Abstract
The facial nerve stimulates the muscles of facial expression and the parasympathetic nerves of the face. Consequently, facial nerve paralysis can lead to facial asymmetry, deformation, and functional impairment. Facial nerve palsy is most commonly idiopathic, as with Bell palsy, but it can also result from a tumor or trauma. In this article, we discuss traumatic facial nerve injury. To identify the cause of the injury, it is important to first determine its location. The location and extent of the damage inform the treatment method, with options including primary repair, nerve graft, cross-face nerve graft, nerve crossover, and muscle transfer. Intracranial proximal facial nerve injuries present a challenge to surgical approaches due to the complexity of the temporal bone. Surgical intervention in these cases requires a collaborative approach between neurosurgery and otolaryngology, and nerve repair or grafting is difficult. This article describes the treatment of peripheral facial nerve injury. Primary repair generally offers the best prognosis. If primary repair is not feasible within 6 months of injury, nerve grafting should be attempted, and if more than 12 months have elapsed, functional muscle transfer should be performed. If the affected nerve cannot be utilized at that time, the contralateral facial nerve, ipsilateral masseter nerve, or hypoglossal nerve can serve as the donor nerve. Other accompanying symptoms, such as lagophthalmos or midface ptosis, must also be considered for the successful treatment of facial nerve injury.
Keywords: Facial nerve, Facial nerve injuries, Facial paralysis, Plastic surgery procedures
INTRODUCTION
Facial nerve palsy, which affects approximately 20 per 100,000 people annually, has a variety of causes. These include tumors, infections, metabolic or congenital diseases, iatrogenic injury, and head and neck trauma [1-3]. The facial nerve controls the muscles of facial expression and stimulates the parasympathetic nerves, which are responsible for the senses of taste and touch as well as secretion by the salivary glands. Consequently, facial nerve paralysis can result in both aesthetic distortion of the face and functional disability. Such outcomes can devastate a patient’s psychological and social life [4-6].
Facial nerve damage occurs in 16% of cases of head and neck trauma and can be caused by traffic accidents, facial stab wounds, gunshot wounds, or iatrogenic damage incurred during head and neck surgery [1,7]. The primary mechanisms of injury include traction, cutting, and compression resulting from edema of the surrounding tissue. As the facial nerve follows a longer pathway than other cranial nerves and traverses a narrow tunnel in the temporal bone, it is particularly susceptible to damage in instances of cranial trauma such as temporal bone fracture [1,8,9]. Consequently, a comprehensive understanding of the anatomy of the facial nerve is crucial, since facial nerve injury produces specific symptoms dependent on the location of the injury. Furthermore, the evaluation and provision of appropriate treatment for traumatic facial nerve injuries play important roles in determining patient prognosis.
CLASSIFICATION OF NERVE DAMAGE
In cases of facial nerve injury, the mechanism of damage at the cellular level is closely related to the functional recovery of the nerve. Seddon and Sunderland devised a classification system for the degree of facial nerve damage according to histological and clinical features. Under this system, nerve damage is classified based on histological changes of the affected nerve, the nerve function, and the potential for spontaneous recovery. Seddon established three categories that corresponded to neurapraxia, axonotmesis, and neurotmesis, based on the degree of nerve damage. Sunderland, then, developed a five-degree classification system based on histological changes of the damaged nerve (Table 1) [10,11].
Table 1.
Classification of nerve damage
| Seddon/Sunderland | Histological changes and responses | Recovery (period) | Treatment | ||
|---|---|---|---|---|---|
| Neurapraxia | I | Transient ischemia, demyelination, intra-bundle edema | Conduction disorder | Reversible (hours to weeks) | Observation |
| Axonotmesis | II | Damaged axon (intact endoneurium) | Wallerian degeneration | Reversible (weeks) | Observation |
| III | Damaged axon, endoneurium (intact perineurium) | Wallerian degeneration | Varies (weeks to months) | Need to check for damage | |
| IV | Damaged axon, endoneurium, and perineurium (intact epineurium) | Wallerian degeneration, death of neuron, neuroma, fibrosis | Irreversible | Microsurgical reconstruction | |
| Neurotmesis | V | Complete nerve transection | Wallerian degeneration, death of neuron, neuroma, fibrosis | Irreversible | Microsurgical reconstruction |
Neurapraxia, the least severe form of nerve damage, aligns with the first degree of the Sunderland classification. This condition involves a local blockage of nerve conduction, often resulting from disorders in blood flow, among other causes. Despite this blockage, nerve conduction is preserved in areas distal or proximal to the lesion, as the continuity of nerve segments remains intact. Neurapraxia typically arises following a mild traction or compression injury, and full functional recovery is generally anticipated within days to weeks. Histologically, axon degeneration does not occur, and the damage is confined to the endoneurium.
Axonotmesis refers to a moderate level of nerve damage. The extent of demyelination and axon damage can vary, and recovery and prognosis differ accordingly [12]. Sunderland categorized axonotmesis into stages 2 to 4, based on the degree of damage to the axonal support structure. In a stage 2 injury, only the axon is damaged, while the histological structures of the endoneurium, perineurium, and epineurium remain intact. Wallerian degeneration occurs at the distal end in this stage. A stage 3 injury involves damage to the axons, myelin, and endoneurium, but no histological changes within the perineurium or epineurium. The recovery pattern in this stage can range from weeks to months. In a stage 4 injury, the axons, myelin, endoneurium, and perineurium are damaged, although the epineurium is not. The likelihood of spontaneous recovery is low for this stage, with a high probability of neuroma or intraneural fibrosis formation. If no recovery is observed after more than 3 months of observation, surgical intervention may be necessary.
