Abstract
Epithelial cell differentiation is regulated by specific combinations of growth factors, hormones, and extracellular matrix (ECM). How these divergent signals are integrated is largely unknown. We used primary cultures of normal human bronchial epithelial cells (NHBEs) to investigate mechanisms of signal integration. In defined, serum-free media, NHBEs undergo mucosecretory differentiation only when grown in the presence of retinoids and on the appropriate substratum (collagen gels). We identified the retinoic acid receptor β (RARβ) gene as an early marker of NHBE differentiation. In contrast to immortalized cell lines, in NHBEs strong retinoid-induced RARβ transcription occurs only when cells are grown on collagen gels, and it requires new protein synthesis and a cis-acting element that maps outside the known RARβ promoter elements. NHBEs grown on collagen gels exhibit reduced epidermal growth factor (EGF)-induced Raf, MEK, and mitogen-activated protein kinase (MAPK) activity. This correlates with a specific inability to achieve high levels of p66SHC tyrosyl phosphorylation and association of p66SHC with GRB2, despite high levels of EGF receptor (EGFR) autophosphorylation. Notably, inhibition of EGFR or MEK/MAPK activation replaces the ECM requirement for RARβ induction. Our results strongly suggest that a key mechanism by which specific ECMs facilitate retinoid-induced mucosecretory differentiation of NHBEs is by restricting the level of EGFR-dependent MEK/MAPK activation evoked by autocrine and/or paracrine EGFR ligands.
Epithelial cell differentiation is a complex process that results from the integration of growth factor-, hormone-, and extracellular matrix (ECM)-derived signals (for reviews see references 17 and 38). The respiratory epithelium provides a good model system for the study of signal integration, since normal bronchial epithelial cell differentiation requires the correct combination of growth factors, retinoids, and ECM (73, 74). In vivo, the upper airways are populated with basal, ciliated, and mucosecretory (goblet) cells (reviewed in reference 24), which rest on a thin basement membrane and a thick lamina propria rich in ECM molecules such as collagens, laminin, fibronectin, and heparan sulfate proteoglycans. Vitamin A (retinol)-deficient animals develop lesions along their upper airways in which mucociliary cells are replaced with highly keratinized squamous epithelia (44, 72), indicating a critical role for retinoids in normal respiratory cell differentiation. When grown ex vivo on plastic dishes in defined serum-free media without retinoids, cultures of normal tracheal/bronchial epithelial cells undergo squamous metaplasia upon reaching confluence (30, 54). Although retinoid treatment inhibits expression of the squamous phenotype under these culture conditions (28, 30, 54), it does not stimulate mucociliary differentiation. For mucosecretory cell differentiation ex vivo, tracheal/bronchial epithelial cells must be cultured on type I collagen gels in the presence of retinoids (29, 55, 73) but with limited amounts of growth factors, since high doses of epidermal growth factor (EGF), for example, are inhibitory (23, 74).
Growth factors signal by binding to and increasing the catalytic activity of receptor tyrosine kinases (RTKs) (for a review, see reference 34). Autophosphorylation of the RTK on its cytoplasmic tail results in the recruitment of several secondary signaling molecules. These usually include the SHC-GRB2-SOS and/or GRB2-SOS complexes, which participate in Ras activation, phosphatidylinositol 3′ kinase, SHP-2, and phospholipase C-γ. Ultimately, several downstream events occur, including activation of the mitogen-activated protein kinases (MAPKs) Erk1 and Erk2.
ECM transmits signals via various cell adhesion molecules (for a review, see reference 57). Integrins are the most widely studied of this group of molecules (for a review, see reference 10). Much is known about the signaling events that are evoked immediately upon integrin ligation and attachment of cells to ECM. Typically, acute engagement of integrins leads to the activation of protein tyrosine kinases (PTKs) such as FAK and Src family PTKs, the subsequent recruitment of many of the same signaling molecules that are recruited to RTKs, and the activation of downstream pathways such as phosphatidylinositol 3′ kinase (35) and MAPK (9, 59). In contrast, little is known about the specific effects of long-term cell-ECM interactions on signaling pathways.
Retinoids signal by binding to two classes of receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs), which belong to the nuclear hormone receptor superfamily of transcription factors (for a review, see reference 8). At least three genes encode each type of receptor (denoted α, β, γ), and typical responses are transduced through binding of RAR-RXR heterodimers to retinoic acid response elements (RAREs) in the promoters of target genes.
Although much is known about how growth factors, retinoids, and ECM signal in isolation, virtually nothing is understood about how these disparate signals are integrated to direct complex responses such as differentiation and/or how signal integration is perturbed under pathological conditions such as cancer. We investigated the molecular basis for signal integration during mucosecretory differentiation of normal human bronchial epithelial cells (NHBEs). We found striking differences in the established paradigms for growth factor, retinoid, and ECM signaling pathways, which largely have been elucidated in studies using immortalized and/or transformed cell lines. These studies stress the importance of using normal primary cell systems to study general epithelial signal transduction pathways and provide new insight into how divergent pathways may cooperate to regulate normal physiologic responses such as differentiation in vivo.
MATERIALS AND METHODS
Cell culture and immunofluorescence staining.
NHBEs were obtained from Clonetics Corp. and were grown as recommended by the manufacturer. Collagen gels were prepared by using Vitrogen 100 (Collagen Corp.) as described elsewhere (73). Fifteen to 24 h prior to experiments, cells were seeded at 15,000 to 25,000 cells/cm2 in retinoid- or growth factor-deficient BEGM (Clonetics). Where indicated, NHBEs were cultured with the anti-EGF receptor (EGFR) neutralizing antibody LA1 (Upstate Biotechnology Inc. [UBI]) or control immunoglobulin G (IgG) (rabbit anti-mouse IgG; product no. 315-005-003; Jackson Laboratories). When necessary, NHBEs were released from collagen gels by treatment with collagenase (type IA; Sigma) or from plastic dishes by trypsin (Clonetics). Cells were collected by centrifugation and either cytospun onto glass slides, frozen in liquid nitrogen, or used immediately for nuclear extract preparation. For immunofluorescence staining, cytospun cells were fixed in methanol and incubated with the antimucin monoclonal antibody (MAb) 17B1 (a gift from R. Wu; 1:1,000) before detection with rhodamine-conjugated goat anti-mouse IgG (Tago).
Plasmid constructions.
The enhancer trap retroviral vector pLEN− was constructed from pLNSX (a gift from D. Miller) by deleting nucleotides 3217 to 3621, which removes the entire U3 region except for 8 bp downstream of the inverted repeat and 21 bp upstream of the repeat region. The luciferase cDNA was amplified by PCR from pXP2 (a gift from S. Nordeen) and subcloned into pLEN− to generate pLEN−LUC. A fusion of the luciferase cDNA to the 5-kbp 5′ flanking region of the human RARβ promoter was amplified by PCR from pX5 (48) and subcloned into pLEN− to generate pLEN−5LUC. Further details regarding plasmid constructions are available upon request.
Immunoblotting.
Cell lysis and immunoblotting were performed as described elsewhere (40) except that Nonidet P-40 buffer contained 20 mM NaF and 20 mM β-glycerophosphate with no ZnCl2. Primary antibodies included αRARβ (antibody directed against RARβ) (537N [66]), 1 μg/ml; αMAPK (Erk1/2) (C1; a gift from J. Blenis), 1:5,000; αphospho-MAPK (New England Biolabs), 1:1,000; αPTP-1B (UBI), 1:1,000; αSHP2 (Transduction Laboratories), 1:1,000; αMEK1 (724; a gift from R. L. Erikson), 1:1,000; antiphosphotyrosine antibody 4G10 (UBI), 1:7,000; αEGFR (sc-03; Santa Cruz), 1:100; αRaf (sc-133; Santa Cruz), 1:100; αSHC (Transduction Laboratories), 1:500; and αGRB2 (sc-255; Santa Cruz), 1:100.
RNase protection assays and RT-PCR.
RNA was isolated by using TRIzol Reagent (GibcoBRL). RNA probes were generated by in vitro transcription (Promega Corp.) from cDNAs for human PTP-1B (63), γ-actin (a gift from T. Maniatis), and RARβ (nucleotides 1280 to 1677) in the presence of [α-32P]UTP (800 Ci/mmol; NEN). RNase protection assays were performed as described previously (3). Each probe (5 × 105 to 10 × 105 cpm) was incubated with 5 μg of total cellular RNA or yeast RNA for 12 to 18 h at 45°C. Following hybridization, reaction products were digested with RNase A (40 μg/ml; Sigma) and RNase T1 (2 μg/ml; Sigma) for 30 min at 30°C. For reverse transcription-PCR (RT-PCR), total cellular RNA (860 ng) was reverse transcribed with Moloney murine leukemia virus reverse transcriptase (GibcoBRL). cDNAs were amplified by PCR with primer set 5′-GACCTGGAGGAGCCCGAAAAAGTG-3′ plus 5′-GGGAGATGGTCAGTCTGCTGCC-3′ (401 bp; for RARγ) or 5′-GCTGCACGTCCACCGGAACAGC-3′ plus 5′-CAGGCAGGGTGGCCAGAACGGG-3′ (450 bp; for RXRα) in reaction mixtures containing 10 μM deoxynucleoside triphosphates and 5 μCi of [α-32P]dCTP (6,000 Ci/mmol; NEN).
EMSAs.
