Abstract
Central nervous system (CNS) metastases can be seen at a rate of 30% in advanced stages for patients with non-small cell lung cancer (NSCLC). Growing evidence indicates the predictive roles of driver gene mutations in the development of brain metastases (BM) in recent years, meaning that oncogene-driven NSCLC have a high incidence of BM at diagnosis. Today, 3rd generation targeted drugs with high intracranial efficacy, which can cross the blood–brain barrier, have made a positive contribution to survival for these patients with an increased propensity to BM. It is important to update the clinical and pathological factors reflected in the survival with real-life data. A multi-center, retrospective database of 306 patients diagnosed with driver mutant NSCLC and initially presented with BM between between November 2008 and September 2022 were analyzed. The median progression-free survival (mPFS) was 12.25 months (95% CI, 10–14.5). While 254 of the patients received tyrosine kinase inhibitor (TKI), 51 patients received chemotherapy as first line treatment. The median intracranial PFS (iPFS) was 18.5 months (95% CI, 14.8–22.2). The median overall survival (OS) was 29 months (95% CI, 25.2–33.0). It was found that having 3 or less BM and absence of extracranial metastases were significantly associated with better mOS and iPFS. The relationship between the size of BM and survival was found to be non-significant. Among patients with advanced NSCLC with de novo BM carrying a driver mutation, long-term progression-free and overall survival can be achieved with the advent of targeted agents with high CNS efficacy with more conservative and localized radiotherapy modalities.
Keywords: Oncogene-driven advanced non-small cell lung cancer, De novo brain metastases, Survival related parameters
Subject terms: Cancer, Oncology
Introduction
Central nervous system (CNS) metastases can be seen at a rate of 30% in advanced stages for patients with non-small cell lung cancer (NSCLC). While 10–25% of patients with stage 4 NSCLC present with BM at diagnosis, the first site of recurrence has been shown to be BM in approximately 20% of patients after definitive treatment for unresectable stage 3 disease1–3. CNS metastasis is one of the main causes of death and contributes to dismal prognosis as an unfortunate site of disease progression. Also, BM is closely associated with poor performance status, quality of life and morbidity in these patients.
Nevertheless, growing evidence indicates the predictive roles of driver gene mutations in the development of BM in recent years, meaning that oncogene-driven NSCLC have a high incidence of BM at diagnosis4–10. A recent retrospective study reported that patients with NSCLC with diverse targetable molecular alterations, except for the KRAS mutation which was excluded in the study and who had received targeted systemic therapies, had a high incidence of BM at diagnosis11. Interestingly, they also found that patients with BM at the time of diagnosis and who subsequently developed BM and receiving local and systemic treatment had similar outcomes when compared to the patients without BM. Today, 3rd generation targeted drugs with high intracranial efficacy, which can cross the blood–brain barrier, have made a positive contribution to survival for these patients with an increased propensity to BM.
Specifically, regarding BM in patients with EGFR mutation or ALK rearrangement, 3rd generation EGFR TKI osimertinib and 2nd and 3rd generation ALK TKIs revealed significant risk reductions for CNS disease progression when compared to their first-generation counterparts12–16.
Stereotactic radiosurgery (SRS), whole-brain irradiation (WBRT) and surgical resection are applied alone or combined/sequentially as local treatment strategies of CNS metastases for the patients with driver mutant NSCLC. In this patient group, it is crucial to investigate factors such as the type of driver mutation, the number of BM, clinicopathological features of the patients, the sequence of the treatment regimens they receive, and the relationship of these factors with life expectancy of the patients and adherence to treatment.
Due to limited data in the literature, we aimed to evaluate the outcome of the patients diagnosed with driver mutant NSCLC who had de novo BM as stage 4 disease and a number of key features including TKI treatments and systemic treatments, patient characteristics, treatments for CNS control, as well as prognostic factors related to intracranial progression-free and overall survival time of these patients.
Materials and methods
The study was initiated as a Turkish Oncology Group (TOG) project. A multi-center (31 tertiary care oncology centers), institutional review board-approved, retrospective database of 306 patients diagnosed with driver mutant NSCLC and initially presented with BM between between November 2008 and September 2022 were analyzed. The patients included were required to receive at least one line of targeted therapy. The patients who subsequently developed BM were excluded. Clinicopathologic characteristics including age at diagnosis, gender, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status (PS), tumor histology, present targetable driver mutations, number and the size of the largest one of intracranial metastasis, extracranial metastatic status, local treatments for BMs and systemic treatments, treatment discontinuation, presence and time of progression and death were noted.
Adverse events obtained from medical records were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. The study protocol was approved by the ethics committee of Ankara Bilkent City Hospital as a multicenter retrospective observational study and all methods were carried out in accordance with Declaration of Helsinki.
Since no experimental procedures were performed on patients and data were retrospectively collected from the medical files of the patients; the need for informed consent was waived by our institutional review board, that is Ankara Bilkent City Hospital, Ethics Committee Number 2, which is deemed unnecessary according to national legislation.
Statistical analysis
The progression-free survival (PFS) was defined as the duration of time from initial first-line treatment to disease progression or the most recent follow-up. And the intracranial PFS (iPFS) was measured from the date of initial first-line treatment to the date of BM progression. The overall survival (OS) was calculated as the time interval in months between de novo BM diagnosis and death or loss of follow-up, whichever was earlier.
Kaplan–Meier method was used to analyse survival data and the log rank test were performed to compare the differences. Multivariable Cox regression analysis was used to determine potential prognostic factors for OS and iPFS. Statistical significance level was determined at P < 0.05. SPSS Statistics version 26.0 was used for the analysis.
Results
Analysis included 306 patients diagnosed with driver mutant, de novo brain metastatic NSCLC. The median age of the patients was 58 (20–85) years. There were 155 female (50.7%), 151 male (49.3%) patients. Most of the patients were never-smoker (58%) and had ECOG PS of 0–2 (94.3%). Regarding tumor-related features, 94.4% of them had adenocarcinoma histology, 1 patient had mixt histology.
