Abstract
Introduction:
Dibutyl phthalate (DBP), a phthalate congener, is widely utilized in consumer products and medication coatings. Women of reproductive age have a significant burden of DBP exposure through consumer products, occupational exposure, and medication. Prenatal DBP exposure is associated with adverse pregnancy/fetal outcomes and cardiovascular diseases in the offspring. However, the role of fetal sex and the general mechanisms underlying DBP exposure-associated adverse pregnancy outcomes are unclear. We hypothesize that prenatal DBP exposure at an environmentally relevant low dosage adversely affects fetal-placental development and function during pregnancy in a fetal sex-specific manner.
Methods:
Adult female CD-1 mice (8-10wks) were orally treated with vehicle (control) or with environmentally relevant low DBP dosages at 0.1 μg/kg/day (refer as DBP0.1) daily from 30 days before pregnancy through gestational day (GD) 18.5. Dam body mass composition was measured non-invasively using the echo-magnetic resonance imaging system. Lipid disposition in fetal labyrinth and maternal decidual area of placentas was examined using Oil Red O staining.
Results:
DBP0.1 exposure did not significantly affect the body weight and adiposity of non-pregnant adult female mice nor the maternal weight gain pattern and adiposity during pregnancy in adult female mice. DBP0.1 exposure does not affect fetal weight but significantly increased the placental weight at GD18.5 (indicative of decreased placental efficiency) in a fetal sex-specific manner. We further observed that DBP0.1 significantly decreased lipid disposition in fetal labyrinth of female, but not male placentas, while it did not affect lipid disposition in maternal decidual.
Conclusions:
Prenatal exposure to environmentally relevant low-dosage DBP adversely impacts the fetal-placental efficiency and lipid disposition in a fetal sex-specific manner.
Keywords: Dibutyl phthalate, Prenatal Exposure, Fetal Sex, Placenta, Lipid Disposition
Introduction
Phthalates are a group of environmental endocrine disruptors that widely exist in the environment and adversely impact cardiovascular and metabolic function in humans1–5. Although general usage of phthalates has gradually reduced in the past 5–10 years, dibutyl phthalate (DBP), a phthalate congener, is still widely utilized in consumer products and medication coatings3, 6–9. Despite decreasing trends in legacy phthalate use in response to enhanced awareness and regulation, a strong need for phthalate research remains as reductions in phthalate burden differ by age, socio-economic, and geographical groups for specific phthalate metabolites10–13. Women of reproductive age have a significant burden of DBP exposure3, 6, 7 through consumer products, occupational exposure, and medication (0.1-233µg/kg/day)8, 9. Higher urinary DBP metabolite levels (indicative of higher exposure) were observed in Hispanic and non-Hispanic black women than in other ethnic groups14, strongly suggesting disparities among women of different backgrounds.
Multiple biomonitoring studies reported DBP exposure in most mothers and babies during pregnancy15–17. Prenatal DBP exposure is associated with adverse pregnancy/fetal outcomes (e.g., fetal growth restriction)15–17, childhood obesity17, 18, and cardiovascular diseases in the offspring5, 19–22. Studies in rats have shown that prenatal DBP exposure at 500mg/kg/day leads to reduced placental weight23, 24. In humans, maternal DBP exposure in 1st trimester is reported to be associated with increased risks of fetal intrauterine growth restriction (which is associated with placental dysfunction) in pregnancies with male but not with female fetuses25. However, mechanisms underlying these adverse effects of prenatal DBP exposure are unclear.
Fetal sex has significant impacts on the effects of prenatal stress exposure-associated adverse perinatal and pregnancy outcomes26–29. Pregnancies with male fetuses are reported to be at a higher risk of preterm birth, while female fetuses are at a higher risk of fetal growth restriction27. Although prenatal exposure to other phthalate congeners (DMP and DEHP) is reported to be associated with fetal sex-specific fetal growth restriction25 in humans, the role of fetal sex in DBP exposure-associated adverse pregnancy/fetal outcomes is still unclear.
