Abstract
Objective
The study was aimed to investigate the influence factor between preoperative inflammatory indicators and drainage tube retention time in patients with breast cancer.
Methods
This retrospective study enrolled 121 patients with breast cancer who were undergoing surgery between October 2020 and June 2021. The enumeration data were used the Chi-square test, and the measurement data were used the t-test analysis. The univariate and multivariate logistic regression models were performed to access the risk factors for affecting drainage tube retention time in patients with breast cancer. The receiver operating characteristic curve (ROC) was performed to test the prediction effect of the model.
Results
Through the median extraction time of postoperative drainage tube retention time, all patients were divided into two groups: drainage tube retention time (DTRT) < 13 (d) and drainage tube retention time (DTRT) ≥ 13 (d). The results showed that type of surgery, total lymph nodes (TLN), pathological T stage, NLR were related to the drainage tube retention time (P<0.05). Moreover, the univariate and multivariate logistic regression analysis performed that Hb, type of surgery, pathological T stage, chest wall drainage tube, NRI were the independent risk predictors of affecting drainage tube retention time. Furthermore, a significant correlation existed between NRI and drainage tube retention at different times (P < 0.05).
Conclusion
NRI is an independent risk factor for postoperative drainage tube extraction time and can effectively predict the probability of drainage tube retention time. Thus, it can also provide personalized nursing intervention for patients with breast cancer after drainage tube retention time and the rehabilitation process.
Keywords: breast cancer, inflammatory indicator, drainage tube, operation, nutritional indicator
Introduction
Breast cancer (BC) is the most common public health threat to females across the global.1 In recent decades, the number of patients with breast cancer shows an upward trend with each passing year, and the age of onset inclines to become younger in average age.2 At the moment, the treatment of breast cancer primarily includes surgery, chemotherapy, radiotherapy, targeted therapy, and endocrine therapy.3 The surgical operation of breast cancer comprises mastectomy and breast-conserving surgery. As a result of mastectomy, patients have extensive trauma, and the drainage tubes are routinely placed after operation.4 Nowadays, clinical pathways are commonly designed as a standardized tool for perioperative management of patients with breast cancer who received surgical operations.5 Through the long-term clinical practice and experience of surgical operation in our department, the drainage tube retention time is about two weeks, and the incision cicatrized time is about three weeks, respectively.
It has been found that inflammatory cell and inflammatory response affects tumor proliferation, invasion, metastasis by transforming the tumor immunization microenvironment.6 The common peripheral blood inflammatory factors contain white blood cell (W), lymphocyte (L), monocyte (M), neutrophils (N), platelet (P), C-reactive protein (CRP), and form the derived ratio, for example, neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (dNLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), are used to forecast the prognosis of tumors.7–10
There are many invasive operations during the operation. The large wound and the length of the operations may bring incision infection complications for the patients, and then cause infection in other parts of the body. Moreover, it also affects the treatment effect, and brings serious physical and mental injury to cancer patients.11,12 Drainage tubes are routinely placed after breast cancer surgery. The drainage volume and drainage time after surgical operations will affect postoperative rehabilitation and functional exercise. The purpose of the current study is aimed at the influence factor between preoperative inflammatory indicators and drainage tube retention time in patients with breast cancer, and to provide personalized nursing intervention and recover limb function.
Materials and Methods
Patients’ Selection and Data Collection
From October 2020 to June 2021, this retrospective study involved 121 patients with breast cancer at Cancer Hospital Chinese Academy of Medical Sciences. All patients were female and underwent surgery. After mastectomy and axillary dissection, two drainage tubes were put in vacuum aspiration: one at the chest wall and the other at the axilla. After breast-conserving surgery and axillary dissection, one drainage tube was placed to provide suction under negative pressure at the axilla. Moreover, surgical dressing and elastic bandages were applied to eliminate the dead chambers in the chest wall and axilla. In addition, dressings and elastic bandages were used to remove dead chambers in the chest wall and armpit after the operation. This study was approved by the ethics committee of Cancer Hospital Chinese Academy of Medical Sciences and was conducted in accordance with the amended Declaration of Helsinki. And all enrolled patients signed informed consent.
Inclusion Criteria and Exclusion Criteria
The inclusion criteria were as follows: 1) Patients were diagnosed with breast cancer by pathology; 2) Surgical treatment of unilateral breast; 3) All patients had indwelling drainage tube after the operation, and with chest wall drainage tube and/or axillary drainage tube. The exclusion criteria were as follows: 1) with metastasis or other tumors; 2) without indwelling drainage tube after surgery; 3) complicated with chronic diseases and difficult to control, for instance, hypertension or diabetes mellitus.
Inflammatory Index
We analyzed the common inflammatory parameters, for instance, white blood cell (W), red blood cell (R), hemoglobin (Hb), neutrophils (N), lymphocyte (L), monocyte (M), platelet (P), and the complex index, such as neutrophil-to-lymphocyte ratio (NLR), derived neutrophil-to-lymphocyte ratio (dNLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), systemic inflammation response index (SIRI), systemic immune-inflammation index (SII), prognostic nutritional index (PNI), nutritional risk index (NRI), albumin-to-fibrinogen ratio (AFR), HALP, controlling nutritional status, and breast immune prognostic index (BIPI).
The dNLR was defined as neutrophil count/(white blood cell count – neutrophil count). The systemic inflammation response index (SIRI) was an indicator that combined the neutrophil, monocyte, and lymphocyte and defined as neutrophil count × monocyte count/lymphocyte count. The systemic immune-inflammation index (SII) was an indicator that combined the platelet, neutrophil, and lymphocyte and defined as platelet count × neutrophil count/lymphocyte count. The PNI was defined as albumin level (g/L) + 5×total lymphocyte count (109/L). The NRI was defined as 1.519× albumin level (g/L) + 0.417×(present weight/usual weight×100). The HALP was determined by hemoglobin level (g/L) × albumin level (g/L) × lymphocyte count (109/L)/platelet count (109/L). The controlling nutritional status score (CONUT) based on serum albumin, total cholesterol concentration, and total peripheral lymphocyte counts. The breast immune prognostic index (BIPI) was an indicator that combined the serum lactate dehydrogenase (LDH) level and the derived neutrophil-to-lymphocyte ratio (dNLR).
