Abstract
The objective of this study was to investigate the effect of bovine milk derived exosomes (MDEs) on the gut microbiota of Dextran sodium sulfate (DSS)-induced colitis mice. Total of 42 specific pathogen free (SPF) male BALB/c mice (3 weeks old) were randomly assigned to three groups including control group, DSS group (DSS) and bovine milk derived exosome group (Exo), with 7 replicates/cages per treatment and two mice in one cage. 16S rRNA gene sequencing of cecal digesta samples was conducted. DSS significantly decreased the average daily feed intake of mice in DSS and Exo groups (P = 0.03). Shannon index of the DSS group was significantly lower than the control group (P < 0.05) whereas no difference between the control group and Exo group was observed. Administration of MDEs tended to increase the relative abundance of Campylobaterota. Compared to the control group, the relative abundance of Roseburia was significantly decreased in the DSS group (P < 0.05) whereas no difference between the Exo group and control group was observed. MDEs also tended to increase the relative abundance of Lachnospiraceae_UCG_006. In conclusion, oral administration of 10 µL MDEs (1 mg/mL) positively affected gut microbiota of DSS-induced colitis mice. The results of this study provided valuable reference for MDEs application in the prevention and treatment of colitis.
Keywords: Bovine milk derived exosomes, Colitis mouse, Microbiota, Dextran sodium sulfate
Introduction
Exosomes are microscopic nanovesicles containing complex RNAs and proteins, approximately 30–100 nm, formed by multiple vesicle bodies from different cells [1, 2]. They can be found in almost all living cells, especially in dendritic cells, lymphocytes, epithelial cells, and endothelial cells [3]. These vesicles are involved in immune responses, cell adhesion, waste management, protection against stress, and inflammation, with their primary role being intercellular signaling and communication [4]. Exosomes were intensively studied as facilitators of the immune systems and the role of exosomes in antigen presentation has been extensively documented [5–7].
The intestine is colonized by about 10 trillion microbes and the gut microbiota play a significant role in maintaining intestinal homeostasis which has been recognized as a major determinant to host health [8, 9]. The intestinal microflora maintains a symbiotic relationship with the host and regulate host metabolism, immunity and intestinal barrier function [10]. Inflammatory bowel disease (IBD) involves long-standing (chronic) inflammation of tissues in the digestive tract. The cause of IBD has been elusive and the pathogenesis of IBD is attributed to an inappropriate and continuing immune response to the commensal bacteria in genetically susceptible host [11]. A diverse spectrum of pathogenic bacteria have been involved in the development of inflammation in case of IBD [12]. Gut microbiota can be affected by dietary interventions and diet changes affect composition of microbiota directly [13]. Using oral administration it was observed that 75% of exosomes not absorbed in the upper intestine entered the large intestine and cecum [14]. A growth advantage of the bacteria cultured with milk exosome was observed in vitro [13]. It is noteworthy that exosomes entering the digestive tract can largely affect absorption as well as their interaction with gut microbiota.
Understanding the biological effects of milk exosomes on gut microbiota is intriguing and studies on effects of milk derived exosome (MDEs) on gut microbiota and their interactions are scarce. It is hypothesized that MDEs orally administered can enter the large intestine and promote the beneficial bacteria growth in colitis mouse. The objective of the current study was to investigate the effects of bovine MDEs on intestinal microbiota of DSS-induced colitis mouse.
Materials and Methods
Experimental Design and Animals
Total of 42 specific pathogen free (SPF) BALB/c mice (male; 3 weeks old) were purchased from Guangdong medical laboratory animal center in Guangzhou, China. A completely randomized design was used for this study. Mice were randomly assigned to three groups including control group, DSS group (DSS) and Exosome group (Exo), with 7 replicates/cages per treatment and two mice in one cage. Mice were housed at 22 ± 2 °C in 12 h light/dark cycles during the whole period. All mice were fed a SPF standard diet and had free access to drinking water in the first six days. From day 7 to 11, the drinking water of DSS group and Exo group were switched to 3.5% DSS solution to establish the colitis model. From day 12 to 17, all mice had free access to drinking water. Each mouse from the Exo group received 10 µL bovine milk derived exosomes (1 mg/mL; product # UR53201, Umibio Science and Technology Group, Shanghai, China) by oral gavage from day 12 to 17. Daily feed intake and body weight were recorded.
Blood Sampling and Analysis
On the last day of the study (day 18), one mouse was randomly selected from each cage, and blood was collected using the eyeball extraction method. The blood sample was left undisturbed at room temperature for 1 h. Then the sample was centrifuged at 3000 r/min for 20 min and the serum was collected and stored at minus 80 ºC for antioxidant parameters and inflammatory factors analysis. Serum total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), total antioxidant capacity (T-AOC), and catalase (CAT) were measured using the kits from Nanjing Jiancheng Research Institute (Nanjing, China). Concentration of tumor necrosis factor-a (TNF-a), Interleukin-10 (IL-10), Interleukin-6 (IL-6), and Interleukin-4 (IL-4) were determined using kits from Shanghai Enzymes Biotechnology Co., Ltd (Shanghai, China).