To date, most DBP studies in the literature evaluated dosages that are much higher than human environmental exposure5, 30. Furthermore, the majority of the limited existing literature investigating environmentally relevant dosages of DBP exposure focused on the gonad toxicity in non-pregnant mice. Although oral ingestion of environmentally relevant DBP exposure results in detection of its active metabolite in the ovary and disruption of various ovarian processes and pathways (e.g., folliculogenesis, steroidogenesis, xenobiotic metabolism, DNA repair and apoptosis), the effects of environmentally relevant low dosages DBP exposure on pregnancy and fetal development are still not clear.
In this study, we tested the hypothesis that environmentally relevant low dosage DBP exposure differentially dysregulates male and female fetal placental function during pregnancy using an established DBP exposure mouse model8, 31. We examined the effect of environmentally relevant DBP exposure on the weight gain and body composition of adult female mice pre-pregnancy and during pregnancy. We further determined the effect of prenatal environmentally relevant low dosage DBP exposure on fetal/placental development as well as placental lipid disposition at gestational day (GD) 18.5.
Methods
Ethical Approval
All animal experiments followed guidance provided in the Guide for the Care and Use of Laboratory Animals by National Research Council32 and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines33 with the approval of the University of Arizona Institutional Animal Care and Use Committee (Protocol# 20–670).
Animals
Adult (8-10wks) female CD-1 mice were obtained from Charles River Laboratories (Charles River, California) and housed in single-use, BPA-free cages at the University of Arizona Animal Facility. Water and food were provided ad libitum, their light cycle maintained at 14 L:10 D, and temperature at 22 ± 1°C. After an acclimation period of at least 24 h, mice were randomly assigned to receive oral tocopherol-stripped corn oil (MP Biomedicals, OH; vehicle; control group) or an environmentally relevant3, 6–9 DBP (Sigma-Aldrich, MO) dosage (100, 10, and 0.1 μg/kg/day; refer as DBP100, DBP10, and DBP0.1).
Oral DBP Dosing in Non-pregnant and Pregnant Mice
Determining Environmentally Relevant DBP Dosages That Does Not Affect Weight Gain in Non-Pregnant Adult Female Mice
To determine an environmentally relevant DBP dosages that does not affect the weight gain in non-pregnant female CD-1 mice, adult female CD-1 mice (8-10wks) were orally treated with vehicle (control group; n=14) or with environmentally relevant DBP dosages (DBP100, DBP10, or DBP0.1; n=8 per group) daily for 30 days. Mice were subjected to daily weighing, vaginal smear collections, and oral dosing with vehicle or DBP treatments. Weight of each mouse before the first day of treatment was used as baseline weight to calculate the percentage of weight gained during treatment.
Environmentally Relevant Low-Dosage DBP Dosing in Adult Female Mice Pre-Pregnant through Pregnancy
Based on our dose finding experiment in non-pregnant mice, DBP0.1 did not affect the weight gain in adult female mice. Hence, DBP0.1 was used in subsequent experiments to determine physiological changes that are independent of weight gain. Adult female CD-1 mice (8-10wks) were orally treated with vehicle (control [CT] group; n=18) or DBP0.1 (n=18) daily for 30 days before mating with male CD-1 mice without DBP exposure and throughout pregnancy until gestational day (GD) 18.5. The weight of each mouse was measured daily. Weight of each mouse before the first day of treatment was used as baseline weight to calculate the percentage of weight-gain during treatment. At GD18.5, mice were euthanized under pre-sedation with inhaled isoflurane followed by decapitation. Major maternal organs (heart, lung, liver, kidney, ovary) were collected and weighted at GD18.5. In addition, all fetus and placentas of each dam were collected and weighted at GD18.5.
Body Mass Composition Measurement
Control and DBP0.1-treated dams were subjected to body mass composition (fat and lean mass) measurement non-invasively while conscious using an echo-magnetic resonance imaging system (EchoMRI-900 body composition analyzer with A10 insert for mice)34, 35 at day 0, 15, 30 of pre-pregnancy treatment as well as GD5.5, GD11.5, and GD18.5 during pregnancy.
