Abstract
Previous studies have implicated persistent innate immune signaling in the pathogenesis of arrhythmogenic cardiomyopathy (ACM), a familial non-ischemic heart muscle disease characterized by life-threatening arrhythmias and progressive myocardial injury. Here, we provide new evidence implicating inflammatory lipid autocoids in ACM. We show that specialized pro-resolving lipid mediators are reduced in hearts of Dsg2mut/mut mice, a well characterized mouse model of ACM. We also found that ACM disease features can be reversed in rat ventricular myocytes expressing mutant JUP by the pro-resolving epoxy fatty acid (EpFA) 14,15-eicosatrienoic acid (14–15-EET), whereas 14,15-EE-5(Z)E which antagonizes actions of the putative 14,15-EET receptor, intensified nuclear accumulation of the desmosomal protein plakoglobin. Soluble epoxide hydrolase (sEH), an enzyme that rapidly converts pro-resolving EpFAs into polar, far less active or even pro-inflammatory diols, is highly expressed in cardiac myocytes in Dsg2mut/mut mice. Inhibition of sEH prevented progression of myocardial injury in Dsg2mut/mut mice and led to recovery of contractile function. This was associated with reduced myocardial expression of genes involved in the innate immune response and fewer pro-inflammatory macrophages expressing CCR2, which mediate myocardial injury in Dsg2mut/mut mice. These results suggest that pro-inflammatory eicosanoids contribute to the pathogenesis of ACM and, further, that inhibition of sEH may be an effective, mechanism-based therapy for ACM patients.
INTRODUCTION
Arrhythmogenic cardiomyopathy (ACM) is a familial non-ischemic heart muscle disease characterized by arrhythmias and progressive myocardial injury, typically involving the right ventricle.1,2 Most cases are caused by pathogenic variants in genes that encode desmosomal proteins.1,2 We have previously reported that inhibition of nuclear factor κB (NFκB) signaling rescues the disease phenotype and reduces myocardial expression of pro-inflammatory cytokines in a well-characterized mouse model of ACM involving homozygous knock-in of a variant in the gene for the desmosomal protein, desmoglein-2 (Dsg2mut/mut mice).3,4 NFκB signaling is also activated in vitro in induced pluripotent stem cell (iPSC)-cardiac myocytes derived from ACM patients with disease-causing variants in PKP2 or DSG2,3,5 and in rat ventricular myocytes expressing a disease-related variant in JUP (all genes that encode desmosomal proteins).3 ACM iPSC-cardiac myocytes and rat myocytes expressing mutant JUP produce and secrete large amounts of pro-inflammatory mediators under basal conditions without prior stimulation or provocation.3,5–7 Taken together, these observations suggest that the pathogenesis of ACM is driven by a persistent, cardiac myocyte-autonomous innate immune response that fails to resolve.
Here, we show that levels of specialized pro-resolving mediators (SPMs)8 are reduced in hearts of Dsg2mut/mut mice compared to wild type (WT) mice. Hearts of Dsg2mut/mut mice also show increased expression of genes activated in endoplasmic reticulum stress (ER stress), a hallmark of unresolved inflammation.9 In addition, ACM disease features are reversed in rat myocytes expressing mutant JUP by the pro-resolving epoxy fatty acid (EpFA) 14,15-EET, whereas 14,15-EE-5(Z)E (a structural analog of 14,15-EET), which antagonizes actions of the putative 14,15-EET receptor, intensifies nuclear accumulation of the desmosomal protein plakoglobin (aka, γ-catenin), which has been implicated in disease pathogenesis in ACM patients.10 Soluble epoxide hydrolase (sEH), an enzyme responsible for metabolizing pro-resolving EpFAs into pro-inflammatory diols,11,12 is highly expressed in cardiac myocytes in hearts of WT and Dsg2mut/mut mice, confirming previous studies.13 Inhibition of sEH prevents progression of the disease in Dsg2mut/mut mice and promotes significant recovery of contractile function associated with reduced myocardial injury, diminished expression of genes activated in the innate immune response, and fewer pro-inflammatory macrophages expressing CCR2. sEH inhibitors are currently being evaluated in early stage clinical safety trials in patients with chronic neuropathic pain as a clinical path14 Our results suggest that inhibition of sEH may be a novel mechanism-based therapy for ACM patients.
