Abstract
The synthesis of cyclin D1 and its assembly with cyclin-dependent kinase 4 (CDK4) to form an active complex is a rate-limiting step in progression through the G1 phase of the cell cycle. Using an activated allele of mitogen-activated protein kinase kinase 1 (MEK1), we show that this kinase plays a significant role in positively regulating the expression of cyclin D1. This was found both in quiescent serum-starved cells and in cells expressing dominant-negative Ras. Despite the observation that cyclin D1 is a target of MEK1, in cycling cells, activated MEK1, but not cyclin D1, is capable of overcoming a G1 arrest induced by Ras inactivation. Either wild-type or catalytically inactive CDK4 cooperates with cyclin D1 in reversing the G1 arrest induced by inhibition of Ras activity. In quiescent NIH 3T3 cells expressing either ectopic cyclin D1 or activated MEK1, cyclin D1 is able to efficiently associate with CDK4; however, the complex is inactive. A significant percentage of the cyclin D1-CDK4 complexes are associated with p27 in serum-starved activated MEK1 or cyclin D1 cell lines. Reduction of p27 levels by expression of antisense p27 allows for S-phase entry from quiescence in NIH 3T3 cells expressing ectopic cyclin D1, but not in parental cells.
Both positive and negative extracellular growth factors exert their influence upon proliferation during the G1 phase of the cell cycle. At a point in late G1, termed the restriction point (R), cells become largely refractory to these factors and once past R are committed to completing the mitotic cycle (62, 87, 97). The action of serum growth factors is mediated, in part, through their activation of receptor tyrosine kinases. Ras, an inner plasma membrane-bound GTPase, plays a significant role in receiving and transducing extracellular signals by functioning as a downstream mediator of several membrane-bound receptor and nonreceptor tyrosine kinases (5, 45, 46, 69). Ras influences, and is required for, both the G0/G1 transition and passage through G1 to a point temporally coincident with the restriction point. In certain situations, Ras has been shown to be sufficient for progression from G0 to S phase, as demonstrated by the ability of microinjected activated or wild-type Ras to induce DNA synthesis from quiescence in the absence of serum factors (19, 84). Furthermore, microinjection of murine fibroblasts with anti-Ras neutralizing antibodies prevents efficient serum-induced S-phase entry from a G0 state (16, 52), and expression of a dominant-negative Ras protein (RasN17) inhibits the proliferation of NIH 3T3 and 32D myeloid cells, resulting in a G1 arrest (8, 18, 59).
In terms of nuclear events influencing progression through the G1 phase of the cell cycle, a key role is played by G1 cyclins and their catalytic subunits, the cyclin-dependent kinases (CDKs). The three D-type cyclins associate with and activate CDK4 and CDK6. Cyclin E is required for the activation of CDK2. The synthesis of D-type cyclins, whose expression is rate limiting for G1 progression, is regulated by various mitogens (79, 80). The assembly of D cyclins with their catalytic partners is also a mitogen-regulated process occurring in early to mid-G1 (48, 49). Cyclin D-CDK4/CDK6 complexes promote G1 progression, at least in part, by phosphorylating the retinoblastoma protein, Rb, thereby inactivating its ability to act as a transcriptional repressor in a complex with E2F (74, 80). In turn, the expression of cyclin E, which is also rate limiting for G1 progression, is controlled in part by Rb (22, 26, 57). Cyclin E-CDK2 complexes then participate in the phosphorylation and inactivation of Rb but are also thought to have other critical substrates whose phosphorylation is required for DNA synthesis (11, 34, 44, 58, 71, 78, 98). The phosphorylation of Rb roughly coincides with the passage of cells from serum dependence to serum independence and, like Ras, has been linked to restriction point control (26, 92).
The activity of G1 cyclin-CDK complexes is regulated, in part, by the CDK inhibitors p21 and p27. These CDK inhibitors can directly inhibit the activity of both cyclin D-CDK4/CDK6 and cyclin E-CDK2 holoenzymes or prevent the activation of these kinases by cyclin-dependent kinase-activating kinase (CAK) (28, 32, 66, 82). Indeed, the accumulation of p27 is required for cells to efficiently exit from the cell cycle and enter a quiescent state (12, 72). Conversely, p21 and p27 may have a positive role in G1 progression. p21, and to a lesser extent p27, can promote the assembly and nuclear localization of cyclin D-CDK4 complexes (35). In many but not all cell systems described elsewhere, p27 levels are high and p21 levels are low during quiescence, with mitogenic stimulation leading to a reciprocal reduction in p27 levels and elevation in p21 levels (21, 24, 39, 55, 56). These observations indicate the physiological importance of the connection between the influence of mitogens on the expression of CDK inhibitors and their actions during G1.
The influence of Ras on G1 progression has recently been shown to be mediated through direct effects on the cell cycle machinery. Ras transduces extracellular signals through multiple downstream pathways, and activation of more than one effector pathway is required for efficient mitogenesis (27). In this context, the Ras effector pathway involving the Raf1, mitogen-activated protein kinase kinase 1 (MEK1), and mitogen-activated protein kinase (p42mapk and p44mapk [also known as ERK1 and ERK2, respectively, and hereafter referred to as MAPK]) cascade is best characterized. Each element of this pathway has been shown to participate in G1 progression (7, 14, 33, 61) and to positively regulate cyclin D1 expression (2, 20, 25, 36, 40, 94). The importance of this link between Ras and cyclin D1 is highlighted by a number of observations: (i) the activity of cyclin D1-CDK4 appears to be required only in cells expressing functional Rb (81); (ii) the CDK4/CDK6-specific inhibitor p16 inhibits Ras-induced mitogenesis (75); (iii) the expression of dominant-negative Ras can inhibit the expression of cyclin D1 (2, 65); and (iv) inactivation of Ras activity leading to a G1 arrest is Rb dependent (38, 50, 65). In addition, Ras-mediated signaling has also been suggested to be involved in regulating the expression of CDK inhibitors. Both Ras-dependent and -independent pathways have been suggested to have a role in downregulating the expression of p27 during G1 progression (1, 86, 93). With Raf-nuclear receptor fusions, the acute induction of Raf kinase activity has been shown to induce the expression of p21 (76, 77, 96).
Here we have reassessed the relative contribution of cyclin D1 expression to Ras-induced G1 progression, with particular focus on the Raf/MEK/MAPK pathway. This is motivated by a number of observations. Previously, we have shown that expression of cyclin D1 plus CDK4, achieved by transient transfection, can reverse the G1 cell cycle arrest induced by dominant-negative Ras (65). This result, together with the observation that the Ras/Raf/MEK/MAPK pathway regulates the expression of cyclin D1, might be taken to suggest that this cyclin is the sole critical target of Ras during mitogenic stimulation. However, recent findings question the physiological relevance of such overexpression studies. For example, although p16 can inhibit Ras-induced mitogenesis, Ras plus Myc can drive cells from G0 to S phase in the absence of the activation of cyclin D1-CDK4 and without inducing Rb phosphorylation (38). In addition, microinjection of active cyclin D-CDK4 complexes, or overexpression of cyclin E, can also promote G1 progression in the absence of Rb phosphorylation (37, 43). We have thus also addressed the potential importance of the coordinate regulation of cyclin D1 and p27. This is motivated by the realization that both downregulation of cyclin D1 and elevated expression of p27, two events which can be brought about through the inhibition of Ras activity, are characteristic of quiescent cells. Together with the finding that p27 can inhibit the activity of, yet paradoxically possibly promote the assembly of, cyclin D-CDK4, we have asked whether regulation of the levels of cyclin D1 and p27 is required to maintain a quiescent state.
MATERIALS AND METHODS
Plasmids and antibodies.
