Abstract
Ischemic stroke is a leading cause of morbidity and mortality and disproportionally affects women, in part due to their higher longevity. Older women have poorer outcomes after stroke with high rates of cognitive deficits, depression, and reduced quality of life. Post-stroke inflammatory responses are also sexually dimorphic and drive differences in infarct size and recovery. Factors that influence sex-specific immune responses can be both intrinsic and extrinsic. Differences in gonadal hormone exposure, sex chromosome compliment, and environmental/social factors can drive changes in transcriptional and metabolic profiles. In addition, how these variables interact, changes across the lifespan. After the onset of ischemic injury, necrosis and apoptosis occur, which activate microglia and other glial cells within the central nervous system, promoting the release of cytokines and chemokines and neuroinflammation. Cells involved in innate and adaptive immune responses also have dual functions after stroke as they can enhance inflammation acutely, but also contribute to suppression of the inflammatory cascade and later repair. In this review, we provide an overview of the current literature on sex-specific inflammatory responses to ischemic stroke. Understanding these differences is critical to identifying therapeutic options for both men and women.
Keywords: Ischemic stroke, Sex differences, Neuroinflammation, Sex hormones
Background
Stroke is a major cause of morbidity and mortality worldwide. Stroke prevalence increases with age and is expected to increase significantly in the next decade as the population ages further [1, 2]. While the mortality from stroke has decreased by over 7% over the last decade, due to improvements in acute care, the number of patients left disabled after a stroke remains high, and there are currently over a million stroke survivors living in the USA [1, 3]. Similarly, compared to 2010, the cost of stroke care is expected to triple to $184.0 billion by 2030 [2].
Females have a higher lifetime risk of having an ischemic stroke, in large part due to their longer life expectancy [4, 5]. Stroke risk is also higher in younger females (≤ 35 years of age) compared with men in the same age group [6]. In middle age, men have a higher risk of stroke. With increasing age, these differences diminish, and the incidence climbs in both sexes [5, 7, 8]. Importantly, the incidence of stroke in women did not decrease significantly over a 25-year period in the Greater Cincinnati/Northern Kentucky Stroke Study, whereas the incidence in men did [9]. Similarly in the Framingham study, the 30-day mortality decreased significantly for men, but not for women over a 50-year period [10]. Females are also more likely to be severely disabled, less likely to be discharged home and more likely to die after their stroke [11]. However, when adjusted for age and other confounding factors, men have similar to higher mortality [11].
There are multiple reasons for these sex differences. Women have a higher incidence of stroke when < 35 years old, and pregnancy and pregnancy-associated hypertension are important risk factors [12]. Other risk factors include use of oral contraceptive pills (OCPs) and a higher incidence of migraines and autoimmune conditions [13]. Older women are also almost twice as likely to have stroke when in atrial fibrillation (AF) compared to men, which is reflected in the CHA2DS2-VASc that allocates an additional point for female sex [14–17]. The CHA2DS2-VASc is routinely used in clinical practice to help physicians risk stratify patients who are at high risk for embolic stroke and should be started on anticoagulation for stroke prevention (Tables 1 and 2). The reason for the higher embolic risk in women withAF is unknown, but recent trials have found higher baseline levels of endogenous factor Xa in women, which may lead to enhanced thrombosis[18]. The lower incidence of stroke in females between the ages of 35 and 75 years old may partially be explained by exposure to sex hormones as discussed later. However, this neuroprotection is lost after menopause [19].
Table 1.
Components of the CHA2DS2VASc score
| Letter | Risk factor | Score |
|---|---|---|
| C | History of congestive heart failure | 1 |
| H | Hypertension | 1 |
| A2 | Age 75 years or older | 2 |
| D | Diabetes | 1 |
| S2 | Stroke/TIA/thromboembolism history | 2 |
| V | Vascular disease history | 1 |
| A | Age 65–74 years | 1 |
| S | Sex, female | 1 |
Table 2.
Annual risk of embolic stroke, transient ischemic attack, and systemic embolism per year in patients with atrial fibrillation [192]
| CHA2DS2-VASc score | Risk of ischemic stroke (%) | Risk of stroke/transient ischemic attack/systemic embolism (%) |
|---|---|---|
| 0 | 0.2 | 0.3 |
| 1 | 0.6 | 0.9 |
| 2 | 2.2 | 2.9 |
| 3 | 3.2 | 4.6 |
| 4 | 4.8 | 6.7 |
| 5 | 7.2 | 10.0 |
| 6 | 9.7 | 13.6 |
| 7 | 11.2 | 15.7 |
| 8 | 10.8 | 15.2 |
| 9 | 12.2 | 17.4 |
There are also sex differences in the inflammatory response to an acute stroke, which include disruption of the blood–brain barrier (BBB) and resultant secondary ischemic, hemorrhagic, and edema-related damage. These differences may account for post-stroke morbidity, and hence, identification of these disparities is important to stroke management and drug development [20]. These differences may be secondary to variations in hormones, sex chromosomal effects, and their differential effects with aging. In this review, we summarize the known sex differences in the inflammatory response to stroke.
Neuroinflammatory responses after stroke
Innate immune responses in stroke
Following arterial occlusion, the innate immune system detects injury with pattern recognition receptors (PRR). PRRs such as toll-like receptors (TLRs) respond to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which are passively released from cells that have died after the induction of cell death pathways and contribute to activation of the complement system and the innate immune system [21–23]. Experimental studies have found that the activation and response to cell death pathways diverge between the sexes, even in cell culture models, which likely contributes to the subsequent immune response [24–27]. Activation of the complement system in the intravascular and intraparenchymal compartments is associated with worse outcomes [28–30]. Microglia migrate towards the area of ischemic damage [31], and macrophages appear at the site of infarction within minutes to hours and peak 1–4 days after stroke [32]. Microglia are brain-resident innate immune cells that are present throughout the CNS and respond quickly to inflammatory stimuli. Previously, microglial activation states were discretely classified into “M1” (inflammatory) and “M2” (reparative) based on a limited number of cell-surface and cytokine markers. More recently, single-cell transcriptomic studies have demonstrated that this simplistic classification does not accurately capture the diverse multifunctional states of microglia, which exist along a continuous transcriptomic trajectory [33]. After ischemic injury, microglia undergo alternative gene transcription that results in the release of pro- or anti-inflammatory cytokines depending on their activation states. Commonly used pro-inflammatory markers include CD16/32, CD86, MHCII, COX-2, NOS2, interleukin (IL)-1β, and tumor necrosis factor (TNF)-α, while CD206, arginase 1, FIZZ1, and YM1 are considered as anti-inflammatory markers [34, 35]. Caution should be taken when using these markers in clinical studies as human microglia show have limited overlap with mice models [35]. Microglia in the nearby area and perivascular macrophages mediate early neurotoxicity as they release reactive oxygen species (ROS); cytokines such as such as IL-6, IL-1β, and TNF-α; nitric oxide (NO); and matrix metalloproteinases (MMPs) [36–38]. Huang et al. demonstrated that the proinflammatory phenotype of microglia and their associated inflammatory cytokines remained elevated up to 28 days after initial ischemic injury in rats [39]. With time, microglia phagocytose dead cells and debris, helping reduce inflammation and clearing the way for reparative processes [40]. Multiple different cytokines, immunocomplexes, apoptotic cells, fatty acids, oxysterols, and 9-cis retinoic acid then regulate transcription factors to induce genes with anti-inflammatory effects [41, 42]. This transition of microglia results in a cascade of anti-inflammatory pathways including IL-4, shown to decrease infarct size [38]. Microglia with a defined antiinflammatory phenotype release IL-10, chitinase-3-like protein 3, and transforming growth factor-β (TGF-β), which promotes angiogenesis and BBB repair [43] and promotes neural stem/progenitor cells differentiation by upregulating TGF-α [44].
As neurons die, they release extracellular RNA, and microglia release cytokines including IL-1α, TNF, and C1q which induces astrocytes to rapidly kill additional neurons and oligodendrocytes [32, 45]. This is mediated by activation of the transcription factor NF-κB, which leads to increased IL-6 and TNF-α levels [45]. Astrocytes also release IL-15 which recruits peripheral immune cells into the brain and CXCL1 which recruits neutrophils to the brain [46, 47]. In parallel to the activation of the inflammatory cascade, the coagulation cascade is also activated resulting in release and transcription of adhesion molecules, which enhance leukocyte adhesion and transmigration into the brain, and provides a chemotactic stimulus for monocytes and neutrophils [48–50].
Neutrophils are recruited early into the infarcted brain and contribute to increased infarct volume stroke severity and hemorrhagic transformation [51, 52]. Interestingly, depletion of microglia with a colony-stimulating factor 1 receptor (CSF1R) inhibitor increased neutrophil numbers and enlarged the ischemic lesion, suggesting that microglial are an important first line of defense against neutrophil entry after stroke [53]. Neutrophils increase BBB disruption by releasing MMPs including MMP-9, which increases cerebral edema secondary to inflammation [54] and induces thrombosis secondary to the formation of neutrophil extracellular traps (NETs) [55, 56]. Similar to microglia, external stimuli can alter gene transcription in neutrophils, so they produce more anti-inflammatory cytokines and chemokines which are associated with reduced infarct volume and improved outcomes [57]. Dendritic cells are also an important component of the innate immune response to stroke and function as antigen presenting cells. They migrate to the infarct core and express MHCII which contributes to activation of lymphocytes [36].
Adaptive immune responses in stroke
T cells are a critical component of adaptive immunity. With BBB compromise, several subsets of T cells enter the brain parenchyma. CD8+ T cells exert a direct cytotoxic effect by releasing granzymes and perforins in target cells which results in apoptosis of these cells [58]. CD4+ T cells release IL-21 which binds to IL-21 receptors on neurons and induces autophagy [59]. Th17 and γδT cells produce IFN-γ and IL-17 which leads to neutrophil recruitment, BBB disruption, neurotoxicity, and microvascular dysfunction. In addition, previous studies have shown that γδT cell depletion improves stroke outcomes [60, 61]. Regulatory T (Treg) cells release IL-10 which inhibits proinflammatory cytokines IL-1β and TNF-α [62], and these cells also inhibit MMP activity, which improves BBB integrity [63]. Tregs also inhibit astrogliosis through amphiregulin, an epidermal growth factor receptor ligand, promote oligodendrogenesis by secreting osteopontin and inducing reparative microglia, and repair the injury by secreting IL-10 which promote neural stem cell proliferation [64–66]. After the acute phase of inflammation, T cells and microglia release growth factors that aid in recovery after stroke [67, 68]. IL-10-secreting CD19+ B cells limit infarct volume by inhibiting activation and recruitment of inflammatory T cells, macrophages, and microglia [69] (Fig. 1).
Fig. 1.
