Abstract
Perioperative organ injury is among the leading causes of morbidity and mortality of surgical patients. Among different types of perioperative organ injury, acute kidney injury occurs particularly frequently and has an exceptionally detrimental effect on surgical outcomes. Currently, acute kidney injury is most commonly diagnosed by assessing increases in serum creatinine concentration or decreased urine output. Recently, novel biomarkers have become a focus of translational research for improving timely detection and prognosis for acute kidney injury. However, specificity and timing of biomarker release continue to present challenges to their integration into existing diagnostic regimens. Despite many clinical trials using various pharmacologic or non-pharmacologic interventions, reliable means to prevent or reverse acute kidney injury are still lacking. Nevertheless, several recent randomized multi-center trials provide new insights into renal replacement strategies, composition of intravenous fluid replacement, goal-directed fluid therapy, or remote ischemic preconditioning in their impact on perioperative acute kidney injury. In this review, we provide an update on the latest progress toward the understanding of disease mechanism, diagnosis, and managing perioperative acute kidney injury, as well as highlight areas of ongoing research efforts for preventing and treating acute kidney injury in surgical patients.
Keywords: acute kidney injury, renal biomarkers, Kidney Disease Improving Global Outcomes, remote ischemic preconditioning, renal replacement therapy, sepsis, creatinine, neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, adenosine
Introduction
Over the past century, considerable progress has been made in anesthesia safety. Advancements in specialty training, improved monitoring modalities, and safer airway management have all contributed to better patient outcomes. However, surgical morbidity and mortality has essentially remained unchanged and continues to be a leading health burden in Western countries.1 Among different types of organ injury, specifically with regard to organ dysfunction in the postoperative period, acute kidney injury remains particularly prominent, occurring in 20 to 40 % of high-risk patients.2 Moreover, patients with a diagnosis of sepsis and acute kidney injury have an associated mortality rate of 70 %.3 Current experimental outcomes suggest acute kidney injury may precipitate a decline in other organ systems, thus impacting rates of multi-organ failure, sepsis, and death.4 Even subclinical acute kidney injury is correlated to an increased likelihood of mortality.5 Preventing and treating acute kidney injury represents multiple obstacles toward improving outcomes among surgical patients.
Historically, treatment modalities toward prevention of acute kidney injury have been limited. For example, prophylactic use of low-dose dopamine (“renal dopamine”) or treatment with high doses of furosemide to block ATP consumption by renal tubular epithelial cells, have proven detrimental instead of protective in perioperative clinical trials.6 In order to make a definitive diagnosis early in the injury process and effectively treat perioperative acute kidney injury, the obstacle of finding reliable identification tools must be overcome. Approaches based on alterations of serum creatinine may delay identification and intervention because of its relatively late diagnostic presentation. Therefore, the search for novel and specific biomarkers toward early identification of acute kidney injury has challenged this field of research.
Based on these unresolved questions, mechanistic insight into acute kidney injury, novel biomarkers, and therapeutic modalities to prevent or treat are intense areas of research. Many ongoing translational studies and clinical trials are aiming to provide a more acute understanding of the disease process, to establish diagnostic, preventive, or therapeutic options for acute kidney injury in perioperative patients. Through this review, we put these recent and ongoing studies into the context of established findings of perioperative acute kidney injury. We hope that these efforts will soon be successful and lead to an improvement in our diagnostic, preventive, or therapeutic options for surgical patients experiencing acute kidney injury.
Acute Kidney Injury Definition
Acute kidney injury is one of a number of acute kidney diseases, occurring in the presence or absence of other acute or chronic renal disease processes.7 A condition which affects kidney structure and function is categorized as acute or chronic, based upon interval of time. An inclusive nomenclature to enhance understanding and communication has been proposed to systematically classify functional and structural criteria for acute kidney injury, acute kidney disease, chronic kidney disease, and no known kidney disease (Supplementary Table 1).7
The publication of Risk, Injury, Failure, Loss, End-stage renal disease criteria (RIFLE) in 2004 created a standard, widely used definition for acute kidney injury.8 The establishment of these standards ended a plethora of no fewer than 60 definitions for acute kidney injury in acute renal failure literature.9 These standardized criteria established a uniform manner to define acute kidney injury, improving accuracy in reporting incidence and outcomes, and allowing comparability of studies regarding the diagnosis of acute kidney injury. This allowed our understanding to evolve from a “simple loss of function” to a more mature reality where acute kidney injury is a multifaceted, heterogeneous disease process.10,11 However, a significant limitation of the Risk, Injury, Failure, Loss, End-stage renal disease standards is that it underestimated the effect of small acute creatinine changes on mortality as part of the criterion.12 In response to this shortcoming, the Acute Kidney Injury Network (AKIN) modified the Risk, Injury, Failure, Loss, End-stage criteria, taking into account small increases in creatinine (≥0.3 mg/dL) over time (≥48 hours) (Table 1).13 In 2012, the Kidney Disease Improving Global Outcomes (KDIGO) taskforce offered a cohesive interpretation of the Risk, Injury, Failure, Loss, End-stage renal disease criteria, Acute Kidney Injury Network (AKIN), and pediatric Risk, Injury, Failure, Loss, End-stage renal disease criteria (pRIFLE) as “a single definition for practice, research, and public health.”14 The Kidney Disease Improving Global Outcomes classification stages the presence of acute kidney injury from an acute increase in serum creatinine or a period of oliguria.15 As it relates to time, the Acute Dialysis Quality Initiative Group recently clarified that “acute kidney injury” occurs within 48 hours or less and “acute kidney disease” when acute kidney injury persists for seven days or longer.15
Table 1:
AKI Classification Systems
| RIFLE (7 Days) | AKIN (48 Hours) | KDIGO |
|---|---|---|
| Risk | Stage 1 | Stage 1 |
| Increased sCr × 1.5 or GFR decrease >25% OR urine output <0.5 ml kg–1 h–1 for 6 h |
Increased sCr × 1.5–2 or sCr increase ≥0.3 mg dl–1 OR urine output <0.5 ml kg–1 h–1 for >6 h |
Increased sCr × 1.5–1.9 within seven days OR sCr increase ≥0.3 mg dl–1 within 48 h OR urine output <0.5 ml kg–1 h–1 for 6-12 h |
| Injury | Stage 2 | Stage 2 |
| Increased sCr × 2 or GFR decrease >50% OR urine output <0.5 ml kg–1 h–1 for 12 h |
Increased sCr × 2–3 OR urine output <0.5 ml kg–1 h–1 for >12h |
Increased sCr × 2–2.9 OR urine output <0.5 ml kg–1 h–1 for ≥12h |
| Failure | Stage 3 | Stage 3 |
| Increased sCr × 3 or GFR decrease by 75% or sCr ≥ 4 mg dl–1 with an acute rise in sCr (≥0.5 mg dl–1) OR urine output <0.3 ml kg–1 h–1 for 24 h or anuria for 12 h |
Increased sCr × 3 or more or sCr ≥ 4 mg dl–1 with an acute rise in sCr (≥0.5 mg dl–1) OR urine output <0.3 ml kg–1 h–1 for >24 h or anuria for 12 h |
Increased sCr × 3 or more or sCr ≥ 4 mg dl–1 or initiation of RRT or GFR decrease to <35 ml min–1 (1.73 m) –2 in patients <18 yr old OR urine output <0.3 ml kg–1 h–1 for ≥24 h or anuria for ≥12 h |
| Loss | ||
| Persistent acute renal failure=complete loss of kidney function >4 weeks | ||
| End-stage Kidney Disease | ||
| ESRD >3 months | ||
Comparison of the three most notable and historic classification systems used to diagnose AKI. The initial system was the RIFLE (Risk, Injury, Failure, Loss of kidney function, End-stage renal failure), which was developed by an international consensus in 2004. It defined 5 stages of renal injury: risk-end stage disease. A short time later the Acute Kidney Injury Network (AKIN) developed their own diagnostic criteria that uses a smaller creatinine change to define AKI. This was based on studies showing that even small changes in serum creatinine resulted in adverse outcomes.2–6 In 2012, the KDIGO (Kidney Disease: Improving Global Outcomes) classification system was produced and has been the main system in use since.9 The KDIGO system was created by combining the two prior systems and utilizing studies showing which patients were missed by the two prior classification system.
Acute kidney injury has two subgroups, “subclinical acute kidney injury” and “functional acute kidney injury,” and has recently been described with the introduction of biomarkers as a diagnostic tool.16 Elevated concentrations of an acute kidney injury biomarker, without meeting Kidney Disease Improving Global Outcomes classifications, is defined as subclinical acute kidney injury. Whereas, functional acute kidney injury meets the Kidney Disease Improving Global Outcomes definition, but fails to demonstrate an increase in biomarker concentration.17 While it is tempting to assume that subclinical acute kidney injury is an innocuous phenomenon with surgical patients, current evidence suggests that even minor increases of perioperative creatinine levels—not meeting Kidney Disease Improving Global Outcomes definition for acute kidney injury—are related to a doubling of perioperative mortality and longer hospital length-of-stay.5 Haase et al. determined even without diagnostic fluctuations in serum creatinine, subclinical AKI is associated with adverse outcomes.18
Epidemiology of Perioperative Acute Kidney Injury
The effect of acute kidney injury on individual outcomes and healthcare systems is remarkable. Acute kidney injury in industrialized countries costs an estimated $1 billion, claims 300,000 lives, and contributes to the development of 300,000 advanced chronic kidney disease cases annually.19,20 Acute kidney injury correlates with elevated mortality rates, longer hospital stays, and increased treatment expenses, with the seriousness of acute kidney injury directly linked to patient-centered outcomes. While multiple studies have since substantiated the impact of acute kidney injury, the prevalence of acute kidney injury is dependent upon the definition, criteria, and study population.
Kork et al. retrospectively examined 37,000 non-cardiac patients in a single-center study utilizing the Kidney Disease Improving Global Outcomes criteria.5 Acute kidney injury occurred in 6% of the study population. After adjusting for multiple variables including age, hospital length of stay, sex, pre-operative creatinine and hemoglobin, the authors determined that minor changes in creatinine levels (25 to 49% above baseline), increased the risk of death by two-fold and increased the length of hospitalization by three days.5 In 2017, O’Connor et al. investigated the association between postoperative acute kidney injury using the Kidney Disease Improving Global Outcomes standards for a retrospective cohort study and found that 6.8% of investigated patients sustained acute kidney injury resulting in a 13.3% in-hospital mortality rate compared with 0.9% without acute kidney injury (P < 0.001). At one year, 26.6% of patients with acute kidney injury died compared to 6.1% of patients without acute kidney injury (P < 0.001) resulting in an adjusted hazard ratio for death of 2.96 (95% C.I. 1.86 to 4.71; P < 0.001) for acute kidney injury.21 Both studies concluded that in non-cardiac surgery patients, the presence of even mild forms of acute kidney injury posed a significant risk of death and increased the length of hospital stay.5,21 The frequency, risk factors, and outcomes following non-cardiac surgery have not been well identified and could offer a multitude of possibilities for further analysis in postoperative acute kidney injury research.
Cardiac surgery-associated acute kidney injury is a frequent source of perioperative acute kidney injury. Recent meta-analysis found the incidence of acute kidney injury in cardiac surgery patients to be as significant as 25 to 30%.22,23 In 2016, Hu et al. analyzed the pooled incidence rates of acute kidney injury in 300,000 post-cardiac surgery patients and determined the incidence of to be 22.3% (95% CI, 19.8-25.1). Utilizing Kidney Disease Improving Global Outcomes criteria, pooled rates for the development were 13.6%, 3.8%, and 2.7% for Stage 1, Stage 2, and Stage 3 respectively, with 2.3% requiring renal replacement therapy.22 The development of acute kidney injury increased monetary cost and length of hospitalization.22 The connection between acute kidney injury and post-cardiac surgery has pronounced short- and long-term morbidity outcomes. In a cohort study looking at over 1,000 patients undertaking elective cardiac surgery, acute kidney injury patients had a 26% 5-year cumulative risk of death, more than double the cumulative risk in patients without acute kidney injury.24 Although the role of acute kidney injury is well established in patients receiving cardiac surgery, less information is available regarding non-cardiac surgery.
To determine if the type of surgery influenced whether a patient develops acute kidney injury, Grams and colleagues performed a retrospective study of 161,185 Veterans Health Administration patients in 2016.25 Cardiac surgery presented the greatest risk for postoperative acute kidney injury (RR, 1.22; 95% CI, 1.17-1.27), proceeded by general thoracic (RR, 0.92; 95% CI, 0.87-0.98), orthopedic (RR, 0.70; 95% CI, 0.67-0.73), vascular (RR, 0.68; 95% CI, 0.64-0.71), urologic (RR, 0.65; 95% CI, 0.61-0.69), and ear, nose, and throat (RR, 0.32; 95% CI, 0.28-0.37).25 Although cardiac surgery posed the greatest postoperative risk, similar risk factors including advanced age, African American race, hypertension, diabetes mellitus, and a lower estimated glomerular filtration rate was observed across surgical types.25 Further, acute kidney injury post-operative patients had longer hospitalization, higher rates of 30-day readmission, heightened risk for developing end-stage renal disease, and higher mortality rates (19% vs 8%). These findings corroborated research from Kork et al. and O’Connor et al. which examined the occurrence of morbidity acute kidney injury among the non-cardiac perioperative population. Taken together, these data indicate the acute kidney injury occurs more frequently than previously anticipated. Even smaller changes in kidney function are associated with higher degrees of morbidity and mortality. Therefore, clinicians should carefully consider clinical and laboratory signs of mild kidney injury in order to be prepared to manage potential clinical complications, such as organ injury, multi-organ failure, or sepsis.