The retroviral plasmid encoding activated MEK1 (MEK-E217/E221) (14) was kindly provided by C. Marshall. pCMV5-asp27 (72) for expression of antisense p27 was kindly provided by N. Rivard and J. Pouysségur. pMT-RasN17 (and its empty vector counterpart, pMT-ΔBam), pDCR-HRasV12, pCMV-Rb, pRc/CMV-cyclin D1, and pcDNA3-p16 plasmids encoding dominant-negative Ras, oncogenic Ras, Rb, murine cyclin D1, and p16, respectively, have been described previously (65). pBABE-puro (51) was kindly provided by J. Morgenstern and H. Land. pCMV-CDK4 (91) encoding human CDK4 was kindly provided by E. Harlow. pRc/CMV-CDK4K35M (30) encoding inactive murine CDK4 was kindly provided by C. Sherr. pcDNA3-GFP encoding green fluorescent protein (GFP) was kindly provided by P. Silver. Polyclonal antibody to cyclin D1 was from Upstate Biotechnology Inc. Polyclonal antibody to CDK4 (C-22) was from Santa Cruz Biotechnology Inc. Monoclonal antibodies to p27 (catalog no. K25020) and MEK1 (catalog no. M17020) were from Transduction Laboratories. Monoclonal antibodies to cyclin D2 (DCS-5.2) and cyclin D3 (DCS-22) were from NeoMarkers.
Cell lines.
NIH 3T3 derivatives expressing activated MEK1 (MEK1-E217/E221, MEK-EE) were made with retroviral supernatants prepared from the packaging cell line Bosc23 (64) transfected with pBABE-puro-MEK-EE or pBABE-puro (control). Pooled populations of infected NIH 3T3 cells were generated by selection in puromycin (2 μg/ml; Sigma) for 4 to 6 days. Cyclin D1 derivatives of NIH 3T3 cells and the parental line (70) were kindly provided by M. Roussel and C. Sherr. NIH 3T3 cells and their derivatives were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 5% bovine calf serum (BCS; HyClone), glutamine, penicillin, and streptomycin. Cell lines were rendered quiescent by culture in DMEM–0.25% BCS for 96 h. Rb−/− 3T3 cells were maintained as described previously (65).
Immunoprecipitations and Western blot analysis.
Cells (2 × 105) were washed twice with phosphate-buffered saline (PBS) and then lysed in DIP buffer (50 mM HEPES [pH 7.2], 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 10% glycerol, 0.1% Tween 20 and 1% Triton X-100 containing 1 mM NaF, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 mM sodium orthovanadate, 10 mM β-glycerophosphate, 1 μg of aprotinin per ml, 1 μg of leupeptin per ml) for 30 min on ice. The samples were centrifuged at 16,000 × g, and supernatants were precipitated with appropriate antibodies as indicated in the figure legends. Immune complexes were collected with protein A-Sepharose beads, then washed three times with DIP buffer containing inhibitors, and then suspended in sample buffer. Boiled samples were electrophoresed through a sodium dodecyl sulfate–10 or 12% polyacrylamide gel electrophoresis gel. The gels were then transferred onto polyvinylidene difluoride membranes. After the transfer, the filter was blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TBST), and the filter was incubated at room temperature for 60 min with primary antibodies in TBST. After three washes with TBST, 60 min of incubation with horseradish peroxidase-conjugated secondary antibody (anti-mouse or anti-rabbit), and three more washes with TBST, detection was performed with enhanced chemiluminescence (Amersham). Signal was detected with XAR5 film (Kodak).
CDK4 kinase assays.
CDK4 kinase assays were performed as described elsewhere (48, 54). In time course assays, aliquots of lysates containing equivalent amounts of total protein, 100 μg, determined by the Bradford assay (Bio-Rad), were used for kinase assays. Magnetic sorting was performed by transfecting cells with a plasmid encoding the CD20 surface marker alone with the other indicated plasmids. Forty-two hours after transfection, cells were trypsinized and incubated with monoclonal antibody to CD20 as described elsewhere (65), followed by incubation with magnetic beads covalently attached to sheep anti-mouse immunoglobulin G antibody (Dynabeads; Dynal, Inc.). The CD20-positive transfected cells were then magnetically separated from the untransfected cell population. The sorted cells were then used to perform CDK4 kinase assays.
Cell cycle analysis.
Cell cycle phase distribution analysis of transfected cells was performed as described elsewhere (17, 65). When these assays are performed, titrations with both RasN17- and p16-encoding plasmids are carried out. The data shown are for the plasmid concentrations which result in a maximal increase in the G1 population for a given cell line.
Microinjection.
Microinjection experiments were performed as described previously (65) with the following modifications. Cells to be microinjected were plated on coverslips and made quiescent by serum starvation. The indicated plasmids were injected into the nuclei of starved cells. Approximately 100 to 150 cells were microinjected per experiment. To monitor injected cells, a plasmid, pcDNA3-GFP, encoding GFP was coinjected. At 24 h after injection, the cells were incubated for 12 to 14 h with 5-bromodeoxyuridine (BrdU) at 10 μM. Cells were fixed in 3% paraformaldehyde–2% glucose in PBS and then washed with PBS. A PBS solution containing 0.2% Triton X-100 was used to permeabilize the cells. After being washed, cells were blocked with 0.1% Triton X-100–5% fetal bovine serum in PBS with subsequent washing in 5 mM MgSO4 in PBS. Cells were then incubated for 1 h in 5 mM MgSO4 in PBS containing 100 U of DNase (Boehringer Mannheim) per ml and anti-BrdU monoclonal antibody (unconjugated; Becton Dickinson) at a 1/10 dilution in a humidified chamber at room temperature. Cells were then washed in 0.1% Triton X-100 in PBS and incubated with rhodamine-conjugated goat anti-mouse antibody (Jackson ImmunoResearch Laboratories, Inc.) at a 1/200 dilution for 15 min at room temperature. After being washed with 0.1% Triton X-100 in PBS, nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole), washed, and mounted. Cells were examined by fluorescence microscopy.
RESULTS
Neither ectopic expression of cyclin D1 nor that of activated MEK1 is sufficient to activate CDK4 in quiescent cells.
NIH 3T3 cells expressing ectopic cyclin D1 or constitutively active MEK1 (MEK1-E217/E221 [14], hereafter referred to as MEK-EE) were characterized for the expression of these exogenously expressed proteins (Fig. 1A). As previously reported (70), compared to parental NIH 3T3 cells the cyclin D1 lines expressed approximately five times more cyclin D1 (Fig. 1A; see also Fig. 5A). MEK-EE was expressed at a level two to three times that of the endogenous protein. These lines were used to investigate the early mitogenic activation of cyclin D1-CDK4 complexes.
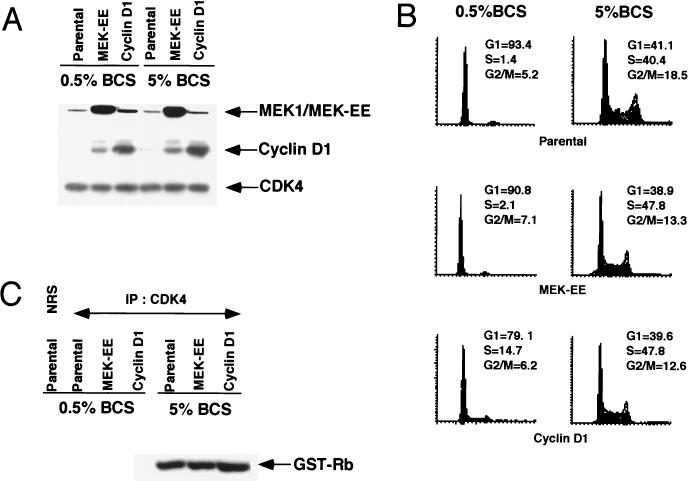
FIG. 1.
Expression of activated MEK1 (MEK-EE) and cyclin D1 in NIH 3T3 cells. (A) Parental NIH 3T3 cells and their cyclin D1 and MEK-EE derivatives were rendered quiescent by serum starvation or maintained in a cycling asynchronous state. Under these two conditions, the levels of endogenous and exogenous MEK1, cyclin D1, and CDK4 were determined by Western blot analysis of whole-cell lysates. (B) Same as panel A, except that the cell cycle distribution of the cells was monitored by fluorescence-activated cell sorting analysis. (C) Lysates were prepared from serum-starved and asynchronous cultures of parental NIH 3T3 cells and the cyclin D1 and MEK-EE derivatives. Immunoprecipitation (IP) with antibody to CDK4 were performed. Immune complexes were assayed for CDK4-associated kinase activity with recombinant glutathione S-transferase (GST)–Rb as substrate. NRS, normal rabbit serum.
