Abstract
Machine learning (ML) has emerged as a promising tool to enhance suicidal prediction. However, as many large-sample studies mixed psychiatric and non-psychiatric populations, a formal psychiatric diagnosis emerged as a strong predictor of suicidal risk, overshadowing more subtle risk factors specific to distinct populations. To overcome this limitation, we conducted a systematic review of ML studies evaluating suicidal behaviors exclusively in psychiatric clinical populations. A systematic literature search was performed from inception through November 17, 2022 on PubMed, EMBASE, and Scopus following the PRISMA guidelines. Original research using ML techniques to assess the risk of suicide or predict suicide attempts in the psychiatric population were included. An assessment for bias risk was performed using the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guidelines. About 1032 studies were retrieved, and 81 satisfied the inclusion criteria and were included for qualitative synthesis. Clinical and demographic features were the most frequently employed and random forest, support vector machine, and convolutional neural network performed better in terms of accuracy than other algorithms when directly compared. Despite heterogeneity in procedures, most studies reported an accuracy of 70% or greater based on features such as previous attempts, severity of the disorder, and pharmacological treatments. Although the evidence reported is promising, ML algorithms for suicidal prediction still present limitations, including the lack of neurobiological and imaging data and the lack of external validation samples. Overcoming these issues may lead to the development of models to adopt in clinical practice. Further research is warranted to boost a field that holds the potential to critically impact suicide mortality.
Subject terms: Bipolar disorder, Prognostic markers
“Is there no way out of the mind?”
-Sylvia Plath
“The person in whom Its invisible agony reaches a certain unendurable level will kill herself the same way a trapped person will eventually jump from the window of a burning high-rise. Make no mistake about people who leap from burning windows. Their terror of falling from a great height is still just as great as it would be for you or me standing speculatively at the same window just checking out the view; i.e., the fear of falling remains a constant. The variable here is the other terror, the fire’s flames: when the flames get close enough, falling to death becomes the slightly less terrible of two terrors.
It’s not desiring the fall; it’s terror of the flames.”
- David Foster Wallace
Introduction
The prediction of suicide has been a challenge for decades, and to date, a method for anticipating individual suicides or stratifying patients according to suicide risk is still lacking [1]. Suicide is a worldwide phenomenon and ranks as the second most frequent cause of premature mortality in individuals between 15 and 29 years (preceded only by traffic accidents), and as the third in the age group 15–44 years [2].
Alarmingly, recent studies suggest that the detection of risk factors and the implementation of interventions are inadequate [3]. The majority of individuals who have attempted suicide are reported to consult with physicians prior to the attempt, suggesting that a possibility to intervene might be possible in these help-seeking subjects. The difficulty in predicting suicidal behaviors relies on the lack of clear psychiatric biomarkers and the poor predictive power of individual risk factors [4]. Suicidal behaviors, as many other psychiatric phenomena, are likely the result of the complex relationship between several environmental and trait variables interacting to modify the actual risk rate [4, 5]. Well-recognized risk factors for suicide encompass mental disorders, previous suicide attempts, early trauma, negative life events, and vulnerable periods, with important differences among sexes in terms of ideation and lethality [6, 7]. However, traditional suicide risk factors have only limited clinical predictive value and show a relatively poor clinical utility in predicting suicide occurrence [8, 9], even in high-risk population, such as depressed patients [10].
That is, to date, a method for anticipating suicides or stratifying patients according to risk for suicidal behaviors remains elusive, and no biomarkers have been yet established [9, 11].
Over the last decades, machine learning (ML) techniques emerged as a potential new tool to improve the management of complex problems in psychiatry [12]. This form of multimodal learning has shown to improve prognostic/predictive performance in various fields of medicine, e.g., cardiology and neurology [13, 14]. As a matter of fact, ML can be used to process high-dimensional sets of variables and determine the optimal model for classification. Importantly, such techniques allow predictions at the individual level, therefore representing a promising tool to accurately characterize the complex nature of suicidal behavior.
In the last few years, several algorithms and procedures have been used to predict suicidal behaviors in different populations [11, 15–17]. Given that suicide is considered a transdiagnostic feature, a number of studies have been conducted in the general population, sometimes with very large and heterogeneous samples [6, 18]. One of the most solid findings emerging from studies focusing on the general population is that a formal psychiatric diagnosis is a strong predictor of suicidal risk in different samples across countries [1, 6, 18, 19]. This is not surprising, as up to 90% of all suicides occur in psychiatric populations [1, 20–22], with mood disorders being considered the leading cause of suicidality among mental disorders [23, 24].
Therefore, the inclusion of both healthy individuals and psychiatric patients into large sample ML studies may prevent the identification of more subtle risk factors specific to distinct psychiatric disorders by merely taking into account a previous psychiatric diagnosis as the driving factor for the analysis. Instead, by targeting vulnerable populations only, ML could uncover predictors of suicidal behaviors specific to distinct disorders and help in better stratifying patients according to the actual risk. This would translate into useful information that can be more easily applied in clinical and forensic settings [25].
In this context, in this work, we provide a systematic review of the results from ML studies in psychiatric clinical populations and discuss crucial issues in ML literature, including employed algorithms, features, and samples, with the aim of providing meaningful considerations to future research in the field of suicide prevention.
Material and method
The current systematic review followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [26].
Search strategy
A systematic literature search was performed for articles published from inception through November 17, 2022 on PubMed, EMBASE, and Scopus, using the following search terms adapted for each database:
(suicid* AND (machine learning OR support vector machine OR deep learning OR neural network OR random forest OR xboost OR gradient boosting OR regression tree OR elastic net) AND (psychiatr* OR schizophren* OR depress* OR obsessive OR bipolar OR mania OR manic OR anxiety OR borderline OR personality)
Database searches were supplemented by hand-search, which encompassed an extensive search through the reference list of included papers, previous reviews, and the “Similar Articles” sections in PubMed (reported in Fig. 1 as “Other sources”).
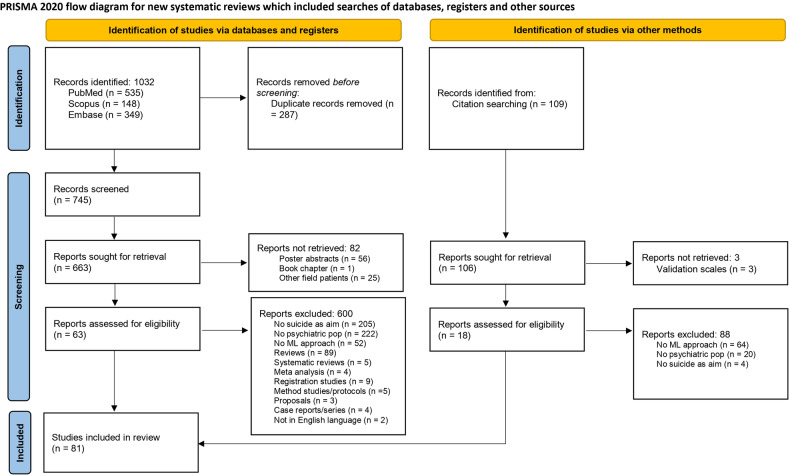
Fig. 1. PRISMA flowchart of the study selection.
Flowchart summary of the study selection process (adapted from PRISMA guidelines; Page et al., 2021).
Two authors (A.P. and G.D.) independently performed the literature search. Documents were assessed according to the following inclusion criteria: (1) journal article available in English, (2) original investigation, (3) employment of ML methodology, (4) evaluation of a suicide risk outcome or self-harm; (5) evaluation of a psychiatric population. Also, we included studies if (a) the sample was composed of individuals with a confirmed psychiatric diagnosis, irrespective of the specific diagnosis and disease severity, and (b) used multiple psychiatric diagnoses or a transdiagnostic framework. The absence of a control group of healthy individuals was not considered an exclusion criterion. To be included, studies must have used ML as a primary or secondary analysis method to predict suicide attempt, suicide risk, or to stratify patients according to risk. No restriction of age was applied. If controversies emerged in the screening processes, they were resolved by discussion between the two authors (A.P. and G.D.) with a third party (P.B.).
Exclusion criteria were the following: (1) non-original investigations (reviews, expert opinions, meta-analyses); (2) article not in English; (3) employment of a methodology other than ML (logistic regression was excluded, except when it was compared to other ML approaches); (4) evaluation of outcomes other than suicide; (5) exclusive evaluation of non-psychiatric populations (e.g., general population, neurologic patients, high-risk populations, emergency department patients). Given that suicidal behaviors are reported across all ages, age-related variables were not considered an exclusion criterion.
We also excluded studies in which the sample was composed by “suicide attempters” without further differentiation in terms of the presence or absence of psychiatric diagnoses. A PRISMA flowchart (Fig. 1) (Page et al., 2021) was created to graphically depict the inclusion/exclusion of studies.
Data extracted
A preliminary data extraction form was designed by A.P.; it was then pilot-tested on five randomly selected studies and fine-tuned accordingly. The search was rerun on a weekly basis, and data from the newly included studies were added to the database accordingly.
For each article, the following variables were extracted:
General information (author, year of publication).
Sample characteristics (demographics, numerosity, clinical data).
Type of ML algorithm(s) employed.
Number and characteristics of features employed for prediction.
ML performance metrics (AUC, Accuracy, Sensitivity, Specificity).
Number of psychiatric diagnoses assessed.
Type of psychiatric disorders assessed.
Findings regarding the prediction of suicide or the classification of risk.
Descriptive analyses
Given the different types of features and algorithms employed, the data were not homogeneous enough to be included in a quantitative meta-analysis. Descriptive analyses were employed to analyze study findings by key design characteristics such as the employed features, sample size, and ML algorithms.
Quality assessment
An assessment for bias risk was performed using the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines [27] (see Supplementary Materials for more details; see Supplementary Table 3 for risk bias results).
Results
Based on the search strings and after the removal of duplicates, 745 unique studies were retrieved and screened for eligibility from direct database search and 109 from other sources (Fig. 1).
During this screening phase, 82 studies were rejected because they failed to fully meet the inclusion criteria. Subsequently, we reviewed the full texts of the remaining 663 studies plus 109 from other sources. Six hundred studies were further excluded since they did not meet the inclusion criteria (see Fig. 1 for a complete description).
As a result, the remaining 81 studies were included in the qualitative synthesis of the review, whose information are summarized in Table 1.
Table 1.
Sociodemographic and clinical characteristics of the reviewed studies.