METHODS
In vivo and in vitro models of ACM:
All animal studies were in full compliance with policies of Beth Israel Deaconess Medical Center and St. George’s University of London, and conformed to the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (NIH publication no. 85–23, revised 1996). Mice for in vivo studies were housed in a 12-hour-light/dark cycle, climate-controlled facility with ad libitum access to water and standard rodent chow. Studies were performed in wild type (WT) mice and mice with homozygous knock-in of a variant in Dsg2, the gene encoding the desmosomal cadherin desmoglein-2 (Dsg2mut/mut mice) as previously described.3,4,6,7 This variant entails loss of exons 4 and 5 which causes a frameshift and premature termination of translation.
In vitro studies were performed in primary cultures of ventricular myocytes prepared from disaggregated ventricles of 1-day-old Wistar rat pups as previously described.3,6,7 Cells were plated on collagen-coated plastic chamber slides at a density of 2.4 × 105 cells/cm2. Two days post-plating, monolayers were transfected in serum-free medium for 1hour with a recombinant adenoviral construct (pAd/CMV/V5-DEST vector) containing 2157del2 JUP, after which the viral solution was replaced with complete medium. 24 hours later, cultures were incubated with 10 μM 14,15-epoxyeicosatrieonic acid (14,15-EET) or 10 μM 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) for an additional 24 hours. Other cultures were incubated for 24 hours with 1 μM TPPU (1-trifluoro-methoxy-phenyl-3-(1-propionylpiperidin-4-yl) urea), a small molecule inhibitor of sEH,16 or 500 nM PTUPB (4-(5-phenyl-3-{3-[3-(4-trifluoromethylphenyl)-ureido]-propyl}-pyrazol-1-yl)-benzenesulfonamide), a dual eicosanoid pathway (COX2/sEH) inhibitor.16 Transfected cultures treated with vehicle only and non-transfected cultures were used as controls. Thereafter, the cultures were rinsed in serum-free medium and fixed in 4% paraformaldehyde at 25°C for 5 minutes in preparation for immunofluorescence microscopy as previously described.3,6,7 Fixed cells were incubated with mouse monoclonal anti-plakoglobin (Sigma), anti-Cx43 (Millipore) and anti-RelA/p65 (LSBio) antibodies. Secondary antibodies included Cy3-conjugated goat anti-mouse or anti-rabbit IgGs (H+L; Jackson Immunoresearch). The cells were counterstained with DAPI and examined by laser scanning confocal microscopy (Nikon A1).
Inhibition of sEH and characterization of disease phenotypes in Dsg2mut/mut mice:
The effects of the small molecule sEH inhibitor TPPU were studied in age-matched WT and Dsg2mut/mut mice. 9-week-old animals underwent echocardiography before being implanted with intraperitoneal osmotic mini-pumps (Alzet, Model 1004) as previously described.3,4,16 They received either vehicle or drug (TPPU dissolved in DMSO/polyethylene glycol). Drug-treated mice received 5mg/kg/day TPPU via continuous infusion (1μL/hour for 28 days); vehicle-treated mice received an equivalent volume of vehicle for 28 days. Final echocardiograms were obtained in both groups at 13 weeks of age. Thereafter, animals were euthanized and hearts were collected for additional studies including histology to measure the amount of ventricular fibrosis; immunohistochemistry to measure the number of CCR2+ cells; western blotting and qPCR to measure amounts of specific proteins and gene expression levels; and ELISA to quantify specific lipid mediators in hearts. Western blots were performed on lysates of hearts from WT and Dsg2mut/mut mice using methods described in previous studies.17,18 ELISA assays were performed on extracts of hearts from WT and Dsg2mut/mut mice using kits and protocols from Cayman Chemicals as described in previous studies.17,19 qPCR was used to measure expression of genes related to the ER-stress response and innate immune signaling as described in previous studies.18
Statistical analysis:
All data are presented as mean ± SEM; n-values and the statistical analyses performed for each experiment are indicated in figure legends. Differences in measured variables were assessed with Mann-Whitney or multiple comparisons ANOVA with Tukey post-hoc analysis. A p-value of <0.05 was considered statistically significant. All statistical analyses were analyzed using GraphPad Prism (v9.2) software.