Nuclear extracts were prepared as described previously (61) except that buffer C was supplemented with 20 mM NaF, 20 mM β-glycerophosphate, 1 mM sodium orthovanadate, leupeptin (10 μg/ml), antipain (1 μg/ml), pepstatin A (1 μg/ml), and aprotinin (1 μg/ml). The βRARE electrophoretic mobility shift assay (EMSA) probe (14), which spans −59 to −33 of the human RARβ promoter, was labeled with [α-32P]dCTP (6,000 Ci/mmol; NEN), and 10 to 20 fmol was used in binding reaction mixtures containing 20 mM HEPES (pH 8.0), 53 mM NaCl, 2.6 mM MgCl2, 0.13 mM EDTA, 0.5 mM dithiothreitol (DTT), 11.3% glycerol, 0.2 mg of salmon testes DNA (Sigma) per ml, and 5 to 10 μg of nuclear extract. Competitor DNAs included either the unlabeled βRARE or mRARE. The mRARE construct (5′-TCGAGGGTAGGGTCTGCAGAAATCGCACTCG-3′ and 5′-TCGACGAGTGCGATTTCTGCAGACCCTACCC-3′) contains seven point mutations (underlined) in the βRARE which disrupt RAR-RXR binding (70). Nuclear extracts were preincubated either in the presence or in the absence of a 100-fold molar excess of competitor DNA or 2 μl of antibodies against RARα1 (sc-551; Santa Cruz), RARγ (sc-773; Santa Cruz), RARβ (537N), or RXRα and RXRβ (gifts from W. Chin) for 10 min at room temperature, followed by the addition of probe and further incubation for 20 min at room temperature. DNA-bound complexes were resolved on 4% 0.5× Tris-borate-EDTA gels.
Transfections, retroviral infections, and reporter gene assays.
NHBEs were transiently transfected by using Lipofectamine (GibcoBRL). Two micrograms of pRARE3tkluc (14) and 500 ng of pEFCAT (47), or 2.5 μg of pUC19 alone, were incubated with 12 μl (24 μg) of Lipofectamine in 800 μl of BEGM for 30 min at room temperature. BEGM (3.2 ml) was subsequently added to the DNA-lipid complexes, and the complete mixture was applied to cells on 100-mm-diameter plastic dishes for 2 h at 37°C. Retroviruses were generated by using the BING amphotropic packaging cell line (a gift from W. Pear) as described previously (51). Infections were carried out in the presence of Polybrene (4 μg/ml) at 37°C for 4 h. Following transfection or infection, cells were subcultured onto plastic or collagen gels and treated with 10 nM Ro 19-0645 15 h later. Twenty-four hours following retinoid treatment, cells were harvested, resuspended in 100 mM Tris-HCl (pH 7.5), and subjected to freeze-thaw lysis, and cellular debris was removed by ultracentrifugation. Luciferase and chloramphenicol acetyltransferase (CAT) activities were measured as described previously (48) except that the luciferase reaction buffer was supplemented with 0.6 mM coenzyme A.
Growth factor stimulations, immunoprecipitations, and kinase assays.
Cells were starved for 20 h in BEGM containing 10 nM Ro 19-0645 but lacking EGF and insulin. Some cultures were treated with 10 μM PD 098059 prior to the addition of EGF (0.1 nM) and/or insulin (5 μg/ml) (Clonetics). Cells were lysed on their respective substrata in Nonidet P-40 buffer, and debris was removed by ultracentrifugation (100,000 × g, 20 min). Immunoprecipitations were performed with 2 μl of anti-MEK1 MAb 3D9 (Zymed)/150 μg of lysate, 3 μl of αErk2 (sc-154; Santa Cruz)/150 μg of lysate, 4 μl of αRaf (sc-133; Santa Cruz)/300 μg of lysate, 2 μl of αSHC (Transduction Laboratories)/200 μg of lysate, 2 μl of αGRB2 (sc-255; Santa Cruz)/200 μg of lysate, or 2 μl of αEGFR (sc-120; Santa Cruz)/200 μg of lysate. Immunoprecipitates were washed in lysis buffer containing 2 mM sodium orthovanadate, 20 mM NaF, and 20 mM β-glycerophosphate. For Erk2 activity assays, immune complexes were washed additionally in kinase buffer (20 mM HEPES [pH 7.4], 10 mM MgCl2, 1 mM DTT, 1 mM EGTA) and resuspended in 30 μl of kinase buffer containing 50 μM ATP, 5 μCi of [γ-32P]ATP (6,000 Ci/mmol; NEN), and 10 μg of myelin basic protein. For MEK activity assays, immune complexes were washed additionally in kinase buffer (50 mM Tris [pH 8.0], 10 mM MgCl2) and resuspended in 30 μl of kinase buffer containing 50 μM ATP, 5 μCi of [γ-32P]ATP (6,000 Ci/mmol; NEN), and 2 μg of glutathione S-transferase (GST)–ERK1(K63M). For Raf activity assays, immune complexes were washed additionally in kinase buffer (25 mM HEPES [pH 7.4], 10 mM MgCl2, 2 mM MnCl2, 1 mM DTT) and resuspended in 50 μl of kinase buffer containing 25 μM ATP, 20 μCi of [γ-32P]ATP (6,000 Ci/mmol; NEN), and 1 μg of GST-MEK(K97A). MEK and Erk2 reactions were carried out at 30°C for 20 and 15 min, respectively, before being terminated by the addition of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Raf activity assays were performed at room temperature for 30 min. Terminated kinase reaction products were resolved by SDS-PAGE and either dried or transferred to Immobilon-P (Millipore) for PhosphorImager (Molecular Dynamics) analysis and immunoblotting.
RESULTS
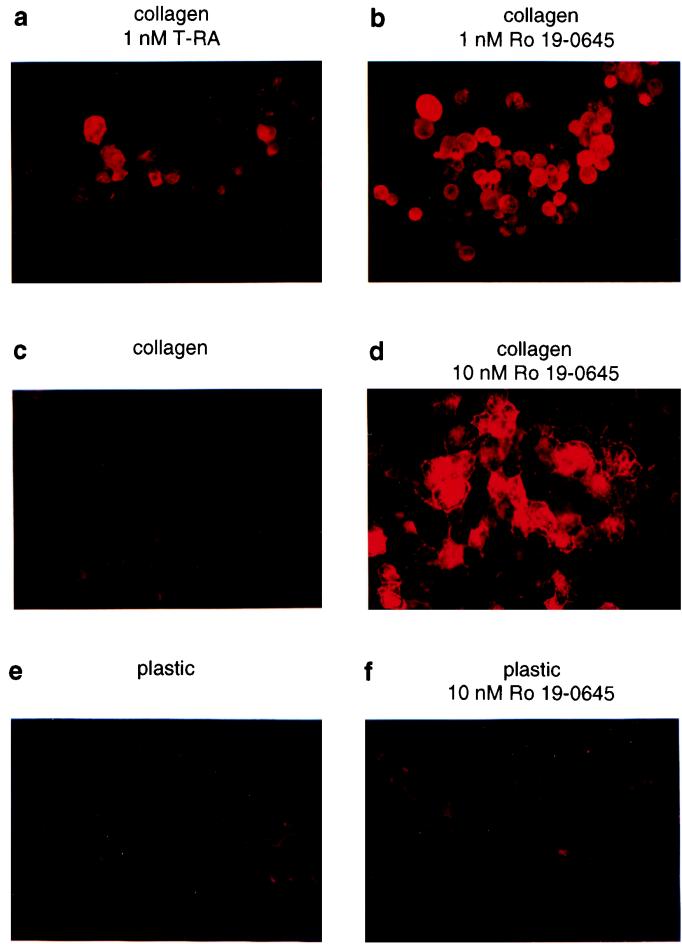
Previous work established that mucosecretory differentiation is induced when NHBEs are grown on type I collagen gels in defined, serum-free media in the presence of all-trans-retinoic acid (T-RA) (73). Differentiation, as assessed by mucous glycoprotein expression, is inefficient under these conditions (Fig. 1a), possibly due to the rapid rate of T-RA metabolism in such epithelial cells. Unfortunately, however, high doses of T-RA are toxic to NHBEs (data not shown). We searched for alternative, synthetic retinoids that might induce differentiation in a large percentage of NHBEs at relatively low doses. Compared to T-RA, the synthetic retinoid Ro 19-0645 (2) markedly enhanced differentiation (Fig. 1b). Importantly, Ro 19-0645-treated NHBEs retained the ECM requirement for mucosecretory differentiation (Fig. 1c to f). Based on these findings, as well as its ability to bind nuclear hormone receptors (2) and stimulate transcription from the RARβ RARE (βRARE) (Fig. 3d), we concluded that Ro 19-0645 is a useful tool for dissecting retinoid signaling in this primary cell system.
FIG. 1.
Ro 19-0645 is a potent inducer of mucosecretory differentiation. Cytospun NHBEs were immunostained with the antimucin MAb 17B1 after growth for 5 (a and b) or 2 (c to f) days.
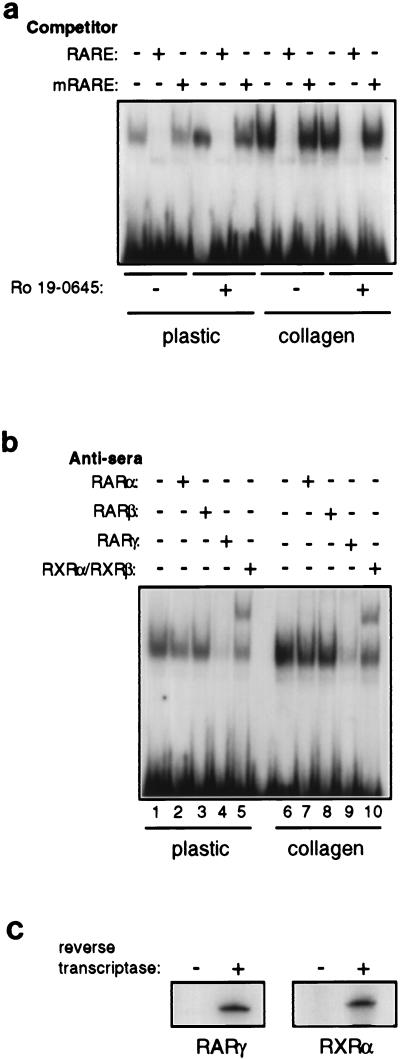
FIG. 3.