The most severe form of nerve injury is neurotmesis, which involves extensive damage to axons and nerve sheaths and is categorized as grade 5 under the Sunderland classification. Neurotmesis is closely related to transection, avulsion, and laceration damage. This condition refers to damage to the entire nervous tissue, including the axon, myelin, endoneurium, perineurium, and epineurium. Immediate surgical intervention is necessary, as spontaneous recovery is not possible.
CAUSES OF TRAUMATIC FACIAL NERVE INJURY
Trauma-induced facial nerve damage can occur at any point along the length of the nerve, from the brainstem to the muscles of facial expression [13]. Traumatic facial nerve injury is most often caused by blunt trauma, with temporal bone fracture being the most common cause [14]. Intracranially, the facial nerve is protected by the petrous bone and the cranial vault within the temporal bone; hence, damage from penetrating trauma is rare. However, a wide variety of penetrating traumas, such as gunshot wounds, can still cause damage. Transection, facial edema, or neurovascular compromise can directly injure the peripherally distributed facial nerve.
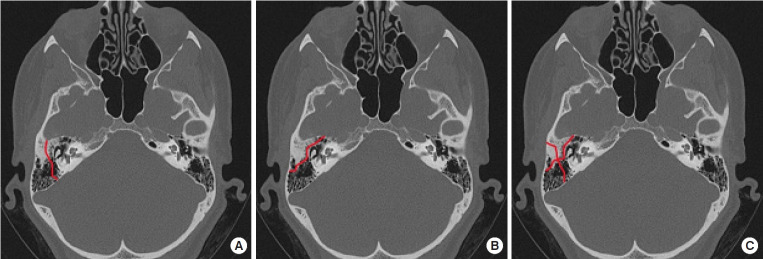
Temporal bone fractures are classified into longitudinal (70%–80%), transverse (10%–20%), and mixed (10%) types, based on the location of occurrence [13,15]. The most common type is longitudinal fracture, which occurs along the long axis of the petrous bone. Facial nerve damage is observed in 10% to 20% of these cases, with delayed paralysis being a frequent symptom. However, normal function is restored in approximately 80% of all cases. Transverse fractures, in contrast, occur perpendicular to the long axis of the petrous bone. In such cases, the geniculate labyrinth and geniculate ganglion are invaded, and hearing loss and vestibular dysfunction are common. Transverse fractures are frequently associated with high-impact cranial injuries, which often result in direct damage to the facial nerve. In fact, approximately half of all transverse fractures result in irreversible facial nerve damage. Finally, mixed fractures are characterized by the concurrent presence of both transverse and longitudinal fractures (Fig. 1).
Fig. 1.
Types of temporal bone fractures. (A) Longitudinal type. (B) Transverse type. (C) Mixed type.
Damage to the peripheral facial nerve often results from penetrating trauma, in which the facial nerve is directly cut, or blunt trauma, which frequently induces temporary neurapraxia. The extent of peripheral facial nerve damage can vary, depending on the location of injury from the stylomastoid foramen to the peripheral muscles. Damage to the pes anserinus, the origin of the facial nerve, can cause complete hemiplegic facial paralysis. In contrast, damage to any point distal to this area can lead to localized facial paralysis or be asymptomatic. Injury to the frontal and marginal mandibular branches can lead to complete local paralysis of the associated muscles. However, damage to the buccal and zygomatic branches typically results in incomplete paralysis of the related muscles, due to their interconnectedness via arborizations.
EVALUATION OF PATIENTS WITH FACIAL NERVE INJURY
History-taking and physical examination
A history of facial injuries and a full physical examination of the head and neck are essential in assessing the extent of facial nerve damage and establishing a treatment plan. Factors such as the mechanism and timing of the injury, the age of the patient, the presence of any comorbidities, and the history of prior surgery should be determined. If a witness is available, this person may be able to describe any changes in facial muscle movement immediately after the injury and upon hospital arrival, although such accounts are frequently inaccurate. For patients presenting with multiple facial injuries, if an otoscope reveals perforation of the tympanic membrane or hematoma, the associated temporal bone fracture should be confirmed. Longitudinal fractures are often accompanied by conductive hearing loss, while transverse fractures frequently present with sensorineural hearing impairment. In cases of facial injury involving the midface, potential damage to the parotid duct (Stensen duct) should be evaluated.
During the assessment of facial muscles, it is essential to examine the tone and movement of all voluntary muscles from the forehead to the neck. Hemifacial palsy, in the absence of bilateral abnormalities of the forehead muscles, indicates a lesion at the level of the upper motor neurons. This stems from bilateral innervation of the facial nerve to the forehead muscles from the cerebral hemispheres. Lesions at the level of the lower motor neurons (i.e., damage below the midbrain nuclei) can cause complete hemiparesis. As the location of the lesion ascends, facial muscle movement disorders are increasingly accompanied by abnormalities in lacrimal secretion, taste, and hearing. Lesions situated further down the nerve pathway frequently manifest as localized abnormal findings. Moreover, in instances of head and neck injury, the accurate evaluation of facial muscle function often poses a challenge due to the patient’s impaired consciousness. Therefore, it is beneficial to induce painful stimuli or observe the patient frowning.
Auxiliary inspection
A computed tomography scan is necessary to confirm the fracture location and the extent of intracranial damage. Moreover, when feasible, a high-resolution computed tomography scan with 1-mm segmentation should be performed to facilitate the identification of the fracture line. Through an intracranial computed tomography scan, the location and type of temporal bone fracture (whether longitudinal, transverse, or mixed) can be confirmed, and any accompanying hematoma or nerve damage can be identified.