Cecal Digesta Sampling, DNA Extraction and Sequencing
On day 18, cecal digesta content was collected from one mouse in each cage and the 16S rRNA gene sequencing of cecal digesta samples was conducted. Genomic DNA was extracted using the cetyltrimethyl ammonium bromide method (CTAB). The DNA quality and concentration were checked using a NanoDrop 2000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE). Samples were diluted to 1 ng/μL to prepare amplicons for high-throughput sequecing. The V3–V4 regions of the 16S rRNA genes were amplified using conventional PCR with primers F341 (5′-CCTAYGGGRBGCASCAG-3′) and R806 (5′-GGACTACNNGGGTATCTAAT-3′). The PCR reaction mix consisted of 15 μL of Phusion High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA), 0.2 mmol of forward and reverse primers and 10 ng template DNA. Reaction condition consisted of initial denaturation at 98 ºC for 1 min, followed by 30 cycles of denaturation at 98 ºC for 10 s, annealing at 50 ºC for 30 s, elongation at 72 ºC for 30 s, and a final extension at 72 ºC for 5 min. Sequencing libraries were generated using TruSeq DNA PCR-Free sample preparation kit (Illumina, San Diego, CA) following manufacturer’s instructions. The library quality was assessed on a Qubit @2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA) and Agilent Bioanalyzer 2100 system (Agilent Technologies, Inc., Santa Clara, CA). The bar-coded amplicons were sequenced on an Illumina NovaSeq system.
Data Analysis
The Fast Length Adjustment of Short reads software (FLASH; V1.2.7) was used to merge paired-end reads. To obtain high-quality clean reads, quality filtering of the raw sequences was conducted on a quality control pipeline using the Quantitative Insight into Microbial Ecology (QIIME) tool kit. By comparing with the Silva database, the chimera sequences were identified and removed using UCHIME algorithm. The obtained high quality reads were assigned to the same operational taxonomic units (OTUs) at ≥ 97% similarity using the QIIME Uclust algorithm. OTUs abundance information was normalized and subsequent diversity analysis was performed using the normalized data. Alpha diversity analysis (Shannon, Simpson, Chao1, Ace, Goods Coverage, and PD whole tree) was conducted to study the complexity of species diversity using QIIME (V1.9.1). Principal coordinate analysis (PCoA) was performed to get principal coordinates with bray–curtis distance algorithm and the data was displayed by WGCNA and ggplot2 packages in R software (V4.0.0; R Core Team, 2013). The linear discriminant analysis effect size (LEfSe) algorithm was applied to analyze microbial funcitions predicted by PICRUSTt at level 3 (LDA > 2).
All the data were analyzed using the PROC GLIMMIX procedure of SAS (SAS Institute, Inc., Cary, NC) including treatment as fixed effect in the model. The model used is: Yij = μ + Ti + εij, where Yij = dependent variable, μ = population mean, Ti = treatment effects, εij = random error. The significance was declared at P < 0.05 and trends at P < 0.1.
Results
Growth and Feed Intake
Average daily feed intake (ADFI), average daily weight gain (ADG) and body weight are listed in Table 1. No treatment effect was observed regarding cecum length, colon length, ADG, and body weight (P > 0.05). Compared to the control group, the ADFI in the DSS and Exo groups significantly decreased (P = 0.03), but no difference was observed between the DSS and Exo groups indicating that administration of MDEs in the current dose amount could not be able to restore the feed intake of colitis mice.
Table 1.
Effects of bovine milk derived exosomes on growth performance of DSS-induced colitis mice
| Item | Control | DSS | Exo | SEM | P |
|---|---|---|---|---|---|
| Cecum length, cm (cm) | 2.74 | 2.64 | 2.57 | 0.074 | 0.28 |
| Colon length,cm | 9.23 | 8.28 | 9.06 | 0.429 | 0.27 |
| ADG, g | 0.63 | 0.53 | 0.56 | 0.067 | 0.57 |
| ADFI, g | 4.11a | 3.52b | 3.46b | 0.181 | 0.03 |
| BW, g | 23.14 | 22.35 | 22.60 | 0.552 | 0.59 |
ADG average daily gain; ADFI average daily feed intake; BW body weight; different lowercase letters within a row indicate significant differences
Serum Antioxidant Indexes and Inflammatory Factors
DSS significantly decreased CAT levels of colitis mice and milk derived exosomes did not reverse this effect (Table 2, P < 0.05). The levels of GSH-Px, T-SOD and MDA were not different among the three groups. Compared to the control group, the T-AOC level was significantly lower in the DSS group, but not different from the Exo group. Compared with the control group, the serum IL-6 content in the DSS and Exo groups was significantly higher (P < 0.05; Table 3).
Table 2.