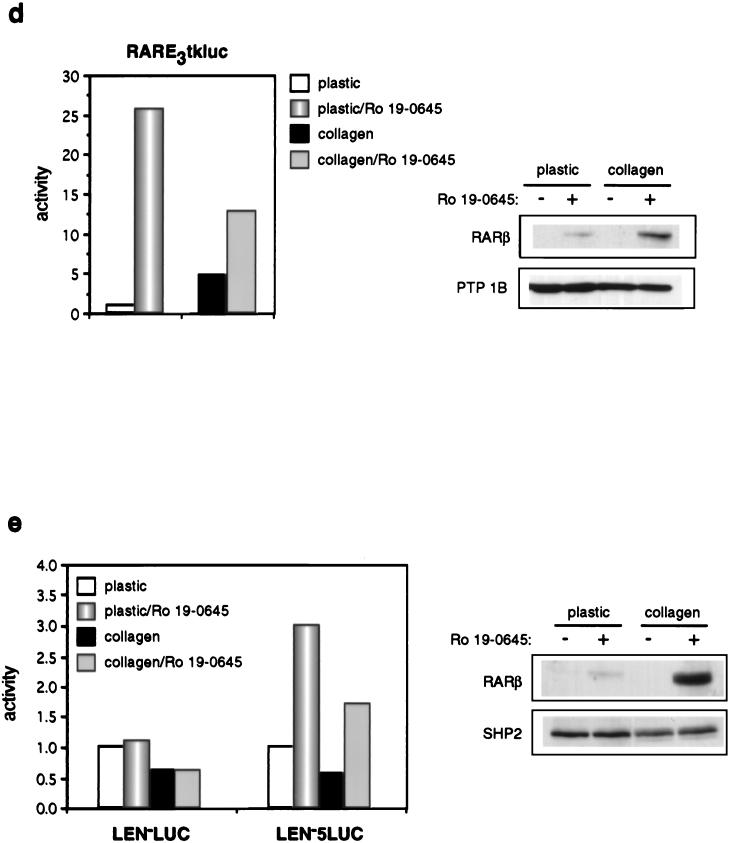
RARs and RXRs are expressed in NHBEs, and their activity is modulated by collagen gels in a promoter-specific manner. (a) EMSA of βRARE binding activity in nuclear extracts from NHBEs growing on plastic or collagen gels in the absence or presence of 10 nM Ro 19-0645 for 48 h. mRARE is a mutated βRARE that cannot bind RARs and RXRs. (b) Nuclear extracts used for panel a were preincubated with RAR- or RXR-specific antisera prior to EMSA. (c) RT-PCR analysis of RARγ and RXRα mRNA expression in NHBEs growing on plastic in the presence of 10 nM Ro 19-0645 for 48 h. (d) Activity (light units normalized to CAT activity) of a transiently transfected minimal βRARE-containing promoter in NHBEs. Synergistic induction of endogenous RARβ by collagen gels and retinoid in transfected cultures was confirmed by immunoblotting lysates from parallel pUC19-transfected cells (right). The blot was reprobed with αPTP-1B to control for loading. (e) Activity (light units normalized to total protein) of a retrovirally integrated 5-kbp RARβ promoter-luciferase fusion (LEN−5LUC) in NHBEs. Synergistic induction of endogenous RARβ by collagen gels and retinoid in mock-infected cultures was confirmed by immunoblotting lysates (right). The blot was reprobed with αPTP-1B to control for loading. We have recently confirmed that individual retrovirally infected cells retain the ability to synergistically induce RARβ in the presence of collagen gels and retinoid (data not shown).
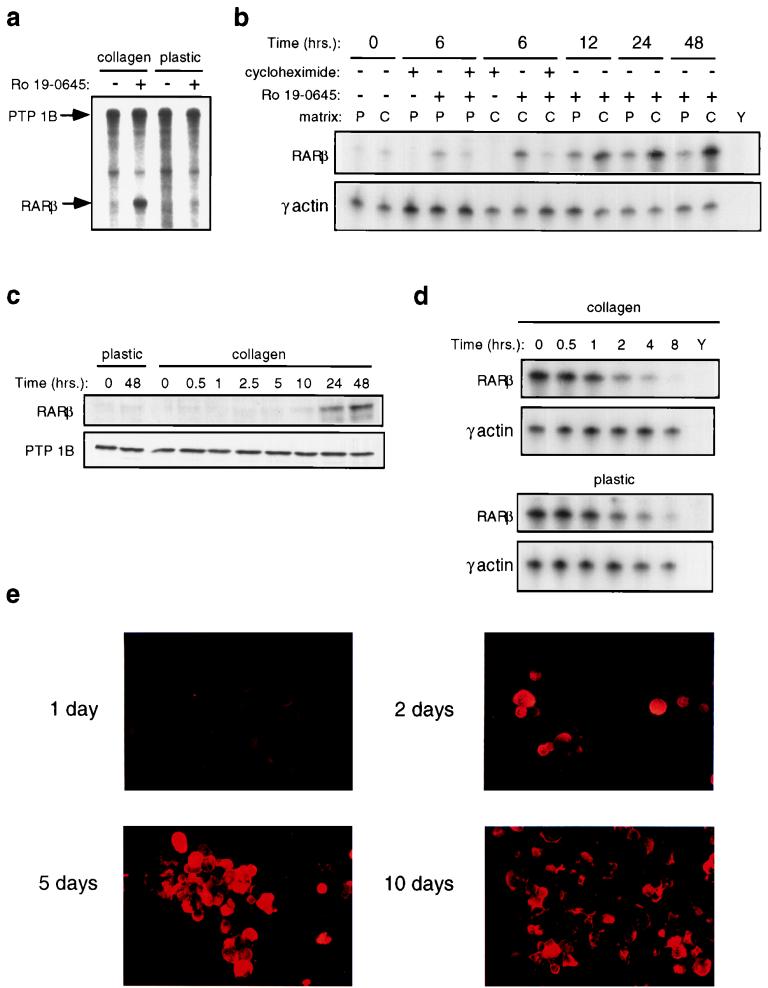
Although MAb 17B1 (39) recognizes an airway-specific mucin, the gene encoding this protein has not been cloned, and no other clearly defined markers for NHBE mucosecretory differentiation exist. We sought to identify additional markers that would facilitate our molecular analysis of signal integration. We suspected that RARβ might be such a marker. In mice, RARβ is highly expressed in the developing respiratory epithelium, and its expression coincides with the onset of cytodifferentiation (15). Loss of RARβ expression has been reported for a number of epithelial cell-derived malignancies (21, 22, 27, 49, 62, 65); notably, it is one of the most frequent events observed in lung cancer (21, 49), in which NHBE differentiation is aberrant. Moreover, defective RARβ expression is an early event in epithelial carcinogenesis (42). RARβ is the prototypical retinoid-inducible gene in cell lines (13, 14, 43): its transcription is induced in an immediate-early fashion upon retinoid treatment, peaking by 6 h and independent of new protein synthesis (13, 43). We observed that RARβ also was induced by retinoids in primary NHBEs. Interestingly, however, unlike in cell lines, retinoid only induced substantial RARβ mRNA (Fig. 2a and b) and protein (Fig. 2c) expression when NHBEs were grown on collagen gels, the appropriate ECM for mucosecretory differentiation. Moreover, unlike in cell lines, RARβ mRNA induction in NHBEs was delayed and required new protein synthesis (Fig. 2b). RARβ mRNA stability was unaffected by ECM (Fig. 2d), implying that growth on collagen gels is required for high levels of retinoid-dependent transcription. Since peak RARβ RNA and protein expression (Fig. 2b and c) occurred well before the peak of mucous glycoprotein expression (5 to 10 days [Fig. 2e]), and only under conditions that allow NHBE differentiation, we used RARβ as an early marker for mucosecretory differentiation.
FIG. 2.
Retinoid induction of RARβ is enhanced by collagen gels and is an early event during mucosecretory differentiation. RARβ mRNA and protein levels at different times following exposure to 10 nM Ro 19-0645 are shown. (a) RNase protection analysis of RARβ mRNA levels 48 h following retinoid treatment. PTP-1B levels were examined to control for loading. (b) RNase protection analysis of RARβ mRNA levels following retinoid treatment. Cycloheximide was used at 10 μg/ml. P, plastic; C, collagen gel; Y, yeast RNA. γ-Actin levels were measured to control for loading. (c) Immunoblot analysis of RARβ protein levels following retinoid treatment. PTP-1B levels were measured by immunoblotting to control for loading. (d) RNase protection analysis of RARβ mRNA stability. Twenty-eight hours following retinoid treatment on the respective substratum, cells were exposed to actinomycin D (5 μg/ml) for the indicated times, and RARβ mRNA levels were then quantitated. P, plastic; C, collagen gel; Y, yeast RNA. The panel displaying RARβ mRNA levels on plastic was exposed much longer than the panel for cells growing on collagen gels. γ-Actin levels were measured as an internal control. (e) Antimucin immunostaining with MAb 17B1 of cytospun NHBEs following growth on collagen gels in the presence of retinoid for the indicated times.