| Author and year | Sample (F/M) | Population | Medication | ML algorithm (CV) | Features | Suicide measure/outcome | Outcome Metrics | Findings |
|---|---|---|---|---|---|---|---|---|
| Wang et al., [77] | 1249 patients with suicide attempts (846/403); 11,851 patients without suicide attempts (6617/5234) | Mood disorders | Not specified |
LR SVM CNN |
Sociodemographic features and Text mining | Suicide attempts |
LR Acc: 0.97 AUC: 0.94 Precision: 0.91 Recall: 0.74 SVM Acc: 0.97 AUC: 0.94 Precision: 0.88 Recall: 0.84 CNN Acc: 0.98 AUC: 0.97 Precision: 0.94 Recall: 0.89 |
Females, the elderly (>59 years old), students, the divorced or widowed, and patients with smoking, psychoactive substance dependence, and a family history of suicide had a higher risk of attempted suicide. CNN resulted the most accurate prediction model. |
| Chen et al., [55] |
36 MDD with low-risk (19/17); 126 MDD with high-risk (86/40) |
MDD | Unmedicated | SVM (LOOCV) | rsMRI | Low vs High suicide risk |
Acc: 0.85 AUC: 0.87 Sens: 0.86 Spec: 0.78 |
intra-network dysconnectivity in the sensorimotor network and inter-network dysconnectivity between the default and dorsal attention network characterized high-risk patients |
| Chen et al., [47] |
32 BD with suicidal ideation (21/11); 18 BD without suicidal ideation (9/9) |
BD | Not specified | SVM (LOOCV, 1000 permutation) |
12 features from 1H-MRS (biochemical metabolite ratios in the bilateral prefrontal white matter and hippocampus) |
Suicide ideation |
Acc: 0.88 AUC: 0.90 Sens: 0.94 Spec: 0.78 |
The most relevant features were NAA/Cr ratios in the bilateral WM, mI/Cr ratios in the bilateral WM, and NAA/Cr ratios and Cho/Cr ratios in the left hippocampus |
| Zhong et al., [80] |
30 BD II with suicide attempts (23/7); 38 BD II without suicide attempts (21/17); 35 HC (19/16) |
BD II | Unmedicated | SVM (10-folds CV) | Dynamic functional network connectivity based on rsMRI | Suicide attempts |
Acc: 0.75 AUC: 0.83 Sens: 0.70 Spec: 0.79 |
Decreased dynamic functional connectivity variability between the left putamen and the right postcentral gyrus was found in non-suicidal compared to suicidal BD II and HC. |
| Xu et al., [60] |
51 MDD with suicidal attempts (39/12); 74 MDD with suicidal ideation (49/25); 48 MDD without ideation/attempts (28/20); 38 HC (25/13) |
MDD | Not specified | SVM (LOOCV) | Dynamic functional network connectivity based on rsMRI (>200 features) | Suicide attempts and ideation |
MDD suicide attemps vs NS Acc: 0.80 AUC: 0.88 Sens: 0.88 Spec: 0. 74 MDD suicide ideation vs NS Acc: 0.75 AUC: 0.78 Sens: 0.78 Spec: 0.74MDD attempts vs ideation Acc: 0.68 AUC: 0.74 Sens: 0.68 Spec: 0.71 |
The features that contributed to stratifying MDD patients with diverse suicide risk levels mainly involved the visual-related and DMN-related inter-network connectivity within the weakly connected state. |
| Zheng et al., [108] |
52 MDD with suicidal attemps (40/12); 61 MDD without suicidal attempts (36/25); 98 HC (49/49) |
MDD | Not specified | XBoost | Sociodemographic, clinical and cognitive features (total: 20 features) | Suicide attempts |
Acc: 0.71 AUC: 0.82 Sens: 0.6 Spec: 0.79 PPV: 0.69 NPV: 0.71 |
Adding cognitive information significantly increased model prediction; the most important feature was HAMD-24 score |
| Shin et al., [70] | 83 MDD (64/19); 83 HC (69/14) | MDD | Not specified |
Naive Bayes classifier (5-folds CV) |
Sociodemographic and text-based | High vs low-risk suicide (based on the MINI interview) |
Acc: 0.75 AUC: 0.80 Sens: 0.82 Spec: 0.65 |
When predicting suicide, only the ensemble analyses (namely, sociodemographic + text) resulted in significant prediction. Demographic alone: AUC 0.5 Text alone: AUC 0.64 |
| Miranda et al., [36] | 38807 PTSD patients | PTSD | Not specified | RNN | EMRs, including sociodemographic, clinical and lab features (>100 features) | Suicide-related events within 3 months | AUC:0.92 | Lab tests (i.e., glucose, glucose urine, chloride, hemoglobin (HGB), hematocrit, mean corpuscular volume, white blood cell, neutrophils, potassium, INR, calcium, mean platelet volume) combined with medications and diagnoses can enhance the prediction of suicide in PTSD patients. |
| Zelkowitz et al., [32] | 3166 (1789/1377) | Mixed diagnoses (not specified) | Not specified |
RF, CART (10-folds CV) |
>700 demographic and clinical features | Nonfatal suicide attempt within 30 days |
RF AUC: 0.86 for men AUC: 0.86 for women CART AUC: 0.79 for men AUC: 0.81 for women |
Women: Histories of self-poisoning, substance-related disorders, and eating disorders were important predictors. Men: Self-poisoning, substance-related disorders, and severe stress reactions were among the most important variables. |
| Tubío-Fungueiriño et al., [52] | 127 (68/59) | OCD |
71 AD 56 AD + AP |
LDA, SVM, SVR (4-folds CV) |
28 Clinical features | Suicidal thoughts |
Sens: 0.79 Spec: 0.88 |
The most relevant features for prediction of suicidal thoughts are: trust, OCD onset, aggressive, previous YBOCS, diagnosis, OCD years, magical thinking, support, previous depression, contamination, sexual, hours info, recreation, and fear. |
| Yang et al., [54] | 59 MDD with suicidal ideation (42/17); 22 MDD without (10/12); 60 HC (33/27) | MDD | Not specified | SVM | Six features functional connectivity between five regions with amygdala (rsMRI) | Suicidal ideation |
Acc: 0.84 AUC: 0.82 |
The reported area that contributed to the prediction are: caudate, superior temporal gyrus, middle temporal gyrus, postcentral gyrus an their connection with amygdala |
| Nock et al., [37] | 1818 (802/1016) | Psychiatric patients presenting in an ED | Not specified | SuperLearner stacked generalization ML method (10-folds CV) | Patient self-report (101 features), EHR, clinician prediction | Suicide attempt within 1 and 6 months | AUC for Patient self-report + EHR + clinician prediction: 0.78 for 1 month prediction; 0.78 for 6 months prediction |
The prediction improved using a combination of patient self-reports and EHR data. The most important predictors resulted: past year suicide, past year suicide plan, >2 lifetime suicide attempts. |
| Shao et al., [58] | 113 (92/21) | Late-life MDD |
SSRI:42 SNRI: 21 Other AD: 41 Other drugs (AP, benzodiazepines etc): 90 |
SVM (LOOCV) | Clinical, sMRI and rsMRI features | Suicide attempts and suicide ideation |
Acc: 0.68–0.85 Sens: 0.57–0.87 Spec: 0.57–0.91 |
The MRI features significantly increased classification performance of suicidal thoughts and actions over that based on clinical and suicide questionnaire variables. Most relevant features: onset age, Parietal, VLPFC/OFC, DMPFC, Precuneus, and DLPFC GMV. |
| Kim et al., [76] | 44 (19/25) high-risk; 80 (34/46) low-risk adolescents | Adolescents with a psychiatric diagnoses (5 psychotic, 40 mood disorders, 42 ANX, 16 ADHD, 35 others) | Not specified |
LR, RF, ANN, SVM, XGB (10-folds CV) |
256 clinical and sociodemographic features | Low vs hig risk, based on suicide scale of the PAI-A |
LR Acc: 0.89 Sens: 0.77 Spec: 0.96 RF Acc: 0.89 Sens: 0.92 Spec: 0.88 ANN Acc: 0.78 Sens: 0.77 Spec: 0.79 SVM Acc: 0.89 Sens: 0.85 Spec: 0.92 XGB Acc: 0.86 Sens: 0.92 Spec: 0.83 |
Most relevant features from the PAI-A contributing to the predictions: anxiety and anxiety-related scores; depression; nonsupport in social life; treatment rejection. |
| Ji et al., [74] | 44 (34/10) 48 (28/20) MDD with suicide; MDD without suicide; 51 (28/23) HC | MDD | Not specified |
SVM, AdaBoost, NB (10-folds CV) |
105 clinical variables from clinical questionnaires (BDI, BSI, BHS, TDPPS, TEPS, STAI, BIS) | Suicide |
SVM Acc: 0.88 Sens: 0.87 Spec: 0.89 AdaBoost Acc: 0.82 Sens: 0.87 Spec: 0.78 NB Acc: 0.82 Sens: 0.75 Spec: 0.89 |
Clinical questionnaires features selected with LR proved to be able to classify the groups with good accuracy. Among the most used features: pain avoidance. |
| Li et al., [83] | 235 Recent suicide MDD (170/65); 111 Long-time suicide MDD (64/47); 1372 no suicide MDD (896/476) | First-episode MDD | Untreated | Gradient-boosted decision trees (5-folds CV) | Clinical, laboratory/biological, and demographic variables (a total of 65 variables) | Recent and long-time suicide |
Recent: Acc: 0.87 Sens: 0.59 Spec: 0.61 Long-time: Acc: 0.88 Sens: 0.83 Spec: 0.50 |
Most relevant features for recent suicide: higher score on excitement, hostility, HAMA, HAMD and higher FT4. Most relevant features for recent suicide: single status, higher score on HAMA and Hostility, higher Low-density lipoprotein cholesterol and lower BMI. |
| Jiang et al., [29] | 2774 (1168/1606) | MDD | Not specified | RF (10-folds CV) | Sociodemographic and clinical variables, assessed in a time-depended manner (total of 780 features for men and 803 for women) | Suicide |
Men AUC: 0.70 Women AUC: 0.75 |
Most important predictors for men: prescriptions of hypnotics and anxiolytics, poisoning diagnoses, prescriptions for analgesics and antipsychotics, and alcohol related disorders. Most important predictors for women: receipt of state pension, prescriptions for psychiatric medications (anxiolytics and antipsychotics), poisoning, prescriptions of analgesics and hormonal contraceptives for systemic use (a marker of relationship status). |
| McMullen et al., [31] | 571 (365/191/15) non-suicide; 20 (16/4) suicide | Psychiatric patients (298 MDD, 80 BD, 45 Anx, 43 SKZ, 64 PTSD, 34 Others) | Not specified |
RF, LR, gradient-boosted trees |
49 clinical features from the SCI and suicidal ideation | Suicide at 1 month follow-up |
RF AUROC: 0.88 Balanced Acc: 0.72 LR AUROC: 0.83 Balanced Acc: 0.58 Gradient boosted trees AUROC: 0.90 Balanced Acc: 0.77 |
The model which performed the best was the combination of the SCI features and current suicidal ideation; however, the increase in the AUROC was not significant, therefore reinforcing the view that suicidal ideation should not be considered a requirement. |
| Coley et al., [35] | 1518968 (563074/955894) | Psychiatric outpatients (74% MDD, 13% BD, 4% SKZ, 9% other) | Not specified |
LR, RF (10-folds CV) |
EHR from single visits | Suicide at 90-days |
RF AUC: 0.85 LR AUC: 0.85 |
Logistic regression and random forest models using a person-level split performed well, accurately estimating prospective discrimination and classification. |
| Chen et al., [59] | 33 (30/3) MDD with suicide attempts, 41 (23/18) MDD with suicide ideation, 54 (31/23) MDD without attempts or ideation, 58 (51/7) HC | MDD | Not Specified | CNN (5-folds CV) | Diffusion-MRI (3 T) | Suicide and suicidal ideation |
AUC: 0.54–0.96 Acc: 0.53–0.91 Sens: 0.31–0.95 Spec: 0.72–0.96 |
Generalized q-sampling imaging- based isotropic value of the orientation distribution function increased the prediction and seemed to possess a gradient pattern moving from ideation to suicide attempt. |
| Liu et al., [82] | 69 (39/30) MDD with suicide; 58 (28/30) MDD without suicide; 50 (26/24) HC | MDD | Not specified | SVM | Network and nodal properties form DTI MRI | Suicide | AUC: 0.79 | The differential structural network connections involved the superior longitudinal fasciculus and the corpus callosum and the feeder serves as a predictor for distinguishing MDD with suicide from MDD without. |
| Nordin et al., [75] | 75 (42/33) | MDD | Not specified |
LR, decision tree, SVM, naïve Bayes, k-nearest neighbors, RF, bagging and voting (3-folds CV) |
36 clinical and demographic features | Suicide |
Acc: 0.79–0.92 AUC: 0.65–0.87 Sens: 0.86–0.92 Spec: 0.50–0.60 PPV: 0.79–0.89 NPV: 0.72–0.76 |
Bagging and voting were the most effective algorithms. Previous history of suicide, suicidal ideation, race, religion, depression severity and medical problems resulted the most important features. |
| Nestsiarovich et al., [64] | 529359 patients with BD (301735/227624) | BD | Not specified |
Tree-based XGboost34 (Balanced-data-model), LR, RF, decision tree, linear SVC (5-folds CV) |
190,919 clinical covariates including age, sex, BD episode index visit characteristics, comorbid mental and physical conditions, medication prescriptions, mental health procedures ER admission in the last 1 year | Self-harm |
AUC: 0.99 Sens: 0.93 Spec: 0.98 |
Higher risk than lithium: tri/tetracyclic antidepressant+SGA, FGA + MSA, FGA, SNRI + SGA, lithium+MSA, and lithium+SGA. Lower risk: lamotrigine, valproate, risperidone, aripiprazole, SNRI, SSRI, “No drug”, bupropion, and bupropion+SSRI. Psychotherapy alone (without medication) had a lower self-harm risk than no treatment. |
| Jiang et al., [29] | 1205 Psychiatric patients | Mixed diagnoses (SUD, SKZ, mood disorders; anxiety, personality disorders) | Not specified |
Classification trees (10-folds CV), RF (2-folds CV) |
509/422 Clinical and demographic feature including diagnoses, surgeries, prescribed medications | Suicide in the 30 days after psychiatric discharge |
AUC 0.70 for men AUC 0.72 for women |
For men: anxiolytics and drugs interacted with other characteristics in the risk profiles (e.g., alcohol-related disorders, hypnotics and sedatives). In women, interaction between recurrent major depression and other characteristics (e.g., poisoning, low income). |
| Adams et al., [73] | 15,953 patients with SUD (5745/10208) | SUD | Not specified |
Classification tree, RF (10-folds CV) |
2563 social and demographic information, mental and physical health diagnoses, surgeries, medications, and poisonings | Suicide attempts |
Classification tree AUC: 0.75 RF AUC: 0.77 |
Among men, most important variables: reaction to severe stress, adjustment disorders, drugs used, age 30+, and prior poisoning. Among women, most important predictors: prior poisonings and reaction to stress and adjustment disorders. |
| Cusick et al., [50] | 600 psychiatric patients (370/230) | patients at risk for suicidal ideation (80% with a diagnoses of MDD) | Not specified |
SVM, CNN, Naive Bayes classifier |
NLP approach to label the training and validation notes (18815 notes) | Current suicidal ideation |
SVM Acc: 0.89% AUC: 0.93 CNN Acc: 94% AUC: 0.94 |
The CNN model outperformed all other methods on documents with “current” suicidal ideation. When applied to a random subset, the algorithm classified 23 for “current” suicidal ideation, of which 87% were truly indicative via manual review. |
| Bohaterewicz et al., [69] |
39 SKZ patients (14/25), 20 HC (10/10) |
SKZ | Not specified |
GB, LASSO, LR, RF, SVM (5-fold CV) |
rsMRI measures: ReHo, ALFF, fALFF, and FC. | Suicide risk measured with SBQ-R |
Best performance (LASSO): AUROC 0.76 Acc: 70% |
The best performance was reached for the LASSO applied to FC. |
| Machado et al., [89] | MDD | Not specified |
Elastic-net regularization, RF, ANN (10-folds CV) |
Stressful life events, and sociodemographic variables | Suicide attempts |
AUC: 0.89 Acc: 81.64% Spec: 85.86% Sens: 77.42% |
Previous suicide attempt, borderline personality disorder, and hospital admission for depressive symptoms were the most predictive features. | |
| Edgcomb et al., [38] | 1628 women with MDD, BD, and chronic psychosis | MDD (46%), BD (16%), psychotic disorder (51%) |
AD (71%) Anxiolytic (59%) AP (45%) Stabilizers (10%) |
Classification tree algorithm (10-folds CV) |
420 electronic health record including: medical comorbidity, history of pregnancy-related mental illness, age, and history of suicide-related behavior |