Effect of bovine milk derived exosomes on serum antioxidant indexes of DSS-induced colitis mice
| Item | Control | DSS | Exo | SEM | P |
|---|---|---|---|---|---|
| CAT, U/mL | 136.82a | 28.44b | 23.64b | 3.105 | < 0.0001 |
| T-AOC, U/mL | 3.94a | 2.51b | 3.26ab | 0.285 | 0.008 |
| GSH-Px, U/mL | 338.30 | 355.40 | 386.94 | 31.789 | 0.55 |
| T-SOD, U/mL | 179.14 | 184.83 | 220.24 | 16.272 | 0.19 |
| MDA, μmol/L | 11.63 | 13.17 | 11.68 | 2.184 | 0.85 |
CAT catalase; T-AOC total antioxidant capacity; GSH-Px glutathione peroxidase; T-SOD total superoxide dismutase; MDA malondialdehyde; different lowercase letters within a row indicate significant differences
Table 3.
Effect of bovine milk derived exosomes on serum inflammatory factors of DSS-induced colitis mice
| Item | Control | DSS | Exo | SEM | P |
|---|---|---|---|---|---|
| IL-4, ng/L | 37.69 | 42.12 | 32.93 | 4.569 | 0.41 |
| IL-10, ng/L | 171.99 | 177.62 | 146.29 | 15.848 | 0.35 |
| IL-6, ng/L | 4.09a | 49.37b | 54.96b | 4.329 | < 0.0001 |
| TNF-α, ng/L | 392.75 | 365.32 | 379.28 | 8.891 | 0.22 |
Different lowercase letters within a row indicate significant differences
OTU Diversity, Similarity Analysis, and Alpha Diversity
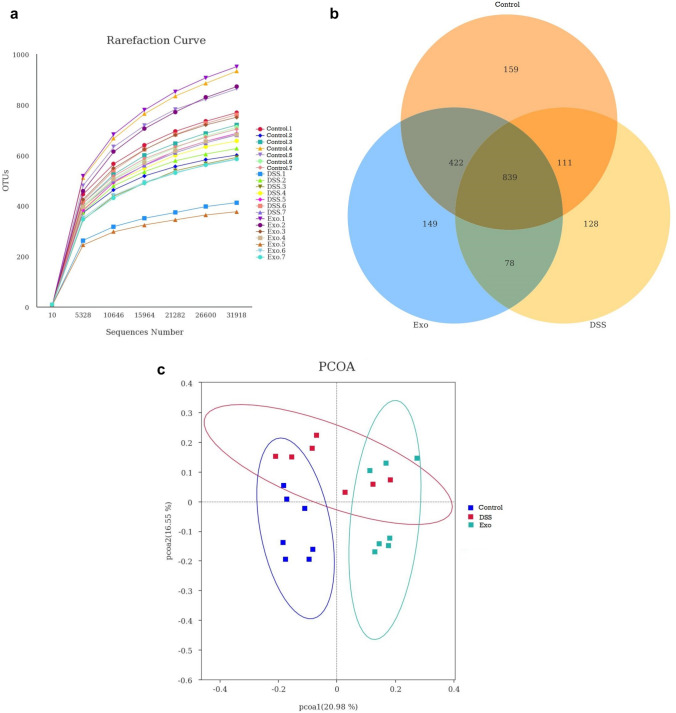
The 16sRNA gene sequencing was performed on cecal digesta samples to compare the microbial differences among three groups. An average of 55,762 effective tags were obtained after data filtering, quality control and chimera sequence removal. The length of the sequences ranged between 413 and 421 bp with an average length of 416 bp. Rarefaction curve revealed that there was sufficient OTU coverage to the bacterial composition within each group (Fig. 1A). The overall number of OTUs was 1886, and 839 shared OTUs were detected in all groups. There were 159, 128 and 149 OTUs unique to the control, DSS, and Exo groups, respectively (Fig. 1B). Principal coordinate analysis revealed that the first principal component and the second principal component explained 20.98% and 16.55% of the variation among samples, respectively. As shown in Fig. 1C, samples from the control group could be distintly separated from Exo group. Both control group and Exo group could not be completely separated from the DSS group. After MDEs were orally gavaged, the samples gradually shifted away from the DSS group indicating the administration of MDEs could affect gut microbiota. The treatment effects on Simpson, Chao1, Ace, Good_Coverage, PD_whole_tree indexes were not observed (Table 4; P > 0.05). The DSS group had significantly lower shannon index (bacterial diversity and eveness) compared to the control group. No difference between the control and Exo group was observed indicating the ability of MDEs to restore the bacterial diversity under colitis condition.
Fig. 1.
Number of operational taxonomic units (OTUs) in each group: a rarefraction curves of OTUs; b Venn diagram of shared OTUs; c. Principle coordinate analysis (PCoA) of the cecal microbiota
Table 4.
