Abstract
Breast cancer continues to be a serious health problem particularly in developed countries. Of particular concern is triple negative breast cancer (TNBC) which does not respond well to standard hormone therapy and is associated with poor overall patient prognosis. Recent studies indicate that Wnt/β-catenin signaling is particularly activated in TNBC, such that the Wnt receptor frizzled-7 (FZD7) and the Wnt co-receptor LRP6 were found to be up-regulated in TNBC. In addition, it has been demonstrated that transcriptional knockdown of LRP6 or FZD7 in TNBC cells suppressed tumor growth in vivo. Furthermore, salinomycin, a selective breast cancer stem cell killer, was recently demonstrated to be an inhibitor of Wnt/β-catenin signaling by inducing LRP6 degradation. Therefore, the Wnt/β-catenin signaling pathway and particularly the Wnt receptors on the cell surface may serve as novel therapeutic targets for the treatment of TNBC.
Breast cancer is the most invasive form of cancer in women and is the second leading cause of death in women in industrialized nations. Three distinct biomarkers including the estrogen receptor (ER), progesterone receptor (PR), and epidermal growth factor receptor-2 (HER2) are used to determine the appropriate breast cancer therapy. Therapies targeting the ER and HER2 have benefitted a subset of breast cancer patients. However, the genetic diversity of this disease varies greatly in that the pathological hallmarks are distinct in each case. Triple-negative breast cancer (TNBC; ER, PR, and HER2-negative breast cancer) is found in approximately 10-20% of breast cancers patients. This form of breast cancer occurs most frequently in premenopausal women, especially women of African-American and Hispanic descent (Carey et al., 2010). Many of the features of TNBC are similar to those identified in the basal-like breast cancer (BLBC). TNBC is clinically characterized as more aggressive and less responsive to standard treatment with a poorer overall patient prognosis (Carey et al., 2010).
The Wnt/β-catenin signaling pathway has been shown to play key roles in both normal development and tumorigenesis (Polakis, 2007; MacDonald et at., 2009). Recent studies indicate that Wnt/β-catenin signaling is particularly over-activated in TNBC. In this review we summarize the current understanding of regulation of the Wnt/β-catenin signaling pathway in breast cancer and provide evidence indicating that the Wnt/β-catenin signaling pathway may represent a new therapeutic target for the treatment of TNBC.
WNT/β-CATENIN SIGNALING
The Wnt/β-catenin signaling pathway is a significant pathway that regulates cell proliferation, migration, and differentiation, thus making it a powerful regulator of embryonic development and tumorigenesis. Wnt proteins, which are secreted glycoproteins, bind to the low-density lipoprotein receptor-related protein5/6 (LRP5/6) and Frizzled (FZD), a seven-pass transmembrane receptor protein, to activate the Wnt/β-catenin signaling pathway (Polakis, 2007; MacDonald et al., 2009). In the absence of Wnts, β-catenin is sequestered in a complex that consists of the adenomatous polyposis coli (APC) tumor suppressor, axin, glycogen synthase kinase-3β (GSK3β), and casein kinase 1 (CK1). This complex formation induces the phosphorylation of β-catenin by CK1 and GSK3β, which results in the ubiquitination and the subsequent degradation of β-catenin by the 26S proteasome (Fig. 1A). Conversely, when Wnt proteins are secreted from cells they can form a ternary complex with the FZD and the LRP5/6 receptors, which results in the inhibition of GSK3β and the stabilization of cytosolic β-catenin. The β-catenin then translocates into the nucleus where it interacts with T-cell factor/lymphoid enhancing factor (TCF/LEF) to induce the expression of downstream target genes that regulate cell cycle, growth, and progression (Fig. 1B) (Polakis, 2007; MacDonald et al., 2009). Wnt/β-catenin signaling can be regulated at the cell surface by various secreted proteins. The extracellular molecules Wnt and R-spondin (Rspo) activate Wnt/β-catenin signaling (Fig. 1B), whereas Wnt inhibitory factor 1 (WIF1), soluble Frizzled-related protein (sFRP), Dickkopf (Dkk), and SOST inhibit it (Fig. 1A) (Kikuchi et al., 2007).