Histology and Ovarian Corpora Lutea (CL) Counts
After the end of the experiment, ovaries from each dam were dissected followed by fat and oviductal tissue removal. Both ovaries were fixed for subsequent histological processing. Fixation was done in neutral buffered formalin overnight at 4°C with shaking. After fixing, ovaries were washed in 70% ethanol, processed, and embedded in paraffin. Embedded ovaries were serially sectioned (5μm thickness) throughout the tissue. Two sequential sections were kept and mounted every 100µm to generate various histological evaluation levels throughout the ovary. The resulting sections were processed for hematoxylin (Richard-Allan, 7211) and eosin (Richard-Allan, 7111) staining using a standard procedure. Stained ovary slides were imaged prior to analysis as described8. Briefly, each ovarian section was imaged at 10x magnification using a Leica DMIL LED microscope. Ovarian sections too large to fit in one view, were imaged and captured in tiles, and then merged using the stitching tool in Adobe Photoshop. Only fully developed, functional CLs were counted and compared between treatments.
Oil Red O Staining of Mouse Placenta
Lipid disposition in placenta is closely linked to placental function and efficiency36, 37. The neutral lipids in mice placenta tissues were stained using an Oil Red O Staining Kit (IHC World Cat# IW-3008) following the manufacturer’s instruction. In brief, mice placenta tissues were embedded in optimal cutting temperature (OCT) compound and stored in −80°C until sectioning. Frozen mice placenta sections (10μm thick, 2 per individual placenta) were fixed in 10% formalin for 5 mins. After fixing, the placental sections were incubated in Pre-Stain Solution for 5 mins at room temperature followed with 10 min Oil Red O solution incubation at 60°C. After rinsing, nuclei of sections were counterstained with Mayer’s Hematoxylin for 1 min. After rinsing, sections were mounted using Aqueous Mounting Medium, and imaged at 40x magnification using a brightfield microscope (Leica DM5500B). Lipid droplets area in mice placental tissue sections were quantified using National Institutes of Health (NIH) ImageJ software (http://rsbweb.nih.gov/ij/).
Statistical Analyses
SigmaPlot software (Systat Software., San Jose, CA) was used for statistical analyses. Data are represented as the medians ± standard deviation (SD). Data analyses were performed using the Student’s t-test, Mann-Whitney Rank Sum test, One-way ANOVA, Kruskal-Wallis test, and two-way ANOVA as appropriate. Differences were considered significant when P <0.05. Benjamini and Hochberg False Discovery Rate (FDR)-adjustment38, 39 was used for multiple comparison correction as appropriate.
Results
Effect of DBP exposure at environmentally relevant dosages on the weight gain of non-pregnant female mice
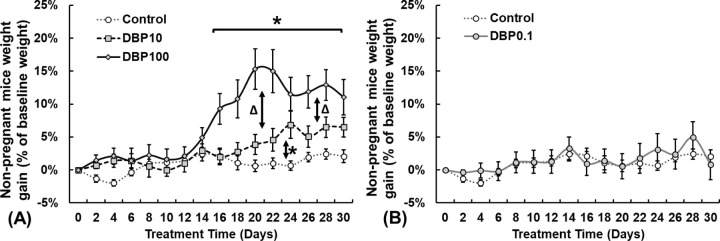
To determine the DBP exposure dosage that does not affect non-pregnant adult female mice, animals were orally treated with vehicle (CT group), DBP100, DBP10, or DBP0.1 daily for 30 days. Compared with control group, 30-day exposure to DBP100 and DBP10 does-dependently increased the weight of non-pregnant female CD-1 mice (Fig.1A). Specifically, female mice with DBP100 exposure significantly gained weight starting after 18 days of treatment (10.8%) and peaked at 20 days of treatment (15.3%). Mice with DBP10 exposure exhibit a trend of weight gain after 20 days of treatment and reach significant weight gain of 5.6%-6.9% by 23 to 25 days after treatment. Similar to the control (CT) group, 30 days of DBP0.1 exposure does not cause significant weight gain of female mice (Fig.1B).
Figure.1.
Adult female CD-1 mice weight gain compared to control over 30 days of DBP exposure at (A) 100, 10, and (B) 0.1 μg/kg/day. Data expressed as median ± standard error of mean. DBP100: 100μg/kg/day (n=8); DBP10: 10μg/kg/day (n=8); DBP0.1: 0.1μg/kg/day (n=8); Control: Vehicle only (n=14). *: Differ from Control (P<0.05). Δ: Differ between DBP100 and DBP10 (P<0.05).