Effects of bovine milk derived exosomes on cecal microbial alpha diversity of DSS-inducedcolitis mice
| Item | Control | DSS | Exo | SEM | P |
|---|---|---|---|---|---|
| Shannon | 6.68a | 6.07b | 6.25ab | 0.130 | 0.01 |
| Simpson | 0.97 | 0.95 | 0.95 | 0.006 | 0.06 |
| Chao1 | 920.0 | 762.8 | 817.9 | 68.85 | 0.28 |
| Ace | 918.6 | 755.6 | 824.4 | 69.49 | 0.27 |
| Goods coverage | 0.99 | 0.99 | 0.99 | 0.0005 | 0.38 |
| PD whole tree | 54.99 | 49.75 | 58.5 | 4.617 | 0.42 |
Different lowercase letters within a row indicate significant differences
Taxonomic Composition of Gut Microbiota
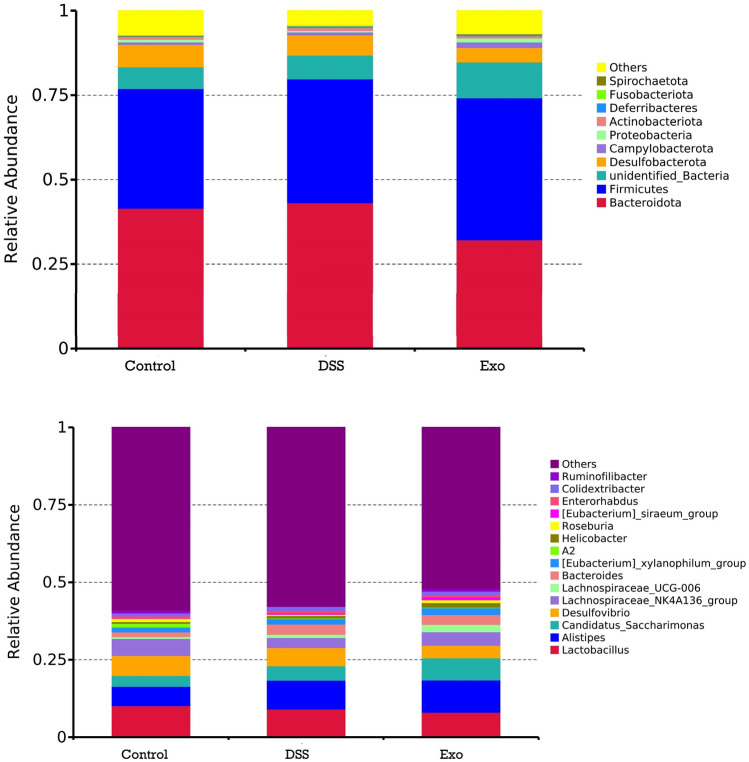
Microbial taxonomic composition data at the phylum and genus levels are presented in Tables 5, 6 and Fig. 2. Taxonomic profiling revealed that gut microbiota mainly composed of nine phyla among the three groups. At the phylum level, Firmicutes and Bacteroidetes are the two dominant phyla and the relative abundance of these two phya occupy approximately 80% of total microbes. Administration of MDEs tended to increase the relative abundance of Campylobaterota. At the genus level, the top two genuses are Lactobacillus and Alistipes. The DSS group had significantly lower relative abundance of Roseburia compared to the control group. Oral gavage of MDEs increased the relative abundance of Roseburia and it is not different from the control group. Both the DSS and Exo group tended to have higher relative abundance of Bacteroides. In addition, administration of MDEs tended to increase the relative abundance of Lachnospiraceae_UCG_006. The data showed that oral administration of MDEs affected the composition of gut microbiota.
Table 5.
Effects of bovine milk derived exosomes on cecal microbial taxonomic composition (phylum level) of DSS-induced colitis mice
| Item | Control | DSS | Exo | SEM | P |
|---|---|---|---|---|---|
| Firmicutes | 35.48 | 36.62 | 42.05 | 2.932 | 0.26 |
| Bacteroidetes | 41.53 | 43.18 | 32.19 | 3.833 | 0.12 |
| Desulfobacterota | 6.68 | 5.92 | 4.35 | 1.056 | 0.30 |
| Campylobacterota | 0.73 | 0.87 | 1.64 | 0.302 | 0.09 |
| Proteobacteria | 0.81 | 0.45 | 1.17 | 0.273 | 0.20 |
| Actinobacteriota | 0.79 | 0.90 | 0.63 | 0.134 | 0.38 |
| Deferribacteres | 0.16 | 0.37 | 0.39 | 0.109 | 0.29 |
| Fusobacteriota | 0.01 | 0.12 | 0.009 | 0.070 | 0.43 |
| Spirochaetota | 0.22 | 0.11 | 0.19 | 0.056 | 0.38 |
| Unidentified_Bacteria | 6.34 | 7.10 | 10.53 | 1.255 | 0.07 |
| Others | 7.22 | 4.33 | 6.84 | 0.810 | 0.04 |
| F/B | 0.89 | 1.01 | 1.39 | 0.176 | 0.14 |
Table 6.
