Abstract
Objective
The treatment of bipolar depression remains challenging due to the limited effective and safe therapeutic options available; thus, developing newer treatments that are effective and well tolerable is an urgent unmet need. The objective of the present trial was to test 150 to 300 mg/day of cannabidiol as an adjunctive treatment for bipolar depression.
Method
A randomized, double-blind, placebo-controlled pilot study to assess the efficacy of adjunctive cannabidiol in bipolar depression was used. Efficacy parameters were changes in the Montgomery-Åsberg Depression Rating Scale (MADRS) from baseline to week 8. Secondary outcomes included response and remission rates, changes in anxiety and psychotic symptoms, and changes in functioning. Patients continued double-blind treatment until week 12 to monitor for adverse effects, laboratory analysis, and manic symptoms. Study registry: NCT03310593.
Results
A total of 35 participants were included. MADRS scores significantly decreased from baseline to the endpoint (placebo, −14.56; cannabidiol, −15.38), but there was no significant difference between the groups. Similarly, there were no other significant effects on the secondary outcomes. However, an exploratory analysis showed a significant effect of cannabidiol 300 mg/day in reducing MADRS scores from week 2 to week 8 (placebo, −6.64; cannabidiol, −13.72). There were no significant differences in the development of manic symptoms or any other adverse effects.
Conclusion
Cannabidiol did not show significantly higher adverse effects than placebo. Despite the negative finding on the primary outcome, an exploratory analysis suggested that cannabidiol should be further studied in bipolar depression in higher doses of at least 300 mg/day and under research designs that could better control for high placebo response.
Keywords: bipolar disorder, mood disorders, bipolar depression, cannabidiol, cannabinoids, clinical trial, randomized controlled trial
Abrégé
Objectif
Le traitement de la dépression bipolaire demeure difficile en raison du nombre limité d’options efficaces et sûres disponibles; ainsi, développer de nouveaux traitements qui sont efficaces et bien tolérés constitue un urgent besoin non comblé. L’objectif du présent essai était de tester 150 à 300 mg/jour de cannabidiol en traitement d'appoint pour la dépression bipolaire.
Méthodes
Une étude pilote randomisée, à double insu, contrôlée par placebo pour évaluer l’efficacité du cannabidiol d’appoint dans la dépression bipolaire. Les paramètres d’efficacité étaient les changements à l’Échelle d'évaluation de la dépression de Montgomery-Åsberg (MADRS) de la base à la semaine 8. Les résultats secondaires étaient notamment les taux de réponse et de rémission, les changements des symptômes anxieux et psychotiques, et les changements de fonctionnement. Les patients ont continué le traitement à double insu jusqu’à la semaine 12 pour vérifier les effets indésirables, l’analyse de laboratoire et les symptômes de manie. Registre des essais : NCT03310593.
Résultats
Un total de 35 participants, inclus dans les scores de MADRS, a diminué significativement de la base au point final (placebo: −14,56; cannabidiol: −15,38), mais il n’y avait pas de différence significative entre les groupes. De même, il n’y avait pas d’autres effets significatifs dans les résultats secondaires. Cependant, une analyse exploratoire a montré un effet significatif du cannabidiol à 300 mg/jour pour réduire les scores de MADRS de la semaine 02 à 08 (placebo : −6,64; cannabidiol : −13). Il n’y avait pas de différences significatives dans le développement de symptômes de manie ou de tout autre effet indésirable.
Conclusion
Le cannabidiol n’a pas montré d’effets indésirables significativement plus élevés que le placebo. Malgré le résultat négatif du résultat primaire, une analyse exploratoire a suggéré que le cannabidiol devrait être étudié plus à fond dans la dépression bipolaire à des doses plus élevées d’au moins 300 mg/jour et selon des conceptions de recherche qui pourraient mieux contrôler la réponse élevée du placebo.
Keywords: Trouble bipolaire, dépression bipolaire, cannabidiol, cannabinoïdes, essai randomisé contrôlé, essai clinique.
Introduction
Bipolar disorder is a severe and chronic mental disorder with a worldwide prevalence of 2.4%, 1 which figures among the top 20 disorders with the highest global burden of disease. 2 Despite manic episodes being more severe and associated with aggressive behaviour, depressive symptoms are responsible for most of the disability associated with this condition since patients spend 3 times a greater amount of time experiencing syndromal/subsyndromal depressive symptoms during symptomatic periods than manic or hypomanic symptoms. 3 In addition, one of the most consistently reported predictors of functional impairment in patients with bipolar disorders is depressive symptoms, even when subthreshold.4,5 Furthermore, bipolar disorder is associated with a high risk of premature death with a suicide rate 20–30 times higher than in the general population. 6
Despite significant morbidity and mortality associated with depression in bipolar disorder, its pharmacological treatment remains challenging. For example, atypical antipsychotics such as quetiapine, lurasidone, cariprazine, and lumateperone are the only agents approved in monotherapy for treating depression in bipolar disorder, and many patients struggle with tolerability with some of these agents. 7 While antidepressant adjunctive therapy has been widely used to treat depression in bipolar disorder, their efficacy remains uncertain, and the risk of manic switches is a matter of concern for psychiatrists. 7 Thus, development of novel, effective, and safe treatments for depression in patients with bipolar disorder is an important clinical unmet need.