Fig. 1.
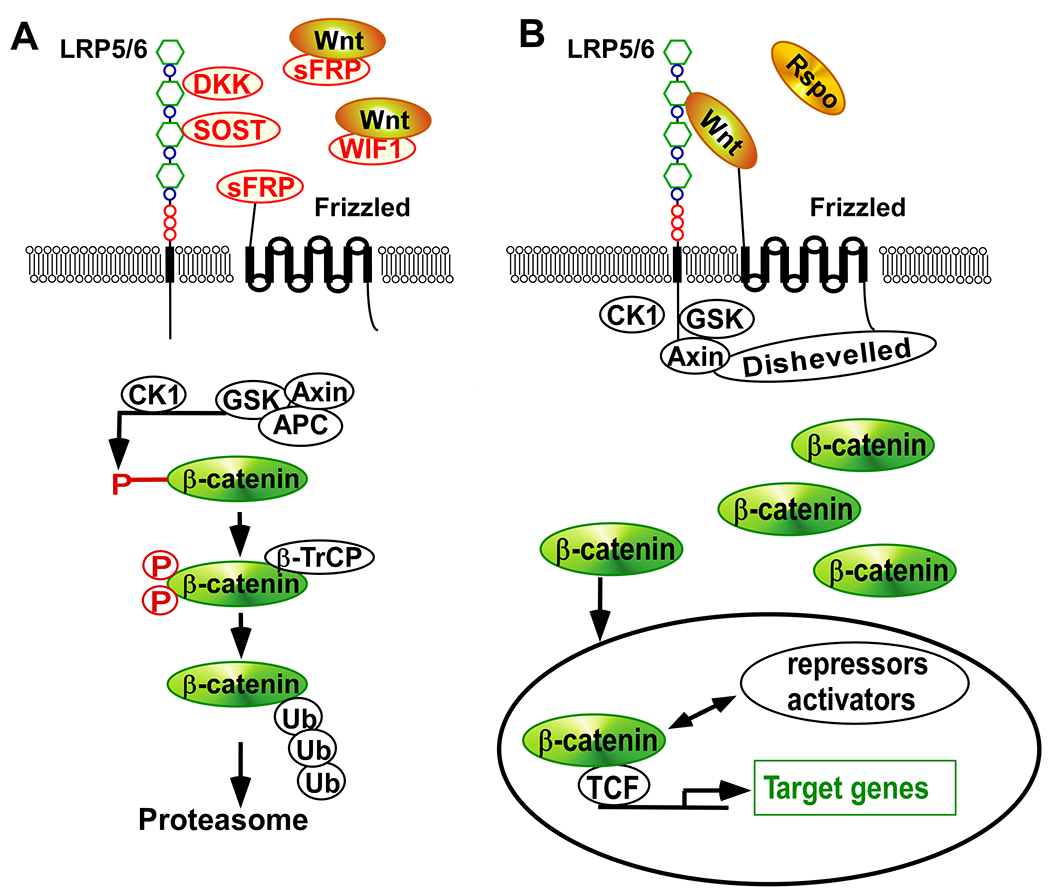
The Wnt/β-catenin signaling pathway. (A) In the absence of interaction between Wnts and LRP5/6, β-catenin levels are efficiently regulated by a complex containing APC, axin, and GSK3β. This complex promotes phosphorylation of β-catenin by Ck1 and GSK3β (P). Phosphorylated β-catenin becomes multi-ubiquitinated (Ub) and subsequently degraded by the 26S proteasome. (B) In the presence of Wnt binding to LRP5/6 and FZD, phosphorylation and degradation of β-catenin is blocked, which results in β-catenin accumulation and the association of β-catenin with TCF transcription factors. The TCF-β-catenin complexes bind to DNA and activate Wnt target genes together with various transcriptional repressors or activators.