Electrophysiological tests, including electroneurography and electromyography, can be used to evaluate local nerve damage and muscle abnormalities [16]. These tests can be particularly useful when the patient is in a full coma and a direct assessment of nerve damage is not feasible. To minimize the risk of false positives, it is advisable to conduct nerve conduction tests at least 3 days post-injury, after Wallerian degeneration has fully developed. Similarly, it is useful to conduct electromyography 10 to 14 days after injury. Electromyography can assist in determining whether nerve transfer using existing muscles is appropriate or whether new muscles need to be transplanted during the reconstruction of facial nerve damage.
TREATMENT OF FACIAL NERVE DAMAGE
When an acute injury to the intracranial facial nerve occurs, surgical intervention can be challenging due to the complex anatomy within the temporal bone. Additionally, most patients are either intentionally sedated or in a comatose state, which often complicates the evaluation of facial nerve damage. In cases of intracranial facial nerve damage, facial nerve compression caused by surrounding structures can be addressed surgically through collaboration between neurosurgeons and otolaryngologists. Decompression surgery in combination with steroids is one option; however, no reports have indicated that steroids are effective in treating traumatic facial nerve palsy, as they are in Bell palsy [14]. Nerve repair or nerve grafting as a general treatment for intracranial facial nerve injury is often difficult, and the literature on this topic is limited. In this review, we discuss the treatment of peripheral facial nerve damage.
Primary repair
If primary suturing can be performed, it typically yields the best prognosis with appropriate treatment [17]. Both the distal and proximal cut sections must be present as a prerequisite for primary suturing, and a sufficient length of the remaining nerve must be secured to allow for tension-free suturing [18]. For local injection at the lesion site, only epinephrine should be used to achieve hemostasis, excluding any anesthetic components. Identifying the branches of the facial nerve is straightforward when using a nerve stimulator. If a patient responds to a 0.05-mA electrical stimulation, the prognosis is likely favorable. Primary suturing is most effective when performed within 3 days following an injury. As time progresses, the formation of scar tissue and fibrosis can lead to nerve deficits, and nerve transplantation becomes inevitable.
Sutures are typically performed using 9-0 or 10-0 nylon threads, with a focus on minimizing damage to the internal fascicle via the use of epineural sutures. It is advisable to isolate the cut surface of the nerve from the surrounding tissue, perform sutures without tension, and trim the unclean cut surface using a blade. Generally, a tension-free suture can be achieved for nerve defects of 17 mm or less. While the application of fibrin glue can lessen the suturing effort, it must be used judiciously, as the glue might infiltrate the cut surfaces and interfere with nerve regeneration.
Nerve graft
If a gap exists between the distal and proximal nerves, nerve grafting can be performed. The fundamental principle mirrors that of primary sutures, in which the distal and proximal nerve endings are connected to the epineurium of the graft. The sural nerve is the most frequently used donor site for nerve transplantation due to its easy sizing and its potential to provide a rich nerve supply in cases of multiple defects. Other potential donor sites include the greater auricular and antecubital cutaneous nerves. As time progresses, the likelihood of successful muscle re-innervation diminishes. Therefore, it is crucial to perform this procedure within 6 months of injury for optimal prognosis.
Cross-face nerve graft
If the proximal facial nerve is not available for use, a cross-face nerve graft can be performed using the healthy facial nerve from the opposite side [19]. This graft is a two-step procedure. The first operation involves cross-transplantation, while the second, which takes place 6 months to 1 year later, entails inducing neurotization in the muscle flap to replace the facial muscle. The cross-face nerve graft is conducted by tunneling the nerve graft subcutaneously through the upper lip. This method of facial nerve cross-transplantation enables a natural and emotional smile by providing similar nerve stimulation. However, compared to connections with other cranial nerves, it requires a longer time for the nerves to reconnect, and the resulting muscle movement is weaker.
Nerve crossover
Nerve crossover is a method of connecting cranial nerves, such as the masseter, hypoglossal, and accessory nerves, to the distal cut surface of the facial nerve on the paralyzed side. This procedure is typically indicated by the presence of an abnormality in the proximal facial nerve due to an intracranial lesion. Recent studies have demonstrated excellent outcomes of nerve crossing procedures utilizing the masseter nerve [20-22]. The nuclei of the trigeminal and facial nerves are known to be closely associated within the pons. Consequently, the masseter nerve, a branch of the trigeminal nerve, transmits similar nerve impulses when linked to the facial nerve, leading to rapid motor recovery. Furthermore, the high motor neuron content of the masseter nerve allows for superior muscle mobility compared to facial nerve cross-transplantation. In general, splitting the hypoglossal nerve can reduce donor site defects such as dysarthria and masticatory disorders [23]. Biofeedback training after nerve crossover can aid in the control of voluntary facial muscle movements and reduce synkinesis.
Muscle transfer
In instances of facial paralysis that persist for more than 12 months, the affected muscle undergoes denervation atrophy, and irreversible nerve fibrosis progresses. This makes it challenging to restore function through primary nerve suture or nerve transplantation. In patients with advanced denervation atrophy, vascularized neuromuscular unit transplantation can be performed for dynamic reconstruction in the form of a local flap or a free flap.
Regional muscle transfer
In this technique, proximal muscles are employed for dynamic reconstruction. The temporalis, masseter, and digastric muscles are connected to the corners of the mouth to form a voluntary smile [24]. Traditionally, the approach involves dissecting the upper insertion of the temporal muscle, transposing it in the reverse direction (orthodromic), and securing it to the modiolus [25-27]. Labbe and Huault [28] modified this existing method and introduced lengthening temporalis myoplasty, in which the inferior insertion, at which the temporal muscle attaches to the muscle process of the mandible, is separated in an orthodromic direction and affixed to the corner of the mouth. The Labbe method enables a voluntary smile due to dynamic muscle movement when the function of the temporal muscle is maintained normally [28].