Therefore, the search for new and more effective pharmacological options for managing bipolar depression has been a focus of recent studies. Of the innovative options, cannabidiol stands out due to its effects on the endocannabinoid system, which has not been a target for any currently available treatments. Cannabidiol is one of the main phytocannabinoids present in the plant Cannabis sativa. 8 While Δ-9-tetrahydrocannabinol (THC), the main component of cannabis, may induce anxiety and psychotic symptoms, clinical studies have supported the potential benefits of cannabidiol in the treatment of neuropsychiatric conditions such as anxiety, 9 schizophrenia,10,11 epilepsy, and substance use disorders12,13 based on preclinical and clinical data. Cannabidiol also shows antidepressant-like properties in several animal models of mood disorders.14–17 Such a pharmacological profile has diverse characteristics in parallel with medications known to benefit bipolar depression. 18
Given the preclinical evidence and preliminary data in humans supporting efficacy in improving depression18,19 and other behavioural symptoms, in addition to its excellent tolerability,18,20 we explored the efficacy and tolerability profile of cannabidiol in bipolar depression in this pilot trial.
Methods
This is a single-site, randomized, double-blind, placebo-controlled, proof-of-concept study to assess the antidepressant efficacy of adjunctive cannabidiol in patients with bipolar disorder who were currently in a major depressive episode. This study was conducted in Brazil from March 2018 to March 2020 and was prematurely terminated due to the coronavirus pandemic outbreak before the full sample could be recruited. The protocol was approved by the Ethics Committee of Hospital de Clínicas de Porto Alegre (HCPA) and registered at clinicaltrials.gov (NCT03310593). After receiving a complete description of the study, all study participants reviewed and provided a written informed consent before study entry. This clinical trial was conducted in accordance with the Helsinki Declaration.
Participants
Participants were outpatients who fulfilled the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) 21 criteria for bipolar I disorder or bipolar II disorder with a current major depressive episode, as confirmed by the Mini-International Neuropsychiatric Interview (MINI). 22 The other inclusion criteria were as follows: (a) 18 years or older; (b) Montgomery-Åsberg Depression Rating Scale (MADRS) 23 total score ≥12, with scores on MADRS items 1 (apparent sadness) and 2 (reported sadness) ≥2 at baseline; (c) Young Mania Rating Scale (YMRS) 24 total score ≤11 at baseline; (d) regular use of either lithium, valproate, or atypical antipsychotics at therapeutic levels for at least 4 weeks before baseline assessment; (e) able to provide informed consent and to comply with the study procedures; and (f) on an effective contraceptive method in the case of heterosexual women of childbearing age.
The exclusion criteria were the following: (a) presence of another active major psychiatric disorder within 6 months before study entry that was requiring acute treatment at the screening or baseline assessment (currently causing more impairment than the depressive symptoms) assessed with MINI and according to DMS-5 criteria; (b) current or past drug sensitivity or intolerance to cannabidiol, assessed through clinical interview; (c) diagnosis of any substance use disorder according to DSM-5 criteria within past 6 months, except for nicotine, assessed with MINI; (d) clinically significant unstable medical illness, neurological disorders or inflammatory/autoimmune diseases, or any autoimmune, inflammatory, or neurologic disorders that required treatment with steroidal anti-inflammatory medications or immunotherapy with biologic drugs at baseline (information were assessed by interview and clinical investigation); (e) current serious suicide risk or risk of injury to self or others as clinically judged by the psychiatrists as well as using the MINI; and (f) being pregnant or breastfeeding, assessed through clinical interview and laboratory exams. Also, participants were not allowed to use cannabis during the clinical trial.
The withdrawal criteria were as follows: (a) treatment-emergent mania or hypomania as clinically defined and with YMRS total score ≥16 on 2 consecutive assessments or if there were any risks associated, as judged by the treating/study psychiatrist; (b) pregnancy or discontinuation of contraceptive methods; (c) severe adverse event or potentially severe adverse events, as assessed by the clinicians; (d) hospitalizations due to medical, surgical, or psychiatric risk; and (e) withdrawal of consent.
Study Design
Eligible and enrolled patients were randomized at the baseline visit to receive either cannabidiol or placebo adjunctive therapy in a 1:1 ratio for 12 weeks, using a block randomization method. The study comprised 1 screening telephone contact and 5 clinical visits (i.e., baseline, weeks 2, 4, 8, and 12). Patients received capsules containing cannabidiol dissolved in corn oil (99.6% purity, free of THC, Biosynthesis Pharma Group, Sandwich, UK) or placebo (corn oil in identical gelatine capsules). All participants initiated the treatment using 1 capsule per day (equivalent to 150 mg of cannabidiol). The dosage was increased to 2 capsules (300 mg of cannabidiol) per day in those patients who did not meet response criteria (≥50% reduction in MADRS scores) at week 2 visit.
