Abstract
OBJECTIVES:
To assess the efficacy of haloperidol in reducing postoperative delirium in individuals undergoing thoracic surgery.
DESIGN:
Randomized double-blind placebo-controlled trial.
SETTING:
Surgical intensive care unit (ICU) of tertiary care center.
PARTICIPANTS:
Individuals undergoing thoracic surgery (N=135).
INTERVENTION:
Low-dose intravenous haloperidol (0.5 mg three times daily for a total of 11 doses) administered postoperatively.
MEASUREMENTS:
The primary outcome was delirium incidence during hospitalization. Secondary outcomes were time to delirium, delirium duration, delirium severity, and ICU and hospital length of stay. Delirium was assessed using the Confusion Assessment Method for the ICU and delirium severity using the Delirium Rating Scale-Revised.
RESULTS:
Sixty-eight participants were randomized to receive haloperidol and 67 placebo. No significant differences were observed between those receiving haloperidol and those receiving placebo in incident delirium (n=15 (22.1%) vs n=19 (28.4%); p = .43), time to delirium (p = .43), delirium duration (median 1 day, interquartile range (IQR) 1–2 days vs median 1 day, IQR 1–2 days; p = .71), delirium severity, ICU length of stay (median 2.2 days, IQR 1–3.3 days vs median 2.3 days, IQR 1–4 days; p = .29), or hospital length of stay (median 10 days, IQR 8–11.5 days vs median 10 days, IQR 8–12 days; p = .41). In the esophagectomy subgroup (n = 84), the haloperidol group was less likely to experience incident delirium (n=10 (23.8%) vs n=17 (40.5%); p = .16). There were no differences in time to delirium (p = .14), delirium duration (median 1 day, IQR 1–2 days vs median 1 day, IQR 1–2 days; p = .71), delirium severity, or hospital length of stay (median 11 days, IQR 10–12 days vs median days 11, IQR 10–15 days; p = .26). ICU length of stay was significantly shorter in the haloperidol group (median 2.8 days, IQR 1.1–3.8 days vs median 3.1 days, IQR 2.1–5.1 days; p = .03). Safety events were comparable between the groups.
CONCLUSION:
Low-dose postoperative haloperidol did not reduce delirium in individuals undergoing thoracic surgery but may be efficacious in those undergoing esophagectomy.
Keywords: delirium, esophagectomy, ICU, haloperidol, cognition
Delirium is a syndrome of disturbance of attention and awareness that develops quickly and fluctuates over the course of the day.1 Individuals with delirium are vulnerable to hospital-acquired complications, leading to prolonged intensive care unit (ICU) and hospital stays, new institutionalization, higher healthcare costs, and greater mortality.2–5 Postoperative delirium is also associated with long-term cognitive decline and dementia.5,6
Incidence of postoperative delirium ranges from 15% to 80%.7–13 For noncardiac thoracic surgery, incidence of delirium could be as high as 50% in individuals undergoing esophagectomies.14 Current theoretical models of delirium pathophysiology posit that a complex interaction between underlying vulnerabilities such as age and preexisting cognitive impairment coupled with an extensive external stressor such as esophagectomy predisposes to delirium.15 As a response to surgery, peripheral macrophages produce pro-inflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor-alpha (TNF-α),16,17 leading to blood–brain barrier disruption with infiltration of leukocytes into the central nervous system.18–20 The resultant microglial activation produces local cytokines (TNF-α, IL-1 β) and reactive oxygen species and promotes cholinergic failure and dopaminergic excess.21,22
Haloperidol is a typical antipsychotic that acts primarily by blocking dopamine receptors.23,24 Haloperidol also inhibits production of pro-inflammatory cytokines IL-1 and TNF-α,25 and increases levels of the IL-1 receptor antagonist (IL-1RA), an anti-inflammatory cytokine.26 Haloperidol has been shown to reduce delirium burden in individuals undergoing abdominal surgery27 and those who have had a hip facture,28 but its role in reducing delirium in individuals undergoing thoracic surgery is unclear. Using the theoretical pathophysiological framework, we designed this randomized trial to assess the feasibility and efficacy of haloperidol prophylaxis in reducing delirium after major thoracic surgery, specifically esophagectomy.
MATERIALS AND METHODS
Study Design
The Indiana University institutional review board approved this randomized double-blind placebo-controlled single-center trial.
Study Setting
The trial was conducted at Indiana University Hospital Simon Cancer Center in Indianapolis, a 257-bed tertiary care center staffed by Indiana University School of Medicine faculty with a referral base from Indiana and neighboring states. Four surgeons from the thoracic surgery department participated in the study.
Enrollment, Eligibility, and Randomization
English-speaking individuals aged 18 and older undergoing thoracic surgery were included. Exclusion criteria were a history of schizophrenia, Parkinson’s disease, severe dementia, alcohol abuse, neuroleptic malignant syndrome, haloperidol allergy, pregnancy, breast feeding, taking cholinesterase inhibitors or levodopa, or a corrected QT interval longer than 500 ms at the time of randomization. Eligible individuals were approached for informed consent before their surgery in the preoperative clinic.
Individuals who provided consent were randomized in a 1:1 ratio to haloperidol or placebo using a computer-generated randomization scheme (permuted block sizes of 6 and 8) stratified according to type of surgery (esophagectomy, pneumonectomy, other thoracic surgery). The investigational pharmacy executed the randomization scheme.