Statistical Analyses
All statistical analyses were conducted using GraphPad Prism 8.0 software and RStudio software version 3.6.0. The enumeration data were used the Chi-square test, and the measurement data were used the t-test analysis. The univariate and multivariate logistic regression models were performed to access the risk factors for affecting drainage tube retention time in patients with breast cancer. The nomogram was validated predicting the risk for drainage tube retention time. Two-sided P values less than 0.05 were considered statistically significant.
Results
Clinicopathological Characteristics of All Enrolled Patients
One hundred and twenty-one patients with breast cancer who received surgical treatment were selected as the study subjects from October 2020 to June 2021. The median drainage duration of chest wall suction drain (DDCSD) of all patients was 13 days, and the median drainage duration of the axillary suction drain (DDASD) of all patients was 13 days, respectively. Based on the postoperative drainage tube retention time, which included drainage duration of chest wall suction drain (DDCSD) and drainage duration of the axillary suction drain (DDASD), we choose the median DDCSD as the cutoff value for drainage tube retention time. And all cases were divided into two groups: drainage tube retention time (DTRT) <13 (d) and drainage tube retention time (DTRT) ≥13 (d). The results showed that type of surgery, total lymph nodes (TLN), pathological T stage were related to the drainage tube retention time (P < 0.05). All detailed information could be found in Table 1.
Table 1.
Clinicopathological Features of Patients with Breast Cancer in the Present Study
| n | Level | Overall | DTRT<13 (d) | DTRT ≥13 (d) | p |
|---|---|---|---|---|---|
| N=121 | N=67 | N=54 | |||
| Age (median [IQR]) | 46.00 [38.00, 56.00] | 46.00 [38.00, 57.00] | 45.00 [38.25, 55.00] | 0.624 | |
| Weight (median [IQR]) | 60.00 [54.00, 66.00] | 59.00 [52.00, 65.00] | 60.50 [55.00, 66.00] | 0.383 | |
| Height (median [IQR]) | 1.61 [1.58, 1.65] | 1.61 [1.58, 1.65] | 1.62 [1.58, 1.65] | 0.738 | |
| BMI (median [IQR]) | 22.83 [20.58, 25.78] | 21.99 [20.56, 25.86] | 23.21 [21.02, 25.75] | 0.274 | |
| Healthy lower arm circumference (median [IQR]) | 19.00 [17.50, 24.00] | 19.00 [18.00, 23.50] | 19.50 [17.50, 23.88] | 0.863 | |
| Healthy upper arm circumference (median [IQR]) | 26.00 [24.00, 28.00] | 26.00 [24.00, 28.00] | 26.50 [24.00, 28.00] | 0.586 | |
| Affected side lower arm circumference (median [IQR]) | 19.00 [18.00, 23.50] | 19.00 [18.00, 23.25] | 19.00 [17.62, 23.38] | 0.712 | |
| Affected side upper arm circumference (median [IQR]) | 26.00 [24.00, 28.00] | 26.00 [24.00, 28.00] | 26.25 [24.00, 28.00] | 0.615 | |
| Chest wall drainage tube (median [IQR]) | 13.00 [10.00, 18.00] | 10.00 [6.00, 12.00] | 18.00 [15.25, 21.00] | <0.001 | |
| Axillary drainage tube (median [IQR]) | 13.00 [10.00, 17.00] | 12.00 [9.00, 13.00] | 16.00 [13.25, 20.00] | <0.001 | |
| Marital status (%) | Married | 107 (88.4) | 60 (89.6) | 47 (87.0) | 0.592 |
| Unmarried | 8 (6.6) | 5 (7.5) | 3 (5.6) | ||
| Divorce | 5 (4.1) | 2 (3.0) | 3 (5.6) | ||
| Widowed | 1 (0.8) | 0 (0.0) | 1 (1.9) | ||
| Occupation (%) | Mental worker | 101 (83.5) | 57 (85.1) | 44 (81.5) | 0.511 |
| Manual worker | 1 (0.8) | 0 (0.0) | 1 (1.9) | ||
| Others | 19 (15.7) | 10 (14.9) | 9 (16.7) | ||
| Type of surgery (%) | MRM | 31 (25.6) | 13 (19.4) | 18 (33.3) | 0.006 |
| M+SLNB | 33 (27.3) | 23 (34.3) | 10 (18.5) | ||
| BCS+SLNB | 15 (12.4) | 13 (19.4) | 2 (3.7) | ||
| BCS+ALND | 7 (5.8) | 4 (6.0) | 3 (5.6) | ||
| BR | 35 (28.9) | 14 (20.9) | 21 (38.9) | ||
| Tumor size (%) | ≤2cm | 54 (44.6) | 34 (50.7) | 20 (37.0) | 0.306 |
| >2 and <5cm | 58 (47.9) | 29 (43.3) | 29 (53.7) | ||
| ≥5cm | 9 (7.4) | 4 (6.0) | 5 (9.3) | ||
| TLN (median [IQR]) | 7.00 [4.00, 19.00] | 6.00 [3.00, 17.00] | 8.00 [5.00, 22.75] | 0.049 | |
| PLN (median [IQR]) | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.75] | 0.683 | |