Pathophysiology of Perioperative Acute Kidney Injury
Historically, acute kidney injury was categorized into pre-renal, renal, and post-renal causes. Pre-renal acute kidney injury is a functional response to renal hypoperfusion, where intrinsic renal tubular function remains intact. Pre-renal acute kidney injury results from a hypovolemic or low circulating volume or low cardiac output state. Post-renal acute kidney injury is caused by the blockage of urinary flow downstream in the urinary tract inducing a backup into the kidney and consequent hydronephrosis. Like pre-renal acute kidney injury, there is no inherent renal disease present, if urinary flow is re-established before permanent structural damage develops. In contrast, intrinsic acute kidney injury results from a disease process of the renal vascular, glomeruli, tubules, or interstitium.26 This traditional classification provides a convenient but somewhat simplistic framework as acute kidney injury often crosses these boundaries. For example, prolonged pre-renal acute kidney injury can lead to secondary intrinsic acute tubular necrosis.27
Perioperative acute kidney injury and the ways in which it develops is multifaceted and complex. Hypoperfusion, inflammation, and neuroendocrine response to surgery are the frequent mechanisms affecting renal perfusion.28,29 Reduction of blood pressure and renal hypoperfusion are frequent consequences of perioperative hypovolemia, as well as the vasodilatory and cardio-depressant effects of anesthesia. In a low perfusion state, the kidneys can exhibit remarkable autoregulation, maintaining constant renal blood flow, and consequently glomerular filtration rate despite fluctuating mean arterial pressure and volume status. Prostaglandin signaling decreases afferent arteriolar resistance, which increases blood flow to the glomeruli and sustains the glomerular capillary pressure in low perfusion state. The activation of the renin-angiotensin-aldosterone systems and release of angiotensin II raises efferent arteriolar resistance, sustaining the glomerular capillary pressure.26 If renal hypoperfusion persists or drops below the autoregulatory range, endogenous vasoconstrictors released from the renal sympathetic system result in afferent arteriolar vasoconstriction. This effectively reduces renal blood flow leading to renal tubular ischemia and reduced glomerular filtration rate.26,30–32 The diminishing of oxygen balance induces renal tissue hypoxia and ATP starvation that stimulates extracellular matrix production, collagen deposition, and fibrosis.33 As a metabolic product of ATP,34 adenosine binds to kidney cell surface receptors to match blood flow with energy consumption.2,35 The interstitial concentration of adenosine rises when neighboring cells are in a negative energy balance.35 To recover from a negative energy balance and high oxygen demand, it is unnecessary to increase blood flow, but rather, to lower the glomerular filtration fraction. This phenomenon has been termed “acute renal success.”36,37 By reducing the filtration rate, the number of sodium ions that must be transported per oxygen delivered is reduced, conserving energy and improving the energy balance.35 Renal perfusion is capitalized to a rate that is adequate to promote healing while maintaining excretory function without the risk of inhibiting volume conservation.36
Renal autoregulation can also be disrupted by using non-steroidal anti-inflammatory drugs (NSAIDs) during the perioperative period. These inhibit the enzyme cyclooxygenase and prohibit the production of renal prostaglandins. This leads to the unopposed constriction of both the afferent and efferent arterioles by angiotensin II in a state of persistent renal hypoperfusion, decreasing renal perfusion flow and glomerular filtration rate (Figure 1).
Figure 1. Glomerular Filtration as a Function of Glomerular Blood Flow.
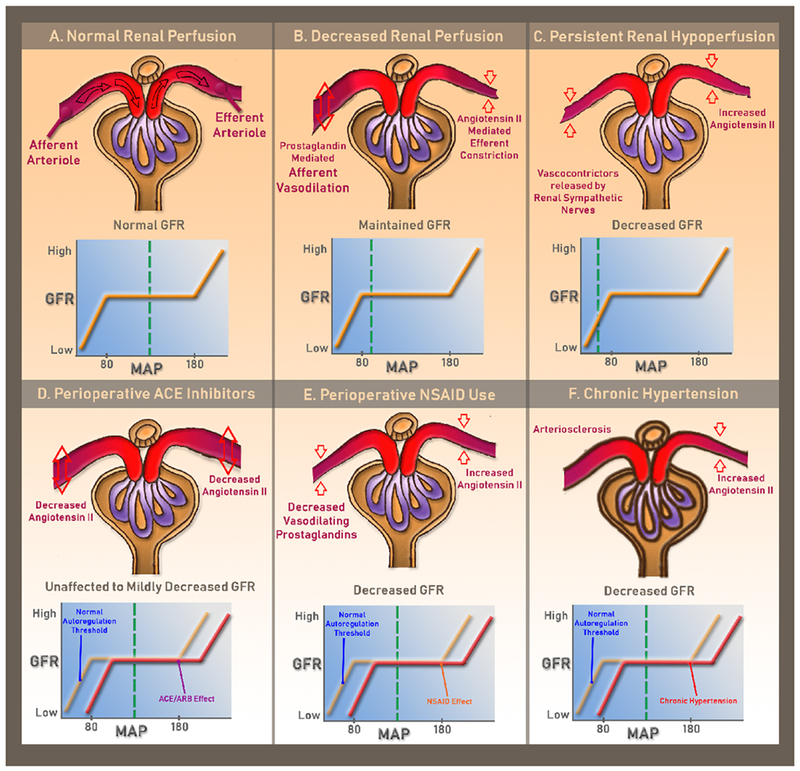
Panel A shows normal glomerular blood flow with normal glomerular filtration rate. Panel B shows reduced renal perfusion pressure within the autoregulatory range, caused by intraoperative conditions such as anesthesia and medication induced hypotension or hypovolemia. Normal glomerular filtration rate is maintained with prostaglandin-mediated afferent arteriolar vasodilation and Angiotensin II-mediated efferent arteriolar vasoconstriction. Panel C shows persistent reduction in renal perfusion pressure below the autoregulatory range. This can be seen intraoperatively with protracted systemic hypotension or severe hypovolemia due to hemorrhage and blood loss. In this state, endogenous vasoconstrictors released from the renal sympathetic nerves increase the afferent arteriolar resistance, which results in a rapid decline in glomerular filtration rate and decrease in renal blood flow. This eventually leads to tubular cell damage and cell death. Panel D shows reduced glomerular filtration rate with nonsteroidal anti-inflammatory drug use due to loss of vasodilatory prostaglandin. Panel E shows the effect of angiotensin-converting-enzyme inhibitor or angiotensin-receptor blocker. Loss of angiotensin II decreases both the afferent and efferent arteriolar resistance, relaxing the efferent arteriole significantly more. The net clinical effect is unchanged or slightly decreased glomerular filtration rate. Panel F shows the effect of chronic hypertension on the preglomerular arterial vessels, primarily the afferent arterioles. Chronic hypertension eventually leads to thickening of arteriole walls and narrowing of lumen, a process known as arteriolosclerosis. This results in inadequate blood flow through the glomeruli and may produce glomerular and tubulointerstitial ischemia. Conditions displayed in Panel D-F can contribute to the development of “normotensive” perioperative acute kidney injury.
In addition to hypoperfusion-induced injury, systemic inflammation and cytokine release caused by trauma and surgical stress directly induce tubular injury and subsequent systemic inflammation.2,34,38 The etiology of this inflammation-induced acute kidney injury is multifactorial, including renin-angiotensin-aldosterone system activation, renal microcirculatory dysfunction, increased oxidative stress, cytokine-induced injury, endothelial cell injury, and activation of pro-apoptotic pathways.27–29,39,40 All these factors predispose the surgical patient to develop acute kidney injury during the perioperative period. In recent years, evidence in both basic science and clinical research has led to a new understanding that acute kidney injury is not a mere singular organ injury. Acute kidney injury is now perceived as a multifaceted systemic disease process that engenders distant organ dysfunctions, including pulmonary,41 cardiac,42 neurologic, immunologic, hepatic, and gastrointestinal dysfunctions (Figure 2).43–45 Recent studies highlight that acute kidney injury can cause remote organ injury, for example, to the intestine. Dr. Lee et al. provides mechanistic insight of the inflammatory activation of Paneth cells that are contained at the bottom of crypts located at the intestinal mucosa.6 Activation of Paneth cells leads to massive release of inflammatory mediators (such as IL-17A), causing a disruption of the intestinal barrier function, translocation of bacteria from the intestinal lumen into the blood stream, promoting sepsis and multi-organ failure (Figure 3).6 These studies highlight in an elegant way a functional role of acute kidney injury beyond its filter function, suggesting that kidney injury and acute kidney injury trigger inflammation and morbidity in distal organ systems.4
Figure 2. Consequences of acute kidney injury on remote organ functions.
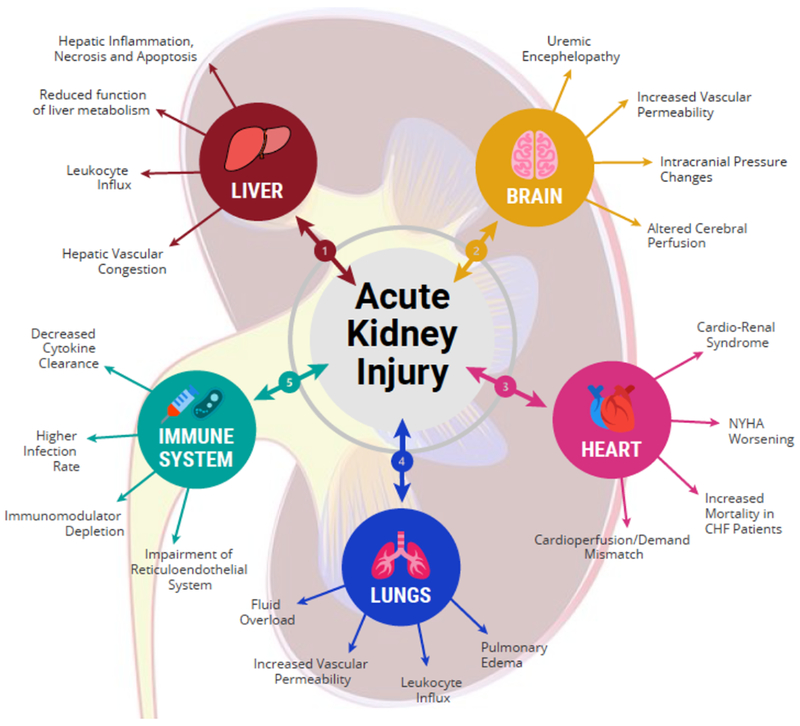
There is increasing evidence that acute kidney injury directly contributes to remote injury in the heart, lung, brain, liver, immunologic, and other organ systems. 1) In the hepatic system, acute kidney injury causes intestinal barrier breakdown and greater gut translocation and delivery of endotoxins and microorganisms to the portal system. This results in hepatic inflammation and apoptosis along with hepatic overproduction and systemic release of pro-inflammatory cytokines. 2) Acute kidney injury is also associated with cerebral dysfunction, including uremic encephalopathy. Activation of neuro-inflammatory cascade results in increase in vascular permeability and breakdown of blood-brain barrier. 3) In the cardiac system, acute kidney injury is associated with cardiorenal syndrome, which is a state of concomitant heart and kidney failure. Suggested mechanisms of acute kidney injury-induced cardiac dysfunction include fluid overload and uremia-induced decrease in myocardial contractility. 4) In the pulmonary system, the remote effect of acute kidney injury is due to activation of inflammatory cascade leading to an increase in pulmonary vascular permeability and lung neutrophil infiltration. This leads to accumulation of fluid within the lung tissue, causing pulmonary edema. 5) In the immunologic system, acute kidney injury has a profound impact on humoral and cellular immunity and overall immunocompetence. This is due to a combination of increase in oxidative stress, impaired clearance of the reticuloendothelial system, and decreased clearance of circulating cytokines, leading to higher rate of infections in patients with acute kidney injury.
Figure 3. Paneth Cell-Mediated Multiorgan Systemic Inflammation after Acute Kidney Injury.
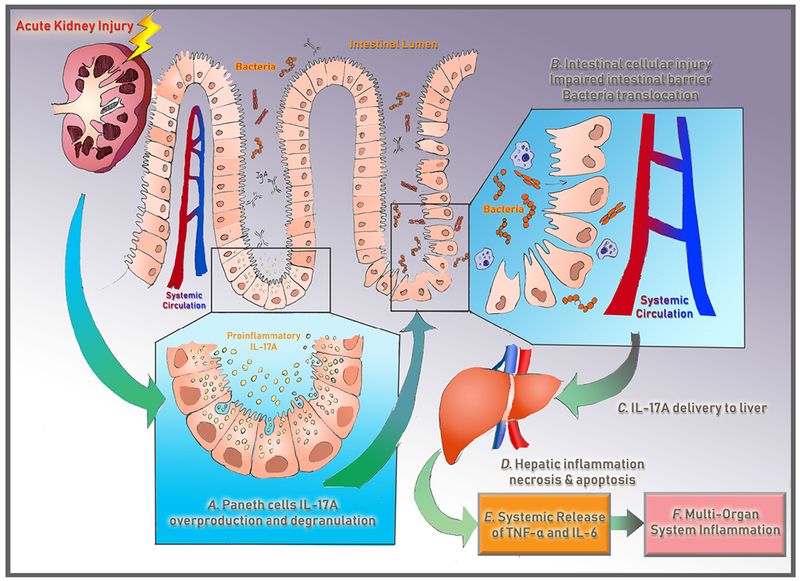
Recent experimental studies indicate that acute loss of kidney function causes small intestinal Paneth cells in the intestinal crypts to generate and degranulate proinflammatory IL-17-A into the intestinal lumen, which directly causes intestinal cellular injury and intestinal barrier breakdown. This allows for bacterial translocation and portal delivery of IL-17-A-containing macrophages, which causes hepatic injury and hepatic release of IL-6 and TNF-alpha into the circulation, leading to further hepatic and systemic inflammation. These studies highlight that acute kidney injury is not merely a bystander but can initiate a downward spiral triggering multi-organ failure and death.6
Many questions regarding organ “cross-talk” during acute kidney injury still remain unclear, for example how communication between the kidneys and distal organs—such as the gut, lungs, heart or the brain—are communicated. Similarly, many mechanistic aspects of acute kidney injury continue to be the focus of basic and translational research. Experimental data suggest that during acute kidney injury, a combination pro-inflammatory cytokine and chemokine release, leukocyte extravasation, induction of remote oxidative stress, and ion channels dysregulation can occur.46,39,47 Similarly, a number of anti-inflammatory pathways that can be targeted for acute kidney injury prevention or treatment have been identified, such as purinergic,48–50 or hypoxia-elicited anti-inflammatory signaling pathways,51 or inflammatory end-points that are under the control of microRNAs.41,52–54
Patient-associated Risk Factors for Acute Kidney Injury
Several elements are correlated with a heightened risk for perioperative acute kidney injury in patients. Pre-existing perioperative elevation of creatinine (>1.2mg/dl or higher) is a significant predictor for post-operative acute kidney injury among both cardiac and non-cardiac surgery populations.31,30,40 Furthermore, independent risk factors for perioperative acute kidney injury include advanced age, African American race, pre-existing hypertension, active congestive heart failure, chronic kidney disease, pulmonary disease, insulin-dependent diabetes mellitus, peripheral vascular disease, presence of ascites and high body mass index.25,29,30,55–58 For example, acute kidney injury prevalence among bariatric surgery cases is 5 to 10%.55,58 In addition to being a general risk factor, a high body mass index may increase the risk of perioperative acute kidney injury. It is hypothesized that an increase in oxidative stress, pro-inflammatory cytokines, and endothelial dysfunction associated with obesity could influence whether a patient develops acute kidney injury.29,59–62 Conflicting data exist regarding the influence of gender on acute kidney injury occurrence. Within cardiac surgery literature, the evidence is inconclusive with conflicting results that female gender may pose an increased perioperative risk for developing acute kidney injury.54,55 However, among general surgery patients, the American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP) 2005 - 2006 national data collection showed that male sex, rather than female, doubles the acute kidney injury threat after general surgery.55,58 From a clinical perspective, many of these factors are not modifiable. Identifying these patient-associated comorbidities may help with individual preoperative risk-stratification and prevention. Such clinical risk factors may include male sex, particularly in general surgery patients, obesity, advanced age, African American race, pre-existing hypertension, active congestive heart failure, chronic kidney disease, pulmonary disease, insulin-dependent diabetes mellitus, and peripheral vascular disease.