The majority had EGFR mutations (68.6%) followed by ALK (27.6%) and ROS1 (2.6%) rearrangements, respectively. EGFR 19 deletion mutation was detected in 131 (61.8%) patients, 61 (28.8%) patients had EGFR exon 21 L858R mutation and other EGFR mutations were present in 20 patients (9.4%) including EGFR exon 20 insertion in 3 of them (1.4%).
One patient had a BRAF V600E mutation and received dabrafenib plus trametinib as first-line therapy. Another one patient had BRAF V600E with EGFR mutation and this patient received chemotherapy, afatinib, chemotherapy, respectively. Another 2 patients had MET amplification with EGFR mutation. One of these patients received crizotinib as first-line treatment, and the other received chemotherapy, afatinib, and crizotinib, respectively.
Local treatment of BM included surgery in 13.4%, radiation in 77% and no local treatment in 9.6%.
Most patients had also extracranial metastasis (86.6%). The clinicopathological characteristics at baseline are listed in Table 1.
Table 1.
The clinicopathological characteristics of the patients.
| Age (median, range) | 58 (20–85) years | |
|---|---|---|
| N | % | |
| < 65 years | 219 | 71.6 |
| ≥ 65 years | 87 | 28.4 |
| Gender | ||
| Female | 155 | 50.7 |
| Male | 151 | 49.3 |
| ECOG PS | ||
| 0 | 77 | 25.5 |
| 1 | 158 | 52.5 |
| 2 | 51 | 17 |
| 3 | 15 | 5 |
| Smoking | ||
| No | 175 | 58 |
| ≤ 10 package/year | 36 | 11.9 |
| > 10 package/year | 91 | 30.1 |
| Tumor histology | ||
| Adenocarcinoma | 289 | 94.4 |
| NOS (not other specified) | 11 | 3.6 |
| Squamous cell carcinoma | 5 | 1.6 |
| Mixt (adeno-squamous) | 1 | 0.4 |
| Present driver mutation status | ||
| EGFR | 210 | 68.6 |
| Exon 21 | 61 | 28.8 |
| Exon 19 del | 131 | 61.8 |
| Rare | 20 | 9.4 |
| ALK | 85 | 27.6 |
| ROS1 | 8 | 2.6 |
| BRAF | 2 | 0.6 |
| Number of BM | ||
| 1–3 | 188 | 61.6 |
| ≥ 4 | 117 | 38.4 |
| Extracranial metastases | ||
| Yes | 265 | 86.6 |
| No | 41 | 13.4 |
| Local treatment for BM | ||
| No | 28 | 9.6 |
| Surgery | 28 | 9.6 |
| WBRT | 123 | 42.4 |
| SRS | 98 | 33.8 |
| WBRT plus SRS | 2 | 0.7 |
| Surgery plus SRS | 6 | 2 |
| Surgery plus WBRT | 5 | 1.7 |
| RT after first BM progression | 78 | |
| SRS | 20 | 25.6 |
| WBRT | 30 | 38.5 |
| Follow-up | 28 | 35.9 |
| RT after second BM progression | 32 | |
| SRS | 10 | 31.3 |
| WBRT | 6 | 18.7 |
| Follow-up | 16 | 50 |
| RT after third BM progression | 14 | |
| SRS | 6 | 42.85 |
| WBRT | 1 | 7.15 |
| Follow-up | 7 | 50 |
| 1st line treatment | ||
| Erlotinib | 115 | 37.7 |
| Gefitinib | 19 | 6.2 |
| Afatinib | 27 | 8.9 |
| Osimertinib | 6 | 2 |
| CT | 51 | 16.7 |
| Crizotinib | 25 | 8.2 |
| Alectinib | 51 | 16.7 |
| Brigatinib | 1 | 0.3 |
| Ceritinib | 4 | 1.3 |
| Dacomitinib | 1 | 0.3 |
| Dabrafenib plus trametinib | 1 | 0.3 |
| Other | 4 | 1.3 |
| 2nd line treatment | 118 | |
| Erlotinib | 27 | 22.9 |
| Afatinib | 6 | 5 |
| Osimertinib | 41 | 34.7 |
| CT | 20 | 16.9 |
| Crizotinib | 4 | 3.4 |
| Alectinib | 10 | 8.5 |
| Brigatinib | 2 | 1.7 |
| Lorlatinib | 8 | 2.3 |
| 3rd line treatment | 38 | |
| Erlotinib | 1 | 2.6 |
| Osimertinib | 5 | 13.1 |
| CT | 23 | 60.5 |
| Crizotinib | 1 | 2.6 |
| Alectinib | 2 | 5.2 |
| Brigatinib | 2 | 5.2 |
| Ceritinib | 1 | 2.6 |
| Lorlatinib | 2 | 5.2 |
| Nivolumab | 1 | 2.6 |
| 4th line treatment | 9 | |
| Erlotinib | 2 | 22.2 |
| Afatinib | 1 | 11.1 |
| CT | 4 | 44.5 |
| Alectinib | 2 | 22.2 |
PS performance status, BM brain metastasis, RT radiotherapy, SRS stereotactic radiosurgery, WBRT whole-brain radiotherapy, CT chemotherapy.
The median duration of follow-up was 33.1 months (95% CI, 26.8–39.5). During the follow-up period, an event of disease progression had occurred in 228 patients and death had occurred in 155 patients. The cause of death was reported as brain progression in 45 patients.
The median PFS was 12.25 months (95% CI, 10–14.5) (Fig. 1A). While 254 of the patients received TKIs, 51 patients received chemotherapy (CT) as first line treatment. After learning the mutation results of 10 patients who had started on CT, CT was stopped and switched to TKI treatment. 118 patients received 2nd line treatment and 88 of them had disease progression. 43 patients received subsequent therapies and 30 of them had disease progression. CNS disease progression developed in 209 patients. The median iPFS was 18.5 months (95% CI, 14.8–22.2) (Fig. 1B). The median OS was 29 months (95% CI, 25.2–33.0) (Fig. 1C).