| Histologic type (%) | Noninvasive carcinoma | 18 (14.9) | 12 (17.9) | 6 (11.1) | 0.479 |
| Invasive special carcinoma | 5 (4.1) | 2 (3.0) | 3 (5.6) | ||
| Invasive nonspecific carcinoma | 98 (81.0) | 53 (79.1) | 45 (83.3) | ||
| Histologic grade (%) | 0 | 6 (5.0) | 3 (4.5) | 3 (5.6) | 0.628 |
| I | 35 (28.9) | 22 (32.8) | 13 (24.1) | ||
| II | 62 (51.2) | 34 (50.7) | 28 (51.9) | ||
| III | 18 (14.9) | 8 (11.9) | 10 (18.5) | ||
| Pathological T stage (%) | Tis/T0 | 28 (23.1) | 16 (23.9) | 12 (22.2) | 0.050 |
| T1 | 56 (46.3) | 37 (55.2) | 19 (35.2) | ||
| T2 | 36 (29.8) | 14 (20.9) | 22 (40.7) | ||
| T3 | 1 (0.8) | 0 (0.0) | 1 (1.9) | ||
| Pathological N stage (%) | N0 | 82 (67.8) | 47 (70.1) | 35 (64.8) | 0.891 |
| N1 | 22 (18.2) | 12 (17.9) | 10 (18.5) | ||
| N2 | 13 (10.7) | 6 (9.0) | 7 (13.0) | ||
| N3 | 4 (3.3) | 2 (3.0) | 2 (3.7) | ||
| Pathological TNM stage (%) | Tis/T0 | 27 (22.3) | 16 (23.9) | 11 (20.4) | 0.746 |
| I | 40 (33.1) | 24 (35.8) | 16 (29.6) | ||
| II | 37 (30.6) | 19 (28.4) | 18 (33.3) | ||
| III | 17 (14.0) | 8 (11.9) | 9 (16.7) | ||
| Molecular subtype (%) | Luminal A | 38 (31.4) | 25 (37.3) | 13 (24.1) | 0.416 |
| Luminal B HER2+ | 56 (46.3) | 30 (44.8) | 26 (48.1) | ||
| Luminal B HER2- | 18 (14.9) | 9 (13.4) | 9 (16.7) | ||
| HER2 enriched | 4 (3.3) | 1 (1.5) | 3 (5.6) | ||
| Triple negative | 5 (4.1) | 2 (3.0) | 3 (5.6) | ||
| ER status (%) | Negative | 28 (23.1) | 16 (23.9) | 12 (22.2) | 1.000 |
| Positive | 93 (76.9) | 51 (76.1) | 42 (77.8) | ||
| PR status (%) | Negative | 24 (19.8) | 14 (20.9) | 10 (18.5) | 0.923 |
| Positive | 97 (80.2) | 53 (79.1) | 44 (81.5) | ||
| HER2 (%) | Negative | 99 (81.8) | 57 (85.1) | 42 (77.8) | 0.425 |
| Positive | 22 (18.2) | 10 (14.9) | 12 (22.2) | ||
| Ki-67 (%) | Negative | 41 (33.9) | 27 (40.3) | 14 (25.9) | 0.142 |
| Positive | 80 (66.1) | 40 (59.7) | 40 (74.1) | ||
| AR (%) | Negative | 29 (24.0) | 20 (29.9) | 9 (16.7) | 0.140 |
| Positive | 92 (76.0) | 47 (70.1) | 45 (83.3) | ||
| CK5/6 (%) | Negative | 114 (94.2) | 62 (92.5) | 52 (96.3) | 0.625 |
| Positive | 7 (5.8) | 5 (7.5) | 2 (3.7) | ||
| E-cad (%) | Negative | 35 (28.9) | 22 (32.8) | 13 (24.1) | 0.393 |
| Positive | 86 (71.1) | 45 (67.2) | 41 (75.9) | ||
| EGFR (%) | Negative | 104 (86.0) | 56 (83.6) | 48 (88.9) | 0.567 |
| Positive | 17 (14.0) | 11 (16.4) | 6 (11.1) | ||
| P53 (%) | Negative | 50 (41.3) | 30 (44.8) | 20 (37.0) | 0.500 |
| Positive | 71 (58.7) | 37 (55.2) | 34 (63.0) | ||
| TOP2A (%) | Negative | 53 (43.8) | 35 (52.2) | 18 (33.3) | 0.058 |
| Positive | 68 (56.2) | 32 (47.8) | 36 (66.7) | ||
| Lymph vessel invasion (%) | Negative | 99 (81.8) | 55 (82.1) | 44 (81.5) | 1.000 |
| Positive | 22 (18.2) | 12 (17.9) | 10 (18.5) | ||
| Neural invasion (%) | Negative | 104 (86.0) | 57 (85.1) | 47 (87.0) | 0.964 |
| Positive | 17 (14.0) | 10 (14.9) | 7 (13.0) |
Abbreviations: BMI, body mass index; TLN, Total lymph nodes; PLN, Positive lymph nodes; ER, estrogen receptor; PR, progesterone receptor; HER2, Human Epidermal Growth Factor Receptor 2; AR, androgen receptor; CK5/6, Cytokeratin 5/6; E-cad, E-cadherin; EGFR, Epidermal Growth Factor Receptor; TOP2A, topoisomerase 2A.
The Effect of the Drainage Tube Retention Time for Inflammatory Parameters
The common inflammatory cells included LDH, ALB, CRP, CHOL, FIB, W, R, Hb, N, L, M, P; and the complex inflammatory index, for instance, NLR, dNLR, MLR, PLR, SIRI, SII, PNI, NRI, AFR, HALP; and the complex inflammatory scores, such as CONUT and BIPI. The cutoff values of inflammatory cells and complex inflammatory index or scores were determined by the ROC curve. Moreover, Figures S1 and S2 were developed to show the ROC curves for these complex inflammatory indexes or scores. Table 2 illustrates the comparison of preoperative clinical inflammatory evaluation indexes between the two groups. The results indicated that NRI was associated with drainage tube retention time (P<0.05).
Table 2.