Effect of DBP0.1 exposure on female fertility efficiency in CD-1 mice
DBP0.1 exposure decreased the pregnancy rate in the female mice from 94.44% to 83.33% (Fig.2A), but it did not affect number of live pups per litter (litter size Fig.2B), male to female fetus ratio (Fig.2C), and ovarian CLs number per dam (Fig.2D) compared to CT group. Interestingly, DBP0.1 exposed mice exhibited significantly lower pup to CL ratio at GD18.5 (defined as number of pups divided by number of CLs, Fig.2E).
Figure.2.
Effect of DBP0.1 exposure on female fertility parameters in control (CT) and DBP0.1 treated CD-1 mice. (A) Pregnancy rate (n=18 for each group), (B) litter size (CT: n=12; DBP0.1: n=14), (C) male/female fetus ratio per litter (CT: n=12; DBP0.1: n=14), and (D) pups to CLs ratio (CT: n=9; DBP0.1: n=13) in CT and DBP0.1 treated CD-1 mice. *: Differ between CT and DBP0.1 groups (P<0.05). Each data point represents the data from an individual dam.
Effect of DBP0.1 exposure on weight gain patterns of pregnant mice
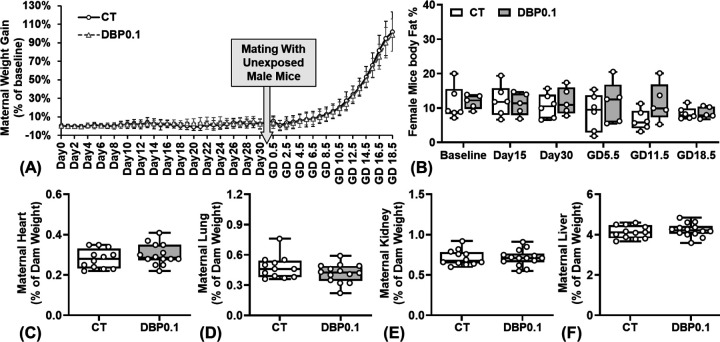
Our daily weight measurement revealed that DBP0.1 exposure does not affect the pre-pregnancy and pregnancy weight gain patterns compared to control group (Fig.3A). Our body composition data (EchoMRI™ LLC, TX; Fig.3B) further showed that DBP0.1 exposure does not significantly alter the body fat percentage of dams (from 30 days pre-pregnancy through GD18.5). Further, DBP0.1 exposure did not affect the weight of the maternal heart, lung, kidney, and liver at GD18.5 compared to control group (Fig.3C–F).
Figure.3.
Effect of DBP0.1 exposure on weight gain patterns of pregnant dams. (A) Daily weight gain (CT: n=15; DBP0.1: n=14), (B) adiposity (body fat %; n=6 for each group), and the weight of maternal (C) heart, (D) lung, (E) kidney, (F) liver at GD18.5 in CD-1 mice (C-F: CT group: n=12; DBP0.1 group: n=14). (A) Data expressed as median ± standard deviation. (B-F) Each data point represents the data from an individual dam.
Effect of Prenatal DBP0.1 exposure on placental efficiency in female and male fetuses
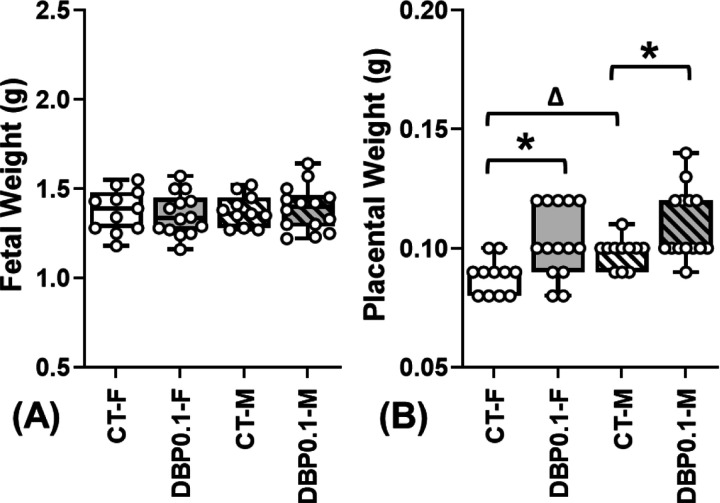
Compared to the CT group, DBP0.1 exposure did not affect fetal weight (Fig.4A) but significantly increased the placental weight (Fig.4B) of both male and female fetuses at GD18.5 (indicative of decreased placental efficiency). There is no difference between the weight of male and female fetuses from both CT and DBP0.1 dams. However, male placental weights are significantly higher than female placentas in CT group. The DBP0.1 exposure-increased placenta weight is more dramatic in female than in male placentas and eliminated the fetal sex-specific placental weight differences observed in CT group.