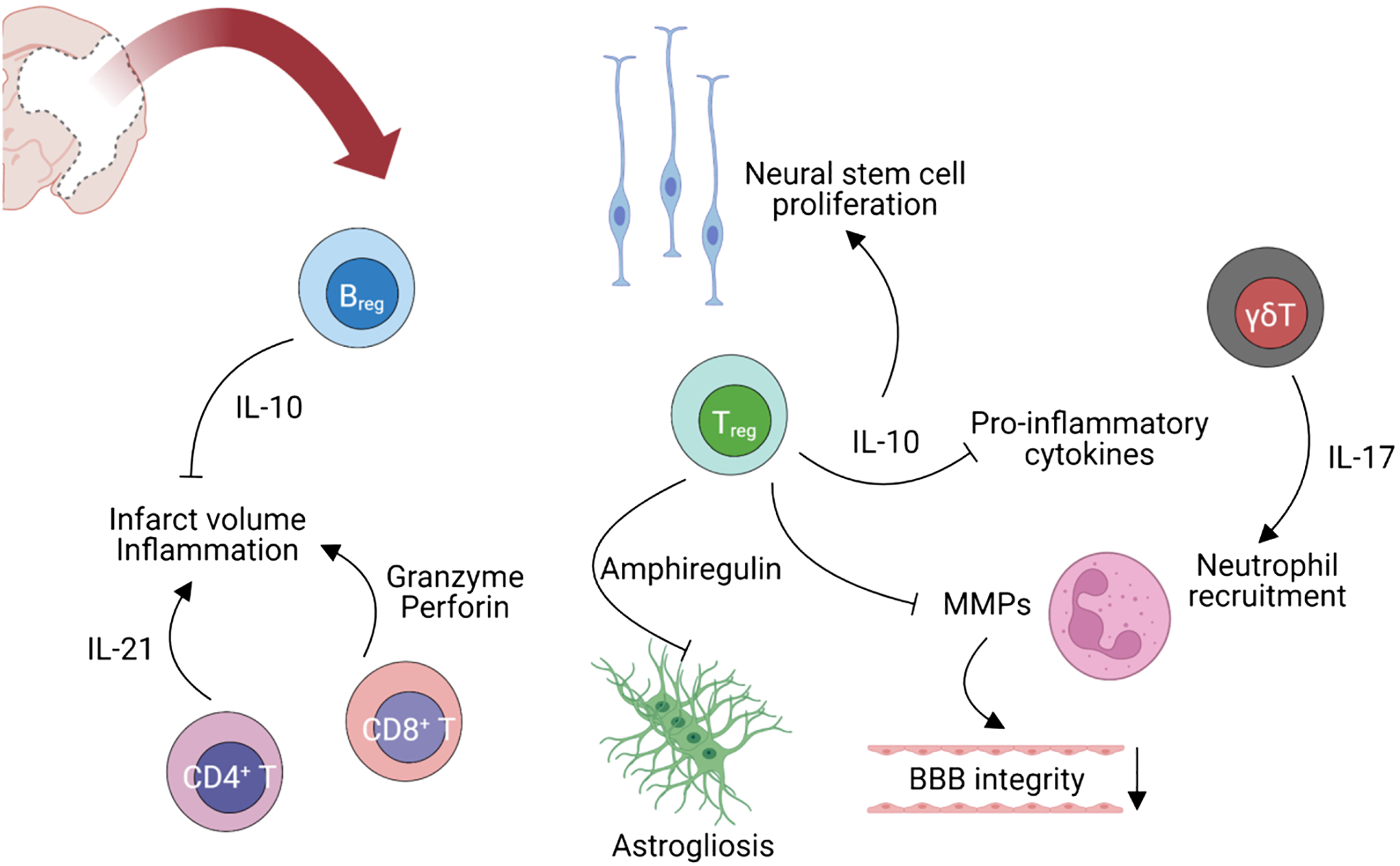
Adaptive immune responses regulated by T and B cells in stroke. (created with BioRender.com)
Systemic responses to stroke
In addition to these local responses, other peripheral organs are also affected by ischemic stroke. Sahota et al. showed that spleen size decreases over the first few days after an ischemic stroke, which is likely due to the exodus of leukocytes [70]. Similarly, the number of monocytes in the spleen decreases and increases in the brain following stroke [71], and monocyte numbers are associated with poorer outcomes [72]. Conversely, splenectomy 2 weeks prior to middle cerebral artery occlusion (MCAO) surgery in rats reduced infarct volumes by 80% and decreased the numbers of macrophages and neutrophils in the brain [73]. However, determining causality can be difficult, as differences in infarct size may mediate the subsequent immune response. In mice, males had larger strokes than females, but after splenectomy, infarct volumes in males were comparable to that seen in females [74]. This may be due to intrinsic differences in splenic T cell composition. More regulatory lymphocytes were found in the spleens of females, whereas male mice had more activated splenic T cells which may contribute to the differential response to splenectomy [75–77]. Although splenectomy decreases infarct volume in animal models of stroke, this is not a practical approach for patients. The contribution of peripheral tissues to post-stroke neuroinflammation (e.g., vagal signals) needs to be investigated as a more translationally relevant target.
Non-splenic immune organs contributing to stroke outcome: gut and bone marrow
The bidirectional communication between the brain, the gut, and the microbiome (often termed the microbiota-gut-brain axis) has been increasingly recognized in neurological diseases including stroke [78]. Stroke-induced dysbiosis (imbalance of the gut microbiota composition) led to infiltration of both anti- and proinflammatory cells (e.g., T cells) into the brain. In addition, post-stroke impairment of intestinal barrier function may lead to the translocation of luminal antigens (e.g., bacteria) evoking inflammatory responses mediated by the immune cells in lamina propria that subsequently exacerbate both systemic and neuroinflammation. In terms of sex differences in the gut response to stroke, it was previously reported that male rats (5–7 months of age) had increased gut permeability and higher systemic proinflammatory cytokines (e.g., IL-17A, MCP-1, and IL-5) and worse neurological outcomes after stroke as compared with females [79]. Likewise, these changes in gut permeability were seen in aged mice and led to an increased risk of hemorrhagic transformation in males [80]. Sex differences in gut dysbiosis have been reported in stroke and other neurodegenerative diseases (reviewed in Korf et al. [81]).
In addition to the involvement of the gut, the hematopoietic stem cell niche in the bone marrow also plays a crucial role in post-stroke inflammation. Courties et al. demonstrated that ischemic stroke causes an efflux of inflammatory monocytes and neutrophils from the bone marrow into the circulation, regulated by sympathetic nerve signaling [82]. Future investigations examining potential sex differences in the role of the bone marrow and other peripheral tissues in post-stroke immune responses are needed.
Thromboinflammation
Thromboinflammation refers to the interaction between thrombotic and inflammatory cascades activated by stroke [83, 84]. Blood components, such as platelets along with innate and adaptive immune cells, play a central role in the pathogenesis of thromboinflammation. Platelets adhere to injured endothelium via glycoprotein (GP) VI, integrin α2β1, and GPIbα subunit binding to collagen and von Willebrand factor (VWF) after which they become activated [84, 85]. After activation, platelets release adenosine diphosphate and thromboxane A2 and platelet aggregation ensues. Platelets activate Factor XII which then initiates coagulation and triggers the kallikrein-kinin system, producing bradykinin. Activation of the bradykinin receptor results in BBB disruption and cerebral edema [86]. T cells interact with platelets through CD40/CD40L which enhances transendothelial migration [83]. Similarly, neutrophils also interact with platelets with MAC-1/GP1ba connections activating neutrophil elastase which induces the production of MMP9, which further disrupts the BBB and triggers the release of DAMPs and ROS.
Epoxyeicosatrienoic (EETs) acid are vasodilators and protect the endothelium [87]. Soluble epoxide hydrolase (sEH) is responsible for the metabolism of EETs, reducing their function. sEH levels are higher in males [88], but these sex differences are lost in sEH knockout mice and after ovariectomy [85]. Data demonstrating differences in thrombus size and composition is conflicting. Male rats had larger thrombus size, and administration of testosterone increased both mortality and thrombus size [89]. In porcine models, ovariectomy resulted in enhanced platelet aggregation and MMP levels [90]. This suggests that estrogen plays a protective role in platelet aggregation and loss of estrogen with menopause may explain the increased incidence of thrombotic events in women, but this has not been recapitulated in humans [91]. While thromboinflammation has been well studied, sex differences in thromboinflammation have been less so. Additional work is needed to elucidate these sex differences.
Sex differences in immunity
Multiple studies have demonstrated sex differences in inflammatory response, which have been reviewed previously [92]. These sex differences vary with age and hormonal exposure. Differences in innate immunity are seen early in the inflammatory cascade, for example, recognition of PRRs. Females have higher levels of TLR7, which results in increased production of IFN-α [93]. Male macrophages express higher levels of TLR4 which results in increased CXC-chemokine ligand 10 production, a proinflammatory chemokine [94]. Adaptive immunity also varies based on age. As women transition from childhood to adulthood and then into old age, their CD4+ T cell count, B cell count, and CD4/CD8 ratio increase in comparison to men [95–97]. Females also show stronger antibody responses and have higher immunoglobulin levels [92]. In addition to hormonal differences, there are also genetic differences between the sexes that influence the transcriptional profile and lead to differential protein expression after ischemic injury, which will be discussed below.
It is important to note that while estrogen has been shown to have protective effects in animal models, hormone therapy (HT) after menopause for primary or secondary prevention of stroke has not been shown to be beneficial. In the WEST trial, post-menopausal women with a recent ischemic stroke or transient ischemic attack (TIA) were randomized to estrogen therapy (1 mg of estradiol-17β per day) or placebo. The incidence of nonfatal stroke and nonfatal ischemic stroke was similar in the two treatment groups although there was a non-significant increase in both risk of death and for more severe stroke in women treated with estrogen (RR; 2.9; 95 percent CI; 0.9 to 9.0) [98]. Other randomized trials confirmed an increase in the risk of ischemic stroke in healthy postmenopausal patients taking HT [99–101]. The reasons for the conflicting results in animal models versus clinical trials have been discussed elsewhere but likely involve the timing of HT and dosing regimens [102]. In the WEST trial, women had established vascular disease, and the average age of enrolled patients was 71. In the WHI, the average age on enrolled subjects was 63, over a decade post-menopause. Animal studies have implicated that extended period of hypoestrogenicity leads to a loss of estrogen-mediated neuroprotection and suppresses its anti-inflammatory actions [103, 104]. Ongoing clinical trials that enrolled women at the time of menopause are currently ongoing [105, 106].
Sex differences in the blood–brain barrier (BBB)
The BBB consists of endothelial cells, astrocytes, and pericytes and with neurons and microglia, forming the neurovascular unit [107]. These cells efficiently provide nutrients to the brain parenchyma while preventing harmful antigens from entering the brain. Endothelial cell tight junctions strictly regulate paracellular and transcellular transport of antigens, with pericytes providing direct contact to the endothelium and lending additional integrity to the BBB [108, 109]. Limited data is available on sex differences in the integrity of BBB, but there is some evidence that females have decreased permeability and hence have a more intact BBB as reviewed by Weber et al. [110]. This may due to protective effects of estrogen as ovariectomy increased the permeability of BBB in mice [111]. Similarly, rats had a higher astrocytic aquaporin-4 expression after ovariectomy which led to vasogenic edema. Estrogen replacement decreased the BBB disruption and restored the expression of aquaporin-4 in adult female rats, further demonstrating the protective role of estrogen [112]. Testosterone is also linked with BBB integrity, and male mice who were castrated showed loss of tight junction protein and increased BBB permeability, which could be reversed with testosterone supplementation [113].
Ischemia further disrupts the BBB barrier. Liu et al. demonstrated that aging female mice have higher BBB permeability compared to young female mice, while aging male mice had decreased permeability compared to young male mice [114]. The difference seen in females with aging is likely secondary to estrogen as mice with ovariectomy had the highest BBB permeability, while male mice continue to metabolize testosterone to estrogen (via aromatase). One small clinical study in stroke patients found no sex differences in MMP-9 levels, a protein that has been shown to disrupt the BBB by degrading tight junctions and the basement membrane, or its inhibitor, tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) [115]. MMP-9-induced disruption of the BBB is associated with hemorrhagic transformation, but sex differences in hemorrhagic transformation incidence remain unclear [116]. While BBB disruption is associated with post-ischemic injury, the role of sex differences in BBB integrity and its implications on infarct size and outcomes is not yet clear.
Cell-specific sex differences in post-stroke immune responses
Microglia
Sex differences in microglia in the healthy brain
Microglia are brain-specific macrophages derived from the fetal yolk sac which account for 5–12% of all brain cells [117]. They are involved in the development and maturation of synapses [118, 119], participate in brain masculinization [120], clear debris, and are involved in regeneration [121]. There is well-documented heterogeneity in the location and functional states of microglia between the sexes [122–126]. Along with differences in density, studies have also shown varying morphologies between males and females. Guneykaya et al. demonstrated that microglia have similar soma sizes in male and female mice at 3 weeks, but at 13 weeks, male microglia were larger [125]. Schwartz et al. showed that at postnatal day 4, male rats had rounder microglia with thick long processes, but this reversed by day 30 [126]. In the prelimbic cortex, female rats had a higher primed/ramified ratio of microglia when compared to males. However, with stress, this ratio increased in males and decreased in females [124]. Another study showed that males had more complex microglia in the dorsal hippocampus, while females had a more complex microglia morphology in the prefrontal cortex [127]. These differences are important to note as they suggest microglia in male rodents are already in a more activated state and are more responsive to neuroinflammatory stimuli, which may play an important role in the response to stroke.