Comparison of Preoperative Inflammatory Parameters with the Drainage Tube Retention Time
| n | Level | Overall | DTRT<13 (d) | DTRT ≥13 (d) | p |
|---|---|---|---|---|---|
| N=121 | N=67 | N=54 | |||
| LDH (median [IQR]) | 158.10 [141.10, 180.00] | 158.10 [141.05, 178.00] | 158.55 [141.85, 181.88] | 0.741 | |
| ALB (median [IQR]) | 44.40 [42.80, 46.60] | 43.90 [42.80, 46.35] | 45.30 [42.85, 46.98] | 0.370 | |
| CRP (median [IQR]) | 0.02 [0.00, 0.08] | 0.03 [0.00, 0.08] | 0.02 [0.00, 0.08] | 0.688 | |
| CHOL (median [IQR]) | 4.83 [4.25, 5.53] | 4.80 [4.24, 5.68] | 4.86 [4.30, 5.46] | 0.942 | |
| FIB (median [IQR]) | 2.73 [2.46, 3.08] | 2.77 [2.48, 3.04] | 2.65 [2.28, 3.10] | 0.289 | |
| W (median [IQR]) | 5.68 [5.02, 6.94] | 5.64 [5.03, 6.50] | 5.84 [5.02, 7.08] | 0.406 | |
| R (median [IQR]) | 4.45 [4.21, 4.69] | 4.54 [4.28, 4.70] | 4.39 [4.08, 4.69] | 0.194 | |
| Hb (median [IQR]) | 133.00 [128.00, 140.00] | 135.00 [129.50, 140.00] | 132.00 [122.25, 139.00] | 0.057 | |
| N (median [IQR]) | 3.56 [2.87, 4.46] | 3.56 [2.89, 4.37] | 3.57 [2.84, 4.78] | 0.778 | |
| L (median [IQR]) | 1.69 [1.37, 2.15] | 1.69 [1.38, 2.15] | 1.70 [1.36, 2.14] | 0.956 | |
| M (median [IQR]) | 0.30 [0.24, 0.36] | 0.29 [0.24, 0.36] | 0.30 [0.25, 0.36] | 0.444 | |
| P (median [IQR]) | 252.00 [208.00, 283.00] | 245.00 [209.00, 284.00] | 255.50 [208.00, 282.75] | 0.802 | |
| NLR (%) | <1.86 | 44 (36.4) | 22 (32.8) | 22 (40.7) | 0.479 |
| ≥1.86 | 77 (63.6) | 45 (67.2) | 32 (59.3) | ||
| dNLR (%) | <1.48 | 45 (37.2) | 22 (32.8) | 23 (42.6) | 0.360 |
| ≥1.48 | 76 (62.8) | 45 (67.2) | 31 (57.4) | ||
| MLR (%) | <0.12 | 24 (19.8) | 14 (20.9) | 10 (18.5) | 0.923 |
| ≥0.12 | 97 (80.2) | 53 (79.1) | 44 (81.5) | ||
| PLR (%) | <159.3 | 77 (63.6) | 40 (59.7) | 37 (68.5) | 0.417 |
| ≥159.3 | 44 (36.4) | 27 (40.3) | 17 (31.5) | ||
| SIRI (%) | <0.63 | 62 (51.2) | 38 (56.7) | 24 (44.4) | 0.246 |
| ≥0.63 | 59 (48.8) | 29 (43.3) | 30 (55.6) | ||
| SII (%) | <262.5 | 14 (11.6) | 5 (7.5) | 9 (16.7) | 0.198 |
| ≥262.5 | 107 (88.4) | 62 (92.5) | 45 (83.3) | ||
| PNI (%) | <55.58 | 88 (72.7) | 53 (79.1) | 35 (64.8) | 0.121 |
| ≥55.58 | 33 (27.3) | 14 (20.9) | 19 (35.2) | ||
| NRI (%) | <109.7 | 44 (36.4) | 31 (46.3) | 13 (24.1) | 0.020 |
| ≥109.7 | 77 (63.6) | 36 (53.7) | 41 (75.9) | ||
| AFR (%) | <17.36 | 78 (64.5) | 48 (71.6) | 30 (55.6) | 0.100 |
| ≥17.36 | 43 (35.5) | 19 (28.4) | 24 (44.4) | ||
| HALP (%) | <34.87 | 44 (36.4) | 27 (40.3) | 17 (31.5) | 0.417 |
| ≥34.87 | 77 (63.6) | 40 (59.7) | 37 (68.5) | ||
| CONUT (%) | <1 | 69 (57.0) | 40 (59.7) | 29 (53.7) | 0.633 |
| ≥1 | 52 (43.0) | 27 (40.3) | 25 (46.3) | ||
| BIPI (%) | Good | 108 (89.3) | 61 (91.0) | 47 (87.0) | 0.412 |
| Intermediate | 12 (9.9) | 5 (7.5) | 7 (13.0) | ||
| Poor | 1 (0.8) | 1 (1.5) | 0 (0.0) |
Abbreviations: LDH, lactic dehydrogenase; ALB, albumin; CRP, C-reactive protein; CHOL, cholesterol; FIB, fibrinogen; W, white blood cell; R, red blood cell; Hb, hemoglobin; N, neutrophils; L, lymphocyte; M, monocyte; P, platelet; NLR, neutrophil-to-lymphocyte ratio; dNLR, derived neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SIRI, systemic inflammation response index; SII, systemic immune-inflammation index; PNI, prognostic nutritional index; NRI, nutritional risk index; AFR, albumin-to-fibrinogen ratio; HALP, hemoglobin × albumin × lymphocyte / platelet; CONUT, controlling nutritional status; BIPI, breast immune prognostic index.
Univariate and Multivariate Logistic Regression Analysis of Factors Affecting Drainage Tube Retention Time in Patients with Breast Cancer
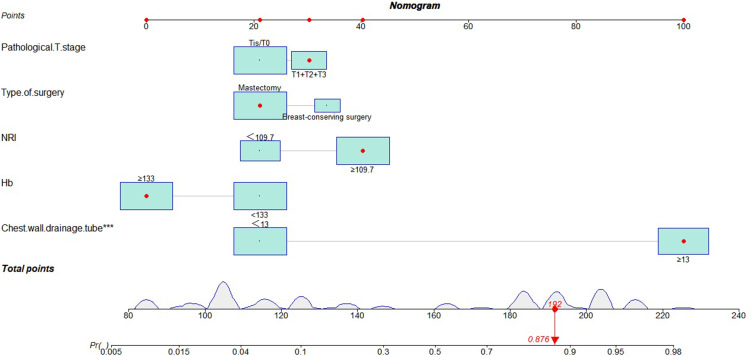
According to the univariate and multivariate logistic regression analysis, the results showed that Hb, type of surgery, pathological T stage, chest wall drainage tube, NRI were independent predictors of affecting drainage tube retention time in patients with breast cancer (Table 3). A nomogram was constructed based on the results of the multivariate Logistic regression analysis to predict the risk for drainage tube retention time in patients with breast cancer (Figure 1).
Table 3.
