dpp expression pattern, which we found to be identical to that observed in wild-type embryos (data not shown). Therefore, the loss of zen refinement is not a consequence of expanded dpp expression.
Dorsal and Dri interact with Gro.
Gro is a transcriptional corepressor necessary for Dorsal-mediated repression of zen and dpp (11). Since Gro does not bind DNA on its own, DNA binding factors must recruit Gro to a regulatory region (36). Previous studies have shown that Dorsal and Gro interact in vitro (11). To determine if Dri may also play a role in recruiting Gro to the zen VRR, a coimmunoprecipitation assay was used to look for a binding interaction between Gro and Dri. These experiments utilized baculovirus-expressed proteins that had been purified to near homogeneity (Fig. 6A, lanes 1 and 2 and data not shown). M2 FLAG-tagged Gro fusion protein was immobilized on M2 monoclonal antibody-linked agarose beads. Recombinant Dorsal and Dri were incubated individually or together with the Gro resin or with a control M2 antibody resin lacking epitope-tagged Gro. After extensive washing, bound proteins were eluted and subjected to SDS-PAGE and immunoblotting with anti-Dorsal and anti-Dri antibodies. Both Dorsal and Dri bound to the Gro resin (Fig. 6B, lanes 3 to 5) but not to the control resin (lanes 1 and 2).
Although Gro can interact with either Dri or Dorsal, it is unlikely that either of these binary interactions is sufficient to recruit Gro to the template in vivo, because repression by the zen VRR requires both Dorsal and Dri binding sites. However, it is possible that Dorsal and Dri function cooperatively to recruit Gro to the template. To test this possibility, Dorsal and Dri were bound separately and together to a DNA affinity resin containing a region from the zen VRR that includes the AT2 and dl2 sites. Radiolabeled Gro was then added to the affinity resin. After extensive washing, bound proteins were eluted and analyzed by SDS-PAGE. In this type of experiment, we would expect to observe maximum cooperativity in the recruitment of Gro at DNA-saturating concentrations of Dorsal and Dri. Thus, the experiments utilized amounts of Dorsal and Dri sufficient to saturate the sites on the affinity resin as described in the legend to Fig. 6.
Immunoblotting with Dorsal and Dri antibodies indicates that the same amount of Dorsal was retained on the column in either the absence or presence of Dri (Fig. 6C, compare lanes 2 and 4) and that the same amount of Dri was retained on the column in either the absence or presence of Dorsal (compare lanes 3 and 4). Autoradiography to detect 35S-labeled Gro shows that, in the absence of both Dorsal and Dri, Gro was not retained on the DNA affinity resin (lane 1). In the presence of Dorsal or Dri alone, a small amount of Gro was retained on the resin (lanes 2 and 3). Dorsal and Dri together bound twice as much Gro as would be expected if the two proteins were functioning independently to recruit Gro (Fig. 6C, lane 4; quantified in Fig. 6D, left half of graph). Although the level of cooperativity is small, it is very reproducible as shown by multiple independent experiments. For example, similar overall results confirming a twofold cooperativity were observed when the amount of Dorsal and Dri added to the beads was increased by a factor of 2.5 (Fig. 6D, right half of graph). In addition to demonstrating the reproducible nature of the cooperativity, this finding confirms that the Dorsal and Dri binding sites were indeed saturated at the lower concentrations of these proteins.
DISCUSSION
Interaction of Ct and Dri with AT2.
A DNA binding activity (ZREB) specific for the AT2 and AT3 elements in the zen VRR was purified extensively from Drosophila embryonic nuclear extracts. Mutagenesis of the AT2 site demonstrates that the DNA binding specificity of ZREB correlates well with the sequence requirements for repression in vivo. ZREB is composed primarily of three polypeptides having apparent masses of 230, 215, and 95 kDa. Partial amino acid sequencing shows that p230 and p215 are products of ct and that p95 is a product of dri. Genetic and biochemical analysis of these genes and their products provides strong support for a role in zen regulation.