Transcriptomic analysis has shown that there are considerable differences in microglia in males and females, and these differences vary based on brain region and age. In 13-week-old mice, there were 1109 differentially expressed genes in the hippocampus and 55 in the cortex. The genes that were more highly transcribed in males included “transcription factor activity” and “histone demethylation and deacetylation” in the cortex and “regulation of defense response to bacteria,” “insulin receptor pathway,” “glia cell differentiation,” and “ATP binding” in the hypothalamus. Females had overrepresentation of “ubiquitin protein activity” and “magnesium ion transport” in their gene set [125]. Similarly, Villa et al. identified 546 differentially expressed genes in mice with increased expression of transcription factors associated with 79% of the 95 inflammatory genes that were more expressed in males. These included genes involved in the inflammatory response, leucocyte migration, regulation of response to wounding, chemotaxis, and regulation of cytokine production. In contrast, microglia from females had increased expression of genes regulated by transcription factors that inhibit inflammation [128]. Other studies demonstrated differential expression of genes at all age groups but showed that as female mice aged, the expression of their inflammatory genes increased [129] including genes involved with the APOE network [130], an important risk factor for neurodegenerative disease. These transcriptomic analyses suggest that genes are differentially expressed in male and female rodents and that males have enhanced expression of transcription factors that induce proinflammatory genes and females have a higher number of transcription factors that induce anti-inflammatory genes. One important caveat is that these studies were performed in young animals, so how the microglial transcriptome changes with aging remains relatively unexplored.
Sex differences in microglial response to stroke
Microglia are highly plastic cells that assume diverse roles in response to different signals [131] and play a central role in the neuroinflammatory response to stroke. As mentioned in previous sections, transcription, phenotype, and location of microglia vary based on sex. Transcriptomics analysis shows that of the differentially expressed genes, proinflammatory genes are more common in males. Similarly, proinflammatory phenotypes of microglia are more dominant in males [131], whereas anti-inflammatory phenotypes of microglia increase significantly in females post-stroke [75] (Fig. 2).
Fig. 2.
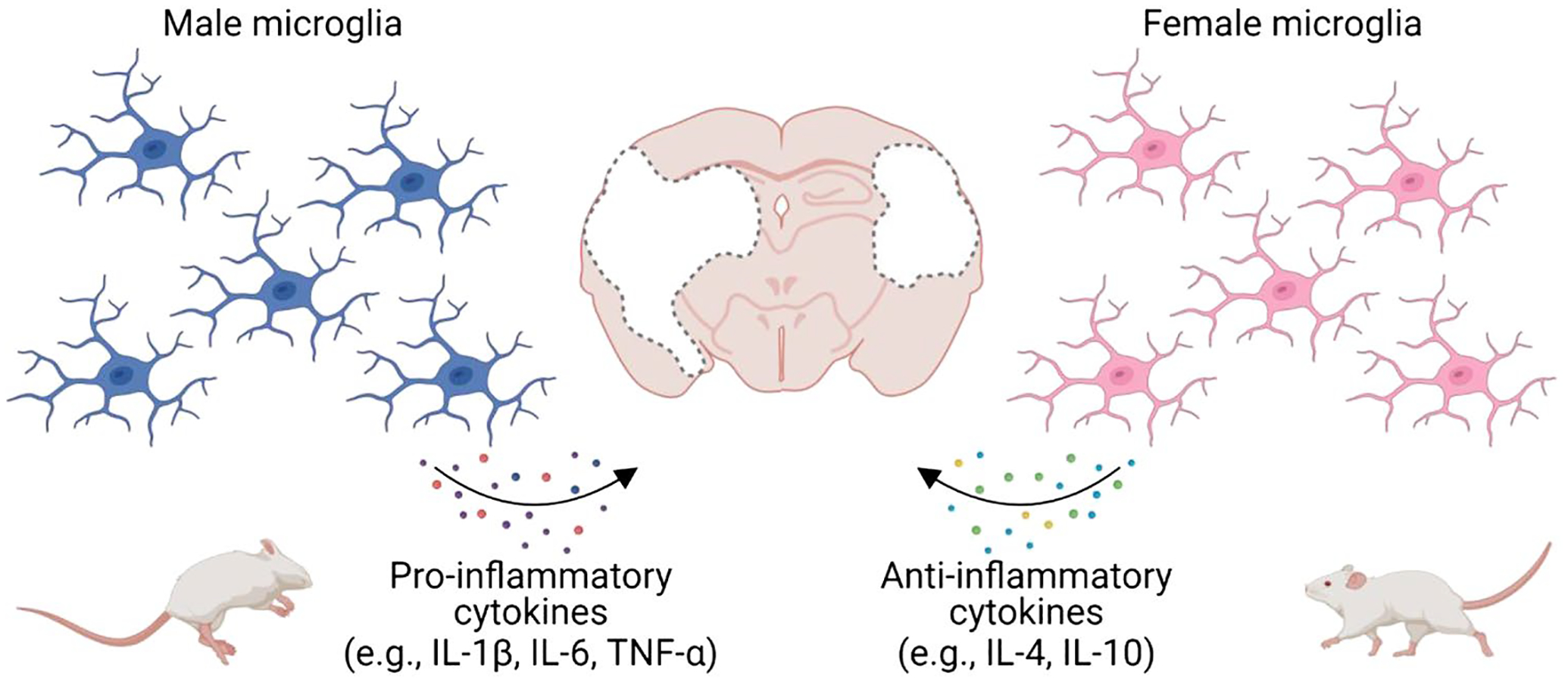
Sex differences in microglial response following stroke. Female microglia confer neuroprotection in stroke by secreting higher levels of anti-inflammatory cytokines including IL-4 and IL-10 and lower proinflammatory cytokines including IL-1β, IL-6, and TNF-α, compared with male microglia (created with BioRender.com)
One important inducer of the anti-inflammatory phenotype of microglia is IL-4, which is more highly expressed in female mice. Deletion of IL-4 using knockout mice led to a loss of sex differences in infarct volume and outcome [131]. In contrast, male-derived microglia have higher expression of S100a8, a TLR4-binding protein that is involved in proinflammatory cytokine regulation [125, 132–134]. With aging, females have an increase in the expression of inflammatory microglial complement proteins, which is consistent with the higher levels of inflammation in this population [55].
Transcriptomic analysis did not demonstrate a difference between male and female expression of estrogen receptors. This suggests that the circulating levels of hormones, rather than the number of estrogen receptors, are responsible for any hormone-related differences [34, 135]. In adult female mice, 17β-estradiol levels are high and induce an anti-inflammatory phenotype in microglia which results in downregulation of IL-1β, IL-6, and TNF-α and upregulation of the anti-inflammatory cytokine, IL-10 (Fig. 2) [135]. Progesterone also induces microglial transition into an antiinflammatory phenotype, decreasing the inflammatory response [136].
Interestingly, with aging and gonadal senescence, the sex chromosome compliment (XX vs. XY) plays an increasingly important role in the response to ischemic injury. Although the second X chromosome in females is “deactivated” for genetic balancing between the sexes, females express a high degree of mosaicism, depending on which X chromosome is silenced and what tissue is examined. It is becoming increasingly clear that many X-linked genes escape X-chromosome inactivation, and this is more common with aging and in the brain [137]. The four-core genotype (FCG) model can be used to dissociate gonadal and chromosomal sex. In this model, the testis determining gene Sry was spontaneously deleted from its normal position on the Y chromosome. A transgenic Sry gene is placed on an autosome (chromosome 3). Breeding XX gonadal female mice with XY –Sry gonadal male mice produces XX females and XY females (with no sry on the Y chromosome), both of which have ovaries, and XXSry-males (with sry on chromosome 3) and XYSry + males (with sry on chromosome 3) that have the testis [138]. In young mice, Manwani et al. found that young FCG mice with ovaries had smaller infarcts than mice with testes. However, this difference was lost after gonadectomy, after which all four genotypes had equivalent infarct size. This suggests that estrogen is the main protective factor in this age group [139]. However, in reproductively senescent aged mice with similar levels of gonadal hormones (18–20 months of age), mice with two X chromosomes had worse outcomes regardless of gonadal sex. All mice with an XX chromosome compliment had enhanced “proinflammatory” microglia, and a downstream increase in proinflammatory cytokines [135, 140]. This was due to X chromosome genes that escape X chromosome activation in a low hormone milieu [140] and was mediated by interferon regulatory factors (IRF). Expression of KDM6A, a gene on the X chromosome that escapes inactivation, led to H3K27me3 demethylation and higher levels of IRF5, which promotes the proinflammatory microglia phenotype. The KDM6A-IRF5 pathway was increased in females, confirming epigenetic involvement in cytokine production [141]. Microglia in aged females also show an increased expression of MHCII and increased proinflammatory cytokine production [140]. Multiple factors influence microglial activation, and the resultant production of cytokines and chemokines (Table 3). Additional studies are needed to further investigate the sex-specific modulation of microglia.
Table 3.
Preclinical studies examining sex-specific differences in microglia and astrocytes in the response to ischemic stroke
| Study | Species and strain | Age | Key findings |
|---|---|---|---|
| Xiong et al. [131] | BALB/cJ and IL-4 KO (BALB/c-IL-4tm2Nnt/J) mice | 10–12 weeks | IL-4 is neuroprotective in female mice. It prompts differentiation to the M2 (anti-inflammatory) microglia phenotype and reduced monocyte/macrophage infiltration into the ischemic brain |
| Seifert et al. [75] | C57BL/6 J mice | 8–10 weeks | Post MCAO, female mice had smaller infarct volumes. This was likely due to higher frequency of anti-inflammatory microglia/macrophages, CD11b+CD206+ in infarcted tissue. Female mice also have decreased anti-inflammatory macrophages and IFNγ expression after MCAO |
| Bodhankar et al. [191] | Wild-type C57BL/6 J | 10–12 weeks | Treatment with rIL-10 B cells inhibited proinflammatory cytokine production and differentiation into M2 microglia in both sexes. However, the inhibition was more pronounced in female mice. rIL-10 B cells also increase the production of the neuroprotectant IL-4 in females. Post-stroke, female mice have higher expression of IL-10R and IL-4Rα |
| Crain et al. [135] | C57Bl/6 mice | P3, P21,7–8 week, 4 months, 12 months | Microglia gene expression changes with age. The expression of inflammatory and anti-inflammatory cytokines is also sex dependent. Female mice had a more anti-inflammatory response at different age groups, but there was shift towards a more inflammatory phenotype in older mice |
| McCullough et al. [140] | C57BL/6 J XYM and wild-type C57BL/6 J mice bred to produce the following genotypes: XYM, XXM, XYF, XXF | 18–20 months | Aged mice with a second X chromosome (XXF and XXM) had larger strokes volumes, with significantly more activated microglia and higher levels of inflammatory cytokines, when gonadal hormones are not present |
| Qi et al. [141] | C57BL/6 J XYM and wild-type C57BL/6 J mice bred to produce the following genotypes: XYM, XXM, XYF, XXF | 18–22 months | The KDM-Histone-IRF pathway, which regulates microglia cytokine production, was more highly expressed in wild-type females and in FCG mice with 2 X chromosomes |
| Manwani et al. [193] | C57BL/6 mice | 5–6 months, 14–15 months, and 20–22 months | Stroke leads to splenic contraction in male mice of all ages and aged female mice. Dendritic cells were recruited in higher numbers to the female compared to the male brain. No differences in the number of macrophages and neutrophils were identified |
| Dotson et al. [74] | C57BL/6 J mice | Not reported | Prior to splenectomy, male mice had larger stroke volumes than female mice. After splenectomy, male mice had reduced stroke volume such that there was no longer a difference in the infarct size between male and female mice. Activated microglia were reduced in females regardless of splenectomy and in males after splenectomy when compared to male mice without splenectomy |
| Morrison et al. [147] | C56BL6/J and C57BL/6 with CX3CR1GFP/+ | 3 months | Female mice have a higher number of CD11b microglia at baseline, whereas these numbers increase in males after MCAO. Astrocyte AQP4 changes polarity in male mice |
| Banerjee et al. [76] | C57BL/6 J mice | Not reported | Post-stroke, male mice have higher numbers of CD45hiCD11b+ microglial cells and macrophages |
| Villapol et al. [194] | C57BL6/J mice | Postnatal day 9 | Male mice had more proinflammatory microglia markers in the ischemic brain compared to female mice, 3 days after stroke. Higher levels of the proinflammatory cytokine, TNF α, was seen in male mice |
| CordeauJr et al. ([195] | Transgenic GFAP-luc mice | 10–17 week | Astrocyte response (GFAP upregulation) results in increased infarct size in males but not females |
KO knock out, IL interleukin, MCAO middle cerebral artery occlusion, IFN interferon, FCG four core genotype, AQP aquaporin, TNF tissue necrosis factor, GFAP glial fibrillary acidic protein
Astrocytes
Sex differences in astrocytes
Astrocytes form tight junctions that contribute the integrity of the BBB, use calcium ion signaling to regulate blood flow, and express aquaporin 4 to regulate water balance [142]. They also serve as a source of estradiol, progesterone, and testosterone in the CNS [143]. Female astrocytes also produce a positive feedback loop, increasing estrogen levels [144]. Astroglial transcriptomic analysis in mice showed 20 to a 100 differentially expressed genes in males compared to females. This varied with age with the peak difference seen at postnatal day 14, with few differences in adulthood. These differences were mostly found in genes related to cell cycle and neurite, dendritic, and synaptic development [145].