Univariate and Multivariate Logistic Regression Analysis of Factors Affecting Drainage Tube Retention Time in Patients with Breast Cancer
| n | Level | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI low | 95% CI high | p | OR | 95% CI low | 95% CI high | p | ||
| Age | <46 | 1(Reference) | 0.935 | ||||||
| ≥46 | 0.971 | 0.473 | 1.991 | ||||||
| Marital status | Married | 1(Reference) | 0.668 | ||||||
| Unmarried + Divorce + Widowed | 1.277 | 0.410 | 3.974 | ||||||
| Occupation | Mental worker | 1(Reference) | 0.597 | ||||||
| Manual worker + Others | 1.295 | 0.491 | 3.425 | ||||||
| Weight | <60 | 1(Reference) | 0.372 | ||||||
| ≥60 | 1.389 | 0.677 | 2.875 | ||||||
| Height | <1.61 | 1(Reference) | 0.837 | ||||||
| ≥1.61 | 0.927 | 0.451 | 1.905 | ||||||
| BMI | <22.83 | 1(Reference) | 0.082 | ||||||
| ≥22.83 | 1.906 | 0.926 | 3.980 | ||||||
| LDH | <158.10 | 1(Reference) | 0.935 | ||||||
| ≥158.10 | 0.971 | 0.473 | 1.991 | ||||||
| ALB | <44.40 | 1(Reference) | 0.168 | ||||||
| ≥44.40 | 1.662 | 0.810 | 3.453 | ||||||
| CRP | <0.02 | 1(Reference) | 0.627 | ||||||
| ≥0.02 | 0.837 | 0.406 | 1.715 | ||||||
| CHOL | <4.83 | 1(Reference) | 0.776 | ||||||
| ≥4.83 | 1.110 | 0.541 | 2.280 | ||||||
| FIB | <2.73 | 1(Reference) | 0.329 | ||||||
| ≥2.73 | 0.699 | 0.338 | 1.433 | ||||||
| W | <5.68 | 1(Reference) | 0.516 | ||||||
| ≥5.68 | 1.269 | 0.619 | 2.614 | ||||||
| R | <4.45 | 1(Reference) | 0.181 | ||||||
| ≥4.45 | 0.611 | 0.294 | 1.253 | ||||||
| Hb | <133 | 1(Reference) | 0.036 | 1(Reference) | 0.035 | ||||
| ≥133 | 0.457 | 0.217 | 0.943 | 0.401 | 0.168 | 0.926 | |||
| N | <3.56 | 1(Reference) | 0.966 | ||||||
| ≥3.56 | 0.985 | 0.480 | 2.022 | ||||||
| L | <1.69 | 1(Reference) | 0.904 | ||||||
| ≥1.69 | 1.045 | 0.510 | 2.147 | ||||||
| M | <0.30 | 1(Reference) | 0.144 | ||||||
| ≥0.30 | 1.719 | 0.834 | 3.591 | ||||||
| P | <252 | 1(Reference) | 0.776 | ||||||
| ≥252 | 1.110 | 0.541 | 2.280 | ||||||
| Type of surgery | Mastectomy | 1(Reference) | 0.019 | 1(Reference) | 0.040 | ||||
| Breast-conserving surgery | 0.278 | 0.086 | 0.759 | 0.273 | 0.071 | 0.883 | |||
| Tumor size | <2 | 1(Reference) | 0.133 | ||||||
| ≥2 | 1.752 | 0.848 | 3.676 | ||||||
| Histologic type | Ductal | 1(Reference) | 0.556 | ||||||
| Lobular + Others | 1.321 | 0.529 | 3.441 | ||||||
| Histologic grade | 0+I | 1(Reference) | 0.376 | ||||||
| II+III | 1.414 | 0.661 | 3.079 | ||||||
| Pathological T stage | Tis/T0 | 1(Reference) | 0.011 | 1(Reference) | 0.039 | ||||
| T1+T2+T3 | 2.809 | 1.278 | 6.363 | 6.684 | 1.155 | 44.961 | |||
| Pathological N stage | 1 | 1(Reference) | 0.533 | ||||||
| 2 | 1.276 | 0.592 | 2.752 | ||||||
| Pathological TNM stage | 1 | 1(Reference) | 0.287 | ||||||
| 2 | 1.481 | 0.720 | 3.070 | ||||||
| TLN | <7 | 1(Reference) | 0.394 | ||||||
| ≥7 | 1.367 | 0.667 | 2.824 | ||||||
| PLN | <1 | 1(Reference) | 0.816 | ||||||
| ≥1 | 1.095 | 0.506 | 2.358 | ||||||
| Molecular subtype | Luminal A + Luminal B (HER2+) + Luminal B (HER2-) | 1(Reference) | 0.181 | ||||||
| HER2 enriched + Triple negative | 2.667 | 0.668 | 13.140 | ||||||
| ER status | Negative | 1(Reference) | 0.830 | ||||||
| Positive | 1.098 | 0.470 | 2.618 | ||||||
| PR status | Negative | 1(Reference) | 0.745 | ||||||
| Positive | 1.162 | 0.473 | 2.939 | ||||||
| HER2 | Negative | 1(Reference) | 0.304 | ||||||
| Positive | 1.629 | 0.643 | 4.203 | ||||||
| Ki-67 | Negative | 1(Reference) | 0.099 | ||||||
| Positive | 1.929 | 0.894 | 4.290 | ||||||
| AR | Negative | 1(Reference) | 0.095 | ||||||
| Positive | 2.128 | 0.897 | 5.372 | ||||||
| CK5/6 | Negative | 1(Reference) | 0.388 | ||||||
| Positive | 0.477 | 0.066 | 2.313 | ||||||
| E-cad | Negative | 1(Reference) | 0.292 | ||||||
| Positive | 1.542 | 0.696 | 3.518 | ||||||
| EGFR | Negative | 1(Reference) | 0.406 | ||||||
| Positive | 0.636 | 0.206 | 1.803 | ||||||
| P53 | Negative | 1(Reference) | 0.391 | ||||||
| Positive | 1.378 | 0.665 | 2.892 | ||||||
| TOP2A | Negative | 1(Reference) | 0.039 | 1(Reference) | 0.226 | ||||
| Positive | 2.188 | 1.051 | 4.656 | 1.945 | 0.672 | 5.920 | |||
| Lymph vessel invasion | Negative | 1(Reference) | 0.931 | ||||||
| Positive | 1.042 | 0.404 | 2.638 | ||||||
| Neural invasion | Negative | 1(Reference) | 0.758 | ||||||
| Positive | 0.849 | 0.288 | 2.382 | ||||||
| Healthy lower arm circumference | <19 | 1(Reference) | 0.655 | ||||||
| ≥19 | 1.178 | 0.575 | 2.423 | ||||||
| Healthy upper arm circumference | <26 | 1(Reference) | 0.329 | ||||||
| ≥26 | 1.431 | 0.698 | 2.955 | ||||||
| Affected side lower arm circumference | <19 | 1(Reference) | 0.966 | ||||||
| ≥19 | 1.016 | 0.495 | 2.085 | ||||||