Efficacy Parameters
The primary efficacy parameter was the change in MADRS scores from baseline to week 8. The secondary efficacy parameters were response rate, defined as a ≥50% reduction from the MADRS baseline score at week 8, and remission rate, defined as MADRS and YMRS scores ≤7 at week 8. Additional outcomes were the changes in the Clinical Global Impression-Severity (CGI-S) scale, 25 Patient Health Questionnaire-9 (PHQ-9), 26 Hamilton Depression Rating Scale (HAMD), 27 Hamilton Anxiety Rating Scale (HAMA), 28 Brief Psychiatric Rating Scale (BPRS), 29 and Functioning Assessment Short Test (FAST) 30 from baseline to week 8. All the clinical rating scales were completed at baseline and weeks 2, 4, 8, and 12.
Safety
Vital signs and body mass index (BMI) were recorded at every clinical visit, and laboratory monitoring was conducted at screening, at week 4, and at the end of week 8. The YMRS, CGI-S for manic symptoms, and Udvalg for Kliniske Undersøgelser (UKU) 31 were administered at every visit to assess the risk of manic switches and the development of adverse effects. Other laboratories or supplementary exams were performed whenever they were necessary according to clinical judgment.
Statistical Analysis
Efficacy assessments were based on the intent-to-treat population. Changes in MADRS scores from baseline to week 8 were analysed with generalized estimation equations using an unstructured working correlation matrix and a robust variance estimator. Group, visit, and the group × visit interaction were included as predictors, controlled for age, gender, years of education, and the baseline MADRS scores. A similar approach was used to investigate the changes in the other scales’ scores (PHQ-9, CGI-S depression, HAM-D, HAM-A, BPRS, and FAST). Fisher exact test was used to assess the response, remission, and dropout rates at the endpoint (week 8). Additionally, we investigated the differences in early response and remission rates, defined as ≥50% reduction from MADRS baseline score at week 2, and remission, defined as MADRS and YMRS scores ≤7 at week 2.
Due to the high initial response rate, an exploratory analysis was performed excluding those participants who met the criteria for an early response at week 2. Thus, only those participants who did not meet early response criteria and had their dose increased to 2 capsules per day from the beginning of the third week of the trial were included in this exploratory analysis which assessed changes in MADRS scores from week 2 to week 8 adjusting for week 2 MADRS scores,
Safety assessments were based on the safety population (randomized patients who took at least 1 dose of cannabidiol or placebo). Changes in CGI-S mania and YMRS scores from baseline to week 12 were assessed using the same procedure as the main efficacy analysis. The UKU scores from baseline to week 12 were assessed using group, visit, and group × visit interaction and controlled for the baseline scores. Longitudinal changes in weight, BMI, vital signs, and laboratory tests were also investigated with generalized estimation equations similar to the procedure used to assess the changes in UKU scores. The laboratory tests included complete blood count, thyroid-stimulating hormone, creatinine, alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyl transferase.
Results
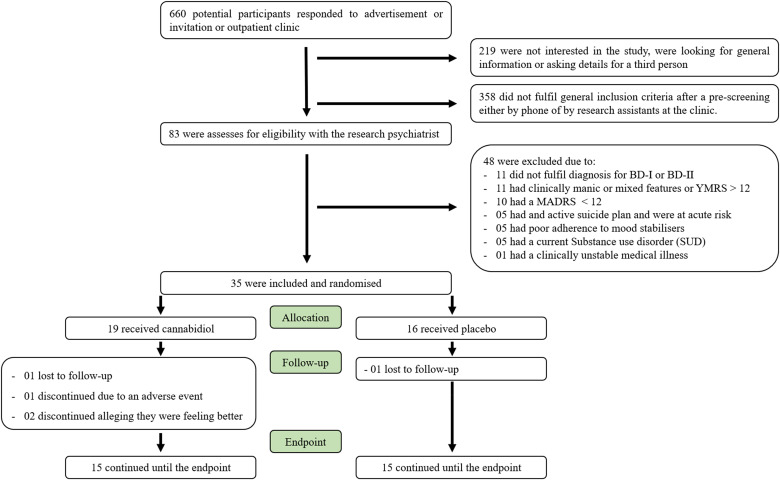
A total of 35 subjects fulfilled the study entry criteria and were randomly assigned to double-blind treatment with cannabidiol (n = 19) or placebo (n = 16). Figure 1 shows the screening and enrolment phase details as per the Consolidated Standards of Reporting Trials (CONSORT). There were no differences in baseline variables between the 2 groups (Table 1). One participant in the cannabidiol group was discontinued due to an adverse event (skin rash without complications). The all-cause dropouts were higher in the cannabidiol group: 2 participants in the cannabidiol group discontinued stating that they felt better and thought it was unnecessary to continue in the study, and 1 participant in each group discontinued before week 8 due to loss to follow-up. The CONSORT checklist is included in Supplemental Table 1.
Figure 1.
Study flowchart of participant enrolment and allocation.
Table 1.