Sex differences in the astrocytic response to stroke
Multiple identified sex differences are seen in the astrocytic response to ischemia (Table 3). Female astrocytes release increased amounts of ionized calcium which is neuroprotective after stroke secondary to a reduction in brain edema from decreased water uptake [146, 147]. Aquaporin-4 supports paravascular flow of CSF. Increased aquaporin-4 polarization is indicated by higher aquaporin-4 presence in perivascular endfeet than in the parenchyma [148]. Aquaporin-4 polarity is associated with increased interstitial solute clearance, and lack of polarity is associated with heightened neuroinflammation [147]. Morrison et al. demonstrated that while astrocytes are equally polarized in males and females at baseline, after stroke, male astrocytes are more likely to have a change in polarity, which could also account for increased inflammation [147]. In the same study, S100β, an astrocyte Ca2+ binding protein that acts as a DAMP and promotes the neuroinflammatory response was found to increase more in males than in females [147].
Similar to what is seen in microglia, estrogen decreases the expression of the transcription factor NF-κB, leading to a reduction in the activation of proinflammatory genes and, thus, a decrease in proinflammatory cytokine levels [149]. Estrogen also blocks oxygen–glucose deprivation-induced astrocyte mitochondrial dysfunction and cell death which results in less ROS release [150]. Estrogen also induces glutamate transporter-1 and glutamate-aspartate transporter expression, which decreases excitotoxicity by removing glutamate and hence decreases the inflammatory response [151]. Further studies are needed to investigate sex differences in astrocyte after stroke.
Monocytes and macrophages
Sex differences in monocytes and macrophages
Monocytes and macrophages are central components of the innate immune response. Transcriptomic analysis showed that in human monocytes, there are 428 differentially expressed genes by sex, but the female-male fold change value was low [152]. Another study on the blood of humans with chronic inflammation showed that the expression of genes involved in the production of Fcγ receptors was increased in females, which are involved in monocyte activation (153). Similarly, females had a stronger IFN-γ response [153]. In murine bone marrow, Fcγ receptors genes were also differentially expressed in macrophages. Female mice also had a higher expression of genes that are stimulated by IFN [152]. On the other hand, analysis of human blood has shown that men have a higher expression of genes involved in phagocytosis and extracellular anti-microbial response [154].
Sex difference in monocytic and macrophagic response to stroke
After ischemic injury, monocytes (e.g., Ly6ChighCD43low cells) are recruited to the brain within a few hours and differentiate into macrophages, releasing IL-1β and TNF-α [155, 156]. Similar to other cells, mononuclear macrophages also show sex differences. After stroke, higher numbers of activated macrophages are seen in the brain of male mice, along with increased percentage of activated microglia and cells expressing the homing marker, VLA-4, that have transmigrated into the infarct [76]. These cells might contribute to larger infarcts size in males but may also be secondary to the increased ischemic damage seen in young male vs. female animals. Female mice exhibited anti-inflammatory phenotypes in microglia/macrophage with higher expression of CD206 compared with males after stroke [75]. Female mice also had more robust anti-inflammatory responses from IL-10-producing CD8 + CD122 + T cells [76]. Notably, this female-specific neuroprotection was not observed in IL-4 KO mice (131).
The spleen exhibits sex differences in stroke outcomes. Dotson et al. showed that splenectomy reduces infarct volumes in stroke males, and infarct volumes between splenectomized males and females were comparable. Interestingly, spleen-intact males had higher levels of circulating CD11b + monocytes compared to male mice with splenectomy, which was not seen in females (74). Implication of sex differences in monocyte function in inflammation after stroke needs additional study.
Neutrophils
Sex differences in neutrophils
Neutrophilic transcriptome analysis identified 106 genes which were upregulated and 128 genes which were down-regulated in women compared to men [157]. Women also had more activated and mature neutrophils (e.g., enhanced type I interferon), which have a lower activation threshold in inflammation [157, 158]. These IFN-primed neutrophils increase ROS production, migration, NET formation, and adhesion molecule expression [157]. In addition, male neutrophils had elevated mitochondrial metabolism compared with female neutrophils. Interestingly, estradiol-treated male neutrophils had lower mitochondrial metabolism, which was similar with female neutrophils, indicating that the sex-specific maturation of neutrophils might be modulated by sex hormones [157].
Sex difference in the neutrophilic response to stroke
Neutrophils are the predominate leukocyte in the blood and one of the first blood-borne immune cell to arrive in the brain following stroke, with possible contributions from the skull bone marrow and the blood via the leptomeningeal vessels [159–161]. They release proinflammatory factors, ROS, proteases, and MMPs, which leads to increased BBB damage, hemorrhagic transformation, and post-stroke edema. This results in increased infarct volumes, higher stroke severity, and poorer outcomes [36, 55]. It is important to note that neutrophils also have beneficial effects on inflammation as they promote angiogenesis, produce MMPs which can break down proinflammatory DAMPs leading to reduced inflammation, and help with recruitment of beneficial cells [162]. Estrogen plays a significant inhibitory role in neutrophil recruitment to the stroke as it inhibits CINC-2, a chemokine associated with neutrophilic chemotaxis [163]. In older mice, males had a significantly higher number of neutrophils in the brain and higher levels of MCP-1 and G-CSF in plasma when compared to females. This, along with elevated CD8+ T and regulatory T cells, resulted in a 25% higher mortality and 55% higher risk of hemorrhagic transformation in male mice (Table 4) [164].
Table 4.
Preclinical studies examining sex-specific differences in neutrophil and lymphocyte responses in ischemic stroke
| Study | Species and strain | Age | Key findings |
|---|---|---|---|
| Xiong et al. [131] | BALB/cJ and IL-4 KO (BALB/c-IL-4tm2Nnt/J) mice | 10–12 weeks | During estrous, WT female mice have significantly lower T cells, but this difference was lost with deletion of IL4 |
| Banerjee et al. [76] | C57BL/6 J mice | Not reported | Post-stroke, spleens of male mice become severely atrophic. Male spleens had higher CD8+CD122+ T-suppressor cells, whereas female spleens had higher IL-10 secreting CD8+CD122+ T-suppressor cells. There was a higher percentage of VLA-4 expression on male mice lymphocytes |
| Manwani et al. [193] | C57BL/6 mice | 5–6 months, 14–15 months, and 20–22 months | Stroke leads to splenic contraction in male mice of all ages and aged female mice. Similarly, male mice with stroke had higher levels of T cells when compared with female mice, except in elderly mice, where the difference disappeared |
| Dotson et al. [74] | C57BL/6 J mice | - | Prior to splenectomy, male mice had larger stroke volumes than female mice. After splenectomy, male mice had reduced stroke volume, and there was no longer a difference in male and female mice stroke volume. Spleen intact females have lower CD4 + T cells and lower number of “activated” T cells than males, a difference lost with splenectomy |
| Brait et al. [184] | C57BL/6 J mice | 6–8 weeks | Male mice have a higher CD3+ T cell response with increased Nox2 expression resulting in larger infarcts than those in female mice |
| Seifert et al. [75] | C57BL/6 J mice | 8–10 weeks | Post-MCAO, female mice had smaller infarct volumes. This was likely due to higher frequency of B10, CD19+CD5+CD1dhi cells. Female mice also have decreased Breg cells and increased Treg in their spleen after MCAO |
| Ahnstedt et al. [80] | C57BL/6 N mice | 20–22 months | Increased CD8+ T cells and Tregs in infarcts of aged males. No sex difference in CD4+ T cells |
| Jackson et al. [196] | Wistar rats | 12–15 weeks | After stroke, the blood of male mice had a higher percentage of circulating γδTCR and TH1 cells, but lower Treg when compared to females In the brain, males had a higher percentage of TH1 cells. Diabetes also had a sexually dimorphic effect on regulation of various T cell subtypes |
IL interleukin, VLA very late antigen, MCAO middle cerebral artery occlusion
Neutrophil extracellular traps (NETs)
Activated neutrophils release fragile fibers composed of chromatin with granular proteins called NETs [165]. NET formation peaks 3–5 days after ischemic injury [166]. NETs can form through nuclear delobulation and envelope disassembly which leads to chromatin decondensation and cellular depolarization induced by cell death. Subsequently the plasma membrane ruptures and NETs are released. NETs can also form via degranulation and expulsion of chromatin followed by extracellular NET assembly [167]. These NETs form primarily in the vessel lumen and act as a scaffold for platelet binding and further thrombosis following stroke [160, 168]. Three clinical studies which cumulatively enrolled more than 150 patients undergoing mechanical thrombectomy that had clots available for histological evaluation had increased markers of NET formation (citrullinated histone and extracellular DNA) [56, 169, 170]. Higher levels of NET markers were associated with worse discharge disposition and poorer outcomes at 1 year after the index stroke [171]. Two studies found no association between sex and the presence of NETs. A higher number of NETs was associated with poorer recanalization times, increased recanalization attempts, and contributed to worse outcomes by stabilizing the thrombus leading to less complete recanalization [169].
Following stroke, NETs release cytotoxic proteases including elastase myeloperoxidase and histones which disrupt the blood–brain barrier and reduce neovascularization [166]. NETs have also been implicated in a reduction of T cell activation threshold increasing the release of the proinflammatory cytokines IL-17 and IFN-γ [172, 173]. These findings suggest that NETs are key targets for improving recanalization with thrombolytics and thrombectomy and to reduce post-stroke inflammation and improve neovascularization.
Few studies have assessed sex differences in NETs, and most of these are confined to pregnant women as aberrant neutrophil activation is known to be involved in complications of pregnancy [174, 175]. Estrogen, granulocyte colony-stimulating factors (G-CSF), and human chorionic gonadotropin promote NETosis by increasing expression of peptidyl arginine deaminase 4 expression, neutrophil elastase, and myeloperoxidase [174]. Neutrophils from cells of healthy women underwent more NETosis after ex vivo calcium stimulation compared those from men [176]. While this suggests that women might have higher NETosis, further studies are needed to see if this impacts neuroinflammation after stroke. While DNAse-I and NET inhibitory peptides are effective in clearing NETs in vitro and ex vivo, further investigations into the role of NETs and stroke are needed with a focus on sex differences in NET pathophysiology [177].
Lymphocytes
Sex differences in T cells
Naïve CD4+ T cells differentiate into T helper cells, including Th1, Th2, Th17, and Tregs. In adults, sex differences in T cell subsets exist; women having a higher CD4+ T cell count and CD4/CD8 ratio in blood [92, 95–97, 178]. These CD4+ T cells also have higher levels of estrogen receptors than CD8+ T cells. Estrogen promotes differentiation of these CD4+ T cells to Treg cells which promotes anti-inflammatory cytokines and to Th2 cells which decreases the production of proinflammatory IL-17 by Th17 cells [179].
The X chromosome also contains the Forkhead box P3 gene which is important in Treg differentiation and the gene for CD40 ligand, which is needed for T cell activation [180]. As discussed previously, some X chromosome genes can escape inactivation in females and can result in a more robust immune response [180]. With age, CD4+ and CD8+ T cell function decreases, but this decline is accelerated in men and leads to a poor inflammatory response [181, 182].