RESULTS
Dsg2mut/mut mice have reduced levels of pro-resolving lipid mediators and express markers of endoplasmic reticulum (ER) stress.
We used ELISA to quantify levels of selected pro-resolving lipid mediators in hearts of Dsg2mut/mut mice. Levels of the specialized pro-resolving mediators (SPMs) resolvin D1, resolvin D2 and maresin-1 were all significantly reduced in hearts of Dsg2mut/mut mice compared to WT mice, as was expression of GRP18, the receptor for resolvin D2 measured by western blotting (Figure 1A). ER stress is a hallmark of unresolved innate immune signaling.9 As shown in Figure 1B, expression of the ER stress response genes BiP, which acts as an ER chaperone, and Pdi, involved in protein folding, was increased in hearts of Dsg2mut/mut mice compared to WT mice. Taken together, these results provide evidence of persistent inflammation in Dsg2mut/mut mice that fails to resolve.
Figure 1:
A. Reduced levels of specialized pro-resolving mediators resolvin D1, maresin 1 and resolvin E2 in hearts of 16-week-old Dsg2mut/mut compared to wildtype (WT) mice measured by ELISA, and of GPR18 in hearts of 16-week-old Dsg2mut/mut measured by western blotting. A sample of spleen was used as a positive control in the western blot; * p<0.05 vs. WT by Mann Whitney U test.
B. qPCR showing increased expression of the endoplasmic reticulum chaperone gene BiP and the protein folding protein disulfide isomerase gene Pdi, both markers of endoplasmic reticulum stress. Gene expression values in wildtype samples were normalized to 1; values in Dsg2mut/mut samples are shown as relative levels; * p<0.05 vs. WT by Mann Whitney U test.
14,15-EET blocks NFκB signaling and rescues ACM disease features in vitro:
We have previously reported that NFκB signaling is activated in a well-characterized in vitro model of ACM involving neonatal rat ventricular myocytes that express a variant in JUP, the gene for the desmosomal protein plakoglobin.3 These cells exhibit characteristic features seen in ACM patients including redistribution of junctional plakoglobin to intracellular and nuclear sites and loss of cell surface signal for Cx43, the major ventricular gap junction protein.3,6,7 They also exhibit activation of NFκB signaling under basal conditions in vitro.3 As shown in Figure 2, junctional plakoglobin and Cx43 distribution was normalized when cells were incubated with the pro-resolving EpFA 14,15-eicosatrienoic acid (14,15-EET). In addition, NFκB signaling, indicated by the presence of nuclear signal for phospho-RelA/p65, was turned off in cells exposed to 14,15-EET. By contrast, nuclear accumulation of plakoglobin (aka γ-catenin) was greatly increased in cells incubated with 10μM 14,15-EE-5(Z)E, which antagonizes actions of 14,15-EET (Figure 2). This was of particular interest, as nuclear translocation of plakoglobin in cardiac myocytes is typically seen in patients with ACM,20 and has been implicated in altered Wnt signaling in the pathogenesis of ACM.10
Figure 2.
Effects of 14,15-eicosatrienoic acid (14,15-EET) and 14,15-eicosa-5(Z)-enoic acid (14,15-EEZE) on the distribution of immunoreactive signals for plakoglobin, connexin43 (Cx43) and RelA/p65 in primary cultures of wildtype (WT) neonatal rat ventricular myocytes and myocyes transfected to express the ACM disease allele JUP2157del2.
sEH is expressed mainly in cardiac myocytes in hearts of Dsg2mut/mut mice.