Surgery-associated risk factors for perioperative acute kidney injury
Acute Kidney Injury is a dangerous complication in both cardiac and non-cardiac surgeries, and independently associated with emergency surgery.58 One-third of acute kidney injury cases which occurred among patients who are critically ill have previous history of major surgery.63
Acute Kidney Injury in Cardiac and Vascular Surgery.
In vascular and cardiac surgeries, acute kidney injury is a well-established obstacle. Within these surgical settings, acute kidney injury is correlated with prolonged aortic cross-clamp and ischemia time, the creation of micro- and macro-emboli, low cardiac output state, prolonged hypotension, and the use of vasopressors and inotropes. In addition to low mean arterial pressure (MAP) during CPB, there are reports of contact-activated systemic inflammation, triggered by blood flow across the artificial surface of the bypass circuit. These damaging elements of CPB compromise renal blood flow and lead to renin-angiotensin-aldosterone system activation, decline of renal perfusion pressure, and worsening renal insult.64 Furthermore, components of CPB (pump, oxygenator, suction, filters) impose mechanical damage to the circulating erythrocytes, leading to intraoperative hemolysis and release of free hemoglobin. Free hemoglobin can cause direct injury to the renal epithelium via generation of free-radical species and obstructive cast formation.65 Strategies to minimize renal injury with CPB have been explored and include a goal-directed oxygen delivery threshold for adjusting arterial pump flow according to the hematocrit value and when necessary by blood transfusion when thresholds cannot be maintained with increased pump flow.66 Similar to a goal-directed oxygen delivery strategy, maintenance of a mixed venous oxygen saturation (SvO2) target above 75% during CPB is hypothesized to optimize system perfusion and may be linked with a lower risk of postoperative acute kidney injury.67 Further investigations are still needed to understand the optimal strategies necessary to reduce acute kidney injury risk during CPB among high-risk cases.
With the apparent renal implications of CPB, it may appear intuitive that off-pump coronary artery bypass (CAB) would exhibit renal sparing effect compared to the traditional on-pump coronary artery bypass graft (CABG) surgery. But to date, clear consensus has not been reached. The CABG Off or On Pump Revascularization Study (CORONARY) randomized 4,752 patients from 2006 through 2011 at 79 centers in 19 countries to on-pump or off-pump technique. The study found no significant difference in new renal failure requiring dialysis at 30 days, but a substantial decrease in the incidence of acute kidney injury was observed (28.0% vs. 32.1%; RR, 0.87; 95% CI, 0.80 to 0.96; P=0.01) in the off-pump group.68 In contrast, the German Off Pump Coronary Artery Bypass Grafting in Elderly Patients (GOPCABE) trial randomized 1,593 elderly patients to either on or off technique from 2008 through 2011 and found that off-pump CABG surgery was not correlated with a decreased incidence or reduced severity of acute kidney injury (P=0.174).69 Current evidence does not demonstrate a consistent reduction in the relative risk of acute kidney injury or dialysis with an off-pump revascularization technique. Given the lack of supporting evidence, off-pump CABG surgery should not routinely be recommended for all CABG patients at risk of perioperative acute kidney injury, especially in light of associated lower off-pump CABG revascularization success rates.68,70
Acute Kidney Injury in Non-Cardiac Surgery.
Occurrence of acute kidney injury among non-cardiac and non-vascular surgeries has been studied less extensively than during cardiac surgery, probably due to its overall lower incidence. According to the American College of Surgeons-National Surgical Quality Improvement Program national data collection, complications due to acute kidney injury occur in approximately 1% of general surgery cases, resulting in an eight-fold increase in all-cause 30-day mortality.58 Within the general surgery category, intraperitoneal surgery is an established risk for developing perioperative acute kidney injury.58 Procedure-related factors in abdominal surgery include intraoperative blood transfusions, episodes of intraoperative hemodynamic instability, and the use of vasopressors and diuretics.28,71 Increase in intra-abdominal pressure, often due to an excessive fluid administration or rapid fluid shift, is predictive of postoperative renal impairment.72,73 The reduction in perfusion pressure is attributed to mechanical compression of renal vasculature, causing a decreasing renal perfusion pressure and inducing renal ischemia.73,72 Of note, laparoscopic surgery with transient elevations in intra-abdominal pressure due to pneumo-peritoneum may result in a clinical decline in urine output, without causing an increase in postoperative acute kidney injury rates.29,71
Diagnosis of Perioperative Acute Kidney Injury
Diagnostic Criteria Utilizing Creatinine and Urine Output.
Multiple definitions and measurements to diagnose acute kidney injury have been explored and studied over the past thirty years. Measurements of serum creatinine and urine output remain the foundation of acute kidney injury diagnosis because they are both easily measurable and distinct to the kidney. The Risk, Injury, Failure, Loss, End-stage renal disease and Acute Kidney Injury Network criteria were each developed with the goal of creating a standardized definition of acute kidney injury that would also allow early and accurate diagnosis and treatment.48, 54, 12,74 In 2012, the Kidney Disease Improving Global Outcomes classification system emerged and remains the primary classification system for acute kidney injury56 (Supplemental Table 2).
Diagnostic Difficulties in the Perioperative Period.
Despite the development of a uniform standard to define acute kidney injury, the Kidney Disease Improving Global Outcomes classification system still has recognized limitations, particularly in the perioperative period. Urine output is a sensitive detection tool for identifying acute kidney injury and is appropriately included in all acute kidney injury definitions. However, the perioperative period has shown to be a unique environment with its own diagnostic challenges. Studies have shown that urine output frequently is decreased in the intraoperative and postoperative period due to the release of aldosterone and vasopressin from stress, hypovolemia, or even anesthesia.75,76 A 2010 study noted during surgery, only 5 to 15% of a crystalloid volume load is excreted in the urine, as opposed to 40 to 75% in a non-anesthetized patient.59 This decrease in urine output was unchanged whether the anesthesia was performed using isoflurane or a propofol infusion. Several studies have examined the relationship between fluid administration and intraoperative urine output and its correlation with postoperative acute kidney injury. One such randomized prospective study examined 107 patients undergoing bariatric surgery who received either high- or low-volume amounts of Ringer’s Lactate. The authors failed to find a correlation between low urine output in the intraoperative period and postoperative acute kidney injury. In conclusion, urine output is a less useful criterion for diagnosing perioperative acute kidney injury, necessitating a need for other diagnostic methodologies.
Serum creatinine is also a flawed diagnostic tool and can be an inaccurate marker for glomerular filtration rate. As a primary diagnostic criterion for acute kidney injury, serum creatinine is problematic because of the temporal delay from injury to the necessary diagnostic rise in creatinine. Creatinine will begin to rise after the glomerular filtration rate is decreased by 50%.77 An initial creatinine rise may occur on postoperative day one, however, the clear majority of patients fail to meet criteria for acute kidney injury until postoperative day two. Due to this “creatinine blind window of acute kidney injury,” perioperative acute kidney injury is frequently recognized late in the kidney injury process. Serum creatinine can also be altered by a variety of other factors than kidney junction, many of which are common in the perioperative period, including muscle injury, volume overload, nutrition, and steroids.77 In summary, the clinical practice of predominantly basing the clinical diagnosis of AKI on measurements of serum creatinine has many limitations, such as a delay in diagnosing early stages of AKI. Research into the efficacy of novel biologic markers is ongoing to improve the identification and treatment of acute kidney injury.
Novel Acute Kidney Injury Biomarkers.
The utility of biomarkers as reliable measurement tools for detecting minor but significant renal injury has been a focus of translational and bench research. As shown in Supplementary Table 2, there are numerous ongoing clinical trials to further sharpen our view and clinical approaches for novel acute kidney injury biomarkers (Supplementary Table 2). A highly precise biomarker would be ideal for renal injury and sensitive from insult to resolution (e.g., accounting for the “creatinine blind” window). Currently, biomarkers showing the most promise all have differing levels of sensitivity and specificity for acute kidney injury, with vastly differing time courses. They are described in more detail below (Figure 4).78
Figure 4. Biomarkers Over Time After Acute Kidney Injury.
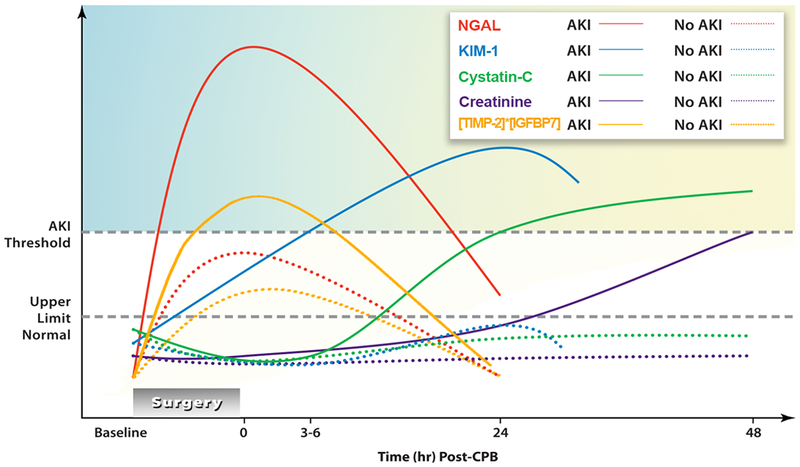
Schematic representation of the levels of several biomarkers over time. The baseline (time 0) is immediately following cardiac bypass (CBP). The lines are a schematic of the predicted rise and fall of the biomarkers following CBP as a function of time and when levels become significant enough to cross the threshold for diagnosing acute kidney injury. These patterns and specifically the timeline for diagnosing acute kidney injury, represent ideal circumstances (the shortest possible time interval shown in a clinical study), and not necessarily what will prove to be clinically verifiable.
neutrophil gelatinase-associated lipocalin = urinary neutrophil-associated lipocalin; kidney injury molecule-1= urinary kidney injury molecule; cystatin C= serum cystatin C; [tissue inhibitor of metalloproteinases-2]*[IGFBP7]= tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein-7 (IGFBP7); serum Cr= serum creatinine.78
Modified from Figure 1, from McIlroy DR, Wagener G, Lee HT: Biomarkers of Acute Kidney Injury: An Evolving Domain. Anesthesiology 2010;112(4):998-1004
The neutrophil gelatinase-associated lipocalin (NGAL) molecule is absent in urine and plasma of healthy individuals. The molecule was initially discovered in a screening study that was designed to identify genes that are differentially expressed in the early periods following renal ischemia. By clamping the renal artery in mice for 45 minutes, cDNA microarrays were used to define changes in renal gene expression. The authors identified seven upregulated genes, including neutrophil gelatinase-associated lipocalin, an easily measurable stable polypeptide found in the urine during the kidney injury process.79,80 However, point-of-care testing is unable to detect the renal isoform of neutrophil gelatinase associated lipocalin. Initial studies showed promise as a predictive indicator of acute kidney injury within cardiac surgery cases, where urine neutrophil gelatinase-associated lipocalin levels are diagnostic of acute kidney injury at two hours post-cardiac bypass. In a pediatric trial, similar findings in cardiopulmonary bypass patients were observed with neutrophil gelatinase-associated lipocalin levels elevated in acute kidney injury patients within two hours, compared to 1 to 3 days for diagnostic creatinine levels.81 Criticism of these early neutrophil gelatinase-associated lipocalin trials was the homogenous study population and comorbidity exclusion criteria.81,82,83 Subsequent studies with heterogeneous surgical populations found no association between neutrophil gelatinase-associated lipocalin and acute kidney injury.84,85 An examination of non-cardiac surgery cases, showed higher levels of urine and serum neutrophil gelatinase-associated lipocalin among those who have diabetes, an infection, and chronic kidney disease, but failed to correlate an association between neutrophil gelatinase-associated lipocalin levels and acute kidney injury.65 Specificity of neutrophil gelatinase-associated lipocalin has been estimated to range between 70 to 80%, however sensitivity is unpredictable, varying from 40 to 90%.78 The reason for such discrepancy is uncertain. However, neutrophil gelatinase-associated lipocalin levels may be affected by a wide range of factors, such as infections, certain tumors, cardiovascular disease, pre-existing renal disease, age, and diabetes. The influence of confounding variables has raised concerns regarding its diagnostic performance.86,87 Research is still ongoing for neutrophil gelatinase-associated lipocalin with several studies evaluating opportunities in contrast-induced nephropathy and utilizing neutrophil gelatinase-associated lipocalin to project whether there will be a need for renal replacement therapy.88,89 Better defining which patient populations will benefit from neutrophil gelatinase-associated lipocalin as a diagnostic tool and its role in combination with other biomarkers is the next step in the research of this important protein.
Another promising biomarker is the kidney injury molecule-1 (KIM-1). Kidney injury molecule-1 is a type-1 membrane glycoprotein that is upregulated after an ischemic or nephrotoxic injury to the proximal tubule epithelial cells.90 Studies show kidney injury molecule-1 functions as a cell adhesion molecule in the reconstruction of injured proximal tubules.78 A trial examining the occurrence of acute kidney injury during cardiac surgery in pediatric cases, found kidney injury molecule-1 to be an excellent diagnostic molecule with elevated urine levels within 6 hours after cardiac bypass.71 However, another study in adult cardiac surgery patients showed good specificity, but only a sensitivity of 50% when using urinary kidney injury molecule-1 to diagnose acute kidney injury.91 This study, along with others, showed that kidney injury molecule-1 is potentially more useful when used as part of a panel combining several biomarkers.91,92 However, there is currently no point-of-care device in the marketplace for immediate assessment of kidney injury molecule-1.
Cystatin C is unique in that it is a very small, charged molecule completely filtered at the glomerulus where it undergoes catabolism by proximal tubule cells. Because of this, there is virtually zero measurable cystatin C found in the urine of healthy kidneys.70 The above characteristics and a short half-life in the serum (two hours), have made several investigators propose that serum cystatin C is an ideal surrogate for glomerular filtration rate and tubular cell integrity.70,93,94,95 Two studies examining the use of serum cystatin C have had mixed results, with one showing its ability to diagnose acute kidney injury occurring within six hours following surgery, whereas the other found it to be no better than creatinine.92,96 However, this study did show that urine cystatin C levels rose within 6 hours after bypass in those with acute kidney injury, necessitating further study to clarify its role. Currently, the number of patients studied utilizing cystatin C is small, making the results and future of this molecule as a novel acute kidney injury biomarker still to be seen.
Tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor binding protein-7 (IGFBP-7) get released during cell cycle arrest and could potentially present sensitive and precise biomarker molecules for diagnosing acute kidney injury. During normal cell proliferation, a cell must go through each stage of the cell cycle (G1-M). However, when cells are damaged, they utilize cell cycle arrest as a protective mechanism to circumvent replication of damaged DNA. When renal cells enter cell cycle arrest, this adaptive response is mediated by surrounding cells through the release of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor binding protein-7. Their presence in the urine is hypothesized to be one of the earliest signs of cellular kidney damage.75,97 Tissue inhibitor of metalloproteinases-2 and insulin-like growth factor binding protein-7 biomarkers were identified and consequently confirmed in the second phase of the SAPHIRE trial which examined samples from over 700 patients across 35 centers.75,97 Univariate analysis showed that tissue inhibitor of metalloproteinases-2 and insulin-like growth factor binding protein-7 >2.0 was correlated to an elevated risk of all-cause mortality or renal replacement therapy (hazard ratio, 2.11; 95%, CI 95% CI, 1.37 to 3.23; P<0.001). In a multivariate analysis adjusted for the clinical model, tissue inhibitor of metalloproteinases-2, and insulin-like growth factor binding protein-7 levels >0.3 was associated with death or renal replacement therapy among subjects who developed acute kidney injury. The SAPHIRE trial also determined that the combination of the two biomarkers proved to be of greater prognostic value for acute kidney injury than either in isolation. Tissue inhibitor of metalloproteinases-2 and insulin-like growth factor binding protein-7 was appreciably more predictive to previously described markers of acute kidney injury (P < 0.002), such as neutrophil gelatinase-associated lipocalin and kidney injury molecule-1, in a heterogeneous population.98 A recent meta-analysis found they accurately predicted the probability to develop acute kidney injury and subsequent necessity for renal replacement therapy with a sensitivity and specificity of 0.69 and 0.81, respectively.99 These numbers are promising, and will be the focus of several upcoming translational studies to determine its clinical application.
Although several studies were able to demonstrate a statistical association between biomarker level and acute kidney injury, it has been more difficult to prove that biomarker measurements alter clinical outcomes. A recent study comparing the use of a furosemide stress test (which involves giving an IV dose of furosemide followed by two hours of close urine output monitoring), was equally or more effective at predicting acute kidney injury and necessity of renal replacement therapy than several biomarkers, including neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and tissue inhibitor of metalloproteinases-2 and insulin-like growth factor binding protein-7.100 This shows that we should not necessarily forget about previously used methods of acute kidney injury diagnosis, such as urine output and creatinine, but potentially find other modalities to complement and enhance their diagnostic capability. This is especially true in the perioperative period where urine output cannot be relied on as an accurate indicator of acute kidney injury.
The future of biomarkers remains an active and dynamic area of intense research, with translational studies merging biomarker “panels” with existing diagnostic criteria to improve detection and intervention. A promising recent study examined several renal biomarkers, including kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, and cystatin C, and failed to show that any of them alone were better at predicting the necessity of renal replacement therapy or hospital mortality than changes in creatinine.101 However, when the biomarkers were combined, along with a measure of change in creatinine, they could predict adverse events with excellent sensitivity and specificity. NephroCheck (Astute Medical, San Diego, CA, USA), was authorized by the US Food and Drug Administration in 2014 as a point-of-care urinary biomarker assay, to evaluate acute kidney injury development.102 This in vitro urine assessment quantitatively measures tissue inhibitor of metalloproteinases-2 and insulin-like growth factor binding protein-7 with reference intervals established in healthy adults and stable chronic morbid conditions without preexisting acute kidney injury.103 Daubin et al. evaluated the NephroCheck in critically ill patients and determined acute kidney injury patients have a significantly higher score than patients without acute kidney injury (0.43 [0.07-2.06] versus 0.15 [0.07-0.35]; P = 0.027). However, the authors noted the NephroCheck was unable to distinguish between temporary and persistent acute kidney injury.104
The future of point-of-care testing is bright, but significant confounding hurdles must be accounted for to validate the integration of point-of-care testing with biomarker panels. As more biomarkers become commercially available, translational research is necessary to evaluate the efficacy of biomarker panels and point-of-care testing to allow for early intervention, risk assessment, and diagnosis of acute kidney injury.
Therapeutic approaches for perioperative Acute Kidney Injury
Developing successful therapies to treat acute kidney injury has been an elusive endeavor. Despite significant advancements in diagnosis, surgical techniques, anesthetic methods, and critical care management, the frequency of perioperative acute kidney injury has remained essentially unchanged.105 Although numerous agents have shown promise, a single strategy toward improving treatment options for acute kidney injury has failed to demonstrate utility in clinical care.106,107,108,109 This continues to be an area of intense research with numerous ongoing translational and clinical trials (Supplementary Table 3). Moreover, many exciting recent studies have found important concepts that are critical for patient management in acute kidney injury prevention.
Pharmacologic Interventions.
Pharmacologic interventions to effectively treat and prevent acute kidney injury have been evaluated extensively across perioperative environments including major non-vascular, cardiovascular, contrast-induced acute kidney injury, and intensive care units. In addition, there is a substantial number of ongoing clinical trials to define and evaluate pharmacologic interventions in the perioperative setting (Supplementary Table 4). Historically, evidence for pharmacological intervention decreasing rates of acute kidney injury has largely been unsupported by quality data. More recent pharmacologic research has investigated the potential benefit of anti-inflammatory, anti-apoptotic, and anti-oxidative interventions to prevent and treat acute kidney injury.
N-acetylcysteine is a precursor of intracellular glutathione which reduces the oxidative burst response of neutrophils by improving oxygen free radical scavenging.110 In prospective randomized controlled trials, intravenous N-acetylcysteine failed to prevent postoperative renal dysfunction or reduce mortality rates in high-risk CPB surgery patients.111 Similarly, a double-blinded randomized controlled trial by Song et al. failed to establish a protective benefit of N-acetylcysteine in off-pump CPB surgery, with similar rates of acute kidney injury observed between treatment and control groups (35% N-acetylcysteine vs. 32% control P = 0.695).112 The anti-oxidative benefit of allopurinol and supplements such as selenium, zinc, and vitamins C, E, and B1, have also failed to show benefit in clinical trials.113,114 Current evidence does not support the role of N-acetylcysteine or supplements such as selenium, zinc, and vitamins C, E, and B1, to prevent acute kidney injury.111,113,114,115
The lipid-lowering, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors or statins are a drug class with increasing research interest because of their potential anti-inflammatory, antioxidative, and endothelial protective properties.29,116,117 A 2018 study involving eight randomized controlled trials, investigated the effect of cardiac surgery-associated acute kidney injury and perioperative statin therapy but did not find a correlation between statin administration and acute kidney injury reduction (RR 1.17, 95% CI: 0.98-1.39; P=0.076). Statin administration both pre- and post-surgery actually increased acute kidney injury risk when compared to preoperative statin therapy alone (p=0.040).118 A meta-analysis by Zhao et al. suggests sufficient evidence has accrued to reject the hypothesis that perioperative statin therapy decreases the prevalence of acute kidney injury and secondary postoperative consequences such as renal replacement therapy, mechanical ventilation, length of stay in the ICU or hospital, and in-hospital death.117 In summary, the present evidence does not substantiate using statin therapy in the treatment or prevention of acute kidney injury.
Dexmedetomidine, a selective α2-adrenergic receptor agonist, was another candidate agent for perioperative kidney protection through increasing renal blood flow and also decreasing oxidative insult to the kidney. In animal models, dexmedetomidine has demonstrated anti-inflammatory, anti-apoptotic, and anti-oxidative properties across organ systems.119,120,121,122 Several studies have also demonstrated inhibition of inflammatory mediators including IL-1, IL-6, and TNF-α.123,124 The clinical application of dexmedetomidine’s renoprotective properties has successfully reduced the incidence of cardiac surgery-associated acute kidney injury in valvular heart surgery populations.125,126,127,128 The presumed beneficial effect includes a reduction in norepinephrine release, enhanced hemodynamic stability, and myocardial oxygen supply/demand balance.29 In a recent meta-analysis involving 19,266 patients, dexmedetomidine was found to lower rates of cardiac surgery-associated acute kidney injury in both randomized controlled trials (RR 0.44, 95% CI 0.26-0.76; P= 0.003) and cohort studies (RR 0.74, 95% CI 0.63-0.86; P = 0.0001).129 However, dexmedetomidine failed to decrease postoperative mortality, duration of mechanical ventilator, and length of stay in the ICU or hospital.129 Based on these studies, dexmedetomidine may have the capacity to attenuate acute kidney injury in surgical patients. However, additional high-quality, multi-center trials will have to confirm these findings in order to establish the basis for its routine clinical use to prevent perioperative AKI.29
Beyond pharmacologic intervention, opportunities for non-pharmacologic therapy such as remote ischemic preconditioning and renal replacement therapy have been researched extensively to treat and prevent acute kidney injury.
Remote Ischemic Preconditioning.
Ischemic preconditioning is an experimental strategy where short, non-lethal episodes of ischemia are applied to provide protection from a subsequent, more lethal ischemic insult.42,130,131 As a more recently discovered form of ischemic preconditioning, remote ischemic preconditioning is achieved through application of brief periods of ischemia and reperfusion of remote tissues or organs, resulting in the protective adaptive response of distant organ systems. Usually, a blood pressure cuff placed around the upper arm is expanded to 200 to 300 mm Hg pressure for a 5-minute duration, and then the pressure is released, followed by several repeat cycles. Remote ischemic preconditioning is thought to activate several pathways including systemic anti-inflammatory, neuronal, and humoral signaling pathways.132 In this regard, remote ischemic preconditioning offers a novel, noninvasive, and inexpensive strategy to decrease the occurrence of acute kidney injury.132,133,134
Clinically, some controversy exists to the effectiveness of remote ischemic preconditioning on acute kidney injury. The outcomes of remote ischemic preconditioning on the kidney has been investigated extensively within the framework of adult vascular and cardiac surgery. In a large multicenter, randomized double-blind clinical trial, Zarbock et al. recently found that remote ischemic preconditioning before cardiac surgery in high-risk patients was effective for decreasing the rate of acute kidney injury (37.5% versus 52.5%) with sham (absolute risk reduction 15%; 95% CI, 2.56-27.44; P= 0.02).134 In addition, a subsequent long-term follow up by the same authors revealed remote ischemic preconditioning lowered the 3-month prevalence of a composite endpoint of major adverse kidney events consisting of death, necessity of renal replacement therapy, and chronic renal dysfunction.135 These findings are supported by similar randomized controlled trials in the cardiac and vascular literature conducted by Ali et al. and Theilmann et al.136,137 Despite these exciting findings, the discussion of remote ischemic preconditioning and kidney protection remains inconclusive with other post-cardiac surgery outcomes trials unable to establish a protective effect.138,139,140 The differences in these outcomes could be related to study design (e.g. the selection of primary endpoints), different patient populations (high risk versus lower risk of acute kidney injury), or the specific protocol used for remote ischemic preconditioning. Additionally, anesthetic type may alter the effect of remote ischemic preconditioning, with reports of Propofol blunting the observed beneficial effects relative to volatile anesthetics.141,142 Due to the very non-invasive nature, unknown side-effects, and promising initial findings, the threshold to introduce remote ischemic preconditioning into routine clinical practice is relatively low. Nevertheless, further multicenter trials are needed to fully establish the clinical benefits, dose, and ideal patient population for remote ischemic preconditioning therapy to prevent acute kidney injury in surgical patients.
Renal Replacement Therapy.
Renal replacement therapy is the only therapy for acute kidney injury to date. The Kidney Disease Improving Global Outcomes criteria advocate initiating renal replacement therapy when fluid accumulation becomes life-threatening or major imbalances (e.g., acidosis, electrolyte abnormalities, and uremia) occur.29,143 The ideal mode of renal replacement therapy, the correct timing of when to begin therapy, and duration are still under debate.29 A recent meta-analysis evaluating renal replacement therapy modalities on clinical outcomes failed to find statistical differences for the pooled mortality results (ICU, in-hospital, or 30-day) and dialysis dependence between continuous renal replacement therapy (CRRT) and sustained low efficiency dialysis (SLED).144 Clinical studies to address the exact timing of when to initiate renal replacement therapy show conflicting data as well. Two large prospective randomized controlled trials, the Artificial Kidney Initiation in Kidney Injury (AKIKI) trial and Early versus Late Initiation of Renal Replacement Therapy in Critically Ill Patients with Acute Kidney Injury (ELAIN) trial, evaluated the influence of renal replacement therapy timing in ICU patients with acute kidney injury.145,146,147 The ELAIN trial reported that early initiation of renal replacement therapy in patients suffering from acute kidney injury resulted in a significant reduction in 90-day mortality. This is in contrast to the AKIKI trial, which failed to show a reduction in mortality as a function of renal replacement therapy timing.145,146,147 Most recently, the IDEAL-ICU Trial examined a relatively homogenous population of patients with early-stage septic shock who had severe acute kidney injury.148 In this multicenter randomized trial, patients with early sepsis were randomized to early (within 12h) or delayed initiation (delay of 48h) of renal replacement therapy. The trial was stopped early after an interim analysis. In this group of patients, early initiation of renal-replacement therapy failed to demonstrate a lowering of mortality at 90 days, compared to the delayed initiation of renal replacement. Importantly, there was overall less use of renal-replacement therapy in the delayed-strategy group, as 75% of patients in the delayed group recovered their kidney function spontaneously. However, it is important to keep in mind that starting treatments late in the chain of acute kidney injury (e.g. at the stage of severe acute kidney injury) does not improve patient outcomes. Therefore, it is important that we detect patients who suffer from a progressive form of acute kidney injury early. In addition, the Acute Disease Quality Initiative (ADQI) workgroup suggested a more personalized approach should be considered for initiation of renal replacement therapy, based on the dynamic assessment of different clinical parameters that reflect the mismatch of demand and capacity.149 Based on the conflicting results from these trials, there continues to be a need for additional research to reduce the variability of timing in the initiation of renal replacement therapy.149
Fluid Replacement Approaches.
The administration of fluids is a mainstay of therapy to prevent hypovolemia and improve renal perfusion. However, the debate of restrictive versus liberal fluid therapy has been an ongoing argument amongst perioperative physicians for many decades. Historically, conventional regimens were characterized by the liberal administration of large intravenous-fluid volumes (e.g. more than 7 liters for open abdominal surgery) to account for fluid deficits, vasodilation, hemorrhage, and fluid accumulation in extravascular spaces.150,151 However with the introduction of Enhanced Recovery After Surgery (ERAS) protocols, a more restrictive pattern of fluid administration has been proposed with the benefit of fewer complications (i.e. pulmonary, acute kidney injury, sepsis, and wound healing), time to recovery, and length of hospital stay.152,153,154–156 To investigate the outcome of liberal versus restrictive fluid administration in the perioperative patient, The Restrictive versus Liberal Fluid Therapy in Major Abdominal Surgery (RELIEF) trial was conducted in 2018.