Sex differences in T cell responses to stroke
CD4+ and CD8+ T cells contribute to the inflammatory response, especially in delayed ischemic injury. Ahnstedt et al. showed that aged male mice had higher CD8+ T cells in the brain 15 days after stroke and higher mortality than age-matched females (20–22 months of age) [80]. In both human and murine studies, females have increased serum levels of IL10, which dampens inflammatory responses but led to an increased risk of post-stroke infection and delayed recovery in females but not in males [79]. However, these elevated levels of IL-10 did not independently predict better outcomes in stroke patients (Table 5) [183]. Similar to IL-10, females also have higher levels of IL-4, which is associated with decreased recruitment of CD4+ and CD8+ T cells and, consequently, smaller infarcts [182]. In transient ischemia models, males had higher Nox2-derived superoxide, Cox-2, and VCAM-1 which are proinflammatory proteins. Nox2-containing NADPH oxidase contributes to infarct development, whereas VCAM assists with leukocyte infiltration into the brain [184]. Females also have higher numbers of regulatory T cells, which are protective in ischemic stroke by releasing anti-inflammatory cytokines including IL-10 [74]. Estrogen plays a major role in the sexual differences in Treg number and function [185]. Despite the enhanced release of anti-inflammatory cytokines, women have worse outcomes after ischemic stroke, which may be due in part to a weaker suppressive response in Tregs to T cell activation in women, leading to enhanced inflammation [186]. Beckmann et al. recently demonstrated sex differences in the Treg response after neonatal hypoxic ischemia in male and female mice, at a time point where levels of sex hormones are similar between [187]. Female mice had increased cerebral Treg infiltration, coinciding with elevated chemokine receptor expression. Treg depletion in females aggravated HI-induced brain tissue injury which paralleled an increase in microglia and endothelial activation and leukocyte infiltration. Surprisingly, Treg depletion in male mice reduced HI-induced brain injury and behavioral deficits. Isolated female Tregs had an increased immunosuppressive activity on effector T cell proliferation ex vivo. This is important as it demonstrates that sex differences in Treg function are not only due to differences in sex hormone levels, but also to differential gene expression (Table 4) [187]. Females also have upregulation of genes mediating the IL-12 cytokine signaling pathways which promotes natural killer cell toxicity, T cell proliferation, and Th1 cell differentiation (Fig. 3) [188, 189]. The full extent of regulatory T cell involvement in post-stroke outcomes remains unclear in aged subjects [190].
Table 5.
Clinical studies examining sex-specific differences in the inflammatory response to ischemic stroke
| Study | Number of study members | Age (years) | Key findings |
|---|---|---|---|
| Conway et al. [183] | 178 patients (76 females and 102 males) | Female—72.9 Male—64.5 |
Women have higher levels of IL-10, but when controlled for other factors associated with post-stroke outcomes, elevated IL-10 level was not associated with outcome |
| Yan et al. [186] | 77 stroke (31 female and 46 males) and 64 controls (31 females and 33 males) | Female controls—63 Female stroke patients—66 Male controls—63 Male stroke patients—65.5 |
Activated T cells were significantly elevated in patients with stroke. Tregs were increased in men. Tregs had a weaker suppressive response in women |
| Stamova et al. [197] | 23 stroke (11 female and 12 males) and 23 controls (11 females and 12 males) | Female controls—59.1 Female stroke patients—71.3 Male controls—56.8 Male stroke patients—72.1 |
Women and men had differential expression of genes after stroke resulting in an earlier increase in inflammatory and anti-inflammatory pathways in women. Women also had higher expression of genes associated with cell death |
| Tian et al. [188] | Control Males: 28 and females: 24 Ischemic stroke Males: 27 and females: 24 |
Control Males: 60.3 and females: 60.6 Ischemic stroke Males: 63.6 and females: 65.1 |
Women and men had differential expression of genes after stroke. Women had increased expression of genes involved with inflammatory pathways and caspase-mediated cell death, while men had higher expression of genes involved with caspase-independent cell death pathway and vessel wall adhesion |
| Xu et al. [198] | Control: 20 Ischemic stroke Male: 10 and female 10 |
Not reported | Women had higher expression of CCL20, ICAM, and PTGS2 after ischemic stroke as compared to men. Both sexes had modulation of inflammatory responses |
| Ross et al. [199] | Males: 96 and females: 96 | Males: 73.2 and females: 77.3 | Women had an increase in monocytes which was not seen in men |
| Zhu et al. [200] | Control: 20 Ischemic stroke Male: 10 and female 10 |
Ischemic stroke: 60.2 | IL1α, IL1β IL6, IL8, CXCL1, CXCL2, CXCL20, CCL4, ICAM1, and PTGS2 genes were down-regulated in women and upregulated in men |
| Trott et al. [201] | Ischemic stroke Male 70 and female 59 |
Ischemic stroke: 60.2 | After thrombectomy, there was a positive association with NIHSS change and in WBC counts in women, a finding not seen in men |
| Stamova et al. [202] | Controls Males: 41 and females: 68 Ischemic stroke Males: 35 and females: 26 |
Controls Males: 50.2 and females: 49 Ischemic stroke Males: 67.1 and females: 67 |
Women and men had differential expression of genes after stroke |
IL interleukin, CCL chemokine ligand, ICAM intercellular adhesion molecule, PTGS2 prostaglandin-endoperoxide synthase, CXCL (C-X-C) motif ligand, WBC white blood cells, NIHSS National Institute of Health Stroke Scale
Fig. 3.
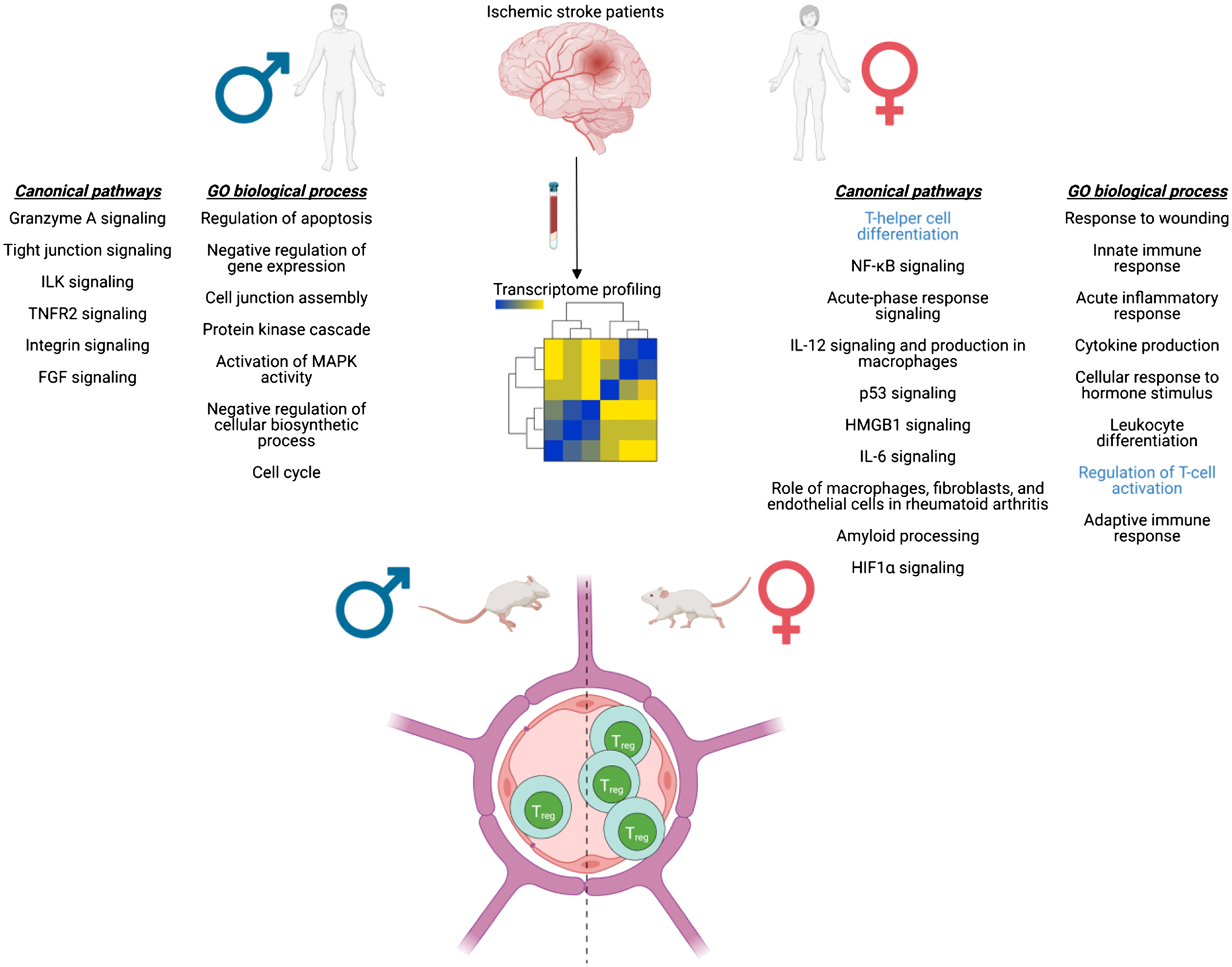
Sex differences in the systemic T cell response following stroke. Tian et al. [188] profiled gene expression in the blood from male and female ischemic stroke patients (≤ 3, 5, and 24 h) and performed functional analysis to identify pathways. Of note, female-specific pathways include T-helper cell differentiation and regulation of T cell activation. Canonical pathways and GO biological process for ≤ 3 h after stroke were shown here. In a mouse model of stroke, females had higher regulatory T cells in the blood compared with males [74] (created with BioRender.com)
Sex differences in the B cell response to stroke
B cells, and in particular IL-10 producing B cells, are associated with decreased infarct volume. Female mice have increased regulatory B (Breg) cells (CD19+CD5+CD1dhigh cells) in the ischemic hemisphere of the brain after stroke, which corresponds to fewer Breg cells in the spleen of females [75]. B cells are also implicated in the increase of anti-inflammatory microglia seen in the female brain post-stroke. IL-10 producing Breg cells promote the anti-inflammatory phenotype in microglial cells, in both males and females. Female mice have higher levels of microglial IL-10 receptor expression, which responds to the IL-10 released by Bregs. This results in sex differences with higher levels of anti-inflammatory microglia in females [191].
Conclusion
Neuroinflammation plays a vital role in post-stroke injury and recovery. Neuronal damage induced by inflammation occurs both acutely and chronically following stroke, hence providing a larger window for intervention as compared to thrombolytics and thrombectomy. The identification of sex differences in post-stroke neuroinflammation is important. There is an urgent need for sex-stratified clinical and preclinical analyses to identify and investigate these differences and implement this knowledge to develop novel sex-specific treatments for both men and women.
Funding
This work was supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) NS08779, NS103592, and NS096493 (to Dr. McCullough).
