Abstract
Background
Reducing the burden of multidrug-resistant organism (MDRO) colonization and infection among renal transplant recipients (RTRs) may improve patient outcomes. We aimed to assess whether the detection of an MDRO or a comparable antibiotic-susceptible organism (CSO) during the early post-transplant (EPT) period was associated with graft loss and mortality among RTRs.
Methods
We conducted a retrospective cohort study of RTRs transplanted between 2005 and 2021. EPT positivity was defined as a positive bacterial culture within 30 days of transplant. The incidence and prevalence of EPT MDRO detection were calculated. The primary outcome was a composite of 1-year allograft loss or mortality following transplant. Multivariable Cox hazard regression, competing risk, propensity score–weighted sensitivity, and subgroup analyses were performed.
Results
Among 3507 RTRs, the prevalence of EPT MDRO detection was 1.3% (95% CI, 0.91%–1.69%) with an incidence rate per 1000 EPT-days at risk of 0.42 (95% CI, 0.31–0.57). Among RTRs who met survival analysis inclusion criteria (n = 3432), 91% (3138/3432) had no positive EPT cultures and were designated as negative controls, 8% (263/3432) had a CSO detected, and 1% (31/3432) had an MDRO detected in the EPT period. EPT MDRO detection was associated with the composite outcome (adjusted hazard ratio [aHR], 3.29; 95% CI, 1.21–8.92) and death-censored allograft loss (cause-specific aHR, 7.15; 95% CI, 0.92–55.5; subdistribution aHR, 7.15; 95% CI, 0.95–53.7). A similar trend was seen in the subgroup and sensitivity analyses.
Conclusions
MDRO detection during the EPT period was associated with allograft loss, suggesting the need for increased strategies to optimize prevention of MDRO colonization and infection.
Keywords: allograft failure, MDROs, renal transplant
In a retrospective study of 3,507 renal transplant recipients (RTRs) the detection of an MDRO during the early post-transplant (EPT) period was associated with death-censored allograft loss. This suggests the need for increased strategies to optimize prevention of MDROs colonization and infection.
The World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have declared antimicrobial resistance (AMR) a priority public health threat [1, 2]. Therapeutic options for patients with multidrug-resistant organism (MDRO) infections are limited, less efficacious, and more toxic compared with those for susceptible infections. For these reasons, rates of mortality are disproportionately increased when compared with infections caused by susceptible bacteria [3–5]. MDRO colonization, defined as the detection of an organism without signs or symptoms of infection, frequently precedes invasive clinical infection [6–8].
Renal transplant recipients (RTRs) represent a vulnerable population at increased risk of MDRO acquisition and colonization [9]. This is especially true in the early post-transplant (EPT) period due to prolonged hospitalization, the need for invasive procedures and indwelling devices, exposure to broad-spectrum antibiotics, and a higher net state of immunosuppression [9–12]. Post-transplant infection, including MDRO infection, is a major cause of mortality and has been linked to allograft loss among RTRs [3, 13, 14].
Understanding the impact of MDRO colonization and infection on RTR patient mortality and allograft function is paramount to mitigating these deleterious outcomes. Prior studies have been limited by lack of control groups, small sample sizes, a focus on 1 taxon/species of MDROs, or a single infection site (such as the urinary tract) [15–19]. To overcome previous limitations, we utilized a cohort of >4000 RTRs followed from 2005 to 2021 to estimate the prevalence and incidence of EPT MDRO detection among RTRs and compare mortality and allograft failure, among RTRs with EPT MDRO detection compared with a control group of RTRs.
METHODS
Study Setting, Design, and Data Sources
The Emory Transplant Center performs ∼250 adult renal transplants annually and provides ongoing care for >4000 renal transplant patients. We performed a retrospective cohort study of RTRs in the Emory Renal Transplant database [20] between 2005 and 2021. Data are prospectively entered into the database directly from Emory's electronic medical records. The study was approved by the Emory University Institutional Review Board.
Study Participants
Adult RTRs undergoing their first episode of renal transplantation (including kidney-pancreas and kidney-liver) at Emory were included. RTRs <18 years old and/or who had their renal transplant performed outside of Emory University Hospital or who were undergoing a repeat renal transplant were excluded.
Exposure, Outcomes, and Covariate Definitions
The EPT period was defined as 30 days from transplant. The exposure variable was defined as an EPT bacterial culture positive for a target MDRO or a comparative antibiotic-susceptible organism (CSO). EPT culture positivity was defined as a positive culture (irrespective of culture specimen site) within 30 days of renal transplant. MDRO selection was guided by the CDC and WHO MDRO priority lists and the American Society of Transplantation (AST) Infectious Diseases Community Guidelines [1, 2, 9, 21, 22]. These included methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), extended-spectrum cephalosporin-resistant Enterobacterales (ESCRE), carbapenem-resistant Enterobacterales (CRE), carbapenem-resistant Acinetobacter baumanii complex (CRAB), and carbapenem-resistant Pseudomonas aeruginosa (CRPA). CSOs included methicillin-susceptible Staphylococcus aureus (MSSA), vancomycin-susceptible Enterococcus (VSE), extended-spectrum cephalosporin-susceptible Enterobacterales (ESCSE), carbapenem-susceptible P. aeruginosa (CSPA), and carbapenem-susceptible A. baumanii complex (CSAB) (Supplementary Table 1). Urine cultures obtained within the EPT period are obtained for cause as determined by the treating team and not in a routine systematic fashion. No systemic MDRO rectal screening of RTRs was performed during the study period. Antimicrobial susceptibility testing was performed at the Emory University Hospital microbiology laboratory as part of routine clinical care on the MicroScan (Beckman Coulter, Brea, CA, USA) initially and then on the Vitek 2 (bioMérieux, Durham, NC, USA) from 2017 onwards. MDROs and CSOs were defined as per Clinical and Laboratory Standards Institute (CLSI) breakpoints each year [23].
The primary outcome was a combined composite outcome of 1-year allograft loss or mortality following renal transplant. One-year allograft loss was defined as renal replacement therapy for >3 months without subsequent improvement or relisting for transplant within 1 year of renal transplant, and 1-year post-transplant mortality was defined as death within 1 year of renal transplant.