Study Procedures and Drug Administration
Participants underwent a thorough preoperative evaluation including neuropsychological testing. Those admitted for surgery received an order for blinded study drug, and the investigational pharmacist referred to the randomization list for treatment assignment. Apart from the investigational pharmacists, all study personnel, participants, and clinicians were blinded to each participant’s treatment assignment.
Randomized participants underwent electrocardiography to confirm that their corrected QT interval was less than 500 ms. The first dose of the study drug was administered immediately postoperatively, followed by a 3-times-daily schedule, for a total of 11 doses. Haloperidol dose was 0.5 mg administered intravenously by bolus injection over 3 minutes. Placebo was identical in route, appearance, and volume.
A delirium rescue protocol was implemented to manage pain, agitation, and delirium in participants. The protocol focused on pain control using fentanyl, morphine, or hydromorphone and sedation and management of agitation using propofol in mechanically ventilated participants and dexmedetomidine in nonmechanically ventilated participants. Participants received a nonpharmacological delirium protocol including reorientation, eyeglasses and hearing aids, and sleep preservation through noise reduction, minimizing sleep interruptions, and illumination during daytime. Open-label antipsychotics were discouraged during the intervention phase, but the primary services were not otherwise restricted in their prescribing of haloperidol or other antipsychotics. An independent data safety monitoring board oversaw trial implementation.
Outcome Measures
The primary outcome measure was incidence of delirium during hospitalization. Secondary outcomes were time to delirium, delirium duration and severity, cognitive scores after surgery, and ICU and hospital lengths of stay. Trained, blinded research assistants collected all data.
Delirium Incidence and Duration
We used the Confusion Assessment Method for the ICU (CAM-ICU) to detect delirium.29 Because delirium is a fluctuating disorder, research assistants conducted 2 delirium assessments each day, one in the morning (9:00–11:00 am) and one in the afternoon (3:00–5:00 pm), to maximize delirium identification. Delirium duration was defined as total number of days a participant was CAM-ICU-positive on the morning or afternoon assessment during their entire hospital stay. The Richmond Agitation Sedation Scale (RASS)30 was used to assess eligibility for delirium assessment. Participants with a RASS score of −4 or −5 with lack of response to verbal or physical stimuli were characterized as comatose and were not eligible for CAM-ICU assessments. Participants with a RASS score of −3 to +4 were considered eligible to be assessed using the CAM-ICU.30
Delirium Severity
The Delirium Rating Scale—Revised (DRS-R-98)31 was administered daily to measure delirium severity. The DRS-R-98 captures impairments in attention; short- and long-term memory; visuospatial ability and orientation; perceptual and sleep–wake cycle disturbances; abnormalities of language, thought process, and content; motor agitation or retardation; and mood lability.31 It is a 16-item scale, with the severity scale having 13 items (rated 0–3 each, maximum 39 points) and higher scores indicating greater delirium severity.
Cognitive Impairment
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)32 was used to assess for cognitive impairment. The RBANS is a validated scale that yields scores for 5 cognitive domains (immediate memory, visuospatial construction, language, attention, delayed memory).32 It was administered during the preoperative assessment and at postsurgery clinic follow-up.
ICU and Hospital Lengths of Stay
Electronic medical records were reviewed to determine ICU and hospital lengths of stay.
Other Data Collection
Information was collected on participant demographic characteristics, chronic comorbidities using the Charlson Comorbidity Index,33 severity of illness using the Acute Physiology and Chronic Health Evaluation (APACHE) II,34 depression using the Patient Health Questionnaire,35 anxiety using the 7-item Generalized Anxiety Disorder Scale,36 posttraumatic stress disorder symptoms using the Post Traumatic Stress Symptoms scale,37 activities of daily living using the Katz scale,38 and instrumental activities of daily living using the Lawton scales.39 Information was also collected on daily sedative and analgesic exposure, duration of mechanical ventilation, and intraoperative data. Supplementary Table S1 describes the timing of data collection.
Drug-Related Adverse Events
Adverse effects related to haloperidol use, including QT prolongation and extrapyramidal symptoms, were monitored and reported to the data safety monitoring board.