In local muscle transplantation, the direction of the smile may be irregular due to the differing pulling vectors of each muscle and the limited lip movement compared to when a distal muscle is utilized. Furthermore, problems with mastication may occur due to the loss of muscle function at the donor site. Regional muscle transfer is suitable for elderly patients, as it allows for a swift recovery and the immediate verification of a response after surgery.
Free functional muscle transfer
The use of a distal muscle in a free flap is a standard treatment option for patients with long-term facial paralysis, typically lasting between 12 and 24 months. When determining the donor site in free functional muscle transfer, the chosen muscle should be capable of providing adequate mobility, possess a vascular-nerve pedicle of sufficient length, and cause minimal complications at the donor site. The gracilis, latissimus dorsi, serratus anterior, and pectoralis minor muscles have been utilized effectively in facial paralysis reconstruction, with the gracilis and latissimus dorsi muscles being the most commonly used [29,30]. The gracilis flap, in particular, is relatively simple to harvest, results in few complications at the donor site, and is not overly bulky, making it suitable for dynamic reconstruction [29].
After a distal muscle is selected, the subsequent consideration is the selection of the donor nerve. The most straightforward approach involves using the contralateral facial nerve through a cross-face nerve graft. Typically, this graft is the initial step; then, after approximately 6 months, the second step of functional muscle transfer is performed. Generally, when the facial nerve serves as the donor nerve, it is possible to induce spontaneous and emotional smiles simultaneously with the contralateral facial nerve; however, this effect is less pronounced in the elderly. Another option for the donor nerve involves the use of the ipsilateral masseter or hypoglossal nerve, which is connected to the nerve of the transplanted muscle [21,23]. Utilizing ipsilateral cranial nerves, such as the masseter or hypoglossal nerve, yields superior results in terms of symmetry and excursion of the corners of the mouth compared with using contralateral facial nerves. However, this approach has drawbacks, including poor synchronicity and the potential for synkinesis.
1) Two-stage technique
1-1) Selection of the donor nerve
The first step of two-step free functional muscle transfer involves the choice of a donor nerve. As previously described, the contralateral facial nerve, accessed via a cross-face nerve graft, is most commonly used [31]. When selecting the donor facial nerve, a nerve stimulator should be used to pinpoint an appropriate nerve, depending on whether the goal is smile reconstruction or eyelid movement reconstruction. The chosen donor nerve must have two or more branches that connect to the same muscle to minimize complications at the donor site. The zygomatic-buccal branches of the facial nerve are interconnected, making the task of locating a donor nerve relatively straightforward. Generally, the thicker the axon bundle of the donor nerve, the more suitable it is for nerve reconnection. Consequently, the prognosis tends to be favorable in older patients when the thickest facial nerve is used. Once the donor facial nerve has been selected, a sural nerve graft is performed from the contralateral facial nerve to the top of the canine tooth (Fig. 2).
Fig. 2.
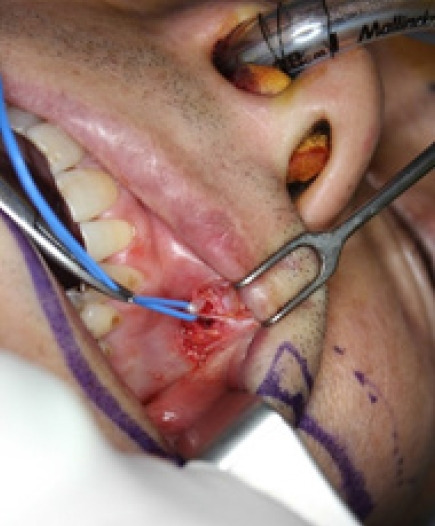
This photograph shows a 43-year-old man with complete left facial paralysis caused by the resection of a parotid tumor. It also illustrates the sural nerve implanted via cross-face nerve graft 6 months prior to functional gracilis muscle transfer. A neurorrhaphy was performed with the obturator nerve, accessed intraorally at the position of the canine tooth on the affected side.
1-2) Functional muscle transfer
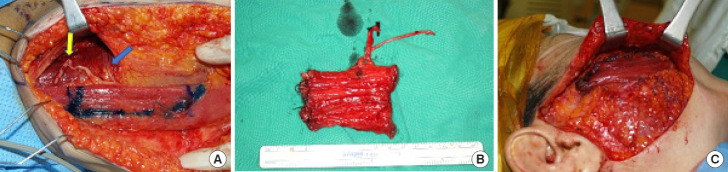
After 6 to 12 months following cross-face nerve grafting, the patient is examined for the presence of the Tinel sign to confirm connection of the nerves; then, functional muscle transfer is performed [31]. Functional gracilis muscle transfer is most common and is associated with minimal donor site complications. In functional muscle transfer, the key factors include the tension-free placement of neurovascular structures and the positioning of muscle flaps to achieve symmetry with the opposite side during facial expression (Fig. 3).
Fig. 3.
A 41-year-old woman underwent facial reanimation surgery due to complete right-sided facial paralysis caused by Bell,s palsy. The gracilis muscle is most commonly utilized as a functional muscle donor. (A) Image depicting the harvesting of a gracilis muscle flap from the left inner thigh, with the obturator nerve (indicated by the yellow arrow) and the vascular pedicle (highlighted by the blue arrow) visible. (B) Harvested functional gracilis muscle. (C) Inset of the gracilis muscle on the paralyzed side.