Analyzed covariates included sex, age at time of renal transplant, race (used as a surrogate for social–demographic effects rather than a biological variable), induction immunosuppressive regimen, etiology of end-stage renal disease (ESRD), type of renal transplant donor (living vs cadaveric donor), pretransplant positive MDRO culture status (any), dual solid organ transplant status (kidney-pancreas or kidney-liver), year of transplantation, CMV serostatus of donor and recipient, and biopsy-proven acute graft rejection. Antibiotic and nephrotoxic drug exposure were not included as covariates. All variables included were obtained from the electronic medical record (EMR).
Exclusion Criteria
RTRs were excluded from the survival analysis if they had a positive EPT culture that did not meet MDRO/CSO criteria or had key susceptibility results missing (eg, vancomycin for Enterococcus spp.). To avoid misclassification bias, RTRs who had both MDROs and CSOs on culture in the EPT period were also excluded.
Statistical Analysis
The prevalence of EPT MDRO detection was estimated using all eligible RTRs as the denominator. The incidence rate of EPT MDRO detection was calculated per 1000 EPT-days at risk (Supplementary Data). Differences in incidence rates pre- and postchange in CLSI breakpoint were assessed using the Wilcoxon signed-rank test. The overall change in incidence over time was assessed using a negative binomial model with adjustment for pre- and postchange in CLSI breakpoint, and model fit was assessed using a log likelihood test (compared with the model without breakpoint change).
To examine the impact of post-transplant MDRO detection on post-transplant outcomes, we examined the hazards of experiencing the primary outcome among 3 mutually exclusive exposure categories: (i) RTRs with EPT MDRO detection, (ii) RTRs with EPT CSO detection, (iii) RTRs with no positive cultures during the EPT period. RTRs with EPT MDRO and CSO detection were compared with a control group of RTRs with no positive cultures (negative control group). Cohort characteristics were described by overall and EPT culture positivity status. Among RTRs with multiple MDROs or CSO detected in the EPT period, the earliest post-transplant organism episode was included. A Kaplan-Meier survival analysis was performed, and differences between survival curves were assessed using the log-rank test. Multivariable Cox proportional hazard regression modeling was performed, with adjustment covariates selection informed by literature review, directed acyclic graph (Supplementary Figure 1), and bivariate analysis. None of the variables violated the proportional hazard assumption by graphical and Schoenfeld residual statistical methods. Given the competing risks of risk of death on allograft loss, a competing risk analysis was performed by fitting a cause-specific and subdistribution hazard model for both outcomes (mortality and allograft loss). The competing cumulative incidences of each outcome (mortality and allograft loss) by exposure group were compared using Gray's test, with the comparator group being the negative control group for both the MDRO and CSO groups. Wilcoxon tests were used to compare these measurements between the treatment groups. Adjusted hazard ratios (aHRs) and corresponding 95% CIs were reported. The following subgroup analyses were performed: (1) among patients with positive urine cultures (urine subgroup), (2) among patients in the post-Enterobacterales breakpoint change period (2011–2021) [24], (3) kidney transplant–only patients (removing those with kidney-pancreas or kidney-liver).
A sensitivity analysis was performed to adjust for the propensity of MDRO acquisition on outcomes. A propensity score (PS)–weighted analysis was performed where a PS was generated based on the probability of each exposure category stratum (Supplementary Data). After PS calculation, 2 methods of weighting that incorporated the PS, inverse probability of treatment weighting (IPTW) and matching weighting (MW), were applied to create weighted cohorts (Supplementary Figure 2) [25]. Weighted multivariable Cox proportional hazard modeling was performed using the MW and IPTW cohorts. Statistical analysis was performed with R using Rstudio, version 2022.12.0.353 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Prevalence and Incidence of Early Post-transplant MDRO Detection
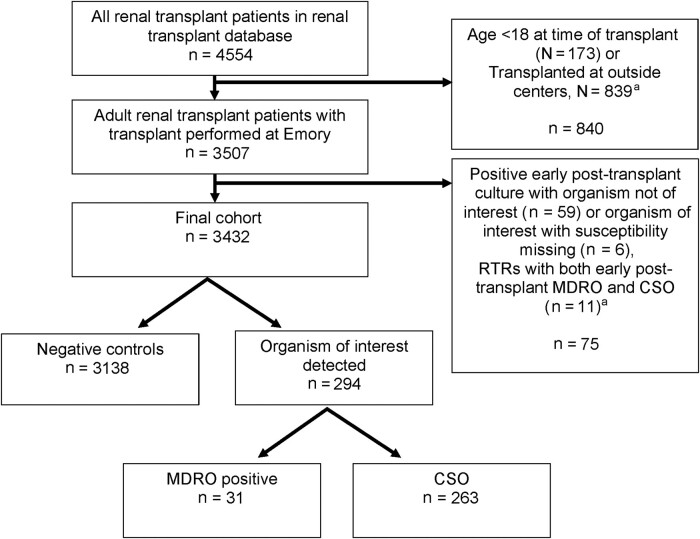
Of the 4554 unique RTRs enrolled in the Emory Renal Transplant database between 2005 and 2021, 3507 met inclusion criteria (Figure 1). Among eligible RTRs (n = 3507), 328 (10.5%) had an EPT-positive culture with any organism. Eighty-three MDROs were detected among 44 unique RTRs, resulting in an EPT MDRO detection prevalence of 1.3% (95% CI, 0.91%–1.69%) (Supplementary Figure 3). ESCRE was the most common MDRO detected (52% [43/83]) followed by MRSA (25% [21/83]), VRE (14% [12/83]), CRPA (5% [4/83]), and CRAB (4% [3/82]). MDROs were most frequently detected in urinary tract samples (48% [40/83]), followed by blood (23% [19/83]), intra-abdominal specimens (18% [15/83]), respiratory tract samples (4% [3/83]), other invasive sites (4% [3/83]), and superficial wounds (2% [2/83]).
Figure 1.