Statistical Analysis
We assessed whether the proportion of participants developing delirium differed between the 2 groups using the Fisher exact test. To assess differences in the number of days in a coma or with delirium at discharge, we used a Poisson regression model that included an offset equal to the log (length of stay after randomization) to account for different lengths of stay between participants. Poisson regression was used to test for group differences in time in the ICU and on mechanical ventilation. Because there were no in-hospital deaths, Kaplan–Meier survival estimates were used to compare length of stay and differences in time to delirium. Time to delirium was calculated as days from the day of surgery until the first CAM-ICU positive result in patients with delirium. In delirium-negative participants, event time was censored at the day of last CAM-ICU screening. We used a mixed model to test whether delirium severity course differed over time. This model included all DRS-R-98 assessment days, a random effect for participant, and fixed effect for day and the interaction between day and randomization group. All other differences between groups were assessed using the Fisher exact test for categorical outcomes and the Wilcoxon rank sum test for continuous measures because the majority of these measures contained skewed data. Power analysis showed that a sample size of 53 individuals undergoing esophagectomy per group would achieve 80% power to detect a reduction of delirium incidence from 50% to 25% using a 1-tailed test at 5% significance. A power analysis for all thoracic surgeries was not performed because the study was designed a priori to answer the question in individuals undergoing esophagectomy. No interim analyses were planned, and there were no prespecified stopping guidelines. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
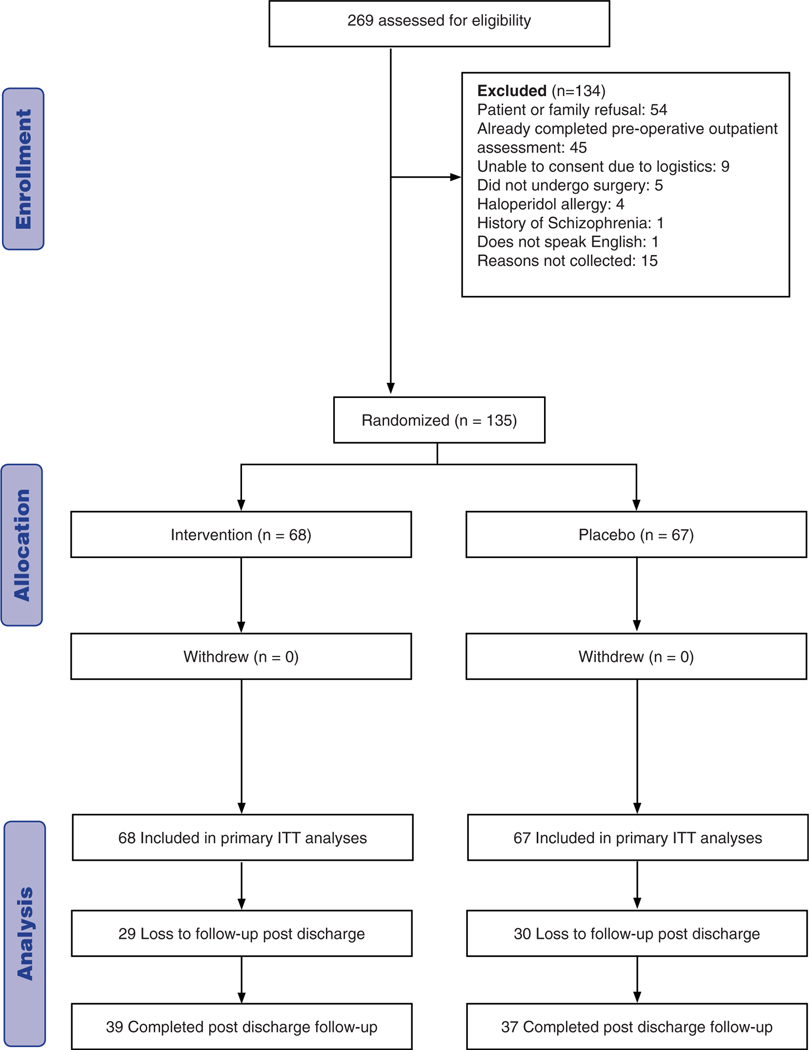
Of 269 individuals undergoing major thoracic surgery screened from October 2013 to June 2015, 135 were enrolled in the trial. Sixty-eight were randomly assigned to haloperidol and 67 to placebo (Figure 1). Individuals undergoing esophagectomy constituted the largest group (n = 84), with 42 assigned to haloperidol and 42 to placebo (Supplementary Figure S1). There were no differences in baseline participant characteristics between the haloperidol and placebo groups (Table 1) or in the intraoperative and postoperative factors except that more individuals undergoing esophagectomy in the placebo group received propofol (Table 2, Supplementary Tables S2 and S3).
Figure 1.
Flow of participants through study.
Table 1.
Baseline Characteristics of All Participants Undergoing Thoracic Surgery and the Subgroup Undergoing Esophagectomy
| Characteristic |
Overall, N = 135 |
Esophagectomy, n = 84 |
||
|---|---|---|---|---|
| Haloperidol, n= 68 | Placebo, n = 67 | Haloperidol, n= 42 | Placebo, n= 42 | |
| Age, median (IQR) | 60 (51.8–68) | 62.3 (52.6–69.2) | 61.0 (52.1–67.9) | 64 (57.2–70.5) |
| African American, n (%) | 2 (3.0) | 3 (4.5) | 0 (0.0) | 3 (7.1) |
| Female, n (%) | 22 (32.4) | 13 (19.4) | 12 (28.6) | 6 (14.3) |
| Education | ||||
| High school graduate, n (%) | 36 (55.4) | 32 (51.6) | 24 (60.0) | 21 (55.3) |
| Some college, n (%) | 16 (24.6) | 22 (35.5) | 8 (20.0) | 12 (31.6) |
| Bachelor’s degree or more, n (%) | 13 (20.0) | 8 (12.9) | 8 (20.0) | 5 (13.2) |
| Preoperative chemotherapy, n (%) | 33 (50.8) | 40 (64.5) | 27 (69.2) | 31 (81.6) |
| Acute Physiology and Chronic Health Evaluation II score, median (IQR) | 16 (13–24) | 17 (11–24) | 16.5 (13–24) | 18 (14–25) |
| Charlson Comorbidity Index, median (IQR) | 2 (2–3) | 3 (2–4) | 2 (2–3) | 3 (2–4) |
| Activity of daily living score, median (IQR) | 6 (6–6) | 6 (6–6) | 6 (6–6) | 6 (6–6) |
| Instrumental activity of daily living score, median (IQR) | 8 (7–8) | 8 (7–8) | 8 (7–8) | 8 (7–8) |
| Preoperative RBANS score, median (IQR) | 88.5 (78.5–101) | 90 (84.5–98) | 95 (76–102) | 90 (87–100) |
| Preoperative RBANS percentile, median (IQR) | 22 (8–52.5) | 25 (15–45) | 37 (7–55) | 25 (19–50) |
| Preoperative Patient Health Questionnaire score, median (IQR) | 3.7 (1–7) | 2 (1–5) | 4 (1–7) | 3 (1–5) |