WNT/β-CATENIN SIGNALING IN TNBC
Wnt/β-catenin signaling has been implicated in different stages of mammary gland development and is important for mammary oncogenesis (Prosperi and Goss, 2010). Initially, it was reported that Wnt/β-catenin signaling activation, as defined by β-catenin nuclear expression and overexpression of the Wnt/β-catenin target cyclin D1, was associated with a poorer prognosis in breast cancer patients (Lin et al., 2000). More recently, two studies demonstrated that Wnt/β-catenin signaling activation is preferentially found in a subgroup of invasive breast cancers of TNBC and is associated with a poor clinical outcome (Geyer et al., 2011; Khramtsov et al., 2010). This suggests that the Wnt/β-catenin signaling pathway plays an important role in TNBC development and progression (Howe and Brown, 2004).
While genetic mutations of the Wnt/β-catenin signaling intracellular components APC and CTNNB1 (β-catenin encoding gene) are major contributing factors for colorectal cancers, they are typically not the key mechanism associated with breast cancer. It has been demonstrated that only 6% of breast tumors contain mutations in the APC gene and there are no mutations in CTNNB1 in breast cancer (Jonsson et al., 2000; Geyer et al., 2011). Therefore, dysfunction of the Wnt/β-catenin pathway at the cell surface could result in the aberrant activation of Wnt/β-catenin signaling, which is thought to drive breast tumorigenesis.
WNT CO-RECEPTOR LRP5 AND LPR6
LRP5 and LRP6 are two members of the expanding low density lipoprotein receptor (LDLR) family and are essential co-receptors for the Wnt/β-catenin signaling pathway. Both LRP5 and LRP6 contain four β-propeller/epidermal growth factor (EGF) modules. It is interesting to note that all of the identified LRP5/6 extracellular ligands including Wnts, Dkk1 and SOST bind to the β-propeller/EGF repeat modules (MacDonald et al., 2009).
There is a correlation between LRP5 expression and BLBC (Badders et al., 2009; Lindvall et al., 2009). In addition, an aberrantly spliced internally truncated LRP5 receptor (LRP5Δ666–809, LRP5Δ), which retains the ability to transduce Wnt/β-catenin signaling, but is resistant to Dkk1 inhibition, is frequently expressed in breast tumors of different cancer stages including in situ and metastatic carcinomas (Bjorklund et al., 2009). In mice, the deletion of LRP5 delays MMTV-Wnt1-induced tumor formation (Lindvall et al., 2006).
LRP6 is over-expressed in a subgroup of invasive breast cancers of TNBC/BLBC. Initially, Lindvall and colleagues (2009) demonstrated that increased expression of LRP6 is associated with human BLBC. Subsequently, Liu et al. (2010) demonstrated that LRP6 overexpression is highly represented in a subset of breast cancers that are negative for ER and/or HER2. Consistently, Yang et al. (2011) reported that LRP6 is over-expressed in TNBC. In mice, mammary gland development and MMTV-Wnt1-induced mammary tumorigenesis are delayed in LRP6+/− mice (Lindvall et al., 2009), while MMTV-LRP6 transgenic mice develop hyperplasia in their mammary glands due to LRP6-mediated Wnt/β-catenin signaling (Zhang et al., 2010). Transcriptional knockdown of LRP6 in TNBC MDA-MB-231 cells significantly decreased Wnt/β-catenin signaling, cell proliferation, and tumor growth in vivo (Liu et al., 2010). Together, these studies indicate that LRP6 is a potential therapeutic target for TNBC.
While mesoderm development (MESD) was discovered as a specialized molecular endoplasmic reticulum (ER) chaperone for the Wnt co-receptors LRP5 and LRP6, recombinant Mesd protein is able to bind to mature LRP5 and LRP6 on the cell surface and inhibit Wnt/β-catenin signaling in LRP5- and LRP6-expressing cells (Li et al., 2005; Lu et al. 2010). Interestingly, in vivo administration of recombinant Mesd protein or its C-terminal peptide, which is necessary and sufficient for Mesd binding to mature LRP6, markedly suppressed growth of MMTV-Wnt1 tumors without causing undesirable side effects (Liu et al., 2010). These results support the concept that LRP5/6 is also a potential therapeutic target for the treatment of breast cancer.