Figure 1.
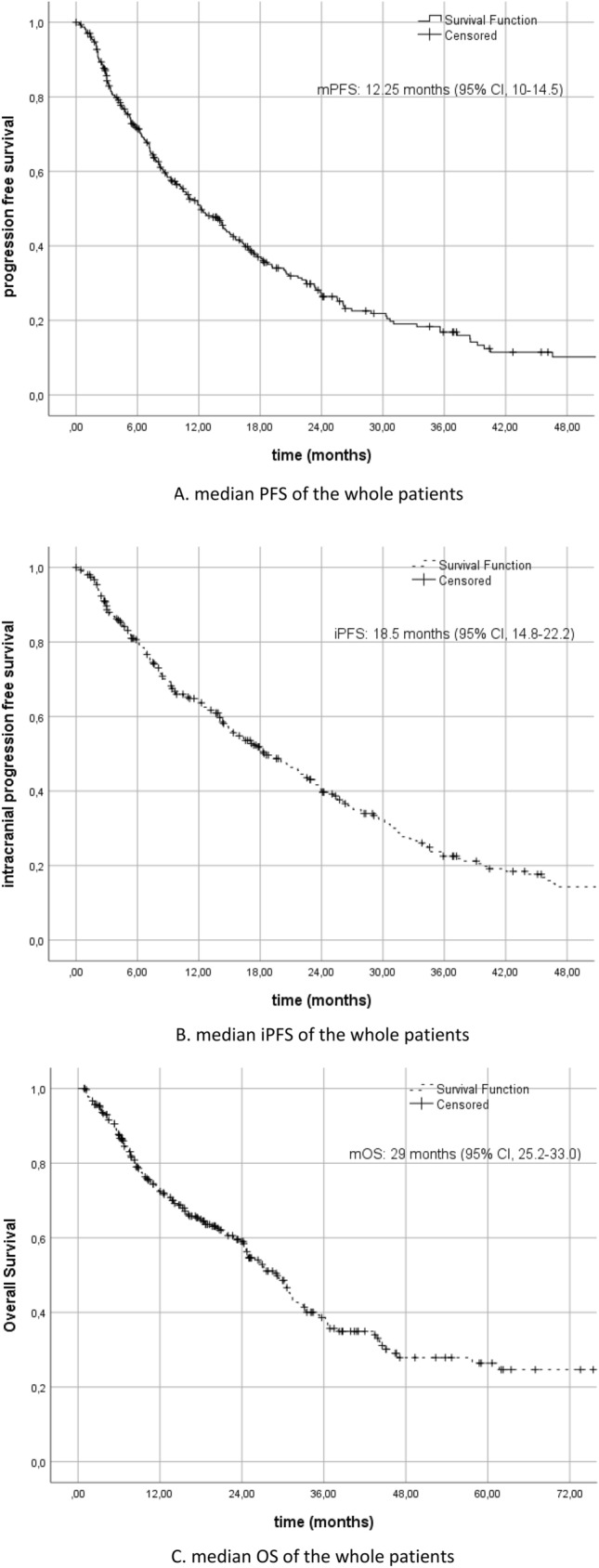
(A) Median PFS of the whole patients. (B) Median iPFS of the whole patients. (C) Median OS of the whole patients.
In this group of patients receiving sequential therapy, there was no difference in terms of iPFS and mOS between patients who received CT as a first-line treatment and those who started with a TKI (p = 0.68 and p = 0.70, respectively) (Fig. 2A,B). However, there was a significant improvement with the first-line TKI treatment in terms of mPFS (p < 0.001) (Fig. 2C).
Figure 2.
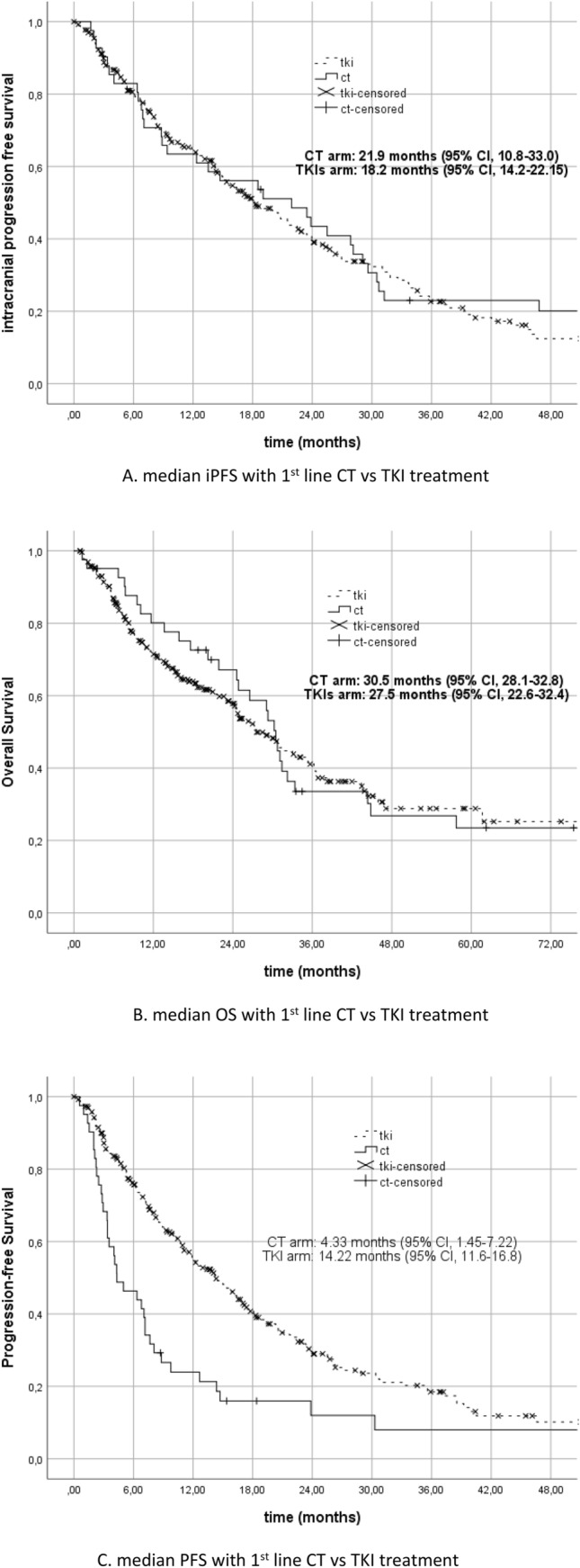
(A) median iPFS with 1st line CT vs TKI treatment. (B) median OS with 1st line CT vs TKI treatment. (C) median PFS with 1st line CT vs TKI treatment.