| Preoperative 7-item Generalized Anxiety Disorder Scale score, median (IQR) | 2 (0–6) | 2 (0–5) | 3 (0–6) | 1.5 (0–5) |
| Preoperative Post-Traumatic Symptom Scale score, median (IQR) | 16.5 (12–22) | 14 (11–20) | 16.5 (12–22) | 13 (11–20) |
| Body mass index, kg/m2, median (IQR) | 27.7 (23.7–32.2) | 29.1 (25.1–32.1) | 25 (22.6–31.5) | 28.5 (24.4–32) |
| Type of surgery | ||||
| Esophagectomy, n (%) | 42 (61.8) | 42 (62.7) | ||
| Ivor Lewis, n | 32 | 39 | ||
| Other, n | 10 | 3 | ||
| Left pneumonectomy, n (%) | 1 (1.5) | 2 (3.0) | ||
| Thoracotomy, n (%) | 25 (36.8) | 23 (34.3) | ||
| Lobectomy or bilobectomy with mediastinal dissection, n | 11 | 10 | ||
| Lobectomy or bilobectomy, n | 6 | 1 | ||
| Mediastinal dissection, n | 2 | 7 | ||
| Other, n | 6 | 5 | ||
| American Society of Anesthesiologists class, n (%) | ||||
| I | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| II | 3 (4.4) | 0 (0.0) | 1 (2.4) | 0 (0.0) |
| III | 65 (95.6) | 66 (98.5) | 41 (97.6) | 42 (100.0) |
| IV | 0 (0.0) | 1 (1.5) | 0 (0.0) | 0 (0.0) |
IQR=interquartile range; RBANS=Repeatable Battery for the Assessment of Neuropsychological Status.
Table 2.
Intra- and Postoperative Data of All Participants Undergoing Thoracic Surgery and the Subgroup Undergoing Esophagectomy
| Variable | Haloperidol | Placebo | P-Value |
|---|---|---|---|
| All thoracic surgeries, n (N = 135) | 68 | 67 | |
| Intraoperative data, median (IQR) | |||
| Duration of surgery, hours | 4.1 (2.3–5.0) | 4.0 (2.6–5.1) | .91 |
| Duration of anesthesia, hours | 5.7 (4.0–6.4) | 5.4 (3.8–6.7) | .99 |
| Estimated blood loss, mL | 150 (100–300) | 150 (100–300) | .71 |
| Volume infused intraoperatively, mL, median (IQR) | |||
| Total | 2,507 (1,400–3,500) | 2,500 (1,500–3,100) | .67 |
| Crystalloid | 2,050 (1,350–3,000) | 2,000 (1,410–2,500) | .51 |
| Colloid | 500 (0–500) | 500 (0–850) | .50 |
| Postoperative transfusion, n (%) | 3 (4.4) | 4 (6.0) | .72 |
| Esophagectomies, n (n = 84) | 42 | 42 | |
| Intraoperative data, median (IQR) | |||
| Duration of surgery, hours | 5.0 (4.2–5.2) | 4.7 (4.1–5.6) | .70 |
| Duration of anesthesia, hours | 6.3 (5.7–6.8) | 6.4 (5.5–7.1) | .93 |
| Estimated blood loss, mL | 200 (100–300) | 300 (100–300) | .48 |
| Volume infused intraoperatively, mL, median (IQR) | |||
| Total | 3,225 (2,514–3,800) | 3,000 (2,500–3,500) | .30 |
| Crystalloid | 2,550 (2,100–3,300) | 2,200 (1,900–3,000) | .10 |
| Colloid | 500 (250–750) | 500 (500–1,000) | .11 |
| Postoperative transfusion, n (%) | 2 (4.8) | 3 (7.1) | >.99 |
IQR = interquartile range.
Primary Outcomes
There was no difference in delirium incidence between the haloperidol (22.1%, 15/68) and placebo (28.4%, 19/67) groups (p = .43) (Table 3).
Table 3.
Outcomes of All Participants Undergoing Thoracic Surgery and the Subgroup Undergoing Esophagectomy
| Outcome | Haldol | Placebo | P-Value |
|---|---|---|---|
| All participants, n (N = 135) | 68 | 67 | |
| Delirium incidence, n (%) | 15 (22.1) | 19 (28.4) | .43 |
| Delirium or coma duration, days, median (IQR) | 1 (0–2) | 1 (0–2) | .94 |
| Delirium duration, days, median (IQR)1 | 1 (1–2) | 1 (1–2) | .71 |
| Delirium or coma duration, days, median (IQR)1 | 2 (2–3) | 2 (2–4) | .14 |
| Length of mechanical ventilation, days, median (IQR) | 0.8 (0.2–1.1) | 1.0 (0.2–1.1) | .67 |
| ICU length of stay, days, median (IQR) | 2.2 (1.0–3.3) | 2.3 (1.0–4.0) | .29 |
| Hospital length of stay, days, median (IQR) | 10 (8–11.5) | 10 (8–12) | .40 |
| Discharged home, n (%) | 61 (89.7) | 59 (88.1) | .79 |
| In-hospital mortality, n (%) | 0 (0.0) | 0 (0.0) | |
| Esophagectomy, n (N = 84) | 42 | 42 | |
| Delirium incidence, n (%) | 10 (23.8) | 17 (40.5) | .16 |
| Delirium or coma duration, days, median (IQR) | 1 (1–2) | 1 (1–2) | .58 |
| Delirium duration, days, median (IQR)1 | 1 (1–2) | 1 (1–2) | .70 |
| Delirium or coma duration, days, median (IQR)1 | 2 (2–3) | 3 (2–4) | .36 |
| Length of mechanical ventilation, days, median (IQR) | 1.0 (0.3–2.2) | 1.1 (0.9–1.2) | .46 |
| ICU length of stay, days, median (IQR) | 2.8 (1.1–3.8) | 3.1 (2.1–5.1) | .03 |
| Hospital length of stay, days, median (IQR) | 11 (10–12) | 11 (10–15) | .25 |
| Discharged home, n (%) | 35 (83.3) | 36 (85.7) | >.99 |
| In-hospital mortality, n (%) | 0 (0.0) | 0 (0.0) |
In participants with delirium.