The RELIEF trial was an international, randomized, assessor-blinded trial which compared a restrictive intravenous-fluid regimen to a liberal regimen in 3,000 patients undergoing major abdominal surgery.151 The median intravenous-fluid intake for the group with restricted fluid was 3.7 liters versus 6.1 liters in the liberal fluid group.151 They found that a regimen consisting of restricted fluid intake had no correlation with higher rates of disability-free survival than a regimen of liberal fluid intake. However, the study did find that a restricted fluid regimen was correlated to higher rates of acute kidney injury (8.6% restrictive versus 5.0% liberal fluid group (P <0.001).151 Brandstrup, in the editorial response to the Myles et al. RELIEF trial, eloquently surmised “a modestly liberal administration of balanced salt solutions does not create substantial fluid retention, and it appears to be safe to administer a fluid volume that slightly exceeds zero fluid balance, although patients for whom an ERAS protocol is used might not need it. In addition, we learn that physiologic principles remain valid: both hypovolemia and oliguria must be recognized and treated with fluid.”157 Based on these findings, a reasonably liberal fluid intake regimen is potentially safer than a highly restricted fluid intake regimen for fluid resuscitation of the perioperative patient.157
Goal-Directed Hemodynamic Therapy.
Maintaining volume status and perfusion pressure are central tenants of the Kidney Disease Improving Global Outcomes recommendations. Early goal-directed hemodynamic therapy was proposed to optimize fluid resuscitation and cardiac output to improve microvascular perfusion pressure and cellular oxygenation while minimizing the harmful effects of fluid overload.107,158,159,160,161 Utilizing goal-directed hemodynamic therapy, predefined algorithms of fluid loading, and inotropic support are utilized to account for variables such as myocardial performance,162,163 vascular tone, regional blood flow distribution,164 venous reservoir capacity, and capillary permeability. 165 A recent randomized controlled trial, the Optimization of Cardiovascular Management to Improve Surgical Outcome trial (OPTIMISE), was conducted in order to analyze the virtues of using goal-directed therapy to prevent acute kidney injury in non-cardiac surgical populations.
The OPTIMISE trial was a pragmatic, multicenter, randomized, observer-blinded trial of 734 high-risk patients. Patients were over the age of 65 undergoing major gastrointestinal surgery with the presence of cardiac or respiratory disease; renal impairment (serum creatinine ≥1.5 mg dl-1); diabetes mellitus; or undergoing emergency surgery.166 Patients were randomized to standard of care or a cardiac output–guided hemodynamic therapy algorithm for intravenous fluid and inotrope support during and after surgery.166 Patients receiving intervention had a RR 0.84, 95% CI, 0.71-1.0; and an ARR, 6.8%, 95% CI, −0.3% to 13.9%; P = 0.07.166 There was no difference in the secondary outcomes of seven-day morbidity, critical care days, all-cause mortality at 30 and 180 days, or acute hospital length of stay.166 The question of whether goal-directed therapy improves postoperative outcome is still under debate. Gelman and Bigatello attribute the inconsistent benefits to an infused fluid volume that serves only to increase the unstressed capacity while the stressed volume and hemodynamics remain largely unchanged.167 Further, variability is influenced by type of hemodynamic monitor, fluid administered algorithms, as well as the type and duration of hemodynamic intervention. Multicenter randomized controlled trials are needed to understand the role of goal-directed therapy in acute kidney injury outcomes. Based on these studies, the value of using goal-directed hemodynamic monitoring approaches to guide specific algorithms for fluid replacement remains questionable. Even in today’s world, perioperative physicians may still be faced with the challenge of utilizing best clinical judgment in a complex clinical environment to make decisions about hemodynamic optimization of surgical patients.
Impact of Intravenous Fluid Composition.
In line with the previous discussion of hemodynamic optimization, an ongoing argument in the field focuses on the type of fluid used in resuscitation. Isotonic crystalloid remains the standard for first-line resuscitation fluid therapy in the perioperative and ICU environments. Globally, the most frequently employed isotonic crystalloid is 0.9% sodium chloride. However, accumulating evidence suggests patients are at risk of adverse effects to acid-base homeostasis, renal vasoconstriction, reduced glomerular filtration rate, increased risk of acute kidney injury, and death.168,169,170–172,173 Based on this research, a supraphysiologic chloride concentration of saline could be a potential contributor to kidney injury.174 Two large-scale studies investigated the effect of balanced crystalloids and saline in critical and noncritical populations: the Isotonic Solutions and Major Adverse Renal Events Trial (SMART), examined using balanced crystalloids versus saline in patients in medical (SMART-MED) and nonmedical (SMART-SURG) ICUs; and the Saline against Lactated Ringer’s or Plasma-Lyte in the Emergency Department (SALT-ED) trial.
The SALT-ED trial was a single-center, pragmatic, multiple-crossover study comparing balanced crystalloids with saline in 13,347 adults treated with intravenous crystalloids during hospitalization outside of the ICU environment.174 There was not a significant difference between the two groups in the number of hospital-free days. However, there was a lower prevalence of major adverse kidney events in the balanced crystalloids group (4.7% vs. 5.6%; AOR 0.82; 95% CI, 0.70 - 0.95; P=0.01).174 In this trial of non-critically ill adult patients, balanced crystalloids treatment did not result in reduced time to hospital discharge but it did find there was a lower prevalence of composite death, new renal replacement therapy, and chronic renal dysfunction.174
The SMART trial was a pragmatic, cluster-randomized, multiple-crossover design study of 15,802 ICU adult patients who received either 0.9% sodium chloride or balanced crystalloid solutions.175 Similar to the SALT-ED trial, the main outcomes were major adverse kidney events within 30 days, new renal replacement therapy, or chronic renal dysfunction.175 A major adverse kidney event occurred in 14.3% of the study population who received balanced-crystalloid solutions; and in the cohort who received 0.9% sodium chloride solution, 15.4% suffered from a major adverse kidney event (marginal OR 0.91; 95% CI, 0.84-0.99; conditional OR 0.90; 95% CI, 0.82-0.99; P=0.04).175 Among critically ill adults, using balanced crystalloids lowered death rates from any cause, new renal replacement therapy, or chronic renal dysfunction.175 Based on these recent, high-quality clinical trials, a balanced crystalloid solution with electrolyte compositions comparable to plasma is preferred for volume resuscitation to minimize adverse kidney events and death.29
In a classical view of microvascular fluid dynamics, colloids were hypothesized to be more effective than crystalloids in reestablishing circulating plasma volume, as their volume of distribution was thought to be comparatively maximized within the intravascular compartment, reducing time to hemodynamic stability with a comparatively smaller volume, with an effective longer duration. Through the investigation of crystalloid resuscitation alternatives, various controlled studies have scrutinized the efficacy of albumin, hydroxyethyl starch, and gelatin-based colloids.
The Crystalloid Versus Hydroxyethyl Starch Trial (CHEST) and Scandinavian Starch for Severe Sepsis/Septic Shock Trial (6S) were landmark trials that compared the effects of resuscitation with hydroxyethyl starch and crystalloid solutions.159,176 Findings from these trials demonstrate an association of acute kidney injury risk, increased rate of renal replacement therapy, and death with hydroxyethyl starch among ICU and septic patient populations.176,177 These findings led drug regulators in Europe and the US to issue black box safety warnings in 2013 against the use of hydroxyethyl starch. However, the use of hydroxyethyl starch-containing solutions remains controversial. As Weiss et al. recently highlighted, “every drug can be used safely and effectively when it is used appropriately, according to its indication and in the right patient population.”178 Currently two prospective, randomized, controlled, double-blind trials are underway, the PHOENICS trial (NCT03278548) and TETHYS trial (NCT03338218). Currently, hydroxyethyl starch should be administered with caution in critically ill patients until new evidence from ongoing trials is made available.
After concerns were raised about the safety profile of hydroxyethyl starch, a renewed interest in the safety and efficacy of gelatin and albumin colloid alternatives occurred in the marketplace. The use of albumin, a natural colloid, is an effective plasma volume expander and has been shown to improve microcirculation.179,180 In the SAFE trial, a double-blinded randomized controlled trial, there was no observed difference in urine output, organ failure, and duration of renal replacement therapy between saline and 4% albumin solutions. While albumin appears safe, it is 2 to 5 times more expensive than crystalloid, and offers no significant difference in patient outcomes.159 Another colloid alternative to crystalloid extensively utilized worldwide is gelatin, a degradation product of collagen.181 Gelatin-based solutions are similarly costly (>10 times crystalloid) with evidence of safety and efficacy from large prospective randomized controlled trials surprisingly limited.182 Moeller et al. found risk ratios after gelatin administration were 1.15 (95% CI, 0.96-1.38) for mortality, 1.10 (0.86-1.41) for requiring allogeneic blood transfusion, 1.35 (0.58-3.14) for acute kidney injury, and 3.01 (1.27-7.14) for anaphylaxis.183 Like hydroxyethyl starch, the authors concluded an increased risk of mortality, renal failure, anaphylaxis, and coagulation impairment with gelatin administration.183 A novel prospective, double-blind randomized controlled trial (NCT02715466) is investigating the therapeutic value and safety of gelatin in patients with early severe sepsis or septic shock.183 Considering cheaper and safer crystalloid alternatives, the administration of gelatins should be undertaken with caution until further evidence from a well-designed trial supporting its use is made available.
Impact of Anemia and Transfusion on Perioperative Acute Kidney Injury.
Preoperative anemia, defined by the World Health Organization as less than 12g/dl for female and less than 13 g/dl for male patients, is linked with perioperative acute kidney injury in both cardiac and non-cardiac surgery patients.184,185,186 Early postoperative decrements in hemoglobin level have also been linked with acute kidney injury.184 Anemia leads to a state of decreased oxygen-carrying capacity, putting the vulnerable renal medulla at an increased risk of hypoxic injury. An anemic state also imposes greater oxidative stress on the renal tubules, in part because of the inherent protective function of red blood cells.186 In addition to preoperative anemic state, perioperative blood transfusion has also been recognized as an independent risk factor for perioperative acute kidney injury. The deleterious effect of allogeneic transfusion has been attributed to the preservation and storage effect of red blood cells, promoting oxidative stress and a pro-inflammatory state.28,76,186,92 It is important to note that both anemia and transfusion are associated with acute kidney injury. Measures to optimize a patient’s overall preoperative status while minimizing surgical bleeding should be employed to reduce hematologic related acute kidney injury risk.
Avoidance of Nephrotoxic Drugs.
Nephrotoxin-induced acute kidney injury is a considerable risk to patients in the perioperative period. Avoidance and minimizing the duration of exposure of these agents reduces the risk of acute kidney injury development.29 Goldstein et al. performed a prospective analysis of implementing an Electronic Health Record screening and decision matrix at Cincinnati Children’s Hospital.187 In 1,749 patients, implementation of a surveillance system reduced the exposure rate and acute kidney injury rate by 38% and 64%, respectively.187 As highlighted in these studies, scrutinizing for nephrotoxic exposure has the potential to reduce avoidable harm and can help to prevent perioperative acute kidney injury.
Glycemic Control and Nutritional Support.
Like nephrotoxic exposure, glycemic control and nutritional support are modifiable, independent predictors of outcome that should be optimized in the perioperative patient. In epidemiological studies, protein-calorie malnutrition is a significant risk factor for in-hospital death among patients suffering from acute kidney injury.188 Such patients frequently have accelerated protein breakdown and increased caloric needs, especially if they are critically ill or undergoing renal replacement therapy.26 Piggot et al. retrospectively reviewed records of neonates who underwent congenital heart surgery at Arnold Palmer Hospital for Children.189 In a multivariable analysis, an inability to reach caloric goal preoperatively was independently associated with stage 2 or 3 acute kidney injury (P = 0.04; OR, 4.48 (95% CI, 1.02-19.63).189 Further, a difference in peak lactate (P = 0.002), inotropic score (P = 0.02), and duration of mechanical ventilation (P = .013) was also observed.189 Nutrition is fundamental for cellular and organ function, with malnutrition potentially worsening the severity of illness and contributory to acute kidney injury. Similarly, hyperglycemia is considered one of the best independent predictors of mortality and worse outcomes.190 The Kidney Disease Improving Global Outcomes criteria recommend maintaining blood glucose concentrations between 110 and 149 mg/dL in critically ill patients, to minimize perioperative hyperglycemia associated with increased mortality, surgical complications, and acute kidney injury risk.110 The Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial investigated restrictive blood glucose targets.191 This parallel-group randomized controlled trial, demonstrated a higher mortality in ICU patients with restrictive targets (OR 1.14, 95% CI, 1.02 to 1.28; P=0.02), without reducing the prevalence of acute kidney injury. Based on the NICE-SUGAR trial and subsequent meta-analysis it seems practical to adopt higher glycemic values, avoiding the inherent risks of hypoglycemia associated with tight glycemic benchmarks. Practical targets should be in accordance with the 2012 guidelines set forth by the Kidney Disease Improving Global Outcomes of (110-149 mg/dl) or the statement from the European Renal Best Practice based on guidelines from the Kidney Disease Improving Global Outcomes of (140-180 mg/dl).143,188
Preventative Acute Kidney Injury Bundle Protocols.
The effectiveness of implementing preventative measures such as nutritional support and glycemic control, minimizing nephrotoxic medication exposure, and hemodynamic optimization have largely been studied independently. In 2017, the PrevAKI trial was conducted to analyze the value of implementing a “Kidney Disease Improving Global Outcomes bundle” in cardiac-associated acute kidney injury.192 In the PrevAKI Trial, Meersch et al. implemented a Kidney Disease Improving Global Outcomes protocol to optimize intravascular volume and hemodynamics, minimize nephrotoxic exposure, and prevent hyperglycemia in 882 high-risk cardiac surgery patients.192 The prevalence of acute kidney injury, identified by tissue inhibitor of metalloproteinases-2 and insulin-like growth factor binding protein-7 biomarkers, was reduced with the Kidney Disease Improving Global Outcomes bundle (55.1 vs. 71.7%; ARR 16.6%, 95% CI 5.5-27.9%; P = 0.004). A Kidney Disease Improving Global Outcomes bundle enhanced hemodynamic parameters (P < 0.05), reduced hyperglycemia (P < 0.001) as well as ephrotoxic agent utilization of (P< 0.001).192 However, the PrevAKI trial was not sufficiently powered to demonstrate a change in secondary outcomes of rate of dialysis, length of stay, and adverse kidney events, with bundle implementation.192 Current therapy has focused on minimizing the progression and optimizing the clinical response of the specific perioperative environment to improve acute kidney injury with limited success in secondary endpoints. Göcze et al. examined urinary cell-cycle arrest biomarkers, insulin-like growth factor-binding protein-7, and tissue inhibitor of metalloproteinase-2 in 107 high-risk surgical patients to predict early identification of acute kidney injury and allow for preventive measures.193 This rapid urinary biomarker assessment panel significantly improved risk stratification (p<0.001). Further, in combination with clinical parameters, Göcze et al. successfully allowed for early initiation of renal protective measures (i.e. hemodynamic optimization) and escalation of care prior to the development of acute kidney injury.193 Based on these studies, combinations of biomarker panels for early detection of perioperative acute kidney injury in conjunction with accelerated intervention protocols have great promise to become a cornerstone of acute kidney injury prevention in surgical patients.