It was found to be significantly associated with the number of BM and mOS. Patients with 3 or less BM had better survival than patients with 4 or more BM (31.1 (95% CI, 25.2–37.0) vs 16.2 (95% CI, 7.3–25.2) months, p < 0.001) (Fig. 3A). And in terms of iPFS, the patients with 3 or less BM had better survival than the patients with 4 or more BM (23.9 (95% CI, 20.4–27.5) vs 10.5 (95% CI, 8–12.9) months, p < 0.001) (Fig. 3B).
Figure 3.
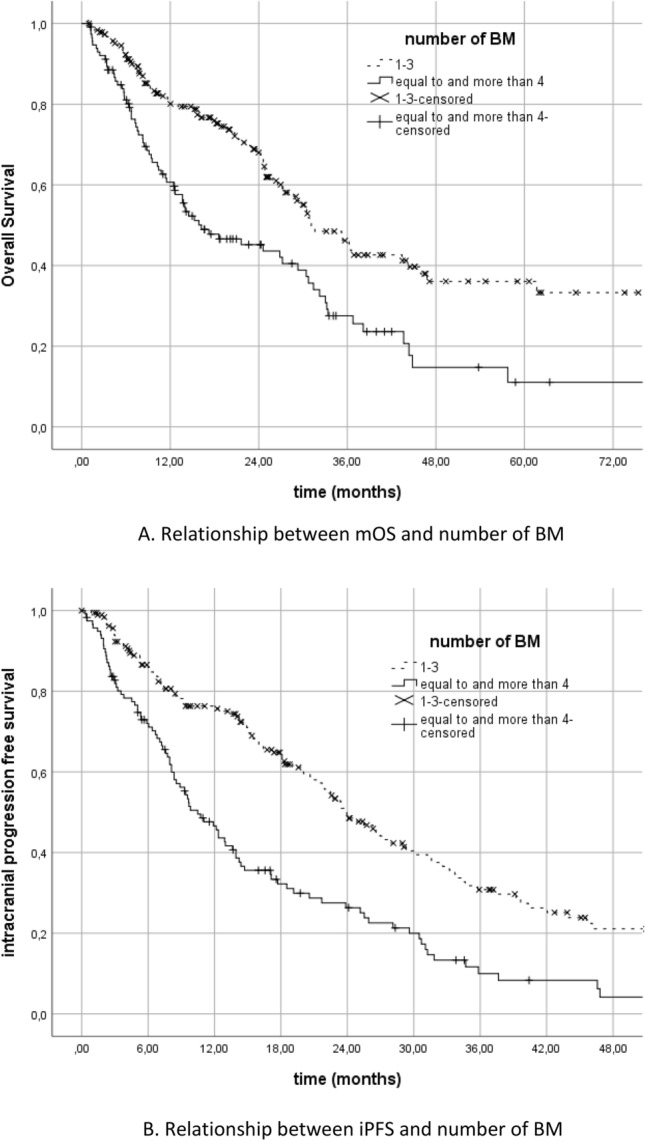
(A) Relationship between mOS and number of BM. (B) Relationship between iPFS and number of BM.
The relationship between the size of BM and survival was found to be non-significant. There was no statistically significant difference in terms of mOS or iPFS when compared between patient groups with the largest BM size less than 3 cm and ≥ 3 cm (p = 0.55 and p = 0.32, respectively) (Fig. 4A,B).
Figure 4.
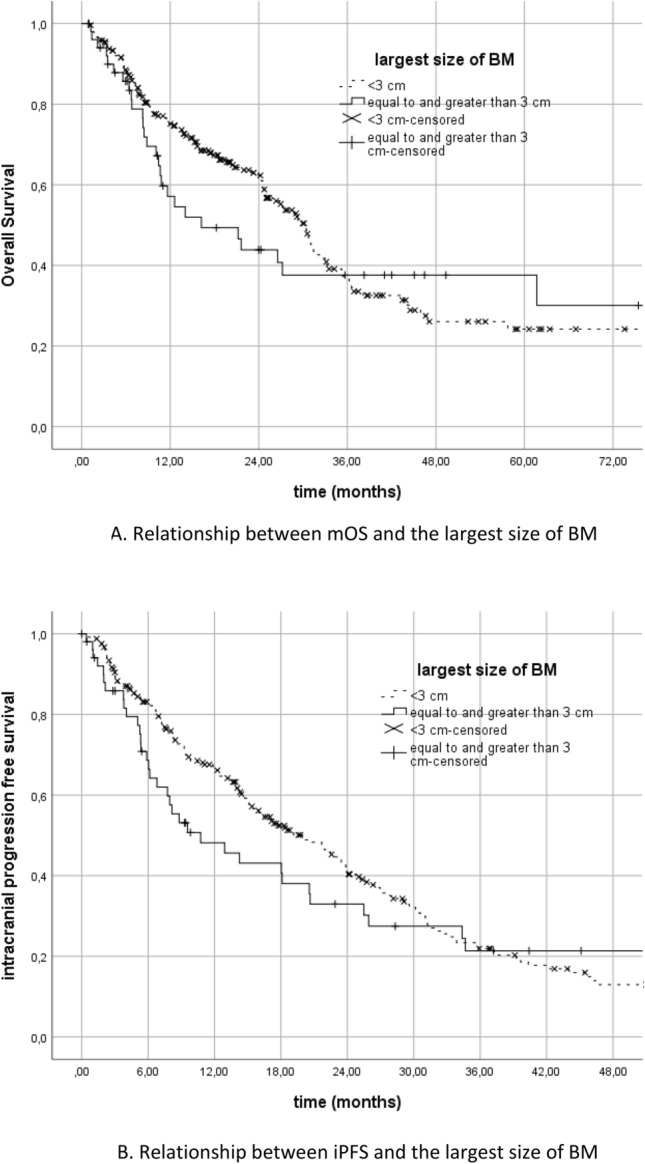
(A) Relationship between mOS and the largest size of BM (B) Relationship between iPFS and the largest size of BM.