Risk factors of suicide attempt and self-harm |
AUC: 0.73 Acc: 0.84 Sens: 73.4 Spec: 84.1 |
Predictors included medical comorbidity, history of pregnancy-related mental illness, age, and history of suicide-related behavior. |
| Iorfino et al., [63] | 1962 young people presenting at mental health services (1184/778) | Mixed diagnoses (32% MDD; 32% Anxiety; 4% BD; 2% psychosis) | Not specified |
AUCRF, Boruta, Lasso regression, Elastic-net regression, BART, LR (10-folds CV) |
37 demographic and clinical variables | Self-harm at 6 months after initial presentation |
AUROC: 0.74–0.76 AUPCR: 0.31–0.35 Sens: 0.67–0.75 Spec: 0.68–0.72 PPV: 0.31–0.32 NPV: 0.91–0.93 |
The strongest predictors were: history of self-harm, age, social and occupational functioning, sex, BD, psychosis-like experiences, treatment with antipsychotics, and a history of suicide ideation. |
| Zhu et al., [79] | 37 BD II depressed with past suicide (27/10); 53 (33/20) BD II without past suicide (33/20); 64 HC (34/28) | BD II |
AD (38%) AP (12%) Stabilizers (6%) Stimulants (8%) |
SVM (5-fold CV, 1000 permutations) |
significantly different frontolimbic rsFCs between the SA and NSA groups for classification analysis | Past attempts | Acc: 84% | Top predictors: frontolimbic rsFCs. |
| Edgcomb et al., [38] | 3091 patients with MDD, BD, and psychotic disorders (1628/1463) | MDD (43%), BD (16%), psychotic disorders (55%) |
AD 67% AP 47% Anxiolytic 59% Stabilizers 9% |
CART (10-fold CV) |
22 categories (for a total of 355 predictors) from electronic health record |
Hospital readmission for suicide attempt or self-harm in 1 yr |
Acc: 79.66% AUC: 0.86 Sens: 81.9% Spec: 79.7% |
Incidence of suicide-related behavior highest after general non-psychiatric hospitalizations. Predictor combinations, rather than single risk factors, explained the majority of risk. |
| Hong et al., [53] | 41 Suicide attempters (28/13) and 25 suicide ideation (18/7) young with MDD | MDD |
AD 54% AP 4% Stabilizers 3% |
SVM - Recursive Feature Elimination (LOOCV) |
142 features including sMRI regions together with age, sex, HAMD score, and intracranial volume |
Attempters vs ideation without attempt |
BAC 78.59% Sens: 73.17% Spec: 84.0% PPV: 88.24% NPV 65.63% |
Right lateral OFC thickness, left caudal anterior cingulate thickness, left fusiform thickness, left temporal pole volume, right rostral anterior cingulate volume, left lateral orbitofrontal thickness, left posterior cingulate thickness, right pars orbitalis thickness, right posterior cingulate thickness, and left medial orbitofrontal thickness were the 10 top-ranked classifiers for suicide attempt. |
| Parghi et al., [30] | 591 high-risk psychiatric inpatients (381/195) |
Mixed diagnoses (MDD 50% Anxiety 8% BD 13% SKZ 7% PTSD 10% OCD 0.2%) |
Not specified |
LR, RF, gradient boosting |
49 features from the Suicide Crisis Inventory | Suicide attempt at a one‐month follow‐up |
RF AUROC 87.8% Gradient Boosting AUROC 89.4% |
The enhanced bootstrap approach considerably outperformed the other sampling approaches, with RF algorithms performing best in predicting positive cases of near-term suicidal behavior. |
| Chen et al., [33] | 126,205 psychiatric inpatients and outpatient (68,151/58,054) |
Mixed diagnoses (MDD 17% BD 8% SKZ 6% SUD 13% Anxiety 20% Borderline 4% ADHD 12%) |
Not specified |
Ensemble learning that combined predictions from elastic net penalized LR, RF, GB, and a NN (10-fold CV) |
425 predictors covering demographic, SES, electronic medical records, criminality, family history of disease and crime | Suicide attempt/death within 90 and 30 days |
AUC: 0.89 (30 days) AUC: 0.88 (90 days) |
Top predictors: Intentional self-harm past 1 year, unplanned psychiatric visit past 1 to 3 months, diagnosis of borderline personality disorder, MDD, antidepressants, anxiolytics, benzodiazepines, and antipsychotics. |
| Dai et al., [68] | 78 MMD (28/50) | MDD | Medication-naïve for at least two weeks | ISODATA clustering algorithm | fMRI connectivity pairs with significant difference over the well-defined groups | Suicide risk stratification | NA | The functional connectivity, locating mostly within the frontal-temporal circuit and involving the default mode network, were integrated to discriminative the gradual susceptibility of suicidal. |
| Fan et al., [57] | 205 suicide (139/66) and 2963 non-suicide patients (2275/688) | Both PTSD and BD | Lithium 8% |
LR, RF, decision tree, K-nearest neighbors, Naïve Bayes, SVM (5-fold CV) |
90 features from EMR including: demographic data, number of emergency department visits and diagnoses, medication usage within one year | Suicide ideation, attempts, and deaths |
Best performance (RF) TPR: 83% PPV: 91% NPV: 98% |
The use of Aripiprazole, Levomilnacipran, Sertraline, Tramadol, Fentanyl, or Fluoxetine, a diagnosis of autistic disorder, schizophrenic disorder, or SUD were strong predictors. |
| Obeid et al., [62] | 835 intentional self-harm and 1670 HC | Intentional self-harm | Not specified |
Naïve Bayes, decision tree, RF, SVM, multilayer perceptron, CNN, long short-term memory with randomly initialized word embeddings. |
Text from clinical notes | Concurrent and future self-harm |
Best model: CNN Concurrent self-harm, AUC: 0.98 Future self-harm AUC: 0.88 |
The AUC for the CNN on the phenotyping task, that is, the detection of intentional self-harm in clinical notes concurrent with the events was 0.999, with an F1 score of 0.985. |
| Agne et al., [88] | 959 OCD patients (546/413) | OCD | Not specified | Elastic net (10-CV) | 89 features from demographic and clinical variables | Suicide attempts |
AUC: 0.95 Sens: 84.61% Spec: 87.32% BAC: 85.97 PPV: 44.89% NPV: 97.89% |
Relevant predictors: previous suicide planning, previous suicide thoughts, lifetime depressive episode, and intermittent explosive disorder. |
| Haines-Delmont et al., [67] | 80 Inpatients (45/35) | Mixed diagnoses (Not specified) | Not specified |
RF, LR, SVM, K-nearest neighbors (10-fold CV) |
172 features from sleep data, journal entries, data usage, mood, and app activity statistics | Low vs high suicide risk based on C-SSRS |
K-nearest neighbors Acc: 68% AUC: 0.65 RF Acc: 60% LR Acc: 59% SVM Acc: 57% |
K-nearest neighbors (k = 2) with uniform weighting and the Euclidean distance metric emerged as the most promising algorithm. |
| Kessler et al., [87] | 391.018 psychiatric patients from US veterans | Mixed diagnoses (psychotic disorders, mood disorders, personality disorders) | Not specified | Ensemble learning, from SVM, RF, NN, LR, elastic net, Bayesian additive regression trees | 57 features from EHR, including history of suicidal behavior, psychopathological risk factors, socio-demographic, physical disorders, medications | Suicide death up to 12 months after psychiatric hospitalization | AUC: 0.79–0.82 | Variable importance analysis shows that 51.1% of model performance is due to psychopathological risk factors, 26.2% to social determinants of health, 14.8% to prior history of suicidal behaviors, and 6.6% to physical disorders. |
| Roglio et al., [99] | 689 cocaine-use disorder (442/247) | Cocaine-use disorder | Not specified |
Descriptive Poisson regression and predictive RF (5 and 10-folds CV) |
57 Features from sociodemographic, ASI-6, the SCID-I and the CTQ | Suicide attempts |
For men: AUC: 0.68, Acc: 0.66, Sens: 0.82, Spec: 0.50, PPV: 0.47 NPV: 0.84 For women: AUC: 0.73, Acc: 0.71, Sens: 0.71, Spec: 0.71, PPV: 0.71 NPV: 0.71 |
This model identified several variables as important predictors, mainly related to drug use severity. |
| Senior et al., [66] | 57 patients with severe mental illness (SKZ spectrum and BD) (23/34) | Mixed diagnoses (SKZ spectrum disorder and BD spectrum) |
AD: 33% AP: 89% |
Named entity recognition based on NN | EHR (free-text): history of violence, self-harm, medication, formal education, benefits recipient, drug/alcohol, parental suicide, psychiatric admission | Extracting concepts related to predictors of suicide in the OxMIS tool | Precision 0.77 | The concept with the best precision and recall was medication and the weakest were suicide, and drug/alcohol use disorder. |
| Ge et al., [51] | 1994 MDD patients with or without suicidal ideation (1377/617) | MDD | Not specified |
NN (10-folds cross training) |
31 predictors from demographic, clinical, and biological variables | Suicidal ideation |
AUC: 0.76 Acc: 70.08% Sen: 70.68% Spec: 67.09% |
The most relevant predictor variables included free thyroxine, the total scores of HAMD, vocational status, and free triiodothyronine. |
| Weng et al., [49] | 41 MDD with suicidal ideation (23/18); 54 MDD without suicidal thoughts (31/23); and 58 HC (51/7) | MDD | Not specified |
CNN-based autoencoder model, XGB, LR (5-folds CV) |
25,168 features extracted from diffusion brain imaging, containing generalized FA, isotropic value of the orientation distribution function, and normalized quantitative anisotropy maps | Suicidal ideation |
CNN ACC: 85% Spec: 92% Sens: 75% AUC: 0.94 LR ACC: 80% Spec: 92% Sens: 62% AUC: 0.93 |
The best pattern of structure across multiple brain locations can classify suicidal ideates from non-suicidal and HC with a prediction accuracy of 85%, a specificity of 100% and a sensitivity of 75%. |
| Tasmim et al., [105] | 189 SKZ patients (76/113) | SKZ | Not specified |
LR, RF, classification tree (10-folds CV) |
23 items from the LEI-2 scale | Suicide attempts |
LR: Acc: 62% Sens: 38% Spec: 77% PPV: 50% NPV: 67% RF: Acc: 62% Sens: 15.3% Spec: 93.2% PPV: 57.9% NPV: 64.1% Classification tree: Acc: 69% Sens: 36% Spec: 89% PPV: 67% NPV: 69% |
The items “suffering from mental illness” and “sexual molestation” were classified as highly important in all three models. |
| Kumar et al., [61] | 10,120,030 patients with major psychiatric diagnoses (6,780,420/3,339,610) | Mixed diagnoses (SKZ, schizoaffective disorder, BD, and MDD) | Not specified |
Tree-based XGboost (Balanced-data-model), LR, RF, decision tree, linear SVC (5-folds CV) |
185,234 unique clinical covariates (including patient age, sex, meta-visit start year, and nine feature classes: Manually curated, Procedure, Condition, Drug, Billing Code Position, Device, Observation, Measurement, and Ancestor terms.) | Self-harm |
Acc: 0.94–0.96 AUC: 0.65–0.99 |
Self-harm undercoding was higher in male than in female and increased with age. Substance abuse, injuries, poisoning, asphyxiation, brain disorders, harmful thoughts, and psychotherapy were the main features. |
| Bhak et al., [106] | 56 MDD with suicide attempts (30/26); 39 MDD without attempts (18/21); 87 HC (44/43) | MDD | AD 93% for attempters; 95% for non-attempters | RF | 69 methylated sites from whole genome methylome, after feature selection (LOOCV) | Suicide attempts |
Acc: 0.92 Sens: 0.98 Spec: 0.85 PPV: 0.90 NPV: 0.97 |
RF classifiers showed good accuracies in distinguishing attempters. |
| Xu et al., [41] | 2323 patients with self-harm (1163/1160) and 46,460 inpatients controls (23,260/23,200) | Self-harm (Not specified) | Not specified | Patient embedding method, Dx2Vec (Diagnoses to Vector) followed by NN | 19 features from comorbidities | Risk prediction at 12-month |
Precision 0.54 Sens: 0.72 for positive cases |
Dx2Vec-based model outperforms the baseline deep learning model in identifying patients who would self-harm within 12 months. |
| Peis et al., [48] | 1023 patients (662/361) | Mixed diagnoses (Mood disorder 23%, anxiety disorders 53%) | Not specified | Recurrent NN | 117 features from EMA in combination with traditional EHR | Suicide ideation |
Acc: 95% AUC 0.94 |
Addition of EMA records boosts the prediction of suicidal ideation diagnosis from 48.13% obtained exclusively from EHR-based state-of-the-art methods to 67.78%. |
| Gosnell et al., [81] | 423 psychiatric inpatients (Not specified) | Mixed diagnoses (mood, anxiety, personality, and SUD) | Not specified | RF (LOOCV) | Structural (316) and resting-state functional connectivity (8256) measures | Suicide attempts |
Sens: 79.4% Spec: 72.3% |
Altered resting-state functional connectivity features from frontal and middle temporal regions, as well as the amygdala, parahippocampus, putamen, and vermis were found to generalize best. |
| Carson et al., [109] | 73 adolescents psychiatric inpatients (45/28) | Mixed diagnoses (Not specified) | Not specified |
RF (5-folds CV) |
Notes from electronic medical records | Suicide attempts in the past year |
AUC: 0.68 Acc: 0.47 Sens: 0.83 Spec: 0.22 PPV: 0.42 NPV: 0.67 |
The terms mostly highly associated clustered around terms related to suicide, family members, psychiatric disorders, and psychotropic medications. |
| Fernandes et al., [84] | Not specified | Mixed diagnoses (Not specified) | Not specified | SVM after NLP | 500 notes from correspondence and questionnaires |
Suicide attempts Suicide ideation |
Attempts PPV: 91.7% Sens: 87.8% Ideation PPV: 82.8% Sens: 98.2% |
Good performance of the two classifiers in the evaluation study suggest they can be used to accurately detect mentions of suicide ideation and attempt within free-text documents. |
| Jordan et al., [40] | 218 psychiatric patients with previous attempts (109/109) | Mixed diagnoses (Psychosis 10%; MDD 79%; BD 11%; personality disorder 73%; alcohol/SUD 62%) | Not specified |
IROC analysis, LR |
Variables from sociodemographic and clinical scales | 1-year suicide attempt after discharge |
AUC: 0.63 Sens: 55.77% Spec: 69.11% PPV: 43.28% NPV: 78.70% |
The cross-validated IROC, but not logistic regression, predicted attempts. Furthermore, participants who made definite plans and underwent extensive preparation were at highest risk. |
| Oh et al., [110] | 573 anxiety or MDD patients (306/267) | Anxiety and MDD | Not specified | NN | 41 variables: 31 psychiatric scales and 10 sociodemographic variables | Lifetime, past year, past month suicide attempts |
1-month: Acc: 93.7% Spec: 99.6% Sens: 12.8% 1-year: Acc: 90.8% Spec: 98.4% Sens: 33.8% Lifetime: Acc: 86.4% Spec: 91.2% Sens: 77.9% |
Among all variables, the Emotion Regulation Questionnaire had the highest contribution, and the positive and negative characteristics of the scales similarly contributed to classification performance. |
| Hettige et al., [111] | 345 patients with SKZ (104/241) | SKZ | Not specified |
LASSO, RF, SVM, elastic net (10-folds CV) |
27 sociocultural and clinical variables | Suicide attempts |
Lasso AUC: 0.71 Acc: 0.67 Sens: 0.64 Spec: 0.68 PPV: 0.67 NPV: 0.66 RF AUC: 0.67 Acc: 0.66 Sens: 0.45 Spec: 0.80 PPV: 0.68 NPV: 0.60 SVM AUC: 0.70 Acc: 0.66 Sens: 0.63 Spec: 0.68 PPV: 0.67 NPV: 0.66 Elastic Net: AUC: 0.71 Acc: 0.65 Sens: 0.65 Spec: 0.65 PPV: 0.65 NPV: 0.66 |
The four models did not differ in pair-comparison as tested using the McNemar’s test. The weighs of the predictors differ a lot between the four models. |
| Setoyama et al., [46] | 94 affective patients (Not specified) | MDD | Both medicated and non-medicated patients |
LR, SVM, RF |
123 metabolites from metabolome analysis | Suicidal ideation | AUC 0.60–0.79 | Kynurenine, Xanthurenate, Xanthosine, Citrate and Alanine correlated with suicide. |
| Pestian et al., [112] |
130 suicidal patients, 126 non-suicidal patients with mental illness, and 123 controls (Not specified) |