Conclusions
The development of acute kidney injury has important implications for recovery and outcomes of surgical patients. Decreased urine output and an increase in serum creatinine concentrations are classically used to diagnose acute kidney injury. The search for earlier and more specific biomarkers is an area of intense research. We believe that their introduction into routine clinical praxis is eminent. A uniform classification of acute kidney injury by the Kidney Disease Improving Global Outcomes workgroup has allowed for advances in medical practice, research, and public health. Promising therapeutic advances to prevent or treat perioperative acute kidney injury continue to be a challenge. Although previous clinical trials have been disappointing, new preclinical and clinical interventions are targeting novel pathophysiology aspects of acute kidney injury inflammatory and oxidative stress response, endothelial dysfunction, microRNA modulators, as well as renal-specific vasodilators. Clinical research continues to explore our evolving understanding of fluid balance and type of fluid used in the resuscitation of the perioperative patient, renal replacement therapy, and alternative therapeutic approaches, such as remote ischemic preconditioning.
Advancements in risk stratification systems, with the use of instantaneous analytics, has the potential to emerge as an effective means of acute kidney injury risk assessment in the perioperative setting.194 This combination of clinical parameters, biomarkers, and electronic medical record risk stratification will allow timely identification of subclinical acute kidney injury and initiation of renal protective measures. Once identified, a phased approach to more complex and costly evaluations can be integrated into existing low-cost and low-risk therapies.194 The importance of rapid diagnosis cannot be overstated. It extends the opportunity for intervention, preventing the progression and development of subclinical injury to acute kidney injury. Further multicenter trials and translational research are needed to further define or establish appropriate therapeutic and diagnostic opportunities in acute kidney injury. The historic premise of “do no harm” continues to be a cornerstone in acute kidney injury management, including hemodynamic monitoring approaches, fluid replacement therapy, and avoidance of nephrotoxic agents.
Supplementary Material
Acknowledgements
Special thanks to Ms. Kelli Wallen, MPH, for her invaluable editorial support throughout.
Sources of Funding, Potential Conflict of Interest:
None of the authors has a conflict of interest. The present manuscript is supported by National Institute of Health Grants R01 DK097075, POI-HL114457, R01-HL109233, R01-DK109574, R01-HL119837 and R01-HL133900 to HKE.
Appendix 1:
Inclusion Criteria to Accompany Supplementary Table 3
| NCT | Inclusion criteria |
|---|---|
| NCT02698930 |
|
| NCT02607163 |
|
| NCT02875873 |
|
| NCT03277677 |
|
| NCT03188614 |
|
| NCT02980003 |
|
| NCT02799368 |
|
| NCT02793661 |
|
| NCT02974946 |
|
| NCT01866800 |
|
| NCT03509935 |
|
| NCT02481518 |
|
| NCT03056248 |
|
| NCT03331146 |
|
| NCT03476460 |
|
| NCT03396770 |
|
| NCT02531412 |
|
| NCT03206658 |
|
| NCT03329443 |
|
| NCT01275729 |
|
| NCT02983422 |
|
| NCT02771509 |
|
| NCT02474667 |
|
| NCT02808351 |
|
| NCT02869347 |
|
| NCT02962102 |
|
| NCT03401710 |
|
| NCT03007537 |
|
| NCT02665377 |
|
| NCT02836899 |
|
| NCT02664753 |
|
| NCT02531724 |
|
| NCT01720030 |
|
| NCT03510897 |
|
| NCT03403751 |
|
| NCT03526367 |
|
| NCT03273751 |
|
| NCT03236441 |
|
| NCT02997748 |
|
| NCT02518087 |
|
| NCT03384875 |
|
| NCT02250131 |
|
| NCT03373318 |
|
| NCT02568722 |
|
| NCT02937935 |
|
| NCT03343340 |
|
| NCT03396757 |
|
| NCT02937961 |
|
| NCT03175328 |
|
| NCT01062984 |
|
| NCT02669589 |
|
| NCT02384525 |
|
| NCT03329313 |
|
| NCT03438877 |
|
| NCT02786277 |
|
| NCT02753751 |
|
| NCT02793167 |
|
| NCT02771977 |
|
| NCT01621152 |
|
| NCT03219398 |
|
| NCT03534141 |
|
| NCT02928887 |
|
| NCT02643745 |
|
| NCT03590028 |
|
| NCT02643745 |
|
| NCT03244514 |
|
| NCT03178435 |
|
| NCT03236103 |
|
| NCT03564314 |
|
| NCT02726620 |
|
| NCT02915575 |
|
| NCT02483039 |
|
| NCT03165552 |
|
| NCT03015623 |
|
| NCT02933892 |
|
References
- 1.Bartels K, Karhausen J, Clambey ET, Grenz A, Eltzschig HK: Perioperative organ injury. Anesthesiology 2013; 119: 1474–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK: Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol 2011; 22: 14–20 [DOI] [PubMed] [Google Scholar]
- 3.Schrier RW, Wang W: Acute renal failure and sepsis. N Engl J Med 2004; 351: 159–69 [DOI] [PubMed] [Google Scholar]
- 4.Park SW, Kim M, Kim JY, Ham A, Brown KM, Mori-Akiyama Y, Ouellette AJ, D’Agati VD, Lee HT: Paneth cell-mediated multiorgan dysfunction after acute kidney injury. J Immunol 2012; 189: 5421–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kork F, Balzer F, Spies CD, Wernecke KD, Ginde AA, Jankowski J, Eltzschig HK: Minor Postoperative Increases of Creatinine Are Associated with Higher Mortality and Longer Hospital Length of Stay in Surgical Patients. Anesthesiology 2015; 123: 1301–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HT, Kim M, Kim JY, Brown KM, Ham A, D’Agati VD, Mori-Akiyama Y: Critical role of interleukin-17A in murine intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 2013; 304: G12–25 [DOI] [PubMed] [Google Scholar]
- 7.Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2: 19–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Kidney F: K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–266 [PubMed] [Google Scholar]
- 9.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup: Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein SL, Chawla LS: Renal angina. Clin J Am Soc Nephrol 2010; 5: 943–9 [DOI] [PubMed] [Google Scholar]
- 11.Kellum JA, Bellomo R, Ronco C: Kidney attack. JAMA 2012; 307: 2265–6 [DOI] [PubMed] [Google Scholar]
- 12.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 2004; 15: 1597–605 [DOI] [PubMed] [Google Scholar]
- 13.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network: Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International 2012; Supplement: 1–138 [Google Scholar]
- 15.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA, Acute Disease Quality Initiative W: Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017; 13: 241–257 [DOI] [PubMed] [Google Scholar]
- 16.de Geus HR, Ronco C, Haase M, Jacob L, Lewington A, Vincent JL: The cardiac surgery-associated neutrophil gelatinase-associated lipocalin (CSA-NGAL) score: A potential tool to monitor acute tubular damage. J Thorac Cardiovasc Surg 2016; 151: 1476–81 [DOI] [PubMed] [Google Scholar]
- 17.Cerda J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, Bagga A, Levin A: Epidemiology of acute kidney injury. Clin J Am Soc Nephrol 2008; 3: 881–6 [DOI] [PubMed] [Google Scholar]
- 18.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR: The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol 2011; 57: 1752–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashani K, Shao M, Li G, Williams AW, Rule AD, Kremers WK, Malinchoc M, Gajic O, Lieske JC: No increase in the incidence of acute kidney injury in a population-based annual temporal trends epidemiology study. Kidney Int 2017; 92: 721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewington AJ, Cerda J, Mehta RL: Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int 2013; 84: 457–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor ME, Hewson RW, Kirwan CJ, Ackland GL, Pearse RM, Prowle JR: Acute kidney injury and mortality 1 year after major non-cardiac surgery. Br J Surg 2017; 104: 868–876 [DOI] [PubMed] [Google Scholar]
- 22.Hu J, Chen R, Liu S, Yu X, Zou J, Ding X: Global Incidence and Outcomes of Adult Patients With Acute Kidney Injury After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth 2016; 30: 82–9 [DOI] [PubMed] [Google Scholar]
- 23.Romagnoli S, Ricci Z: Postoperative acute kidney injury. Minerva Anestesiol 2015; 81: 684–96 [PubMed] [Google Scholar]
- 24.Hansen MK, Gammelager H, Mikkelsen MM, Hjortdal VE, Layton JB, Johnsen SP, Christiansen CF: Post-operative acute kidney injury and five-year risk of death, myocardial infarction, and stroke among elective cardiac surgical patients: a cohort study. Crit Care 2013; 17: R292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, Szabo Z, Kalantar-Zadeh K, Kovesdy CP: Acute Kidney Injury After Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am J Kidney Dis 2016; 67: 872–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abuelo JG: Normotensive ischemic acute renal failure. N Engl J Med 2007; 357: 797–805 [DOI] [PubMed] [Google Scholar]
- 27.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 2012; 380: 756–66 [DOI] [PubMed] [Google Scholar]
- 28.Gameiro J, Fonseca JA, Neves M, Jorge S, Lopes JA: Acute kidney injury in major abdominal surgery: incidence, risk factors, pathogenesis and outcomes. Ann Intensive Care 2018; 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meersch M, Schmidt C, Zarbock A: Perioperative Acute Kidney Injury: An Under-Recognized Problem. Anesth Analg 2017; 125: 1223–1232 [DOI] [PubMed] [Google Scholar]
- 30.Calvert S, Shaw A: Perioperative acute kidney injury. Perioper Med (Lond) 2012; 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sear JW: Kidney dysfunction in the postoperative period. Br J Anaesth 2005; 95: 20–32 [DOI] [PubMed] [Google Scholar]
- 32.Carmichael P, Carmichael AR: Acute renal failure in the surgical setting. ANZ J Surg 2003; 73: 144–53 [DOI] [PubMed] [Google Scholar]
- 33.Singh P, Ricksten SE, Bragadottir G, Redfors B, Nordquist L: Renal oxygenation and haemodynamics in acute kidney injury and chronic kidney disease. Clin Exp Pharmacol Physiol 2013; 40: 138–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riegel AK, Faigle M, Zug S, Rosenberger P, Robaye B, Boeynaems JM, Idzko M, Eltzschig HK: Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood 2011; 117: 2548–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallon V, Miracle C, Thomson S: Adenosine and kidney function: potential implications in patients with heart failure. Eur J Heart Fail 2008; 10: 176–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thurau K, Boylan JW: Acute renal success. The unexpected logic of oliguria in acute renal failure. Am J Med 1976; 61: 308–15 [DOI] [PubMed] [Google Scholar]
- 37.Redfors B, Bragadottir G, Sellgren J, Sward K, Ricksten SE: Acute renal failure is NOT an “acute renal success”--a clinical study on the renal oxygen supply/demand relationship in acute kidney injury. Crit Care Med 2010; 38: 1695–701 [DOI] [PubMed] [Google Scholar]
- 38.Idzko M, Ferrari D, Riegel AK, Eltzschig HK: Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood 2014; 124: 1029–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grams ME, Rabb H: The distant organ effects of acute kidney injury. Kidney Int 2012; 81: 942–948 [DOI] [PubMed] [Google Scholar]
- 40.Zafrani L, Ince C: Microcirculation in Acute and Chronic Kidney Diseases. Am J Kidney Dis 2015; 66: 1083–94 [DOI] [PubMed] [Google Scholar]
- 41.Neudecker V, Brodsky KS, Clambey ET, Schmidt EP, Packard TA, Davenport B, Standiford TJ, Weng T, Fletcher AA, Barthel L, Masterson JC, Furuta GT, Cai C, Blackburn MR, Ginde AA, Graner MW, Janssen WJ, Zemans RL, Evans CM, Burnham EL, Homann D, Moss M, Kreth S, Zacharowski K, Henson PM, Eltzschig HK: Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci Transl Med 2017; 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koeppen M, Lee JW, Seo SW, Brodsky KS, Kreth S, Yang IV, Buttrick PM, Eckle T, Eltzschig HK: Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury. Nat Commun 2018; 9: 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan X, Lee JW, Bowser JL, Neudecker V, Sridhar S, Eltzschig HK: Targeting Hypoxia Signaling for Perioperative Organ Injury. Anesth Analg 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neudecker V, Yuan X, Bowser JL, Eltzschig HK: MicroRNAs in mucosal inflammation. J Mol Med (Berl) 2017; 95: 935–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowser JL, Lee JW, Yuan X, Eltzschig HK: The hypoxia-adenosine link during inflammation. J Appl Physiol (1985) 2017; 123: 1303–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yap SC, Lee HT: Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology 2012; 116: 1139–48 [DOI] [PubMed] [Google Scholar]
- 47.Druml W: Systemic consequences of acute kidney injury. Curr Opin Crit Care 2014; 20: 613–9 [DOI] [PubMed] [Google Scholar]
- 48.Idzko M, Ferrari D, Eltzschig HK: Nucleotide signalling during inflammation. Nature 2014; 509: 310–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colgan SP, Eltzschig HK: Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol 2012; 74: 153–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eltzschig HK: Adenosine: an old drug newly discovered. Anesthesiology 2009; 111: 904–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartels K, Grenz A, Eltzschig HK: Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci U S A 2013; 110: 18351–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan X, Berg N, Lee JW, Le TT, Neudecker V, Jing N, Eltzschig H: MicroRNA miR-223 as regulator of innate immunity. J Leukoc Biol 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neudecker V, Haneklaus M, Jensen O, Khailova L, Masterson JC, Tye H, Biette K, Jedlicka P, Brodsky KS, Gerich ME, Mack M, Robertson AAB, Cooper MA, Furuta GT, Dinarello CA, O’Neill LA, Eltzschig HK, Masters SL, McNamee EN: Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med 2017; 214: 1737–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colbert JF, Ford JA, Haeger SM, Yang Y, Dailey KL, Allison KC, Neudecker V, Evans CM, Richardson VL, Brodsky KS, Faubel S, Eltzschig HK, Schmidt EP, Ginde AA: A model-specific role of microRNA-223 as a mediator of kidney injury during experimental sepsis. Am J Physiol Renal Physiol 2017; 313: F553–F559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 2005; 16: 162–8 [DOI] [PubMed] [Google Scholar]
- 56.Kim M, Brady JE, Li G: Variations in the risk of acute kidney injury across intraabdominal surgery procedures. Anesth Analg 2014; 119: 1121–32 [DOI] [PubMed] [Google Scholar]
- 57.Biteker M, Dayan A, Tekkesin AI, Can MM, Tayci I, Ilhan E, Sahin G: Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014; 207: 53–9 [DOI] [PubMed] [Google Scholar]
- 58.Kheterpal S, Tremper KK, Heung M, Rosenberg AL, Englesbe M, Shanks AM, Campbell DA Jr.: Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology 2009; 110: 505–15 [DOI] [PubMed] [Google Scholar]