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on E, Stroke Statistics S (2020) Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141:e139–e596 [DOI] [PubMed] [Google Scholar]
- 2.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG, American Heart Association Advocacy Coordinating C, Stroke C (2013) Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke 44:2361–75 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease C, Prevention (2009) Stroke 37:345–50 [Google Scholar]
- 4.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA (2006) The lifetime risk of stroke: estimates from the Framingham Study. Stroke 37:345–350 [DOI] [PubMed] [Google Scholar]
- 5.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L (2008) Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 7:915–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leppert MH, Burke JF, Lisabeth LD, Madsen TE, Kleindorfer DO, Sillau S, Schwamm LH, Daugherty SL, Bradley CJ, Ho PM, Poisson SN (2022) Systematic review of sex differences in ischemic strokes among young adults: are young women disproportionately at risk? Stroke 53:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sealy-Jefferson S, Wing JJ, Sanchez BN, Brown DL, Meurer WJ, Smith MA, Morgenstern LB, Lisabeth LD (2012) Age- and ethnic-specific sex differences in stroke risk. Gend Med 9:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewsey JD, Gillies M, Jhund PS, Chalmers JW, Redpath A, Briggs A, Walters M, Langhorne P, Capewell S, McMurray JJ, Macintyre K (2009) Sex differences in incidence, mortality, and survival in individuals with stroke in Scotland, 1986 to 2005. Stroke 40:1038–1043 [DOI] [PubMed] [Google Scholar]
- 9.Madsen TE, Khoury J, Alwell K, Moomaw CJ, Rademacher E, Flaherty ML, Woo D, Mackey J, De Los Rios La Rosa F, Martini S, Ferioli S, Adeoye O, Khatri P, Broderick JP, Kissela BM, Kleindorfer D (2017) Sex-specific stroke incidence over time in the Greater Cincinnati/Northern Kentucky Stroke Study. Neurol 89:990–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA (2006) Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA 296:2939–2946 [DOI] [PubMed] [Google Scholar]
- 11.Turtzo LC, McCullough LD (2008) Sex differences in stroke. Cerebrovasc Dis 26:462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poorthuis MH, Algra AM, Algra A, Kappelle LJ, Klijn CJ (2017) Female- and male-specific risk factors for stroke: a systematic review and meta-analysis. JAMA Neurol 74:75–81 [DOI] [PubMed] [Google Scholar]
- 13.Chang BP, Wira C, Miller J, Akhter M, Barth BE, Willey J, Nentwich L, Madsen T (2018) Neurology concepts: young women and ischemic stroke-evaluation and management in the emergency department. Acad Emerg Med 25:54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ (2014) Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129:837–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D’Agostino RB, Larson MG, Kannel WB, Benjamin EJ (2003) A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA 290:1049–1056 [DOI] [PubMed] [Google Scholar]
- 16.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137:263–272 [DOI] [PubMed] [Google Scholar]
- 17.Piccini JP, Simon DN, Steinberg BA, Thomas L, Allen LA, Fonarow GC, Gersh B, Hylek E, Kowey PR, Reiffel JA, Naccarelli GV, Chan PS, Spertus JA, Peterson ED, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation I, Patients (2016) Differences in clinical and functional outcomes of atrial fibrillation in women and men: two-year results from the ORBIT-AF Registry. JAMA Cardiol 1:282–91 [DOI] [PubMed] [Google Scholar]
- 18.Zelniker TA, Ardissino M, Andreotti F, O’Donoghue ML, Yin O, Park J-G, Murphy SA, Ruff CT, Lanz HJ, Antman EM, Braunwald E, Giugliano RP, Merlini PA (2021) Comparison of the efficacy and safety outcomes of edoxaban in 8040 women versus 13 065 men with atrial fibrillation in the ENGAGE AF-TIMI 48 Trial. Circulation 143:673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullough LD, Hurn PD (2003) Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab 14:228–235 [DOI] [PubMed] [Google Scholar]
- 20.Spychala MS, Honarpisheh P, McCullough LD (2017) Sex differences in neuroinflammation and neuroprotection in ischemic stroke. J Neurosci Res 95:462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anthony S, Cabantan D, Monsour M, Borlongan CV (2022) Neuroinflammation, stem cells, and stroke. Stroke 53:1460–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuhmann MK, Kollikowski AM, Marz AG, Bieber M, Pham M, Stoll G (2021) Danger-associated molecular patterns are locally released during occlusion in hyper-acute stroke. Brain Behav Immun Health 15:100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roh JS, Sohn DH (2018) Damage-Associated molecular patterns in inflammatory diseases. Immune Netw 18:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patrizz AN, Moruno-Manchon JF, O’Keefe LM, Doran SJ, Patel AR, Venna VR, Tsvetkov AS, Li J, McCullough LD (2021) Sex-specific differences in autophagic responses to experimental ischemic stroke. Cells 10(7):1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD (2009) Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol 217:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD (2005) Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab 25:502–512 [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Ge Y, Liu Y, Zheng Y, Hu R, Ren C, Liu Q (2022) Identification of the key genes and immune infiltrating cells determined by sex differences in ischaemic stroke through co-expression network module. IET Syst Biol 16:28–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szeplaki G, Szegedi R, Hirschberg K, Gombos T, Varga L, Karadi I, Entz L, Szeplaki Z, Garred P, Prohaszka Z, Fust G (2009) Strong complement activation after acute ischemic stroke is associated with unfavorable outcomes. Atherosclerosis 204:315–320 [DOI] [PubMed] [Google Scholar]
- 29.Schafer MK, Schwaeble WJ, Post C, Salvati P, Calabresi M, Sim RB, Petry F, Loos M, Weihe E (2000) Complement C1q is dramatically up-regulated in brain microglia in response to transient global cerebral ischemia. J Immunol 164:5446–5452 [DOI] [PubMed] [Google Scholar]
- 30.Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, Lambris JD, Huber-Lang M (2008) Interaction between the coagulation and complement system. Adv Exp Med Biol 632:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubart A, Benbenishty A, Har-Gil H, Laufer H, Gdalyahu A, Assaf Y, Blinder P (2021) Single cortical microinfarcts lead to widespread microglia/macrophage migration along the white matter. Cereb Cortex 31:248–266 [DOI] [PubMed] [Google Scholar]
- 32.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19:987–991 [DOI] [PubMed] [Google Scholar]
- 34.Ugidos IF, Pistono C, Korhonen P, Gómez-Budia M, Sitnikova V, Klecki P, Stanová I, Jolkkonen J, Malm T (2022) Sex differences in poststroke inflammation: a focus on microglia across the lifespan. Stroke 53:1500–1509 [DOI] [PubMed] [Google Scholar]
- 35.Jurga AM, Paleczna M, Kuter KZ (2020) Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci 14:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA (2019) Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation 16:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neher JJ, Cunningham C (2019) Priming microglia for innate immune memory in the brain. Trends Immunol 40:358–374 [DOI] [PubMed] [Google Scholar]
- 38.Xiong XY, Liu L, Yang QW (2016) Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol 142:23–44 [DOI] [PubMed] [Google Scholar]
- 39.Huang M, Wan Y, Mao L, He QW, Xia YP, Li M, Li YN, Jin HJ, Hu B (2017) Inhibiting the migration of M1 microglia at hyperacute period could improve outcome of tMCAO rats. CNS Neurosci Ther 23:222–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neumann H, Kotter MR, Franklin RJ (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colonna M, Butovsky O (2017) Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 35:441–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saijo K, Crotti A, Glass CK (2013) Regulation of microglia activation and deactivation by nuclear receptors. Glia 61:104–111 [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Cao D, Guo C, Liu M, Tao Y, Zhou J, Wang F, Zhao Y, Wei J, Zhang Y, Fang W, Li Y (2019) Berberine facilitates angiogenesis against ischemic stroke through modulating microglial polarization via AMPK signaling. Cell Mol Neurobiol 39:751–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi JY, Kim JY, Kim JY, Park J, Lee WT, Lee JE (2017) M2 phenotype microglia-derived cytokine stimulates proliferation and neuronal differentiation of endogenous stem cells in ischemic brain. Exp Neurobiol 26:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer S, Nasyrov E, Brosien M, Preissner KT, Marti HH, Kunze R (2021) Self-extracellular RNA promotes pro-inflammatory response of astrocytes to exogenous and endogenous danger signals. J Neuroinflammation 18:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M, Li Z, Yao Y, Jin WN, Wood K, Liu Q, Shi FD, Hao J (2017) Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity. Proc Natl Acad Sci U S A 114:E396–E405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O, Thom V, Friese MA, Prinz I, Holscher C, Glatzel M, Korn T, Gerloff C, Tolosa E, Magnus T (2012) Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 120:3793–3802 [DOI] [PubMed] [Google Scholar]
- 48.Anrather J, Iadecola C (2016) Inflammation and stroke: an overview. Neurotherapeutics 13:661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rezaie AR (2014) Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost 112:876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ (2016) Inflammatory disequilibrium in stroke. Circ Res 119:142–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy-O’Reilly MA, Ahnstedt H, Spychala MS, Munshi Y, Aronowski J, Sansing LH, McCullough LD (2020) Aging exacerbates neutrophil pathogenicity in ischemic stroke. Aging (Albany NY) 12:436–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T, ShaoBJJoS, Diseases C. (2017) Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis 26:650–7 [DOI] [PubMed] [Google Scholar]
- 53.Otxoa-de-Amezaga A, Miró-Mur F, Pedragosa J, Gallizioli M, Justicia C, Gaja-Capdevila N, Ruíz-Jaen F, Salas-Perdomo A, Bosch A, Calvo M, Márquez-Kisinousky L, Denes A, Gunzer M, Planas AM (2019) Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol 137:321–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perl M, Chung CS, Perl U, Biffl WL, Cioffi WG, Ayala A (2007) Beneficial versus detrimental effects of neutrophils are determined by the nature of the insult. J Am Coll Surg 204:840–852 (discussion 52–3) [DOI] [PubMed] [Google Scholar]
- 55.Banerjee A, McCullough LD (2022) Sex-specific immune responses in stroke. Stroke 53:1449–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laridan E, Denorme F, Desender L, Francois O, Andersson T, Deckmyn H, Vanhoorelbeke K, De Meyer SF (2017) Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol 82:223–232 [DOI] [PubMed] [Google Scholar]
- 57.Sas AR, Carbajal KS, Jerome AD, Menon R, Yoon C, Kalinski AL, Giger RJ, Segal BM (2020) A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat Immunol 21:1496–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Catalfamo M, Henkart PA (2003) Perforin and the granule exocytosis cytotoxicity pathway. Curr Opin Immunol 15:522–527 [DOI] [PubMed] [Google Scholar]
- 59.Clarkson BD, Ling C, Shi Y, Harris MG, Rayasam A, Sun D, Salamat MS, Kuchroo V, Lambris JD, Sandor M, Fabry Z (2014) T cell-derived interleukin (IL)-21 promotes brain injury following stroke in mice. J Exp Med 211:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konoeda F, Shichita T, Yoshida H, Sugiyama Y, Muto G, Hasegawa E, Morita R, Suzuki N, Yoshimura AJB, communications br (2010) Therapeutic effect of IL-12/23 and their signaling pathway blockade on brain ischemia model. Biochem Biophys Res Commun 402:500–506 [DOI] [PubMed] [Google Scholar]
- 61.Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R (2012) The immunology of acute stroke. Nat Rev Neurol 8:401–410 [DOI] [PubMed] [Google Scholar]
- 62.de Bilbao F, Arsenijevic D, Moll T, Garcia-Gabay I, Vallet P, Langhans W, Giannakopoulos P (2009) In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J Neurochem 110:12–22 [DOI] [PubMed] [Google Scholar]
- 63.Li P, Mao L, Liu X, Gan Y, Zheng J, Thomson AW, Gao Y, Chen J, Hu X (2014) Essential role of program death 1-ligand 1 in regulatory T-cell-afforded protection against blood-brain barrier damage after stroke. Stroke 45:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, Nakatsukasa H, Chikuma S, Shichita T, Yoshimura A (2019) Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 565:246–250 [DOI] [PubMed] [Google Scholar]
- 65.Shi L, Sun Z, Su W, Xu F, Xie D, Zhang Q, Dai X, Iyer K, Hitchens TK, Foley LM, Li S, Stolz DB, Chen K, Ding Y, Thomson AW, Leak RK, Chen J, Hu X (2021) Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity 54(1527–42):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Xie L, Yang C, Ren C, Zhou K, Wang B, Zhang Z, Wang Y, Jin K, Yang G-Y (2015) Activated regulatory T cell regulates neural stem cell proliferation in the subventricular zone of normal and ischemic mouse brain through interleukin 10. Front Cell Neurosci 9:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aarum J, Sandberg K, Haeberlein SL, Persson MA (2003) Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A 100:15983–15988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lambiase A, Bracci-Laudiero L, Bonini S, Bonini S, Starace G, D’Elios MM, De Carli M, Aloe L (1997) Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol 100:408–414 [DOI] [PubMed] [Google Scholar]