ECM-derived signaling pathways could regulate the activity of the βRARE either through direct regulation of retinoid receptors or indirectly through heterologous elements in the RARβ promoter. Nuclear extracts prepared from NHBEs growing on either collagen gels or plastic demonstrated βRARE binding activity in vitro, although binding activity was reduced in cells growing on plastic. In both cases, a single specific shifted species was observed (Fig. 3a). Complex formation was inhibited by preincubation of nuclear extracts with RARγ antiserum and supershifted by preincubation with RXRα and RXRβ antisera (Fig. 3b), suggesting that these receptors are in the complex. Moreover, the EMSA complex migrated in a manner similar to that of a complex generated with in vitro-translated RARs and RXRs, suggesting that retinoid receptors were the sole components of the EMSA complex (data not shown). RT-PCR analysis confirmed the expression of both RARγ and RXRα mRNAs in cells growing on plastic (Fig. 3c). These data indicate that NHBEs growing on plastic express RARs and RXRs that are capable of forming appropriate heterodimeric complexes on the βRARE in vitro, but they suggest that the efficiency of complex formation may be reduced.
To determine whether this difference in the level of βRARE binding activity contributes to reduced RARβ transcription, we transiently transfected a βRARE/heterologous tk promoter-luciferase construct into NHBEs. Notably, cells growing on plastic actually supported higher levels of retinoid-dependent transcription from this promoter than cells growing on collagen (Fig. 3d, left). Importantly, the transfection conditions did not interfere with the synergistic induction of endogenous RARβ by collagen gels and retinoid (Fig. 3d, right). Thus, the reduced βRARE binding activity observed in nuclear extracts from cells growing on plastic is still sufficient to confer high levels of retinoid-dependent transcription to minimal βRARE-containing promoters in NHBEs. Moreover, these data suggest that elements mapping outside the βRARE are required to observe ECM-dependent effects on RARβ transcription. Conceivably, stable integration of reporter constructs may be necessary for observation of proper transcriptional regulation of larger fragments of the RARβ promoter. Since the limited life span of primary NHBE cultures does not allow selection of stably transfected clones, we introduced reporter constructs into NHBEs by using enhancer trap retroviruses (see Materials and Methods). Following infection of NHBEs on plastic with retroviruses carrying luciferase alone (LEN−LUC) or a fusion of 5 kbp of the RARβ 5′ flanking region to luciferase (LEN−5LUC), cells were subcultured onto either plastic or collagen gels. Retinoid treatment of LEN−5LUC-infected cells, but not LEN−LUC-infected cells, resulted in a threefold increase in luciferase activity regardless of the substratum (Fig. 3e, left). Under the same conditions, control mock-infected cells demonstrated strong synergy between ECM and retinoid for induction of endogenous RARβ expression (Fig. 3e, right). These data strongly suggest that sequences outside the 5 kbp 5′ flanking region of the RARβ gene are required to reproduce correct transcriptional regulation by collagen gels and retinoid. Consistent with our results, a 3.8-kbp fragment of the 5′ flanking region of the murine RARβ gene previously was found to be unable to direct β-galactosidase expression to the bronchi of transgenic mice (45).
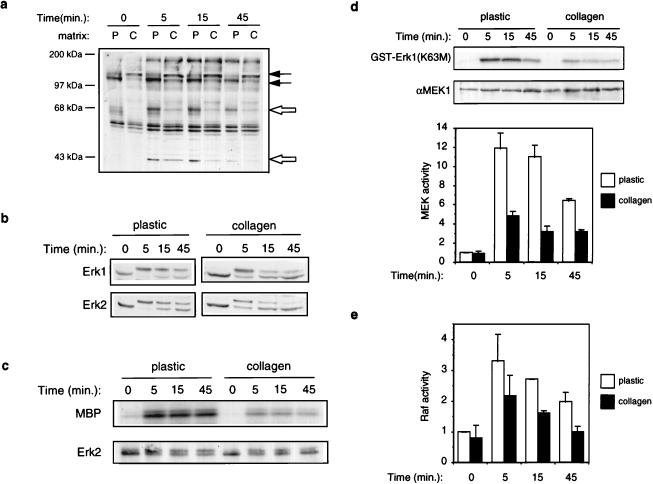
Since the effects of ECM on RARβ expression did not map exclusively to the βRARE, we suspected that collagen gels might instead modulate a heterologous signaling pathway that indirectly communicated with RARs and RXRs on the endogenous RARβ gene. NHBE cell growth is regulated by a number of autocrine and paracrine factors besides retinoids. Some of these factors include agonists for RTKs, such as EGF, insulin, platelet-derived growth factor (PDGF), and hepatocyte growth factor (68, 74, 75). We therefore asked whether RTK signaling might be modulated by differentiation-promoting collagen gels. We examined the ability of a combination of EGF and insulin, the only two exogenously supplied RTK agonists in the NHBE culture medium, to evoke downstream signaling events. NHBEs were grown in the presence of retinoid for 2 days, starved for exogenous EGF and insulin during the last 20 h, and then subjected to acute stimulation with both growth factors. Antiphosphotyrosine immunoblotting of whole-cell lysates indicated a number of differences in basal and growth factor-induced protein tyrosyl phosphorylation that were substratum dependent. In starved cells, a 120-kDa protein was hypophosphorylated and a 130-kDa protein was hyperphosphorylated on collagen gels (Fig. 4a, closed arrows). When grown on plastic, growth factors induced the tyrosyl phosphorylation of four major proteins, with molecular masses of 180, 68, 52, and 43 kDa (Fig. 4a). Strikingly, however, growth factors selectively failed to promote extensive tyrosyl phosphorylation of the 68- and 43-kDa proteins (Fig. 4a, open arrows) in cells growing on collagen gels. Thus, growth on collagen gels diminishes specific aspects of RTK signaling in NHBEs.
FIG. 4.
Collagen gels inhibit activation of the MAPK pathway by growth factors. Cells were stimulated with both EGF and insulin for the indicated times (unless otherwise indicated). (a) Antiphosphotyrosine blot of total cell lysates. Closed arrows indicate proteins that are basally differentially tyrosyl phosphorylated; open arrows indicate proteins that are differentially tyrosyl phosphorylated upon growth factor stimulation. P, plastic; C, collagen gels. (b) MAPK activation examined by immunoblotting total cell lysates with anti-Erk1/2 antibodies. (c) Erk2 activity measured directly in immune complex kinase assays. Top, phosphorylation of the myelin basic protein (MBP) substrate; bottom, immunoblot of levels of Erk2 in the immune complexes. (d) MEK activity measured in immune complex kinase assays. Top, phosphorylation of the kinase-inactive Erk1 substrate [GST-Erk1(K63M)]; middle, immunoblot of MEK levels in the immune complexes; bottom, graphic representation of MEK activity as assessed by PhosphorImager quantitation of 32P incorporation into the GST-Erk1 substrate. The data represent means and standard deviations from two independent experiments. (e) Raf activity measured in immune complex kinase assays using kinase-inactive MEK1 as a substrate [GST-MEK(K97A)]. Shown is graphic representation of Raf activity as assessed by PhosphorImager quantitation of 32P incorporation into the GST-MEK1 substrate. The data represent means and standard deviations from two independent experiments. Growth on collagen gels did not affect levels of Raf protein (data not shown).
In most other systems, the MAPKs Erk1 and Erk2 are the major 42- to 44-kDa tyrosyl phosphoproteins upon growth factor stimulation (reviewed in reference 52), which led us to believe that the 43-kDa species that was hypophosphorylated on collagen gels might be a MAPK. To directly test this hypothesis, we analyzed growth factor-stimulated Erk1 and Erk2 activation by immunoblotting. As evidenced by the characteristic reduction of electrophoretic mobility of their activated forms, we observed less growth factor-induced Erk1 and Erk2 activation over time in NHBEs cultured on collagen gels than in those cultured on plastic dishes (Fig. 4b). Immune complex Erk2 kinase assays supported the immunoblotting data and directly demonstrated that following growth factor stimulation, Erk2 had a lower specific activity in cells growing on collagen gels than in cells growing on plastic dishes (Fig. 4c). Immune complex kinase assays revealed that the specific activity of MEK1 was comparably reduced in cells growing on collagen gels (Fig. 4d). In NHBEs, the extent to which Raf was activated upon growth factor stimulation was consistently lower than the fold activation of MEK and MAPK (on either plastic dishes or collagen gels). Nevertheless, growth on collagen gels, compared to plastic dishes, also reduced the ability of growth factors to activate Raf (Fig. 4e).
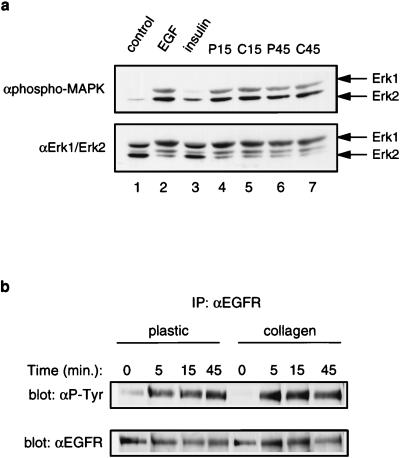
Since our stimulations were performed in the presence of both exogenous EGF and insulin, we asked whether one of these signaling pathways might predominantly activate MAPK in NHBEs. Immunoblotting with an activation-specific pan-Erk1/2 antibody revealed that even at low doses (0.1 nM), EGF was a much more potent activator of MAPK than insulin (Fig. 5a, lanes 2 and 3). Since some ECMs bind growth factors tightly, collagen gels conceivably could lower the response to exogenously supplied EGF by sequestering it from the EGFR. To address this possibility, serum-free media were incubated with cells growing on collagen gels or plastic dishes; after a 15- or 45-min stimulation with growth factors, media were removed and used to stimulate NHBEs growing on plastic dishes. Immunoblotting with the activation-specific pan-Erk1/2 antibody revealed equivalent abilities of growth factors to stimulate MAPK activation regardless of whether they had been preincubated with cultures growing on plastic dishes or collagen gels (Fig. 5a, lanes 4 to 7). These data indicate that during the time course of our stimulations, exogenously supplied EGF is not sequestered by the collagen gel to the point that it is limiting. Consistent with these data, NHBEs growing on either plastic dishes or collagen gels bound similar levels of 125I-EGF (data not shown). We next examined whether growth on collagen gels impaired the ability of the EGFR to autophosphorylate. Immunoprecipitation and immunoblotting experiments following growth factor stimulation revealed comparable levels of tyrosyl-phosphorylated EGFR regardless of the substratum (Fig. 5b). These data are consistent with antiphosphotyrosine immunoblots of whole-cell lysates, which demonstrated equal amounts of a major growth factor-induced 180-kDa tyrosyl phosphoprotein (Fig. 4a).