FRIZZLED PROTEINS
Wnt binds both FZD and LRP5/6 to activate Wnt/β-catenin signaling. Among the 10 FZD proteins, FZD7 is likely the most important one involved in breast cancer Wnt/β-catenin signaling and tumorigenesis. Very recently, Yang et al. (2011) demonstrated that FZD7 plays a critical role in cell proliferation in TNBC. It was demonstrated that FZD7 was over-expressed in TNBC and TNBC-derived cell lines and that downregulation of FZD7 inactivates Wnt/β-catenin signaling in TNBC MDA-MB-231 and BT-20 cells, leading to impaired cell growth and tumor transformation (Yang et al., 2011). Furthermore, in vivo studies revealed that FZD7 shRNA significantly suppressed tumor formation through reduced cell proliferation in mice bearing xenografts without FZD7 expression (Yang et al., 2011).
WNT AND R-SPONDIN PROTEINS
There are 19 Wnt proteins in humans and many of them are able to activate Wnt/β-catenin signaling and cause transformation of mammary epithelial cells (Shimizu et al., 1997). Wnt1, the founding member of the Wnt gene family, is a strong oncogene in the mouse mammary gland tumorigenesis (Tsukamoto et al., 1988). Fusion of the Wnt1 allele with the mouse mammary tumor virus (MMTV) long terminal repeat and subsequent generation of MMTV-Wnt1 transgenic mice has been shown to cause mammary gland hyperplasia and an increase in adenocarcinomas (Tsukamoto et al., 1988). Many Wnt proteins including Wnt2, Wnt7b, and Wnt10b are up-regulated in human breast carcinomas (Howe and Brown, 2004).
The Rspo protein family is another group of secreted proteins that can activate Wnt/β-catenin signaling. This family consists of four members (Rspo1-4) that are structurally similar and share 40-60% homology (Kim et al., 2006). The Wnt receptors FZD and LRP5/6 are involved in the action of Rspo proteins on Wnt/β-catenin signaling. While the direct binding between Rspo and LRP5/6 is controversial (Kazanskaya et al., 2004; Kim et al., 2005; Nam et al., 2006; Wei et al., 2007; Li et al. 2010), two very recent studies demonstrated that Rspo proteins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling (Carmon et al., 2011; de Lau et al., 2011).
It is still unclear whether Rspo proteins are up-regulated in human breast carcinoma, however insertional activation of the Rspo2 and Rspo3 genes has been observed in the MMTV model system (Lowther et al., 2005; Gattelli et al., 2006; Theodorou et al., 2007). Furthermore, it has been recently demonstrated that mouse mammary epithelial cells expressing Wnt1 and Rspo2 independently or together can develop into tumors in nude mice. In addition, Rspo2-derived tumors exhibit a strong epithelial mesenchymal transformation (EMT) phenotype and are highly metastatic to the lung as well as the spleen (Klauzinska et al., 2011). These studies suggest a potential positive contribution of Rspos to mammary gland tumorigenesis.
Although alterations of Wnt and Rspo proteins contribute to activation of Wnt/β-catenin signaling in breast cancer, it is unclear whether dysregulation of these Wnt/β-catenin signaling agonists preferentially occurs in TNBC. Furthermore, Rspo proteins could synergize with Wnt proteins in activating Wnt/β-catenin signaling in breast cancer.
WNT ANTAGONISTS WIF1 AND sFRPs
WIF1 is a secreted protein that binds to Wnt proteins and inhibits their interaction with the FZD receptor. This leads to the termination of transcription of the genes activated by the β-catenin/TCF/LEF transcriptional complex (MacDonald et al., 2009). The sFRP family consists of five secreted glycoproteins in humans (sFRP1-5) that are able to interact with both Wnt and FDZ proteins. The interaction between sFRPs and Wnt proteins prevents the latter from binding the FDZ receptors. The sFRPs are also able to downregulate Wnt/β-catenin signaling by the formation of an inhibitory complex with the FZD receptor (MacDonald et al., 2009).