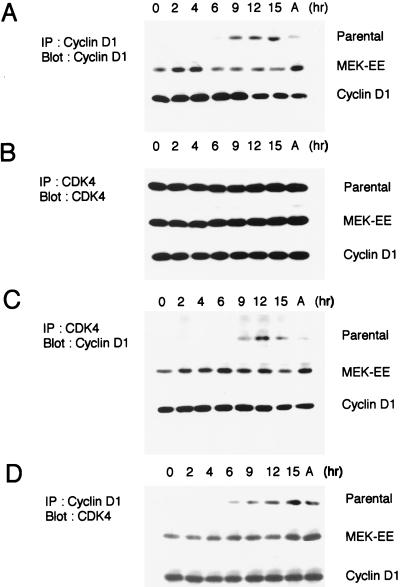
FIG. 5.
Association between cyclin D1 and CDK4. (A) Parental NIH 3T3 cells and their MEK-EE and cyclin D1 derivatives were rendered quiescent by serum starvation. Cells were then restimulated by serum addition. At the indicated times, lysates were prepared and immunoprecipitations (IP) for cyclin D1 were performed. Immune complexes were resolved in a denaturing gel, and Western blot analysis was performed for cyclin D1. Lysates prepared from cycling asynchronous cultures (designated A) were also analyzed. (B) Same as panel A except that CDK4 was analyzed. (C and D) Same as above except that CDK4 immunoprecipitates were analyzed for associated cyclin D1 and cyclin D1 immunoprecipitates were analyzed for associated CDK4. The experiments performed for each of the blots shown in panels A, B, C, and D were performed in parallel. Indicated proteins were detected by enhanced chemiluminescence, and the exposure time in each of the panels is the same; thus, the relative amounts of cyclin D1 associated with CDK4 and vice versa can be compared. The results are representative of at least five independent experiments.
The parental, cyclin D1, and MEK-EE lines were rendered quiescent by serum starvation for a period of 96 h. The increase in the G0/G1 population (50%), under these conditions, was approximately the same for the MEK-EE and parental lines (Fig. 1B). By comparison, the cyclin D1 line did not arrest quite as efficiently (40% increase in G0/G1) as the other lines under these conditions. Cyclin D1 was undetectable in the parental line. By contrast, a significant amount of cyclin D1 was detected in the MEK-EE line, consistent with the role of the Ras/Raf/MEK/MAPK pathway in the positive regulation of cyclin D1 synthesis. The levels of cyclin D1 in the starved MEK-EE lines were comparable to that found in an asynchronous culture of parental or MEK-EE cycling cells (Fig. 1A). The levels of CDK4 were similar in both starved and asynchronous cultures in each of the lines.
To begin to understand why both the cyclin D1 and MEK-EE lines could be arrested in G0/early G1 by serum withdrawal, the ability of CDK4 to support kinase activity under these conditions was determined. In serum-starved cyclin D1 and MEK-EE lines, CDK4 immune complexes did not show associated kinase activity against a recombinant Rb substrate (Fig. 1C). Thus, despite the continued expression of cyclin D1, CDK4 was inactive. These results provide a possible explanation for why neither the ectopic expression of activated MEK1 nor that of cyclin D1 is sufficient to efficiently drive NIH 3T3 cells from a quiescent state into S phase.
Expression of activated MEK1 or cyclin D1 and CDK4, but not cyclin D1 alone, can overcome G1 arrest induced by dominant-negative Ras.
In cells stimulated out of a quiescent state, either serum withdrawal or inhibition of Ras activity in the presence of serum, prior to the restriction point, prevents S-phase entry. Likewise, in an asynchronous population Ras inhibition leads to a G1 arrest. Given that neither the serum-starved cyclin D1 line nor the MEK-EE line showed detectable amounts of CDK4 activity, a characteristic of mid- to late G1 cells, they have likely not progressed beyond the G0/G1 transition under these conditions. This motivated us to next determine if ectopic expression of cyclin D1 or MEK-EE has similar effects on the cell cycle in an asynchronous population of cycling cells with respect to Ras-mediated signaling. Specifically, we compared the effects of serum withdrawal (noted above) and inhibition of Ras activity. We also reinvestigated the requirements of cyclin D1 and CDK4 with respect to their participation in the override of a G1 arrest induced by the expression of dominant-negative Ras.
We have previously demonstrated that expression of dominant-negative Ras, RasN17, results in an Rb-dependent G1 arrest that is mediated, at least in part, by the downregulation of cyclin D1 expression (65). This mutant of Ras has a preferential affinity for GDP and is thought to function by sequestering guanine nucleotide exchange factors, thereby inhibiting endogenous Ras (8, 18, 83, 85), and blocking the activation of MAPKs in response to receptor tyrosine kinases (15, 73, 89, 95). Furthermore, we had shown that transient transfection of both cyclin D1 and CDK4 could override a G1 arrest caused by inhibition of Ras activity (65). Given that transient overexpression studies can sometimes lead to aphysiological results, we determined whether stable expression of cyclin D1 could override a G1 arrest induced by RasN17 and whether there was a requirement for the coexpression of CDK4. This would also allow us to more accurately compare the outcomes when analyzing the cyclin D1 and MEK-EE lines, which, as opposed to transient transfection of cyclin D1, express roughly the same amount of cyclin D1.
Either stable or transient expression of cyclin D1 alone only partially overrode a G1 arrest induced by RasN17 (Fig. 2A), by the same experimental approach we had previously employed (65). Ectopic expression of CDK4 caused a significant reversal in the G1 arrest induced by dominant-negative Ras in the cyclin D1 lines but not in the parental line, consistent with our previous results. Given that the expression of cyclin D1 and not that of CDK4 is thought to be rate limiting for G1 progression and that the expression of the former but not of the latter is inhibited by expression of RasN17, we also investigated the requirement for CDK4 activity in these override experiments. Expression of a catalytically inactive allele of CDK4, CDK4K35M (30), also allowed the cyclin D1 line, but not parental NIH 3T3 cells, to override the RasN17-induced G1 arrest (Fig. 2A). Thus, expression of cyclin D1 alone is not sufficient to override a cell cycle arrest mediated by the inhibition of Ras activity. That there is a requirement for either wild-type or catalytically inactive CDK4 suggests the possibility that titration of CDK inhibitors, an established function of cyclin D-CDK4 complexes (10, 66), participates in the override.
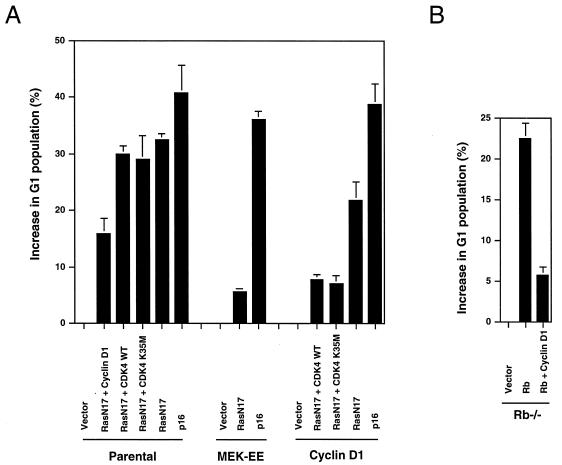
FIG. 2.
Relative ability of stable expression of cyclin D1 or MEK-EE to reverse the cell cycle arrest induced by RasN17. (A) The indicated cell lines were transfected with pMT-ΔBam (control plasmid; similar results were obtained with pcDNA3 and pCMV), pMT-RasN17, pCMV-CDK4, pRc/CMV-CDK4K35M (catalytically inactive CDK4), or pcDNA3-p16 together with a plasmid encoding the CD20 cell surface marker. The transfected population was identified by staining with fluorescein isothiocyanate-conjugated anti-CD20 antibody, and DNA content (2N, 4N) was monitored by staining with propidium iodide. The cell cycle distribution of the transfected population was determined by two-color flow cytometry. The cells were cultured in DMEM–5% BCS throughout the experiment. The absolute changes in the percentages of cells in G1 compared to control transfections are shown with the mean standard error from at least three independent experiments. In control transfected cultures, the G1 population was approximately 40%. WT, wild type. (B) Same as panel A except that Rb−/− 3T3 cells, cultured in DMEM–10% fetal bovine serum, were used. The expression plasmid for Rb was pCMV-Rb. The absolute changes in the percentages of cells in G1 compared to control transfections are shown with the mean standard error from at least three independent experiments.