Figure.4.
Effect of DBP0.1 exposure on fetal and placental weight at GD18.5. DBP0.1 exposure did not alter fetal weight (A), while it significantly increased placental weight (B) in female (F) and male (M) fetuses in CD-1 mice by GD18.5 (CT-F: n=12; CT-M: n=12; DBP0.1-F: n=14; DBP0.1-M: n=14). *:Differ between CT and DBP0.1 group within the same fetal sex; Δ:Differ between F and M fetuses within the same treatment group (P <0.05). Median value of all female or male fetuses from the same dam was shown as a single data point in figures.
Effect of prenatal DBP0.1 exposure on female and male placental lipid disposition pattern
In placentas from control dams, lipid disposition (calculated as the neutral lipid area relative to tissue area) in the fetal labyrinth area of the placenta is significantly higher in female than in male placentas (Fig.5A). The fetal sex-specific fetal labyrinth lipid disposition pattern observed in control placentas was diminished in placentas from the DBP0.1 group. DBP0.1 exposure significantly reduced the lipid disposition in the fetal labyrinth area of female placentas but did not significantly affect the lipid disposition in male placentas. DBP0.1 exposure does not affect the lipid disposition in the maternal decidual area in both female and male placentas (Fig.5B).
Figure.5.
Lipid disposition in (A) fetal labyrinth and (B) maternal decidual of mice placental tissues from CT and DBP0.1 groups. *: Differ between CT and DBP0.1 within the same fetal sex; Δ: Differ between female and male fetuses within the same treatment group (P <0.05 Two-way ANOVA). The median value of all images from the same placenta was shown as a single data point (CT-F: n=15; DBP0.1-F: n=10; CT-M: n=8); DBP0.1-M: n=11).
Discussion
In this study, we have demonstrated that although daily dosing with a low, environmentally relevant exposure to DBP of 0.1 µg/kg/day does not affect the weight (both pre-pregnant and during pregnancies) of adult female CD-1 mice, it significantly increases the placental weight and reduces the placental efficiency at GD18.5. Further, we have shown for the first time that prenatal DBP0.1 (0.1μg/kg/day) exposure caused fetal sex-specific reductions in the fetal labyrinth lipid disposition in the placenta while not affecting the lipid disposition in the maternal decidual area at GD18.5 in CD-1 mice. These data clearly indicate that environmentally relevant low-dosage DBP exposure at DBP0.1 causes fetal sex-specific placental dysfunction.
Consistent with previous reports8, we observed that 30 days of environmentally relevant dosages of DBP exposure (DBP100 and DBP10) dose-dependently increased the weight of non-pregnant adult female mice. However, our data showed that 30 days of environmentally relevant DBP exposure at a much lower environmentally relevant dosage at DBP0.1 causes no significant weight gain nor body fat percentage changes in non-pregnant adult female mice compared to control. DBP is known to be associated with obesity40–42, metabolic disorders43, and cardiovascular diseases25, 44 in humans. Maternal obesity is a prevalent risk factor for pregnancy complications (e.g., preterm birth, fetal growth restriction)45–49. In studies utilizing higher DBP exposure dosages that cause significant maternal weight gains, it would be very difficult to separate the adverse pregnancy outcomes caused by DBP exposure and by the maternal weight gain caused by DBP exposure. In this study, we specifically chosen an environmentally relevant low DBP dosage (DBP0.1) that does not significantly affect the weight gain in female mice, which allows us to evaluate the effect of maternal exposure to environmentally relevant low-dosage DBP during pregnancy without being affected by the maternal weight gain that would have occurred with higher DBP dosages.