IQR=interquartile range; ICU=intensive care unit.
Secondary Outcomes
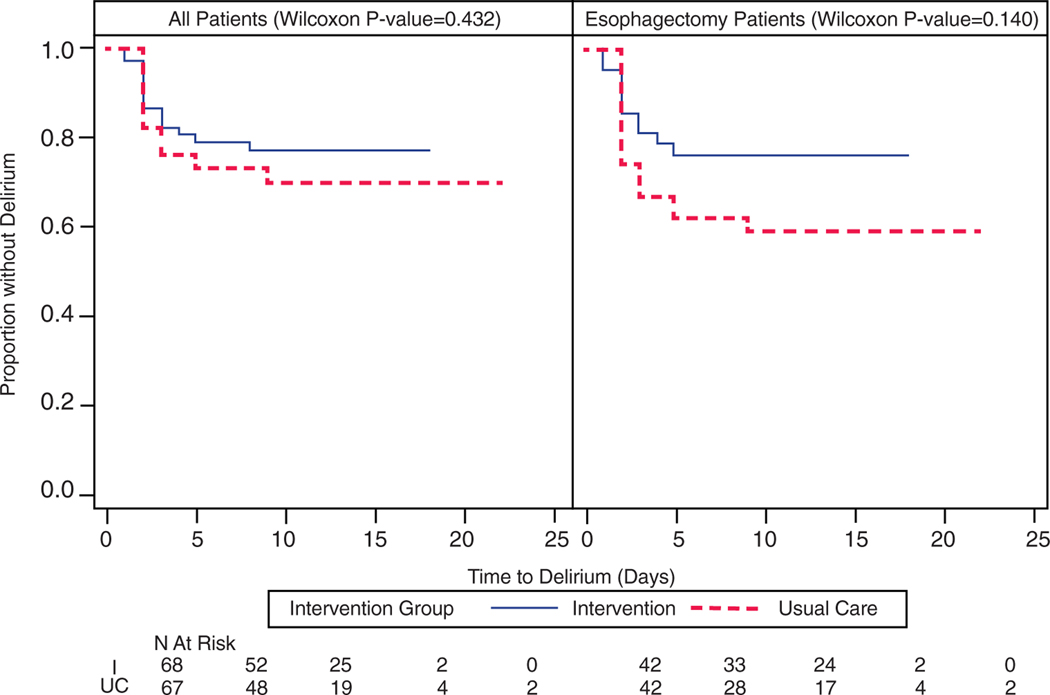
There were no differences in time to delirium (p = .43) (Figure 2) or median number of delirium days in participants with delirium (haloperidol: median 1 day, interquartile range (IQR) 1–2 days; placebo: 1 day, IQR 1–2 days; p = .71). There was a similar magnitude of reduction in delirium severity scores over time in both groups (Supplementary Figure S2).
Figure 2.
Time to delirium in all participants undergoing thoracic surgery and the subgroup undergoing esophagectomy.
There were no differences in ICU (haloperidol: median 2.2 days, IQR 1–3.3 days; placebo: median 2.3 days, IQR 1–4 days; p = .29) or hospital (haloperidol: median 10 days, IQR 8–11.5 days; placebo: median 10 days, IQR 8–12 days; p = .40) length of stay (Table 3, Supplementary Figure S3). When the analysis was limited to participants with delirium, the haloperidol group had a shorter hospital stay (median 10 days, IQR 8–14 days vs median 11 days, IQR 11–16 days; p = .03) (Supplementary Figure S4).
A small subset of participants underwent pre- and post-operative RBANS cognitive assessments (haloperidol, n = 17; placebo, n = 16). Postoperatively, the RBANS percentile change scores improved in the placebo group (haloperidol: median 6, IQR 0–23; placebo: median −9.25, IQR −17.5–0; p = .008).
Esophagectomy Subgroup
Primary Outcome
Delirium incidence was lower in the haloperidol (23.8%, 10/42) than the placebo (40.5%, 17/42) group, but the difference was not statistically significant (p = .16) (Table 3).
Secondary Outcomes
There was no difference in time to delirium between the groups (p = .14) (Figure 2) and no difference in number of days with delirium in participants with delirium (haloperidol: median 1 day, IQR 1–2 days, placebo median 1 day, IQR 1–2 days; p = .70). Delirium severity reduction was similar in both groups (Supplementary Figure S5).