Clinical and Sociodemographic Characteristics.
| Cannabidiol (n = 19) | Placebo (n = 16) | |
|---|---|---|
| Age, mean (SD) | 42.2 (13.8) | 45.9 (13.0) |
| Gender, female (%) | 13 (68.4) | 11 (68.8) |
| Ethnicity, white (%) | 15 (78.9) | 9 (56.3) |
| Years of education, mean (SD) | 11.6 (4.2) | 13.5 (3.7) |
| Working or studying, yes (%) | 9 (47.4) | 7 (43.8) |
| Bipolar disorder type I, yes (%) | 13 (68.4) | 11 (68.8) |
| Past cannabis use, yes (%) | 02 (10.5) | 04 (25.0) |
| Current medication | ||
| Lithium, yes (%) | 10 (52.6) | 10 (62.5) |
| Anticonvulsants, yes (%) | 13 (68.4) | 8 (50.0) |
| Antipsychotics, yes (%) | 14 (73.7) | 11 (68.8) |
| Antidepressants, yes (%) | 13 (68.4) | 8 (50.0) |
SD: standard deviation.
Efficacy Results
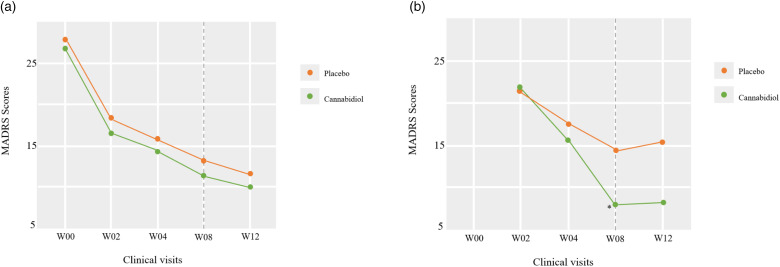
MADRS scores decreased from baseline to the endpoint across the full sample, but with no significant differences between cannabidiol and placebo groups (Figure 2A). In the placebo group, there was a change of −14.56 in MADRS scores from baseline to week 8, while in the cannabidiol group, there was a change of −15.38 (Table 2). There were no significant differences between the 2 groups on all the other clinical rating scales assessed (Table 2). In addition, there were no significant differences between groups in response rates at week 2 (cannabidiol = 42.1%, placebo = 31.3%, p = 0.7270) or at week 8 (cannabidiol = 57.9%, placebo = 62.5%, p = 1.0). There was no significant difference in remission rate at week 8 (cannabidiol = 47.4%, placebo = 31.3%, p = 0.4910), but the remission rate at week 2 was marginally significant in favour of cannabidiol (cannabidiol = 23.3%, placebo = 0%, p = 0.0493). About one-third of patients (5 out of 16 in the placebo group and 8 out of 19 in the cannabidiol group) met the criteria for response at week 2, and these patients continued 1 capsule of study medication for the remainder of the study. No further significant changes were noted in depressive symptoms in this subgroup.
Figure 2.
Changes in Montgomery-Åsberg Depression Rating Scale (MADRS) scores. (A) Shows the results of the main analysis. (B) Shows the results of the complementary analysis. *p < 0.05.
Table 2.
Main Analysis of Efficacy From Baseline to Week 8.
| Baseline | Change | Endpoint | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SE | Difference | 95% CI | P value | |
| MADRS | – | – | – | – | −0.82 | [–7.81 to 6.17] | 0.8176 |
| Placebo | 26.4 | 6.85 | –14.56 | 2.21 | – | – | –- |
| Cannabidiol | 26.2 | 5.49 | –15.38 | 2.82 | – | – | – |
| CGI severity | – | – | – | – | 0.52 | [–0.59 to 1.63] | 0.3573 |
| Placebo | 4.69 | 0.95 | -2.12 | 0.43 | – | – | – |
| Cannabidiol | 4.50 | 0.92 | -1.60 | 0.37 | – | – | – |
| HAMD | – | – | – | – | –2.47 | [–7.24 to 2.30] | 0.3106 |
| Placebo | 16.7 | 6.25 | –7.82 | 1.58 | – | – | – |
| Cannabidiol | 18.4 | 4.88 | –10.29 | 1.85 | – | – | – |
| PHQ-9 | – | – | – | – | -0.67 | [–5.49 to 4.16] | 0.7860 |
| Placebo | 17.2 | 4.01 | –8.65 | 1.51 | – | – | – |
| Cannabidiol | 17.2 | 4.01 | –9.32 | 1.94 | – | – | – |
| HAMA | – | – | – | – | –0.51 | [–5.55 to 4.54] | 0.8435 |
| Placebo | 16.3 | 7.20 | -8.03 | 1.51 | – | – | – |
| Cannabidiol | 19.1 | 10.5 | -8.54 | 2.08 | – | – | – |
| BPRS | – | – | – | – | –3.24 | [–7.45 to 0.97] | 0.1314 |
| Placebo | 33.7 | 5.16 | –7.16 | 1.68 | – | – | – |
| Cannabidiol | 36.8 | 6.25 | –10.40 | 1.40 | – | – | – |
| FAST | – | – | – | – | 0.52 | [–10.1 to 11.2] | 0.9246 |
| Placebo | 38.1 | 13.9 | –12.05 | 4.57 | – | – | – |
| Cannabidiol | 35.8 | 14.7 | –11.53 | 2.86 | – | – | – |
Note. The main analysis of efficacy shows the changes in scale scores from baseline (week 0) to endpoint (week 8). Mean changes are represented by the estimated mean changes. BPRS = Brief Psychiatric Rating Scale; CGI-S = Clinical Global Impression-Severity scale for depression; FAST = Functioning Assessment Short Test; HAMA = Hamilton Anxiety Rating Scale; HAMD = Hamilton Depression Rating Scale 17 items; MADRS = Montgomery-Åsberg Depression Rating Scale; PHQ-9 = Patient Health Questionnaire-9.