The effect of local treatment types given for BM on survival was evaluated and the patients treated with surgery and SRS had better survival than the patients receiving WBRT (Fig. 5). While 80 of the patients who received WBRT treatment had 1 to 3 BM, 42 of them had ≥ 4 BM, and iPFS was significantly better for the group of patients with 1–3 BM (Table 2).
Figure 5.
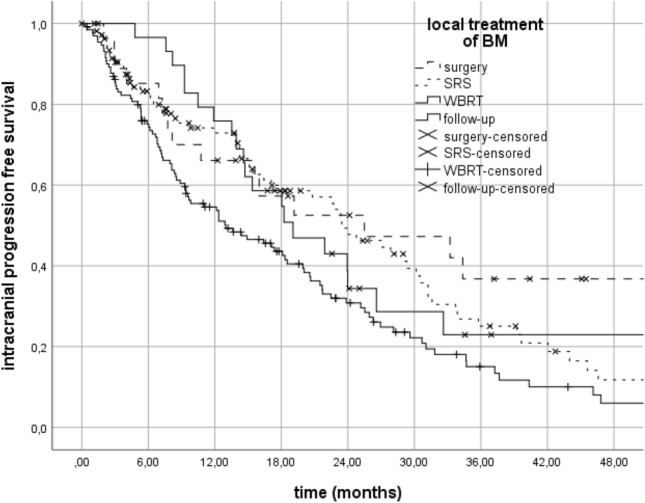
Relationship between iPFS and local treatments of BM.
Table 2.
median iPFS according to local treatment modalities of BM.
| Local treatment of BM | iPFS (months) | p |
|---|---|---|
| Surgery | 25.5 (2.5–48.4) | S vs WBRT: 0.01 |
| SRS | 23.4 (18.1–28.7) | SRS vs WBRT: 0.008 |
| 1–3 BM | 24.8 (18.8–30.7) | 0.08 |
| ≥ 4 BM | 14.2 (4.8–23.6) | |
| WBRT | 13 (7–19) | |
| 1–3 BM | 20.6 (16.3–25) | 0.01 |
| ≥ 4 BM | 9.5 (6–13) | |
| Follow-up | 19 (11.3–26.8) |
BM brain metastasis, S surgery, SRS stereotactic radiosurgery, WBRT whole-brain radiotherapy.
Regarding the patients with EGFR mutation, while iPFS (p = 0.14) and mOS (p = 0.30) were not statistically different between the patient groups carrying exon 19 deletion, exon 21 mutation or other rare EGFR mutations; both iPFS and mOS were numerically shorter in rare EGFR mutation carriers (Table 3).
Table 3.
median iPFS and mOS according to EGFR mutations.
| EGFR mutation | iPFS | mOS |
|---|---|---|
| Exon 21 | 14.9 (13.3–16.5) | 24.8 (16.2–33.4) |
| Exon 19 | 20.8 (15.8–25.8) | 27.2 (22.3–32) |
| Rare | 3 (0.1–9.1) | 12.5 (5.5–19.5) |
In EGFR mutant population, number of BM was associated with iPFS (22.7 vs 9.7 months, p < 0.001) and mOS (29.6 vs 14.0 months, p < 0.001). No correlation was shown between size of BM and survival parameters. While no statistical difference was found between the presence of extracranial metastases and mOS, iPFS was better in patients without extracranial metastases than in those having extracranial metastases.
In ALK positive population, number of BM was associated with iPFS (37.2 vs 14.4 months, p = 0.002) and mOS (61.7 vs 33.2, p = 0.004). No correlation was shown between size of BM and survival parameters. Presence of extracranial metastases was associated with survival outcomes and iPFS and mOS were better in patients without extracranial metastases than in those having extracranial metastases. In Table 4, the results of subgroup analysis of EGFR mutant and ALK positive patients according to BM status were summarized.
Table 4.
Subgroup analysis of EGFR and ALK mutant patients regarding iPFS and mOS and associated variables.