Effects of bovine milk derived exosomes on cecal microbial taxonomic composition (genus level) of DSS-induced colitis mice
| Item | Control | DSS | Exo | SEM | P |
|---|---|---|---|---|---|
| Lactobacillus | 10.19 | 9.10 | 8.07 | 2.811 | 0.86 |
| Alistipes | 6.24 | 9.31 | 10.43 | 1.517 | 0.16 |
| Desulfovibrio | 6.45 | 5.91 | 4.05 | 1.053 | 0.26 |
| Lachnospiraceae_NK4A136_group | 5.47 | 3.24 | 4.38 | 0.716 | 0.11 |
| Candidatus_Saccharimonas | 3.49 | 4.62 | 7.15 | 1.196 | 0.11 |
| Bacteroides | 1.54 | 3.31 | 3.18 | 0.537 | 0.06 |
| A2 | 1.18 | 0.19 | 0.15 | 0.405 | 0.15 |
| Roseburia | 1.01a | 0.33b | 0.93ab | 0.167 | 0.02 |
| Colidextribacter | 0.93 | 1.30 | 1.29 | 0.133 | 0.12 |
| Enterorhabdus | 0.69 | 0.82 | 0.55 | 0.127 | 0.33 |
| Lachnospiraceae_UCG_006 | 0.61 | 1.02 | 2.35 | 0.502 | 0.06 |
| Helicobacter | 0.59 | 0.84 | 1.49 | 0.302 | 0.12 |
| Escherichia_Shigella | 0.25 | 0.27 | 0.43 | 0.170 | 0.72 |
| Others | 59.63 | 57.52 | 52.40 | 3.291 | 0.30 |
Different lowercase letters within a row indicate significant differences
Fig. 2.
Phylum-level (top) and genus-level (bottom) taxonomic composition of the cecal bacterial communities
LEfSe Analysis of Microbial Functions Predicted by PICRUSTt Based on KEGG Database
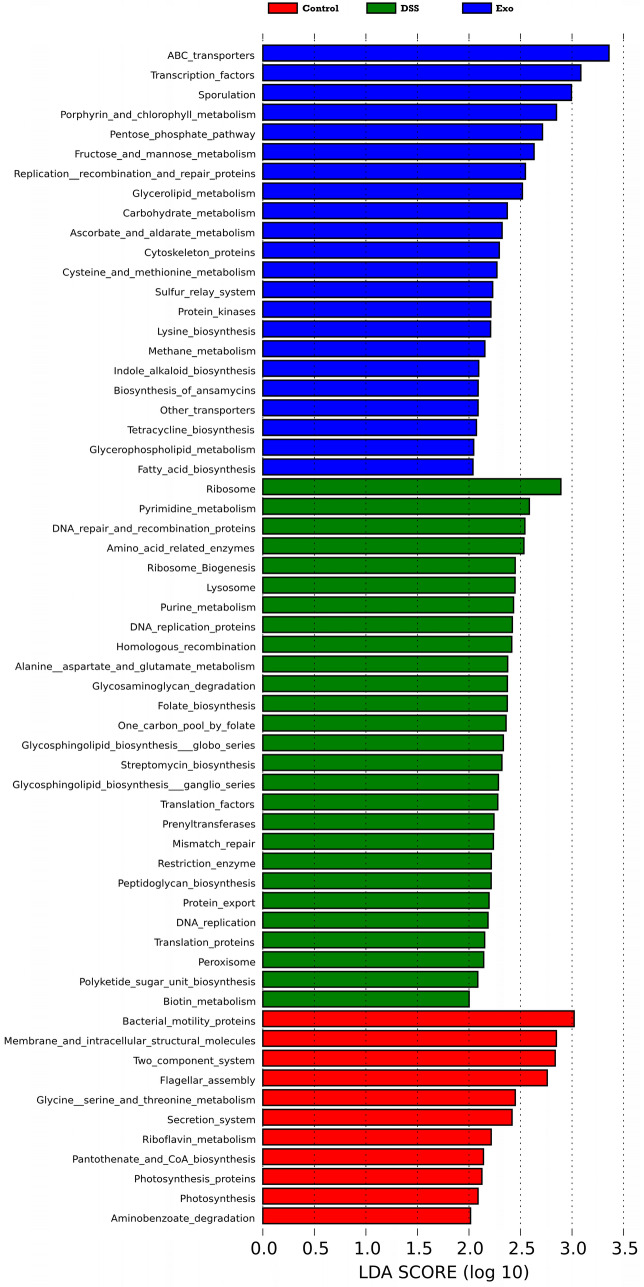
Microbial functions were predicted by using PICRUSTt at level 3 based on KEGG database. LefSe analysis (Linear discriminant analysis effect size; LDA > 2.0) was applied to discriminate the microbial functions among all groups. The microbial functions shown in each group are functions that are highly enriched in the corresponding group (Fig. 3). For the control, DSS and Exo groups, the number of microbial functions having a LDA score higher than 2.0 are 22, 27, and 11, respectively. For control group, the top three microbial functions were ABC transporters, transcription facotors,and sporulation. The top three microbial functions in the DSS group are ribosome, pyrimidine-metabolism, and DNA repair and recombination production. MDEs could increase bacterial motility proteins, membrane and intracellular structural molecules, two-component system, fragellar assembly, secretion system. Compared to the DSS group, the Exo group samples had more microbial funcition having LDA score higher than 2.0 in the metabolism section. These findings suggested that administration of MDEs not only affected the community compositions of gut microbiota, but also affected the microbial functions as well.
Fig. 3.