ICU stay was significantly shorter in the haloperidol (median 2.8 days, IQR 1.1–3.8 days) than the placebo (median 3.1 days, IQR 2.1–5.1 days) group (p = .03) (Table 3). No differences were found in hospital length of stay (haloperidol: median 11 days, IQR 10–12 days; placebo: median 11 days, IQR 10–15 days; p = .25) (Table 3). When the analysis was limited to participants with delirium, the haloperidol group had a trend toward significantly fewer hospital days (haloperidol: median 11 days, IQR 10–14 days; placebo: median 12 days, IQR 11–16 days; p = .06] (Supplementary Figure S6).
A small subset of participants underwent pre- and post-operative RBANS cognitive assessments (haloperidol, n = 9; placebo, n = 9). Postoperatively, RBANS percentile change scores improved in the placebo group (haloperidol: median 13, IQR 0–24; placebo: median −2, IQR −18–0; p = .05).
Esophagectomy Versus Other Thoracic Surgeries
Individuals undergoing esophagectomy were slightly older (median 62, IQR 53–69 vs median 61 IQR 45–67; p = .16), were more likely to undergo preoperative chemotherapy (75.3% vs 30.0%, p <.001), had greater severity of illness (APACHE 11 score: median 17, IQR 13.5–24.5 vs median 14, IQR 11–20; p = .008), had longer surgeries (median 4.9 hours, IQR 4.1–5.4 hours vs median 2.1 hours, IQR 1.4–3 hours; p <.001), and received a higher intraoperative volume (median 3,000, IQR 2,500–3,775 mL vs median 1,400, IQR 1,000–2,000 mL; p <.001) and more benzodiazepines (diazepam equivalents: median 20 mg, IQR 11.3–62.5 mg vs median 12.5, IQR 5–22.5 mg; p = .003) than those undergoing other thoracic surgeries.
Individuals undergoing esophagectomy had higher delirium incidence (32.1%, 27/84), than those undergoing other thoracic surgeries (13.7%, 7/51) (p = .02), longer duration of mechanical ventilation (median 1 day, IQR 0.4–1.2 days vs median 0.2 days, IQR 0.1–0.8 days; p = .01), and longer ICU (median 2.9 days, IQR 2–4 days vs median 1.1 days, IQR 0–2.2 days; p < .001) and hospital (median 11 days, IQR 10–13 days vs median 7 days, IQR 5–8 days; p < .001) stays and were less likely to be discharged home (84.5% vs 96.1%, p = .048).
Safety
There were no differences in adverse events between the groups (Supplementary Tables S4–S6).
DISCUSSION
Our results demonstrate the feasibility of conducting a randomized, placebo-controlled clinical trial in individuals undergoing major thoracic surgery. The low-dose postoperative haloperidol intervention did not reduce delirium incidence, duration, or severity in the overall population, although prespecified esophagectomy subgroup experienced modest benefits; the haloperidol intervention reduced the incidence of delirium by 17% and reduced ICU length of stay. Similar to the overall group, hospital length of stay was shorter, although not statistically significant, in the esophagectomy subgroup when the analysis was limited to participants with delirium.
Our study results had similarities to and differences from those of other delirium prevention studies in the surgical literature27,28. One study27 showed a decrease in delirium incidence, delirium duration, and ICU length of stay with haloperidol prophylaxis in elderly individuals—predominantly individuals undergoing abdominal surgery. The other study28 did not show a reduction in delirium incidence in elderly individuals undergoing hip surgery, but prophylaxis reduced delirium duration, severity, and hospital length of stay in individuals with delirium. Both studies used low-dose haloperidol (1.2 mg/d for 3 days,28 1.7-mg 1-time postoperative infusion27). We used a similar low-dose strategy based on the evidence from the aforementioned studies and prior work from our group40 demonstrating that low-dose haloperidol conferred efficacy similar to that of higher haloperidol doses with less risk of extrapyramidal symptoms. Our results were similar to those of the Hope-ICU41 and (REDUCE) trials.42 Both compared haloperidol with placebo in traditional medical and surgical ICU populations and used delirium- and coma-free days as one of the outcomes. They tested a slightly higher dose of haloperidol (Hope-ICU: 2.4 mg every 8 hours, REDUCE: 2 mg 3 times daily) than the 0.5 mg 3 times daily that we used. No differences in delirium- or coma-free days were observed in either trial between the intervention and placebo groups. REDUCE also evaluated the effect of haloperidol on delirium incidence and did not find any difference between the intervention and placebo groups. The incidence rate was 33% in both groups. Our low-dose haloperidol approach could not reduce delirium incidence either, but we were able to reduce the length of ICU stay in individuals undergoing esophagectomy, in contrast to the Hope-ICU and REDUCE studies. Because there were no differences in delirium duration between the intervention and control groups, the reduction in the ICU length of stay could not be attributed to shorter delirium duration. Other factors, although statistically nonsignificant, such as high benzodiazepine use in the placebo group and ondansetron in the intervention group, may have contributed to these results. A potential protective effect of haloperidol that could have been mediated through its antiinflammatory and immunomodulatory properties could not be discounted25; especially in individuals undergoing esophagectomy, who may have a higher cytokine burden.