We previously performed single nucleus RNA sequencing (sn-RNAseq) in cells isolated from hearts of 16-week-old Dsg2mut/mut and WT mice.4 Here, we analyzed the sn-RNAseq data to identify the specific cell types that expressed Ephx2, the major sEH gene in mice. We found that in and Dsg2mut/mut mouse hearts, sEH is expressed mainly in cardiac myocytes and to a lesser extent in endothelial and myeloid cells (Figure 3). Similar data were seen in WT mouse hearts. These observations are in accordance with and confirm a recent report showing that selective disruption of sEH in cardiac myocytes (but not endothelial cells) improves recovery following ischemia/reperfusion injury in mouse hearts.13
Figure 3.
UMAPs showing populations of cells isolated from hearts of 16-week-old Dsg2mut/mut mice and the relative density of Ephx2 expression in each population.
Inhibition of sEH normalizes ACM disease features in vitro and prevents myocardial injury in Dsg2mut/mut mice.
We have previously reported that inhibition of NFκB corrects ACM disease features in cultured rat myocytes expressing mutant JUP.3 It also rescues the disease phenotype in Dsg2mut/mut mice.3,4 To determine if inhibition of sEH also mitigates the ACM disease phenotype and turns off NFκB signaling in vitro, we incubated cultures of rat myocytes expressing JUP2157del2 with the sEH inhibitor TPPU or the dual COX-2/sEH inhibitor PTUPB. As shown in Figure 4, TPPU and PTUPB both restored the normal cell surface distribution of plakoglobin and Cx43 in rat myocytes expressing mutant JUP and eliminated nuclear signal for RelA/p65 indicating that NFκB signaling was reduced. Thus, inhibition of sEH in primary cultures of cardiac myocytes in vitro reverses characteristic features of the ACM disease phenotype seen in patients. These data further suggest that cyclooxygenase inhibitors can synergize with sEH inhibitors in reversing disease features in ACM and support previous observations regarding multiple pro-inflammatory pathways in ACM.3,4
Figure 4.
Effects of 1 μM 1-trifluoro-methoxy-phenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU) and 500 nM 4-(5-phenyl-3-{3-[3-(4-trifluoromethylphenyl)-ureido]-propyl}-pyrazol-1-yl)-benzenesulfonamide (PTUPB) on the distribution of of immunoreactive signals for plakoglobin, connexin43 (Cx43) and RelA/p65 in primary cultures of wildtype (WT) neonatal rat ventricular myocytes and myocyes transfected to express the ACM disease allele JUP2157del2.
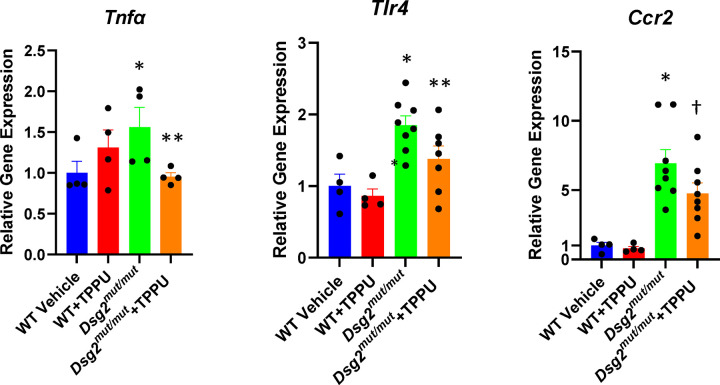
To determine if inhibition of sEH can promote resolution of inflammation and limit myocardial injury in vivo, we treated 9-week old Dsg2mut/mut mice for 4 weeks with TPPU and monitored effects on left ventricular contractile function and immune signaling. As shown in Figure 5, disease progressed during the 4-week treatment interval in Dsg2mut/mut mice given vehicle. These mice exhibited significant deterioration of LV ejection fraction and reduced LV fractional shortening. By contrast, Dsg2mut/mut mice treated with TPPU showed significant improvement in left ventricular ejection fraction and LV fractional shortening to levels roughly equivalent to those seen in age-matched WT mice. Every treated Dsg2mut/mut mouse showed enhanced contractile performance