LEfSe analysis of microbial functions predicted by PICRUSt at level 3 (LDA > 2)
Discussion
The exact etiology of IBD is unclear, but an abnormal and prolonged inflammatory response to intestinal microbiota might be the cause [15]. There is an urgent need to develop an effective treatment for IBD. Exosomes are vesicular carriers of the large molecules including RNA and proteins and can influence gene expression. These fascinating vesicles are capable of traveling from one cell to another, easily passing their contents across the cell membrane and delivering the macromolecular message in a biologically active form [16]. Milk derived exosomes can be absorbed in the upper intestine and play an important role in the digestive tract developement. Exosomal cargos, such as miRNAS, are involved in gut health by exerting certain physiological functions [17]. Many studies have demonstrated that MDEs can enter the cytoplasma and then release the contents across the basolateral membrane. Oral administration of MDEs can modify the genes which are critical for intestinal barrier function and immune regulation [18]. It was reported that MDEs can promote goblet cell expression, increased villus height, stimulate intestinal epithelial cells’ viability and stem cell activity, among others [19–23]. Lower expression of miRNAs related to intestinal goblet cell differentiation and intestinal epithelium intact was observed in inflammatory intestinal tissues. Many miRNAs, such as miR-148, miR-155, miR-200b as well as miR-146b, were reported to be able to prevent colorectal cancer carcinogenesis, alleviate intestinal inflammation and improve epithelial barrier function [24–26]. Tong et al. reported that the dose amount of 0.6 mg/kg of BW had better biological effects on healthy mice [18]. So this amount was chosen in current study to investigate its effects on gut microbiota of colitis mice.
Several studies have shown that mice with IBD have significantly lower body weight, higher DAI scores, and shortened colonic length [27]. In the present study, it was found that DSS significantly decreased the average daily feed intake (Table 1) and increased DAI scores indicating successful establishment of colitis model. However, oral gavage of MDEs did not improve ADFI as expected. Using healthy mice, oral gavage of MDEs increased the size and surface area of cecum compared to the control group [18]. In current study, administration of MDEs did not affect the cecum length as expected. This might be because the dose amount is low and there is not enough MDEs to positively increase the intestinal length in colitis mice.
The ability of increasing antioxidant capacity by milk derived exosmes has been reported previously [28–30]. Catalase catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species. DSS treatment significantly decreased the CAT levels and milk exosomes did not reverse this effect. This might be due to the low dose used in this study. Total antioxidant capacity is normally used to assess the antioxidant status of biological samples and can evaluate the antioxidant response against the ROS produced in disease. In current study, milk derived exosomes significantly increased the T-AOC levels compared to the DSS group. Imbalance of inflammatory factors is associated with colitis development [31]. TNF-α and IL-6 enhance the migration of granulocytes to the intestinal mucosa, which induces an inflammatory response [32]. IL-10 has anti-inflammatory and anti-allergic effects and inhibits the release of pro-inflammatory cytokines and antigen transmission. IL-4 is a subpopulation of Th2 cells polarized mainly by CD4+ T-cell cytokines secreted by inflammation and can reduce inflammation in epithelial cells to some extent [33]. In current experiment, serum IL-6 were higher in the DSS and Exo groups than in the control group, indicating that DSS can promote inflammation. The serum IL-6 levels were not significantly different between the Exo and the DSS group, indicating that MDEs administered in current dose did not help decrease the IL-6 in serum.
There are trillions of fungi, bacteria and other microorganisms in the animals’ digestive tract. The gut microflora not only have digestive and immune functions, but also play an important role in maintaining homeostasis and promoting metabolism and development of the intestine. Alpha diversity is a common indicator of species abundance in community ecology, and is also a composite indicator of species abundance and evenness, mainly including Chao1, Ace, Shannon and index. In current study, the Shanon index significantly decreased in the DSS group mice. However, no difference between the control group and Exo group was observed, indicating MDEs has a positive regulatory effect on the diversity of the intestinal flora in colitis mice. This is consistent with the results from Tian et al. [34]. Goods coverage is used as an indicator of sequencing depth, and the closer its value is to 1, the more reasonable the sequencing depth is. The basic sequencing depth has covered all species in the samples, and it can be seen from Table 4, indicating that the sequencing depth is sufficient.
Increased or decreased Firmicutes/Bacteroidetes (F/B) ratio is regarded as dysbiosis, whereby the former is usually observed with obesity, and the latter with inflammatory bowel disease (IBD). In current study, the Exo group mice had numerically higher F/B ratio, but not statistically significant. Roseburia can prevent intestinal inflammation and maintain energy homeostasis by producing metabolites-butyric acid [35]. Tong et al. (2020) reported that MDEs can alter the gut microbiota composition and modulate microbial metabolites such as short chain fatty acids [18]. Hans et al. found that the intestinal flora of colitis mice was disturbed, showing an increase in Bacillus and Clostridium [36]. The hypothesis of current study was that oral administration of MDEs would exert a positive effect on the gut microbiota of colitis mice. In current study, DSS siginificantly decreased the relative abundance of Roseburia compared to the control group. By oral administration of MDEs the relative abundance of Roseburia returned back to the same level as observed in the control group, indicating the positive effects of MDEs on regulation of gut microbiota. Lachnospiraceae have the ability to promote health by producing nutrients and energy for host colonic epithelium and maintainning host immune homeostasis [37]. Administration of MDEs also tended to increase the relative abundance of Campylobaterota and Lachnospiraceae_UCG_006. Furthermore, administration of MDEs also affected the microbial functions by regulating the composition of gut microbiota. PICRUSTt functin prediction demonstrated that a higher metabolism capacity of gut microbiota was observed in the Exo group. It was expect that MDEs would exert a dramatic effect on the gut microbiota of the DSS colitis mouse. However, the effects are limited in current study and that may be related to the dose amount used and the length of the administration period.