The findings noted above differ from an Rb-mediated G1 arrest in Rb-deficient fibroblasts. Here ectopic expression of cyclin D1 alone was sufficient to significantly reverse the cell cycle block induced by Rb (Fig. 2B). Together with our previous results, these new data suggest that, though a G1 arrest caused by Ras inactivation is Rb dependent, there are different requirements with respect to cyclin D1 and CDK4 in reversing an Rb- versus a RasN17-induced G1 arrest.
In contrast to the cyclin D1 lines, the MEK-EE lines efficiently reversed a G1 arrest induced by expression of RasN17. Similar results were obtained by transient transfection of plasmids encoding MEK-EE and RasN17 (data not shown). Since the levels of cyclin D1 in both starved and asynchronous MEK-EE cells did not exceed those observed in the cyclin D1 line, the result cannot be entirely attributable to MEK1-mediated regulation of cyclin D1, and additional events play a significant role in the ability of MEK-EE to reverse the G1 arrest induced by dominant-negative Ras. Together with the results noted above, these data suggest that ectopic expression of cyclin D1 and that of MEK-EE have different cellular responses to serum withdrawal and Ras inactivation.
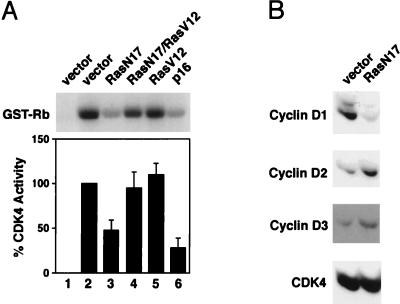
To begin to understand the different abilities of ectopic cyclin D1 and MEK-EE to override a G1 arrest induced by RasN17, we analyzed its effect on CDK4 activity in the various cell lines. Consistent with the notion that a RasN17-induced G1 arrest is caused in part by inhibition of cyclin D1 expression, the expression of RasN17 in cycling NIH 3T3 cells resulted in an inhibition of CDK4 kinase activity (Fig. 3A). This was determined by analyzing the transfected subpopulation after magnetic sorting. The degree of inhibition was greater than 50% compared to approximately 75% for p16-transfected cells. The difference may be in part to due to our observations that the RasN17 expression specifically inhibits the expression of cyclin D1 but does not affect the synthesis of cyclin D2 and cyclin D3 (Fig. 3B). With the available antibodies, we have not been able to accurately assess whether the above-noted difference in CDK4 activity is due to cyclin D2- and cyclin D3-associated kinase activity.
FIG. 3.
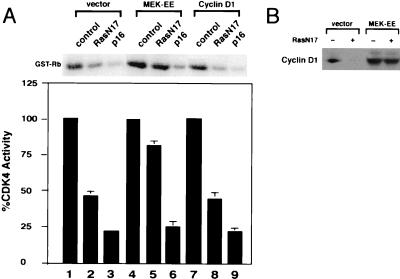
Effect of RasN17 expression on CDK4 activity and D cyclin levels. (A) NIH 3T3 cells, cultured in DMEM–5% BCS, were transfected with plasmids encoding the indicated proteins or vector alone together with a plasmid encoding the surface marker CD20. Forty-two hours later, transfected cells were isolated by magnetic sorting with Dynabeads (see Materials and Methods). Extracts were prepared, normalized for protein content, and subjected to immunoprecipitation for CDK4. CDK4-associated kinase was measured by using glutathione S-transferase (GST)–Rb as a substrate. In lane 1, control normal rabbit serum was used. Lane 2 is the control (pMT-ΔBam, vector-alone transfection; the same results were obtained when pcDNA3 was used as the vector control) set to 100% kinase activity. The bar graph represents the relative kinase activity with the mean standard error from at least three independent experiments. A representative autoradiogram is shown. (B) Same as panel A except that cell extracts were used for Western blot analysis for cyclin D1, cyclin D2, cyclin D3, and CDK4. Indicated proteins were detected by enhanced chemiluminescence.
Expression of MEK-EE largely prevented the downregulation of cyclin D1 and the inhibition of CDK4 kinase activity caused by expression of RasN17 (Fig. 4). Transient transfection of plasmids encoding activated Ras, RasV12 (5), or MEK-EE was also able to prevent the inhibition of CDK4 kinase activity caused by expression of RasN17 (Fig. 3 and data not shown). By contrast, in the cyclin D1 line, CDK4 activity was inhibited to a similar degree as that found for the parental NIH 3T3 line (Fig. 4A). Thus, the relative ability of ectopic cyclin D1 and MEK-EE to override a RasN17-induced G1 arrest correlates, to a significant extent, with their ability to prevent the inhibition of CDK4 activity following Ras inactivation.
FIG. 4.
The effect of activated MEK1 on CDK4 activity and cyclin D1 levels in cells transiently expressing RasN17. (A) NIH 3T3 cells expressing ectopic cyclin D1 or MEK-EE or cells infected with control virus were transiently transfected with control plasmid (pMT-ΔBam), pMT-RasN17, or pcDNA3-p16. The transfected population, cultured in DMEM–5% BCS, was magnetically sorted, and CDK4 kinase assays were performed as described for Fig. 3B. Kinase activity for vector control transfections was set to 100% as indicated. The bar graph represents the relative kinase activity with the mean standard error from at least three independent experiments. A representative autoradiogram is shown. GST, glutathione S-transferase. (B) Same as panel A except that cell lysates were used to assess the relative amounts of cyclin D1 protein present as a function of RasN17 expression by Western blot analysis.
Cyclin D1 and CDK4 complex formation in cell lines that constitutively express cyclin D1 during G1 progression.
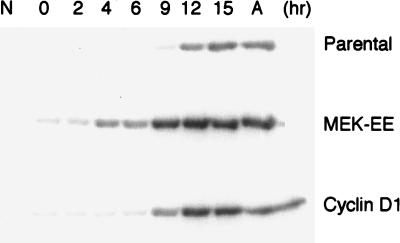
The above results suggest that cyclin D1 and activated MEK1 differ in their abilities to overcome Ras inactivation in cycling cells, while both the cyclin D1 and activated MEK1 lines responded similarly to serum deprivation and subsequently to quiescence; both lines could be arrested in G0/G1 with CDK4 inactive. As a first step in determining why CDK4 might be inactive in the cyclin D1 and MEK-EE lines, we assayed the ability of cyclin D1 and CDK4 to form complexes in serum-starved cells. Cyclin D1 immunoprecipitates were found to contain CDK4, and conversely, CDK4 immune complexes were found to contain cyclin D1. The degree to which complexes between cyclin D1 and CDK4 could be detected in serum-starved cells was comparable to that observed in mid- to late G1 or in cycling cells (Fig. 5). The parental line, used as a control, did not show complex formation between cyclin D1 and CDK4 in starved and early G1 cells, simply due to the lack of cyclin D1 expression under these conditions. Cyclin D1 was detected at 6 to 9 h after serum stimulation of quiescent parental NIH 3T3 cells. At this time in mid- to late G1, complexes could be detected between cyclin D1 and CDK4 (Fig. 5) (48), commensurate with the appearance of kinase activity (see below) (48). The results suggest that the inability to detect CDK4-associated kinase activity in starved cyclin D1 and MEK-EE lines was not due to the inability of cyclin D1 to associate with CDK4.
We also measured CDK4-associated kinase activity during G1 following serum stimulation. In parental NIH 3T3 cells, CDK4 activity was first detected at 9 h after stimulation at the time when complexes between cyclin D1 could be detected (Fig. 5 and 6). The cyclin D1 and MEK-EE lines reproducibly displayed CDK4 activity at 9 and 4 h poststimulation, respectively (Fig. 6).
FIG. 6.