FIG. 5.
Collagen gels do not impair general EGFR autophosphorylation. (a) EGF, rather than insulin, is a potent activator of MAPK in NHBEs. Cells growing on plastic were subjected to a 5-min stimulation with either EGF or insulin (lanes 2 and 3). Collagen gels do not sequester EGF from the EGFR. NHBEs growing on either plastic (P) or collagen gels (C) were stimulated for either 15 or 45 min with growth factors, after which the media were removed and used to stimulate cells growing on plastic for 5 min (lanes 4 to 7). Growth factor activity after preincubation with cultures growing on plastic or collagen gels was assessed by measuring MAPK activation by immunoblotting with α-phospho-MAPK (top); the blot was reprobed with αErk1/2 to control for loading (bottom). (b) General EGFR autophosphorylation is not impaired by growth on collagen gels. NHBEs were stimulated with EGF and insulin for the indicated times, and EGFR tyrosyl phosphorylation was examined by immunoprecipitation with αEGFR and antiphosphotyrosine (α-P-Tyr) immunoblotting (top). Equal amounts of EGFR in the immunoprecipitates were confirmed by immunoblotting with αEGFR (bottom).
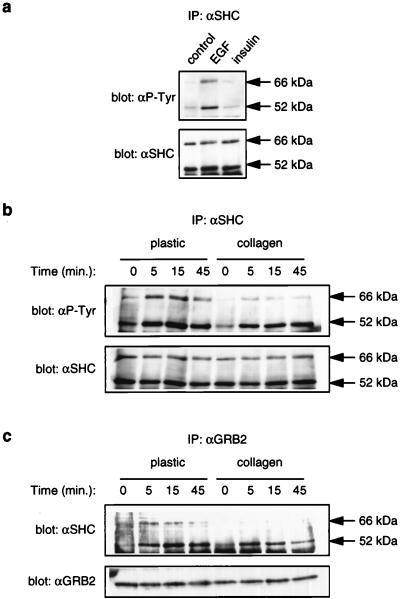
Our results indicate that the reduced MAPK activation on collagen gels reflects a specific reduction in the ability of an activated EGFR to transduce a strong signal to MEK. Reduced Raf activity (Fig. 4e) is likely to account, at least partially, for the differences in MEK activation, suggesting that one point of action of ECM is upstream of Raf. Interestingly, besides the MAPKs, the only other protein that was differentially tyrosyl phosphorylated upon growth factor stimulation was 68 kDa (Fig. 4a, open arrows). We sought to identify this protein, since it might be an important component of EGFR-dependent MAPK activation in epithelial cells. In other cell systems, SHP-2 and p66SHC are the two major phosphotyrosyl proteins in this molecular weight range upon EGF stimulation (18, 36, 53), and both of these proteins are implicated in the control of MAPK activation by EGF (4, 46, 50). In NHBEs, SHP-2 was not significantly tyrosyl phosphorylated upon EGF stimulation (data not shown). In contrast, EGF induced significant tyrosyl phosphorylation of p66SHC in NHBEs (Fig. 6a). Strikingly, however, like the 68-kDa tyrosyl phosphoprotein in whole-cell lysates, p66SHC was hypophosphorylated on collagen gels upon growth factor stimulation (Fig. 6b). Notably, EGF-induced p52SHC tyrosyl phosphorylation was not impaired significantly on collagen gels (Fig. 6b); we were unable to analyze p46SHC phosphorylation in anti-SHC immunoprecipitates from NHBEs due to its close migration with the IgG heavy chain during SDS-PAGE. Both p52SHC and p66SHC can bind to the EGFR through their PTB domains, and once tyrosyl phosphorylated, they can bind to GRB2 (reviewed in reference 7). Consistent with the decrease in EGF-induced tyrosyl phosphorylation of p66SHC, growth on collagen gels diminished the ability of EGF to promote coimmunoprecipitation of p66SHC, but not p52SHC, with GRB2 (Fig. 6c). Thus, in NHBEs, extensive tyrosyl phosphorylation of both p52SHC and p66SHC, and their association with GRB2, correlates with strong MAPK activation by EGF. In contrast, tyrosyl phosphorylation of only p52SHC, and association of p52SHC alone with GRB2, correlates with weaker MAPK activation.
FIG. 6.
Collagen gels inhibit EGF-dependent tyrosyl phosphorylation of p66SHC and its association with GRB2. (a) EGF stimulates tyrosyl phosphorylation of p52SHC and p66SHC in NHBEs growing on plastic. Cells growing on plastic were stimulated for 5 min with the indicated growth factor, and SHC tyrosyl phosphorylation examined by immunoprecipitation (IP) with αSHC and antiphosphotyrosine (αP-Tyr) immunoblotting. Equal levels of SHC in the immunoprecipitates were confirmed by immunoblotting with αSHC. (b) p66SHC is hypophosphorylated in response to growth factor stimulation of cells growing on collagen gels. Cells were stimulated for the indicated times with a combination of EGF and insulin. SHC tyrosyl phosphorylation was examined as for panel a. (c) Growth factors fail to promote extensive association of p66SHC with GRB2 in cells growing on collagen gels. Cells were stimulated for the indicated times with a combination of EGF and insulin, lysed, and subjected to immunoprecipitation with αGRB2. The level of SHC in the αGRB2 immunoprecipitates was determined by immunoblotting with αSHC. Equal levels of GRB2 in the immunoprecipitates were confirmed by immunoblotting with αGRB2 (bottom).
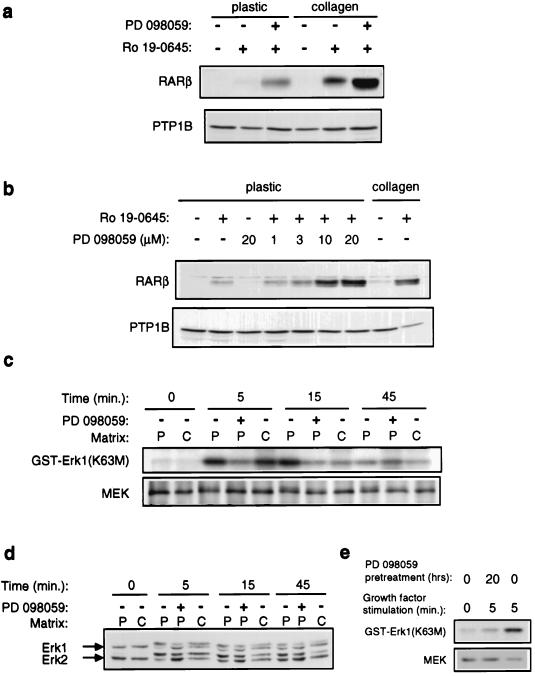
Our findings that collagen gels inhibit EGFR-dependent MAPK activation and promote differentiation suggested that elevated MAPK activity might inhibit mucosecretory differentiation. To test this hypothesis, we treated randomly growing cultures of NHBEs with PD 098059, a highly specific inhibitor of the activation of MEK by Raf (1, 16). Strikingly, PD 098059 treatment permitted high levels of RARβ induction by retinoid in the absence of exogenous ECM (Fig. 7a). PD 098059 did not induce RARβ expression in the absence of retinoid, suggesting that it acts on the same signaling pathway activated by growth on collagen gels (Fig. 7b). The dose dependence for its effect on RARβ expression was comparable to its reported dose dependence for inhibiting MAPK activation (1, 16) (Fig. 7b). Furthermore, we confirmed that PD 098059 inhibited MEK (Fig. 7c) and MAPK (Fig. 7d) activation by growth factors and that this drug is extremely stable in the culture medium, since pretreatment of cells with PD 098059 for up to 20 h still potently inhibited MEK activation (Fig. 7e). Consistent with our observations with PD 098059, recent preliminary experiments indicate that infection of NHBEs with retroviruses carrying an activated allele of MEK inhibits RARβ induction on collagen gels (data not shown).
FIG. 7.
Inhibition of MEK and MAPK signaling promotes retinoid-induced RARβ expression in the absence of collagen gels. NHBEs were grown for 48 (a) or 24 (b) h in the presence of PD 098059 and retinoid. RARβ expression was determined by immunoblotting total cell lysates. Anti-PTP-1B immunoblotting was used to confirm equal loading. (c and d) Cells growing on either plastic (P) or collagen gels (C) were stimulated for the indicated times with EGF and insulin. In some cases, cultures were treated with 10 μM PD 098059 for 45 min prior to growth factor stimulation. (c) MEK activity was measured in immune complex kinase assays. Top, phosphorylation of the kinase-inactive Erk1 substrate [GST-Erk1(K63M)]; bottom, immunoblot of MEK levels in the immune complexes. (d) MAPK activation examined by immunoblotting total cell lysates with anti-Erk1/2 antibodies. (e) Stability of PD 098059 in NHBE cultures. NHBEs growing on plastic were preincubated with PD 098059 for 20 h prior to stimulation with growth factors and measurement of MEK activation as described for panel c. Note that the drug remains fully able to inhibit MEK activity even after 20 h of incubation.