Although the reproductive toxicity of DBP on male fertility is well established50, 51, the effect of DBP exposure on female fertility is not fully understood. Existing literature on the effects of DBP exposure on pregnancy outcomes using animal models often utilizes DBP dosages that are much higher than the human environment5, 30, 52. Nonetheless, these studies have identified various endpoints of interest including increased offspring adiposity and body weight52, and lack of glucose homeostasis53. Despite this, the effect of environmentally relevant low-dosage DBP exposure during pregnancy on fetal development and pregnancy outcomes remains elusive.
Our data have shown that although 30 days of DBP0.1 exposure did not affect the body weight or body fat composition of non-pregnant adult female mice, it significantly reduced the reproductive efficiency of the female mice. When mating with untreated CD-1 male mice, the DBP0.1 exposure reduced the pregnancy rate in female CD-1 mice from 94.44% to 83.33%. In addition, although DBP0.1 exposure did not significantly affect the litter size, fetal sex ratio, and ovarian CL count per dam, it significantly reduced the pup-to-CL ratio which is indicative of a disconnect between ovulation and live pups. Although DBP has been shown to disrupt ovarian folliculogenesis in mice8, 54, 55, this disconnect between ovulation and pups is likely not due to a defect in ovarian release of eggs but rather due to embryo loss during pregnancy. This possibility is supported by previous reports describing that DBP is an endocrine disruptor that adversely affects pregnancy outcomes30, 42. Specifically, human epidemiological studies report associations between phthalate exposures and increased risk of recurrent pregnancy loss56; thus, the disconnect between CL number and pups could be due to embryo loss but further studies will be needed to address this possibility directly. Observations in this current study extended the DBP exposure-associated adverse effects on female fertility to an environmentally relevant DBP exposure dosage to as low as 0.1μg/kg/day (DBP0.1).
Our data have shown that DBP0.1 exposure allows for examination of the effect the environmentally relevant DBP exposure during pregnancy independent of the effect of maternal weight gain that would usually cause by higher dosages of DBP. Specifically, adult female mice with 30-days pre-pregnancy DBP0.1 exposure and continued daily DBP0.1 exposure throughout pregnancy did not have significant changes in weight gain pattern and the body fat composition when compared with control animals. Further, this DBP0.1 treatment did not change the weight of major maternal organs at GD18.5. We further observed that the prenatal DBP0.1 exposure did not affect fetal weight but significantly increased placental weight and reduced fetal/placental weight ratio at GD18.5, indicative of reduced placental efficiency in pups from DBP0.1 exposed dams. Further, our data showed that DBP0.1 causes more dramatic placental weight increase in female than male placentas and eliminated the sex dimorphisms of placental weight seen in control animals, which indicates more dramatic reduction in placental efficiency in female fetuses. All these data show that although DBP exposure at an environmentally relevant low dosage (DBP0.1) seems not to affect maternal weight and body composition during pregnancy, it still fetal sex-specifically affects placental development and placental efficiency.
Lipids play a crucial role in placental function57, 58. Dysregulation in placental lipid dynamics, as observed in our study, indicates disrupted nutrient transportation and overall placental function58–61. We observed a notable sexual dimorphism in fetal labyrinth lipid disposition in placentas from control mice, with female placentas exhibiting significantly higher fetal labyrinth lipid accumulation compared to their male counterparts. Strikingly, DBP0.1 exposure led to a significant reduction in fetal labyrinth lipid disposition in female placentas but does not affect the fetal labyrinth lipid disposition in male placentas. Our observation that DBP0.1 exposure abolishes the fetal sex-specific difference in fetal labyrinth lipid disposition, highlighting the disruptive effects of DBP on normal placental development and function. This is further supported by the fetal sex-specific dysregulation of placental weight in DBP0.1 exposed animals that observed in the current study.