| Affected side upper arm circumference | <26 | 1(Reference) | 0.368 | ||||||
| ≥26 | 1.393 | 0.678 | 2.879 | ||||||
| Chest wall drainage tube | <13 | 1(Reference) | 0.000 | 1(Reference) | 0.000 | ||||
| ≥13 | 92.188 | 29.083 | 374.069 | 127.427 | 25.938 | 1139.723 | |||
| Axillary drainage tube | <13 | 1(Reference) | 0.000 | 1(Reference) | 0.480 | ||||
| ≥13 | 5.298 | 2.441 | 12.090 | 1.872 | 0.311 | 11.822 | |||
| NLR | <1.86 | 1(Reference) | 0.370 | ||||||
| ≥1.86 | 0.711 | 0.336 | 1.498 | ||||||
| dNLR | <1.48 | 1(Reference) | 0.271 | ||||||
| ≥1.48 | 0.659 | 0.312 | 1.383 | ||||||
| MLR | <0.12 | 1(Reference) | 0.745 | ||||||
| ≥0.12 | 1.162 | 0.473 | 2.939 | ||||||
| PLR | <159.3 | 1(Reference) | 0.317 | ||||||
| ≥159.3 | 0.681 | 0.317 | 1.438 | ||||||
| SIRI | <0.63 | 1(Reference) | 0.181 | ||||||
| ≥0.63 | 1.638 | 0.798 | 3.398 | ||||||
| SII | <262.5 | 1(Reference) | 0.124 | ||||||
| ≥262.5 | 0.403 | 0.117 | 1.248 | ||||||
| PNI | <55.58 | 1(Reference) | 0.082 | ||||||
| ≥55.58 | 2.055 | 0.918 | 4.698 | ||||||
| NRI | <109.7 | 1(Reference) | 0.013 | 1(Reference) | 0.025 | ||||
| ≥109.7 | 2.716 | 1.256 | 6.116 | 2.837 | 1.164 | 7.292 | |||
| AFR | <17.36 | 1(Reference) | 0.068 | ||||||
| ≥17.36 | 2.021 | 0.954 | 4.345 | ||||||
| HALP | <34.87 | 1(Reference) | 0.317 | ||||||
| ≥34.87 | 1.469 | 0.695 | 3.159 | ||||||
| CONUT | <1 | 1(Reference) | 0.508 | ||||||
| ≥1 | 1.277 | 0.619 | 2.645 | ||||||
| BIPI | Good | 1(Reference) | 0.481 | ||||||
| Intermediate + Poor | 1.514 | 0.473 | 4.990 | ||||||
Abbreviations: BMI, body mass index; LDH, lactic dehydrogenase; ALB, albumin; CRP, C-reactive protein; CHOL, cholesterol; FIB, fibrinogen; W, white blood cell; R, red blood cell; Hb, hemoglobin; N, neutrophils; L, lymphocyte; M, monocyte; P, platelet; TLN, Total lymph nodes; PLN, Positive lymph nodes; ER, estrogen receptor; PR, progesterone receptor; HER2, Human Epidermal Growth Factor Receptor 2; AR, androgen receptor; CK5/6, Cytokeratin 5/6; E-cad, E-cadherin; EGFR, Epidermal Growth Factor Receptor; TOP2A, topoisomerase 2A; NLR, neutrophil-to-lymphocyte ratio; dNLR, derived neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SIRI, systemic inflammation response index; SII, systemic immune-inflammation index; PNI, prognostic nutritional index; NRI, nutritional risk index; AFR, albumin-to-fibrinogen ratio; HALP, hemoglobin × albumin × lymphocyte / platelet; CONUT, controlling nutritional status; BIPI, breast immune prognostic index.
Figure 1.
Nomogram constructed by the multivariate Logistic regression analysis. ***Statistical significance of P value.
The Useful Factors by Univariate Logistic Regression Analysis for Drainage Tube at Different Times
Through the univariate Logistic regression analysis, the results indicated that Hb, type of surgery, pathological T stage, TOP2A, chest wall drainage tube, axillary drainage tube, NRI were independent predictors of affecting drainage tube retention time in patients with breast cancer. There were significant differences in the type of surgery, pathological T stage, chest wall drainage tube, NRI by removing the drainage tube at different times (Table 4).
Table 4.
The Useful Factors by Univariate Logistic Regression Analysis for Drainage Tube at Different Times
| n | Level | Overall | DTRT≤7 (d) | DTRT≤14 (d) | DTRT≤21 (d) | DTRT>21 (d) | p |
|---|---|---|---|---|---|---|---|
| 121 | 24 | 48 | 38 | 11 | |||
| Hb (%) | <133 | 61 (50.4) | 10 (41.7) | 21 (43.8) | 23 (60.5) | 7 (63.6) | 0.271 |
| ≥133 | 60 (49.6) | 14 (58.3) | 27 (56.2) | 15 (39.5) | 4 (36.4) | ||
| Type of surgery (%) | Mastectomy | 98 (81.0) | 10 (41.7) | 44 (91.7) | 34 (89.5) | 10 (90.9) | <0.001 |
| Breast-conserving surgery | 23 (19.0) | 14 (58.3) | 4 (8.3) | 4 (10.5) | 1 (9.1) | ||
| Pathological T stage (%) | Tis/T0 | 84 (69.4) | 20 (83.3) | 37 (77.1) | 21 (55.3) | 6 (54.5) | 0.041 |
| T1+T2+T3 | 37 (30.6) | 4 (16.7) | 11 (22.9) | 17 (44.7) | 5 (45.5) | ||
| TOP2A (%) | Negative | 53 (43.8) | 13 (54.2) | 23 (47.9) | 14 (36.8) | 3 (27.3) | 0.341 |
| Positive | 68 (56.2) | 11 (45.8) | 25 (52.1) | 24 (63.2) | 8 (72.7) | ||
| Chest wall drainage tube (%) | <13 | 63 (52.1) | 18 (75.0) | 41 (85.4) | 4 (10.5) | 0 (0.0) | <0.001 |
| ≥13 | 58 (47.9) | 6 (25.0) | 7 (14.6) | 34 (89.5) | 11 (100.0) | ||
| Axillary drainage tube (%) | <13 | 70 (57.9) | 12 (50.0) | 31 (64.6) | 20 (52.6) | 7 (63.6) | 0.557 |
| ≥13 | 51 (42.1) | 12 (50.0) | 17 (35.4) | 18 (47.4) | 4 (36.4) | ||
| NRI (%) | <109.7 | 44 (36.4) | 13 (54.2) | 19 (39.6) | 11 (28.9) | 1 (9.1) | 0.047 |
| ≥109.7 | 77 (63.6) | 11 (45.8) | 29 (60.4) | 27 (71.1) | 10 (90.9) |
Abbreviations: Hb, hemoglobin; TOP2A, topoisomerase 2A; NRI, nutritional risk index.