Study flow diagram. aGroups not mutually exclusive. Abbreviations: CSO, comparative antibiotic-susceptible organism; MDRO, multidrug-resistant organism; RTR, renal transplant recipient.
The incidence rate of EPT MDRO detection during the study period was 0.42 (95% CI, 0.31–0.57) per 1000 EPT-days at risk. The incidence during the pre-Enterobacterales cephalosporin and carbapenem breakpoint change period was 0.33 (95% CI, 0.14–0.71), which was similar to the incidence during the postbreakpoint change period (0.44; 95% CI, 0.31–0.61; P = .79). There was no association with time (year) in a negative binomial model adjusted for breakpoint change (P > .05) (Supplementary Table 2; Supplementary Figure 4).
Impact of Early Post-transplant MDRO Detection on RTR Outcomes
Among eligible RTRs (n = 3507), 75 (2%, 75/3507) were excluded from survival analysis (Figure 1). Among RTRs who met survival analysis inclusion criteria (n = 3432), 91% (3138/3432) had no positive EPT cultures and were designated as negative controls, 8% (263/3432) had a CSO detected, and 1% (31/3432) had an MDRO detected in the EPT period (Figure 1, Table 1). Compared with negative controls, RTRs with positive EPT cultures were older (MDRO: median age [interquartile range {IQR}], 55 [43–64] years; CSO: median [IQR], 53 [43–64] years; negative controls: median [IQR], 50 [40–60] years), had a higher proportion of females (MDRO: 55% [17/31]; CSO: 62% [162/263]; negative controls: 41% [1274/3138]), had diabetes mellitus as the primary etiology of their ESRD (MDRO: 32% [10/31]; CSO: 38% [99/263]; negative controls: 30% [945/3138]), were dual organ transplant recipients (MDRO: 13% [4/31]; CSO: 9% [23/263]; negative controls: 7% [204/3138]), were deceased donor recipients (MDRO: 81% [25/31]; CSO: 76% [200/263]; negative controls: 67% [2107/3138]), had pretransplant MDRO detection (MDRO: 7% [2/31]; CSO: 3% [7/263]; negative controls: 2% [5/3138]), were on dialysis longer (MDRO: median [IQR], 7 [3.8–10.3] years; CSO: median [IQR], 4.7 [2.4–7.4] years; negative controls: median [IQR], 4.4 [2.2–7.3] years), and had a longer transplant admission length of stay (LOS; MDRO: median [IQR], 7 [4–15] days; CSO: median [IQR], 5 [4–8] days; negative controls: 4 [3–6] days). Remaining demographic and transplant-related characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics of Renal Transplant Recipients by Early Post-transplant Positive Culture Status, 2005–2021 (n = 3432)
| Variables | Study Population, n = 3432 | Negative Controls, n = 3138 | Early Post-transplant Positive Culture, n = 294 | |
|---|---|---|---|---|
| CSO n = 263 | MDRO, n = 31 | |||
| Age, median [IQR], y | 51 [40–60] | 50 [40–60] | 53 [43–63] | 55 [46–63] |
| Sex, No. (%) | ||||
| Female | 1453 (42) | 1274 (41) | 162 (62) | 17 (55) |
| Race, No. (%) | ||||
| Black | 1749 (51) | 1607 (51) | 128 (49) | 14 (45) |
| White | 1395 (41) | 1271 (41) | 109 (41) | 15 (48) |
| Biracial | 13 (0.4) | 12 (0.4) | 1 (0.4) | 0 (0) |
| Other | 252 (8) | 226 (8) | 4 (11) | 22 (9) |
| Unknown | 134 (4) | 122 (4) | 11 (4) | 1 (3) |
| Primary ESRD etiology, No. (%) | ||||
| Diabetes mellitus | 1054 (31) | 945 (30) | 99 (38) | 10 (32) |
| Hypertension | 858 (25) | 796 (25) | 49 (19) | 13 (42) |
| Glomerular disease | 665 (21) | 611 (21) | 4 (11) | 50 (19) |
| Polycystic kidney disease | 308 (9.0) | 293 (9.3) | 13 (5) | 2 (7) |
| Other | 494 (14) | 443 (14) | 48 (18) | 3 (10) |
| Transplant type, No. (%) | ||||
| Kidney | 3201 (93) | 2934 (93) | 240 (91) | 27 (87) |
| Kidney-pancreas | 229 (6.7) | 202 (6) | 23 (9) | 4 (13) |
| Kidney-liver | 2 (<0.01) | 2 (<0.01) | 0 (0) | 0 (0) |
| Transplant year, No. (%) | ||||
| 2005–2009 | 674 (20) | 620 (20) | 50 (19) | 4 (13) |
| 2010–2015 | 1188 (35) | 1056 (34) | 118 (45) | 14 (45) |
| 2016–2020 | 1570 (46) | 1462 (47) | 95 (36) | 13 (42) |
| Donor type, No. (%) | ||||
| Deceased | 2332 (68) | 2107 (67) | 200 (76) | 25 (81) |
| Living | 1100 (32) | 1031 (33) | 63 (24) | 6 (19) |
| Years on dialysis, median [IQR] | 4.4 [2.2–7.3] | 4.4 [2.2–7.3] | 4.7 [2.4–7.4] | 7.0 [3.8–10.3] |
| Missing, No. (%) | 1021 (29) | 933 (30) | 80 (30) | 8 (26) |
| Immune induction protocol, No. (%) | ||||
| Basiliximab | 2466 (72) | 2256 (72) | 188 (71) | 22 (71) |
| Thymoglobulin | 605 (18) | 541 (17) | 56 (21) | 8 (26) |
| Other | 361 (11) | 341 (11) | 19 (7) | 1 (3) |
| CMV status, No. (%) | ||||
| D-/R- | 402 (12) | 376 (12) | 260 (9) | 3 (10) |
| D-/R+ or D+/R+ | 2454 (72) | 2228 (72) | 201 (77) | 25 (83) |
| D+/R- | 529 (16) | 491 (16) | 36 (14) | 2 (7) |
| Missing | 47 (1) | 43 (1) | 3 (1) | 1 (3) |
| Rejection within first year post-transplant | 441 (13) | 399 (13) | 38 (14) | 4 (13) |
| MDRO detected pretransplant | 67(2) | 58(2) | 7(3) | 2(7) |
| Transplant admission LOS, median [IQR], d | 4.0 [3.0–6.0] | 4.0 [3.0–6.0] | 5.0 [4.0–8.0] | 7.0 [4.0–15.0] |
| Missing, No. (%) | 1 | 1 | 0 | 0 |
Abbreviations: CSO, comparative antibiotic-susceptible organism; D, donor; ESRD, end-stage renal disease; IQR, interquartile range; LOS, length of stay; MDRO, multidrug-resistant organism; R, recipient.