CDK4 kinase activity during G1. Parental NIH 3T3 cells and their MEK-EE and cyclin D1 derivatives were rendered quiescent by serum starvation. Cells were stimulated to reenter the cell cycle by serum addition. At the indicated times, lysates were prepared and normalized for protein content, and immunoprecipitations for CDK4 were performed. Immune complexes were then assayed for CDK4-associated kinase activity with glutathione S-transferase–Rb as substrate. Cycling asynchronous cultures (designated A) were also assayed. Control immunoprecipitations from asynchronous cultures with normal rabbit serum (N) are shown. The results are representative of at least three independent experiments.
p27 is bound to inactive CDK4 in quiescent cyclin D1 and MEK-EE lines.
We next sought to determine why CDK4 was inactive in the serum-starved NIH 3T3 cells expressing cyclin D1 and MEK-EE. Three possibilities were considered. First, under conditions of serum starvation, CDK4 might be rendered inactive due to tyrosine phosphorylation at position 17 (88). Second, cyclin D1 when expressed in serum-starved cells might fail to localize to the nucleus. In some cell systems, cyclin D1 has been reported to be localized to the cytoplasm in starved cells (4). Third, one or more CDK inhibitors might be responsible for maintaining cyclin D1-CDK4 in an inactive state. In terms of the first possibility, we were unable to detect the presence of tyrosine phosphate in CDK4 either in the parental NIH 3T3 cells or in the cyclin D1 or MEK-EE derivatives, with an antiphosphotyrosine antibody. Similarly, we did not detect significant amounts of cyclin D1 in the cytoplasm of starved cyclin D1 or MEK-EE lines by indirect immunofluorescence.
We next investigated the possibility that CDK inhibitors might be involved in the inhibition of CDK4 in the cyclin D1 and MEK-EE lines under conditions of serum starvation. The CDK inhibitor p27 is present at comparatively high levels in starved cells, and the levels decrease upon mitogenic stimulation (56, 82). p27 has the ability to inhibit active cyclin D-CDK4 complexes (67, 90). In addition, an approximately twofold elevation in p27 levels has been reported to inhibit CAK-dependent activation of CDK4 in murine macrophages (28). CAK is active in serum-starved cells (29, 47); thus, p27 association with cyclin D1-CDK4 complexes might prevent the access of CAK to CDK4. Together, these observations suggested that p27 might participate in the inhibition of CDK4 in starved cyclin D1 and MEK-EE lines.
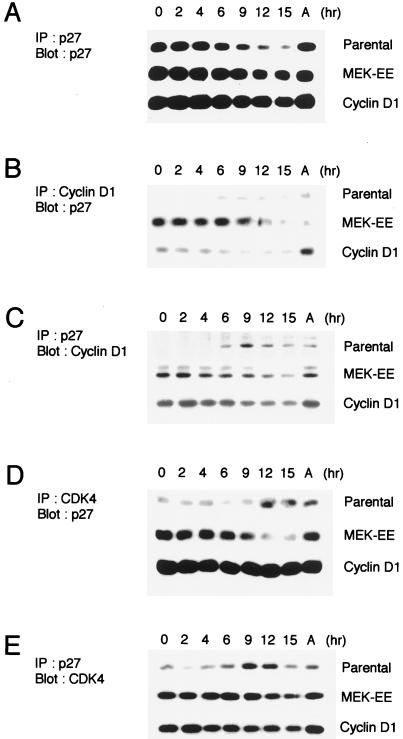
In the parental line, the levels of p27 were found to be highest in starved cells and decreased during passage through G1 (Fig. 7A). In the cyclin D1 and MEK-EE lines, the levels of p27 were approximately twofold higher than that in the parental line, as determined by densitometric scanning, under conditions of serum starvation. For these lines, the levels of p27 decreased during G1 progression, although not as dramatically as in the parental cells (Fig. 7A).
FIG. 7.
Association of p27 with cyclin D1 and CDK4. (A) Parental NIH 3T3 cells and their MEK-EE and cyclin D1 derivatives were rendered quiescent by serum starvation. Cells were then restimulated by serum addition. At the indicated times, lysates were prepared and immunoprecipitations (IP) for p27 were performed. Immune complexes were resolved in a denaturing gel, and Western blot analysis was performed for p27. Lysates prepared from cycling asynchronous cultures (designated A) were also analyzed. (B, C, D, and E) Same as panel A except that antibodies used for immunoprecipitation and Western blot analysis (Blot) were as indicated. The experiments for each of the blots shown in panels A, B, C, D, and E were performed in parallel. Indicated proteins were detected by enhanced chemiluminescence, and the exposure time in each of the panels is the same; thus, a comparison of the relative amounts of p27 associated with cyclin D1 and CDK4 can be made. The results are representative of at least five independent experiments.
We next determined to what extent cyclin D1 and CDK4 could be found associated with p27 in NIH 3T3 cells and their cyclin D1 and MEK-EE derivatives. In both the cyclin D1 and MEK-EE lines, a significant proportion of the detectable cyclin D1 and CDK4 was found associated with p27 under conditions of serum starvation (Fig. 7B, C, D, and E). The blots shown in Fig. 7 were from the same lysates used in Fig. 5; thus, a comparison of the relative amounts of total cyclin D1 and CDK4 associated with p27 can be made. By contrast, in starved parental NIH 3T3 cells, low levels of CDK4 could be found associated with p27 (Fig. 7D and E). The results are consistent with the observation that cyclin D1 and CDK4 can independently associate with p27 in vitro (90). The results shown here suggest that cyclin D1-CDK4 complexes have a higher affinity for p27 than do the monomeric subunits, consistent with the observation made with p21 (9). Thus, ectopic expression of cyclin D1 in quiescent cells results in a significant proportion of p27 being bound to CDK4 in comparison to the parental line.
When the cyclin D1 and MEK-EE lines were released from a G0/G1 state by serum addition, the amount of p27 found associated with cyclin D1 and CDK4 decreased, despite the relatively high levels of p27 in these lines (Fig. 7). In the cyclin D1 line, the increase in CDK4 kinase activity accompanies a reduction in p27 binding to cyclin D1 (compare Fig. 6 and 7B). By contrast, in the parental NIH 3T3 cells, a significant amount of p27 was found associated with cyclin D1-CDK4 complexes in late G1, coincident with the formation of cyclin D1-CDK4 complexes and their activation (Fig. 5 and 6). In Fig. 7, in comparing panels B and C, it is noteworthy that reciprocal immunoprecipitations with cyclin D1 and p27 show differing amounts of coprecipitating protein, i.e., in early G1, more p27 precipitates with cyclin D1 in the MEK-EE lines than in the cyclin D1 lines but comparable amounts of cyclin D1 precipitated with p27 in both lines. Similar observations were noted in comparing panels D and E (Fig. 7). This likely reflects the differing abilities of the antibodies employed to detect pairwise interactions between the proteins. However, over a time course the trends in the relative amounts of associated protein are similar in the interactions measured. Given these observations, it is difficult to firmly establish a causal relationship between the relative amounts of p27 associated with cyclin D1 and CDK4 and the activation of CDK4 in the cyclin D1 and MEK-EE lines.
Antisense p27 cooperates with ectopic cyclin D1 to promote progression from quiescence to S phase.
The above results suggest the possibility that increased expression of p27 found in serum-starved cyclin D1 and MEK-EE lines may be responsible for the lack of detectable CDK4-associated kinase activity under these conditions. Theoretically, lowering p27 levels might lead to the activation of cyclin D1-CDK4 complexes. To test this possibility indirectly, we determined whether microinjection of a plasmid expressing antisense p27 into the cyclin D1 lines, and two clonal derivatives, would allow these cells to progress through G1 and enter S phase.