- 69.Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H (2011) Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci 31:8556–8563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sahota P, Vahidy F, Nguyen C, Bui TT, Yang B, Parsha K, Garrett J, Bambhroliya A, Barreto A, Grotta JC, Aronowski J, Rahbar MH, Savitz S (2013) Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int J Stroke 8:60–67 [DOI] [PubMed] [Google Scholar]
- 71.Rasouli J, Lekhraj R, Ozbalik M, Lalezari P, Casper D (2011) Brain-spleen inflammatory coupling: a literature review. Einstein J Biol Med 27:74–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anthony S, Cabantan D, Monsour M, Borlongan CV (2022) Neuroinflammation, stem cells, and stroke. Stroke 53:1460–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ajmo CT Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR (2008) The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res 86:2227–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dotson AL, Wang J, Saugstad J, Murphy SJ, Offner H (2015) Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J Neuroimmunol 278:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seifert HA, Benedek G, Liang J, Nguyen H, Kent G, Vandenbark AA, Saugstad JA, Offner H (2017) Sex differences in regulatory cells in experimental stroke. Cell Immunol 318:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H (2013) Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res 4:554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seifert HA, Offner H (2018) The splenic response to stroke: from rodents to stroke subjects. J Neuroinflammation 15:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Honarpisheh P, Bryan RM, McCullough LD (2022) Aging micro-biota-gut-brain axis in stroke risk and outcome 130:1112–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El-Hakim Y, Mani KK, Eldouh A, Pandey S, Grimaldo MT, Dabney A, Pilla R, Sohrabji F (2021) Sex differences in stroke outcome correspond to rapid and severe changes in gut permeability in adult Sprague-Dawley rats. Biol Sex Differ 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahnstedt H, Patrizz A, Chauhan A, Roy-O’Reilly M, Furr JW, Spychala MS, D’Aigle J, Blixt FW, Zhu L, Bravo Alegria J, McCullough LD (2020) Sex differences in T cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain Behav Immun 87:556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Korf JM, Ganesh BP, McCullough LD (2022) Gut dysbiosis and age-related neurological diseases in females. Neurobiol Dis 168:105695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Courties G, Herisson F, Sager HB, Heidt T, Ye Y, Wei Y, Sun Y, Severe N, Dutta P, Scharff J, Scadden DT, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M (2015) Ischemic stroke activates hematopoietic bone marrow stem cells. Circ Res 116:407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C (2016) Thromboinflammation in Stroke Brain Damage. Stroke 47:1165–1172 [DOI] [PubMed] [Google Scholar]
- 84.De Meyer SF, Langhauser F, Haupeltshofer S, Kleinschnitz C, Casas AI (2022) Thromboinflammation in brain ischemia: recent updates and future perspectives. Stroke 53:1487–1499 [DOI] [PubMed] [Google Scholar]
- 85.Roy-O’Reilly M, McCullough LD (2014) Sex differences in stroke: the contribution of coagulation. Exp Neurol 259:16–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nokkari A, Abou-El-Hassan H, Mechref Y, Mondello S, Kindy MS, Jaffa AA, Kobeissy F (2018) Implication of the Kallikrein-Kinin system in neurological disorders: quest for potential bio-markers and mechanisms. Prog Neurobiol 165–167:26–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davis CM, Fairbanks SL, Alkayed NJ (2013) Mechanism of the sex difference in endothelial dysfunction after stroke. Transl Stroke Res 4:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gupta NC, Davis CM, Nelson JW, Young JM, Alkayed NJ (2012) Soluble epoxide hydrolase: sex differences and role in endothelial cell survival. Arterioscler Thromb Vasc Biol 32:1936–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Emms H, Lewis GP (1985) Sex and hormonal influences on platelet sensitivity and coagulation in the rat. Br J Pharmacol 86:557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jayachandran M, Owen WG, Miller VM (2003) Effects of ovariectomy on aggregation, secretion, and metalloproteinases in porcine platelets. Am J Physiol Heart Circ Physiol 284:H1679–H1685 [DOI] [PubMed] [Google Scholar]
- 91.Aldrighi JM, Oliveira RL, D’Amico E, Rocha T, Gebara OE, Rosano GM, Ramires JA (2005) Platelet activation status decreases after menopause. Gynecol Endocrinol 20:249–257 [DOI] [PubMed] [Google Scholar]
- 92.Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638 [DOI] [PubMed] [Google Scholar]
- 93.Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H (2006) TLR7 ligands induce higher IFN-alpha production in females. J Immunol 177:2088–2096 [DOI] [PubMed] [Google Scholar]
- 94.Marriott I, Bost KL, Huet-Hudson YM (2006) Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol 71:12–27 [DOI] [PubMed] [Google Scholar]
- 95.Abdullah M, Chai PS, Chong MY, Tohit ER, Ramasamy R, Pei CP, Vidyadaran S (2012) Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol 272:214–219 [DOI] [PubMed] [Google Scholar]
- 96.Lee BW, Yap HK, Chew FT, Quah TC, Prabhakaran K, Chan GS, Wong SC, Seah CC (1996) Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry 26:8–15 [DOI] [PubMed] [Google Scholar]
- 97.Lisse IM, Aaby P, Whittle H, Jensen H, Engelmann M, Christensen LB (1997) T-lymphocyte subsets in West African children: impact of age, sex, and season. J Pediatr 130:77–85 [DOI] [PubMed] [Google Scholar]
- 98.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI (2001) A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med 345:1243–1249 [DOI] [PubMed] [Google Scholar]
- 99.Wassertheil-Smoller S, Hendrix S, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ, Investigators ftW (2003) Effect of Estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA 289:2673–84 [DOI] [PubMed] [Google Scholar]
- 100.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S (2004) Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291:1701–1712 [DOI] [PubMed] [Google Scholar]
- 101.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J (2006) Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation 113:2425–2434 [DOI] [PubMed] [Google Scholar]
- 102.Bhupathiraju SN, Grodstein F, Rosner BA, Stampfer MJ, Hu FB, Willett WC, Manson JE (2017) Hormone therapy use and risk of chronic disease in the nurses’ health study: a comparative analysis with the Women’s Health Initiative. Am J Epidemiol 186:696–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD (2012) Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J Neuroendocrinol 24:319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM (2007) Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A 104:6013–6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP (2016) Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 374:1221–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller VM, Naftolin F, Asthana S, Black DM, Brinton EA, Budoff MJ, Cedars MI, Dowling NM, Gleason CE, Hodis HN, Jayachandran M, Kantarci K, Lobo RA, Manson JE, Pal L, Santoro NF, Taylor HS, Harman SM (2019) The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause 26:1071–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Solar P, Zamani A, Lakatosova K, Joukal M (2022) The blood-brain barrier and the neurovascular unit in subarachnoid hemorrhage: molecular events and potential treatments. Fluids Barriers CNS 19:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dion-Albert L, Bandeira Binder L, Daigle B, Hong-Minh A, Lebel M, Menard C (2022) Sex differences in the blood-brain barrier: implications for mental health. Front Neuroendocr 65:100989. [DOI] [PubMed] [Google Scholar]
- 110.Weber CM, Clyne AM (2021) Sex differences in the blood-brain barrier and neurodegenerative diseases. APL Bioeng 5:011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cipolla MJ, Godfrey JA, Wiegman MJ (2009) The effect of ovariectomy and estrogen on penetrating brain arterioles and blood-brain barrier permeability. Microcirculation 16:685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tomas-Camardiel M, Venero JL, Herrera AJ, De Pablos RM, Pintor-Toro JA, Machado A, Cano J (2005) Blood-brain barrier disruption highly induces aquaporin-4 mRNA and protein in perivascular and parenchymal astrocytes: protective effect by estradiol treatment in ovariectomized animals. J Neurosci Res 80:235–246 [DOI] [PubMed] [Google Scholar]
- 113.Atallah A, Mhaouty-Kodja S, Grange-Messent V (2017) Chronic depletion of gonadal testosterone leads to blood-brain barrier dysfunction and inflammation in male mice. J Cereb Blood Flow Metab 37:3161–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu F, Yuan R, Benashski SE, McCullough LD (2009) Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab 29:792–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, El-Zammar Z, Alam S, Hallenbeck JM, Kidwell CS, Warach S (2010) Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke 41:e123–e128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J (2008) MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 39:1121–1126 [DOI] [PubMed] [Google Scholar]
- 117.Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39:151–170 [DOI] [PubMed] [Google Scholar]
- 118.Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B (2015) Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol 36:605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schafer DP, Lehrman EK, Stevens B (2013) The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia 61:24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lenz KM, Nugent BM, Haliyur R, McCarthy MM (2013) Microglia are essential to masculinization of brain and behavior. J Neurosci 33:2761–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neumann H, Kotter M, Franklin RJB (2009) Debris clearance by microglia: an essential link between degeneration and regeneration 132:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kerr N, Dietrich DW, Bramlett HM, Raval AP (2019) Sexually dimorphic microglia and ischemic stroke. CNS Neurosci Ther 25:1308–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK (2002) Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res 956:30–35 [DOI] [PubMed] [Google Scholar]
- 124.Bollinger JL, Bergeon Burns CM, Wellman CL (2016) Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun 52:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guneykaya D, Ivanov A, Hernandez DP, Haage V, Wojtas B, Meyer N, Maricos M, Jordan P, Buonfiglioli A, Gielniewski B, Ochocka N, Comert C, Friedrich C, Artiles LS, Kaminska B, Mertins P, Beule D, Kettenmann H, Wolf SA (2018) Transcriptional and translational differences of microglia from male and female brains. Cell Rep 24(2773–83):e6. [DOI] [PubMed] [Google Scholar]
- 126.Schwarz JM, Sholar PW, Bilbo SD (2012) Sex differences in microglial colonization of the developing rat brain. J Neurochem 120:948–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simoes-Henriques C, Mateus-Pinheiro M, Gaspar R, Pinheiro H, Mendes Duarte J, Baptista FI, Canas PM, Fontes-Ribeiro CA, Cunha RA, Ambrosio AF, Gomes CA (2020) Microglia cytoarchitecture in the brain of adenosine A2A receptor knockout mice: brain region and sex specificities. Eur J Neurosci 51:1377–1387 [DOI] [PubMed] [Google Scholar]
- 128.Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, Lolli F, Marcello E, Sironi L, Vegeto E, Maggi A (2018) Sex-specific features of microglia from adult mice. Cell Rep 23:3501–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mangold CA, Wronowski B, Du M, Masser DR, Hadad N, Bixler GV, Brucklacher RM, Ford MM, Sonntag WE, Freeman WM (2017) Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation 14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kang SS, Ebbert MTW, Baker KE, Cook C, Wang X, Sens JP, Kocher JP, Petrucelli L, Fryer JD (2018) Microglial translational profiling reveals a convergent APOE pathway from aging, amyloid, and tau. J Exp Med 215:2235–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xiong X, Xu L, Wei L, White RE, Ouyang YB, Giffard RG (2015) IL-4 is required for sex differences in vulnerability to focal ischemia in mice. Stroke 46:2271–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eldahshan W, Fagan SC, Ergul A (2019) Inflammation within the neurovascular unit: focus on microglia for stroke injury and recovery. Pharmacol Res 147:104349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma L, Sun P, Zhang JC, Zhang Q, Yao SL (2017) Proinflammatory effects of S100A8/A9 via TLR4 and RAGE signaling pathways in BV-2 microglial cells. Int J Mol Med 40:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, Lledo PM, Greter M, Chan JKY, Amit I, Beyer M, Schultze JL, Schlitzer A, Pettersson S, Ginhoux F, Garel S (2018) Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172(500–16):e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Crain JM, Nikodemova M, Watters JJ (2013) Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J Neurosci Res 91:1143–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Won S, Lee JK, Stein DG (2015) Recombinant tissue plasminogen activator promotes, and progesterone attenuates, microglia/macrophage M1 polarization and recruitment of microglia after MCAO stroke in rats. Brain Behav Immun 49:267–279 [DOI] [PubMed] [Google Scholar]