Among RTRs with a positive EPT culture, the median (IQR) number of days between transplant and positive culture was 13 (7–20); this was similar across RTRs, with an MDRO and a CSO (median [IQR], 10 [18–18] days vs 13 [7–20] days; P = .40) detected. The most common organisms detected among RTRs were Enterobacterales (62% [183/294]), followed by Enterococcus spp. (24%, 72/294). The urinary tract (80%, 234/294) was the most common site of EPT-positive cultures, followed by blood (11%, 31/294). Trimethoprim-sulfamethoxazole resistance was common and similar among MDROs and CSOs in terms of which trimethoprim-sulfamethoxazole antimicrobial susceptibility testing is appropriate (59% [16/27] vs 66 [120/181]; P = .50) (Table 2).
Table 2.
Microbiological Characteristics of Renal Transplant Recipients With Positive Early Post-transplant Cultures, 2005–2021 (n = 294)
| Variables | Overall, n = 294 | CSO, n = 263 | MDRO, n = 31 |
|---|---|---|---|
| Species, No. (%)a | |||
| Staphylococcus aureus | 12 (4) | 6 (2) | 6 (19) |
| Enterococcus spp.b | 72 (24) | 67 (25) | 5 (16) |
| Enterobacterales c | 183 (62) | 164 (62) | 19 (61) |
| Pseudomonas aeruginosa | 26 (9) | 26 (10) | 0 (0) |
| Acinetobacter baumannii | 1 (0.3) | 0 (0) | 1 (3) |
| Trimethoprim-sulfamethoxazole resistance | 136 (46) | 120 (66) | 16 (59) |
| Anatomic source, No. (%)d | |||
| Urinary tract | 234 (80) | 214 (81) | 20 (65) |
| Blood/endovascular | 31 (11) | 25 (10) | 6 (19) |
| Respiratory | 9 (3.1) | 9 (3) | 0 (0) |
| Superficial wound | 3 (1.0) | 3 (1) | 0 (0) |
| Intra-abdominal | 9 (3.1) | 5 (2) | 4 (13) |
| Superficial wound | 3 (1) | 3 (1) | 0 (0) |
| Other invasive site | 7 (2) | 6 (2) | 1 (3) |
| Stool | 1 (0.3) | 1 (0.4) | 0 (0) |
| Time between transplant and positive culture, median [IQR], d | 13 [7–20] | 13 [7–20] | 10 [8–18] |
Abbreviations: CSO, comparative antibiotic-susceptible organism; IQR, interquartile range; MDRO, multidrug-resistant organism.
aIf an RTR had multiple MDROs or susceptible organisms detected in the early post-transplant period, only the first organism was recorded.
b Enterococcus spp. group includes Enterococcus faecalis (n = 65), Enterococcus faecium (n = 7).
cEnterobacterales group includes Citrobacter freundii complex (n = 8), Enterobacter cloacae complex (n = 7), Enterobacter spp. (n = 2), Escherichia coli (n = 111), Hafnia alvei (n = 1), Klebsiella aerogenes (n = 3), Klebsiella oxytoca (n = 9), Klebsiella pneumoniae (n = 28), Proteus mirabilis (n = 8), Providencia rettgeri (n = 1), Serratia marcescens (n = 5). None were carbapenem resistant.
dCalculated for bacteria in which trimethoprim-sulfamethoxazole antimicrobial susceptibility testing is appropriate.
Survival Analysis
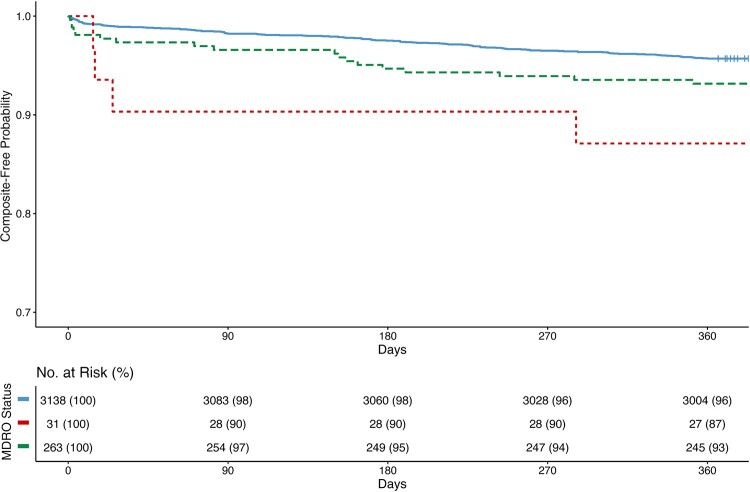
One hundred fifty-seven (5% [157/3432]) RTRs experienced the primary composite outcome. The median (IQR) number of days from transplant to primary composite outcome was 152 (24-235). There was a significant difference between time from transplantation to the composite outcome across the exposure categories (MDROs: median [IQR], 20 [15–90] days; CSO: median [IQR], 116 [8-174] days; negative controls: median [IQR], 155 [32–242] days; log-rank P = .01) (Figure 2). A higher proportion of RTRs in with MDRO EPT detection experienced the primary composite outcome compared with RTRs with CSO EPT detection and negative controls (MDRO: 13% [4/31]; CSO: 8% [18/263]; negative controls: 4% [135/3138]; P = .02). A higher proportion of RTRs with MDRO EPT detection had 1-year allograft failure compared with RTRs with CSO EPT detection and negative controls (10% [3/31] vs 3% [9/263] vs 2% [63/3138]; P = .01). One-year mortality was similar across the exposure categories (3% [1/31] vs 4% vs [11/263] vs 3% [86/3138]) (Supplementary Table 3).