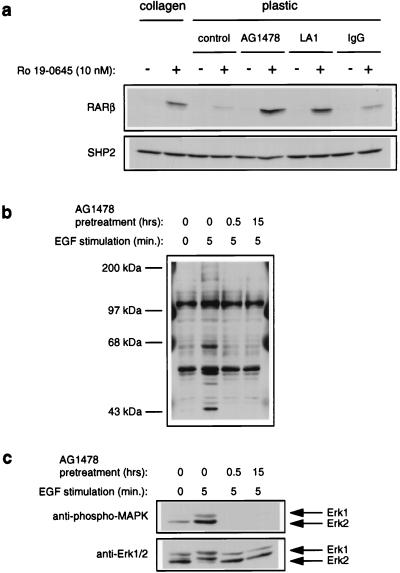
Since (i) high doses of EGF have been reported to interfere with mucosecretory differentiation ex vivo (23, 74) and (ii) we found that collagen gels inhibit EGF-dependent activation of MAPK, we asked whether excessive EGFR signaling specifically contributed to the MAPK-dependent inhibition of retinoid signaling observed in NHBEs. We cultured NHBEs in the presence of retinoid but in the absence of exogenous EGF. Unlike treatment with PD 098059, removal of exogenous EGF did not promote RARβ expression in the absence of collagen (data not shown). However, NHBEs secrete transforming growth factor α (TGF-α) and amphiregulin, two endogenous ligands for the EGFR (69). We therefore suspected that an autocrine EGFR signaling loop may be predominantly responsible for the elevated MAPK activity and inhibition of RARβ expression observed in cells growing on plastic. Consistent with our hypothesis, treatment of NHBEs with AG1478, a specific inhibitor of EGFR kinase activity (37), strongly promoted RARβ expression by retinoid in the absence of collagen (Fig. 8a). We confirmed that AG1478 was stable in our NHBE cultures and was a potent antagonist of EGFR signaling. Pretreatment of cultures with AG1478 for up to 15 h strongly inhibited the appearance of all major EGF-induced tyrosyl phosphoproteins (Fig. 8b) and MAPK activation (Fig. 8c). To independently confirm the role of the EGFR in the inhibition of retinoid signaling in NHBEs, we cultured cells in the presence of the anti-EGFR neutralizing antibody LA1 (31). In agreement with our data for assays using AG1478, LA1, but not control IgG, strongly promoted the expression of RARβ by retinoid in the absence of collagen (Fig. 8a). Together, our data support a model wherein collagen gels suppress an autocrine loop involving EGFR-induced activation of MAPK and suggest that this attenuation is critical for retinoids to induce at least some of the markers associated with NHBE differentiation.
FIG. 8.
Inhibition of EGFR activity promotes retinoid-induced RARβ expression in the absence of collagen gels. (a) NHBEs were grown for 24 h in the presence of Ro 19-0645 (10 nM), AG1478 (300 nM), anti-EGFR neutralizing antibody LA1 (13 μg/ml), or control IgG (13 μg/ml) as indicated. RARβ levels were determined by immunoblotting total cell lysates. The blot was reprobed with anti-SHP2 antibodies to control for loading. (b and c). AG1478 is stable in NHBE cultures and inhibits all major EGF-dependent tyrosyl phosphorylation events and MAPK activation. Cells growing on plastic were preincubated with AG1478 (300 nM) for the indicated times and then subjected to stimulation with EGF. Cell lysates were immunoblotted with antibodies specific for phosphotyrosine (b), phospho-MAPK (c, top) or Erk1/2 (c, bottom) to control for loading.
DISCUSSION
Using primary cultures of NHBEs that respond to growth factors, retinoids, and ECM by undergoing normal differentiation ex vivo, we have uncovered significant differences in some established paradigms for mammalian cell signal transduction. Retinoid-mediated induction of the prototypical retinoid-inducible gene, RARβ, does not proceed in an immediate-early fashion in these primary cells. Instead, its ability to respond to retinoids requires new protein synthesis and is regulated by an ECM/MAPK-dependent signaling pathway(s). Furthermore, although previous work in other systems demonstrated the ability of ECM to enhance RTK-dependent activation of MAPK (60), we provide the first example of a cell-substratum interaction that results in inhibition of RTK-dependent MAPK activation.
We identified RARβ as the first defined early marker for mucosecretory differentiation of NHBEs (Fig. 2). This finding is consistent with studies of lung cancer cells, which frequently display loss of RARβ expression (21, 49), and studies in which RARβ expression suppresses growth and tumorigenesis (20, 25) and correlates with responsiveness to chemotherapy (42). In addition, transgenic mice expressing RARβ antisense RNA develop lung tumors (6). However, unlike in multiple immortalized cell lines, this prototypical retinoid-inducible gene is not an immediate-early gene in primary NHBEs (Fig. 2b), and its transcriptional induction by retinoids is dramatically enhanced by growth on type I collagen gels (Fig. 2a and d). Since growth on collagen gels does not appear to regulate the transcriptional activity of either the isolated βRARE (Fig. 3e) or a 5-kbp fragment of the RARβ 5′ flanking region containing the βRARE (Fig. 3f), we propose that an ECM-derived signaling pathway controls the activity of a factor distinct from the retinoid receptors and their general coactivators.
Our finding that the MEK inhibitor PD 098059 permits high levels of RARβ expression in cells growing on plastic (Fig. 5a) suggests that phosphorylation by MAPK (or conceivably by MEK itself) either inhibits the activity of retinoid receptors directly or promotes the inhibitory activity of a factor distinct from these receptors. In cell lines, MAPK directly phosphorylates two other nuclear hormone receptors, the estrogen receptor (33) and peroxisome proliferator-activated receptor gamma (PPARγ) (26), and modulates their activity on isolated hormone response elements. Since growth on collagen gels, where there is less MEK and MAPK activation (Fig. 4c and d), does not enhance the retinoid inducibility of a minimal βRARE-containing promoter (Fig. 3e) or the 5-kbp fragment of the RARβ promoter (Fig. 3f), we favor a model in which MAPK does not directly affect retinoid receptors but instead promotes the inhibitory properties of a novel factor that likely requires cis-acting sequences outside the 5-kbp promoter region for its inhibitory activity. Notably, and consistent with our proposal, MAPK does not affect RARα phosphorylation in COS cells (56), and a 3.8-kbp fragment of the murine RARβ 5′ flanking region cannot confer bronchial cell-specific expression in transgenic mice (45).
The ability of ECM to promote MAPK activation by acute engagement of integrins has been studied extensively in assays using cell lines. Immediately upon integrin engagement or attachment to ECM, PTKs are activated, leading to MAPK activation (reviewed in reference 10). Consistent with the above studies, tyrosine kinase signaling was acutely activated during the initial stages of NHBE attachment to collagen gels (data not shown). However, NHBEs, like other epithelial cells, do not continuously detach and reattach to their underlying substratum in vivo. Therefore, a more physiologically relevant question is how long-term growth on an ECM that supports differentiation modulates the ability of cells to activate MAPKs in response to growth factors. Indeed, under such conditions (long-term growth on collagen gels), NHBEs exhibit a decreased ability to activate MEK and MAPK in response to EGF (Fig. 4c, 4d, and 5a). This inhibition cannot be attributed to limiting amounts of EGF in the culture medium (Fig. 5a) or a general inability of EGF to promote high levels of EGFR autophosphorylation (Fig. 5b). However, the inhibition of MEK activation does correlate with a reduced ability to activate Raf (Fig. 4e) and the inability of EGF to promote extensive tyrosyl phosphorylation of a major 68-kDa protein (Fig. 4a), which we have identified as p66SHC (Fig. 6b).
SHC proteins have been implicated in Ras activation by a number of growth factors (for a review, see reference 7), but little is known about the role of specific SHC proteins during normal, physiologic responses. The recently cloned p66SHC (46, 50) differs from p52SHC by virtue of an additional collagen homology domain (CH2) in its amino terminus. Like p52SHC, p66SHC binds to the EGFR and GRB2 (46, 50). In CHO/IR/ER cells, overexpression of p66SHC inhibits EGF-dependent MAPK activation (50), while in COS and HeLa cells, overexpression of p66SHC has no effect on EGF-dependent MAPK activation but does inhibit EGF-dependent c-fos promoter activation (46). In contrast, in HC-11 mouse mammary epithelial cells, cripto-1-dependent association of SOS with SHC proteins and activation of MAPK is correlated more strongly with p66SHC tyrosyl phosphorylation than with p52SHC tyrosyl phosphorylation (32). We observe that in NHBEs growing on plastic, where EGF promotes extensive tyrosyl phosphorylation of both p52SHC and p66SHC and association of both SHC proteins with GRB2, MEK and MAPK activation is strong (Fig. 4 and 6). In contrast, on collagen gels, where EGF selectively fails to promote extensive tyrosyl phosphorylation of p66SHC and its association with GRB2, there is less Raf, MEK and MAPK activation (Fig. 4 and 6). In NHBEs, the total level of Ras activation integrated over time may depend on the recruitment of both pools of SHC to the EGFR. By virtue of its unique CH2 domain, the tyrosyl phosphorylation status of p66SHC, and hence its ability to regulate EGFR signaling, may be sensitive to novel signaling pathways activated in epithelial cells by growth on differentiation-promoting ECMs. One attractive possibility is that the 120- and/or 130-kDa tyrosyl phosphoproteins that exhibit different levels of basal phosphorylation on plastic and collagen gels (Fig. 4a) may be part of a signaling pathway that regulates p66SHC phosphorylation. Preliminary experiments indicate that the 120-kDa protein is not FAK and the 130-kDa protein is not p130CAS (data not shown). Due to reagent limitations, we have not yet been able to determine whether the 130-kDa protein that is hyperphosphorylated on collagen gels is either of the newly identified collagen receptors DDR1 and DDR2 (64, 71).