- 59.Billings FTt, Pretorius M, Schildcrout JS, Mercaldo ND, Byrne JG, Ikizler TA, Brown NJ: Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol 2012; 23: 1221–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suneja M, Kumar AB: Obesity and perioperative acute kidney injury: a focused review. J Crit Care 2014; 29: 694 e1–6 [DOI] [PubMed] [Google Scholar]
- 61.Thakar CV, Kharat V, Blanck S, Leonard AC: Acute kidney injury after gastric bypass surgery. Clin J Am Soc Nephrol 2007; 2: 426–30 [DOI] [PubMed] [Google Scholar]
- 62.Weingarten TN, Gurrieri C, McCaffrey JM, Ricter SJ, Hilgeman ML, Schroeder DR, Kendrick ML, Greene EL, Sprung J: Acute kidney injury following bariatric surgery. Obes Surg 2013; 23: 64–70 [DOI] [PubMed] [Google Scholar]
- 63.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning, Ending Supportive Therapy for the Kidney I: Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813–8 [DOI] [PubMed] [Google Scholar]
- 64.Aronson S, Phillips-Bute B, Stafford-Smith M, Fontes M, Gaca J, Mathew JP, Newman MF: The association of postcardiac surgery acute kidney injury with intraoperative systolic blood pressure hypotension. Anesthesiol Res Pract 2013; 2013: 174091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Neal JB, Shaw AD, Billings FTt: Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care 2016; 20: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranucci M, Johnson I, Willcox T, Baker RA, Boer C, Baumann A, Justison GA, de Somer F, Exton P, Agarwal S, Parke R, Newland RF, Haumann RG, Buchwald D, Weitzel N, Venkateswaran R, Pistuddi V: Goal-directed perfusion to reduce acute kidney injury: A randomized trial. J Thorac Cardiovasc Surg 2018 [DOI] [PubMed] [Google Scholar]
- 67.Svenmarker S, Hannuksela M, Haney M: A retrospective analysis of the mixed venous oxygen saturation as the target for systemic blood flow control during cardiopulmonary bypass. Perfusion 2018; 33: 453–462 [DOI] [PubMed] [Google Scholar]
- 68.Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, Noiseux N, Padmanabhan C, Bahamondes JC, Novick RJ, Vaijyanath P, Reddy S, Tao L, Olavegogeascoechea PA, Airan B, Sulling TA, Whitlock RP, Ou Y, Ng J, Chrolavicius S, Yusuf S, Investigators C: Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366: 1489–97 [DOI] [PubMed] [Google Scholar]
- 69.Reents W, Hilker M, Borgermann J, Albert M, Plotze K, Zacher M, Diegeler A, Boning A: Acute kidney injury after on-pump or off-pump coronary artery bypass grafting in elderly patients. Ann Thorac Surg 2014; 98: 9–14; discussion 14–5 [DOI] [PubMed] [Google Scholar]
- 70.Shroyer AL, Hattler B, Grover FL: Five-Year Outcomes after On-Pump and Off-Pump Coronary-Artery Bypass. N Engl J Med 2017; 377: 1898–1899 [DOI] [PubMed] [Google Scholar]
- 71.Teixeira C, Rosa R, Rodrigues N, Mendes I, Peixoto L, Dias S, Melo MJ, Pereira M, Bicha Castelo H, Lopes JA: Acute kidney injury after major abdominal surgery: a retrospective cohort analysis. Crit Care Res Pract 2014; 2014: 132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demarchi AC, de Almeida CT, Ponce D, e Castro MC, Danaga AR, Yamaguti FA, Vital D, Gut AL, Ferreira AL, Freschi L, Oliveira J, Teixeira UA, Christovan JC, Grejo JR, Martin LC: Intra-abdominal pressure as a predictor of acute kidney injury in postoperative abdominal surgery. Ren Fail 2014; 36: 557–61 [DOI] [PubMed] [Google Scholar]
- 73.Dalfino L, Tullo L, Donadio I, Malcangi V, Brienza N: Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med 2008; 34: 707–13 [DOI] [PubMed] [Google Scholar]
- 74.Praught ML, Shlipak MG: Are small changes in serum creatinine an important risk factor? Curr Opin Nephrol Hypertens 2005; 14: 265–70 [DOI] [PubMed] [Google Scholar]
- 75.Hahn RG: Volume kinetics for infusion fluids. Anesthesiology 2010; 113: 470–81 [DOI] [PubMed] [Google Scholar]
- 76.Goren O, Matot I: Perioperative acute kidney injury. Br J Anaesth 2015; 115 Suppl 2: ii3–14 [DOI] [PubMed] [Google Scholar]
- 77.Uchino S: Creatinine. Curr Opin Crit Care 2010; 16: 562–7 [DOI] [PubMed] [Google Scholar]
- 78.McIlroy DR, Wagener G, Lee HT: Biomarkers of acute kidney injury: an evolving domain. Anesthesiology 2010; 112: 998–1004 [DOI] [PubMed] [Google Scholar]
- 79.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P: Differential gene expression following early renal ischemia/reperfusion. Kidney Int 2003; 63: 1714–24 [DOI] [PubMed] [Google Scholar]
- 80.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003; 14: 2534–43 [DOI] [PubMed] [Google Scholar]
- 81.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005; 365: 1231–8 [DOI] [PubMed] [Google Scholar]
- 82.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J: Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 2008; 148: 810–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P: Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 2008; 3: 665–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shavit L, Dolgoker I, Ivgi H, Assous M, Slotki I: Neutrophil gelatinase-associated lipocalin as a predictor of complications and mortality in patients undergoing non-cardiac major surgery. Kidney Blood Press Res 2011; 34: 116–24 [DOI] [PubMed] [Google Scholar]
- 85.Cullen MR, Jhanji S, Pearse RM, Fitzgibbon MC: Neutrophil gelatinase-associated lipocalin and albuminuria as predictors of acute kidney injury in patients treated with goal-directed haemodynamic therapy after major abdominal surgery. Ann Clin Biochem 2014; 51: 392–9 [DOI] [PubMed] [Google Scholar]
- 86.Cai L, Rubin J, Han W, Venge P, Xu S: The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol 2010; 5: 2229–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Makris K, Rizos D, Kafkas N, Haliassos A: Neurophil gelatinase-associated lipocalin as a new biomarker in laboratory medicine. Clin Chem Lab Med 2012; 50: 1519–32 [DOI] [PubMed] [Google Scholar]
- 88.Tong J, Li H, Zhang H, Luo Z, Huang Y, Huang J, He F, Fu J: Neutrophil Gelatinase-associated Lipocalin in the Prediction of Contrast-induced Nephropathy: A Systemic Review and Meta-analysis. J Cardiovasc Pharmacol 2015; 66: 239–45 [DOI] [PubMed] [Google Scholar]
- 89.Hjortrup PB, Haase N, Wetterslev M, Perner A: Clinical review: Predictive value of neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care patients. Crit Care 2013; 17: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huo W, Zhang K, Nie Z, Li Q, Jin F: Kidney injury molecule-1 (KIM-1): a novel kidney-specific injury molecule playing potential double-edged functions in kidney injury. Transplant Rev (Orlando) 2010; 24: 143–6 [DOI] [PubMed] [Google Scholar]
- 91.Han WK, Wagener G, Zhu Y, Wang S, Lee HT: Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 2009; 4: 873–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M: Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery--a prospective cohort study. Crit Care Med 2009; 37: 553–60 [DOI] [PubMed] [Google Scholar]
- 93.Ahlstrom A, Tallgren M, Peltonen S, Pettila V: Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol 2004; 62: 344–50 [DOI] [PubMed] [Google Scholar]
- 94.Mazul-Sunko B, Zarkovic N, Vrkic N, Antoljak N, Bekavac Beslin M, Nikolic Heitzler V, Siranovic M, Krizmanic-Dekanic A, Klinger R: Proatrial natriuretic peptide (1–98), but not cystatin C, is predictive for occurrence of acute renal insufficiency in critically ill septic patients. Nephron Clin Pract 2004; 97: c103–7 [DOI] [PubMed] [Google Scholar]
- 95.Villa P, Jimenez M, Soriano MC, Manzanares J, Casasnovas P: Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit Care 2005; 9: R139–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Kasza KE, O’Connor MF, Konczal DJ, Trevino S, Devarajan P, Murray PT: Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int 2008; 74: 1059–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, Haase M, Shi J, Kellum JA, Sapphire I: Tissue Inhibitor Metalloproteinase-2 (TIMP-2)IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J Am Soc Nephrol 2015; 26: 1747–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmele T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA: Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013; 17: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jia HM, Huang LF, Zheng Y, Li WX: Prognostic value of cell cycle arrest biomarkers in patients at high risk for acute kidney injury: A systematic review and meta-analysis. Nephrology (Carlton) 2017; 22: 831–837 [DOI] [PubMed] [Google Scholar]
- 100.Koyner JL, Davison DL, Brasha-Mitchell E, Chalikonda DM, Arthur JM, Shaw AD, Tumlin JA, Trevino SA, Bennett MR, Kimmel PL, Seneff MG, Chawla LS: Furosemide Stress Test and Biomarkers for the Prediction of AKI Severity. J Am Soc Nephrol 2015; 26: 2023–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McIlroy DR, Farkas D, Pan K, Pickering JW, Lee HT: Combining Novel Renal Injury Markers with Delta Serum Creatinine Early after Cardiac Surgery and Risk-Stratification for Serious Adverse Outcomes: An Exploratory Analysis. J Cardiothorac Vasc Anesth 2017 [DOI] [PubMed] [Google Scholar]
- 102.Lameire N, Vanmassenhove J, Van Biesen W, Vanholder R: The cell cycle biomarkers: promising research, but do not oversell them. Clin Kidney J 2016; 9: 353–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chindarkar NS, Chawla LS, Straseski JA, Jortani SA, Uettwiller-Geiger D, Orr RR, Kellum JA, Fitzgerald RL: Reference intervals of urinary acute kidney injury (AKI) markers [IGFBP7][TIMP2] in apparently healthy subjects and chronic comorbid subjects without AKI. Clin Chim Acta 2016; 452: 32–7 [DOI] [PubMed] [Google Scholar]
- 104.Daubin D, Cristol JP, Dupuy AM, Kuster N, Besnard N, Platon L, Buzancais A, Brunot V, Garnier F, Jonquet O, Klouche K: Urinary Biomarkers IGFBP7 and TIMP-2 for the Diagnostic Assessment of Transient and Persistent Acute Kidney Injury in Critically Ill Patients. PLoS One 2017; 12: e0169674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Siddiqui NF, Coca SG, Devereaux PJ, Jain AK, Li L, Luo J, Parikh CR, Paterson M, Philbrook HT, Wald R, Walsh M, Whitlock R, Garg AX: Secular trends in acute dialysis after elective major surgery−-1995 to 2009. CMAJ 2012; 184: 1237–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 2013; 61: 649–72 [DOI] [PubMed] [Google Scholar]
- 107.Romagnoli S, Ricci Z, Ronco C: Therapy of acute kidney injury in the perioperative setting. Curr Opin Anaesthesiol 2017; 30: 92–99 [DOI] [PubMed] [Google Scholar]
- 108.Goren O, Matot I: Update on perioperative acute kidney injury. Curr Opin Crit Care 2016; 22: 370–8 [DOI] [PubMed] [Google Scholar]
- 109.Vanmassenhove J, Kielstein J, Jorres A, Biesen WV: Management of patients at risk of acute kidney injury. Lancet 2017; 389: 2139–2151 [DOI] [PubMed] [Google Scholar]
- 110.Alsabbagh MM, Asmar A, Ejaz NI, Aiyer RK, Kambhampati G, Ejaz AA: Update on clinical trials for the prevention of acute kidney injury in patients undergoing cardiac surgery. Am J Surg 2013; 206: 86–95 [DOI] [PubMed] [Google Scholar]
- 111.Burns KE, Chu MW, Novick RJ, Fox SA, Gallo K, Martin CM, Stitt LW, Heidenheim AP, Myers ML, Moist L: Perioperative N-acetylcysteine to prevent renal dysfunction in high-risk patients undergoing cabg surgery: a randomized controlled trial. JAMA 2005; 294: 342–50 [DOI] [PubMed] [Google Scholar]
- 112.Song JW, Shim JK, Soh S, Jang J, Kwak YL: Double-blinded, randomized controlled trial of N-acetylcysteine for prevention of acute kidney injury in high risk patients undergoing off-pump coronary artery bypass. Nephrology (Carlton) 2015; 20: 96–102 [DOI] [PubMed] [Google Scholar]
- 113.Berger MM, Soguel L, Shenkin A, Revelly JP, Pinget C, Baines M, Chiolero RL: Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Crit Care 2008; 12: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nouri-Majalan N, Ardakani EF, Forouzannia K, Moshtaghian H: Effects of allopurinol and vitamin E on renal function in patients with cardiac coronary artery bypass grafts. Vasc Health Risk Manag 2009; 5: 489–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adabag AS, Ishani A, Bloomfield HE, Ngo AK, Wilt TJ: Efficacy of N-acetylcysteine in preventing renal injury after heart surgery: a systematic review of randomized trials. Eur Heart J 2009; 30: 1910–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Campese VM, Nadim MK, Epstein M: Are 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors renoprotective? J Am Soc Nephrol 2005; 16 Suppl 1: S11–7 [DOI] [PubMed] [Google Scholar]
- 117.Zhao BC, Shen P, Liu KX: Perioperative Statins Do Not Prevent Acute Kidney Injury After Cardiac Surgery: A Meta-analysis of Randomized Controlled Trials. J Cardiothorac Vasc Anesth 2017; 31: 2086–2092 [DOI] [PubMed] [Google Scholar]
- 118.He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX: Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14: 475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kocoglu H, Karaaslan K, Gonca E, Bozdogan O, Gulcu N: Preconditionin effects of dexmedetomidine on myocardial ischemia/reperfusion injury in rats. Curr Ther Res Clin Exp 2008; 69: 150–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF: Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology 1991; 75: 328–32 [DOI] [PubMed] [Google Scholar]
- 121.Yao H, Chi X, Jin Y, Wang Y, Huang P, Wu S, Xia Z, Cai J: Dexmedetomidine Inhibits TLR4/NF-kappaB Activation and Reduces Acute Kidney Injury after Orthotopic Autologous Liver Transplantation in Rats. Sci Rep 2015; 5: 16849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frumento RJ, Logginidou HG, Wahlander S, Wagener G, Playford HR, Sladen RN: Dexmedetomidine infusion is associated with enhanced renal function after thoracic surgery. J Clin Anesth 2006; 18: 422–6 [DOI] [PubMed] [Google Scholar]
- 123.Kurt A, Ingec M, Isaoglu U, Yilmaz M, Cetin N, Calik M, Polat B, Akcay F, Gundogdu C, Suleyman H: An investigation about the inhibition of acute ischemia/reperfusion damage by dexmedetomidine in rat ovarian tissue. Gynecol Endocrinol 2013; 29: 222–5 [DOI] [PubMed] [Google Scholar]
- 124.Eser O, Fidan H, Sahin O, Cosar M, Yaman M, Mollaoglu H, Songur A, Buyukbas S: The influence of dexmedetomidine on ischemic rat hippocampus. Brain Res 2008; 1218: 250–6 [DOI] [PubMed] [Google Scholar]
- 125.Ji F, Li Z, Young JN, Yeranossian A, Liu H: Post-bypass dexmedetomidine use and postoperative acute kidney injury in patients undergoing cardiac surgery with cardiopulmonary bypass. PLoS One 2013; 8: e77446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xue F, Zhang W, Chu HC: Assessing perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int 2016; 89: 1164. [DOI] [PubMed] [Google Scholar]
- 127.Kwiatkowski DM, Axelrod DM, Sutherland SM, Tesoro TM, Krawczeski CD: Dexmedetomidine Is Associated With Lower Incidence of Acute Kidney Injury After Congenital Heart Surgery. Pediatr Crit Care Med 2016; 17: 128–34 [DOI] [PubMed] [Google Scholar]