Previous studies demonstrated that sFRP1-5 and WIF1 are silenced in several types of cancers including breast cancer (Klarmann et al., 2008). It was also shown that overexpression of sFRP1, sFRP2, and sFRP5 dramatically decreases the proliferation of breast cancer cells, while transcriptional knockdown of sFRP1 robustly increases Wnt/β-catenin signaling in breast cancer cells (Suzuki et al., 2008). Furthermore, a recent study showed that stable overexpression of sFRP1 in human breast cancer MDA-MB-231 cells blocks Wnt/β-catenin signaling with ensuing decreases in cell proliferation and suppression of tumor growth and metastasis in xenograft mouse models (Matsuda et al., 2009). Thus, epigenetic silencing of the Wnt antagonists sFRPs and WIF1 leads to aberrant regulation of Wnt/β-catenin signaling in breast cancer (Klarmann et al., 2008), although it is unclear whether epigenetic silencing of sFRP and WIF1 preferentially happens in TNBC.
WNT/β-CATENIN SIGNALING IN BREAST CANCER STEM CELLS
The Wnt/β-catenin signaling pathway has been implicated in the control over various types of stem cells and may act as a niche factor to maintain stem cells in a self-renewing state (Reya and Clevers, 2005; Nusse, 2008). There is mounting evidence that many cancers, including breast cancer, contain populations of cells that display stem-cell properties. Expression of Wnt1 and stabilized β-catenin (ΔN89β-catenin) under the MMTV promoter induces Wnt/β-catenin signaling in distinct progenitor compartments in mouse mammary tumors (Teissedre et al. 2009). Wnt/β-catenin signaling also mediates the radiation resistance of mouse mammary progenitor cells (Woodward et al., 2007).
Breast cancer stem cells (CSCs) are characterized by the expression of several cell surface markers including CD44+/CD24−. The CD44+/CD24− breast cancer cells have tumor-initiating properties (Al-Hajj et al., 2003; Ponti et al., 2005). It has been demonstrated that the CD44+/CD24− phenotype is enriched in BLBC (Honeth et al., 2008), and that increased cytoplasmic and nuclear localization of β-catenin in BLBC overlaps with CD44+/CD24− staining (Khramtsov et al., 2010), suggesting that CSC populations with activated Wnt/β-catenin signaling could exist in BLBC/TNBC.
Recently, Gupta et al. (2009) developed and implemented a high-throughput screening method to identify agents with specific toxicity for epithelial CSCs, and identified salinomycin as a selective inhibitor of breast CSCs. Salinomycin is able to reduces the proportion of CSCs by >100-fold relative to paclitaxel, inhibit mammary tumor growth in vivo and induce increased epithelial differentiation of tumor cells (Gupta et al., 2009). More recently, it has been demonstrated that salinomycin is a Wnt/β-catenin signaling inhibitor through induction of Wnt co-receptor LRP6 degradation (Lu et al., 2011; Tang et al., 2011). This suggests that the inhibitory effect of salinomycin on Wnt/β-catenin signaling contributes to its toxicity toward breast CSCs.
SUMMARY
TNBC is one of the most difficult subtypes of breast cancer to treat due to a lack of a targeted therapy, although poly (ADP-ribose) polymerase, angiogenesis, EGFR, src kinase, and mTOR inhibitors are currently under investigation in patients with TNBC/BLBC (Carey et al., 2010). Over activation of Wnt/β-catenin signaling with the up-regulation of Wnt receptor expression in TNBC/BLBC suggests that the Wnt/β-catenin pathway could be an attractive novel therapeutic target for TNBC/BLBC. The identification of salinomycin as an inhibitor of LRP6 expression and Wnt/β-catenin signaling and a selective killer of breast CSCs suggests that LRP6 may offer a unique opportunity to target breast CSCs. Specific LRP6 antibodies or the LRP6 inhibitor Mesd were shown to block tumor growth in Wnt1- or Wnt3A-driven xenografts (Ettenberg et al., 2010, Gong et al., 2010, Liu et al., 2010). These interesting findings indicate that further studies to examine whether Mesd and LRP6 antibodies have therapeutic potential in TNBC are warranted. Furthermore, small molecule inhibitors disrupting the Wnt/β-catenin pathway, particularly those targeting Wnt receptors LRP6 and FZD7, could represent a novel therapeutic treatment for TNBC.