- 137.Peeters SB, Cotton AM, Brown CJ (2014) Variable escape from X-chromosome inactivation: identifying factors that tip the scales towards expression. BioEssays 36:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arnold AP, Chen X (2009) What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD (2015) Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab 35:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F (2016) Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging (Albany NY) 8:1432–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qi S, Al Mamun A, Ngwa C, Romana S, Ritzel R, Arnold AP, McCullough LD, Liu F (2021) X chromosome escapee genes are involved in ischemic sexual dimorphism through epigenetic modification of inflammatory signals. J Neuroinflammation 18:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chisholm NC (2016) Sohrabji FJNod. Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging 85:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang T, Hu L, Liu Y, Fu X, Li J, Yan F, Cao S, Chen G (2022) Sex-associated differences in neurovascular dysfunction during ischemic stroke. Front Mol Neurosci 15:860959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rurak GM, Simard S, Freitas-Andrade M, Lacoste B, Charih F, Van Geel A, Stead J, Woodside B, Green JR, Coppola G, Salmaso N (2022) Sex differences in developmental patterns of neocortical astroglia: a mouse translatome database. Cell Rep 38:110310. [DOI] [PubMed] [Google Scholar]
- 146.Zheng W, Talley Watts L, Holstein DM, Wewer J, Lechleiter JD (2013) P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J Cereb Blood Flow Metab 33:600–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morrison HW, Filosa JA (2016) Sex differences in astrocyte and microglia responses immediately following middle cerebral artery occlusion in adult mice. Neuroscience 339:85–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76:845–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guo H, Yang J, Liu M, Wang L, Hou W, Zhang L, Ma Y (2020) Selective activation of estrogen receptor beta alleviates cerebral ischemia neuroinflammatory injury. Brain Res 1726:146536. [DOI] [PubMed] [Google Scholar]
- 150.Guo J, Duckles SP, Weiss JH, Li X, Krause DN (2012) 17beta-Estradiol prevents cell death and mitochondrial dysfunction by an estrogen receptor-dependent mechanism in astrocytes after oxygen-glucose deprivation/reperfusion. Free Radic Biol Med 52:2151–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pawlak J, Brito V, Kuppers E, Beyer C (2005) Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res 138:1–7 [DOI] [PubMed] [Google Scholar]
- 152.Gal-Oz ST, Maier B, Yoshida H, Seddu K, Elbaz N, Czysz C, Zuk O, Stranger BE, Ner-Gaon H, Shay T (2019) ImmGen report: sexual dimorphism in the immune system transcriptome. Nat Commun 10:4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.So J, Tai AK, Lichtenstein AH, Wu D, Lamon-Fava S (2021) Sexual dimorphism of monocyte transcriptome in individuals with chronic low-grade inflammation. Biol Sex Differ 12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bongen E, Lucian H, Khatri A, Fragiadakis GK, Bjornson ZB, Nolan GP, Utz PJ, Khatri P (2019) Sex differences in the blood transcriptome identify robust changes in immune cell proportions with aging and influenza infection. Cell Rep 29:1961–73.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miró-Mur F, Pérez-de-Puig I, Ferrer-Ferrer M, Urra X, Justicia C, Chamorro A, Planas AM (2016) Immature monocytes recruited to the ischemic mouse brain differentiate into macrophages with features of alternative activation. Brain Behav Immun 53:18–33 [DOI] [PubMed] [Google Scholar]
- 156.Clausen BH, Lambertsen KL, Babcock AA, Holm TH, Dagnaes-Hansen F, Finsen B (2008) Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J Neuroinflammation 5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gupta S, Nakabo S, Blanco LP, O’Neil LJ, Wigerblad G, Goel RR, Mistry P, Jiang K, Carmona-Rivera C, Chan DW, Wang X, Pedersen HL, Gadkari M, Howe KN, Naz F, Dell’Orso S, Hasni SA, Dempsey C, Buscetta A, Frischmeyer-Guerrerio PA, Kruszka P, Muenke M, Franco LM, Sun HW, Kaplan MJ (2020) Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc Natl Acad Sci U S A 117:16481–16491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Blazkova J, Gupta S, Liu Y, Gaudilliere B, Ganio EA, Bolen CR, Saar-Dover R, Fragiadakis GK, Angst MS, Hasni S, Aghaeepour N, Stevenson D, Baldwin N, Anguiano E, Chaussabel D, Altman MC, Kaplan MJ, Davis MM, Furman D (2017) Multicenter systems analysis of human blood reveals immature neutrophils in males and during pregnancy. J Immunol 198:2479–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim JY, Park J, Chang JY, Kim SH, Lee JE (2016) Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol 25:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Perez-de-Puig I, Miro-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM (2015) Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol 129:239–257 [DOI] [PubMed] [Google Scholar]
- 161.Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, Wojtkiewicz GR, Masson GS, Vinegoni C, Kim J, Kim DE, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M (2018) Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci 21:1209–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR (2015) Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 35:888–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S (2004) Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation 110:1664–1669 [DOI] [PubMed] [Google Scholar]
- 164.Ahnstedt H, Patrizz A, Roy-O’Reilly M, Spychala M, Bravo Alegria J, Chauhan A, McCullough LD (2018) Abstract TMP36: sex differences in neutrophil-T cell Immune responses and outcome after ischemic stroke in aged mice. Stroke 49. 10.1161/str.49.suppl_1.TMP36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535 [DOI] [PubMed] [Google Scholar]
- 166.Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, Cao Y, Xu H, Luo H, Lu L, Shi MJ, Tian Y, Fan W, Zhao BQ (2020) Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun 11:2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Papayannopoulos V (2018) Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18:134–147 [DOI] [PubMed] [Google Scholar]
- 168.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD (2010) Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 107:15880–15885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Novotny J, Oberdieck P, Titova A, Pelisek J, Chandraratne S, Nicol P, Hapfelmeier A, Joner M, Maegdefessel L, Poppert H, Pircher J, Massberg S, Friedrich B, Zimmer C, Schulz C, Boeckh-Behrens T (2020) Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology 94:e2346–e2360 [DOI] [PubMed] [Google Scholar]
- 170.Ducroux C, Di Meglio L, Loyau S, Delbosc S, Boisseau W, Deschildre C, Ben Maacha M, Blanc R, Redjem H, Ciccio G, Smajda S, Fahed R, Michel JB, Piotin M, Salomon L, Mazighi M, Ho-Tin-Noe B, Desilles JP (2018) Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 49:754–757 [DOI] [PubMed] [Google Scholar]
- 171.Valles J, Lago A, Santos MT, Latorre AM, Tembl JI, Salom JB, Nieves C, Moscardo A (2017) Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb Haemost 117:1919–1929 [DOI] [PubMed] [Google Scholar]
- 172.Strecker JK, Schmidt A, Schabitz WR, Minnerup J (2017) Neutrophil granulocytes in cerebral ischemia - evolution from killers to key players. Neurochem Int 107:117–126 [DOI] [PubMed] [Google Scholar]
- 173.Tillack K, Breiden P, Martin R, Sospedra M (2012) T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol 188:3150–3159 [DOI] [PubMed] [Google Scholar]
- 174.Giaglis S, Stoikou M, Sur Chowdhury C, Schaefer G, Grimolizzi F, Rossi SW, Hoesli IM, Lapaire O, Hasler P, Hahn S (2016) Multimodal regulation of NET formation in pregnancy: progesterone antagonizes the Pro-NETotic effect of estrogen and G-CSF. Front Immunol 7:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Giaglis S, Stoikou M, Sur Chowdhury C, Schaefer G, Grimolizzi F, Rossi SW, Hoesli IM, Lapaire O, Hasler P, Hahn S (2016) Multimodal regulation of NET formation in pregnancy: progesterone antagonizes the Pro-NETotic effect of estrogen and G-CSF. Front Immunol 7:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yuan XZ (2018) Sex differences in neutrophil extracellular trap formation. https://tspace.library.utoronto.ca/handle/1807/91738. Accessed 15 Jul 2022
- 177.Denorme F, Portier I, Rustad JL, Cody MJ, de Araujo CV, Hoki C, Alexander MD, Grandhi R, Dyer MR, Neal MD, Majersik JJ, Yost CC, Campbell RA (2022) Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest 132:e154225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Uppal SS, Verma S, Dhot PS (2003) Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Cytometry B Clin Cytom 52:32–36 [DOI] [PubMed] [Google Scholar]
- 179.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H (2004) Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol 173:2227–2230 [DOI] [PubMed] [Google Scholar]
- 180.Libert C, Dejager L, Pinheiro I (2010) The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 10:594–604 [DOI] [PubMed] [Google Scholar]
- 181.Hirokawa K, Utsuyama M, Hayashi Y, Kitagawa M, Makinodan T, Fulop T (2013) Slower immune system aging in women versus men in the Japanese population. Immun Ageing 10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ahnstedt H, McCullough LD (2019) The impact of sex and age on T cell immunity and ischemic stroke outcomes. Cell Immunol 345:103960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Conway SE, Roy-O’Reilly M, Friedler B, Staff I, Fortunato G, McCullough LD (2015) Sex differences and the role of IL-10 in ischemic stroke recovery. Biol Sex Differ 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, Guida E, Broughton BR, Drummond GR, Sobey CG (2010) Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab 30:1306–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Luo CY, Wang L, Sun C, Li DJ (2011) Estrogen enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell Mol Immunol 8:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yan J, Read SJ, Henderson RD, Hull R, O’Sullivan JD, McCombe PA, Greer JM (2012) Frequency and function of regulatory T cells after ischaemic stroke in humans. J Neuroimmunol 243:89–94 [DOI] [PubMed] [Google Scholar]
- 187.Beckmann L, Obst S, Labusek N, Abberger H, Koster C, Klein-Hitpass L, Schumann S, Kleinschnitz C, Hermann DM, Felder-hoff-Muser U, Bendix I, Hansen W, Herz J (2022) Regulatory T cells contribute to sexual dimorphism in neonatal hypoxic-ischemic brain injury. Stroke 53:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tian Y, Stamova B, Jickling GC, Liu D, Ander BP, Bushnell C, Zhan X, Davis RR, Verro P, Pevec WC, Hedayati N, Dawson DL, Khoury J, Jauch EC, Pancioli A, Broderick JP, Sharp FR (2012) Effects of gender on gene expression in the blood of ischemic stroke patients. J Cereb Blood Flow Metab 32:780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN (1996) Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382:171–174 [DOI] [PubMed] [Google Scholar]
- 190.Phan HT, Blizzard CL, Reeves MJ, Thrift AG, Cadilhac DA, Sturm J, Heeley E, Otahal P, Rothwell P, Anderson CS, Parmar P, Krishnamurthi R, Barker-Collo S, Feigin V, Gall S (2019) Sex differences in long-term quality of life among survivors after stroke in the INSTRUCT. Stroke 50:2299–2306 [DOI] [PubMed] [Google Scholar]
- 191.Bodhankar S, Lapato A, Chen Y, Vandenbark AA, Saugstad JA, Offner H (2015) Role for microglia in sex differences after ischemic stroke: importance of M2. Metab Brain Dis 30:1515–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Friberg L, Rosenqvist M, Lip GYH (2012) Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 33:1500–1510 [DOI] [PubMed] [Google Scholar]
- 193.Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD (2013) Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol 249:120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Villapol S, Faivre V, Joshi P, Moretti R, Besson VC, Charriaut-Marlangue C (2019) Early sex differences in the immune-inflammatory responses to neonatal ischemic stroke. Int J Mol Sci 20:3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cordeau P Jr, Lalancette-Hebert M, Weng YC, Kriz J (2008) Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke 39:935–942 [DOI] [PubMed] [Google Scholar]
- 196.Jackson L, Li W, Abdul Y, Dong G, Baban B, Ergul A (2019) Diabetic stroke promotes a sexually dimorphic expansion of T cells. Neuromolecular Med 21:445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stamova B, Jickling GC, Ander BP, Zhan X, Liu D, Turner R, Ho C, Khoury JC, Bushnell C, Pancioli A, Jauch EC, Broderick JP, Sharp FR (2014) Gene expression in peripheral immune cells following cardioembolic stroke is sexually dimorphic. PLoS ONE 9:e102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu H, Ge Y, Liu Y, Zheng Y, Hu R, Ren C, Liu QJISB (2022) Identification of the key genes and immune infiltrating cells determined by sex differences in ischaemic stroke through co-expression network module 16:28–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ross AM, Lee CS, Lutsep H (2016) Influence of gender and age on the peripheral immune response in stroke. J Cardiovasc Nurs 31:331–335 [DOI] [PubMed] [Google Scholar]
- 200.Zhu W, Nan Y, Wang S, Liu W (2019) Bioinformatics analysis of gene expression profiles of sex differences in ischemic stroke. Biomed Res Int 2019:2478453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Trott S, Vsevolozhskaya O, Pennypacker K, Alhajeri A, Fraser JF (2019) Immune system activation in perioperative thrombectomy patients: preliminary retrospective study. World Neurosurg 128:e966–e969 [DOI] [PubMed] [Google Scholar]
- 202.Stamova B, Tian Y, Jickling G, Bushnell C, Zhan X, Liu D, Ander BP, Verro P, Patel V, Pevec WC, Hedayati N, Dawson DL, Jauch EC, Pancioli A, Broderick JP, Sharp FR (2012) The X-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke 43:326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]