- 128.Cho JS, Shim JK, Soh S, Kim MK, Kwak YL: Perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int 2016; 89: 693–700 [DOI] [PubMed] [Google Scholar]
- 129.Shi R, Tie HT: Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Crit Care 2017; 21: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK: Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol 2006; 291: H2533–40 [DOI] [PubMed] [Google Scholar]
- 131.Eltzschig HK, Eckle T: Ischemia and reperfusion--from mechanism to translation. Nat Med 2011; 17: 1391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zarbock A, Kellum JA: Remote Ischemic Preconditioning and Protection of the Kidney--A Novel Therapeutic Option. Crit Care Med 2016; 44: 607–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Er F, Nia AM, Dopp H, Hellmich M, Dahlem KM, Caglayan E, Kubacki T, Benzing T, Erdmann E, Burst V, Gassanov N: Ischemic preconditioning for prevention of contrast medium-induced nephropathy: randomized pilot RenPro Trial (Renal Protection Trial). Circulation 2012; 126: 296–303 [DOI] [PubMed] [Google Scholar]
- 134.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Gorlich D, Kellum JA, Meersch M, Renal RI: Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313: 2133–41 [DOI] [PubMed] [Google Scholar]
- 135.Zarbock A, Kellum JA, Van Aken H, Schmidt C, Kullmar M, Rosenberger P, Martens S, Gorlich D, Meersch M: Long-term Effects of Remote Ischemic Preconditioning on Kidney Function in High-risk Cardiac Surgery Patients: Follow-up Results from the RenalRIP Trial. Anesthesiology 2017; 126: 787–798 [DOI] [PubMed] [Google Scholar]
- 136.Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, Boyle JR, Varty K, Kharbanda RK, Dutka DP, Gaunt ME: Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation 2007; 116: I98–105 [DOI] [PubMed] [Google Scholar]
- 137.Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G: Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 2010; 105: 657–64 [DOI] [PubMed] [Google Scholar]
- 138.Kork F, Eltzschig HK: The Devil Is in the Detail: Remote Ischemic Preconditioning for Perioperative Kidney Protection. Anesthesiology 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM, Investigators ET: Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med 2015; 373: 1408–17 [DOI] [PubMed] [Google Scholar]
- 140.Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K, Collaborators RIS: A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N Engl J Med 2015; 373: 1397–407 [DOI] [PubMed] [Google Scholar]
- 141.Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J: Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol - a clinical trial. Acta Anaesthesiol Scand 2012; 56: 30–8 [DOI] [PubMed] [Google Scholar]
- 142.Xie J, Zhang X, Xu J, Zhang Z, Klingensmith NJ, Liu S, Pan C, Yang Y, Qiu H: Effect of Remote Ischemic Preconditioning on Outcomes in Adult Cardiac Surgery: A Systematic Review and Meta-analysis of Randomized Controlled Studies. Anesth Analg 2018; 127: 30–38 [DOI] [PubMed] [Google Scholar]
- 143.Group KAW: KDIGO clinical practice guideline for acute kidney injury. Kidney Inr Suppl 2012: 1–138 [Google Scholar]
- 144.Nash DM, Przech S, Wald R, O’Reilly D: Systematic review and meta-analysis of renal replacement therapy modalities for acute kidney injury in the intensive care unit. J Crit Care 2017; 41: 138–144 [DOI] [PubMed] [Google Scholar]
- 145.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N, Carpentier D, de Prost N, Lautrette A, Bretagnol A, Mayaux J, Nseir S, Megarbane B, Thirion M, Forel JM, Maizel J, Yonis H, Markowicz P, Thiery G, Tubach F, Ricard JD, Dreyfuss D, Group AS: Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med 2016; 375: 122–33 [DOI] [PubMed] [Google Scholar]
- 146.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstadt H, Boanta A, Gerss J, Meersch M: Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 2016; 315: 2190–9 [DOI] [PubMed] [Google Scholar]
- 147.Shiao CC, Huang TM, Spapen HD, Honore PM, Wu VC: Optimal timing of renal replacement therapy initiation in acute kidney injury: the elephant felt by the blindmen? Crit Care 2017; 21: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyere R, Lebert C, Bohe J, Badie J, Eraldi JP, Rigaud JP, Levy B, Siami S, Louis G, Bouadma L, Constantin JM, Mercier E, Klouche K, du Cheyron D, Piton G, Annane D, Jaber S, van der Linden T, Blasco G, Mira JP, Schwebel C, Chimot L, Guiot P, Nay MA, Meziani F, Helms J, Roger C, Louart B, Trusson R, Dargent A, Binquet C, Quenot JP, Investigators I-IT, the CTN: Timing of Renal-Replacement Therapy in Patients with Acute Kidney Injury and Sepsis. N Engl J Med 2018; 379: 1431–1442 [DOI] [PubMed] [Google Scholar]
- 149.Zarbock A, Mehta RL: Timing of Kidney Replacement Therapy in Acute Kidney Injury. Clin J Am Soc Nephrol 2019; 14: 147–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shires T, Williams J, Brown F: Acute change in extracellular fluids associated with major surgical procedures. Ann Surg 1961; 154: 803–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, Christophi C, Leslie K, McGuinness S, Parke R, Serpell J, Chan MTV, Painter T, McCluskey S, Minto G, Wallace S, Australian, New Zealand College of Anaesthetists Clinical Trials N, the A, New Zealand Intensive Care Society Clinical Trials G: Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N Engl J Med 2018; 378: 2263–2274 [DOI] [PubMed] [Google Scholar]
- 152.Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, MacFie J, Liberman AS, Soop M, Hill A, Kennedy RH, Lobo DN, Fearon K, Ljungqvist O, Enhanced Recovery After Surgery S: Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr 2012; 31: 783–800 [DOI] [PubMed] [Google Scholar]
- 153.Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH, Gramkow CS, Okholm M, Blemmer T, Svendsen PE, Rottensten HH, Thage B, Riis J, Jeppesen IS, Teilum D, Christensen AM, Graungaard B, Pott F, Danish Study Group on Perioperative Fluid T: Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003; 238: 641–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I: Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005; 103: 25–32 [DOI] [PubMed] [Google Scholar]
- 155.Ratner AJ, Lysenko ES, Paul MN, Weiser JN: Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. Proc Natl Acad Sci U S A 2005; 102: 3429–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lang K, Boldt J, Suttner S, Haisch G: Colloids versus crystalloids and tissue oxygen tension in patients undergoing major abdominal surgery. Anesth Analg 2001; 93: 405–9 , 3rd contents page [DOI] [PubMed] [Google Scholar]
- 157.Brandstrup B: Finding the Right Balance. N Engl J Med 2018; 378: 2335–2336 [DOI] [PubMed] [Google Scholar]
- 158.Jhanji S, Vivian-Smith A, Lucena-Amaro S, Watson D, Hinds CJ, Pearse RM: Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Crit Care 2010; 14: R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Prowle JR, Kirwan CJ, Bellomo R: Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 2014; 10: 37–47 [DOI] [PubMed] [Google Scholar]
- 160.Goldstein SL: Fluid management in acute kidney injury. J Intensive Care Med 2014; 29: 183–9 [DOI] [PubMed] [Google Scholar]
- 161.Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD, National Heart L, Blood Institute Acute Respiratory Distress Syndrome N: Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol 2011; 6: 966–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bouhemad B, Nicolas-Robin A, Arbelot C, Arthaud M, Feger F, Rouby JJ: Isolated and reversible impairment of ventricular relaxation in patients with septic shock. Crit Care Med 2008; 36: 766–74 [DOI] [PubMed] [Google Scholar]
- 163.Rudiger A, Singer M: Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 2007; 35: 1599–608 [DOI] [PubMed] [Google Scholar]
- 164.Ruokonen E, Takala J, Kari A, Saxen H, Mertsola J, Hansen EJ: Regional blood flow and oxygen transport in septic shock. Crit Care Med 1993; 21: 1296–303 [DOI] [PubMed] [Google Scholar]
- 165.Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, Calman KC: Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet 1985; 1: 781–4 [DOI] [PubMed] [Google Scholar]
- 166.Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, Grocott MP, Ahern A, Griggs K, Scott R, Hinds C, Rowan K, Group OS: Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014; 311: 2181–90 [DOI] [PubMed] [Google Scholar]
- 167.Gelman S, Bigatello L: The physiologic basis for goal-directed hemodynamic and fluid therapy: the pivotal role of the venous circulation. Can J Anaesth 2018; 65: 294–308 [DOI] [PubMed] [Google Scholar]
- 168.Yunos NM, Kim IB, Bellomo R, Bailey M, Ho L, Story D, Gutteridge GA, Hart GK: The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med 2011; 39: 2419–24 [DOI] [PubMed] [Google Scholar]
- 169.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M: Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012; 308: 1566–72 [DOI] [PubMed] [Google Scholar]
- 170.Raghunathan K, Shaw A, Nathanson B, Sturmer T, Brookhart A, Stefan MS, Setoguchi S, Beadles C, Lindenauer PK: Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Crit Care Med 2014; 42: 1585–91 [DOI] [PubMed] [Google Scholar]
- 171.McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS: Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg 2013; 117: 412–21 [DOI] [PubMed] [Google Scholar]
- 172.Yunos NM, Bellomo R, Story D, Kellum J: Bench-to-bedside review: Chloride in critical illness. Crit Care 2010; 14: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jaynes MP, Murphy CV, Ali N, Krautwater A, Lehman A, Doepker BA: Association between chloride content of intravenous fluids and acute kidney injury in critically ill medical patients with sepsis. J Crit Care 2018; 44: 363–367 [DOI] [PubMed] [Google Scholar]
- 174.Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, Slovis CM, Lindsell CJ, Ehrenfeld JM, Siew ED, Shaw AD, Bernard GR, Rice TW, Investigators S-E: Balanced Crystalloids versus Saline in Noncritically Ill Adults. N Engl J Med 2018; 378: 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, Kumar AB, Hughes CG, Hernandez A, Guillamondegui OD, May AK, Weavind L, Casey JD, Siew ED, Shaw AD, Bernard GR, Rice TW, Investigators S, the Pragmatic Critical Care Research G: Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med 2018; 378: 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, Bendtsen A, Winding R, Steensen M, Berezowicz P, Soe-Jensen P, Bestle M, Strand K, Wiis J, White JO, Thornberg KJ, Quist L, Nielsen J, Andersen LH, Holst LB, Thormar K, Kjaeldgaard AL, Fabritius ML, Mondrup F, Pott FC, Moller TP, Winkel P, Wetterslev J, Group ST, Scandinavian Critical Care Trials G: Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367: 124–34 [DOI] [PubMed] [Google Scholar]
- 177.Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, McGuinness S, Rajbhandari D, Taylor CB, Webb SA, Investigators C, Australian, New Zealand Intensive Care Society Clinical Trials G: Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367: 1901–11 [DOI] [PubMed] [Google Scholar]
- 178.Weiss R, Wenk M, Van Aken H, Zwissler B, Chappell D, Zarbock A: HES or How to End Science. Anesth Analg 2018 [DOI] [PubMed] [Google Scholar]
- 179.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P: Human serum albumin: from bench to bedside. Mol Aspects Med 2012; 33: 209–90 [DOI] [PubMed] [Google Scholar]
- 180.Langer T, Ferrari M, Zazzeron L, Gattinoni L, Caironi P: Effects of intravenous solutions on acid-base equilibrium: from crystalloids to colloids and blood components. Anaesthesiol Intensive Ther 2014; 46: 350–60 [DOI] [PubMed] [Google Scholar]
- 181.Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, Du B, McArthur C, Myburgh J, Investigators ST: Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care 2010; 14: R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rueddel T, Kneser M, Tost F: Impact of exercise on retinal microvascular regulation measured by dynamic vessel analysis in healthy individuals. Clin Physiol Funct Imaging 2012; 32: 158–61 [DOI] [PubMed] [Google Scholar]
- 183.Moeller C, Fleischmann C, Thomas-Rueddel D, Vlasakov V, Rochwerg B, Theurer P, Gattinoni L, Reinhart K, Hartog CS: How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J Crit Care 2016; 35: 75–83 [DOI] [PubMed] [Google Scholar]
- 184.Walsh M, Garg AX, Devereaux PJ, Argalious M, Honar H, Sessler DI: The association between perioperative hemoglobin and acute kidney injury in patients having noncardiac surgery. Anesth Analg 2013; 117: 924–31 [DOI] [PubMed] [Google Scholar]
- 185.Fowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA, Pearse RM: Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg 2015; 102: 1314–24 [DOI] [PubMed] [Google Scholar]
- 186.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B, Laflamme C, Lamy A, Legare JF, Mazer CD, McCluskey SA, Rubens FD, Sawchuk C, Beattie WS: Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119: 495–502 [DOI] [PubMed] [Google Scholar]
- 187.Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, Kirkendall ES: A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 2016; 90: 212–21 [DOI] [PubMed] [Google Scholar]
- 188.Ad-hoc working group of E, Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, Van Biesen W: A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant 2012; 27: 4263–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Piggott KD, Liu A, Monczka J, Fakioglu H, Narasimhulu SS, Pourmoghadam K, DeCampli W: Inadequate preoperative nutrition might be associated with acute kidney injury and greater illness severity postoperatively. J Thorac Cardiovasc Surg 2018; 155: 2104–2109 [DOI] [PubMed] [Google Scholar]
- 190.Basi S, Pupim LB, Simmons EM, Sezer MT, Shyr Y, Freedman S, Chertow GM, Mehta RL, Paganini E, Himmelfarb J, Ikizler TA: Insulin resistance in critically ill patients with acute renal failure. Am J Physiol Renal Physiol 2005; 289: F259–64 [DOI] [PubMed] [Google Scholar]
- 191.Investigators N-SS, Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ: Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283–97 [DOI] [PubMed] [Google Scholar]
- 192.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A: Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017; 43: 1551–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gocze I, Koch M, Renner P, Zeman F, Graf BM, Dahlke MH, Nerlich M, Schlitt HJ, Kellum JA, Bein T: Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS One 2015; 10: e0120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hobson C, Ruchi R, Bihorac A: Perioperative Acute Kidney Injury: Risk Factors and Predictive Strategies. Crit Care Clin 2017; 33: 379–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