2) One-stage technique
The one-stage method bears technological resemblance to the two-stage method, except for differences in the donor nerve. This method is indicated for cases in which cross-face nerve grafting cannot be performed due to bilateral facial nerve damage, those in which a broad face reduces the efficiency of cross-face nerve grafting, and those in which the nerve reconnection rate is comparatively low, such as in the elderly. This also includes cases in which the patient does not desire prolonged treatment. The use of ipsilateral cranial nerves, such as the masseter or hypoglossal nerve, allows for the transmission of a greater axonal load compared with using the facial nerve. According to one study, 1,542 axons were available for the flat muscle in the masseter nerve, 834 in the facial nerve, and 100 to 200 in the distal portion of the transplanted cross-face nerve graft.
The one-stage method has the advantage of faster motor recovery relative to the two-stage approach, as the period of muscle neurotization is approximately 2 to 4 months. However, this method does have its drawbacks, including a lack of synchronicity in facial expression movements and the potential for synkinesis. Since the masseter nerve is under the control of the fifth cranial nerve nucleus, masticatory motions of the masseter precede facial expression movements. However, Manktelow et al. [32] suggested that even when the masseter nerve is used as the donor nerve, independent facial expression movements can be recovered without masseter muscle movement in approximately 60% of patients through consistent practice and biofeedback education. This outcome is achievable through cerebral adaptation. The signal transmitted by the trigeminal nerve is a result of the activation of the cerebral cortex, which controls the facial nerve through a cerebral cortical pathway.
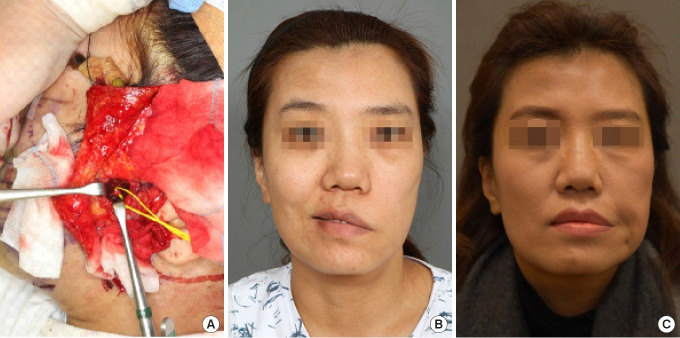
Each type of muscle flap grafted carries both advantages and disadvantages. Koshima et al. [33] performed a one-stage transplant using the rectus femoris muscle, but they found the flap to be excessively bulky. Harii et al. [30] employed a latissimus dorsi muscle flap and a 15-cm-long thoracodorsal nerve to connect directly to the contralateral facial nerve; however, the contractile force of the graft was weakened due to denervation. Finally, Zuker et al. [34] conducted a one-stage functional muscle transfer to the masseter nerve using the gracilis muscle in a child with Möbius syndrome. Those authors confirmed rapid nerve reconnection occurring between 2 and 4 months (Fig. 4).
Fig. 4.
A 46-year-old woman with complete left facial palsy underwent facial reanimation through functional gracilis muscle transfer. (A) The masseter nerve on the affected side was used as the donor nerve. (B) A clinical photograph of the patient smiling before surgery. (C) Six months after surgery, movement of the transplanted gracilis muscle was observed during mastication, and relatively rapid nerve reconnection was noted.
3) Double-innervated functional muscle transfer
To minimize the disadvantages of the two-stage method, Biglioli et al. [35] proposed a method of double-innervated functional muscle transfer involving both ipsilateral cranial nerves. This technique entails the simultaneous use of the contralateral facial nerve and the ipsilateral cranial nerve as donor nerves. The reconnection of the cross-face nerve graft can lead to a reduction in muscle atrophy due to denervation, as well as axon loss. Through the stimulation of both nerves, voluntary movements of the facial expression muscles and a natural smile can be concurrently obtained. Biglioli et al. [35] demonstrated that within 2 to 5 months after surgery, all patients were able to voluntarily contract the transplanted muscle flap. Furthermore, a natural and spontaneous smile was observed within 6 to 8 months.
In a clinical setting, during functional gracilis muscle transfer, the obturator nerve is coaptated to the ipsilateral masseter nerve via end-to-end neurorrhaphy and to the contralateral cross-face nerve graft via end-to-side neurorrhaphy, thereby receiving dual innervation (Fig. 5) [36]. In certain cases, the obturator nerve can be divided in two through nerve splitting; one division is then coaptated to the ipsilateral masseter nerve and the other to the contralateral cross-face nerve graft through end-to-end neurorrhaphy. The range of techniques available is due to the diversity of nerves that can serve as the primary source for neurotization. The authors favor the induction of axonal regeneration via end-to-end neurorrhaphy with the masseter nerve [37]. They also posit that the stimulation of cross-face nerves, connected through end-to-side neurorrhaphy, will demonstrate significant axonal input [36].
Fig. 5.