| iPFS (months) | p | mOS (months) | p | |
|---|---|---|---|---|
| EGFR mutant patients | ||||
| Number of BM | ||||
| 1–3 | 22.7 (19.2–26.2) | < 0.001 | 29.6 (26–33.2) | < 0.001 |
| ≥ 4 | 9.7 (7.5–11.9) | 14 (10.5–17.5) | ||
| Size of BM | ||||
| < 3 cm | 17.7 (12.9–22.5) | 0.35 | 27.2 (22.8–31.5) | 0.78 |
| ≥ 3 cm | 10.7 (4.3–17.2) | 16.2 (3.7–28.7) | ||
| Presence of extracranial metastases | ||||
| No | 27.1 (13.2–41) | 0.03 | 24.9 (11.1–38.7) | 0.59 |
| Yes | 16.4 (12.6–20.2) | 26.5 (22.9–30.1) | ||
| ALK mutant patients | ||||
| Number of BM | ||||
| 1–3 | 37.2 (23.9–50.6) | 0.002 | 61.7 (NE-NE) | 0.004 |
| ≥ 4 | 14.4 (3.9–24.9) | 33.2 (21.8–44.6) | ||
| Size of BM | ||||
| < 3 cm | 23.4 (16.1–30.8) | 0.65 | 44.3 (31.7–57) | 0.58 |
| ≥ 3 cm | 52.5 (1.8–103.3) | 61.7 (0.1–140.1) | ||
| Presence of extracranial metastases | ||||
| No | 87.16 (NE-NE) | 0.001 | NE | 0.008 |
| Yes | 19.15 (10.7–27.6) | NE | ||
Cox analysis showed that age, ECOG PS, and presence of extracranial metastases were associated with mOS. Age and presence of extracranial metastases were associated with iPFS, but no correlation was found between ECOG PS and iPFS (Table 5).
Table 5.
Factors associated with iPFS and mOS (cox analysis).
| HR | 95% CI | p | |
|---|---|---|---|
| mOS associated variables | |||
| Age | |||
| < 65 | 0.001 | ||
| > 65 | 1.8 | 1.3–2.5 | |
| ECOG PS | |||
| 0–1 | 0.02 | ||
| > 2 | 1.54 | 1.01–2.21 | |
| Extracranial metastasis | |||
| No | 0.048 | ||
| Yes | 1.7 | 1.0–2.87 | |
| iPFS associated variables | |||
| Age | |||
| < 65 | 0.005 | ||
| ≥ 65 | 1.5 | 1.1–2.1 | |
| ECOG PS | |||
| 0–1 | 0.32 | ||
| ≥ 2 | 1.18 | 0.85–1.63 | |
| Extracranial metastasis | |||
| No | < 0.001 | ||
| Yes | 2.4 | 1.5–3.9 | |
Discussion
For NSCLC patients presenting with BM, knowledge of the driver gene mutation status is particularly important because such as EGFR, ALK, and RET has reported to be the risk factors contributing to BM development in advanced NSCLC17. It is currently possible to talk about long survival data, especially thanks to the effectiveness of TKIs that crosses the blood–brain barrier and delays both the emergence and progression of BM. In addition, improvements in the field of radiotherapy techniques for BM have resulted in a positive contribution to survival18.
In our study, upfront TKIs and starting chemotherapy as the first-line therapy were not different in terms of long-term CNS-related or overall survival. In contrast, superior efficacy of EGFR-TKIs than chemotherapy as the first-line therapy has been shown19,20. The potential difference may have been obscured by the factors that the patients in this cohort must have received at least 1 line of TKI therapy, the ability to switch to TKI treatment immediately after progression during chemotherapy and also application of local treatment for BM despite shorter mPFS with chemotherapy.
90% of our patients, all of whom presented with BM at the time of diagnosis, initially received local treatment for BMs and all patients were started on systemic treatment. The reason why iPFS was longer than systemic progression in our study can be interpreted as the effect of local treatments and systemic treatment together on BM control. In case of progression of BM during follow-up, if the isolated brain metastasis progressed, they were followed with a second local treatment without changing the systemic treatment. However, if prominently symptomatic, multiple BM or accompanying systemic progression occurred, systemic treatments were changed and the next treatment step was started. Since reflecting our real-life practice in the general population, the results of our study are valuable.
In the context of combining/sequencing local treatments and TKI treatments for BM in the patients with driver mutant NSCLC, when survival-related factors are evaluated either considering the EGFR mutant and ALK positive patient groups separately or the entire group, having 1–3 BMs and isolated BMs were associated with significantly better iPFS and OS data, in line with the literature21. In general, in this the group of patients with measurable BM, the size was not at all associated with survival. As a limitation of our study, the BM volumes of the patients were not recorded. We believe that assessing the relationship between number, size and volume of BMs with prospective studies would be valuable for the patients with driver mutant NSCLC with BMs.
Regarding the efficacy of local treatments on survival parameters, the patients who underwent surgery and SRS had similar iPFS and mOS, while the survival of the patients who received WBRT was inferior when compared to surgery/SRS. Although two third of the patients who were treated WBRT had 1–3 BM, it was observed that the results of WBRT were not better. For the patient group with a low number of BM, WBRT does not seem to provide additional contribution compared to SRS. A recent retrospective Korean study reported that RT was associated with a survival difference among Korean patients with BM, especially among patients with BM from lung cancer and also that SRS was associated with better overall survival, relative to WBRT22.
When evaluating the patients with EGFR mutation subtypes in particular, mutation subtypes did not seem to have an independent prognostic impact on survival. Although the survival results of exon 19 deletion mutation carriers were numerically better, there was no statistical difference between exon 19 deletion and exon 21 mutation carriers in terms of iPFS or mOS. On the contrary, the patients with other EGFR mutations (such as exon 18, exon 20 mutation, overexpression, amplification) had numerically inferior survival results, however no statistically significant difference was found again.
According to the results of another retrospective, non-interventional study with a similar design to ours, EGFR mutations other than exon 19 deletion EGFR mutations were associated with earlier intracranial progression and iPFS was significantly better in patients with EGFR exon 19 deletions compared to patients with other EGFR mutations23. However, it would be more informative to specify the specific subtypes of EGFR mutations for 43% of patients reported to have non-deletion 19 EGFR mutation. Results of the ongoing prospective studies evaluating more potent monoclonal antibody and TKIs for the patients with other EGFR mutation (especially other than exon 19 deletion and exon 21 mutation and for exon 20 ins mut) will be instructive in regard to CNS metastasis.
For the whole patient group, BM less than 4, absence of extracranial metastases, good performance status at the time of admission (asymptomatic or minimally symptomatic) and age below 65 were observed as predictive markers for better survival data. The fact that number of BM is a prognostic factor rather than size may be related to better local control of BM in these patients.