Exploratory Analysis
The remaining patients (11 per group) had their study medication dose increased to 2 capsules per day at the beginning of week 3. The exploratory analysis in these patients who were not early responders showed that participants in the cannabidiol group had a significantly greater reduction in MADRS scores compared with the placebo group to the week 8 endpoint (estimated mean change difference = 7.09, 95% CI: −12.5 to −1.69, p = 0.0100), as a well as in HAMD scores (estimated mean change difference: −6.18, 95% CI: −9.58 to −2.77, p = 0.0004). In the placebo group, there was a change of −6.64 in MADRS scores from week 2 to week 8, while in the cannabidiol group, there was a change of −13.72 (Table 3 and Figure 2B). There were also almost significant reductions in PHQ-9 and BPRS scores (Table 3). In this subgroup, the remission rate at week 8 was nearly 2.5 times numerically higher in the cannabidiol group (cannabidiol = 45.5%, placebo = 18.2%, p = 0.3615). There were no other significant differences between the groups.
Table 3.
Exploratory Analysis of Efficacy From Week 2 to Week 8.
| Baseline | Change | Endpoint | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SE | Difference | 95% CI | P value | |
| MADRS | – | – | – | – | −7.08 | [–12.50 to –1.69] | 0.0100 |
| Placebo | 21.2 | 3.38 | –6.64 | 1.43 | – | – | – |
| Cannabidiol | 21.5 | 2.95 | –13.72 | 2.38 | – | – | – |
| CGI severity | – | – | – | – | –0.50 | [–1.74 to 0.74] | 0.4303 |
| Placebo | 4.13 | 0.70 | –1.06 | 0.45 | – | – | – |
| Cannabidiol | 4.09 | 0.60 | –1.56 | 0.46 | – | – | – |
| HAMD | – | – | – | – | –6.18 | [–9.58 to –2.77] | 0.0004 |
| Placebo | 13.80 | 2.12 | –2.62 | 1.29; | – | – | – |
| Cannabidiol | 14.30 | 2.22 | –8.80 | 1.16 | – | – | – |
| PHQ-9 | – | – | – | – | –4.09 | [–8.17 to 0.01] | 0.0501 |
| Placebo | 14.02 | 2.39 | –4.33 | 1.28 | – | – | – |
| Cannabidiol | 14.46 | 1.96 | –8.42 | 5.1 | – | – | – |
| HAMA | – | – | – | – | –2.94 | [–7.08 to 1.19] | 0.1625 |
| Placebo | 14.99 | 2.22 | –4.52 | 1.27 | – | – | – |
| Cannabidiol | 15.33 | 2.82 | –7.46 | 1.69; | – | – | – |
| BPRS | – | – | – | – | –4.02 | [–8.07 to 0.03] | 0.0518 |
| Placebo | 31.4 | 3.18 | –2.62 | 1.10 | – | – | – |
| Cannabidiol | 32.1 | 3.48 | –6.64 | 1.77 | – | – | – |
| FAST | – | – | – | – | –2.02 | [–14.0 to 9.97] | 0.7413 |
| Placebo | 38.4 | 3.81 | –8.44 | 4.67 | – | – | – |
| Cannabidiol | 38.5 | 4.21 | –10.46 | 3.76 | – | – | – |
Note. Exploratory analysis of efficacy showing the changes in scale scores from baseline (week 2) to endpoint (week 8) in those patients who were not early responders. Mean and mean changes are represented by the estimated mean changes. BPRS = Brief Psychiatric Rating Scale; CGI-S = Clinical Global Impression-Severity scale for depression; FAST = Functioning Assessment Short Test; HAMA = Hamilton Anxiety Rating Scale; HAMD = Hamilton Depression Rating Scale 17 items; MADRS = Montgomery-Åsberg Depression Rating Scale; PHQ-9 = Patient Health Questionnaire-9.
Safety Results
There were no significant differences between cannabidiol and placebo groups in YMRS, CGI-S mania, and UKU scores. There was a statistically significant difference in BMI between the groups, reflecting a reduction of BMI in the cannabidiol group and an increase in the placebo group from baseline to week 12. There were no significant findings in blood pressure or any laboratory tests (Table 4 and Supplemental Table 2).
Table 4.