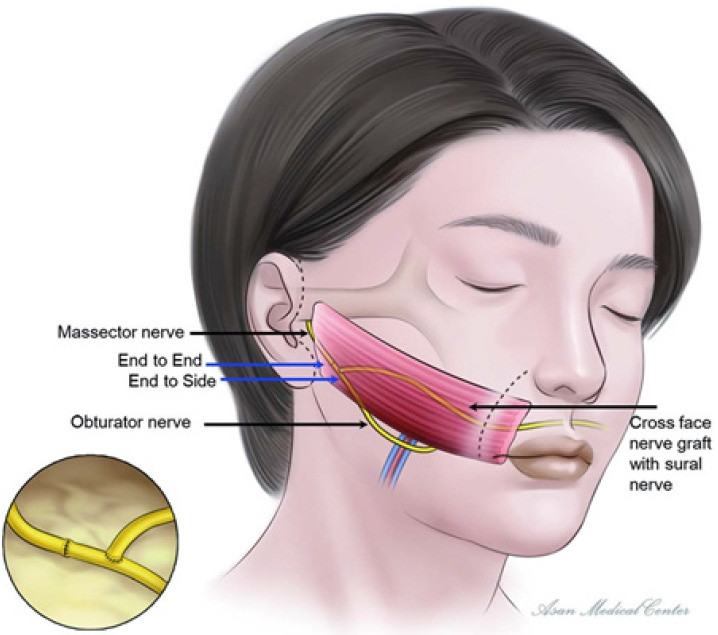
Schematic of double-innervated functional muscle transfer. The obturator nerve and the masseter nerve are sutured end-to-end, while a concurrent cross-face nerve graft is performed using the sural nerve and sutured end-to-side with the obturator nerve. Reprinted from Kim et al. Ann Plast Surg 2020;84:188-95, with permission from Wolters Kluwer Health, Inc. [36].
ADJUVANT TREATMENT OF FACIAL NERVE INJURY (ANCILLARY PROCEDURE)
Patients with complete facial paralysis typically exhibit lagophthalmos and downward displacement of the lower eyelid. Keratitis due to prolonged ocular exposure is common and may progress to corneal ulceration or blindness. Additionally, the occurrence of both facial paralysis and decreased lacrimal gland secretion can promote visual deterioration. Therefore, it is crucial to protect the eyeball through auxiliary treatment, even in the absence of an eyelid nerve graft.
The most common treatment for lagophthalmos is the insertion of platinum or gold deep within the orbicularis oculi muscle, which induces downward displacement of the upper eyelid [38,39]. The implant can be placed either in the deep section of the pretarsal orbicularis or within the post-septal orbicularis. Opting for the latter location can minimize side effects such as diminished visibility or implant extrusion, which may occur with the former placement [39,40].
Downward displacement of the lower eyelid usually occurs in elderly patients rather than in young individuals or in those who have had prolonged atonia. In young patients without horizontal laxity, lateral canthoplasty is performed, while in older patients with horizontal laxity, tarsal strip suspension is effective in correcting lower eyelid displacement and horizontal laxity [39].
In patients with facial paralysis, correction of midface ptosis is also necessary. This can be achieved through rhytidectomy, either as a standalone procedure or in conjunction with facial muscle reconstruction surgery, to rectify the midfacial asymmetry. Typically, the side affected by paralysis appears younger due to the increased volume resulting from muscle transplantation. Consequently, it is necessary to adjust the normal or unaffected side to reestablish symmetry. In elderly patients, a nasolabial fold reset procedure can be performed to restore lost nasolabial fold volume on the affected side [3]. Likewise, this procedure can be performed independently or simultaneously with facial muscle reconstruction surgery. It thereby addresses the excess soft tissue on the affected side and restores the nasolabial fold symmetry. Scars resulting from the incision of the nasolabial fold are not a concern for most middle-aged or older patients, as they are concealed by the movement of the facial flap. These authors have previously documented the usefulness of the nasolabial fold reset procedure in managing excess soft tissue following facial palsy reconstruction and in restoring nasolabial fold symmetry (Fig. 6) [3]. The nasolabial fold incision method can be performed concurrently with facial palsy reconstruction to provide an additional surgical field of view. Alternatively, it can be performed as a secondary procedure at a later stage to address midfacial sagging and the excess soft tissue that may arise after reconstruction.
Fig. 6.
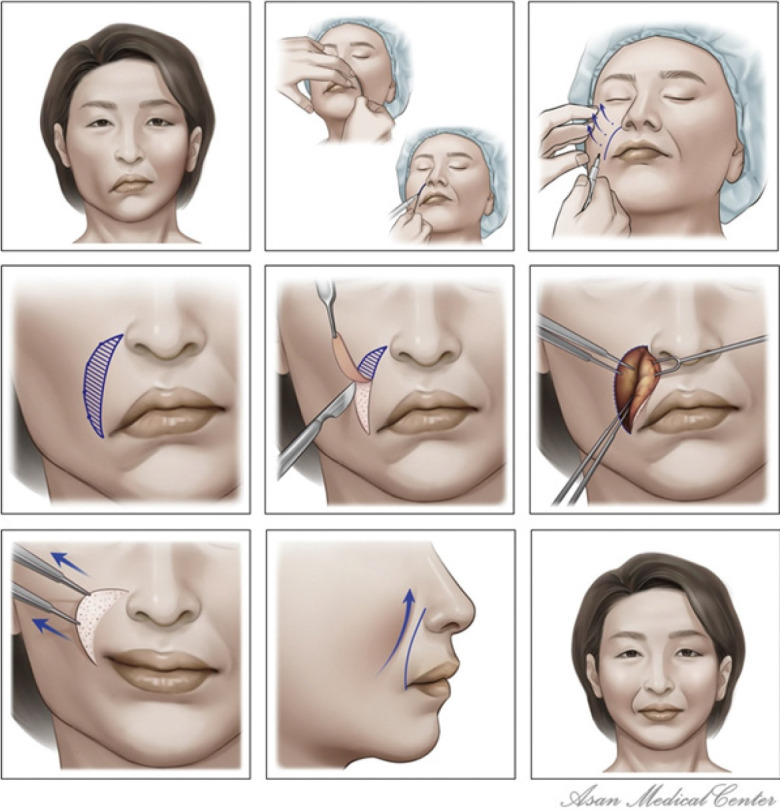
Nasolabial fold reset procedure, performed to restore the symmetry of the nasolabial folds and soft tissues. Reprinted from Kim et al. J Craniomaxillofac Surg 2020;48:162-9, with permission from Elsevier [3].