As shown by gel shift, DNase I footprinting, and UV cross-linking assays, Ct and Dri bind DNA with similar, although not identical, specificity. Both proteins interact with the conserved TATTGAT element found in both AT2 and AT3. Mutations within this conserved element greatly reduce binding, while mutations outside this conserved element have relatively small effects on binding. From UV cross-linking assays, it appears that Ct, a homeodomain-containing protein, interacts directly with the major groove of the DNA helix. This is in accord with high-resolution structural analysis of a number of other homeodomain DNA binding motifs (27). Dri binding was not detected in the UV cross-linking assay, which is specific for binding to the major groove of DNA. This is consistent with the minor groove binding activity reported for a mammalian Dri homolog (16).
ct was identified over 70 years ago and was cloned more recently by a positional gene cloning approach (4, 20). ct mutations result in a number of phenotypes, including cut wings, kinked femurs, embryonic lethality, and homeotic transformations in the peripheral nervous system. dri was identified as a gene whose product binds specifically to the Engrailed homeodomain recognition element through a newly recognized DNA binding domain that has no similarity to the homeodomain (15). dri plays additional roles in segmentation and hindgut development (40a). Previous studies of ct and dri have failed to reveal roles for these genes in dorsoventral pattern formation. As described below, this is most likely due to the redundant nature of the zen regulatory region.
Several other factors with homology to Ct or Dri appear to function as transcriptional repressors. For example, mammalian homologs of Ct have been shown to repress transcription in cultured cells (12, 31). Human Ct can repress transcription by either direct competition for binding site occupancy or by active repression. The active repression domain of human Ct has been mapped to an alanine-rich domain in the C terminus of the protein. This alanine-rich C-terminal region is also present in Drosophila Ct as well as a number of other known transcriptional repressors, namely Krüppel, Engrailed, Even-skipped, and the murine Msx-1. Dri is a member of the recently defined ARID family of DNA binding proteins, a family that includes the B-cell-specific factor Bright (16) and the Drosophila factor Eyelid (47). Although Bright is thought to function as a transcriptional activator, genetic data suggest that Eyelid functions to repress transcription in response to activation of the wingless pathway.
Genetic analysis of the role of Ct and Dri in zen regulation.
Genetic analysis indicates that maternal and zygotic Ct and zygotic Dri participate in the ventral repression of zen. Mutations in either ct or dri result in the nearly complete loss of ventral repression of a zen VRR MSE/lacZ transgene. In dri embryos, we also observe VRR-directed ventral activation. Thus, the phenotype of a ct mutation is nearly as severe, while that of a dri mutation is as severe as the phenotype resulting from a mutation in the AT2 site. These findings strongly suggest that Ct and Dri are both essential for the activity of the AT2 site. The observation that the dri mutation results in ventral activation in addition to ventral derepression, while the ct mutation results only in ventral derepression could simply reflect the different relative importance of the two factors in AT2 site function. Alternatively, however, it is possible that two mechanisms are operating, one involving both Ct and Dri that actively represses transcription and another involving Dri, but not Ct, that inhibits Dorsal’s intrinsic activation function.
Although dri is maternally expressed (15), the finding of a zygotic dri effect is in accord with recent evidence indicating that dri is activated very early during embryogenesis (probably prior to syncytial blastoderm formation [40a]). The lack of a maternal effect may mean that the zygotic protein represents the predominant species by nuclear cycle 13. Alternatively, it is conceivable that maternal Dri is not fully equivalent to zygotic Dri and cannot substitute for zygotic Dri in zen repression. The finding of a maternal ct effect is in accord with our finding that Ct protein is present in extracts of 0- to 1.5-h embryos.