whereas untreated Dsg2mut/mut mice showed no improvement or exhibited functional deterioration (Figure 5). TPPU had no effect on left ventricular contractile function in WT mice. As previously reported, Dsg2mut/mut mice show little if any fibrosis at ~8 weeks of age.3,4,6,7 However, marked ventricular fibrosis was seen in 13-week vehicle-treated Dsg2mut/mut mice, whereas significantly less fibrosis was present in hearts of Dsg2mut/mut mice treated with TPPU (Figure 6A). Similarly, as previously reported,4 the number of cells expressing CCR2 was increased by ~5-fold in hearts of untreated Dsg2mut/mut mice, but they were significantly reduced in number in hearts of treated Dsg2mut/mut mice (Figure 6B). These observations are of particular interest as we have shown that pro-inflammatory myeloid cells expressing CCR2 are highly injurious to the heart in ACM.4 Lastly, we have previously reported increased expression of key genes involved in the innate immune response in hearts of Dsg2mut/mut mice including Tnfα (the gene for the pro-inflammatory cytokine tumor necrosis factor-alpha), Tlr4 (which encodes Toll-like receptor 4, the major pattern recognition receptor on cardiac myocytes), and Ccr2 which is expressed by pro-inflammatory macrophages.3,4 These findings reflect a state of persistent innate immune signaling. As shown in Figure 7, we confirmed upregulation of these genes in Dsg2mut/mut mice and, further, showed that inhibiton of sEH reduced myocardial expression of these genes. Taken together with data in Figure 6, these results show that inhibition of sEH promotes resolution of inflammation leading to significant functional recovery and cessation of ACM disease progression.
Figure 5.
Effects of 1-trifluoro-methoxy-phenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU) on left ventricular ejection fraction (A) and fractional shortening (B) in wildtype (WT) and Dsg2mut/mut mice. Baseline echocardiography was performed in 9-week-old mice and then repeated after treatment for 4 weeks with TPPU or vehicle (Veh). Data are shown for each group (left) and each individual animal (right); * p<0.0001 vs. WT-Veh by multiple comparisons ANOVA.
Figure 6.
Effects of 1-trifluoro-methoxy-phenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU) on the amount of myocardial fibrosis (A) and the number of cells expressing CCR2 (B) in wildtype (WT) and Dsg2mut/mut mice (positive cells identified by arrows in representative immunostained tissue sections). Hearts were excised from animals after treatment for 4 weeks with TPPU or vehicle (Veh) and analyzed by histology in trichrome stained sections or by immunohistochemistry in sections stained with an anti-CCR2 antibody; * p<0.05 vs. WT; ** p<0.05 vs. vehicle-treated Dsg2mut/mut mice by multiple T test.
Figure 7.
Effects of 1-trifluoro-methoxy-phenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU) on expression of Tnfα, Tlr4 and Ccr2 in hearts of wildtype (WT) and Dsg2mut/mut mice treated with TPPU or vehicle. Gene expression values in wildtype samples treated with vehicle were normalized to 1; values in all other groups are shown as relative levels; * p<0.05 vs. WT Veh; ** p<0.05 vs. Dsg2mut/mut mice; † p=0.0579 vs. Dsg2mut/mut mice, all by multiple comparisons ANOVA.