Figure 2.
Kaplan-Meier analysis of composite outcome of renal transplant recipients by early post-transplant positive culture status, 2005–2021 (n = 3432). Kaplan-Meier analysis of composite outcome comparing renal transplant recipients with a multidrug-resistant organism detected on early post-transplant culture (red), a comparative antibiotic-susceptible organism detected on post-transplant culture (green), and negative controls (blue) (B). Time is measured from transplant until event. Log-rank P = .01. Abbreviation: MDRO, multidrug-resistant organism.
The adjusted hazard ratio (aHR) of experiencing the primary composite outcome was higher among RTRs with EPT MDRO detection (aHR, 3.29; 95% CI, 1.21–9.92; P = .02) and EPT CSO detection (aHR, 1.54; 95% CI, 0.94–2.53; P = .09) compared with negative controls (Table 3). Similar trends were seen among RTRs with EPT MDRO detection in the postbreakpoint change subgroup (aHR, 3.46; 95% CI, 1.09–10.9; P = .04)) and kidney transplant–only subgroup (aHR, 2.70; 95% CI, 0.85–8.51; P = .09), but not among the urine-positive subgroup (aHR, 1.30; 95% CI, 0.18–9.39; P = .80). A similar trend was seen in the sensitivity analysis of RTRs with EPT MDRO detection in the MW cohort (aHR, 3.51; 95% CI, 1.26–9.78; P = .02) and IPTW cohort (aHR, 2.53; 95% CI, 0.94–6.83; P = .07) (Supplementary Table 4).
Table 3.
Results of Cox Proportional Hazards Regression Model for Primary Outcome by Early Post-transplant Culture Positivity Status (n = 3432)
| Composite Outcome HR | ||||
|---|---|---|---|---|
| HR (95% CI) | P Value | aHR (95% CI)a | P Value | |
| Negative controls | Ref | - | Ref | - |
| CSO | 1.62 (0.99–2.65) | .05 | 1.54 (0.94–2.53) | .09 |
| MDRO | 3.19 (1.18–8.63) | .02 | 3.29 (1.21–8.92) | .02 |
Abbreviations: aHR, adjusted hazard ratio; CSO, comparative antibiotic-susceptible organism; ESRD, end-stage renal disease; HR, hazard ratio; MDRO, multidrug-resistant organism.
aAdjusted for age, sex, year of transplant, 1-year post-transplant, deceased donor status, diabetes as the primary etiology of ESRD, and category of induction therapy.
Competing Risk Analysis
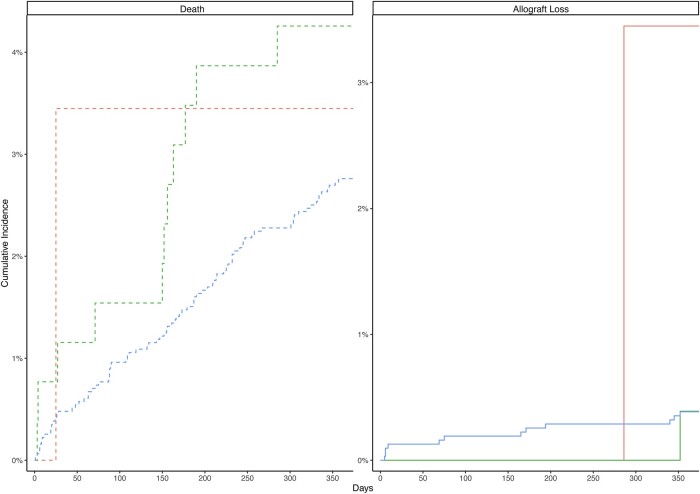
Across the exposure categories, the cumulative incidence of 1-year allograft loss was significantly different across exposure groups (P = .04) (Figure 3; Supplementary Figure 5). When accounting for death as a competing risk, an increased adjusted cause-specific hazard (aHR, 7.15; 95% CI, 0.92–55.5; P = .06) and subdistribution hazard (aHR, 7.15; 95% CI, 0.95–53.7; P = .06) of 1-year allograft loss was observed among RTRs with EPT MDRO detection compared with negative control RTRs. This finding was not seen among RTRs with EPT CSO detection (cause-specific aHR, 0.84; 95% CI, 0.11–6.54; P = .9; subdistribution aHR, 0.84; 95% CI, 0.12–6.03; P = .9) (Table 4).
Figure 3.
Cumulative incidence curves for 1-year mortality and 1-year allograft loss of renal transplant recipients by early post-transplant positive culture status, 2005–2021 (n = 3432). Cumulative incidence curves for 1-year mortality (left panel, dashed line) and 1-year allograft loss (right panel, solid line) comparing renal transplant recipients with a multidrug-resistant organism detected on early post-transplant culture (red), a comparative antibiotic-susceptible organism detected on post-transplant culture (green), and negative controls (blue). Time is measured from transplant until event.
Table 4.
Competing Risk Analysis Results Stratified by Early Post-transplant Positive Culture Status (n = 3432)
| Cause-Specific | Subdistribution | |||||
|---|---|---|---|---|---|---|
| One-Year Mortality | One-Year Mortality | |||||
| HR (95% CI) | aHRa (95% CI) | P Value* | HR (95% CI) | aHRa (95% CI) | P Value* | |
| Negative controls | Ref | Ref | - | Ref | Ref | - |
| CSO | 1.56 (0.83–2.92) | 1.38 (0.73–2.59) | .3 | 1.56 (0.83–2.93) | 1.38 (0.72–2.64) | .30 |
| MDRO | 1.26 (0.18–9.08) | 1.34 (0.19–9.66) | .8 | 1.26 (0.17–9.26) | 1.34 (0.18–10.1) | .80 |
| … | Cause-Specific | Subdistribution | ||||
|---|---|---|---|---|---|---|
| … | One-Year Allograft Loss | One-Year Allograft Loss | ||||
| … | HR (95% CI) | aHRa (95% CI) | P Value | HR (95% CI) | aHRa (95% CI) | P Value |
| Negative controls | Ref | Ref | - | Ref | Ref | … |
| CSO | 1.02 (0.13–7.82) | 0.84 (0.11–6.54) | .9 | 1.02 (0.13–7.79) | 0.84 (0.12–6.03) | .9 |
| MDRO | 9.41 (1.16 68.8) | 7.15 (0.92–55.5) | .06 | 8.95 (1.18–67.9) | 7.15 (0.95–53.7) | .06 |
Abbreviations: aHR, adjusted hazard ratio; CSO, comparative antibiotic-susceptible organism; ESRD, end-stage renal disease; HR, hazard ratio; MDRO, multidrug-resistant organism.
aAdjusted for age, sex, year of transplant, deceased donor status, diabetes as the primary etiology of ESRD, and category of induction therapy.