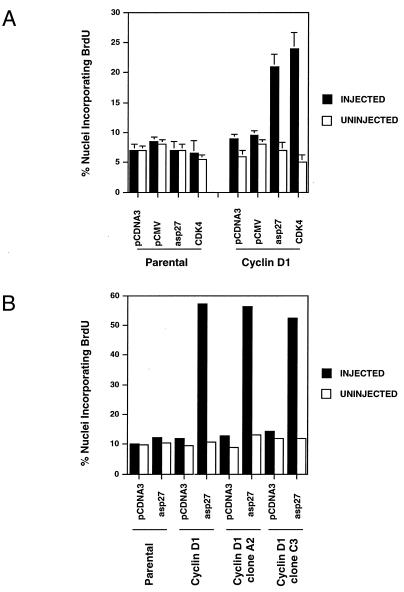
The p27 plasmid construct used in these studies has previously been shown to significantly reduce the levels of p27 in transfected cells (72). DNA replication was monitored by incorporation of BrdU. The MEK-EE lines failed to adhere sufficiently well to the coverslips to be microinjected; thus, only the cyclin D1 and parental lines were used. Microinjection of antisense p27 plasmid led to a significant increase in DNA synthesis in NIH 3T3 cells expressing exogenous cyclin D1 but not in the parental NIH 3T3 cells (Fig. 8).
FIG. 8.
Effect of microinjection of antisense p27-encoding plasmid into quiescent cells on S-phase entry. (A) Vector plasmids, pcDNA3 and pCMV, or plasmids encoding either antisense p27 (pCMV5-asp27) or CDK4 (pCMV-CDK4 together with a plasmid encoding GFP [pcDNA3-GFP]) were microinjected into serum-starved NIH 3T3 cells (Parental) or their cyclin D1 derivative. BrdU was added to the cultures, and cells were subsequently stained for incorporated BrdU as described in Materials and Methods. The percentages of nuclei staining positive for both BrdU and GFP are shown (filled bars). Also shown is the percent BrdU incorporation for uninjected cells (open bars). Shown are the means plus standard errors for three independent experiments. (B) Same as panel A except that two clonal derivatives of the NIH 3T3-cyclin D1 lines, A2 and C3, were used. The results are for one experiment.
The above results suggest that inhibition of p27 synthesis in quiescent cells, containing assembled but inactive cyclin D1-CDK4 complexes, facilitates DNA replication. These observations prompted us to determine if enforced expression of CDK4 might allow the cyclin D1 NIH 3T3 derivatives to proceed from quiescence to S phase in the absence of added serum. In this setting, CDK4 would be predicted to titrate p27, thereby allowing cyclin D1-CDK4 complexes to become active. Indeed, microinjection of a plasmid encoding CDK4 into serum-starved cyclin D1 lines, but not the parental NIH 3T3 cells, resulted in a significant induction of DNA synthesis (Fig. 8A).
DISCUSSION
Progression from G0 through G1 and into S phase is thought to require the integration of extracellular signals that coordinately regulate the cell cycle machinery. Two major pathways identified that participate in this process are the assembly and activation of cyclin D-dependent kinase, and the Ras/Raf/MEK/MAPK kinase cascade. The data presented by our group and by others suggest that these pathways are, in part, linked in that cyclin D1 expression is a downstream effect of MEK1 activation. The expression of the CDK inhibitor p27 is also regulated by extracellular signaling. p27 plays a critical role in entrance into G0, and the data presented here suggest that p27 has a role in maintaining a G0 state. Furthermore, our data suggest that elevated expression of cyclin D1 and reduced expression of p27 may cooperate to deregulate the G0/G1 transition. Activating mutations in Ras, amplification or overexpression of cyclin D1, and low levels of p27 are found in a significant percentage of human cancers (6, 23, 41, 68). These data are consistent with the observation that deregulation of the signal transduction pathways that control progression through G1 is a major target in the development of cancer (63).
We show here that ectopic expression of cyclin D1 alone is insufficient to override a G1 arrest induced by expression of dominant-negative Ras in cycling cells in the presence of serum. Wild-type or catalytically inactive CDK4 is able to cooperate with cyclin D1 to reverse the arrest caused by RasN17 expression. In contrast, cyclin D1 alone can override the G1 arrest induced by Rb. Rb accumulates in its unphosphorylated growth-suppressive form when reintroduced into Rb-negative cells, and ectopic expression of cyclin D1 alone can reverse this effect (data not shown). Likewise, we and others had previously shown that expression of RasN17 also results in the accumulation of unphosphorylated Rb (38, 65). Together, these results suggest that ectopic expression of cyclin D1 can promote the activation of CDK4 in the setting of Rb overexpression in Rb-negative cells but not in cells expressing dominant-negative Ras. Indeed, RasN17 expression in the cyclin D1 derivatives of NIH 3T3 cells results in a significant inhibition of CDK4 activity (Fig. 3). These results suggest that the Ras inactivation brings about other events in addition to the inhibition of cyclin D1 synthesis. Our observation that catalytically inactive CDK4 can cooperate with cyclin D1 to reverse the G1 arrest brought about by Ras inactivation suggests that one or more CDK inhibitors may be involved. Thus far, we have not been able to demonstrate that the inhibition of CDK4 in the cyclin D1 lines is caused by p27 due to our inability to accurately assess the degree to which this or other CDK inhibitors are associated with CDK4 in transfected and sorted cells. Consistent with the idea that CDK inhibitors are involved is the observation that expression of RasN17 in NIH 3T3 cells during mitogenic stimulation out of a quiescent state prevents the downregulation of p27 normally seen during G1 (1, 86).
In contrast to ectopic cyclin D1, expression of an activated form of MEK1 effectively overcame the G1 arrest induced by inactivation of Ras (Fig. 2). Our data suggest that MEK-EE overrides a G1 arrest induced by expression of RasN17 in part by positively regulating cyclin D1 expression (Fig. 4) but also through another pathway(s) that remains to be identified. It has been shown that p27 can be phosphorylated in vitro by MAPK and that this phosphorylation event may play a role in p27 degradation and/or regulate its ability to bind CDK2 (3, 31). These data are consistent with our interpretation that catalytically inactive CDK4 cooperates with cyclin D1 to override a G1 arrest induced by Ras inactivation by titrating CDK inhibitors. Furthermore, the data support our finding that CDK4 is activated earlier after mitogenic stimulation in the MEK-EE lines than in the cyclin D1 lines, although our data do not allow us to directly suggest the involvement of p27.
The results presented here suggest that, in serum-starved derivatives of NIH 3T3 cells, under conditions where cyclin D1 is constitutively expressed, cyclin D1 is associated with CDK4 (Fig. 5). This contrasts with previous observations indicating that ectopic cyclin D3 and CDK4 fail to assemble in serum-starved NIH 3T3 cells (48). NIH 3T3 cells from a variety of sources yielded similar results (data not shown). The results are consistent with a number of observations. First, we have noted that, in serum-starved cells that express cyclin D1, there are elevated p27 levels compared to those in parental cells and significant amounts of cyclin D1 and CDK4 are found associated with p27. Recently, it has been demonstrated that members of the p21 family of CDK inhibitors can function as cyclin D-CDK4 assembly factors (35). Thus, the presence of elevated levels of p27 in the quiescent cyclin D1 and MEK-EE derivatives of NIH 3T3 cells, compared to those in the parental line, may be responsible for promoting the assembly of cyclin D1 and CDK4 in this setting. Second, our results obtained with microinjection of antisense p27 plasmid indirectly support the observation that cyclin D1 and CDK4 can assemble in vivo in serum-starved cells. Expression of antisense p27 was effective in driving the cyclin D1 lines, but not parental NIH 3T3 cells, into S phase. Assuming that the reduction in p27 levels allows CDK4 to become active and in turn promote S-phase entry suggests that cyclin D1 and CDK4 were assembled in serum-starved cells. We do not rule out the possibility that reduction of p27 levels also promotes S-phase entry, in part, through activation of cyclin E-CDK2.