Mixed diagnoses (Not specified) | Not specified | SVM (LOOCV) | Linguistic and Acoustic Features extracted from open-ended questions | Suicide attempts |
Suicidal vs HC ROC: 0.92 Suicidal vs non-suicidal Patients: ROC: 0.82 Suicidal vs All: ROC: 0.87 |
By combining linguistic and acoustic characteristics, subjects could be classified into one of the three groups. |
| Barros et al., [65] | 707 mental health patients (564/143) |
Mixed diagnoses (MDD 53% BD 18% Anxiety 10% Adjustment 10% Dysthymia 1% Others 8%) |
Not specified |
CART, k-nearest neighbor, RF, AdaBoost, NN multilayer perceptron, SVM (10-folds CV) |
343 sociodemographic and clinical variables | Suicide risk (current suicidal behavior – attempts o ideation) |
SVM Acc: 0.78 Sens: 0.77 Spec: 0.79 CART Acc: 0.72 Sens: 0.71 Spec: 0.74 RF Acc: 0.78 Sens: 0.78 Spec: 0.77 AdaBoost Acc: 0.76 Sens: 0.75 Spec: 0.76 KNN Acc: 0.73 Sens: 0.74 Spec: 0.73 |
The model shows that the variables of a suicide risk zone are related to individual unrest, personal satisfaction, and reasons for living, particularly related to beliefs in one’s own capacities and coping abilities. |
| Cook et al., [45] | 1453 self-harming patients (944/509) | Self-harm (Not specified) | Not specified | NLP-Based Machine Learning (linear classifier) | Open-ended question from a medical app | Suicidal ideation |
Sens: 0.56 Spec: 0.57 PPV: 0.61 |
The top ten words associated with suicidal ideation were conté (I told), monotona (monotony), Equasim (Ritalin), acosado (harassed), trabajamos (we work), raza (race), aseos (restrooms), resfriado (congested/sick), pronuncio (I pronounce), and rechaza (rejects). |
| Kessler et al., [44] | Veterans with a mental disorder diagnosis (Not specified) | Mixed diagnoses (Not specified) | Not specified |
naive Bayes, RF, SVR, elastic net penalized regression |
Nearly 1000 variables from sociodemographic, career, medical records | Suicide deaths after a psychiatric visit | AUC: 0.61–0.75 | An elastic net classifier with 10–14 predictors optimized sensitivity (45.6% of suicide deaths occurring after the 15% of visits with highest predicted risk). |
| Walsh et al., [4] | 5167 patients with a self-injury diagnosis (2432/2706/29) | Self-harm (Not specified) | Not specified | RF | >200 features Demographic and clinical | Suicide attempts | AUC: 0.84 | Recurrent depression with psychosis, SKZ, and schizoaffective disorder were ranked highly in importance. |
| Morales et al., [56] | 707 affective patients (564/143) |
Mixed diagnoses (MDD 53% BD 18% Anxiety 10% Adjustment 10% Dysthymia 1% Others 8%) |
Not specified | Decision tree | 345 variables from clinical scales | Suicidal behavior (attempt or ideation) |
Acc: 0.67–0.71 Spec: 0.65–0.79 |
Suicide risk configuration variables: thought about taking one’s life, frequent headaches, dissatisfied or not very satisfied with life, empty inside, and not feeling happy, depressive lifestyle. |
| Passos et al., [91] | 144 patients with mood disorders (Not specified) | MDD or BD types I or II | Not specified |
LASSO, SVM, RVM (LOOCV) |
17 clinical and demographic variables | Suicide attempts |
RVM AUC: 0.77 BAC: 72% Sens: 72% Spec: 71% PPV: 51% NPV 86% SVM AUC: 0.65 BAC: 65% Sens: 58% Spec: 71% PPV: 46% NPV 80% LASSO AUC: 0.73 BAC: 68% Sens: 56% Spec: 80% PPV: 55% NPV 81% |
All algorithms had significant prediction accuracy (64.7%–72%). RVM yielded the highest result. The most relevant predictor variables included: number of previous hospitalizations for depression, psychosis, cocaine dependence, PTSD. |
| Levey et al., [43] | 51 women with psychiatric disorders | Mood disorders and SKZ | Not specified | Convergent Functional Genomics followed by ROC curve | 50 genetic derived biomarkers combined with clinical scales | Suicidal ideation and future hospitalization for suicide | AUC: 0.94 for the genetic predictors |
50 validated biomarkers predicted future hospitalizations due to suicidality. Best predictors: BCL2, GSK3B, and PIK3C3, circadian clock genes (PER1 and CSNK1A1), docosahexaenoic acid signaling pathways genes. |
| Niculescu et al., [42] | 37 males with psychiatric disorders | Mood disorders (BD 15, MDD 7), SKZ (6), schizoaffective (4), PTSD (3), Others (2) | mood stabilizers; AD; AP; benzodiazepines; and others (percentages not available) | Convergent Functional Genomics followed by ROC curve | 76 genetic derived biomarkers | Suicidal ideation and future hospitalization for suicide |
Transdiagnostic AUC: 0.92; BD AUC: 0.98 |
The best individual biomarker across psychiatric diagnoses for predicting suicidal ideation was SLC4A4. |
| Kessler et al., [39] | Soldiers with a psychiatric diagnoses and hospitalization (Not specified) | Mixed diagnoses (Not specified) | Not specified | Elastic net (10-folds CV) | 421 predictors from demographic, career and clinical variables | Suicides in the 12 months after hospital discharge | AUC:0.71–0.84 | The strongest predictors included sociodemographics (male sex and age), criminal offenses, prior suicidality, prior psychiatric treatment, and diagnosis. |
| Tran et al., [34] | 7578 mental health patients (3842/3736) | Mixed diagnoses (Not specified) | Not specified | Non-negative restricted Boltzmann machines followed by SVM (10-folds CV) | EMR | Suicide risk stratification 3 months | NA | The derived representation not only shows clinically meaningful feature grouping but also facilitates short-term risk stratification. |
| Poulin et al., [113] | 70 HC; 69 suicide attempters; 70 psychiatric patients (Not specified) | Mixed diagnoses (Not specified) | Not specified |
Bags of words by Meta-Optimizing Semantic Evolutionary Search (5-folds CV) |
Words from clinical records (27–77 clinical records for each subject) | Suicide attempts | Acc: 65% |
For single-word models, the accuracy was 59%, and scores for individual candidate models ranged from 46–65%. For pre-selected word pairs, the individual model scores ranged from 52–69%, with an average of 64%. The combined Cohorts ‘1v2v3 classifier’ had a peak performance of 67%, and an average performance of 65%. |
| Delgado-Gomez et al., [114] | 345 suicide attempters, 150 psychiatric inpatients and 384 HC (Not specified) | Mixed diagnoses (Not specified) | Not specified |
Linear discriminant analysis, Fisher linear discriminant analysis, boosting, SVM |
30 features from the BIS-11 and 77 from the IPDE-SQ | Suicide attempts |
SVM Acc: 79% Sens: 66% Spec: 87% Boosting Acc: 77% Sens: 64% Spec: 85% Linear discriminant Acc: 77% Sens: 65% Spec: 85% Fisher linear Acc: 78% Sens: 65% Spec: 88% |
The most discriminative BIS-11 and IPDE-SQ items are “I am self controlled” (Item 6) and “I often feel empty inside” (item 40), respectively. |
| Lopez-Castroman et al., [71] | 1349 suicide attempters (1140/209) |
Mixed diagnoses (MDD 72% BD 15% Anxiety 52% Psychotic disorders 3% Eating disorder 12% OCD 5%) |
Not specified |
SVM (10-folds CV) |
Ten clinical and demographic variables selected through Markov Blanket | Number of suicide attempts |
Sens: 51–67% Spec: 68–98% |
Risk of frequent suicide attempt was highest among middle-aged subjects and diminished with advancing age. Anxiety disorders significantly increased the risk. Frequent suicide attempts were linked to alcohol and SUD and more intensive treatment. |
| Baca-Garcia et al., [98] | 277 male psychiatric patients |
Mixed diagnoses (Attempters: SUD 47%, SKZ 13%, MDD 55%, BD 7%, Anxiety 32%, OCD 2%, Adjustment 5%, somatoform 2%, eating disorders 1%. Non-attempters: SUD 27%, SKZ 42%, MDD 22%, BD 10%, Anxiety 34%, OCD 24%, Adjustment 1%, eating disorders 1%. |
Not specified | SVM | 840 SNPs | Suicide attempts |
Sens: 0.50 Spec: 0.82 positive likelihood ratio: 2.80 negative likelihood ratio: 1.64 |
Three SNPs of three genes (rs10944288, HTR1E; hCV8953491, GABRP; and rs707216, ACTN2) correctly classified 67% of male suicide attempters and non-attempters. |
| Ilgen et al., [92] | 887,859 veterans with depression (Not specified) | MDD | Not specified | Decision tree based on Bayesian Dirichlet Equivalent | Clinical and demographic features | Suicide attempts | NA | Most relevant factors: a co-occurring SUD diagnosis, male sex, African American race, and psychiatric hospitalization in the past year. |
| Mann et al., [86] | 408 patients with mood, SKZ spectrum, or personality disorders (Not specified) |
Mixed diagnoses (Attempters: mood disorders, 54%; SKZ spectrum disorders, 34%; others 22%. Non-attempters: mood disorders, 58%; SKZ spectrum disorders. 37%; others 16% |
Not specified |
CART (LOOCV) |
25 features from sociodemographic, diagnostic and clinical scales | Recent or remote suicide attempts |
Recent attempt: Sens: 73% Spec: 80% PPV: 58% Remote suicide attempt: Sens: 89% Spec: 36% PPV: 44% |
In equally weighted trees, a recent past suicide attempt was best predicted by current suicidal ideation and no adequate model was found for remote attempts. In unequally weighted models, recent attempters were identified by suicidal ideation and comorbid borderline personality disorder. Remote attempters were identified by lifetime aggression and current subjective depression. |
| Baca-Garcia et al., [72] | 539 suicide attempters (343/196) |
Mixed diagnoses (Mood disorders 41%, SUD 24%, SKZ 8%, eating disorders 5%, Anxiety 5%) |
Not specified |
RF, Forward Selection |
101 clinical variables | Family history of suicide |
Acc: 92.1–98.7% Sens: 78.4%–88.2% Spec: 92.5–96.6% |
A classificatory model for family history of attempted suicide included the use of alcohol in the intent and family history of completed suicide. |
| Tiet et al., [28] | 34,251 SUD veterans (33,242/1009) | SUD | Not specified | Decision tree | Demographic factors, diagnoses, and the core ASI items | 30-day risk of an actual suicide attempt |
Sens: 0.33–0.89 Spec: 0.42–0.87 |
The factors included encompass history of prior attempts, drinking to intoxication, cocaine use, first occasion of suicidal ideation, and difficulty controlling violent behavior. |
| Modai et al., [21] | 987 psychiatric inpatients (310/677) |
Mixed diagnoses (SKZ 56%, Schizoaffective 12%, MDD 3%, BD 3%, personality disorders 7%, anxiety 2%, others 18%) |
Not specified | FALCON NN | 20 suicide risk factors variables | Medically serious suicide attempts |
Sens: 91%–94% Spec: 82%–85% |
Trained FALCON, a nonlinear neural network, achieves respectable accuracy in detecting patients based on 20 suicide risk factors. |
| Modai et al., [21] | 612 psychiatric patients (185/427) |
Mixed diagnoses (SKZ 72%, schizoaffective 11%, MDD 1%, BD 3%, anxiety 0.5%, personality disorders 4%, organic syndromes 4%, others 15%) |
Not specified | FALCON NN | 21 features from CSRS-III | Medically serious suicide attempts |
Acc: 71.5% Sens: 83% Spec: 70% |
The influence of the various risk factors differed for diagnoses. |
| Modai et al., [115] | 197 inpatients (Not specified) | Mixed diagnoses (Not specified) | Not specified |
FALCON NN; Backpropagation enhanced with fuzzy logic |
59 suicide-associated variables | Medically serious suicide attempts |
Sens: 94 + 6.9% Spec: 69 + 6.9% |
BP-fuzzy logic trained with programmer input sets, with mean + SD results of 97 + 3.4% sensitivity and 69.25 + 6.9% specificity. The second was the FALCON trained with 15 suicide-associated clinical variables where mean + SD results were 94 + 6.9% sensitivity and 69 + 6.9% specificity. |
| Modai et al., [93] | 198 patients (Not specified) | Mixed diagnoses (Not specified) | Not specified |
Backpropagation NN; LR |
44 demographic and clinical variables | Medically serious suicide attempts |
TSR: 91.8% PPV: 92% NPV: 95.6% |
Living alone, treatment compliance, drug abuse or dependence, GAF score, non-paranoid delusions and suicide of first degree relative were highly associated. |
| Modai et al., [116] | 161 hospitalized psychiatric patients (Not specified) | Mixed diagnoses (Not specified) | Not specified | Backpropagation NN | 54–150 records | Medically serious suicide attempts |
Sens: 63–71% Spec: 60–95% PPV: 30–75% NPV: 90–91% |
At present, neural networks are not reliable instruments for evaluating suicidal risk due to the significant number of false positive results. |
Acc: accuracy, AD antidepressants, ADHD attention deficit hyperactivity disorder, ALFF amplitude of low frequency fluctuations, ANN artificial neural networks, AP antipsychotics, ASI addiction severity index, AUC area under the curve, AUCRF area under the curve RFs, AUROC area under the receiving operator curve, AUDIT alcohol use disorders identification test, AUPRC area under the precision-recall curve, AUROC area under receiver operating curve, BAC balanced accuracy, BART Bayesian additive regression trees, BD bipolar disorder, BDI Beck depression scale, BHS Beck hopelessness scale, BIS-11 Barratt’s impulsiveness scale version 11, BSI: Beck scale for suicidal ideation, CART classification and regression tree algorithm, CNN convolutional neural network, CT childhood trauma questionnaire, CV cross-validation, C-SSRS Columbia-suicide severity rating scale, CSRS-III computerized suicide risk scales, DLPFC dorsolateral prefrontal cortex, EMA ecological momentary assessment, EHR electronic health records, EMR electronic medical records, FA fractional anisotropy, FALCON fuzzy adaptive learning control network, FC functional connectivity, FGA first generation antipsychotic, GAF global assessment of functioning, GB gradient boosting, HAMA Hamilton anxiety scale, HAMD Hamilton depression scale, HC healthy control, 1H-MRS proton magnetic resonance spectroscopy, IPDE-SQ international personality disorder evaluation screening questionnaire, ISODATA iterative self-organizing data analysis techniques, IROC iterative receiver operator characteristic analysis, KNN k-nearest neighbor LEI-2 life event inventory, LDA linear discriminant analysis, LOOCV leave-one-out cross-validation, LR logistic regression, MDD major depressive disorder, MIN: mini-international neuropsychiatric interview, MSA mood-stabilizing anticonvulsant, NLP natural language processing, NN neural network, NPV negative predictive value, NSSI non-suicidal self-injury, OCD obsessive-compulsive disorder, OFC orbitofrontal cortex, OxMIS Oxford mental illness and suicide tool, PAI-A personality assessment inventory for adolescent, PPV positive predictive value, PTSD post-traumatic stress disorder, ReHo regional homogeneity, RF random forest, RNN recurrent neural network RVM relevant vector machine, SBQ-R suicide behavior questionnaire—revised. SCI suicide crisis inventory, SCID-I structured clinical interview for DSM-IV axis I disorders, Sens sensitivity, SES socioeconomic status, SGA: second-generation antipsychotic, SNP single nucleotide polymorphism, SNRI serotonin and noradrenaline reuptake inhibitors, SKZ schizophrenia, SNP single nucleotide polymorphism, Spec specificity, SSRI selective serotonin reuptake inhibitors, STAI state-trait anxiety inventory, SUD substance-use disorder, SVM support vector machine, SVR support vector regression, TDPPS three-dimensional psychological pain scale, TEPS temporal experience of pleasure scale, TPR true positive rate, TSR total success rate, VLPFC ventrolateral prefrontal cortex, XGB extreme gradient boosting.