ACKNOWLEDGMENT
This work was completed with the support of a grant from the National Institute of Health (R01CA124531). Yonghe Li is one of two inventors on a patent for the use of Mesd in bone disease and cancer. Raptor Pharmaceutical has licensed this patent from Washington University in St. Louis.
REFERENCES
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. 2003. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 100:3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, Lindvall C, Williams BO, Alexander CM. 2009. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One 4:e6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund P, Svedlund J, Olsson AK, Akerström G, Westin G. 2009. The internally truncated LRP5 receptor presents a therapeutic target in breast cancer. PLoS One 4:e4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey L, Winer E, Viale G, Cameron D, Gianni L. 2010. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol 7:683–692. [DOI] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. 2011. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc Natl Acad Sci U S A 108:11452–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es J, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H. 2011. Lgr5 homologuesassociate with Wnt receptors and mediate R-spondin signalling. Nature 476:293–297. [DOI] [PubMed] [Google Scholar]
- Ettenberg SA, Charlat O, Daley MP, Liu S, Vincent KJ, Stuart DD, Schuller AG, Yuan J, Ospina B, Green J, Yu Q, Walsh R, Li S, Schmitz R, Heine H, Bilic S, Ostrom L, Mosher R, Hartlepp KF, Zhu Z, Fawell S, Yao YM, Stover D, Finan PM, Porter JA, Sellers WR, Klagge IM, Cong F. 2010. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci U S A 107:15473–15478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattelli A, Zimberlin MN, Meiss RP, Castilla LH, Kordon EC. 2006. Selection of early-occurring mutations dictates hormone-independent progression in mouse mammary tumor lines. J Virol 80:11409–11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, Natrajan R, Reis-Filho JS. 2011. β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 24:209–231. [DOI] [PubMed] [Google Scholar]
- Gong Y, Bourhis E, Chiu C, Stawicki S, DeAlmeida VI, Liu BY, Phamluong K, Cao TC, Carano RA, Ernst JA, Solloway M, Rubinfeld B, Hannoush RN, Wu Y, Polakis P, Costa M. 2010. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS One 5:e12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. 2009.Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138:645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, Grabau D, Ferno M, Borg A, Hegardt C. 2008. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res 10:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe LR, Brown AM. 2004. Wnt signaling and breast cancer. Cancer Biol Ther 3:36–41. [DOI] [PubMed] [Google Scholar]
- Jönsson M, Borg A, Nilbert M, and Andersson T. 2000. Involvement of adenomatous polyposis coli (APC)/β-catenin signalling in human breast cancer. Eur J Cancer. 36 (2): 242–248. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. 2004. R-Spondin2 is a secreted activator of Wnt/β-catenin signaling and is required for Xenopus myogenesis. Dev Cell 7:525–534. [DOI] [PubMed] [Google Scholar]
- Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. 2010. Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol 176:2911–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Kishida S. 2007. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal 19:659–671. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. 2005. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309: 256–1259. [DOI] [PubMed] [Google Scholar]
- Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD. 2006. R-Spondin proteins: a novel link to β-catenin activation. Cell Cycle 5:23–26. [DOI] [PubMed] [Google Scholar]
- Klarmann GJ, Decker A, Farrar WL. 2008. Epigenetic gene silencing in the Wnt pathway in breast cancer. Epigenetics 3:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauzinska M, Baljinnyam B, Raafat A, Rodriguez-Canales J, Strizzi L, Greer YE, Rubin JS, Callahan R. 2011. Rspo2/Int7 regulates invasiveness and tumorigenicproperties of mammary epithelial cells. J Cell Physiol. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen J, Lu W, McCormick LM, Wang J, Bu G. 2005. Mesd binds to mature LDL-receptor related protein-6 and antagonizes ligand binding. J Cell Sci 118:5305–5314. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu W, King TD, Liu CC, Bijur GN, Bu G. 2010. Dkk1 stabilizes Wnt co-receptor LRP6: implication for Wnt ligand-induced LRP6 down-regulation. PLoS One 5:e11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. 2000. β-Catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A 97:4262–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. 2006. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem 281:35081–35087. [DOI] [PubMed] [Google Scholar]