During the completion of this work, Cheng et al. (10) reported that MEK1 plays a significant role in both the synthesis of cyclin D1 and its assembly with CDK4. By contrast, we find that cyclin D1 and CDK4 assemble in the presence or absence of MEK1 activation, although these complexes are inactive in the absence of mitogenic stimulation. There are a number of differences between our experimental approach and those of Cheng et al. that may account for the different experimental outcomes. The most significant difference is that they used cell clones harboring an inducible activated MEK1, whereas we utilized pooled populations that constitutively express activated MEK1. The Ras/Raf/MEK/MAPK pathway is known to be subject to a negative feedback loop limiting the amount of total activated MAPK. In this regard, it has been noted that in NIH 3T3 cells expressing activated MEK1 (the same retroviral vector used in this study) there is only a modest increase in overall MAPK activity (14). It is likely that in an inducible system the degree of transient activation of MAPK is greater than that in a constitutive system. Second, the antibodies used in this study to detect cyclin D1-CDK4 complexes are different from those used by Cheng et al., possibly accounting for a relative difference in the ability to detect complexes. Lastly and independent of studying MEK1, we compared the assembly of cyclin D1 and CDK4 in cyclin D1 lines and MEK-EE lines. Cheng et al. utilized cell lines overexpressing both cyclin D1 and CDK4. There is the possibility that the relative levels of cyclin D1 and CDK4 may influence the ability of CDK inhibitors such as p27 to facilitate their assembly. It is also noteworthy that a requirement for MAPK in the assembly of active CDK4 may not be universal. Work with breast epithelial cells suggests that MAPK activation is not required for the activation of cyclin D1-CDK4 (42).
Our finding that, in serum-starved MEK-EE lines, CDK4 kinase is inactive despite the formation of cyclin D1-CDK4 complexes (Fig. 6 and 7) suggests that an additional mitogenic event is required to activate CDK4 which is not provided by activated MEK1. Given that in the MEK-EE lines the timing of CDK4 activation appeared to precede a significant reduction in p27 associated with cyclin D1 and CDK4, this additional event probably does not solely involve p27 binding. Furthermore, our results suggest that mitogens, possibly operating in a Ras-independent manner, may contribute to CDK4 activation. This follows from the observation that, in the MEK-EE line, CDK4 is inactive following serum starvation but remains active upon introduction of RasN17. Consistent with this interpretation, recently it has been demonstrated that the effect of activated Ras or MEK1 on the cell cycle machinery and G1 progression can be altered by extracellular signaling (60).
In human diploid fibroblasts, expression of antisense p21 has been reported to allow cells to enter S phase from a quiescent state (53). Consistent with the possible requirement for cyclin D1 to achieve this effect, in this study cyclin D1 was reported to be present in quiescent human fibroblasts, in contrast to the situation in quiescent NIH 3T3 cells. Similar to our results obtained with p27, in the cyclin D1 and MEK-EE lines, both cyclin D1 and CDK4 were found associated with p21 in serum-starved cells (data not shown). In the parental line, p21 was found associated with cyclin D1 only at times when complexes between cyclin D1 and CDK4 could be detected (data not shown). Whether expression of antisense p21 will induce S-phase entry in NIH 3T3 cells expressing ectopic cyclin D1 remains to be determined. Together, these data suggest that both p27 and p21 play an active role in maintaining a quiescent state. In addition, our data suggest that downregulation of cyclin D1 expression participates in maintaining a state of quiescence.
CDK4 normally becomes activated in mid-G1 and is thought to promote DNA synthesis by phosphorylating and inactivating Rb at this time. Our data suggest, but do not prove, that activation of cyclin D1-CDK4 in serum-starved cells, by lowering p27 levels, is sufficient to allow for entrance into and progression through G1 with subsequent S-phase entry. This is consistent with the recent demonstration that microinjection of active cyclin D1-CDK4 complexes, prepared from insect cells, is sufficient to initiate DNA synthesis from a quiescent state in human diploid fibroblasts (13).
ACKNOWLEDGMENTS
We thank Martine Roussel and Charles Sherr for the cyclin D1 lines, Christopher Marshall for the activated MEK1 retroviral vector, Jacques Pouysségur and Natalie Rivard for the antisense p27 plasmid, Geoffrey Cooper for the RasN17 plasmid, Pamela Silver for the GFP plasmid, Warren Pear and David Baltimore for the Bosc23 packaging line, and Edward Harlow for the CDK4 plasmid. We thank Othon Iliopoulos, Christine McMahon, Justin Lamb, Martine Roussel, and Peter Adams for critical review of the manuscript.
This work was supported by National Institutes of Health grant CA65842 to M.E.E. M.E.E. is a Scholar for the Leukemia Society of America.
REFERENCES
- 1.Aktas H, Cai H, Cooper G M. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol Cell Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 3.Alessandrini A, Chiaur D S, Pagano M. Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia. 1997;11:342–345. doi: 10.1038/sj.leu.2400581. [DOI] [PubMed] [Google Scholar]
- 4.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 5.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 6.Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 7.Brunet A, Pages G, Pouysségur J. Constitutive active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene. 1994;9:3379–3387. [PubMed] [Google Scholar]
- 8.Cai H, Szeberenyi J, Cooper G M. Effect of a dominant inhibitory Ha-ras mutation on mitogenic signal transduction in NIH 3T3 cells. Mol Cell Biol. 1990;10:5314–5323. doi: 10.1128/mcb.10.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Saha P, Kornbluth S, Dynlacht B D, Dutta A. Cyclin-binding motifs are essential to the function of p21CIP1. Mol Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng M, Sexl V, Sherr C J, Roussel M F. Assembly of cyclin D-dependent kinase and titration of p27Kip1 by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clurman B E, Sheaff R J, Thress K, Groudine M, Roberts J M. Turnover of cyclin E by the ubiquitin-proteosome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 1996;10:1979–1990. doi: 10.1101/gad.10.16.1979. [DOI] [PubMed] [Google Scholar]
- 12.Coats S, Flanagan W M, Nourse M, Roberts J M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 13.Connell-Crowley L, Elledge S J, Harper W J. G1 cyclin-dependent kinases are sufficient to initiate DNA synthesis in quiescent human fibroblasts. Curr Biol. 1998;8:65–68. doi: 10.1016/s0960-9822(98)70021-1. [DOI] [PubMed] [Google Scholar]
- 14.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 15.de Vries-Smits A M M, Burgering B M T, Leevers S J, Marshall C J, Bos J L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature. 1992;357:602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]
- 16.Dobrowolski S, Harter M, Stacey D W. Cellular ras activity is required for passage through multiple points of the G0/G1 phase in BALB/c 3T3 cells. Mol Cell Biol. 1994;14:5441–5449. doi: 10.1128/mcb.14.8.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J-Y, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 18.Feig L A, Cooper G M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feramisco J R, Gross M, Kamata T, Rosenberg M, Sweet R W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984;38:109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- 20.Filmus J, Robles A I, Shi W, Wong M J, Colombo L L, Conti C J. Induction of cyclin D1 overexpression by activated ras. Oncogene. 1994;9:3627–3633. [PubMed] [Google Scholar]
- 21.Firpo E J, Koff A, Solomon M J, Roberts J M. Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994;14:4889–4901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng Y, Eaton E N, Picon M, Roberts J M, Lundberg A S, Gifford A, Sardet C, Weinberg R A. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;16:2402–2407. [PubMed] [Google Scholar]
- 23.Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67–108. doi: 10.1016/s0065-230x(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 24.Hengst L, Dulic V, Slingerland J M, Lees E, Reed S I. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:5291–5295. doi: 10.1073/pnas.91.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herber B, Truss M, Beato M, Muller R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene. 1994;9:1295–1304. [PubMed] [Google Scholar]
- 26.Herrera R E, Sah V P, Williams B O, Makela T P, Weinberg R A, Jacks T. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol. 1996;16:2402–2407. doi: 10.1128/mcb.16.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joneson T, White M A, Wigler M H, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 28.Kato J-Y, Matsuoka M, Polyak K, Massague J, Sherr C J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 29.Kato J-Y, Matsuoka M, Strom D K, Sherr C J. Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol Cell Biol. 1994;14:2713–2721. doi: 10.1128/mcb.14.4.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato J-Y, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase cdk4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 31.Kawada M, Yamagoe S, Murakami Y, Suzuki K, Mizuno S, Uehara Y. Induction of p27Kip1 degradation and anchorage independence through the MAP kinase signaling pathway. Oncogene. 1997;15:629–637. doi: 10.1038/sj.onc.1201228. [DOI] [PubMed] [Google Scholar]