Description of outcome employed
Regarding the predicted outcome, 41 (51%) studies used ML to predict lifetime suicide attempts (e.g., retrospective assessed past attempts), while only 16 (19.7%) longitudinally assessed the risk of suicide using future risk/attempts as an outcome. Specifically, five studies [28–32] predicted the attempts/death at 1 month after the actual evaluation, the study by Chen and colleagues [33] predicted suicide attempts at both one and 3 months from the assessment, while three studies [34–36] predicted suicide risk at three months, and Nock and colleagues [37] predicted suicide between 1 and 6 months. Three studies [38–40] predicted suicide attempts at 12 months, and one study [41] stratified suicide risk at 12 months after the actual assessment. Finally, three studies [42–44] predicted future hospitalization for suicide or future suicide attempts without defining a precise temporal window.
Moreover, 14 studies predicted suicide ideation alone [45–55] or in combination with suicide attempts [56–60]. Finally, other studies predicted self-harm [61–64], suicide risk [38, 55, 65–70], the number of suicide attempts [71], and the presence of a familiar history of suicide [72].
Description of ML algorithms used
Regarding the number and type of ML approaches employed in the studies, 46 (57%) of the retrieved papers used a single ML algorithm, while 35 (43%) employed more than one. Among those employing more than one ML method, the average number of ML algorithms used was 3.8, with a range from 2 to 7. The most used algorithms were random forest (RF) and support vector machine (SVM), which were employed 29 times each, followed by neural networks-based approaches and decision tree-based approaches, employed 22 and 18 times, respectively. Other ML approaches were used more scarcely: elastic net eight times, Bayesian-based approaches six times, and clustering methods only four times.
Among studies adopting only one ML algorithm, neural networks were used 12 times, SVM 11, RF 5, tree-based approaches 4 times, and elastic nets three times.
In the studies that compared more than one algorithm, ML methods always performed better than LR. Moreover, RF [32, 57, 73] and SVM [74, 75] resulted among the best-performing algorithms, often with comparable results [65, 76], when compared to other methods. Finally, when present, CNN outperformed other ML methods [49, 50, 62, 77], including SVM and RF (please see Supplementary Table 4 for further details).
Description of the sample sizes and most assessed diagnoses
Sample sizes varied substantially across studies, ranging from 37 [42] to 10,120,030 [61] individuals, with an average of 230,074.5 and a standard deviation of 1,392,637. More in detail, twelve studies (14.8%) enrolled less than 100 participants, 27 studies (33.3%) enrolled between 100 and 500 individuals, 12 studies (14.8%) between 500 and 1000, 15 studies (18.5%) between 1000 and 10,000 and the remaining ten studies (12.3%) more than 10,000 subjects. For six studies, it was not possible to retrieve the exact number of participants included in the analysis.
Given the relatively low prevalence of the event of interest (i.e., suicide), most of the samples were unbalanced in terms of the number of subjects in each group. For instance, in the studies conducted by Fan and colleagues [57] and Wang and colleagues [77], the difference in size between the suicidal group and the non-suicidal control group was tenfold (i.e., 205 subjects in the “suicide” group and 2963 in the “no suicide” group). Similarly, the difference in Xu et al. [41] was 20-fold, with 2323 patients reporting self-harm and 46,460 patients with no self-harm characteristics. It is important to note that, on the one hand, very large differences in sample size require significant corrections in the predictive algorithm (e.g., the weighting of the hyperplane for uneven group sizes), whereas, on the other hand, they reflect real data, as the prevalence of suicidal events in the assessed population is typically low.
Regarding the psychiatric diagnoses, 45 studies (55.5%) included more than one diagnosis in their sample and assessed the risk of suicide in a transdiagnostic manner, whereas 36 studies (45.5%) focused on patients with a single specific diagnosis. Not all the studies reported full details regarding the diagnostic status of the included sample, with some of them only referring to “psychiatric patients” to describe the sample.
Among reports detailing patients’ diagnosis, mood disorders were prevalent in 64 studies (79%). Specifically, major depressive disorder (MDD) was studied in 37 investigations, and bipolar disorder (BD) in 21 publications. Six studies simply reported “mood disorders” to characterize the sample. Patients affected by schizophrenia were included in 14 studies, whereas four enrolled patients diagnosed with schizoaffective disorder and five simply reported “psychosis” as a sample description. Thirteen studies focused on anxiety disorders, eight on substance-use disorders and four on obsessive-compulsive disorders.
Finally, among studies focusing on a single diagnosis, MDD was the most represented one (16 times), followed by BD, schizophrenia, and substance-use disorders represented three times each.
Description of the number and types of features
The number of features employed in the prediction of suicidal behaviors varied considerably across studies, ranging from 10 [71] to 190,919 [64]. Specifically, 20 studies (24.7%) predicted suicide with less than 50 features, seven studies (8.6%) employed between 50 and 100 features, 11 (13.6%) between 100 and 200, ten (12.3%) between 200 and 500, and, lastly, 11 studies (13.6%) employed more than 500 features. In addition, 22 studies (27.1%) did not report the exact number of features being fed to the algorithm for suicide prediction.
As far as the feature types are concerned, the majority of the studies (54, 66.6%) used clinical and sociodemographic variables. Among these, ten studies were based on electronic health records (EHR), which are becoming an important source of data in the last few years [78].
Ten studies employed brain imaging data to predict suicide: seven studies used resting-state MRI (rsMRI) [54, 55, 60, 68, 69, 79, 80], two used both rsMRI and structural MRI [58, 81], three used diffusion tensor imaging (DTI) [49, 59, 82], and one structural MRI in combination with clinical and demographic data [53], and one single study employed measures from spectroscopy [47]. Eight studies (13.6%) analyzed the text obtained from interviews and EHR using natural language processing (NLP).
Only four studies (4.9%) focused on genetics and epigenetics features in order to predict suicide, and a single study [46] explored the predictive value of the human metabolome, employing 123 plasma metabolites, to predict suicide. Lastly, three studies [36, 51, 83] used blood biochemistry in association with clinical and sociodemographic data.
Description of AUC and accuracy ranges
A total of 62 studies (76.5%) reported at least the accuracy or the area under the curve (AUC) of their prediction, while the remaining studies reported different metrics (e.g., positive predictive value, sensitivity F1 score [84]), also because of the methods employed (e.g., clustering and neural networks [41, 68]).
Interestingly, 87% of studies (i.e., 54 out of 62) focusing on either prediction accuracy or AUC reported values above 70% or 0.70, respectively. Specifically, eleven studies reported an accuracy between 70 and 80%, 14 between 80 and 90%, and six studies above 90%. Regarding AUC, 14 studies showed AUC between 0.70 and 0.80, 16 between 0.80 and 0.90, and eleven studies reported AUC above 0.90. The AUC of selected studies is reported in Fig. 2 as a function of sample sizes and number of features. Nonetheless, besides a few notable exceptions [38, 42, 43], no studies tested their prediction on independent validation samples. However, it is noticed that in highly unbalanced samples, the lack of an independent validation sample greatly reduces the overall generalizability. Therefore, these findings are likely to suffer from overfitting and should be regarded with caution [85].
Fig. 2. Graphical representation of the AUCs as a function of the number of features and the sample size.
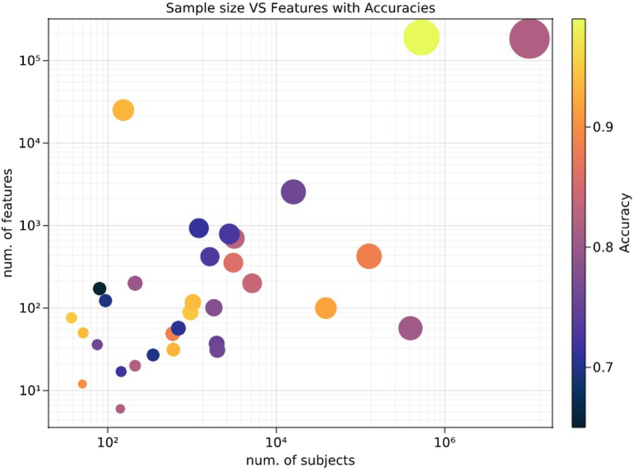
When the authors performed more than one analyses using the same features and sample, the highest prediction value was used for the present graph. Features number and sample size are reported in a logarithmic scale. The color bar indicates the prediction rate. Good predictions are reached even with a limited number of subjects and features. However, this graph does not hold any meta-analytic value, given the differences between the studies.
Most relevant features
Studies employing clinical and sociodemographic variables confirmed previous suicide risk factors. Previous suicide attempts, suicidal behaviors, or self-harm acts were among the strongest and most replicated predictors [28, 32, 33, 37–39, 61, 63, 71, 73, 75, 86–90]. Similarly, the type and severity of the psychiatric diagnosis seem to be associated with an increased risk of suicide. In detail, diagnosis and severity of MDD [4, 33, 56, 86, 88, 89, 91], psychotic features alone or accompanied by mood disorder [4, 63, 91], borderline personality disorder [33, 86, 89] and previous psychiatric hospitalizations [91, 92], ranked among the most relevant features. Moreover, also comorbidity with alcohol or substance use or abuse emerged as relevant features, irrespectively of the initial diagnosis [28, 57, 71–73, 90–93]. Interestingly, a significant effect on suicide prediction was reported for the use and dosage of psychiatric pharmacotherapy, specifically antipsychotics [33, 63, 64] and antidepressants, especially tricyclics [33, 64, 73]. Moreover, variable importance analysis in a sample of 390,000 US veterans showed that 51.1% of model performance was driven by psychopathological risk factors, 26.2% by social determinants of health, 14.8% by prior history of suicidal behaviors, and 6.6% by physical disorders [87].
In line with this result, other ML studies highlighted the importance of socio-occupational status and well-being [56, 63, 65, 87, 93]. Similarly, non-psychiatric health issues have been reported among the features able to predict suicide [38, 56, 94]; moreover, one study reported the use of commonly prescribed opioids (e.g., Fentanyl) as a relevant feature in the prediction [57].
Regarding demographic variables, sex, and age differences also emerged. Sex resulted in a significant predictor in five studies, showing either increased risk for males [39, 63, 92] or more complex relationships between biological sex and risk factors [29, 73]. Moreover, age ranked among the most predictive features in five studies [38, 39, 63, 71, 73, 94], with Lopez-Castroman and colleagues [71] also suggesting that the risk increases until middle-aged, but then tends to decrease in the elderly. Lastly, only two studies [72, 93] reported family history of suicide among the most relevant features assessed, whereas criminal or violent behavior were listed as predictive in two other investigations [28, 39].
Regarding the studies that assessed the predictive power of brain imaging data, the thickness and volume of the orbitofrontal, the anterior and posterior cingulate, and the temporal areas were selected by the algorithm as best predictors of suicide attempts in a group of young individuals and MDD patients [53], while in late-life depression sample, frontal areas and precuneus emerges as the strongest predictors [58]. Moreover, measures of functional connectivity [69] of frontolimbic [79, 81] and fronto-temporal circuits, as well as of the default mode network (DMN) [54, 68, 81], the amygdala, the parahippocampus and the putamen [54, 81], attained classification accuracies above 70%.
Regarding clinical predictors in MDD populations, Ilgen and colleagues [92] reported that co-occurring substance use, male sex, and previous psychiatric hospitalizations increased the risk of suicide. Similarly, in a more recent publication [89], hospitalization, previous suicide attempts, and co-diagnosis with a personality disorder resulted in the most relevant features to predict suicide, yielding an accuracy above 80%. Moreover, thyroxine plasma level and the severity of depression (measured via the Hamilton scale for depression - HAMD) were able to predict suicide with an accuracy of 70% [51].
In studies that involved a broader spectrum of diagnoses of mood disorders (including MDD, BD and also anxiety disorders), previous history of suicide or suicidal thoughts [56, 63], presence of psychotic features [63, 91], and socio-occupational functioning [56, 63, 65] ranked among the most important features in the prediction (all scoring above 70% accuracy). Lastly, Passos and colleagues [91] showed a significant contribution of substance use or dependence and of the number of previous hospitalizations to suicide risk, whereas Iorfino and colleagues [63] found that treatment with antipsychotics, sex, and age were relevant features in the prediction. A brief summary of the most important features is reported in Supplementary Table 5.
Discussion
The objective of our review was to summarize the results of ML studies in predicting suicidal behaviors in psychiatric clinical populations. Although the earliest publication in our review dates back to 1998, more than half of the reports were published between 2019 and 2022, ultimately suggesting that ML approaches in psychiatry, and especially in suicide prediction, are becoming more and more frequent nowadays. It is, therefore, important to constantly update the literature evaluation in order to keep pace with an exponentially increasing field. This translates into the opportunity to critically guide the nascent field and address key gaps in the existing literature. Compared to previous literature [95], our review focused only on psychiatric samples, in order to reduce the bias given by the diagnoses in general population. When focusing on broader samples, studies tend to find the presence of a psychiatric diagnosis as one of the most predictive features. Since it is well-known that the psychiatric population are at higher risk for suicidal behaviors, using general population often does not add knowledge in suicide prevention, while on the other side might mask more subtle risk factors. Moreover, compared to previous reviews in the field [95], we gave a more in-depth analysis of predictive features and also employed two different scoring ranking especially designed for ML studies (see Supplementary materials), in order to give the most precise overview of the literature. Critically, all these aspects might serve as a starting point for future studies.