Since (i) collagen gels inhibit EGFR-dependent activation of MEK and MAPK and promote the induction of RARβ by retinoids, (ii) direct inhibition of EGFR signaling by AG1478 or anti-EGFR neutralizing antibodies promotes RARβ induction by retinoids, and (iii) specific inhibition of Raf-dependent MEK/MAPK activation by treatment with PD 098059 also promotes retinoid inducibility of the RARβ gene, we propose that collagen gels promote the retinoid responsiveness of the RARβ gene by reducing the level of EGFR-dependent MEK/MAPK activity (Fig. 9). Thus, in vivo, the role of ECM in modulating MAPK activation in response to growth factors is likely to be cell type dependent. For example, fibroblasts, which normally respond to growth factors at sites of wounding by undergoing proliferation, display enhanced MAPK activation and growth in response to PDGF when cultured on ECMs such as vitronectin (60). In contrast, NHBEs are designed to undergo differentiation when growing on their normal ECM, and accordingly display reduced activation of MAPK by growth factors, when cultured on differentiation-promoting collagen gels.
FIG. 9.
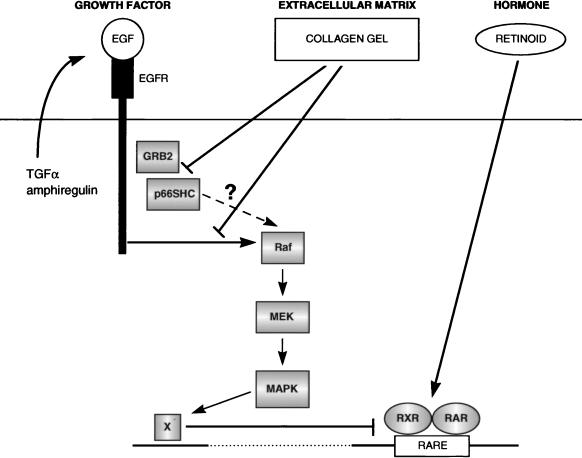
Model for integration of growth factor-, retinoid-, and ECM-derived signaling pathways during normal mucosecretory cell differentiation. Randomly growing NHBEs express and secrete TGF-α and amphiregulin, one or both of which participate in an autocrine loop, signaling via the endogenous EGFR. In addition, NHBEs respond to exogenous EGF present in growth media or produced by neighboring cells in vivo. Differentiation-promoting ECMs (e.g., collagen) suppress autocrine and paracrine activation of the MAPK pathway by the EGFR, acting downstream of the EGFR and upstream of Raf. This is correlated with an inability to achieve high levels of tyrosyl-phosphorylated p66SHC and its association with GRB2. However, it remains possible that as yet unidentified differences in EGFR-dependent signaling may actually account for the ECM-dependent inhibition of Raf, MEK, and MAPK activation. Ultimately, reduction in MAPK activity inactivates an inhibitor of retinoid receptor activity and results in the strong induction of RARβ expression in the presence of retinoids.
Although treatment with PD 098059, AG1478, or LA1 promoted retinoid-dependent induction of RARβ, none of these agents could promote the induction of mucous glycoprotein expression in the absence of collagen gels (data not shown). This most likely reflects the involvement of MAPK-dependent and independent pathways in regulating mucosecretory differentiation. Given that NHBEs secrete TGF-α and amphiregulin, we propose that ECM restricts the extent of EGFR-dependent MEK/MAPK activation that occurs in the bronchial epithelium in response to both autocrine (TGF-α and amphiregulin) and paracrine (EGF) growth factors (Fig. 9). This would be critical for minimizing the inhibitory effects of MAPK on retinoid-dependent transcription of target genes such as RARβ, which are likely to be involved in differentiation.
The antidifferentiative role of MAPKs in NHBE mucosecretory differentiation contrasts with their role in PC12 cell neuronal differentiation (12, 67) but is similar to their role in 3T3-L1 adipocyte (19) and myoblast differentiation (5, 11). Strikingly, specific restriction of the amount of EGFR-dependent MAPK activation is critical for retinoids to induce at least some of the target genes normally induced during NHBE differentiation. This would suggest that part of the mechanism of carcinogenesis in the lung, in which overexpression of the EGFR and/or other EGFR family members is frequent (reviewed in reference 58), may involve antagonism of the differentiation-promoting activity of retinoids. This may also explain the limited success of retinoid therapy in treating lung cancer (41). Accordingly, combination therapy of EGFR antagonists and retinoids might be more effective. Further study of primary cell systems such as NHBEs should continue to provide important insight into epithelial cell signaling pathways and their perturbation in disease states like cancer.
ACKNOWLEDGMENTS
We thank M. Klaus for Ro 19-0645, R. Wu for antimucin antibody 17B1, W. Pear for BING cells, W. Chin for αRXR antibodies, R. L. Erikson, B. Brott, A. Alessandrini, and Y. Gotoh for αMEK antibodies and GST-Erk1(K63M) fusion protein, K. L. Guan for GST-MEK(K97A) fusion protein, J. Blenis for αErk1/2 antiserum, S. Soltoff for AG1478, and J. Timms for assistance with the final stages of this work. We also thank Y. Gotoh, L. Klaman, C. Carpenter, and K. Carraway for helpful discussions.
This work was supported by grant CA-49152 from the National Institutes of Health to B.G.N.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Apfel C, Bauer F, Crettaz M, Forni L, Kamber M, Kaufmann F, LeMotte P, Pirson W, Klaus M. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci USA. 1992;89:7129–7133. doi: 10.1073/pnas.89.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: Greene Publishing Associates; 1987. [Google Scholar]
- 4.Bennett A M, Hausdorff S E, O’Reilly A M, Freeman R M, Neel B G. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett A M, Tonks N K. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- 6.Berard J, Laboune F, Mukuna M, Masse S, Kothary R, Bradley W E C. Lung tumors in mice expressing an antisense RARβ2 transgene. FASEB J. 1996;10:1091–1097. doi: 10.1096/fasebj.10.9.8801172. [DOI] [PubMed] [Google Scholar]
- 7.Bonfini L, Migliaccio E, Pelicci G, Lanfrancone L, Pelicci P G. Not all Shc’s roads lead to Ras. Trends Biochem Sci. 1996;21:257–261. [PubMed] [Google Scholar]
- 8.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 9.Chen Q, Kinch M S, Lin T H, Burridge K, Juliano R L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 10.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 11.Coolican S A, Samuel D S, Ewton D Z, McWade F J, Florini J R. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- 12.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 13.de The H, Marchio A, Tiollais P, Dejean A. Differential expression and ligand regulation of the retinoic acid receptor α and β genes. EMBO J. 1989;8:429–433. doi: 10.1002/j.1460-2075.1989.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de The H, Vivanco-Ruiz M D M, Tiollais P, Stunnenberg H, Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor β gene. Nature (London) 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- 15.Dolle P, Ruberte E, Leroy P, Morriss-Kay G, Chambon P. Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development. 1990;110:1133–1151. doi: 10.1242/dev.110.4.1133. [DOI] [PubMed] [Google Scholar]
- 16.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards G, Streuli C. Signaling in extracellular matrix-mediated control of epithelial cell phenotype. Biochem Soc Trans. 1995;23:464–468. doi: 10.1042/bst0230464. [DOI] [PubMed] [Google Scholar]
- 18.Feng G S, Hui C C, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 19.Font de Mora J, Porras A, Ahn N, Santos E. Mitogen-activated protein kinase activation is not necessary for, but antagonizes, 3T3-L1 adipocyte differentiation. Mol Cell Biol. 1997;17:6068–6075. doi: 10.1128/mcb.17.10.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frangioni J V, Moghal N, Stuart-Tilley A, Neel B G, Alper S L. The DNA binding domain of retinoic acid receptor β is required for ligand-dependent suppression of proliferation. J Cell Sci. 1994;107:827–838. doi: 10.1242/jcs.107.4.827. [DOI] [PubMed] [Google Scholar]
- 21.Gebert J F, Moghal N, Frangioni J V, Sugarbaker D J, Neel B G. High frequency of retinoic acid receptor β abnormalities in human lung cancer. Oncogene. 1991;6:1859–1868. [PubMed] [Google Scholar]
- 22.Geisen C, Denk C, Gremm B, Baust C, Karger A, Bollag W, Schwarz E. High-level expression of the retinoic acid receptor beta gene in normal cells of the uterine cervix is regulated by the retinoic acid receptor alpha and is abnormally down-regulated in cervical carcinoma cells. Cancer Res. 1997;57:1460–1467. [PubMed] [Google Scholar]
- 23.Gray T E, Guzman K, Davis C W, Abdullah L H, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 24.Harkema J, Mariassy A, St. George J, Hyde D M, Plopper C G. Epithelial cells of the conducting airways. In: Farmer S G, Hay D W P, editors. The airway epithelium. New York, N.Y: Marcel Dekker, Inc.; 1991. pp. 3–39. [Google Scholar]
- 25.Houle B, Rochette-Egly C, Bradley W E C. Tumor-suppressive effect of the retinoic acid receptor β in human epidermoid lung cancer cells. Proc Natl Acad Sci USA. 1993;90:985–989. doi: 10.1073/pnas.90.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu E, Kim J B, Sarraf P, Spiegelman B M. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 27.Hu L, Crowe D L, Rheinwald J G, Chambon P, Gudas L. Abnormal expression of retinoic acid receptors and keratin 19 by human oral and epidermal squamous cell carcinoma cell lines. Cancer Res. 1991;51:3972–3981. [PubMed] [Google Scholar]