In contrast to the strong ventral derepression of the minimal zen VRR MSE/lacZ transgene resulting from ct or dri mutations, the effects of these mutations on the ventral repression of zen in the stage 4 embryo are weak. Apparently, the endogenous gene must contain additional redundant repression regions outside the minimal VRR that function in a Ct- and Dri-independent (but a Dorsal- and Gro-dependent) manner. Such redundancy appears to be a common feature of developmentally important genes in Drosophila. For example, the Ultrabithorax (Ubx) locus contains multiple enhancers capable of independently directing the normal spatially regulated expression of this gene (42). Furthermore, Zeste, which is encoded by a nonessential gene, is nonetheless an important regulator of Ubx. The role of Zeste in Ubx regulation is readily observed only under conditions in which Ubx expression is dependent upon transvection or when Ubx regulation is studied through the use of reporter gene under the control of a minimal Ubx regulatory module (28). Other examples of genes with apparently redundant regulatory modules include twist, which contains two redundant enhancers that independently direct ventral-specific transcription (21, 34, 35); dpp, which contains at least two partially redundant ventral repression regions (17); and tailless, which contains two regions capable of independently directing terminal-specific gene expression (30). The biological rationale for this kind of redundancy is not clear. However, as has been suggested previously (28), redundancy could serve to buffer the organism against variations in regulatory factor activity resulting from developmental miscues, thereby ensuring the invariant spatially controlled expression of developmentally important genes. This could, in turn, result in a small, but evolutionarily significant increase in reproductive success.
These observations imply that the Drosophila embryo must contain other factors capable of converting Dorsal from an activator to a repressor when it is bound near and is in proper alignment with Dorsal. One such factor may be NTF-1/Grainyhead, which binds to important repression elements in a dpp ventral-repression region (18). Thus, NTF-1/Grainyhead may play a role in dpp repression that is analogous to the role played by Ct and/or Dri in zen repression.
Although ct and dri mutations have only minor effects on the expression of zen in syncytial (stage 4) embryos, dramatic defects are observed on the refinement of the zen expression pattern that normally occurs during cellularization (stage 5). In these embryos, zen is not refined to the characteristic three- to five-cell wide stripe of expression on the dorsal-most side of the embryo. This suggests a role for the AT2 site in ventral refinement. Alternatively, the role of Ct and Dri in zen refinement could be indirect.
Cooperative recruitment of Gro.
Both Dorsal and Dri bind to the corepressor Gro in vitro, suggesting a possible mechanism for repression in which Dorsal and Dri recruit Gro to the template. This model is strengthened by results showing that Dorsal and Dri bound to DNA can cooperatively recruit Gro to the zen VRR in vitro. However, the magnitude of the cooperativity observed in vitro is small (twofold) and therefore does not completely account for the absolute requirement for the Dorsal and AT2 sites observed in germ line transformation assays. This suggests that factors in addition to Dorsal and Dri are required for the efficient recruitment of Gro in vivo. For example, it is possible that the addition of Ct would enhance cooperative recruitment, an idea we have been unable to test due to difficulty obtaining sufficient amounts of recombinant Ct. It is also likely that elements in addition to Dorsal sites and AT2 are required for efficient Gro recruitment and therefore for efficient repression, since previous experiments indicate that, while these sites are required for repression, they are not sufficient for repression (references 23 and 25 and data not shown). Finally, it is possible that the cooperativity of Gro recruitment would be enhanced in the context of chromatin templates rather than naked DNA templates.
In conclusion, ventral repression of genes such as zen and dpp appears to involve the formation of a multiprotein complex at the ventral silencer. The two obligate components of this complex are Dorsal and Gro. In addition, other DNA binding repressor proteins (which vary from gene to gene and from VRR to VRR) apparently bind to VRRs and assist Dorsal in the recruitment of Gro to the template. These findings together with recent results on the mechanism of Dorsal-mediated activation may provide the basis for understanding what determines whether Dorsal will function as an activator or repressor when it is bound to a particular target gene. In addition to interacting with the Gro corepressor, Dorsal has been found to interact with CREB binding protein (CBP), a well-studied coactivator (1). In both cases, the Dorsal rel homology domain has been implicated in the interaction (1, 11). Thus, there may be a competition between Gro and CBP for binding to Dorsal. When Dorsal binding sites are placed in the context of binding sites for proteins such as Dri that can assist in the recruitment of Gro, the result is ventral repression. In contrast, when sites for factors such as Dri are absent, CBP wins the competition for the protein binding interface on the rel homology domain, and the result is ventral activation.