- Lindvall C, Zylstra CR, Evans N, West RA, Dykema K, Furge KA, Williams BO. 2009. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS One 4:e5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Prior J, Piwnica-Worms D, Bu G. 2010. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci U S A 107:5136–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther W, Wiley K, Smith GH, Callahan R. 2005. A new common integration site, Int7, for the mouse mammary tumor virus in mouse mammary tumors identifies a gene whose product has furin-like and thrombospondin-like sequences. J Virol 79:10093–10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. 2011. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A 108:13253–13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Liu CC, Thottassery JV, Bu G, Li Y. 2010. Mesd is a universal inhibitor of Wnt coreceptors LRP5 and LRP6 and blocks Wnt/β-catenin signaling in cancer cells. Biochemistry 49:4635–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. 2009. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Schlange T, Oakeley EJ, Boulay A, and Hynes NE. 2009. WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppressesMDA-MB-231 xenograft growth. Breast Cancer Res 11:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Yoon JK. 2006. Dynamic expression of R-spondin family genes in mouse development. Gene Expr Patterns 7:306–312. [DOI] [PubMed] [Google Scholar]
- Nusse R 2008. Wnt signaling and stem cell control. Cell Res 18:523–527. [DOI] [PubMed] [Google Scholar]
- Polakis P 2007. The many ways of Wnt in cancer. Curr Opin Genet Dev 17:45–51. [DOI] [PubMed] [Google Scholar]
- Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. 2005. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 65:5506–5511. [DOI] [PubMed] [Google Scholar]
- Prosperi JR, Goss KH. 2010. A Wnt-ow of opportunity: targeting the Wnt/β-catenin pathway in breast cancer. Curr Drug Targets 11:1074–1088. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. 2005. Wnt signalling in stem cells and cancer. Nature 434:843–850 [DOI] [PubMed] [Google Scholar]
- Shimizu H, Julius MA, Giarré M, Zheng Z, Brown AM, Kitajewski J. 1997. Transformation by Wnt family proteins correlates with regulation of β-catenin. Cell Growth Differ 8:1349–1358. [PubMed] [Google Scholar]
- Suzuki H, Toyota M, Carraway H, Gabrielson E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T, Mori M, Hirata K, Imai K, Shinomura Y, Baylin SB, Tokino T. 2008. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer 98:1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ, Zou CY, Xie XB, Zeng YX, Shen JN, Kang T, Wang J. 2011. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Teissedre B, Pinderhughes A, Incassati A, Hatsell SJ, Hiremath M, and Cowin P. 2009. MMTV-Wnt1 and -ΔN89β-catenin induce canonical signaling in distinctprogenitors and differentially activate Hedgehog signaling within mammary tumors. PLoS One 4: e4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou V, Kimm MA, Boer M, Wessels L, Theelen W, Jonkers J, Hilkens J. 2007. MMTV insertional mutagenesis identifies genes, gene families and pathways involved in mammary cancer. Nat Genet 39:759–769. [DOI] [PubMed] [Google Scholar]
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, and Varmus HE. 1988. Expression of theint-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 55:619–625. [DOI] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. 2007. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and β-catenin signaling. J Biol Chem 282:15903–15911. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Medina A, Swain RK, Steinbeisser H. 2001. Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature 413:856–860. [DOI] [PubMed] [Google Scholar]
- Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. 2007. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA 104:618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, Somlo G, Yen Y. 2011. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Liu Q, Lu W, Bu G. 2010. Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene 29:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]