Interestingly, although DBP0.1 exposure leads to fetal sex-specific dysregulation of fetal labyrinth lipid disposition, it does not affect lipid disposition in maternal decidual areas of placental tissues both female and male. This data indicates that the DBP0.1 exposure mainly dysregulates lipid disposition in the fetal labyrinth area of the placentas in a fetal sex-specific manner. This observation aligns with the established role of the labyrinth as the primary nutrient exchange structure in the placenta62, 63, where dynamic lipid trafficking and metabolism is essential to sustain fetal growth and development. This data also agrees with previous reports showing that DBP and its metabolite can cross the placenta and play important roles in lipid metabolism and transportation64–67.
In conclusion, our data have demonstrated that environmentally relevant low dosage of DBP exposure at 0.1μg/kg/day reduces the reproductive efficiency in female mice and fetal sex-specifically dysregulates the placental efficiency and lipid disposition. Findings in this study highlight the significance of including fetal sex as a biological variable when examining the impact of environmental endocrine disruptor exposures on placental function and fetal development.
Perspectives
To date, mechanisms underlying adverse effects of prenatal DBP exposure are unclear. Further, most studies in the literature evaluated DBP dosages that are much higher than the human environmental exposure. Here we reported that DBP exposure as low as 0.1μg/kg/day during pregnancy, though does not affect maternal weight gain, still causes fetal sex-specific dysregulation of placental development and fetal labyrinth lipid disposition. These observations highlight the significance of further studying the effect of environmental endocrine disruptor exposure during pregnancy on fetal development at a human environment relevant dosage as well as the necessity to include fetal sex as a biological variable in future investigations.
Acknowledgments
We would like to thank Dr. Xiaosong Liu for technical assistance.
Sources of Funding
This study is supported by the American Heart Association (AHA) Transformative Project Award 23TPA1066252 (Zhou), and Pilot grant (Zhou, Craig) from the Southwest Environmental Health Sciences Center (National Institutes of Health [NIH] P30 ES006694). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This study is also supported by the Research, Innovation & Impact (RII) and the Technology Research Initiative Fund/Improving Health initiative at the University of Arizona (Zhou).
Funding Statement
This study is supported by the American Heart Association (AHA) Transformative Project Award 23TPA1066252 (Zhou), and Pilot grant (Zhou, Craig) from the Southwest Environmental Health Sciences Center (National Institutes of Health [NIH] P30 ES006694). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This study is also supported by the Research, Innovation & Impact (RII) and the Technology Research Initiative Fund/Improving Health initiative at the University of Arizona (Zhou).
Footnotes
Conflict of Interest/Disclosure Statement
The authors have no conflict of interest.
References
- 1.Jaimes R 3rd, Swiercz A, Sherman M, Muselimyan N, Marvar PJ and Posnack NG. Plastics and cardiovascular health: phthalates may disrupt heart rate variability and cardiovascular reactivity. Am J Physiol Heart Circ Physiol. 2017;313:H1044–h1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaimes R 3rd, McCullough D, Siegel B, Swift L, McInerney D, Hiebert J, Perez-Alday EA, Trenor B, Sheng J, Saiz J, Tereshchenko LG and Posnack NG. Plasticizer Interaction With the Heart: Chemicals Used in Plastic Medical Devices Can Interfere With Cardiac Electrophysiology. Circ Arrhythm Electrophysiol. 2019;12:e007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saravanabhavan G, Guay M, Langlois É, Giroux S, Murray J and Haines D. Biomonitoring of phthalate metabolites in the Canadian population through the Canadian Health Measures Survey (2007–2009). International journal of hygiene and environmental health. 2013;216:652–661. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Zhu H and Kannan K. A Review of Biomonitoring of Phthalate Exposures. Toxics. 2019;7:21. doi: 10.3390/toxics7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariana M and Cairrao E. Phthalates Implications in the Cardiovascular System. Journal of cardiovascular development and disease. 2020;7:26. doi: 10.3390/jcdd7030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitro SD, Chu MT, Dodson RE, Adamkiewicz G, Chie L, Brown FM and James-Todd TM. Phthalate metabolite exposures among immigrants living in the United States: findings from NHANES, 1999–2014. Journal of exposure science & environmental epidemiology. 