Regarding our results, most studies classified lifetime suicide attempts, and fewer assessed suicidal attempts in a follow-up time window [28–32, 38, 39, 96]. Moreover, some studies classified their sample for death by suicide [44], suicidal ideation [45, 46, 48–51, 56, 57], or risk stratification [38, 41, 65–69]. Differences in the outcomes and in the definition of risk pose a problem for the interpretation of the results, as risk factors for suicide are reported to be different from those for self-harm and suicidal ideation [1, 97]. In addition, studies also varied in terms of sample selection. Indeed, while most of the publications assessed suicide as a transdiagnostic outcome [38, 40, 63, 66, 67, 81, 98], only a few authors focused on patients with a specific diagnosis, mostly mood disorders [46, 51, 53, 58, 68, 75, 89, 92]. These differences limit the translation of the findings into clinical practice. Prediction models will likely improve prediction accuracy and inform clinical decisions if tailored not just for specific diagnostic groups but also on a dimensional approach to psychiatric disorders [16], as every diagnosis has a different and specific type of assessment and disease trajectory. This means that different patients’ groups might have different predictive features, with probable overlaps between diagnoses. Therefore, a focus on specific diagnostic groups should not divert attention from a comprehensive evaluation of the patient, given that both physical and psychiatric (especially substance abuse disorder) comorbidities proved among the most important predictive features.
Furthermore, another main issue regarding the reviewed studies is the imbalance between the prediction groups, given the low prevalence of the event of interest, with some studies including a larger control group, even tenfold bigger, than the suicidal group [41, 57, 77]. Although an imbalance is intrinsic to this kind of studies, given the prevalence of suicide in psychiatric disorders, some methods can be deployed to reduce the risk of false positive. Fan and colleagues [57] opted for an oversampling in the training phase, a procedure that creates new samples by connecting inliers and outliers from the original dataset. This technique allows the creation of dummy subjects to balance the sample, to foster the reliability of the ML analysis. Other analytical procedures to overcome the issue of imbalanced samples imply weighting of the hyperplane for uneven group sizes, selecting a specific “weight” based on the difference between the groups.
Notably, in most of the cases, the variables employed as predictors were clinical and sociodemographic [48, 57, 87]. Several of the strongest predictors in ML studies are well-known risk factors for suicide, such as previous suicide attempts, previous hospitalizations, and severity of depression [28, 38, 51, 89, 91, 94, 96, 99]. Moreover, the presence of psychosis and a higher amount of pharmacological treatments, especially antipsychotics, resulted to be highly predictive features in many investigations [4, 63, 64, 91, 100, 101]. Interestingly, also presence of psychiatric comorbidities was one of the most valuable predictive features, in particular substance or alcohol use disorders [57, 61, 71, 72, 92]. These results emphasize the importance of a comprehensive evaluation of psychiatric patients and of the burden that comorbidities represent, also given their frequent occurrence [102]. This is particularly important for the comorbid use of alcohol and drug abuse, since they can reduce compliance to treatments [103] and increase impulsive behaviors [104], which in turn may act as risk factors for suicide. Besides the well-known suicide risk factors (i.e., history of suicide attempts, hospitalizations, etc.), more subtle risk factors emerged from the reviewed studies. More in detail, comorbidities resulted in important features in different studies, suggesting that not only psychiatric comorbidities but also physical health is important. Similarly, the use of specific drugs (i.e., antipsychotics), illness severity, and psychosis seemed to be highly predictive of suicide attempts. Finally, some studies suggested that also laboratory tests, such as thyroid hormones, might play a role in predicting suicidal behaviors, even at a subclinical level [51, 83].
Although most of the significant features identified by ML are well-known risk factors for suicide [6, 7], ML demonstrate a greater predictive ability when compared with classical univariate statistics (i.e., logistic regression) and clinician assessment of risk factors [8, 9]. In particular, ML attained higher accuracies as compared to logistic regression [46, 49, 57, 61, 63, 67, 69, 87, 105]. These results suggest that advanced methods may inform the clinical decision-making processes in a more precise manner, likely overcoming the poor predictive value provided by classical statistics and expert assessment of the same risk factors [8, 9]. Interestingly, when present, CNN seemed to perform better than other ML algorithms, including SVM and RF. This might indicate the possibility of using deep learning to better stratify suicide risk, at the cost of a slight loss of interpretability.
Lastly, only a few studies employed biological features, such as genes, SNPs, epigenetic loci [42, 43, 98, 106], and neuroimaging measures [47, 49, 53, 68, 69, 79, 81] to predict suicide. Surprisingly, just a single study [53] combined brain imaging with clinical data to predict suicidal behaviors. As one of the major strengths of ML is the possibility to combine data obtained through different modalities (e.g., genetics, brain imaging, clinical features) to increase prediction accuracy, this approach should be exploited in future suicide research, since it is already occurring in other field of medicine [14].
Limitations and future challenges
A number of limitations should be highlighted. Methods varied widely across studies in terms of ML approach, sample selection, features employed, and preprocessing pipeline. Moreover, distinct investigations focused on a variety of different outcomes, from lifetime attempts to death by suicide, from cross-sectional to longitudinal evaluations. Such differences call for increased uniformity in the assessment of suicidal behaviors and in the design of ML protocols to enhance predictions of risk that may translate into clinical practice.
For instance, the decision to use either a specific and unique ML framework or different algorithms should be motivated: the testing of several approaches at once seems confusing and rather exploratory, especially in the absence of an external validation dataset. Regarding the different algorithms, it is noteworthy to mention that, from our results, it emerged that deep learning methods (such as CNN) performed better than other ML algorithms in direct comparisons. Although important from a research point of view, deep learning algorithms tend to be less interpretable (more “black boxes”), and this aspect might prove crucial in the further development of AI techniques in medicine and psychiatry. This is true, especially in the field of mental health and suicide prediction, where AI tools should assist clinicians and not introduce further complexity. For an AI to become useful in clinical practice, it should prove to be trustworthy, therefore not only valid and reliable, but also easily understandable [107]. In the last years, the concept of explainable AI (“XAI”) emerged, as a possibility to close the gap between the algorithms and the clinicians, creating a human-understandable correspondence between inputs and outputs of the black-box model either through intrinsic transparency of the model or through post-hoc techniques. Given that clinical applications are high-stakes, we require understandability from the prediction tools, or either AI tools will grow in distrust [107].
Moreover, features should be accurately selected, and their number should not be excessive (e.g., curse of dimensionality), as in some of the studies [44, 61]. Collecting such a huge amount of data could be feasible only in university centers, thus reducing the translational value of the results. This comprehensive review should also help in the choice of the right type and number of features. For example, pharmacological treatments, especially antipsychotics, were among the most important features in those studies who included them in the models. However, the pharmacological status of patients is often not reported (see Table 1), and in most cases type and dosage of different drugs are not included in the models. Based on the results of our review, it might be beneficial to include data related to pharmacological therapy in the models, since it could potentially enhance the predictive power and clinical applicability of these models. Moreover, the inclusion of pharmacological information might also help in defining protective features, not just risk factors, as suggested by studies showing that some stabilizers and antidepressants might actually reduce the risk of suicide [64]. Also, both psychiatric and physical comorbidities seem to have a predictive role in the presented models; especially, substance abuse as a comorbid disorder resulted to be highly predictive. This aspect suggests a comprehensive evaluation of the patient in order to define the clinical risk.
In addition, most of the studies addressed the prediction of suicide using a cross-sectional approach, disregarding the temporal aspects. Yet, time may represent a crucial feature for predictive models of suicide [17]. In this regard, defining in advance one or more prediction windows after the assessment is fundamental, as the prediction of short-term suicide risk may rely on different features as compared with long-term risk. Similarly, the temporal characteristics of a feature with respect to the assessment point might impact differentially the accuracy of prediction. For instance, suicide attempts in the year prior to the assessment, but not those that occurred several years before, may be a stronger predictor for new short-time suicidal behaviors.
Finally, despite the high heterogeneity, most of the studies (>80%) obtained a good accuracy, namely 70% or higher. However, many studies did not report additional key metrics (e.g., PPV, F1-score) that are paramount to interpret the actual usefulness of prediction models. Moreover, only few studies tested their prediction on external validation samples; therefore, caution is needed when interpreting these findings, since it is possible that they suffer from overfitting.
Finally, it is evident the importance of further studies also examining the role of neurocognitive variables, dimensions of social support, loneliness, extent and type of medical comorbidity and associated disability, the type of pharmacological interventions used in the context of specific diagnoses as well as the presence of psychotherapies and their combination with medications on suicidal risk. Similarly, a call for a more consistent use of ML is of paramount importance. CNN, RF, and SVM proved to perform better against other algorithms, but these results should be further tested in the future.
Conclusions
The results that emerged from the reviewed studies lead to the conclusion that ML approaches attain greater accuracies in predicting suicidal behaviors across a variety of psychiatric disorders as compared to classical analysis methods. From the reviewed ML studies, well-known risk factors for suicide emerged as relevant predictors, along with new subtle aspects, such as physical and psychiatric comorbidities, presence of psychotic symptoms, and subclinical lab tests, that should be further analyzed and confirmed in future studies. However, additional work is needed to improve the predictive strength of ML algorithms, resolve the systemic lack of external validation, and finally make them become of use in clinical psychiatry. To do so, ML should integrate genetics, neurobiological, brain imaging, psychometric and clinical data to achieve better predictions. Then, algorithms should be presented in an intuitive way for both psychiatrists and patients to foster their adoption and easiness of use in the clinical setting. Although some attempts have been made, to date, ML approaches are not routinely part of clinical practice in psychiatry. We believe ML development should aim to gain the trust of clinicians, by proving to be valid, reliable, and understandable, to be realistically included in decision processes. Our review proved they can be valid in the context of suicide risk stratification; future studies should demonstrate that ML tools are reliable and, even more importantly, easy to understand by clinicians. Multifactorial disorders require multifaceted approaches, and ML could really help in this aspect; however, AI tools should not introduce further complexity in the decision processes, and therefore explainable AI will be a crucial point in further clinical development of predictive tools.
Supplementary information
Acknowledgements
No acknowledgments.
Author contributions
AP, PP, LC, and PB conceptualized the study. AP and GD performed the research and wrote the manuscript. NT performed the analysis and critically revised the manuscript. DM, PB, LC, and PP critically revised the manuscript. All authors approved the manuscript.
Funding
This study was partially supported by the Italian Ministry of Health (GR-2019-12369479 to AP and Ricerca Corrente 2024 to PB, and and Hub Life Science- Diagnostica Avanzata, HLS-DA, PNC-E3-2022-23683266– CUP: C43C22001630001 / MI-0117 to PB), the Italian Ministry of Education and Research (Dipartimenti di Eccellenza Program 2023–2027 - Dept. of Pathophysiology and Transplantation, University of Milan to PB), Fondazione Cariplo (Award Number 2019–3415), and ERANET NEURON JTC2018 “Mental Disorders” (UNMET project - Neuron-051 to PB).
Data availability
All data will be made available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alessandro Pigoni, Giuseppe Delvecchio.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-024-02852-9.