- 28.Jetten A M. Multistep process of squamous differentiation in tracheobronchial epithelial cells in vitro: analogy with epidermal differentiation. Environ Health Perspect. 1989;80:149–160. doi: 10.1289/ehp.8980149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jetten A M, Brody A R, Deas M A, Hook G E R, Rearick J I, Thacher S M. Retinoic acid and substratum regulate the differentiation of rabbit tracheal epithelial cells into squamous and secretory phenotype. Lab Investig. 1987;56:654–664. [PubMed] [Google Scholar]
- 30.Jetten A M, Shirley J E. Characterization of transglutaminase activity in rabbit tracheal epithelial cells. J Biol Chem. 1986;261:15097–15101. [PubMed] [Google Scholar]
- 31.Johnson G R, Kannan B, Shoyab M, Stromberg K. Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. J Biol Chem. 1993;268:2924–2931. [PubMed] [Google Scholar]
- 32.Kannan S, De Santis M, Lohmeyer M, Riese D J I, Smith G H, Hynes N, Seno M, Brandt R, Bianco C, Perisco G, Kenney N, Normanno N, Martinez-Lacaci I, Ciardiello F, Stern D F, Gullick W J, Salomon D J. Cripto enhances the tyrosine phosphorylation of Shc and activates mitogen-activated protein kinase (MAPK) in mammary epithelial cells. J Biol Chem. 1997;272:3330–3335. doi: 10.1074/jbc.272.6.3330. [DOI] [PubMed] [Google Scholar]
- 33.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 34.Kazlauskas A. Receptor tyrosine kinases and their targets. Curr Opin Genet Dev. 1994;4:5–14. doi: 10.1016/0959-437x(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 35.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lechleider R J, Freeman R M, Neel B G. Tyrosyl phosphorylation and growth factor receptor association of the human corkscrew homologue, SH-PTP2. J Biol Chem. 1993;268:13434–13438. [PubMed] [Google Scholar]
- 37.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 38.Lin C Q, Bissell M J. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- 39.Lin H, Carlson D M, St. George J, Plopper C G, Wu R. An ELISA method for the quantitation of tracheal mucins from human and nonhuman primates. Am J Respir Cell Mol Biol. 1989;1:41–48. doi: 10.1165/ajrcmb/1.1.41. [DOI] [PubMed] [Google Scholar]
- 40.Lorenz U, Ravichandran K S, Pei D, Walsh C T, Burakoff S J, Neel B G. Lck-dependent tyrosyl phosphorylation of the phosphotyrosine phosphatase SH-PTP1 in murine T cells. Mol Cell Biol. 1994;14:1824–1834. doi: 10.1128/mcb.14.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lotan R. Retinoids in cancer chemoprevention. FASEB J. 1996;10:1031–1039. doi: 10.1096/fasebj.10.9.8801164. [DOI] [PubMed] [Google Scholar]
- 42.Lotan R, Xu X-C, Lippman S M, Ro J Y, Lee J S, Lee J J, Hong W K. Suppression of retinoic acid receptor-β in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332:1405–1410. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- 43.Martin C A, Ziegler L M, Napoli J L. Retinoic acid, dibutyryl-cAMP, and differentiation affect the expression of retinoic acid receptors in F9 cells. Proc Natl Acad Sci USA. 1990;87:4804–4808. doi: 10.1073/pnas.87.12.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDowell E M, Keenan K P, Huang M. Effects of vitamin A-deprivation on hamster tracheal epithelium: a quantitative morphologic study. Virchows Arch Cell Pathol. 1984;45:197–219. doi: 10.1007/BF02889865. [DOI] [PubMed] [Google Scholar]
- 45.Mendelsohn C, Ruberte E, LeMeur M, Morriss-Kay G, Chambon P. Developmental analysis of the retinoic acid-inducible RAR-β2 promoter in transgenic animals. Development. 1991;113:723–734. doi: 10.1242/dev.113.3.723. [DOI] [PubMed] [Google Scholar]
- 46.Migliaccio E, Mele S, Salcini A E, Pelicci G, Lai K-M V, Superti-Furga G, Pawson T, Di Fiore P P, Lanfrancone L, Pelicci P G. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signaling pathway. EMBO J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moghal N, Neel B G. Evidence for impaired retinoic acid receptor-thyroid hormone receptor AF-2 cofactor activity in human lung cancer. Mol Cell Biol. 1995;15:3945–3959. doi: 10.1128/mcb.15.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nervi C, Vollberg T M, George M D, Zelent A, Chambon P, Jetten A M. Expression of nuclear retinoic acid receptors in normal tracheobronchial cells and in lung carcinoma cells. Exp Cell Res. 1991;195:163–170. doi: 10.1016/0014-4827(91)90512-s. [DOI] [PubMed] [Google Scholar]
- 50.Okada S, Kao A W, Ceresa B P, Blaikie P, Margolis B, Pessin J E. The 66-kDa Shc isoform is a negative regulator of the epidermal growth factor-stimulated mitogen-activated protein kinase pathway. J Biol Chem. 1997;272:28042–28049. doi: 10.1074/jbc.272.44.28042. [DOI] [PubMed] [Google Scholar]
- 51.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pelech S L, Sanhera J S. Mitogen-activated protein kinases: versatile transducers for cell signaling. Trends Biochem Sci. 1992;17:233–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- 53.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Grignani F, Pawson T, Pelicci P G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 54.Rearick J I, Albro P W, Jetten A M. Increase in cholesterol sulfotransferase activity during in vitro squamous differentiation of rabbit tracheal epithelial cells and its inhibition by retinoic acid. J Biol Chem. 1987;262:13069–13074. [PubMed] [Google Scholar]
- 55.Rearick J I, Deas M, Jetten A M. Synthesis of mucous glycoproteins by rabbit tracheal cells in vitro. Modulation by substratum, retinoids and cyclic AMP. Biochem J. 1987;242:19–25. doi: 10.1042/bj2420019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rochette-Egly C, Adam S, Rossignol M, Egly J-M, Chambon P. Stimulation of RARα activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell. 1997;90:97–107. doi: 10.1016/s0092-8674(00)80317-7. [DOI] [PubMed] [Google Scholar]
- 57.Rosales C, O’Brien V, Kornberg L, Juliano R. Signal transduction by cell adhesion receptors. Biochim Biophys Acta. 1995;1242:77–98. doi: 10.1016/0304-419x(95)00005-z. [DOI] [PubMed] [Google Scholar]
- 58.Salomon D S, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 59.Schlaepfer D D, Hanks S K, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature (London) 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 60.Schneller M, Vuori K, Ruoslahti E. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sever C E, Locker J. Expression of retinoic acid α and β receptor genes in liver and hepatocellular carcinoma. Mol Carcinog. 1991;4:138–144. doi: 10.1002/mc.2940040209. [DOI] [PubMed] [Google Scholar]
- 63.Shifrin V I, Neel B G. Growth factor-inducible alternative splicing of nontransmembrane phosphotyrosine phosphatase PTP-1B pre-mRNA. J Biol Chem. 1993;268:25376–25384. [PubMed] [Google Scholar]
- 64.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan T E, Davis S, Goldfarb M P, Glass D J, Lemke G, Yancopoulos G D. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 65.Swisshelm K, Ryan K, Lee X, Tsou H C, Peacocke M, Sager R. Down-regulation of retinoic acid receptor β in mammary carcinoma cell lines and its up-regulation in senescing normal mammary epithelial cells. Cell Growth Differ. 1994;5:133–141. [PubMed] [Google Scholar]
- 66.Tang T L. Signal transduction by the SH2-containing protein tyrosine phosphatase SH-PTP2. Thesis. Cambridge, Mass: Harvard University; 1994. [Google Scholar]
- 67.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsao M S, Zhu H, Giaid A, Viallet J, Nakamura T, Park M. Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial epithelial and lung carcinoma. Cell Growth Differ. 1993;4:571–579. [PubMed] [Google Scholar]
- 69.Tsao M S, Zhu H, Viallet J. Autocrine growth loop of the epidermal growth factor receptor in normal and immortalized human bronchial epithelial cells. Exp Cell Res. 1996;223:268–273. doi: 10.1006/excr.1996.0081. [DOI] [PubMed] [Google Scholar]
- 70.Vivanco Ruiz M M, Bugge T H, Hirschmann P, Stunnenberg H G. Functional characterization of a natural retinoic acid response element. EMBO J. 1991;10:3829–3838. doi: 10.1002/j.1460-2075.1991.tb04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vogel W, Gish G D, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 72.Wong Y C, Buck R C. An electronic microscopic study of metaplasia of the rat tracheal epithelium in vitamin A deficiency. Lab Investig. 1971;24:55–66. [PubMed] [Google Scholar]
- 73.Wu R, Martin W R, Robinson C B, St. George J A, Plopper C G, Kurland G, Last J A, Cross C E, McDonald R J, Boucher R. Expression of mucin synthesis and secretion in human tracheobronchial epithelial cells grown in culture. Am J Respir Cell Mol Biol. 1990;3:467–478. doi: 10.1165/ajrcmb/3.5.467. [DOI] [PubMed] [Google Scholar]
- 74.Wu R, Zhao Y H, Chang M M J. Growth and differentiation of conducting airway epithelial cells in culture. Eur Respir J. 1997;10:2398–2403. doi: 10.1183/09031936.97.10102398. [DOI] [PubMed] [Google Scholar]
- 75.Yi E S, Lee H, Yin S, Piguet P, Sarosi I, Kaufmann S, Tarpley J, Wang N S, Ulich T R. Platelet-derived growth factor causes pulmonary cell proliferation and collagen deposition in vivo. Am J Pathol. 1996;149:539–548. [PMC free article] [PubMed] [Google Scholar]