Discussion
After the operation, the complications of breast cancer mainly include subcutaneous effusion, wound infection, delayed healing, bleeding, and skin flap necrosis.13,14 The subcutaneous effusion and wound infection cause a huge gap among the wounds, the flap and the surface of the wound cannot be appressed effectively. This will affect the quality of life and survival time of breast cancer patients. The drainage tubes are usually to prevent the complications of subcutaneous effusion and infection. The drainage tube removal time is determined on the basis of drainage volume and color, and the postoperative recovery condition. The preoperative and postoperative nursing care of breast cancer patients is a comprehensive procedure. The drainage tube retention time and nutritional status may influence the wound healing time, and this may be related to the poor nutritional status and immune function after the operation. It is very critical to control basic diseases (diabetes mellitus, hypertension), strengthen nutritional support, and enhance immunity.
Inflammation is a critical component of the tumor microenvironment (TME) and is an indispensable participant in the development, progression, and metastasis of cancer.15 The TME is largely orchestrated by inflammatory cells and also selected some signal molecules of the innate immune system.16 It is an attractive strategy to make a profound study of inflammation for cancer prevention and treatment. A number of reports have confirmed that in many types of cancers, for instance, digestive tract cancers, lung cancer, liver cancer, and breast cancer, the inflammatory reactions are abnormal, and related to the prognosis of tumors.17–24 Yet, the relationship between the duration of suction drainage and the inflammatory reactions has been rarely studied.
This study systematically analyzed the effects of common inflammatory cells and the composite inflammatory indexes on the postoperative drainage tube extraction time. Our drainage tube retention time is determined by the median DDCSD time. The results showed that the type of surgery, total lymph nodes, pathological T stage, NRI were related to the drainage tube retention time. Moreover, the univariate and multivariate Logistic regression analysis performed that Hb, type of surgery, pathological T stage, chest wall drainage tube, NRI were the independent risk predictors of affecting drainage tube retention time. Furthermore, we also found that a significant correlation existed between NRI and drainage tube retention at different times. These results go a step further to suggest that NRI is an important risk factor affecting drainage tube retention time and emphasizes the major impact of nutrition on breast cancer patients.
The NRI is a clinical biological index that combines the strength of two nutritional indicators-albumin and weight loss.25 Serum albumin is an important indicator of nutritional status and immunological functioning. The albumin level decreases upon the occurrence of inflammatory reactions. Albumin can be used as a non-specific host defense substance and used to fight against various toxic metabolites during infection, so as to reduce the harmful substances in the body.26,27 The doctors can identify patients who are more likely to retain suction drainage by the serum albumin levels and take early interventions. Obesity has been proved to be a risk factor for postoperative recovery for cancer patients.28 Owing to the special anatomical position of the breast, the use of high-frequency electric knives in operation leads to the liquefaction of adipose tissue for obese women with rich subcutaneous adipose tissue. After the operation, it is easy to have an insufficient level of blood supply, which could result in subcutaneous effusion, and subsequent delays in the extraction time of the drainage tube. The blockage of the drainage tube by adipose tissue leads to the flap stays in a free state. The flap cannot establish a normal blood supply with the thorax, which further leads to flap necrosis.
Observing the nature of drainage fluid is an important part of nursing after breast surgery. The drainage volume and color are ideal clinical observation indicators that can reflect the incision exudation. The drainage tube retention time can be determined by the flow and the color of the drainage tube decreasing. At the same time, it can also assist clinicians to understand the condition of the healing of the incision, so as to formulate the next diagnosis and treatment plan. Negative pressure drainage can form continuous negative pressure, make the wound cavity narrow, and then make the flap close to the chest wall and armpit. On the one hand, continuous negative pressure suction can promote the formation of the capillary, provide sufficient blood supply and establish blood circulation.29 On the other hand, continuous negative pressure suction can reduce skin tension, promote wound healing, and prevent flap necrosis.29 At the same time, continuous negative pressure suction can also effectively prevent the spread of bacteria into the incision, and prevent the spread of bacteria into the incision.30
The difficulty in predicting the drainage tube retention time in these patients poses an uncertainty that may complicate the development of a suitable clinical pathway. This study had some limitations. Firstly, this study had insufficient data, and bring about a bias. Secondly, the patients are from a single research site, which means that the findings of our study may not be applicable to other research contexts. Furthermore, follow-up data are not available.
Conclusions
The preoperative inflammatory indicators are related to the drainage tube retention time in patients with breast cancer. NRI is an independent risk factor for postoperative drainage tube extraction time and can provide personalized nursing intervention of patients with breast cancer after drainage tube retention time and rehabilitation process.
Funding Statement
This research was supported by grants from the Hubei Province Postdoctoral Innovation Research Post Fund Project (No.0106540096), Open Fund for the Key Laboratory of Organ Transplantation of Ministry of Education and National Health Commission (No.2021QYKF03), Tongji Hospital Cultivation Project (No.2022B03), Chen Xiao-ping Foundation for the Development of Science and Technology of Hubei province, Youth Science Special Fund (No.CXPJJH123001-2308).
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author (Li Chen) upon reasonable request.