* P value of adjusted model.
Similar results were seen among RTRs with EPT MDRO detection in the postbreakpoint change subgroup (cause-specific aHR, 9.98; 95% CI, 1.26–79.2; P = .03), the urine-positive subgroup (cause-specific aHR, 10.1; 95% CI, 1.28–79.9; P = .03), the kidney transplant–only subgroup (aHR, 7.44; 95% CI, 0.95–58.3; P = .06), RTRs in the MW cohort (cause-specific aHR, 15.1; 95% CI, 1.61–142.00; P = .02), and RTRs in the IPTW cohort (cause-specific aHR, 7.18; 95% CI, 0.97–53.3; P = .05) (Supplementary Table 5).
DISCUSSION
Our study of >3500 RTRs over a 16-year time period represents one of the largest studies to estimate the burden of MDRO detection during this critical EPT period and to examine MDRO detection the association of MDRO EPT detection with key outcomes. We found the incidence and prevalence of EPT MDRO detection to be low. However, MDRO detection during the EPT period was significantly associated with an increased hazard of 1-year mortality or allograft loss (the composite primary outcome) and of death-censored allograft loss, adjusting for important variables when compared with negative control RTRs. These findings were robust across the various subgroup and PS-weighted sensitivity analyses.
The first 30 days in the post-transplant period represent a time when RTRs are most vulnerable to nosocomial MDRO infection [26–28]. We found MDRO culture positivity during this period predominated by uropathogenic bacteria. Our findings are in concordance with the epidemiology described in other large cohort studies [27, 29] and reflects the practice of frequent urine sampling [30]. Consistent with prior literature, RTRs with EPT MDROs were more frequently of older age, female, diabetic, recipients of a kidney from a deceased donor, and had a longer transplant admission length of stay [31, 32]. While our prevalence and incidence of MDRO detection were low, these findings likely represent the tip of the iceberg, as clinical culture positivity underestimates rates of MDRO colonization compared with active surveillance [33]. Hence, further prospective active surveillance studies to determine the rates of MDRO colonization among RTRs are needed [9].
We found that EPT MDRO detection resulted in an increased hazard of the composite outcome of 1-year death or allograft loss, which was driven by an increase in death-censored allograft loss. A similar signal was seen among RTRs with a CSO detected. Prior studies have previously demonstrated poor outcomes in RTRs with MDRO infections [4]. Our study builds on previous findings by examining the specific impact of MDRO detection, regardless of symptoms (ie, inclusive of those with MDRO colonization), among a large cohort during the critical EPT period [27]. Our cohort was primarily comprised of RTRs with positive urinary tract cultures. Among this subgroup, we found an increased hazard of death-censored allograft loss but not death, likely due to the removal of those with bloodstream infections. Bacterial invasion of the urinary tract in RTRs is hypothesized to lead to an inflammatory response and cytokine activation, contributing to allograft dysfunction [34]. The association of allograft loss and urinary tract infections (UTIs) and/or asymptomatic bacteriuria (ASB) in the EPT remains undetermined [14]. Most prior studies included patients with UTI/ASB in the post–early transplant (after 30 days) period or had a wide inclusion period [30, 35]. The lack of association in the CSO group suggests the additive impact of antimicrobial resistance on RTR outcomes [4, 5]. This may be due to different treatment regimens of RTRs with a MDRO vs CSO detected. Current AST recommendations do not endorse the routine treatment of ASB [30]. Multicenter studies using standardized definitions to parse the impact of ASB and UTI on graft function and to guide management are required. In such studies, stratification based on susceptibility profile or adjusting for inappropriate empiric therapy will be helpful to assess the impact of resistance on outcomes [36].
Increased stewardship and infection prevention–based interventions to decrease MDRO acquisition in ESRD and RTRs are of importance. These include both system- and patient-level interventions such as limiting the duration of urinary catheters and stents, avoiding broad-spectrum nephrotoxic agents when possible, and vigilance in standard infection prevention practices [37–39]. Pretransplant MDRO infection/colonization has been associated with high rates of EPT MDRO invasive infection [3, 40, 41]. If our findings are replicated in prospective studies, active surveillance of RTRs for MDROs before transplantation and decolonization using novel techniques such as microbiome therapeutics may be considered in the future [42, 43]. We recently demonstrated the safety and efficacy of fecal microbiota transplantation (FMT) in a phase 1 randomized controlled trial of FMT administered for MDRO decolonization among RTRs [44]. Moreover, in a post hoc analysis, the FMT-treated participants in that trial had a longer time to recurrent MDRO infection compared with matched RTRs who met FMT eligibility criteria but were not treated with FMT.