DISCUSSION
Inflammation is usually a self-limited process, designed to eliminate the cause of injury and restore homeostasis.8,21 If left uncontrolled, however, inflammation can cause progressive tissue damage. Indeed, diverse diseases such as sepsis, acute respiratory distress syndrome, cancer and COVID-19 are driven by unresolved inflammation.22,23 Resolution of inflammation is an active regulated process, orchestrated in part by various endogenous specialized pro-resolving mediators (SPMs) synthesized from ω-3 polyunsaturated fatty acids (PUFAs) by lipoxygenase, cyclooxygenase and/or cytochrome P450 mono-oxygenase enzymes (CYP450s).24 EpFAs not only increase SPM production but are also direct pro-resolving mediators.25,26 SPMs also promote resolution of pathological endoplasmic reticulum (ER)-stress, a hallmark of unresolved inflammation.9 However, many pro-resolving EpFAs are short-lived. For example, EETs are rapidly metabolized by sEH to their corresponding diols, including dihydroxyeicosatrienoic (DiHETES) and dihydroxyoctadecenoic (DiHOMES) acids, which may exert pro-inflammatory effects.11,12,25 Small molecule inhibitors of sEH stabilize levels of pro-resolving EpFAs and reduce tissue injury in various animal models.25–28 sEH inhibitors shift arachidonic acid metabolic cascades from a pattern of initiation of inflammation to one of resolution.29 Importantly, both SPMs and EpFAs, synergized by inhibition of sEH, potently down-regulate NFκB pathways,29,30 which are persistently activated in cardiac myocytes in a cell-autonomous fashion in ACM.3–7
Increasing evidence suggests that chronic inflammation in ACM is the result of deficient resolution. The consistent presence of inflammatory infiltrates in the hearts of ACM patients obviously implicates immune mechanisms.1,2 Moreover, ACM patients have elevated circulating levels of cytokines and their cardiac myocytes express multiple pro-inflammatory mediators.31 Recurrent bouts of inflammation (rigorously defined as troponin elevation with normal coronary arteries, and typical 18F-fluoro-deoxyglucose PET findings) occur in ACM patients with variants in DSP, the gene for the desmosomal protein desmoplakin.32
Here, we provide new evidence that 1) ACM is driven by a persistent innate immune response associated with reduced levels of pro-resolving bioactive lipid mediators; 2) the pro-resolving EpFA 14,15-EET can reverse disease features in an in vitro model of ACM; and 3) inhibition of the sEH enzyme mitigates the disease phenotype in a well characterized animal model of ACM. sEH inhibitors are currently being evaluated in phase 1 clinical trials. sEH inhibitors are in clinical development for hypertension,33 chronic obstructive pulmonary disease,34 chronic pain and other conditions.14,35 Dual COX-2/sEH inhibitors are also currently in clinical development for multiple inflammatory diseases.36,37 Should these small molecule drug candidates be found to be safe and free of toxic side-effects, our observations suggest that they may be of benefit in patients with ACM.
Acknowledgments
This work was supported by NIH grant R01HL148348 (JES) and 1R01CA276107-01A1 DP, and grants from Credit Unions Kids at Heart and the Carter Joseph Buckley Pediatric Brain Tumor Fund (DP). Partial support was provided by NIH – NIEHS (RIVER Award) R35 ES030443-01, NIH-NINDS U54 NS127758 (Counter Act Program), and NIH – NIEHS (Superfund Award) P42 ES004699 (all to BDH). Additional support came from a Washington University in St. Louis Rheumatic Diseases Research Resource-Based Center grant (NIH P30AR073752, KL), a National Institutes of Health grant (R35 HL161185, KL), a Leducq Foundation Network grant (#20CVD02, KL, a Burroughs Welcome Fund grant (1014782, KL), a Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital grant (CH-II-2015-462, CH-II-2017-628, PM-LI-2019-829, KL), a Foundation of Barnes-Jewish Hospital grant (8038-88, KL), and gifts from Washington University School of Medicine (KL). V. Penna was supported by a National Institutesof Health grant (5T32AI007163-44). Additional support came from the British Heart Foundation (PG/18/27/33616; CBB, AA).
Footnotes
Author Conflicts of Interest Disclosures: Dr. Saffitz is a consultant to Implicit Biosciences and Rejuvenate Bio. Dr. Hammock holds patents related to the commercial development of soluble epoxide hydrolase inhibitors for cardiovascular disease. He is Chief Scientific Officer of EicOsis Human Health, currently in human 1b safety trials of the soluble epoxide hydrolase inhibitor EC5026. Dr. Lavine is a consultant for Kiniksa, Cytokinetics, Implicit Biosciences, and SUN Pharmaceuticals. Other authors have no relevant conflicts or financial relationships to disclose.