As we know that almost 30–40% of patients presenting with de novo BM are included and evaluated in pivotal studies; our study is important because all patients included were diagnosed with oncogene-driven NSCLC presented with de novo brain metastases and reflects real-life data despite possible biases regarding retrospective nature. Another limitation of our study is that the evaluation of BM with radio-imaging modality was performed in different tertiary centers and radiologist and not provided by a single center standard. Additionally, it could not provide information about the optimal timing of radiotherapy. Additionally, only clinical information of patients with existing driver mutations was included. Therefore, there is no extensive knowledge on whether patients received NGS, the presence of additional mutations, and its relationship with clinical factors.
However, according to our knowledge, this study represents one of the largest real-world studies evaluating advanced NSCLC patients with de novo BM. In line with the literature, the number of BM less than 4 and the absence of extracranial metastases have been found as the factors associated with better survival outcomes.
ALK-positive patients received crizotinib, alectinib, brigatinib, and ceritinib for the first-line. Patients could receive lorlatinib in the 2nd and 3rd line due to the lack of reimbursement in our country. The results of our study which included first line chemotherapy, crizotinib and sequential ALK inhibitor treatment receiving patient population, could be interpreted as consistent with the literature. In further studies, to what extend local treatments will contribute to survival in conditions where newer generation TKIs are used more intensively may be the subject of research.
As a therapeutic target, epidermal growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK) rearrangements, c-ros oncogene 1 (ROS1) rearrangements, v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) mutations, Kirsten rat sarcoma viral oncogene homologue (KRAS) mutations, neurotrophic receptor tyrosine kinase (NTRK) 1/2/3 rearrangements, rearranged during transfection (RET) rearrangements, N-methyl-N0-nitroso-guanidine human osteosarcoma transforming gene (MET) exon14 skipping mutations, and activating human epidermal growth factor receptor 2 (HER2) mutations are sought as treatment targets and TKIs are preferred for the advanced stage NSCLC treatment even BM presence24. Cytotoxic chemotherapy and early generation TKIs generally failed to achieve therapeutically relevant concentrations in the CNS due to their inability to cross the blood–brain barrier. Several novel and newer-generation TKIs with improved CNS penetrance are currently used in the clinical practice. Erlotinib, gefitinib (1st generation), and afatinib (2nd generation) have intracranial activity in NSCLC patients with EGFR mutations, with objective response rates ranging from 60–80%25. However, Osimertinib (3rd generation) was significantly associated with better hazard ratio (HR) for CNS progression-free survival (0.48; 95% CI: 0.26–0.86) compared with gefitinib or erlotinib as the first-line treatment26.
Currently, there are five ALK-TKIs for the treatment of ALK-positive NSCLC, namely crizotinib (1st generation), alectinib, ceritinib, brigatinib (2nd generation), and lorlatinib (3rd generation). Ceritinib (45%) and brigatinib (42–67%) demonstrated high intracranial ORR in patients who relapsed after first line treatment with crizotinib27. Alectinib demonstrated an 81% of intracranial response among the patients with previously untreated BM28. Finally, lorlatinib had 42–48% intracranial response in patients with recurrence after first line crizotinib and reached 82% as a first line treatment16,29. According to the results of the CROWN study, the HR for time to intracranial progression for lorlatinib versus crizotinib was 0.10 (95% CI 0.04–0.27) in patients with baseline BM, and 0.02 (95% CI 0.002–0.14) in patients without baseline BM16. Studies are encouraged to clarify the sequence in which ALK-TKIs should be used for effective BM control.
Novel therapeutic strategies following osimertinib resistance are also being investigated. Beyond BM progression, osimertinib-based combination strategy can be considered as one of them. In addition, efforts are being made to develop biomarker-focused treatment strategies for patients harboring a definite acquired resistance alteration, such as MET amplification30.
In the MARIPOSA trial, the risk of disease progression or death was significantly reduced in patients who received amivantamab (the EGFR-MET bispecific antibody) combined with a brain-penetrant irreversible third-generation EGFR TKI, lazertinib, compared with patients received osimertinib (hazard ratio [HR] 0.70; 95% CI, 0.58–0.85; p < 0.001)31. FLAURA 2 reported that osimertinib plus platinum-pemetrexed demonstrated improved CNS efficacy compared with osimertinib monotherapy (HR for disease progression or death, 0.47 (95% CI, 0.33–0.66)32. Primary results from the MARIPOSA 2 trial announced a median intracranial PFS of 12.5 months for amivantamab plus chemotherapy and 12.8 months for amivantamab plus Lazertinib plus chemotherapy versus 8.3 months for chemotherapy following disease progression with osimertinib treatment (HRs of 0.55 and 0.58; p = 0.001 and p < 0.001, respectively)33. Phase II SAVANNAH study, evaluating the combination of osimertinib with the selective MET TKI, savolitinib (NCT03778229), phase III SAFFRON study, evaluating osimertinib plus savolitinib versus platinum-doublet chemotherapy (NCT05261399) in patients with MET-mediated resistance to osimertinib and, other strategies such as development of antibody–drug conjugates (i.e. patritumab deruxtecan) to overcome resistance following EGFR TKI treatment are in progress34. The results of further investigations to unravel the complexity of brain metastatic EGFR-mutated NSCLC and optimal treatment sequence are eagerly awaited.
Last of all, multimodality therapy has come into prominence among the patients with advanced NSCLC patients with de novo BMs who carry a driver mutation and received at least one line of targeted therapy and long-term progression-free and overall survival can be achieved with the advent of targeted agents with high CNS efficiency with more conservative and localized radiotherapy methods. In our study, we discussed and revealed the prognostic factors reflected in the survival of these patients with real-life data.