Safety Analysis.
| Baseline | Change | Endpoint | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SE | Difference | 95% CI | P value | |
| YMRS | – | – | – | – | 0.13 | [–3.64 to 3.91] | 0.9460 |
| Placebo | 2.88 | 3.30 | –0.35 | 1.45 | – | – | – |
| Cannabidiol | 2.47 | 2.59 | –0.22 | 1.22 | – | – | – |
| CGI severity – mania | – | – | – | – | –0.02 | [–0.89 to 0.93] | 0.9679 |
| Placebo | 1.25 | 0.78 | –0.20 | 0.33 | – | – | – |
| Cannabidiol | 1.17 | 0.38 | –0.22 | 0.30 | – | – | – |
| UKU | – | – | – | – | –1.87 | [–12.3 to 8.61] | 0.7273 |
| Placebo | 24.9 | 12.6 | –6.87 | 2.56 | – | – | – |
| Cannabidiol | 25.1 | 9.23 | –8.74 | 4.67 | – | – | – |
| Body mass index | – | – | – | – | –0.72 | [–1.21 to –0.22] | 0.0044 |
| Placebo | 28.4 | 4.49 | 0.55 | 0.22 | – | – | – |
| Cannabidiol | 30.8 | 6.13 | –0.17 | 0.13 | – | – | – |
| Mean arterial pressure | – | – | – | – | –3.02 | [–11.4 to 5.34] | 0.4786 |
| Placebo | 93.1 | 14.0 | –3.65 | 3.22 | – | – | – |
| Cannabidiol | 95.1 | 14.2 | –6.67 | 2.75 | – | – | – |
Note. Safety analysis showing the changes from baseline (week 0) to endpoint (week 12) using generalized estimating equations. CGI-S = Clinical Global Impression-Severity scale for mania; UKU = Udvalg for Kliniske Undersøgelser; YMRS = Young Mania Rating Scale.
Discussion
The present study is the first placebo-controlled trial to test the efficacy, safety, and tolerability of cannabidiol as an add-on treatment to mood stabilizers for acute bipolar depression. In fact, this was the first clinical trial to test cannabidiol for the treatment of any mood disorder.18,19 The results showed significant improvements in depressive symptoms at as early as 2 weeks with no significant difference between the 2 groups. Further, there were no significant differences between the 2 groups on the primary endpoint of MADRS changes scores at week 8. However, the remission rates were marginally significantly higher in the cannabidiol group at week 2 and numerically higher, albeit not significant, at week 8.
While the results on the primary endpoint were negative, the early and rapid improvement in depressive symptoms required further exploration of the data as there was a 10-point mean reduction in MADRS scores at week 2. Given that such rapid reduction in depressive symptoms is likely to be due to a placebo response and as the placebo response often obscures the detection of signal for the efficacy of a potential therapeutic agent, we explored the data further by excluding all patients that responded to placebo or cannabidiol treatment (more than 50% reduction from the MADRS baseline score) in the first 2 weeks of the trial. In this subgroup of early nonresponders, there was a significantly greater improvement in MADRS and HAMD scores to week 8 endpoint in the cannabidiol group compared with the placebo group.
As observed in our trial, a high placebo response rate makes it harder to find significant differences against active drugs. 32 The present trial had an unusually high response to the advertisement for recruitment of patients with many subjects eager to participate in the trial. At the time of commencement of this trial, cannabidiol was approved in Brazil for use in research settings only but not for routine clinical use. However, the positive media coverage had made it a popular substance in the country, as described elsewhere. 33 A recent study showed that a positive view on cannabidiol effects could interfere with its psychological effects, 34 which is in line with its high placebo effect observed even in treatment-resistant epilepsy patients. 35 It is possible that a similar effect might have contributed to a high placebo response rate in our study.
An alternative explanation is that 150 mg of cannabidiol is ineffective in treating bipolar depression as significant improvements in MADRS scores relative to placebo were seen only in patients whose dose increased to cannabidiol 300 mg daily but not in those that continued 150 mg daily. Indeed, a previous trial of cannabidiol in cannabis use disorder showed that a 200 mg dose of cannabidiol was ineffective and that 400 mg and 800 mg doses were effective. 12 Further, cannabidiol 400 mg or 800 mg daily was shown to be effective in reducing drug cue-induced craving and anxiety in heroin use disorder 13 and that 900 mg of cannabidiol daily was effective in treating symptoms of psychosis in patients with schizophrenia. Therefore, future studies of cannabidiol in bipolar depression should use a minimum of 300 mg daily to examine its efficacy in improving depressive symptoms.
The research recruitment for our trial was interrupted due to the coronavirus pandemic outbreak, given that our institution, the Hospital de Clínicas de Porto Alegre, became a referral centre to treat the coronavirus disease 2019 (COVID-19). It was not possible to postpone the recruitment during the pandemic due to operational limitations.
The current study essentially, therefore, is an early terminated first trial of cannabidiol in bipolar depression conducted under peculiar circumstances due to the ongoing COVID-19 pandemic outbreak. Thus, the findings need to be seen as preliminary, given the limitations of the study. In addition, the subject sample was modest, and the early termination limited the study's statistical power. Despite these limitations and the negative finding on the primary outcome, our exploratory analysis suggests that cannabidiol can be further studied in bipolar disorder under research designs that better control for high placebo response. 19 Also, the exploratory analysis indicated that further studies should investigate higher doses of cannabidiol, at least 300 mg/day. The search for validating an efficacious treatment for bipolar depression, with a different mechanism of action than other medications, is highly warranted given the chronic and severe burden of depressive symptoms in bipolar disorder.