References
- 1.Corrado D, Link M, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. New Eng J Med, 2017; 376:61–72. [DOI] [PubMed] [Google Scholar]
- 2.Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G, Camerini F. Spectrum of clinico-pathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol 1997; 30:1512–1520. [DOI] [PubMed] [Google Scholar]
- 3.Chelko SP, Asimaki A, Lowenthal J, Bueno-Beti C, Bedja D, Scalco A, Amat-Codina N, Andersen P, Judge DP, Tung L, Saffitz JE. Therapeutic modulation of the immune response in arrhythmogenic cardiomyopathy. Circulation 2019; 140:1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chelko SP, Penna V, Engle M, Landim-Vieira ML, Cannon EN, Lavine K, Saffitz JE. Mechanisms of innate immune injury in arrhythmogenic cardiomyopathy. bioRxiv. 2023. Jul 13.2023.07.12.548682, doi: 10.1101/2023.07.12.548682. [DOI] [Google Scholar]
- 5.Hawthorne RN, Blazeski A, Lowenthal J, Kannan S, Teuben R, DiSilvestre D, MorrissetteMcAlmon J, Saffitz JE, Boheler KR, James CA, Chelko SP, Tomaselli G, Tung L. Altered electrical, biomolecular, and immunologic phenotypes in a novel patient-derived stem cell model of desmoglein-2 mutant ARVC. J Clin Med 2021; 10;10(14):3061. doi: 10.3390/jcm10143061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelko SP, Asimaki A, Andersen P, Bedja D, Amat-Alarcon N, DeMazumder D, Jasti R MacRae CA, Leber R, Kleber AG, Saffitz JE, Judge DP. Central role for GSK3β in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight 2016; 1(5). pii: e85923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, Wu JC, MacRae CA, Kléber AG, Saffitz JE. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med 2014; 6:240ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014; 510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inceoglu B, Bettaieb A, Haj FG, Gomes AV, Hammock BD. Modulation of mitochondrial dysfunction and endoplasmic reticulum stress are key mechanisms for the wide-ranging actions of epoxy fatty acids and soluble epoxide hydrolase inhibitors. Prostaglandins Other Lipid Mediat 2017; 133:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythymogenic right ventricular cardiomyopathy. J Clin Invest 2006; 116:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ota K, Hammock BD. Cytosolic and microsomal epoxide hydrolases: differential properties in mammalian liver. Science 1980; 207:1479–1481. [DOI] [PubMed] [Google Scholar]
- 12.Moghaddam MF, Grant DF, Cheek JM, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med 1997; 3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edin ML, Gruzdev A, Bradbury JA, Graves JP, Lih FB, DeGraff LM, Fleming I, Zeldin DC. Disruption of Ephx2 in cardiomyocytes but not endothelial cells improves functional recovery after ischemia-reperfusion in isolated mouse hearts. J Biol Chem 2023; 299:103049. doi: 10.1016/j.jbc.2023.103049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du F, Cao R, Chen L, Sun J, Shi Y, Fu Y, Hammock BD, Zheng Z, Liu Z, Chen G. Structure-guided discovery of potent and oral soluble epoxide hydrolase inhibitors for the treatment of neuropathic pain. Acta Pharm Sin B. 2022; 12:1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An G, Lee KSS, Yang J, Hammock BD. Target-mediated drug disposition – a class effect of soluble epoxide hydrolase inhibitors. J Clin Pharmacol 2021; 61:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gartung A, Yang J, Sukhatme VP, Bielenberg DR, Fernandes D, Chang J, Schmidt BA, Hwang SH, Zurakowski D, Huang S, Kieran MW, Hammock BD, Panigrahy D. Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proc Natl Acad Sci USA 2019; 116:1698–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng J, et al. Eicosanoid regulation of debris-stimulated metastasis. Proc Natl Acad Sci USA 2021; 118(41)e2107771118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishbein, et al. Resolution of eicosanoid/cytokine storm prevents carcinogen and inflammation-initiated hepartocellular cancer progression. Proc Natl Acad Sci USA 2020; 117:21576–21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prashar K, Schulte F, Hardt M, Baker OJ. Sex-mediated elevation of the specialized pro-resolving lipid mediator levels in a Sjögren’s syndrome mouse model. FASEB J 2020; 34:7733–7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asimaki A, Tandri J, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. New Eng J Med 2009:360:1075–84. [DOI] [PubMed] [Google Scholar]