By showing how the activity of a gene-specific transcription factor can be critically dependent upon the context of its binding site, our findings have general implications for the developmental regulation of transcription. For example, the products of the homeotic genes all bind to similar target elements, and yet they all have dramatically different functions in development (3). Perhaps the differential developmental effects of different homeoproteins are determined not only by the identity of the target genes to which they bind but also by the way a particular homeoprotein functions (e.g., as an activator or repressor) once it is bound to a particular target gene. As has been shown for Dorsal, this could, in turn, be determined by the proximity of the bound factor to other DNA-bound factors that can assist in the recruitment of coactivators or corepressors.
ACKNOWLEDGMENTS
We thank Karen Blochlinger and Steve Jackson for providing us with the ct mutant and antibodies.
This work was supported by a National Institutes of Health research grant (GM44522) to A.J.C. and by a National Institutes of Health training grant (GM07185) to S.A.V.
REFERENCES
- 1.Akimaru H, Hou D X, Ishii S. Drosophila CBP is required for dorsal-dependent twist gene expression. Nat Genet. 1997;17:211–214. doi: 10.1038/ng1097-211. [DOI] [PubMed] [Google Scholar]
- 2.Belvin M P, Anderson K V. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 3.Biggin M D, McGinnis W. Regulation of segmentation and segmental identity by Drosophila homeoproteins: the role of DNA binding in functional activity and specificity. Development. 1997;124:4425–4433. doi: 10.1242/dev.124.22.4425. [DOI] [PubMed] [Google Scholar]
- 4.Blochlinger K, Bodmer R, Jack J, Jan L Y, Jan Y N. Primary structure and expression of a product from cut, a locus involved in specifying sensory organ identity in Drosophila. Nature. 1988;333:629–635. doi: 10.1038/333629a0. [DOI] [PubMed] [Google Scholar]
- 5.Blochlinger K, Jan L Y, Jan Y N. Transformation of sensory organ identity by ectopic expression of Cut in Drosophila. Genes Dev. 1991;5:1124–1135. doi: 10.1101/gad.5.7.1124. [DOI] [PubMed] [Google Scholar]
- 6.Bodmer R, Barbel S, Shepard S, Jack J W, Jan L Y, Jan Y N. Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell. 1987;51:293–307. doi: 10.1016/0092-8674(87)90156-5. [DOI] [PubMed] [Google Scholar]
- 7.Cai H N, Arnosti D N, Levine M. Long-range repression in the Drosophila embryo. Proc Natl Acad Sci USA. 1996;93:9309–9314. doi: 10.1073/pnas.93.18.9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos-Ortega J A, Hartenstein V. The embryonic development of Drosophila melanogaster. Berlin, Germany: Springer-Verlag; 1985. [Google Scholar]
- 9.Chou T B, Perrimon N. Use of a yeast site-specific recombinase to produce female germ line chimeras in Drosophila. Genetics. 1992;131:643–653. doi: 10.1093/genetics/131.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courey A J, Huang J. The establishment and interpretation of transcription factor gradients in the Drosophila embryo. Biochim Biophys Acta. 1995;1261:1–18. doi: 10.1016/0167-4781(94)00234-t. [DOI] [PubMed] [Google Scholar]
- 11.Dubnicoff T, Valentine S A, Chen G, Shi T, Lengyel J A, Paroush Z, Courey A J. Conversion of dorsal from an activator to a repressor by the global corepressor Groucho. Genes Dev. 1997;11:2952–2957. doi: 10.1101/gad.11.22.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupfort D, Nepveu A. The human Cut homeodomain protein represses transcription from the c-myc promoter. Mol Cell Biol. 1994;14:4251–4257. doi: 10.1128/mcb.14.6.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez J, Andrews L, Mische S M. An improved procedure for enzymatic digestion of polyvinylidene difluoride-bound proteins for internal sequence analysis. Anal Biochem. 1994;218:112–117. doi: 10.1006/abio.1994.1148. [DOI] [PubMed] [Google Scholar]