From the safety perspective, we reported only 1 adverse event that led to discontinuation, a skin rash in the cannabidiol group, which remitted without other complications. Also, our results did not show treatment-emergent mania since there was no significant change in YMRS or CGI-S mania scores over visits. There was no significant difference in discontinuation rates between groups and no significant differences in adverse effects and laboratory exams. Previous findings showed that cannabidiol is a safe and well-tolerated medication when used to treat other health conditions and it is rarely associated with severe adverse effects.18,36,37 Our results suggest that cannabidiol may be a safe option to be used in bipolar disorder; however, the present clinical trial is limited, and further studies are needed to confirm these findings.
Therefore, considering the preliminary results of our study, we believe that cannabidiol should further be studied in bipolar depression in higher doses of at least 300 mg/day, in larger clinical trials and under research designs that could better control for high placebo response.
Supplemental Material
Supplemental material, sj-docx-1-cpa-10.1177_07067437231209650 for Cannabidiol as an Adjunctive Treatment for Acute Bipolar Depression: A Pilot Study by Jairo Vinícius Pinto, José Alexandre S. Crippa, Keila Maria Ceresér, Miréia Fortes Vianna-Sulzbach, Érico de Moura Silveira Júnior, Gabriel Santana da Rosa, Manoella Guatimuzim Testa da Silva, Gabriel Henrique Hizo, Leonardo Simão Medeiros, Carlos Eduardo Santana de Oliveira, Giovana Bristot, Alline Cristina Campos, Francisco Silveira Guimarães, Jaime E. C. Hallak, Antonio W. Zuardi, Lakshmi N. Yatham, Flávio Kapczinski and Márcia Kauer-Sant’Anna in The Canadian Journal of Psychiatry
Acknowledgments
We thank BSPG-Pharm (Sandwich, UK) who kindly donated cannabidiol.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JAC is a member of the International Advisory Board of the Australian Centre for Cannabinoid Clinical and Research Excellence (ACRE)—National Health and Medical Research Council (NHMRC). JAC and JEH have received travel support to attend scientific meetings and personal consultation fees from BSPG-Pharm in the past. JAC, JEH, FK, FSG, and AWZ are coinventors of the patent “Fluorinated CBD compounds, compositions and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023,” Def. US number Reg. 62193296; 29 July 2015; INPI on 19 August 2015 (BR1120150164927; Mechoulam R, Zuardi AW, Kapczinski F, Hallak JEC, Guimarães FS, Crippa JAS, Breuer A). Universidade de São Paulo (USP) has licensed this patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1) and has an agreement with Prati-Donaduzzi to “develop a pharmaceutical product containing synthetic CBD and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson’s disease, and anxiety disorders.” JAC, JEH, FSG, ACC, and AWZ are coinventors of the patent “Cannabinoid-containing oral pharmaceutical composition, method for preparing and using same,” INPI on 16 September 2016 (BR 112018005423-2). MKS reports research grants from SMRI, from the National Council for Scientific and Technological Development, Ministry of Science and Technology, Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq), and Coordination of Superior Level Staff Improvement (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES), and personal fees from SHIRE and Daichii-Sankyo, from FIPE-HCPA (Fundo de Incentivo à Pesquisa e Eventos – HCPA), and from CNPq Produtividade em Pesquisa outside of the submitted work. LNY is a consultant and/or has received speaker fees and/or sits on the advisory board and/or receives research funding from Abbvie, Alkermes, Allergan, Canadian Network for Mood and Anxiety Treatments (CANMAT), Canadian Institutes of Health Research (CIHR), Dainippon Sumitomo Pharma, Gedeon Richter, Intracellular Therapies, Lundbeck, Merck, Otsuka, Sanofi and Sunovion over the past 3 years. All other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2020/12110-9) and by the Instituto Nacional de Ciência e Tecnologia Translational em Medicina (INCT-TM; CNPq/FAPESP; 2008/09009-2). JAC received a grant from the University Global Partnership Network (UGPN) – Global Priorities in Cannabinoid Research Excellence Program. JAC, JEH, FSG, and AWZ are recipients of CNPq research fellowships. MKS was supported by CAPES and CNPq Produtividade em Pesquisa, INCT-TM CNPq, Brazil. JVP received a scholarship from CNPq.