Conclusions
Administration of MDEs significantly increased the relative abundance of Roseburia and tended to increase the relative abundance of Campylobaterota and Lachnospiraceae_UCG_006. In conclusion, oral gavage of 10 µL MDEs (1 mg/mL) positively affected gut microbiota of DSS-induced colitis mouse. The results provided valuable referecences for application of bovine milk derived exosomes in the prevention and treatment of colitis. However, only one dose amount was investigated in current study. Future studies are needed to further determine the optimum dose amount of MDEs on colitis.
Funding
The financial support from Guangdong Basic and Applied Basic Research Foundation (2019A1515110780), Discipline Construction Program of Foshan University (CGZ0400162), the research start-up fund for Postdoctoral Fellows from Foshan City (BKS209059), the Scientific research start-up fund for high-level talents of Foshan University (Gg07145), the National Natural Science Foundation of China (Grant No. 31902228) were acknowledged.
Availability of Data and Materials
The datasets generated and/or analysed during the current study are available in the NCBI Sequence Read Archive (SRA) repository, [Accession Number: PRJNA824449] https://www.ncbi.nlm.nih.gov/bioproject/PRJNA824449.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics Statement
This experimental protocol was approved by the Ethical Committee and conducted under the supervision of the Institutional Animal Care and Use Committee of Foshan University (Foshan, China).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Caiyu Luo, Tonghao Li and Xiaolin Chen contributed equally to this work.
References
- 1.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Wang L, Li C, Yu Y, Yi Y, Wang J, Chen D. Exosome-induced regulation in inflammatory bowel disease. Front Immunol. 2019;10:1464. doi: 10.3389/fimmu.2019.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteom. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Smith VL, Cheng Y, Bryant BR, Schorey JS. Exosomes function in antigen presentation during an in vivo Mycobacterium tuberculosis infection. Sci Rep. 2017;7:43578. doi: 10.1038/srep43578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arima Y, Liu W, Takahashi Y, Nishikawa M, Takakura Y. Effects of localization of antigen proteins in antigen-loaded exosomes on efficiency of antigen presentation. Mol Pharm. 2019;16:2309–2314. doi: 10.1021/acs.molpharmaceut.8b01093. [DOI] [PubMed] [Google Scholar]
- 7.Shenoda BB, Ajit SK. Modulation of immune responses by exosomes derived from antigen-presenting cells. Clin Med Insights Pathol. 2016;9:1–8. doi: 10.4137/CPath.S39925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol. 2011;17:557–566. doi: 10.3748/wjg.v17.i5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15:630–638. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 10.Lobionda S, Sittipo P, Kwon HY, Lee YK. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms. 2019 doi: 10.3390/microorganisms7080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang C-S, Ban M, Choi E-J, Moon H-G, Jeon J-S, Kim D-K, Park S-K, Jeon SG, Roh T-Y, Myung S-J, Gho YS, Kim JG, Kim Y-K. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE. 2013;8:e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dogan B, Simpson KW. Microflora in Crohn's disease: the emergence of adherent and invasive Escherichia coli. Expert Rev Clin Immunol. 2008;4:133–137. doi: 10.1586/1744666X.4.2.133. [DOI] [PubMed] [Google Scholar]
- 13.Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A, Hutchins E, Mu J, Deng Z, Luo C, Sundaram K, Sriwastva MK, Zhang L, Hsieh M, Reiman R, Haribabu B, Yan J, Jala VR, Miller DM, Van Keuren-Jensen K, Merchant ML, McClain CJ, Park JW, Egilmez NK, Zhang HG. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637–652. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manca S, Upadhyaya B, Mutai E, Desaulniers AT, Cederberg RA, White BR, Zempleni J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep. 2018;8:11321. doi: 10.1038/s41598-018-29780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94:155–165. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Feng X, Chen X, Zheng X, Zhu H, Qi Q, Liu S, Zhang H, Che J. Latest trend of milk derived exosomes: cargos, functions, and applications. Front Nutr. 2021;8:747294. doi: 10.3389/fnut.2021.747294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong L, Hao H, Zhang X, Zhang Z, Lv Y, Zhang L, Yi H. Oral administration of bovine milk-derived extracellular vesicles alters the gut microbiota and enhances intestinal immunity in mice. Mol Nutr Food Res. 2020;64:e1901251. doi: 10.1002/mnfr.201901251. [DOI] [PubMed] [Google Scholar]