While our study builds on the limitations of prior studies in terms of sample size, the expanded inclusion of multiple MDROs, and time periods analyzed, some key limitations exist. First, our study is an observational study with relatively infrequent MDRO positivity, and thus subject to residual confounding. While we attempted to account for confounding by multivariable adjustment and PS-weighted sensitivity analysis, important potential confounders were not available in the data set. Of greatest importance is the lack of antibiotic exposure data in the EPT period as these may have driven the difference in outcomes seen among the exposure groups through inappropriate empiric therapy or increased exposure to nephrotoxic agents [36]. Other important variables that were unavailable include severity of illness, intensive care unit admission, timing of ureteral stent removal, postoperative complications, and intraoperative variables such as cold ischemic time and need for surgical re-exploration. Second, we were unable to use raw minimal inhibitory concentration data for our classification of MDROs, which may lead to misclassification bias due to changes in MDRO definitions over time. To account for this and for clinical practice changes over time, we included transplantation year as an adjustment variable in the model and in the PS creation. Moreover, the results of the subgroup analysis of the postbreakpoint change cohort were similar to our primary findings. Third, like prior studies [39, 45], our case definition was based on culture positivity in the absence of symptoms or clinical indicators of infection. While this may be a reflection of severity and/or chronicity of underlying illness and could be considered a surrogate for health care exposure, we believe that culture positivity (colonization) represents an important dysbiotic phenotype associated with poor outcomes and subsequent invasive infections [40–42, 46]. Fourth, our MDRO definitions are not inclusive of all clinically relevant MDRO phenotypes such as carbapenem-susceptible multidrug-resistant Pseudomonas and other difficult-to-treat resistant phenotypes [47, 48]. Finally, as center-level characteristics are associated with RTR outcomes, our findings in a single center may not be generalizable to other centers and require further validation in multicenter studies [49].
Overall, our findings reveal that the acquisition or presence of MDROs in this early post-transplant period, while infrequent, was associated with the study primary composite outcome. These results underline the need for continued studies to elucidate causal pathways and confirm our findings. If replicated, these findings call for increased stewardship and infection prevention efforts in this vulnerable population.
Supplementary Material
Acknowledgments
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Patient consent. The design of the work has been approved by local ethical committees. The work conforms to standards currently applied in the country of origin and includes the name of the authorizing body.
Financial support. A.B. was supported in part by an Antibacterial Resistance Leadership Group Early Faculty Seedling Award (National Institute of Allergy and Infectious Diseases UM1AI104681), and M.H.W. was supported in part by K23AI144036.
Contributor Information
Ahmed Babiker, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Geeta Karadkhele, Emory Transplant Center and Department of Surgery, Emory University School of Medicine, Atlanta, Georgia, USA.
Andrei Bombin, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Rockford Watkins, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Chad Robichaux, Department of Biomedical Informatics, Emory University School of Medicine, Atlanta, Georgia, USA.
Gillian Smith, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Georgia Emerging Infections Program, Atlanta, Georgia, USA; Atlanta Veterans Affairs Medical Center, Atlanta, Georgia, USA.
Vivek B Beechar, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Danielle B Steed, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Jesse T Jacob, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Timothy D Read, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Sarah Satola, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Christian P Larsen, Emory Transplant Center and Department of Surgery, Emory University School of Medicine, Atlanta, Georgia, USA.
Colleen S Kraft, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Stephanie M Pouch, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Michael H Woodworth, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 2. World Health Organization . Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discover, and Development of New Antibiotics. World Health Organization; 2017. [Google Scholar]
- 3. Pouch SM, Kubin CJ, Satlin MJ, et al. Epidemiology and outcomes of carbapenem-resistant Klebsiella pneumoniae bacteriuria in kidney transplant recipients. Transpl Infect Dis 2015; 17:800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anesi JA, Lautenbach E, Thom KA, et al. Clinical outcomes and risk factors for carbapenem-resistant Enterobacterales bloodstream infection in solid organ transplant recipients. Transplantation 2023; 107:254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murray CJL, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399:629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isendahl J, Giske CG, Hammar U, et al. Temporal dynamics and risk factors for bloodstream infection with extended-spectrum β-lactamase-producing bacteria in previously-colonized individuals: national population-based cohort study. Clin Infect Dis 2019; 68:641–9. [DOI] [PubMed] [Google Scholar]
- 7. Howard-Anderson JR, Bower CW, Smith G, et al. Carbapenem-resistant Enterobacterales bacteriuria and subsequent bacteremia: a population-based study. Infect Control Hosp Epidemiol 2021; 42:962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong Nguyen M, Shields RK, Chen L, et al. Molecular epidemiology, natural history, and long-term outcomes of multidrug-resistant Enterobacterales colonization and infections among solid organ transplant recipients. Clin Infect Dis 2021; 74:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pouch SM, Patel G. Multidrug-resistant gram-negative bacterial infections in solid organ transplant recipients—guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13594. [DOI] [PubMed] [Google Scholar]
- 10. Rodríguez-Baño J, Picón E, Gijón P, et al. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli. J Clin Microbiol 2010; 48:1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pouch SM, Patel G. Multidrug-resistant gram-negative bacterial infections in solid organ transplant recipients-guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13594. [DOI] [PubMed] [Google Scholar]
- 12. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007; 357:2601–14. [DOI] [PubMed] [Google Scholar]
- 13. Almond PS, Matas A, Gillingham K, et al. Risk factors for chronic rejection in renal allograft recipients. Transplantation 1993; 55:752–6; discussion 756–757. [DOI] [PubMed] [Google Scholar]
- 14. Hollyer I, Ison MG. The challenge of urinary tract infections in renal transplant recipients. Transpl Infect Dis 2018; 20:e12828. [DOI] [PubMed] [Google Scholar]