Author contributions
Conception and design: M.A.N.S. and A.M.T.; Development of methodology, analysis and interpretation of data, and writing of the article: S.K. and M.A.N.S.; Data acquisition: All authors; Manuscript writing: All authors; Final approval of manuscript: All authors. All authors revised the manuscript critically for important intellectual content and approved the version to be published.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Komaki, R.U., & Ghia, A. J., Brain Metastasis from Lung Cancer, in Lung Cancer p. 572–589. (2014)
- 2.Yamanaka R. Medical management of brain metastases from lung cancer (Review) Oncol. Rep. 2009;22(6):1269–1276. doi: 10.3892/or_00000564. [DOI] [PubMed] [Google Scholar]
- 3.Germain F, et al. Brain metastasis is an early manifestation of distant failure in stage III nonsmall cell lung cancer patients treated with radical chemoradiation therapy. Am. J. Clin. Oncol. 2008;31(6):561–566. doi: 10.1097/COC.0b013e318172d5f9. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, et al. Driver genes as predictive indicators of brain metastasis in patients with advanced NSCLC: EGFR, ALK, and RET gene mutations. Cancer Med. 2020;9(2):487–495. doi: 10.1002/cam4.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomasini P, et al. EGFR and KRAS mutations predict the incidence and outcome of brain metastases in non-small cell lung cancer. Int. J. Mol. Sci. 2016;17(12):2132. doi: 10.3390/ijms17122132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iuchi T, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int. J. Clin. Oncol. 2015;20(4):674–679. doi: 10.1007/s10147-014-0760-9. [DOI] [PubMed] [Google Scholar]
- 7.Patil T, et al. The incidence of brain metastases in stage IV ROS1-rearranged non-small cell lung cancer and rate of central nervous system progression on crizotinib. J. Thorac. Oncol. 2018;13(11):1717–1726. doi: 10.1016/j.jtho.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, et al. Genes associated with increased brain metastasis risk in non-small cell lung cancer: Comprehensive genomic profiling of 61 resected brain metastases versus primary non-small cell lung cancer (Guangdong Association Study of Thoracic Oncology 1036) Cancer. 2019;125(20):3535–3544. doi: 10.1002/cncr.32372. [DOI] [PubMed] [Google Scholar]
- 9.Shih DJH, et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat. Genet. 2020;52(4):371–377. doi: 10.1038/s41588-020-0592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rangachari D, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–111. doi: 10.1016/j.lungcan.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakshit S, B.R., Desai A, et al., Brain metastases in non-small cell lungcancer in era of molecularly driven therapy., in European Lung Cancer Virtual Congress 2021.
- 12.Soria JC, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 13.Reungwetwattana T, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J. Clin. Oncol. 2018;2018:2018783118. doi: 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 14.Peters S, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2017;377(9):829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 15.Camidge DR, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2018;379(21):2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 16.Shaw AT, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N. Engl. J. Med. 2020;383(21):2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 17.Kang Y, et al. Advances in lung cancer driver genes associated with brain metastasis. Front Oncol. 2020;10:606300. doi: 10.3389/fonc.2020.606300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora, R.D., et al., Palliative Radiation Therapy for Brain Metastases, in StatPearls. 2022: Treasure Island.
- 19.Rosell R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 20.Yang JC, et al. Symptom control and quality of life in LUX-Lung 3: A phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013;31(27):3342–3350. doi: 10.1200/JCO.2012.46.1764. [DOI] [PubMed] [Google Scholar]
- 21.Sperduto PW, et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA) JAMA Oncol. 2017;3(6):827–831. doi: 10.1001/jamaoncol.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park K, et al. Radiotherapy for brain metastasis and long-term survival. Sci. Rep. 2021;11(1):8046. doi: 10.1038/s41598-021-87357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Shafie RA, et al. Effect of timing, technique and molecular features on brain control with local therapies in oncogene-driven lung cancer. ESMO Open. 2021;6(3):100161. doi: 10.1016/j.esmoop.2021.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Carlo E, et al. Brain metastases management in oncogene-addicted non-small cell lung cancer in the targeted therapies era. Int. J. Mol. Sci. 2022;23(12):6477. doi: 10.3390/ijms23126477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billena C, et al. The role of targeted therapy and immune therapy in the management of non-small cell lung cancer brain metastases. Front. Oncol. 2023;13:1110440. doi: 10.3389/fonc.2023.1110440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramalingam SS, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 2019;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 27.Nishino M, Soejima K, Mitsudomi T. Brain metastases in oncogene-driven non-small cell lung cancer. Transl. Lung Cancer Res. 2019;8(Suppl 3):S298–s307. doi: 10.21037/tlcr.2019.05.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadgeel S, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann. Oncol. 2018;29(11):2214–2222. doi: 10.1093/annonc/mdy405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw AT, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Araki T, et al. Current treatment strategies for EGFR-mutated non-small cell lung cancer: from first line to beyond osimertinib resistance. Jpn. J. Clin. Oncol. 2023;53(7):547–561. doi: 10.1093/jjco/hyad052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho BC, et al. MARIPOSA: Phase 3 study of first-line amivantamab + lazertinib versus osimertinib in EGFR-mutant non-small-cell lung cancer. Fut. Oncol. 2022;18(6):639–647. doi: 10.2217/fon-2021-0923. [DOI] [PubMed] [Google Scholar]
- 32.Jänne PA, et al. CNS efficacy of osimertinib with or without chemotherapy in epidermal growth factor receptor-mutated advanced non-small-cell lung cancer. J. Clin. Oncol. 2023;2023:Jco2302219. doi: 10.1200/JCO.23.02219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Passaro A, et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase III MARIPOSA-2 study. Ann. Oncol. 2024;35(1):77–90. doi: 10.1016/j.annonc.2023.10.117. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, et al. Combatting acquired resistance to osimertinib in EGFR-mutant lung cancer. Ther. Adv. Med. Oncol. 2022;14:17588359221144099. doi: 10.1177/17588359221144099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