- 19.Vella LJ, Hill AF, Cheng L. Focus on extracellular vesicles: exosomes and their role in protein trafficking and biomarker potential in Alzheimer’s and Parkinson’s disease. Int J Mol Sci. 2016 doi: 10.3390/ijms17020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng PC, Chan KY, Leung KT, Tam YH, Ma TP, Lam HS, Cheung HM, Lee KH, To KF, Li K. Comparative MiRNA expressional profiles and molecular networks in human small bowel tissues of necrotizing enterocolitis and spontaneous intestinal perforation. PLoS ONE. 2015;10:e0135737. doi: 10.1371/journal.pone.0135737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao HN, Guo HY, Zhang H, Xie XL, Wen PC, Ren FZ. Yak-milk-derived exosomes promote proliferation of intestinal epithelial cells in an hypoxic environment. J Dairy Sci. 2019;102:985–996. doi: 10.3168/jds.2018-14946. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Hock A, Wu RY, Minich A, Botts SR, Lee C, Antounians L, Miyake H, Koike Y, Chen Y, Zani A, Sherman PM, Pierro A. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE. 2019;14:e0211431. doi: 10.1371/journal.pone.0211431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hock A, Miyake H, Li B, Lee C, Ermini L, Koike Y, Chen Y, Maattanen P, Zani A, Pierro A. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg. 2017;52:755–759. doi: 10.1016/j.jpedsurg.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Melnik BC, Schmitz G. Exosomes of pasteurized milk: potential pathogens of Western diseases. J Transl Med. 2019;17:3. doi: 10.1186/s12967-018-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Xiao Y, Ge W, Zhou K, Wen J, Yan W, Wang Y, Wang B, Qu C, Wu J, Xu L, Cai W. miR-200b inhibits TGF-beta1-induced epithelial-mesenchymal transition and promotes growth of intestinal epithelial cells. Cell Death Dis. 2013;4:e541. doi: 10.1038/cddis.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nata T, Fujiya M, Ueno N, Moriichi K, Konishi H, Tanabe H, Ohtake T, Ikuta K, Kohgo Y. MicroRNA-146b improves intestinal injury in mouse colitis by activating nuclear factor-kappaB and improving epithelial barrier function. J Gene Med. 2013;15:249–260. doi: 10.1002/jgm.2717. [DOI] [PubMed] [Google Scholar]
- 27.Torres J, Mehandru S, Colombel J-F, Peyrin-Biroulet L. Crohn's disease. The Lancet. 2017;389:1741–1755. doi: 10.1016/s0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 28.Xiang X, Chen J, Jiang T, Yan C, Kang Y, Zhang M, Xiang K, Guo J, Jiang G, Wang C, Xiang X, Yang X, Chen Z. Milk-derived exosomes carrying siRNA-KEAP1 promote diabetic wound healing by improving oxidative stress. Drug Deliv Transl Res. 2023 doi: 10.1007/s13346-023-01306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han G, Kim H, Kim DE, Ahn Y, Kim J, Jang YJ, Kim K, Yang Y, Kim SH. The potential of bovine colostrum-derived exosomes to repair aged and damaged skin cells. Pharmaceutics. 2022 doi: 10.3390/pharmaceutics14020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashidi M, Bijari S, Khazaei AH, Shojaei-Ghahrizjani F, Rezakhani L. The role of milk-derived exosomes in the treatment of diseases. Front Genet. 2022;13:1009338. doi: 10.3389/fgene.2022.1009338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ML, Sundrud MS. Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:1157–1167. doi: 10.1097/MIB.0000000000000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oeser K, Maxeiner J, Symowski C, Stassen M, Voehringer D. T cells are the critical source of IL-4/IL-13 in a mouse model of allergic asthma. Allergy. 2015;70:1440–1449. doi: 10.1111/all.12705. [DOI] [PubMed] [Google Scholar]
- 34.Tian M, Li D, Ma C, Feng Y, Hu X, Chen F. Barley leaf insoluble dietary fiber alleviated dextran sulfate sodium-induced mice colitis by modulating gut microbiota. Nutrients. 2021 doi: 10.3390/nu13030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nie K, Ma K, Luo W, Shen Z, Yang Z, Xiao M, Tong T, Yang Y, Wang X. A beneficial gut organism from the discoveries in genus and species. Front Cell Infect Microbiol. 2021;11:757718. doi: 10.3389/fcimb.2021.757718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hans W, Scholmerich J, Gross V, Falk W. The role of the resident intestinal flora in acute and chronic dextran sulfate sodium-induced colitis in mice. Eur J Gastroenterol Hepatol. 2000;12:267–273. doi: 10.1097/00042737-200012030-00002. [DOI] [PubMed] [Google Scholar]
- 37.Shi S, Qi Z, Jiang W, Quan S, Sheng T, Tu J, Shao Y, Qi K. Effects of probiotics on cecal microbiome profile altered by duck Escherichia coli 17 infection in Cherry Valley ducks. Microb Pathog. 2020;138:103849. doi: 10.1016/j.micpath.2019.103849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the NCBI Sequence Read Archive (SRA) repository, [Accession Number: PRJNA824449] https://www.ncbi.nlm.nih.gov/bioproject/PRJNA824449.