- 15. Taglietti F, Di Bella S, Galati V, Topino S, Iappelli M, Petrosillo N. Carbapenemase-producing Klebsiella pneumoniae-related mortality among solid organ-transplanted patients: do we know enough? Transpl Infect Dis 2013; 15:E164–5. [DOI] [PubMed] [Google Scholar]
- 16. Qiao B, Wu J, Wan Q, Zhang S, Ye Q. Factors influencing mortality in abdominal solid organ transplant recipients with multidrug-resistant gram-negative bacteremia. BMC Infect Dis 2017; 17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moreno A, Cervera C, Gavalda J, et al. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant 2007; 7:2579–86. [DOI] [PubMed] [Google Scholar]
- 18. Venditti M, Falcone M, Micozzi A, et al. Staphylococcus aureus bacteremia in patients with hematologic malignancies: a retrospective case-control study. Haematologica 2003; 88:923–30. [PubMed] [Google Scholar]
- 19. Yuan X, Liu T, Wu D, Wan Q. Epidemiology, susceptibility, and risk factors for acquisition of MDR/XDR gram-negative bacteria among kidney transplant recipients with urinary tract infections. Infect Drug Resist 2018; 11:707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karadkhele G, Hogan J, Magua W, et al. CMV high-risk status and posttransplant outcomes in kidney transplant recipients treated with belatacept. Am J Transplant 2021; 21:208–21. [DOI] [PubMed] [Google Scholar]
- 21. Nellore A, Huprikar S. Vancomycin-resistant Enterococcus in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13549. [DOI] [PubMed] [Google Scholar]
- 22. Pereira MR, Rana MM. Methicillin-resistant Staphylococcus aureus in solid organ transplantation—guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13611. [DOI] [PubMed] [Google Scholar]
- 23. Clinical and Laboratory Standards Institute . M100-S27, Performance Standards for Antimicrobial Susceptibility Testing, 27th Informational Supplement. Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 24. Wang P, Hu F, Xiong Z, et al. Susceptibility of extended-spectrum-beta-lactamase-producing Enterobacteriaceae according to the new CLSI breakpoints. J Clin Microbiol 2011; 49:3127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li L, Greene T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat 2013; 9:215–34. [DOI] [PubMed] [Google Scholar]
- 26. Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med 1998; 338:1741–51. [DOI] [PubMed] [Google Scholar]
- 27. San Juan R, Aguado JM, Lumbreras C, et al. Incidence, clinical characteristics and risk factors of late infection in solid organ transplant recipients: data from the RESITRA study group. Am J Transplant 2007; 7:964–71. [DOI] [PubMed] [Google Scholar]
- 28. Lanini S, Costa AN, Puro V, et al. Incidence of carbapenem-resistant gram negatives in Italian transplant recipients: a nationwide surveillance study. PLoS One 2015; 10:e0123706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sommerer C, Schröter I, Gruneberg K, et al. Incidences of infectious events in a renal transplant cohort of the German Center of Infectious Diseases (DZIF). Open Forum Infect Dis 2022; 9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldman JD, Julian K. Urinary tract infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13507. [DOI] [PubMed] [Google Scholar]
- 31. Summers DM, Johnson RJ, Hudson A, Collett D, Watson CJ, Bradley JA. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet 2013; 381:727–34. [DOI] [PubMed] [Google Scholar]
- 32. Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients—an analysis of USRDS data. Am J Transplant 2007; 7:653–61. [DOI] [PubMed] [Google Scholar]
- 33. Smith RM, Lautenbach E, Omulo S, et al. Human colonization with multidrug-resistant organisms: getting to the bottom of antibiotic resistance. Open Forum Infect Dis 2021; 8:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2002; 2:970–4. [DOI] [PubMed] [Google Scholar]
- 35. Origüen J, López-Medrano F, Fernández-Ruiz M, et al. Should asymptomatic bacteriuria be systematically treated in kidney transplant recipients? Results from a randomized controlled trial. Am J Transplant 2016; 16:2943–53. [DOI] [PubMed] [Google Scholar]
- 36. Kadri SS, Lai YL, Warner S, et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis 2021; 21:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Babiker A, Clarke L, Doi Y, Shields RK. Fosfomycin for treatment of multidrug-resistant pathogens causing urinary tract infection: a real-world perspective and review of the literature. Diagn Microbiol Infect Dis 2019; 95:114856. [DOI] [PubMed] [Google Scholar]
- 38. So M, Yang DY, Bell C, Humar A, Morris A, Husain S. Solid organ transplant patients: are there opportunities for antimicrobial stewardship? Clin Transplant 2016; 30:659–68. [DOI] [PubMed] [Google Scholar]
- 39. Babiker A, Clarke LG, Saul M, et al. Changing epidemiology and decreased mortality associated with carbapenem-resistant gram-negative bacteria, 2000–2017. Clin Infect Dis 2021; 73:e4521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Macesic N, Gomez-Simmonds A, Sullivan SB, et al. Genomic surveillance reveals diversity of multidrug-resistant organism colonization and infection: a prospective cohort study in liver transplant recipients. Clin Infect Dis 2018; 67:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taimur S, Pouch SM, Zubizarreta N, et al. Impact of pre-transplant carbapenem-resistant Enterobacterales colonization and/or infection on solid organ transplant outcomes. Clin Transplant 2021; 35:e14239. [DOI] [PubMed] [Google Scholar]
- 42. Woodworth MH, Hayden MK, Young VB, Kwon JH. The role of fecal microbiota transplantation in reducing intestinal colonization with antibiotic-resistant organisms: the current landscape and future directions. Open Forum Infect Dis 2019; 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Piewngam P, Khongthong S, Roekngam N, et al. Probiotic for pathogen-specific Staphylococcus aureus decolonisation in Thailand: a phase 2, double-blind, randomised, placebo-controlled trial. Lancet Microbe 2023; 4:e75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Woodworth MH, Conrad RE, Haldopoulos M, et al. Fecal microbiota transplantation promotes reduction of antimicrobial resistance by strain replacement. Sci Transl Med 2023; 15:eabo2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jernigan JA, Hatfield KM, Wolford H, et al. Multidrug-resistant bacterial infections in U.S. hospitalized patients, 2012–2017. N Engl J Med 2020; 382:1309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Isendahl J, Giske CG, Hammar U, et al. Temporal dynamics and risk factors for bloodstream infection with extended-spectrum β-lactamase–producing bacteria in previously-colonized individuals: national population-based cohort study. Clin Infect Dis 2018; 68:641–9. [DOI] [PubMed] [Google Scholar]
- 47. Kadri SS, Lai YL, Ricotta EE, et al. External validation of difficult-to-treat resistance prevalence and mortality risk in gram-negative bloodstream infection using electronic health record data from 140 US hospitals. Open Forum Infect Dis 2019; 6:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 49. Tsampalieros A, Knoll GA, Fergusson N, Bennett A, Taljaard M, Fergusson D. Center variation and the effect of center and provider characteristics on clinical outcomes in kidney transplantation: a systematic review of the evidence. Can J Kidney Health Dis 2017; 4:2054358117735523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.