The esophagectomy subgroup differed from participants undergoing other thoracic surgeries in multiple aspects. Individuals undergoing esophagectomy had higher APACHE II scores and poorer function at baseline. Surgery was twice as long in the esophagectomy group (mean 5 hours), and participants undergoing esophagectomy had twice the blood loss during surgery and received higher doses of opioids and benzodiazepines intraoperatively. The high risk profile explains the higher delirium incidence of 32.1% in esophagectomy subgroup than the 13.7% in other thoracic surgeries. In addition, the duration of ICU stay was twice as long in the esophagectomy subgroup. These differences suggest a targeted strategy of low-dose haloperidol for the high-risk esophagectomy subgroup and do not justify use of prophylaxis in all individuals undergoing thoracic surgery, especially uncomplicated cases with short ICU stays. The mechanisms for a potential therapeutic benefit are unclear, but the extent of surgery combined with the severity of illness predisposing to high cytokine levels might respond better to the antiinflammatory properties of haloperidol. Larger studies with serial biomarkers focusing on high-risk subgroups such as individuals undergoing esophagectomy could elucidate the efficacy of haloperidol prophylaxis and underlying pathogenic mechanisms.
One of the main lessons we learned from our pilot investigation is the inherent difficulty in conducting longitudinal postsurgical follow-up. We could not achieve adequate postdischarge follow-up to collect the neurocognitive outcomes. Hence, improvements in cognitive scores in the placebo group could represent a lack of regression of the scores to the mean given the small sample size. Many of our participants were referred from out of the hospital system and did not return for follow-up and in-person cognition assessments. In designing future studies, researchers should consider allocating extra resources for study personnel travel to conduct these assessments at homes of trial participants. With the increasing availability of Internet-connected telephones, better options could be to use Telehealth for cognitive assessments (Skype, Zoom, Face Time) or conduct assessments that could be performed over the telephone.
Our study had several limitations. We had a small sample size for a prevention study. We were unable to achieve the target of 106 esophagectomies as specified in our power calculation because of the limited resources afforded to us through a small local grant. We observed lower delirium incidence rate in the low dose haloperidol group (23.8%) compared to delirium incidence in the placebo group (40.5%) in esophagectomy patients. We estimate that a larger study with 266 total participants would have 80% power to detect the effect size observed in our study. It was a single-site study with results shaped by local practice patterns, which may not be replicable at other institutions. The CAM-ICU was used throughout hospitalization, which may have resulted in missing cases of delirium outside the ICU.
Our study had several strengths. We used a double-blind placebo-controlled design with validated data assessment tools. Trained research staff monitored for delirium twice daily. Information on all relevant baseline, intraoperative, and postoperative variables was collected, including baseline cognitive and psychological assessments, information not routinely collected. We achieved high fidelity to our intervention, with a mean of 10 administered postoperative doses. Inclusion of a prespecified esophagectomy subgroup provides relevant contrast and comparison with individuals undergoing other types of thoracic surgery, which could allow development of surgery-specific approaches.
In conclusion, use of low-dose haloperidol postoperatively does not reduce delirium incidence, duration, or severity in individuals undergoing major thoracic surgery. Low-dose haloperidol prophylaxis may be efficacious in reducing delirium incidence in individuals undergoing esophagectomy, but this finding needs to be confirmed in a multiinstitutional randomized trial with adequate sample size.
Supplementary Material
Supplementary Material S1: Supporting Information.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Jennifer Hurr, Dr. Naomi Burns, the clinical staff at the preoperative and thoracic surgery clinics, and the nursing staff in the surgical ICU at Indiana University Health University Hospital.
Financial Disclosure:
The study was supported by Indiana Health Values Fund Grant VFR 398 awarded to Dr. Babar Khan.
Sponsor’s Role:
The funding agency had no role in study design, data collection, analysis, data interpretation, or the decision to submit the paper for publication.
Conflict of Interest:
The study was supported by Indiana Health Values Fund Grant VFR 398 awarded to Dr. Babar Khan.
Footnotes
ClinicalTrials.Gov Identifier: NCT02213900.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Ed. Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- 2.Lin SM, Liu CY, Wang CH et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med 2004;32:2254–2259. [DOI] [PubMed] [Google Scholar]
- 3.Jackson JC, Gordon SM, Hopkins RO et al. The association between delirium and cognitive decline: A review of the empirical literature. Neuropsychol Rev 2004;14:87–98. [DOI] [PubMed] [Google Scholar]
- 4.Milbrandt EB, Deppen S, Harrison PL et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med 2004;32:955–962. [DOI] [PubMed] [Google Scholar]
- 5.Khan BA, Zawahiri M, Campbell NL et al. Delirium in hospitalized patients: Implications of current evidence on clinical practice and future avenues for research—a systematic evidence review. J Hosp Med 2012;7:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saczynski J, Marcantonio E, Quach L et al. Cognitive trajectories after post-operative delirium. N Engl J Med 2012;367:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demeure MJ, Fain MJ. The elderly surgical patient and post-operative delirium. J Am Coll Surg 2006;203:752–757. [DOI] [PubMed] [Google Scholar]