CONCLUSION
The causes of and treatment approaches for facial nerve injury are diverse. Since the choice of treatment method depends on the location and extent of the damage, the duration of paralysis, and the availability of donor nerves, it is crucial to tailor the treatment to the individual circumstances of each patient. In addition, successful reconstruction should involve the consideration of both functional recovery (such as movement of facial expression muscles) and aesthetic aspects (including facial symmetry, soft tissue sagging, and wrinkle shape).
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Patient consent
The patients provided written informed consent for the publication and use of their images.
Author contributions
Conceptualization: Young Chul Kim, Tae Suk Oh. Methodology: Soo Hyun Woo. Visualization: Young Chul Kim. Writing - original draft: Soo Hyun Woo. Writing - review & editing: Soo Hyun Woo, Young Chul Kim, Tae Suk Oh. Resources: Tae Suk Oh. Supervision: Tae Suk Oh.
REFERENCES
- 1.Brown S, Isaacson B, Kutz W, Barnett S, Rozen SM. Facial nerve trauma: clinical evaluation and management strategies. Plast Reconstr Surg. 2019;143:1498–512. doi: 10.1097/PRS.0000000000005572. [DOI] [PubMed] [Google Scholar]
- 2.Hohman MH, Hadlock TA. Etiology, diagnosis, and management of facial palsy: 2000 patients at a facial nerve center. Laryngoscope. 2014;124:E283–93. doi: 10.1002/lary.24542. [DOI] [PubMed] [Google Scholar]
- 3.Kim MJ, Oh TS. A nasolabial fold reset technique for enhancing midface lifts in facial reanimation: three-dimensional volumetric analysis. J Craniomaxillofac Surg. 2020;48:162–9. doi: 10.1016/j.jcms.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Fattah A, Borschel GH, Manktelow RT, Bezuhly M, Zuker RM. Facial palsy and reconstruction. Plast Reconstr Surg. 2012;129:340e–352e. doi: 10.1097/PRS.0b013e31823aedd9. [DOI] [PubMed] [Google Scholar]
- 5.Ho AL, Scott AM, Klassen AF, Cano SJ, Pusic AL, Van Laeken N. Measuring quality of life and patient satisfaction in facial paralysis patients: a systematic review of patient-reported outcome measures. Plast Reconstr Surg. 2012;130:91–9. doi: 10.1097/PRS.0b013e318254b08d. [DOI] [PubMed] [Google Scholar]
- 6.Kleiss IJ, Hohman MH, Susarla SM, Marres HA, Hadlock TA. Health-related quality of life in 794 patients with a peripheral facial palsy using the FaCE Scale: a retrospective cohort study. Clin Otolaryngol. 2015;40:651–6. doi: 10.1111/coa.12434. [DOI] [PubMed] [Google Scholar]
- 7.May M. Trauma to the facial nerve. Otolaryngol Clin North Am. 1983;16:661–70. [PubMed] [Google Scholar]
- 8.Baumann BM, Jarecki J. Posttraumatic delayed facial nerve palsy. Am J Emerg Med. 2008;26:115. doi: 10.1016/j.ajem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Diaz RC, Cervenka B, Brodie HA. Treatment of temporal bone fractures. J Neurol Surg B Skull Base. 2016;77:419–29. doi: 10.1055/s-0036-1584197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seddon HJ. A classification of nerve injuries. Br Med J. 1942;2:237–9. doi: 10.1136/bmj.2.4260.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunderland S. The anatomy and physiology of nerve injury. Muscle Nerve. 1990;13:771–84. doi: 10.1002/mus.880130903. [DOI] [PubMed] [Google Scholar]
- 12.Han SW, Kim JH, Kim SW, Kim SH, Kang DR, Kim J. Sensory change and recovery of infraorbital area after zygomaticomaxillary and orbital floor fractures. Arch Craniofac Surg. 2022;23:262–8. doi: 10.7181/acfs.2022.01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordin E, Lee TS, Ducic Y, Arnaoutakis D. Facial nerve trauma: evaluation and considerations in management. Craniomaxillofac Trauma Reconstr. 2015;8:1–13. doi: 10.1055/s-0034-1372522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hohman MH, Bhama PK, Hadlock TA. Epidemiology of iatrogenic facial nerve injury: a decade of experience. Laryngoscope. 2014;124:260–5. doi: 10.1002/lary.24117. [DOI] [PubMed] [Google Scholar]
- 15.Cannon CR, Jahrsdoerfer RA. Temporal bone fractures: review of 90 cases. Arch Otolaryngol. 1983;109:285–8. doi: 10.1001/archotol.1983.00800190007002. [DOI] [PubMed] [Google Scholar]
- 16.Gantz BJ, Rubinstein JT, Gidley P, Woodworth GG. Surgical management of Bell’s palsy. Laryngoscope. 1999;109:1177–88. doi: 10.1097/00005537-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Humphrey CD, Kriet JD. Nerve repair and cable grafting for facial paralysis. Facial Plast Surg. 2008;24:170–6. doi: 10.1055/s-2008-1075832. [DOI] [PubMed] [Google Scholar]
- 18.Agnew SP, Dumanian GA. Technical use of synthetic conduits for nerve repair. J Hand Surg Am. 2010;35:838–41. doi: 10.1016/j.jhsa.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Scaramella LF. Anastomosis between the two facial nerves. Laryngoscope. 1975;85:1359–66. doi: 10.1288/00005537-197508000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Hontanilla B, Marre D. Comparison of hemihypoglossal nerve versus masseteric nerve transpositions in the rehabilitation of short-term facial paralysis using the Facial Clima evaluating system. Plast Reconstr Surg. 2012;130:662e–672e. doi: 10.1097/PRS.0b013e318267d5e8. [DOI] [PubMed] [Google Scholar]