References
- 1.Fazel S, Runeson B. Suicide. N. Engl J Med. 2020;382:266–74. doi: 10.1056/NEJMra1902944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann S. Epidemiology of suicide and the psychiatric perspective. Int J Environ Res Public Health. 2018. 10.3390/IJERPH15071425. [DOI] [PMC free article] [PubMed]
- 3.Sanderson M, Bulloch AG, Wang JL, Williams KG, Williamson T, Patten SB. Predicting death by suicide following an emergency department visit for parasuicide with administrative health care system data and machine learning. EClinicalMedicine. 2020. 10.1016/j.eclinm.2020.100281. [DOI] [PMC free article] [PubMed]
- 4.Walsh CG, Ribeiro JD, Franklin JC. Predicting risk of suicide attempts over time through machine learning. Clin Psychol Sci. 2017;5:457–69. doi: 10.1177/2167702617691560. [DOI] [Google Scholar]
- 5.Bauer BW, Law KC, Rogers ML, Capron DW, Bryan CJ. Editorial overview: analytic and methodological innovations for suicide-focused research. Suicide Life Threat Behav. 2021;51:5–7. doi: 10.1111/sltb.12664. [DOI] [PubMed] [Google Scholar]
- 6.Gradus JL, Rosellini AJ, Horváth-Puhó E, Street AE, Galatzer-Levy I, Jiang T, et al. Prediction of sex-specific suicide risk using machine learning and single-Payer Health Care Registry Data from Denmark. JAMA Psychiatry. 2020;77:25–34. doi: 10.1001/jamapsychiatry.2019.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voros V, Tenyi T, Nagy A, Fekete S, Osvath P. Crisis concept re-loaded?-The recently described suicide-specific syndromes may help to better understand suicidal behavior and assess imminent suicide risk more effectively. Front Psychiatry. 2021. 10.3389/FPSYT.2021.598923. [DOI] [PMC free article] [PubMed]
- 8.Galynker I, Yaseen ZS, Cohen A, Benhamou O, Hawes M, Briggs J. Prediction of suicidal behavior in high risk psychiatric patients using an assessment of acute suicidal state: the suicide crisis inventory. Depress Anxiety. 2017;34:147–58. doi: 10.1002/da.22559. [DOI] [PubMed] [Google Scholar]
- 9.Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017;143:187–232. doi: 10.1037/bul0000084. [DOI] [PubMed] [Google Scholar]
- 10.Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: a 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry. 1985;142:559–63. doi: 10.1176/ajp.142.5.559. [DOI] [PubMed] [Google Scholar]
- 11.McHugh CM, Large MM. Can machine-learning methods really help predict suicide? Curr Opin Psychiatry. 2020;33:369–74. doi: 10.1097/YCO.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 12.Porcelli S, Marsano A, Caletti E, Sala M, Abbiati V, Bellani M, et al. Temperament and character inventory in bipolar disorder versus healthy controls and modulatory effects of 3 key functional gene variants. Neuropsychobiology. 2017;76:209–21. doi: 10.1159/000490955. [DOI] [PubMed] [Google Scholar]
- 13.Grassi M, Perna G, Caldirola D, Schruers K, Duara R, Loewenstein DA. A clinically-translatable machine learning algorithm for the prediction of Alzheimer’s disease conversion in individuals with mild and premild cognitive impairment. J Alzheimer’s Dis. 2018;61:1555–73. doi: 10.3233/JAD-170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russak AJ, Chaudhry F, De Freitas JK, Baron G, Chaudhry FF, Bienstock S, et al. Machine learning in cardiology-ensuring clinical impact lives up to the hype. J Cardiovasc Pharm Ther. 2020;25:379–90. doi: 10.1177/1074248420928651. [DOI] [PubMed] [Google Scholar]
- 15.Corke M, Mullin K, Angel-Scott H, Xia S, Large M. Meta-analysis of the strength of exploratory suicide prediction models; from clinicians to computers. BJPsych Open. 2021. 10.1192/BJO.2020.162. [DOI] [PMC free article] [PubMed]
- 16.Fazel S, O’Reilly L. Machine learning for suicide research-can it improve risk factor identification? JAMA Psychiatry. 2020;77:13–14. doi: 10.1001/jamapsychiatry.2019.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudreaux ED, Rundensteiner E, Liu F, Wang B, Larkin C, Agu E, et al. Applying machine learning approaches to suicide prediction using healthcare data: overview and future directions. Front Psychiatry. 2021. 10.3389/FPSYT.2021.707916. [DOI] [PMC free article] [PubMed]
- 18.Jacobson NC, Yom-Tov E, Lekkas D, Heinz M, Liu L, Barr PJ. Impact of online mental health screening tools on help-seeking, care receipt, and suicidal ideation and suicidal intent: evidence from internet search behavior in a large U.S. cohort. J Psychiatr Res. 2022;145:276–83. doi: 10.1016/j.jpsychires.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmstrand C, Bogren M, Mattisson C, Brådvik L. Long-term suicide risk in no, one or more mental disorders: the Lundby Study 1947–1997. Acta Psychiatr Scand. 2015;132:459–69. doi: 10.1111/acps.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modai I, Kuperman J, Goldberg I, Goldish M, Mendel S. Suicide risk factors and suicide vulnerability in various major psychiatric disorders. Med Inform Internet Med. 2009;29:65–74. [DOI] [PubMed]
- 21.Modai I, Kuperman J, Goldberg I, Goldish M, Mendel S. Fuzzy logic detection of medically serious suicide attempt records in major psychiatric disorders. J Nerv Ment Dis. 2004;192:708–10. doi: 10.1097/01.nmd.0000142020.20038.dd. [DOI] [PubMed] [Google Scholar]
- 22.O’Rourke MC, Siddiqui W. Suicide screening and prevention. StatPearls. 2019. http://www.ncbi.nlm.nih.gov/pubmed/30285348. [PubMed]
- 23.McIntyre RS, Berk M, Brietzke E, Goldstein BI, López-Jaramillo C, Kessing LV, et al. Bipolar disorders. Lancet. 2020;396:1841–56. doi: 10.1016/S0140-6736(20)31544-0. [DOI] [PubMed] [Google Scholar]
- 24.Wiebenga JXM, Dickhoff J, Mérelle SYM, Eikelenboom M, Heering HD, Gilissen R, et al. Prevalence, course, and determinants of suicide ideation and attempts in patients with a depressive and/or anxiety disorder: a review of NESDA findings. J Affect Disord. 2021;283:267–77. doi: 10.1016/j.jad.2021.01.053. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell SM, Cero I, Littlefield AK, Brown SL. Using categorical data analyses in suicide research: considering clinical utility and practicality. Suicide Life Threat Behav. 2021;51:76–87. doi: 10.1111/sltb.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed]
- 27.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015. 10.1136/bmj.g7594. [DOI] [PubMed]
- 28.Tiet QQ, Ilgen MA, Byrnes HF, Moos RH. Suicide attempts among substance use disorder patients: an initial step toward a decision tree for suicide management. Alcohol Clin Exp Res. 2006;30:998–1005. doi: 10.1111/j.1530-0277.2006.00114.x. [DOI] [PubMed] [Google Scholar]
- 29.Jiang T, Rosellini AJ, Horváth-Puhó E, Shiner B, Street AE, Lash TL, et al. Using machine learning to predict suicide in the 30 days after discharge from psychiatric hospital in Denmark. Br J Psychiatry. 2021;219:440–7. [DOI] [PMC free article] [PubMed]
- 30.Parghi N, Chennapragada L, Barzilay S, Newkirk S, Ahmedani B, Lok B, et al. Assessing the predictive ability of the Suicide Crisis Inventory for near-term suicidal behavior using machine learning approaches. Int J Methods Psychiatr Res. 2021. 10.1002/MPR.1863. [DOI] [PMC free article] [PubMed]
- 31.McMullen L, Parghi N, Rogers ML, Yao H, Bloch-Elkouby S, Galynker I. The role of suicide ideation in assessing near-term suicide risk: a machine learning approach. Psychiatry Res. 2021. 10.1016/J.PSYCHRES.2021.114118. [DOI] [PubMed]
- 32.Zelkowitz RL, Jiang T, Horváth-Puhó E, Street AE, Lash TL, Sørensen HT, et al. Predictors of nonfatal suicide attempts within 30 days of discharge from psychiatric hospitalization: sex-specific models developed using population-based registries. J Affect Disord. 2022;306:260–8. doi: 10.1016/j.jad.2022.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q, Zhang-James Y, Barnett EJ, Lichtenstein P, Jokinen J, D’Onofrio BM, et al. Predicting suicide attempt or suicide death following a visit to psychiatric specialty care: a machine learning study using Swedish national registry data. PLoS Med. 2020. 10.1371/JOURNAL.PMED.1003416. [DOI] [PMC free article] [PubMed]
- 34.Tran T, Luo W, Phung D, Harvey R, Berk M, Kennedy RL, et al. Risk stratification using data from electronic medical records better predicts suicide risks than clinician assessments. BMC Psychiatry. 2014. 10.1186/1471-244X-14-76. [DOI] [PMC free article] [PubMed]
- 35.Coley RY, Walker RL, Cruz M, Simon GE, Shortreed SM. Clinical risk prediction models and informative cluster size: Assessing the performance of a suicide risk prediction algorithm. Biom J. 2021;63:1375–88. doi: 10.1002/bimj.202000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miranda O, Fan P, Qi X, Yu Z, Ying J, Wang H, et al. DeepBiomarker: identifying important lab tests from electronic medical records for the prediction of suicide-related events among PTSD patients. J Pers Med. 2022;12:524. doi: 10.3390/jpm12040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nock MK, Millner AJ, Ross EL, Kennedy CJ, Al-Suwaidi M, Barak-Corren Y, et al. Prediction of suicide attempts using clinician assessment, patient self-report, and electronic health records. JAMA Netw Open. 2022. 10.1001/JAMANETWORKOPEN.2021.44373. [DOI] [PMC free article] [PubMed]
- 38.Edgcomb JB, Thiruvalluru R, Pathak J, Brooks JO. Machine learning to differentiate risk of suicide attempt and self-harm after general medical hospitalization of women with mental illness. Med Care. 2021;59:S58–S64. doi: 10.1097/MLR.0000000000001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessler RC, Warner CH, Ivany C, Petukhova MV, Rose S, Bromet EJ, et al. Predicting suicides after psychiatric hospitalization in US army soldiers: the Army study to assess risk and resilience in servicemembers (Army STARRS) JAMA Psychiatry. 2015;72:49–57. doi: 10.1001/jamapsychiatry.2014.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jordan JT, McNiel DE. Characteristics of a suicide attempt predict who makes another attempt after hospital discharge: a decision-tree investigation. Psychiatry Res. 2018;268:317–22. doi: 10.1016/j.psychres.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 41.Xu Z, Zhang Q, Yip PSF. Predicting post-discharge self-harm incidents using disease comorbidity networks: a retrospective machine learning study. J Affect Disord. 2020;277:402–9. doi: 10.1016/j.jad.2020.08.044. [DOI] [PubMed] [Google Scholar]
- 42.Niculescu AB, Levey DF, Phalen PL, Le-Niculescu H, Dainton HD, Jain N, et al. Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry. 2015;20:1266–85. doi: 10.1038/mp.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levey DF, Niculescu EM, Le-Niculescu H, Dainton HL, Phalen PL, Ladd TB, et al. Towards understanding and predicting suicidality in women: biomarkers and clinical risk assessment. Mol Psychiatry. 2016;21:768–85. doi: 10.1038/mp.2016.31. [DOI] [PubMed] [Google Scholar]
- 44.Kessler RC, Stein MB, Petukhova MV, Bliese P, Bossarte RM, Bromet EJ, et al. Predicting suicides after outpatient mental health visits in the Army study to assess risk and resilience in servicemembers (Army STARRS) Mol Psychiatry. 2017;22:544–51. doi: 10.1038/mp.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook BL, Progovac AM, Chen P, Mullin B, Hou S, Baca-Garcia E. Novel use of natural language processing (NLP) to predict suicidal ideation and psychiatric symptoms in a text-based mental health intervention in Madrid. Comput Math Methods Med. 2016. 10.1155/2016/8708434. [DOI] [PMC free article] [PubMed]
- 46.Setoyama D, Kato TA, Hashimoto R, Kunugi H, Hattori K, Hayakawa K, et al. Plasma metabolites predict severity of depression and suicidal ideation in psychiatric patients-a multicenter pilot analysis. PLoS ONE. 2016. 10.1371/journal.pone.0165267. [DOI] [PMC free article] [PubMed]
- 47.Chen J, Zhang X, Qu Y, Peng Y, Song Y, Zhuo C, et al. Exploring neurometabolic alterations in bipolar disorder with suicidal ideation based on proton magnetic resonance spectroscopy and machine learning technology. Front Neurosci. 2022. 10.3389/FNINS.2022.944585. [DOI] [PMC free article] [PubMed]
- 48.Peis I, Olmos PM, Vera-Varela C, Barrigon ML, Courtet P, Baca-Garcia E, et al. Deep sequential models for suicidal ideation from multiple source data. IEEE J Biomed Heal Inform. 2019;23:2286–93. doi: 10.1109/JBHI.2019.2919270. [DOI] [PubMed] [Google Scholar]
- 49.Weng J-C, Lin T-Y, Tsai Y-H, Cheok MT, Chang Y-PE, Chen VC-H. An autoencoder and machine learning model to predict suicidal ideation with brain structural imaging. J Clin Med. 2020;9:658. doi: 10.3390/jcm9030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cusick M, Adekkanattu P, Campion TR, Sholle ET, Myers A, Banerjee S, et al. Using weak supervision and deep learning to classify clinical notes for identification of current suicidal ideation. J Psychiatr Res. 2021;136:95–102. doi: 10.1016/j.jpsychires.2021.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge F, Jiang J, Wang Y, Yuan C, Zhang W. Identifying suicidal ideation among chinese patients with major depressive disorder: evidence from a real-world hospital-based study in China. Neuropsychiatr Dis Treat. 2020;16:665–72. doi: 10.2147/NDT.S238286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tubío-Fungueiriño M, Cernadas E, Gonçalves ÓF, Segalas C, Bertolín S, Mar-Barrutia L, et al. Viability study of machine learning-based prediction of COVID-19 pandemic impact in obsessive-compulsive disorder patients. Front Neuroinform. 2022. 10.3389/FNINF.2022.807584. [DOI] [PMC free article] [PubMed]
- 53.Hong S, Liu YS, Cao B, Cao J, Ai M, Chen J, et al. Identification of suicidality in adolescent major depressive disorder patients using sMRI: a machine learning approach. J Affect Disord. 2021;280:72–76. doi: 10.1016/j.jad.2020.10.077. [DOI] [PubMed] [Google Scholar]
- 54.Yang J, Palaniyappan L, Xi C, Cheng Y, Fan Z, Chen C, et al. Aberrant integrity of the cortico-limbic-striatal circuit in major depressive disorder with suicidal ideation. J Psychiatr Res. 2022;148:277–85. doi: 10.1016/j.jpsychires.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Chen S, Zhang X, Lin S, Zhang Y, Xu Z, Li Y, et al. Suicide risk stratification among major depressed patients based on a machine learning approach and whole-brain functional connectivity. J Affect Disord. 2022;322:173–9. doi: 10.1016/j.jad.2022.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Morales S, Barros J, Echávarri O, García F, Osses A, Moya C, et al. Acute mental discomfort associated with suicide behavior in a clinical sample of patients with affective disorders: ascertaining critical variables using artificial intelligence tools. Front Psychiatry. 2017. 10.3389/fpsyt.2017.00007. [DOI] [PMC free article] [PubMed]
- 57.Fan P, Guo X, Qi X, Matharu M, Patel R, Sakolsky D, et al. Prediction of suicide‐related events by analyzing electronic medical records from PTSD patients with bipolar disorder. Brain Sci. 2020;10:1–30. doi: 10.3390/brainsci10110784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shao R, Gao M, Lin C, Huang CM, Liu HL, Toh CH, et al. Multimodal neural evidence on the corticostriatal underpinning of suicidality in late-life depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021. 10.1016/J.BPSC.2021.11.011. [DOI] [PubMed]
- 59.Chen VC-H, Wong F-T, Tsai Y-H, Cheok MT, Chang Y-PE, McIntyre RS, et al. Convolutional neural network-based deep learning model for predicting differential suicidality in depressive patients using brain generalized q-sampling imaging. J Clin Psychiatry. 2021. 10.4088/JCP.19M13225. [DOI] [PubMed]
- 60.Xu M, Zhang X, Li Y, Chen S, Zhang Y, Zhou Z, et al. Identification of suicidality in patients with major depressive disorder via dynamic functional network connectivity signatures and machine learning. Transl Psychiatry. 2022. 10.1038/S41398-022-02147-X. [DOI] [PMC free article] [PubMed]