- 8.Robinson TN, Eiseman B. Post-operative delirium in the elderly: Diagnosis and management. Cin Interven Aging 2008;3:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisani MA, McNicoll L, Inouye SK. Cognitive impairment in the intensive care unit. Clin Chest Med 2003;24:727–737. [DOI] [PubMed] [Google Scholar]
- 10.Edlund A, Lundstrom M, Lundstrom G et al. Clinical profile of delirium in patients treated with femoral neck fractures. Dement Geriatr Cogn Disord 1999;10:325–329. [DOI] [PubMed] [Google Scholar]
- 11.Lee KH, Ha YC, Lee YK et al. Frequency, risk factors, and prognosis of prolonged delirium in elderly patients after hip fracture surgery. Clin Orthop Relat Res 2011;469:2612–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammeke TA, Hastings JE. Neuropsychologic alterations after cardiac operation. J Thorac Cardiovasc Surg 1988;96:326–331. [PubMed] [Google Scholar]
- 13.Rudolph JL, Babikian VL, Birjiniuk V et al. Atherosclerosis is associated with delirium after coronoary artery bypass graft surgery. J Am Geriatr Soc 2005;53:462–466. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi M, Takeuchi H, Fujisawa D et al. Incidence and risk factors of postoperative delirium in patients with esophageal cancer. Ann Surg Oncol 2012;19:3963–3970. [DOI] [PubMed] [Google Scholar]
- 15.Khan BA, Zawahiri M, Campbell N et al. Biomarkers for delirium—a review. J Am Geriatr Soc 2011;59:S256–S261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornsson I, Thorsteinsson L, Gudmundsson KO et al. Inflammatory cytokines in relation to adrenal response following total hip replacement. Scand J Immunol 2007;65:99–105. [DOI] [PubMed] [Google Scholar]
- 17.Kragsbjerg P, Holmberg H, Vikerfors T. Serum concentrations of interleukin-6, tumor necrosis factor alpha, and C-reactive protein in patients undergoing major operations. Eur J surg 1995;161:17–22. [PubMed] [Google Scholar]
- 18.Hofer S, Bopp C, Hoerner C et al. Injury of the blood brain barrier and upregulation of ICAM-1 in polymicrobial sepsis. J Surg Res 2008;146:276–281. [DOI] [PubMed] [Google Scholar]
- 19.Nishioku T, Sohgu S, Takata F et al. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol 2009;29:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semmler A, Okulla T, Sastre M et al. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat 2005;30:144–157. [DOI] [PubMed] [Google Scholar]
- 21.Block ML, Zecca L, Hong JS. Microglia mediated neurotoxicity: Uncovering the molecular mechanisms. Nat Rev Neuroscience 2007;8:57–69. [DOI] [PubMed] [Google Scholar]
- 22.Hshieh TT, Fong TG, Marcantonio ER et al. Cholinergic deficiency hypothesis in delirium: A synthesis of current evidence. J Gerontol A Biol Sci Med Sci 2008;63:764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapur S, Remington G, Jones C et al. High levels of dopamine d2 receptor occupancy with low-dose haloperidol treatment: A PET study. Am J Psychiatry 1996;153:948–950. [DOI] [PubMed] [Google Scholar]
- 24.Tauscher J, Tauscher-Wisniewski S, Kasper S. Treatment of patients with delirium. Am J Psychiatry 2000;157:1711. [DOI] [PubMed] [Google Scholar]
- 25.Moots RJ, Al Saffar Z, Hutchinson D et al. Old drug, new tricks: Haloperidol inhibits secretion of proinflammatory cytokines. Ann Rheum Dis 1999; 58:585–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song C, Lin A, Kenis G et al. Immunosuppressive effects of clozapine and haloperidol: Enhanced production of the interleukin-1 receptor antagonist. Schizophr Res 2000;42:157–164. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, LI HL, Wang DX et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: A randomized controlled trial. Crit Care Med 2012;40:731–739. [DOI] [PubMed] [Google Scholar]
- 28.Kalisvaart KJ, de Jonghe JF, Bogaards MJ et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: A randomized placebo-controlled study. J Am Geriatr Soc 2005;53:1658–1666. [DOI] [PubMed] [Google Scholar]
- 29.Ely EW, Inouye SK, Bernard GR et al. Delirium in mechanically ventilated patients: Validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). J Am Med Assoc 2001;286:2703–2710. [DOI] [PubMed] [Google Scholar]
- 30.Sessler CN, Gosnell MS, Grap MJ et al. The Richmond Agitation–Sedation Scale. Validity and reliability in adult intensive care unit patients. Am J Resp Crit Care Med 2002;166:1338–1344. [DOI] [PubMed] [Google Scholar]
- 31.Trzepacz PT, Mittal D, Torres R et al. Validation of the delirium rating scale-revised-98. J Neuropsychiatry Clin Neurosci 2001;13:229–242. [DOI] [PubMed] [Google Scholar]
- 32.Randolph C, Tierney MC, Mohr E et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsyc 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 33.Charlson ME, Sax FL, MacKenzie CR et al. Assessing illness severity: Does clinical judgment work? J Chronic Dis 1986;39:439–452. [DOI] [PubMed] [Google Scholar]
- 34.Knaus WA, Draper EA, Wagner DP et al. APACHE II: A severity of disease classification system. Crit Care Med 1985;13:818–829. [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitzer RL, Kroenke K, Williams JB et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 37.Stoll C, Kapfhammer HP, Rothenhausler HB et al. Sensitivity and specificity of a screening test to document traumatic experiences and to diagnose post-traumatic stress disorder in ARDS patients after intensive care treatment. Intensive Care Med 1999;25:697–704. [DOI] [PubMed] [Google Scholar]
- 38.Katz S, Ford AB, Moskowitz RW et al. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 39.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 40.Campbell N, Boustani MA, Ayub A et al. Pharmacological management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med 2009;24:848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Page JV, Ely EW, Gates S et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): A randomized double-blind placebo controlled trial. Lancet Resp Med 2013;1:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Boogaard M, Slooter AJC, Brüggemann RJM et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium. The REDUCE randomized clinical trial. JAMA 2018;319:680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1: Supporting Information.