- 21.Klebuc MJA. Facial reanimation using the masseter-to-facial nerve transfer. Plast Reconstr Surg. 2011;127:1909–15. doi: 10.1097/PRS.0b013e31820e9138. [DOI] [PubMed] [Google Scholar]
- 22.Park H, Jeong SS, Oh TS. Masseter nerve-based facial palsy reconstruction. Arch Craniofac Surg. 2020;21:337–44. doi: 10.7181/acfs.2020.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo WY, Park SO, Ahn HC, Ryu SR. Facial reanimation using the hypoglossal nerve and ansa cervicalis: a short-term retrospective analysis of surgical outcomes. Arch Craniofac Surg. 2021;22:303–9. doi: 10.7181/acfs.2021.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon BS, Sun H, Kim JW. Modified temporalis tendon transfer extended with periosteum for facial paralysis patients. Arch Craniofac Surg. 2020;21:351–6. doi: 10.7181/acfs.2020.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conley J, Baker DC, Selfe RW. Paralysis of the mandibular branch of the facial nerve. Plast Reconstr Surg. 1982;70:569–77. doi: 10.1097/00006534-198211000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Conley J. Facial rehabilitation: new potentials. Clin Plast Surg. 1979;6:421–31. [PubMed] [Google Scholar]
- 27.Boahene KD. Dynamic muscle transfer in facial reanimation. Facial Plast Surg. 2008;24:204–10. doi: 10.1055/s-2008-1075835. [DOI] [PubMed] [Google Scholar]
- 28.Labbe D, Huault M. Lengthening temporalis myoplasty and lip reanimation. Plast Reconstr Surg. 2000;105:1289–98. [PubMed] [Google Scholar]
- 29.Harii K, Ohmori K, Torii S. Free gracilis muscle transplantation, with microneurovascular anastomoses for the treatment of facial paralysis: a preliminary report. Plast Reconstr Surg. 1976;57:133–43. doi: 10.1097/00006534-197602000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Harii K, Asato H, Yoshimura K, Sugawara Y, Nakatsuka T, Ueda K. One-stage transfer of the latissimus dorsi muscle for reanimation of a paralyzed face: a new alternative. Plast Reconstr Surg. 1998;102:941–51. doi: 10.1097/00006534-199809040-00001. [DOI] [PubMed] [Google Scholar]
- 31.Chuang DC. Free tissue transfer for the treatment of facial paralysis. Facial Plast Surg. 2008;24:194–203. doi: 10.1055/s-2008-1075834. [DOI] [PubMed] [Google Scholar]
- 32.Manktelow RT, Tomat LR, Zuker RM, Chang M. Smile reconstruction in adults with free muscle transfer innervated by the masseter motor nerve: effectiveness and cerebral adaptation. Plast Reconstr Surg. 2006;118:885–99. doi: 10.1097/01.prs.0000232195.20293.bd. [DOI] [PubMed] [Google Scholar]
- 33.Koshima I, Moriguchi T, Soeda S, Hamanaka T, Tanaka H, Ohta S. Free rectus femoris muscle transfer for one-stage reconstruction of established facial paralysis. Plast Reconstr Surg. 1994;94:421–30. doi: 10.1097/00006534-199409000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Zuker RM, Goldberg CS, Manktelow RT. Facial animation in children with Mobius syndrome after segmental gracilis muscle transplant. Plast Reconstr Surg. 2000;106:1–9. doi: 10.1097/00006534-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Biglioli F, Colombo V, Tarabbia F, Pedrazzoli M, Battista V, Giovanditto F, et al. Double innervation in free-flap surgery for long-standing facial paralysis. J Plast Reconstr Aesthet Surg. 2012;65:1343–9. doi: 10.1016/j.bjps.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Kim MJ, Kim HB, Jeong WS, Choi JW, Kim YK, Oh TS. Comparative study of 2 different innervation techniques in facial reanimation: cross-face nerve graft-innervated versus double-innervated free gracilis muscle transfer. Ann Plast Surg. 2020;84:188–95. doi: 10.1097/SAP.0000000000002034. [DOI] [PubMed] [Google Scholar]
- 37.Oh TS, Kim HB, Choi JW, Jeong WS. Facial reanimation with masseter nerve-innervated free gracilis muscle transfer in established facial palsy patients. Arch Plast Surg. 2019;46:122–8. doi: 10.5999/aps.2018.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gire A, Kwok A, Marx DP. PROSE treatment for lagophthalmos and exposure keratopathy. Ophthalmic Plast Reconstr Surg. 2013;29:e38. doi: 10.1097/IOP.0b013e3182674069. [DOI] [PubMed] [Google Scholar]
- 39.Kim MJ, Oh TS. Treatment for ophthalmic paralysis: functional and aesthetic optimization. Arch Craniofac Surg. 2019;20:3–9. doi: 10.7181/acfs.2019.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh TS, Min K, Song SY, Choi JW, Koh KS. Upper eyelid platinum weight placement for the treatment of paralytic lagophthalmos: a new plane between the inner septum and the levator aponeurosis. Arch Plast Surg. 2018;45:222–8. doi: 10.5999/aps.2017.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]