- 21.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 2005; 6: 1191–7. [DOI] [PubMed] [Google Scholar]
- 22.Panigrahy D, Gilligan MM, Serhan CN, Kashfi K. Resolution of inflammation: an organizing principle in biology and medicine. Pharmacol Ther 2021; 227:10789. doi: 10.1016/j/pharmthera.2021.107879. [DOI] [PubMed] [Google Scholar]
- 23.Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem 2020; 64:443–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999; 285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdalla HB, Alvarez C, Wu YC, Rojas P, Hammock BD, Maddipati KR, Trindade-da-Silva CA, Soares MQS, Clemente-Napimoga JT, Kantarci A, Napimoga MH, Van Dyke TE. Soluble epoxide hydrolase inhibition enhances production of specialized pro-resolving lipid mediator and promotes macrophage plasticity. Br J Pharmacol 2023: 180:1597–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdalla HB, et al. Modulating the sEH/EETs axis restrains specialized proresolving mediator impairment and regulates T cell imbalance in experimental periodontitis. J Immunol 2024; 212:433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ono E, Dutile S, Kazani S, Wechsler ME, Yang J, Hammock BD, Douda DN, Tabet Y, Khaddaj-Mallat R, Sirois M, Sirois C, Rizcallah E, Rousseau E, Marin R, Sutherland ER, Castro M, Jarpur NN, Israel E, Levy BD. Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med 2014; 190:886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flitter BA, Hvorecny KL, Ono E, Eddens T, Yang J, Kwak DH, Bahl CD, Hampton TH, Morisseau C, Hammock BD, Liu X, Lee JS, Kolls JK, Levy BD, Madden DR, Bomberger JM. Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proc Natl Acad Sci USA 2017; 114:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong L, Zhou Y, Zhu ZQ, Liu T, Duan JX, Zhang J, Li P, Hammock BD, Guan CX. Soluble epoxide hydrolase inhibitor suppresses the expression of triggering receptor expressed on myeloid cells by inhibiting NF-κB activation in murine macrophage. Inflammation 2017; 40:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sham HP, Walker KH, Abdulmour RE, Krishnamoorthy N, Douda DN, Norris PC, Barkas I, Benito-Figueroa S, Colby JK, Serhan CN, Levy BD. 15-epi-lipoxin A4, resolving D2, and resolving D3 induce NF-κB in bacterial pneumonia. J Immunol 2018; 200:2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asimaki A, Tandri H, Duffy ER, Winterfield JR, Mackey-Bojack S, Picken MM, Cooper LT, Wilber DJ, Marcus FI, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, Stevenson WG, McKenna WJ, Gautam S, Remick DG, Calkins, Saffitz JE. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhyth Electrophysiol 2011; 4:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith ED, et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation 2020; 141:1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulu A, Harris TR, Morisseau C, Miyabe C, Inoue H, Schuster G, Dong H, Iosif AM, Lie JY, Weiss RH, Chiamvimonvat N, Imig JD, Hammock BD. Anti-inflammatory effects of ω-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J Cardiovasc Pharamacol 2013; 62:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazaar AL, Yang L, Boardley RL, Goyal NS, Robertson J, Baldwin SJ, Newby DE, Wilkinson IB, Tal-Singer R, Mayer RJ, Cheriyan J. Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br J Clin Pharmacol 2016; 81:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodani SD, Hammock BD. The 2014 Bernard B. Brodie award lecture – epoxide hydrolases: drug metabolism to therapeutics for chronic pain. Drug Metab Dispos 2015; 43:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dilepan M, Rastle-Simpson S, Greenberg Y, Wijesinghe DS, Kumar NG, Yang J, Hwang SH, Hammock BD, Sriramaroa P, Rao SP. Effect of dual sEH-COX-2 inhibition on allergen-induced airway inflammation. Front Pharmacol 2019; 10:1118. doi: 10.3389/fphar.2019.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang HH, Duan JX, Liu SK, Xiong JB, Guan XX, Zhong WJ, Sun CC, Zhang CY, Luo XQ, Zhang YF, Chen P, Hammock BD, Hwang SH, Jiang JX, Zhou Y, Guan CX. A COX-2/sEH dual inhibitor PTUPB alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting NLFP3 inflammason activation. Theranostics 2020; 10:4749–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]