Ethics Statement
This study was approved by the ethics committee of Cancer Hospital Chinese Academy of Medical Sciences and was conducted in accordance with the amended Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol. 2019;12(1):140. PMID: 31864424; PMCID: PMC6925497. doi: 10.1186/s13045-019-0828-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radecka B, Litwiniuk M. Breast cancer in young women. Ginekol Pol. 2016;87(9):659–663. PMID: 27723074. doi: 10.5603/GP.2016.0062 [DOI] [PubMed] [Google Scholar]
- 3.Shien T, Iwata H. Adjuvant and neoadjuvant therapy for breast cancer. Jpn J Clin Oncol. 2020;50(3):225–229. PMID: 32147701. doi: 10.1093/jjco/hyz213 [DOI] [PubMed] [Google Scholar]
- 4.Chen CF, Lin SF, Hung CF, Chou P. Risk of infection is associated more with drain duration than daily drainage volume in prosthesis-based breast reconstruction: a cohort study. Medicine. 2016;95(49):e5605. PMID: 27930584; PMCID: PMC5266056. doi: 10.1097/MD.0000000000005605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Héquet D, Huchon C, Baffert S, et al. Preoperative clinical pathway of breast cancer patients: determinants of compliance with EUSOMA quality indicators. Br J Cancer. 2017;116(11):1394–1401. PMID: 28441385; PMCID: PMC5520093. doi: 10.1038/bjc.2017.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. PMID: 18650914. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 7.Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis--tracing the accessory. Oncogene. 2014;33(25):3217–3224. Epub 2013 Jul 15. PMID: 23851506. doi: 10.1038/onc.2013.272 [DOI] [PubMed] [Google Scholar]
- 8.Dong X, Liu C, Yuan J, et al. Prognostic roles of neutrophil-to-lymphocyte ratio and stromal tumor-infiltrating lymphocytes and their relationship in locally advanced triple-negative breast cancer treated with neoadjuvant chemotherapy. Breast Care. 2021;16(4):328–334. PMID: 34602938; PMCID: PMC8436630. doi: 10.1159/000509498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison L, Laukkanen JA, Ronkainen K, Kurl S, Kauhanen J, Toriola AT. Inflammatory biomarker score and cancer: a population-based prospective cohort study. BMC Cancer. 2016;16:80. PMID: 26860264; PMCID: PMC4748611. doi: 10.1186/s12885-016-2115-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M, Chen R, Liu L, et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: the Dongfeng-Tongji cohort study. Atherosclerosis. 2021;323:20–29. PMID: 33773161. doi: 10.1016/j.atherosclerosis.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 11.Browne JP, Jeevan R, Gulliver-Clarke C, Pereira J, Caddy CM, van der Meulen JHP. The association between complications and quality of life after mastectomy and breast reconstruction for breast cancer. Cancer. 2017;123(18):3460–3467. Epub 2017 May 17. PMID: 28513834. doi: 10.1002/cncr.30788 [DOI] [PubMed] [Google Scholar]
- 12.Yfantis A, Sarafis P, Moisoglou I, et al. How breast cancer treatments affect the quality of life of women with non-metastatic breast cancer one year after surgical treatment: a cross-sectional study in Greece. BMC Surg. 2020;20(1):210. PMID: 32957940; PMCID: PMC7507267. doi: 10.1186/s12893-020-00871-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Han X. Clinical significance of breast-conserving surgery for early breast cancer and its impact on patient life quality of life. J BUON. 2019;24(5):1898–1904. PMID: 31786853. [PubMed] [Google Scholar]
- 14.Palve JS, Luukkaala TH, Kääriäinen MT. Predictive risk factors of complications in different breast reconstruction methods. Breast Cancer Res Treat. 2020;182(2):345–354. Epub 2020 May 28. PMID: 32468337; PMCID: PMC7297836. doi: 10.1007/s10549-020-05705-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. PMID: 12490959; PMCID: PMC2803035. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D, Zhang X, Li Z, Zhu B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics. 2021;11(3):1016–1030. PMID: 33391518; PMCID: PMC7738889. doi: 10.7150/thno.51777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Shao Y, Bai L, Zhou X. Increased derived neutrophil-to-lymphocyte ratio and breast imaging-reporting and data system classification predict poor survival in patients with non-distant metastatic HER2+ breast cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. 2018;10:3841–3847. PMID: 30288115; PMCID: PMC6161733. doi: 10.2147/CMAR.S174537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Pretreatment systemic inflammation response index in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Cancer Manag Res. 2020;12:1543–1567. PMID: 32184659; PMCID: PMC7060771. doi: 10.2147/CMAR.S235519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Sun Y, Zhang Q. Prognostic value of the systemic immune-inflammation index in patients with breast cancer: a meta-analysis. Cancer Cell Int. 2020;20:224. PMID: 32528232; PMCID: PMC7282128. doi: 10.1186/s12935-020-01308-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua X, Long ZQ, Huang X, et al. The Value of Prognostic Nutritional Index (PNI) in predicting survival and guiding radiotherapy of patients with T1-2N1 breast cancer. Front Oncol. 2020;9:1562. PMID: 32083015; PMCID: PMC7002465. doi: 10.3389/fonc.2019.01562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki M, Miyoshi N, Fujino S, et al. The geriatric nutritional risk index predicts postoperative complications and prognosis in elderly patients with colorectal cancer after curative surgery. Sci Rep. 2020;10(1):10744. PMID: 32612136; PMCID: PMC7329855. doi: 10.1038/s41598-020-67285-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Mo Z, Zhang L, Qin S, Qin S, Li S. Diagnostic value of albumin to fibrinogen ratio in cervical cancer. Int J Biol Markers. 2020;35(2):66–73. Epub 2020 May 11. PMID: 32389031. doi: 10.1177/1724600820915916 [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Shi D, Zhang J, et al. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) score is a novel significant prognostic factor for patients with metastatic prostate cancer undergoing cytoreductive radical prostatectomy. J Cancer. 2019;10(1):81–91. PMID: 30662528; PMCID: PMC6329846. doi: 10.7150/jca.27210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahiko Y, Shida D, Horie T, et al. Controlling nutritional status (CONUT) score as a preoperative risk assessment index for older patients with colorectal cancer. BMC Cancer. 2019;19(1):946. PMID: 31690275; PMCID: PMC6833132. doi: 10.1186/s12885-019-6218-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagou K, Ozeki K, Ukai S, Adachi Y, Fukushima N, Kohno A. Impact of a nutritional risk index on clinical outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(11):2287–2296. Epub 2019 Jul 5. PMID: 31284071. doi: 10.1016/j.bbmt.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 26.Van de Sande L, Cosyns S, Willaert W, Ceelen W. Albumin-based cancer therapeutics for intraperitoneal drug delivery: a review. Drug Deliv. 2020;27(1):40–53. PMID: 31858848; PMCID: PMC6968566. doi: 10.1080/10717544.2019.1704945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev. 2018;130:73–89. Epub 2018 Jul 27. PMID: 30012492; PMCID: PMC6200408. doi: 10.1016/j.addr.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397. Epub 2017 Aug 1. PMID: 28763097; PMCID: PMC5591063. doi: 10.3322/caac.21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin W, Yang Y, Zhong W, et al. The effect of low and high vacuum drainage on the postoperative drainage of breast cancer: insights from a prospective, non-inferiority, randomized clinical trial. Cancer Manag Res. 2020;12:12487–12496. PMID: 33299355; PMCID: PMC7721110. doi: 10.2147/CMAR.S283031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matusiak D, Wichtowski M, Pieszko K, Kobylarek D, Murawa D. Is negative-pressure wound therapy beneficial in modern-day breast surgery? Contemp Oncol. 2019;23(2):69–73. Epub 2019 May 27. PMID: 31316287; PMCID: PMC6630394. doi: 10.5114/wo.2019.85199 [DOI] [PMC free article] [PubMed] [Google Scholar]