- 14.Govind S, Steward R. Dorsoventral pattern formation in Drosophila: signal transduction and nuclear targeting. Trends Genet. 1991;7:119–125. doi: 10.1016/0168-9525(91)90456-z. [DOI] [PubMed] [Google Scholar]
- 15.Gregory S L, Kortschak R D, Kalionis B, Saint R. Characterization of the dead ringer gene identifies a novel, highly conserved family of sequence-specific DNA-binding proteins. Mol Cell Biol. 1996;16:792–799. doi: 10.1128/mcb.16.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrscher R F, Kaplan M H, Lelsz D L, Das C, Scheuermann R, Tucker P W. The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: a B cell-specific trans-activator that describes a new DNA-binding protein family. Genes Dev. 1995;9:3067–3082. doi: 10.1101/gad.9.24.3067. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Schwyter D H, Shirokawa J M, Courey A J. The interplay between multiple enhancer and silencer elements defines the pattern of decapentaplegic expression. Genes Dev. 1993;7:694–704. doi: 10.1101/gad.7.4.694. [DOI] [PubMed] [Google Scholar]
- 18.Huang J D, Dubnicoff T, Liaw G J, Bai Y, Valentine S A, Shirokawa J M, Lengyel J A, Courey A J. Binding sites for transcription factor NTF-1/Elf-1 contribute to the ventral repression of decapentaplegic. Genes Dev. 1995;9:3177–3189. doi: 10.1101/gad.9.24.3177. [DOI] [PubMed] [Google Scholar]
- 19.Ip Y T, Kraut R, Levine M, Rushlow C A. The dorsal morphogen is a sequence-specific DNA-binding protein that interacts with a long-range repression element in Drosophila. Cell. 1991;64:439–446. doi: 10.1016/0092-8674(91)90651-e. [DOI] [PubMed] [Google Scholar]
- 20.Jack J W. Molecular organization of the cut locus of Drosophila melanogaster. Cell. 1985;42:869–876. doi: 10.1016/0092-8674(85)90283-1. [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Kosman D, Ip Y T, Levine M. The dorsal morphogen gradient regulates the mesoderm determinant twist in early Drosophila embryos. Genes Dev. 1991;5:1881–1891. doi: 10.1101/gad.5.10.1881. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Rushlow C A, Zhou Q, Small S, Levine M. Individual dorsal morphogen binding sites mediate activation and repression in the Drosophila embryo. EMBO J. 1992;11:3147–3154. doi: 10.1002/j.1460-2075.1992.tb05387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang J, Cai H, Zhou Q, Levine M. Conversion of a dorsal-dependent silencer into an enhancer: evidence for dorsal corepressors. EMBO J. 1993;12:3201–3209. doi: 10.1002/j.1460-2075.1993.tb05989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadonaga J T, Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci USA. 1986;83:5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirov N, Zhelnin L, Shah J, Rushlow C. Conversion of a silencer into an enhancer: evidence for a co-repressor in dorsal-mediated repression in Drosophila. EMBO J. 1993;12:3193–3199. doi: 10.1002/j.1460-2075.1993.tb05988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirov N C, Lieberman P M, Rushlow C. The transcriptional corepressor DSP1 inhibits activated transcription by disrupting TFIIA-TBP complex formation. EMBO J. 1996;15:7079–7087. [PMC free article] [PubMed] [Google Scholar]
- 27.Kissinger C R, Liu B, Martin-Blanco E, Kornberg T B, Pabo C O. Crystal structure of an engrailed homeodomain-DNA complex at 2.8 A resolution: a framework for understanding homeodomain-DNA interactions. Cell. 1990;63:579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- 28.Laney J D, Biggin M D. Redundant control of Ultrabithorax by zeste involves functional levels of zeste protein binding at the Ultrabithorax promoter. Development. 1996;122:2303–2311. doi: 10.1242/dev.122.7.2303. [DOI] [PubMed] [Google Scholar]
- 29.Lehming N, Thanos D, Brickman J M, Ma J, Maniatis T, Ptashne M. An HMG-like protein that can switch a transcriptional activator to a repressor. Nature. 1994;371:175–179. doi: 10.1038/371175a0. [DOI] [PubMed] [Google Scholar]