ORCID iDs: Jairo Vinícius Pinto https://orcid.org/0000-0001-6990-6749
Leonardo Simão Medeiros https://orcid.org/0000-0002-0276-7817
Márcia Kauer-Sant’Anna https://orcid.org/0000-0003-2548-6230
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Merikangas KR, He JR, ping J, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2012;68(3):241-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3:171-178. [DOI] [PubMed] [Google Scholar]
- 3.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537. [DOI] [PubMed] [Google Scholar]
- 4.Judd LL, Akiskal HS, Schettler PJ, et al. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005;62(12):1322-1330. [DOI] [PubMed] [Google Scholar]
- 5.Kauer-Sant’Anna M, Bond DJ, Lam RW, Yatham LN. Functional outcomes in first-episode patients with bipolar disorder: a prospective study from the systematic treatment optimization program for early mania project. Compr Psychiatry. 2009;50(1):1-8. [DOI] [PubMed] [Google Scholar]
- 6.Pompili M, Gonda X, Serafini G, et al. Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar Disord. 2013;15:457-490. [DOI] [PubMed] [Google Scholar]
- 7.Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood And Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crippa JA, Guimaraes FS, Campos AC, Zuardi AW. Translational investigation of the therapeutic potential of Cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright M, Di Ciano P, Brands B. Use of cannabidiol for the treatment of anxiety: a short synthesis of pre-clinical and clinical evidence. Cannabis Cannabinoid Res. 2020;5(3):191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231. [DOI] [PubMed] [Google Scholar]
- 11.Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman TP, Hindocha C, Baio G, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. 2020;7(10):865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurd YL, Spriggs S, Alishayev J, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: A double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176(11):911-922. [DOI] [PubMed] [Google Scholar]
- 14.de Morais H, Chaves YC, Waltrick APF, et al. Sub-chronic treatment with cannabidiol but not with URB597 induced a mild antidepressant-like effect in diabetic rats. Neurosci Lett. 2018;682:62-68. [DOI] [PubMed] [Google Scholar]
- 15.Sales AJ, Crestani CC, Guimaraes FS, Joca SRL. Antidepressant-like effect induced by Cannabidiol is dependent on brain serotonin levels. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:255-261. [DOI] [PubMed] [Google Scholar]
- 16.Sales AJ, Fogaca Mv, Sartim AG, et al. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol Neurobiol. 2019;56(2):1070-1081. [DOI] [PubMed] [Google Scholar]
- 17.Shbiro L, Hen-Shoval D, Hazut N, et al. Effects of cannabidiol in males and females in two different rat models of depression. Physiol Behav. 2019;201:59-63. [DOI] [PubMed] [Google Scholar]
- 18.Pinto JV, Ziak ML, Schaffer A, Yatham LN. Cannabidiol in the Treatment of Mood Disorders. Curr Treat Options Psychiatry. 2022;9:140-150. [Google Scholar]
- 19.Pinto JV, Saraf G, Frysch C, et al. Cannabidiol as a treatment for mood disorders: a systematic review. Can J Psychiatry. 2020;65(4):213-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesney E, Oliver D, Green A, et al. Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology. 2020;45(11):1799-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.APA. Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry. 1997;12(5):232-241. [Google Scholar]
- 23.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. [DOI] [PubMed] [Google Scholar]
- 24.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435. [DOI] [PubMed] [Google Scholar]
- 25.Guy W. ECDEU assessment manual for psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. DHEW publication no. (ADM) 76-338. [Google Scholar]
- 26.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282(18):1737-1744. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1): 56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. [DOI] [PubMed] [Google Scholar]
- 29.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10(3):799-812. [Google Scholar]
- 30.Rosa AR, Sanchez-Moreno J, Martinez-Aran A, et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health. 2007;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1-100. [DOI] [PubMed] [Google Scholar]
- 32.Parker G, Ricciardi T, Hadzi-Pavlovic D. Placebo response rates in trials of antidepressant drugs in adults with clinical depression: increasing, decreasing, constant or all of the above? J Affect Disord. 2020;271:139-144. [DOI] [PubMed] [Google Scholar]
- 33.Leas EC, Nobles AL, Caputi TL, Dredze M, Smith DM, Ayers JW. Trends in internet searches for Cannabidiol (CBD) in the United States. JAMA Netw Open. 2019;2(10):e1913853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spinella TC, Stewart SH, Naugler J, Yakovenko I, Barrett SP. Evaluating cannabidiol (CBD) expectancy effects on acute stress and anxiety in healthy adults: a randomized crossover study. Psychopharmacology. 2021;238(7):1965-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devinsky O, Thiele EA, Wright S, et al. Cannabidiol efficacy independent of clobazam: meta-analysis of four randomized controlled trials. Acta Neurol Scand. 2020;142(6):531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dos Santos RG, Guimarães FS, Crippa JAS, et al. Serious adverse effects of cannabidiol (CBD): a review of randomized controlled trials. Expert Opin Drug Metab Toxicol. 2020;16(6):517-526. [DOI] [PubMed] [Google Scholar]
- 37.Bergamaschi MM, Queiroz RHC, Zuardi AW, Crippa JAS. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6(4):237-249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cpa-10.1177_07067437231209650 for Cannabidiol as an Adjunctive Treatment for Acute Bipolar Depression: A Pilot Study by Jairo Vinícius Pinto, José Alexandre S. Crippa, Keila Maria Ceresér, Miréia Fortes Vianna-Sulzbach, Érico de Moura Silveira Júnior, Gabriel Santana da Rosa, Manoella Guatimuzim Testa da Silva, Gabriel Henrique Hizo, Leonardo Simão Medeiros, Carlos Eduardo Santana de Oliveira, Giovana Bristot, Alline Cristina Campos, Francisco Silveira Guimarães, Jaime E. C. Hallak, Antonio W. Zuardi, Lakshmi N. Yatham, Flávio Kapczinski and Márcia Kauer-Sant’Anna in The Canadian Journal of Psychiatry