- 61.Kumar P, Nestsiarovich A, Nelson SJ, Kerner B, Perkins DJ, Lambert CG. Imputation and characterization of uncoded self-harm in major mental illness using machine learning. J Am Med Inform Assoc. 2020;27:136–46. doi: 10.1093/jamia/ocz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Obeid JS, Dahne J, Christensen S, Howard S, Crawford T, Frey LJ, et al. Identifying and predicting intentional self-harm in electronic health record clinical notes: deep learning approach. JMIR Med Informatics. 2020. 10.2196/17784. [DOI] [PMC free article] [PubMed]
- 63.Iorfino F, Ho N, Carpenter JS, Cross SP, Davenport TA, Hermens DF, et al. Predicting self-harm within six months after initial presentation to youth mental health services: a machine learning study. PLoS ONE. 2020. 10.1371/journal.pone.0243467. [DOI] [PMC free article] [PubMed]
- 64.Nestsiarovich A, Kumar P, Lauve NR, Hurwitz NG, Mazurie AJ, Cannon DC, et al. Drug-dependent risk of self-harm in patients with bipolar disorder: a comparative effectiveness study using machine learning imputed outcomes. JMIR Ment Health. 2020. 10.2196/24522. [DOI] [PMC free article] [PubMed]
- 65.Barros J, Morales S, Echávarri O, García A, Ortega J, Asahi T, et al. Suicide detection in Chile: proposing a predictive model for suicide risk in a clinical sample of patients with mood disorders. Rev Bras Psiquiatr. 2017;39:1–11. doi: 10.1590/1516-4446-2015-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Senior M, Burghart M, Yu R, Kormilitzin A, Liu Q, Vaci N, et al. Identifying predictors of suicide in severe mental illness: a feasibility study of a clinical prediction rule (Oxford Mental Illness and Suicide Tool or OxMIS) Front Psychiatry. 2020;11:268. doi: 10.3389/fpsyt.2020.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haines-Delmont A, Chahal G, Bruen AJ, Wall A, Khan CT, Sadashiv R, et al. Testing suicide risk prediction algorithms using phone measurements with patients in acute mental health settings: feasibility study. JMIR mHealth uHealth. 2020. 10.2196/15901. [DOI] [PMC free article] [PubMed]
- 68.Dai Z, Shen X, Tian S, Yan R, Wang H, Wang X, et al. Gradually evaluating of suicidal risk in depression by semi-supervised cluster analysis on resting-state fMRI. Brain Imaging Behav. 2020. 10.1007/s11682-020-00410-7. [DOI] [PubMed]
- 69.Bohaterewicz B, Sobczak AM, Podolak I, Wójcik B, Mȩtel D, Chrobak AA, et al. Machine learning-based identification of suicidal risk in patients with schizophrenia using multi-level resting-state fMRI features. Front Neurosci. 2021. 10.3389/fnins.2020.605697. [DOI] [PMC free article] [PubMed]
- 70.Shin D, Kim K, Lee SB, Lee C, Bae YS, Cho WI, et al. Detection of depression and suicide risk based on text from clinical interviews using machine learning: possibility of a new objective diagnostic marker. Front Psychiatry. 2022. 10.3389/FPSYT.2022.801301. [DOI] [PMC free article] [PubMed]
- 71.Lopez-Castroman J, Perez-Rodriguez M, de lasM, Jaussent I, Alegria AA, Artes-Rodriguez A, et al. Distinguishing the relevant features of frequent suicide attempters. J Psychiatr Res. 2011;45:619–25. doi: 10.1016/j.jpsychires.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 72.Baca-Garcia E, Perez-Rodriguez MM, Saiz-Gonzalez D, Basurte-Villamor I, Saiz-Ruiz J, Leiva-Murillo JM, et al. Variables associated with familial suicide attempts in a sample of suicide attempters. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1312–6. doi: 10.1016/j.pnpbp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 73.Adams RS, Jiang T, Rosellini AJ, Horváth‐Puhó E, Street AE, Keyes KM, et al. Sex‐specific risk profiles for suicide among persons with substance use disorders in Denmark. Addiction. 2021;116:2882–92. doi: 10.1111/add.15455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ji X, Zhao J, Fan L, Li H, Lin P, Zhang P, et al. Highlighting psychological pain avoidance and decision-making bias as key predictors of suicide attempt in major depressive disorder-a novel investigative approach using machine learning. J Clin Psychol. 2022;78:671–91. doi: 10.1002/jclp.23246. [DOI] [PubMed] [Google Scholar]
- 75.Nordin N, Zainol Z, Mohd Noor MH, Lai Fong C. A comparative study of machine learning techniques for suicide attempts predictive model. Health Informatics J. 2021. 10.1177/1460458221989395. [DOI] [PubMed]
- 76.Kim KW, Lim JS, Yang CM, Jang SH, Lee SY. Classification of adolescent psychiatric patients at high risk of suicide using the personality assessment inventory by machine learning. Psychiatry Investig. 2021;18:1137–43. doi: 10.30773/pi.2021.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Wang C, Yao J, Fan H, Wang Q, Ren Y, et al. Comparisons of deep learning and machine learning while using text mining methods to identify suicide attempts of patients with mood disorders. J Affect Disord. 2022;317:107–13. doi: 10.1016/j.jad.2022.08.054. [DOI] [PubMed] [Google Scholar]
- 78.Graham S, Depp C, Lee EE, Nebeker C, Tu X, Kim H-C, et al. Artificial intelligence for mental health and mental illnesses: an overview. Curr Psychiatry Rep. 2019. 10.1007/S11920-019-1094-0. [DOI] [PMC free article] [PubMed]
- 79.Zhu R, Tian S, Wang H, Jiang H, Wang X, Shao J, et al. Discriminating suicide attempters and predicting suicide risk using altered frontolimbic resting-state functional connectivity in patients with bipolar II disorder. Front Psychiatry. 2020. 10.3389/fpsyt.2020.597770. [DOI] [PMC free article] [PubMed]
- 80.Zhong S, Chen P, Lai S, Chen G, Zhang Y, Lv S, et al. Aberrant dynamic functional connectivity in corticostriatal circuitry in depressed bipolar II disorder with recent suicide attempt. J Affect Disord. 2022;319:538–48. doi: 10.1016/j.jad.2022.09.050. [DOI] [PubMed] [Google Scholar]
- 81.Gosnell SN, Fowler JC, Salas R. Classifying suicidal behavior with resting-state functional connectivity and structural neuroimaging. Acta Psychiatr Scand. 2019;140:20–29. doi: 10.1111/acps.13029. [DOI] [PubMed] [Google Scholar]
- 82.Liu X, He C, Fan D, Zang F, Zhu Y, Zhang H, et al. Alterations of core structural network connectome associated with suicidal ideation in major depressive disorder patients. Transl Psychiatry. 2021. 10.1038/S41398-021-01353-3. [DOI] [PMC free article] [PubMed]
- 83.Li XY, Tabarak S, Su XR, Qin Z, Chai Y, Zhang S, et al. Identifying clinical risk factors correlate with suicide attempts in patients with first episode major depressive disorder. J Affect Disord. 2021;295:264–70. doi: 10.1016/j.jad.2021.08.028. [DOI] [PubMed] [Google Scholar]
- 84.Fernandes AC, Dutta R, Velupillai S, Sanyal J, Stewart R, Chandran D. Identifying suicide ideation and suicidal attempts in a psychiatric clinical research database using natural language processing. Sci Rep. 2018. 10.1038/s41598-018-25773-2. [DOI] [PMC free article] [PubMed]
- 85.Dwyer DB, Falkai P, Koutsouleris N. Machine learning approaches for clinical psychology and psychiatry. Annu Rev Clin Psychol. 2018;14:91–118. doi: 10.1146/annurev-clinpsy-032816-045037. [DOI] [PubMed] [Google Scholar]
- 86.Mann JJ, Ellis SP, Waternaux CM, Liu X, Oquendo MA, Malone KM, et al. Classification trees distinguish suicide attempters in major psychiatric disorders: a model of clinical decision making. J Clin Psychiatry. 2008;69:23–31. doi: 10.4088/JCP.v69n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kessler RC, Bauer MS, Bishop TM, Demler OV, Dobscha SK, Gildea SM, et al. Using administrative data to predict suicide after psychiatric hospitalization in the veterans health administration system. Front Psychiatry. 2020. 10.3389/fpsyt.2020.00390. [DOI] [PMC free article] [PubMed]
- 88.Agne NA, Tisott CG, Ballester P, Passos IC, Ferrão YA. Predictors of suicide attempt in patients with obsessive-compulsive disorder: An exploratory study with machine learning analysis. Psychol Med. 2020. 10.1017/S0033291720002329. [DOI] [PubMed]
- 89.MacHado CDS, Ballester PL, Cao B, Mwangi B, Caldieraro MA, Kapczinski F, et al. Prediction of suicide attempts in a prospective cohort study with a nationally representative sample of the US population. Psychol Med. 2020. 10.1017/S0033291720004997. [DOI] [PubMed]
- 90.Jiang T, Nagy D, Rosellini AJ, Horváth-Puhó E, Keyes KM, Lash TL, et al. Suicide prediction among men and women with depression: a population-based study. J Psychiatr Res. 2021;142:275–82. doi: 10.1016/j.jpsychires.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Passos IC, Mwangi B, Cao B, Hamilton JE, Wu MJ, Zhang XY, et al. Identifying a clinical signature of suicidality among patients with mood disorders: a pilot study using a machine learning approach. J Affect Disord. 2016;193:109–16. doi: 10.1016/j.jad.2015.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ilgen MA, Downing K, Zivin K, Hoggatt KJ, Kim HM, Ganoczy D, et al. Exploratory data mining analysis identifying subgroups of patients with depression who are at high risk for suicide. J Clin Psychiatry. 2009;70:1495–1500. doi: 10.4088/JCP.08m04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Modai I, Valevski A, Solomish A, Kurs R, Hines IL, Ritsner M, et al. Neural network detection of files of suicidal patients and suicidal profiles. Med Inf Internet Med. 1999;24:249–56. doi: 10.1080/146392399298276. [DOI] [PubMed] [Google Scholar]
- 94.Edgcomb JB, Shaddox T, Hellemann G, Brooks JO. Predicting suicidal behavior and self-harm after general hospitalization of adults with serious mental illness. J Psychiatr Res. 2020. 10.1016/j.jpsychires.2020.10.024. [DOI] [PMC free article] [PubMed]
- 95.Bernert RA, Hilberg AM, Melia R, Kim JP, Shah NH, Abnousi F. Artificial intelligence and suicide prevention: a systematic review of machine learning investigations. Int J Environ Res Public Health. 2020;17:1–25. doi: 10.3390/ijerph17165929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen C, Wang GH, Wu SH, Zou JL, Zhou Y, Wang HL. Abnormal local activity and functional dysconnectivity in patients with schizophrenia having auditory verbal hallucinations. Curr Med Sci. 2020;40:979–84. doi: 10.1007/s11596-020-2271-4. [DOI] [PubMed] [Google Scholar]
- 97.Turecki G, Brent DA, Gunnell D, O’Connor RC, Oquendo MA, Pirkis J, et al. Suicide and suicide risk. Nat Rev Dis Prim. 2019. 10.1038/S41572-019-0121-0. [DOI] [PubMed]
- 98.Baca-Garcia E, Vaquero-Lorenzo C, Perez-Rodriguez MM, Gratacòs M, Bayés M, Santiago-Mozos R, et al. Nucleotide variation in central nervous system genes among male suicide attempters. Am J Med Genet Part B Neuropsychiatr Genet. 2010;153:208–13. doi: 10.1002/ajmg.b.30975. [DOI] [PubMed] [Google Scholar]
- 99.Roglio VS, Borges EN, Rabelo-Da-Ponte FD, Ornell F, Scherer JN, Schuch JB, et al. Prediction of attempted suicide in men and women with crack-cocaine use disorder in Brazil. PLoS ONE. 2020. 10.1371/journal.pone.0232242. [DOI] [PMC free article] [PubMed]
- 100.Husain MO, Chaudhry IB, Khan Z, Khoso AB, Kiran T, Bassett P, et al. Depression and suicidal ideation in schizophrenia spectrum disorder: a cross-sectional study from a lower middle-income country. Int J Psychiatry Clin Pract. 2021;25:245–51. [DOI] [PubMed]
- 101.de Cates AN, Catone G, Marwaha S, Bebbington P, Humpston CS, Broome MR. Self-harm, suicidal ideation, and the positive symptoms of psychosis: cross-sectional and prospective data from a national household survey. Schizophr Res. 2021;233:80–88. doi: 10.1016/j.schres.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cullen C, Kappelmann N, Umer M, Abdolizadeh A, Husain MO, Bonato S, et al. Efficacy and acceptability of pharmacotherapy for comorbid anxiety symptoms in bipolar disorder: a systematic review and meta-analysis. Bipolar Disord. 2021;23:754–66. doi: 10.1111/bdi.13125. [DOI] [PubMed] [Google Scholar]
- 103.Anugwom GO, Oladunjoye AO, Basiru TO, Osa E, Otuada D, Olateju V, et al. Does cocaine use increase medication noncompliance in bipolar disorders? A United States nationwide inpatient cross-sectional study. Cureus. 2021;13:e16696. doi: 10.7759/cureus.16696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moriarity DP, Bart CP, Stumper A, Jones P, Alloy LB. Mood symptoms and impairment due to substance use: a network perspective on comorbidity. J Affect Disord. 2021;278:423–32. doi: 10.1016/j.jad.2020.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tasmim S, Dada O, Wang KZ, Bani-Fatemi A, Strauss J, Adanty C, et al. Early-life stressful events and suicide attempt in schizophrenia: Machine learning models. Schizophr Res. 2020;218:329–31. doi: 10.1016/j.schres.2019.11.061. [DOI] [PubMed] [Google Scholar]
- 106.Bhak Y, Jeong HO, Cho YS, Jeon S, Cho J, Gim JA, et al. Depression and suicide risk prediction models using blood-derived multi-omics data. Transl Psychiatry. 2019;9:262. doi: 10.1038/s41398-019-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joyce DW, Kormilitzin A, Smith KA, Cipriani A. Explainable artificial intelligence for mental health through transparency and interpretability for understandability. npj Digit Med. 2023;6:1–7. doi: 10.1038/s41746-023-00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng S, Zeng W, Xin Q, Ye Y, Xue X, Li E, et al. Can cognition help predict suicide risk in patients with major depressive disorder? A machine learning study. BMC Psychiatry. 2022;22(1):580.. doi: 10.1186/s12888-022-04223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carson NJ, Mullin B, Sanchez MJ, Lu F, Yang K, Menezes M, Cook BL. Identification of suicidal behavior among psychiatrically hospitalized adolescents using natural language processing and machine learning of electronic health records. PLoS One. 2019;14(2):e0211116. doi: 10.1371/journal.pone.0211116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oh J, Yun K, Hwang JH, Chae JH. Classification of Suicide Attempts through a Machine Learning Algorithm Based on Multiple Systemic Psychiatric Scales. Front Psychiatry. 2017;8:192. doi: 10.3389/fpsyt.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hettige NC, Nguyen TB, Yuan C, Rajakulendran T, Baddour J, Bhagwat N,, et al. Classification of suicide attempters in schizophrenia using sociocultural and clinical features: A machine learning approach. Gen Hosp Psychiatry. 2017;47:20–28. doi: 10.1016/j.genhosppsych.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 112.Pestian JP, Sorter M, Connolly B, Bretonnel Cohen K, McCullumsmith C, et al. STM Research Group. A Machine Learning Approach to Identifying the Thought Markers of Suicidal Subjects: A Prospective Multicenter Trial. Suicide Life Threat Behav. 2017;47(1):112–21. [DOI] [PubMed]
- 113.Poulin C, Shiner B, Thompson P, Vepstas L, Young-Xu Y, Goertzel B, et al. Predicting the risk of suicide by analyzing the text of clinical notes. PLoS One. 2014;9(1):e85733. [DOI] [PMC free article] [PubMed]
- 114.Delgado-Gomez D, Blasco-Fontecilla H, Alegria AA, Legido-Gil T, Artes-Rodriguez A, Baca-Garcia E. Improving the accuracy of suicide attempter classification. Artif Intell Med. 2011;52(3):165–8. doi: 10.1016/j.artmed.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 115.Modai I, Kurs R, Ritsner M, Oklander S, Silver H, Segal A,, et al. Neural network identification of high-risk suicide patients. Med Inform Internet Med. 2002;27(1):39–47. doi: 10.1080/14639230110119243. [DOI] [PubMed] [Google Scholar]
- 116.Modai I, Greenstain S, Weizman A, Mendel S. Backpropagation and adaptive resonance theory in predicting suicidal risk. Med Inform (Lond) 1998;23:325–30. doi: 10.3109/14639239809025368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be made available upon request.