- 30.Liaw G J, Lengyel J A. Control of tailless expression by bicoid, dorsal and synergistically interacting terminal system regulatory elements. Mech Dev. 1993;40:47–61. doi: 10.1016/0925-4773(93)90087-e. [DOI] [PubMed] [Google Scholar]
- 31.Mailly F, Berube G, Harada R, Mao P L, Phillips S, Nepveu A. The human cut homeodomain protein can repress gene expression by two distinct mechanisms: active repression and competition for binding site occupancy. Mol Cell Biol. 1996;16:5346–5357. doi: 10.1128/mcb.16.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palaparti A, Baratz A, Stifani S. The Groucho/transducin-like enhancer of split transcriptional repressors interact with the genetically defined amino-terminal silencing domain of histone H3. J Biol Chem. 1997;272:26604–26610. doi: 10.1074/jbc.272.42.26604. [DOI] [PubMed] [Google Scholar]
- 33.Pan D J, Huang J D, Courey A J. Functional analysis of the Drosophila twist promoter reveals a dorsal-binding ventral activator region. Genes Dev. 1991;5:1892–1901. doi: 10.1101/gad.5.10.1892. [DOI] [PubMed] [Google Scholar]
- 34.Pan D J, Courey A J. The same dorsal binding site mediates both activation and repression in a context-dependent manner. EMBO J. 1992;11:1837–1842. doi: 10.1002/j.1460-2075.1992.tb05235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan D, Valentine S A, Courey A J. The bipartite D. melanogaster twist promoter is reorganized in D. virilis. Mech Dev. 1994;46:41–53. doi: 10.1016/0925-4773(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 36.Paroush Z, Finley R, Jr, Kidd T, Wainwright S, Ingham P, Brent R, Ish-Horowicz D. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with Hairy-related bHLH proteins. Cell. 1994;79:805–815. doi: 10.1016/0092-8674(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 37.Ray R P, Arora K, Nüsslein-Volhard C, Gelbart W M. The control of cell fate along the dorsal-ventral axis of the Drosophila embryo. Development. 1991;113:35–54. doi: 10.1242/dev.113.1.35. [DOI] [PubMed] [Google Scholar]
- 38.Roth S, Stein D, Nüsslein-Volhard C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989;59:1189–1202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- 39.Rubin G M, Spradling A C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 40.Rushlow C A, Han D, Manley J S, Levine M. The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell. 1989;59:1165–1177. doi: 10.1016/0092-8674(89)90772-1. [DOI] [PubMed] [Google Scholar]
- 40a.Shandala, T., and R. Saint. Unpublished data.
- 41.Shirokawa J M, Courey A J. A direct contact between the Dorsal rel homology domain and Twist may mediate transcriptional synergy. Mol Cell Biol. 1997;17:3345–3355. doi: 10.1128/mcb.17.6.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon J, Peifer M, Bender W, O’Connor M. Regulatory elements of the bithorax complex that control expression along the anterior-posterior axis. EMBO J. 1990;9:3945–3956. doi: 10.1002/j.1460-2075.1990.tb07615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soeller W C, Poole S J, Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988;2:68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- 44.Steward R. Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell. 1989;59:1179–1188. doi: 10.1016/0092-8674(89)90773-3. [DOI] [PubMed] [Google Scholar]
- 45.Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- 46.Thisse C, Perrin-Schmitt F, Stoetzel C, Thisse B. Sequence-specific transactivation of the Drosophila twist gene by the dorsal gene product. Cell. 1991;65:1191–1201. doi: 10.1016/0092-8674(91)90014-p. [DOI] [PubMed] [Google Scholar]
- 47.Treisman J E, Luk A, Rubin G M, Heberlein U. Eyelid antagonizes wingless signaling during Drosophila development and has homology to the Bright family of DNA-binding proteins. Genes Dev. 1997;11:1949–1962. doi: 10.1101/gad.11.15.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]