- 32.Koff A, Ohtsuki M, Polyak K, Roberts J M, Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-β. Science. 1993;260:536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- 33.Kolch W, Heidecker G, Lloyd P, Rapp U R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991;12:426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- 34.Krude T, Jackman M, Pines J, Lasky R A. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 1997;88:109–119. doi: 10.1016/s0092-8674(00)81863-2. [DOI] [PubMed] [Google Scholar]
- 35.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 36.Lavoie J N, L’Allemain G, Brunet A, Muller R, Pouysségur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 37.Leng X, Connell-Crowley L, Goodrich D, Harper J W. S-phase entry upon ectopic expression of G1 cyclin-dependent kinases in the absence of retinoblastoma protein phosphorylation. Curr Biol. 1997;7:709–712. doi: 10.1016/s0960-9822(06)00301-0. [DOI] [PubMed] [Google Scholar]
- 38.Leone G, DeGregori J, Sears R, Jakoi L, Nevins J R. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387:422–426. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Jenkins C W, Nichols M A, Xiong Y. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene. 1994;9:2261–2268. [PubMed] [Google Scholar]
- 40.Liu J-J, Chao J-R, Jiang M-C, Ng S-Y, Yen J J-Y, Yang-Yen H F. Ras transformation results in an elevated level of cyclin D1 and acceleration of G1 progression in NIH 3T3 cells. Mol Cell Biol. 1995;15:3654–3663. doi: 10.1128/mcb.15.7.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loda M, Cukor B, Tam S W, Lavin P, Fiorentino M, Draetta G F, Jessup J M, Pagano M. Increased proteosome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 42.Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D–cyclin-dependent kinase–pRb-controlled G1 checkpoint. Mol Cell Biol. 1996;16:6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 44.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 46.Marshall C J. Ras effectors. Curr Opin Cell Biol. 1997;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 47.Matsuoka M, Kato J-Y, Fisher R P, Morgan D O, Sherr C J. Activation of cyclin-dependent kinase 4 (cdk4) by mouse MO15-associated kinase. Mol Cell Biol. 1994;14:7265–7275. doi: 10.1128/mcb.14.11.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mittnacht S, Paterson H, Olson M F, Marshall C J. Ras signalling is required for inactivation of tumour suppressor pRb cell-cycle control protein. Curr Biol. 1997;7:219–221. doi: 10.1016/s0960-9822(97)70094-0. [DOI] [PubMed] [Google Scholar]
- 51.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mulcahy L S, Smith M R, Stacey D W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH3T3 cells. Nature. 1985;313:241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- 53.Nakanashi M, Adami G R, Robetorye R S, Noda A, Venable S F, Dimitrov D, Pereira-Smith O M, Smith J R. Exit from G0 and entry into the cell cycle of cells expressing p21Sdi1 antisense RNA. Proc Natl Acad Sci USA. 1995;92:4352–4356. doi: 10.1073/pnas.92.10.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neuman E, Ladha M H, Lin N, Upton T M, Miller S J, DiRenzo J, Pestell R G, Hinds P W, Dowdy S F, Brown M, Ewen M E. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17:5338–5347. doi: 10.1128/mcb.17.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 56.Nourse J, Firpo E, Flanagan W M, Coats S, Polyak K, Lee M H, Massague J, Crabtree G R, Roberts J M. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 57.Ohtsubo M, Roberts J M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 58.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okuda K, Ernst T J, Griffin J D. Inhibition of p21ras activation blocks proliferation but not differentiation of interleukin-3-dependent myeloid cells. J Biol Chem. 1994;269:24602–24607. [PubMed] [Google Scholar]
- 60.Olson M F, Paterson H F, Marshall C J. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 61.Pages G, Lenorman P, L’Allemain G, Chambard J-C, Meloche S, Pouysségur J. Mitogen-activated protein kinase p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pardee A B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci USA. 1974;71:1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pardee A B. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 64.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peeper D S, Upton T M, Ladha M H, Neuman E, Zalvide J, Bernards R, DeCaprio J A, Ewen M E. Ras signalling linked to the cell-cycle machinery by the retinoblastoma protein. Nature. 1997;386:177–181. doi: 10.1038/386177a0. [DOI] [PubMed] [Google Scholar]
- 66.Polyak K, Kato J-Y, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. p27Kip1, a cyclin-cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 67.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signal. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 68.Porter P L, Malone K E, Heagerty P J, Alexander G M, Gatti L A, Firpo E J, Daling J R. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlates with survival in young breast cancer patients. Nat Med. 1997;3:222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 69.Pronk G J, Bos J L. The role of p21ras in receptor tyrosine kinase signalling. Biochim Biophys Acta. 1994;1198:131–147. doi: 10.1016/0304-419x(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 70.Quelle D E, Ashmun R A, Shurtleff S A, Kato J, Bar-Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 71.Resnitzky D, Reed S I. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rivard N, L’Allemain G, Bartek J, Pouysségur J. Abrogation of p27Kip1 by cDNA antisense suppresses quiescence (G0 state) in fibroblasts. J Biol Chem. 1996;271:18337–18341. doi: 10.1074/jbc.271.31.18337. [DOI] [PubMed] [Google Scholar]
- 73.Robbins D J, Cheng M, Zhen E, Vanderbilt C A, Feig L A, Cobb M H. Evidence for a Ras-dependent extracellular signal-related protein kinase (ERK) cascade. Proc Natl Acad Sci USA. 1992;89:6924–6928. doi: 10.1073/pnas.89.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sellers W R, Kaelin W G. RB as a modulator of transcription. Biochim Biophys Acta. 1996;1288:M1–M5. doi: 10.1016/0304-419x(96)00014-5. [DOI] [PubMed] [Google Scholar]
- 75.Serrano M, Gomez-Lohoz E, DePinho R A, Beach D, Bar-Sagi D. Inhibition of Ras-induced proliferation and transformation by p16INK4. Science. 1995;267:249–252. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- 76.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 77.Sewing A, Wiseman B, LLoyd A, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Cyclin E-cdk2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 79.Sherr C J. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 80.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 81.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 82.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 83.Stacey D W, Feig L A, Gibbs J B. Dominant inhibitor Ras mutants selectively inhibit the activity of either cellular or oncogenic Ras. Mol Cell Biol. 1991;11:4053–4064. doi: 10.1128/mcb.11.8.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stacey D W, Kung H F. Transformation of NIH 3T3 cells by microinjection of Ha-ras protein. Nature. 1984;310:508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- 85.Szeberenyi J, Cai H, Cooper G M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990;10:5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takuwa N, Takuwa Y. Ras activity late in G1 phase required for p27kip1 downregulation, passage through the restriction point, and entry into S phase in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:5348–5358. doi: 10.1128/mcb.17.9.5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Temin H. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971;78:161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]
- 88.Terada Y, Tatsuka M, Jinno S, Okayama H. Requirement for tyrosine phosphorylation of Cdk4 in G1 arrest induced by ultraviolet radiation. Nature. 1995;376:358–362. doi: 10.1038/376358a0. [DOI] [PubMed] [Google Scholar]
- 89.Thomas S M, DeMarco M, D’Arcangelo G, Helegoua S, Brugge J S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992;68:1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- 90.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 91.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 92.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 93.Winston J, Dong F, Pledger W J. Differential modulation of G1 cyclins and the cdk inhibitor p27Kip1 by platelet-derived growth factor and plasma factors in density-arrested fibroblasts. J Biol Chem. 1996;271:11253–11260. doi: 10.1074/jbc.271.19.11253. [DOI] [PubMed] [Google Scholar]
- 94.Winston J T, Coats S R, Wang Y-Z, Pledger W J. Regulation of the cell cycle machinery by oncogenic Ras. Oncogene. 1996;12:127–134. [PubMed] [Google Scholar]
- 95.Wood K W, Sarnecki C, Roberts T M, Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992;68:1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 96.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zetterberg A, Larsson O, Wiman K G. What is the restriction point? Curr Opin Cell Biol. 1995;7:835–842. doi: 10.1016/0955-0674(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 98.Zhao J, Dynlacht B, Imai T, Hori T-A, Harlow E. Expression of NPAT, a novel substrate of cyclin E-cdk2, promotes S-phase entry. Genes Dev. 1998;12:456–461. doi: 10.1101/gad.12.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]