2019;29:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL and Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental health perspectives. 2004;112:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X and Craig ZR. Environmentally relevant exposure to dibutyl phthalate disrupts DNA damage repair gene expression in the mouse ovary†. Biology of reproduction. 2019;101:854–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernández-Díaz S, Su YC, Mitchell AA, Kelley KE, Calafat AM and Hauser R. Medications as a potential source of exposure to phthalates among women of childbearing age. Reproductive toxicology (Elmsford, NY). 2013;37:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zota AR, Calafat AM and Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect. 2014;122:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin HM, Dhar U, Calafat AM, Nguyen V, Schmidt RJ and Hertz-Picciotto I. Temporal Trends of Exposure to Phthalates and Phthalate Alternatives in California Pregnant Women during 2007–2013: Comparison with Other Populations. Environ Sci Technol. 2020;54:13157–13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, Kupsco AJ, Deierlein AL, Just AC, Calafat AM, Oken E, Braun JM, Mercado-Garcia A, Cantoral A, Téllez-Rojo MM, Wright RO and Baccarelli AA. Trends and Patterns of Phthalates and Phthalate Alternatives Exposure in Pregnant Women from Mexico City during 2007–2010. Environ Sci Technol. 2020;54:1740–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domínguez-Romero E, Komprdová K, Kalina J, Bessems J, Karakitsios S, Sarigiannis DA and Scheringer M. Time-trends in human urinary concentrations of phthalates and substitutes DEHT and DINCH in Asian and North American countries (2009–2019). J Expo Sci Environ Epidemiol. 2023;33:244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James-Todd TM, Meeker JD, Huang T, Hauser R, Seely EW, Ferguson KK, Rich-Edwards JW and McElrath TF. Racial and ethnic variations in phthalate metabolite concentration changes across full-term pregnancies. Journal of exposure science & environmental epidemiology. 2017;27:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Li J, Garcia JM, Lin H, Wang Y, Yan P, Wang L, Tan Y, Luo J, Qiu Z, Chen JA and Shu W. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PloS one. 2014;9:e87430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Lin L, Cao Y, Chen B, Zheng L and Ge RS. Phthalate levels and low birth weight: a nested case-control study of Chinese newborns. The Journal of pediatrics. 2009;155:500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK, Perera FP, Andrews H, Just AC, Hoepner L, Tang D and Hauser R. Prenatal di(2-ethylhexyl)phthalate exposure and length of gestation among an inner-city cohort. Pediatrics. 2009;124:1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F and Mazzeo P. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environmental health perspectives. 2003;111:1783–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariana M, Feiteiro J, Verde I and Cairrao E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environment international. 2016;94:758–776. [DOI] [PubMed] [Google Scholar]
- 20.Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, Sunyer J and Vrijheid M. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environmental health perspectives. 2015;123:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amin MM, Ebrahimpour K, Parastar S, Shoshtari-Yeganeh B, Hashemi M, Mansourian M, Poursafa P, Fallah Z, Rafiei N and Kelishadi R. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere. 2018;211:547–556. [DOI] [PubMed] [Google Scholar]
- 22.Vafeiadi M, Myridakis A, Roumeliotaki T, Margetaki K, Chalkiadaki G, Dermitzaki E, Venihaki M, Sarri K, Vassilaki M, Leventakou V, Stephanou EG, Kogevinas M and Chatzi L. Association of Early Life Exposure to Phthalates With Obesity and Cardiometabolic Traits in Childhood: Sex Specific Associations. Frontiers in public health. 2018;6:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahaboob Basha P and Radha MJ. Gestational di-n-butyl phthalate exposure induced developmental and teratogenic anomalies in rats: a multigenerational assessment. Environ Sci Pollut Res Int. 2017;24:4537–4551. [DOI] [PubMed] [Google Scholar]
- 24.Strakovsky RS and Schantz SL. Using Experimental Models to Assess Effects of Bisphenol A (BPA) and Phthalates on the Placenta: Challenges and Perspectives. Toxicological sciences : an official journal of the Society of Toxicology. 2018;166:250–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Chen L, Li LX, Xie CM, Li D, Shi HJ and Zhang YH. Gender-specific relationship between prenatal exposure to phthalates and intrauterine growth restriction. Pediatric research. 2014;76:401–408. [DOI] [PubMed] [Google Scholar]
- 26.Wainstock T, Shoham-Vardi I, Glasser S, Anteby E and Lerner-Geva L. Fetal sex modifies effects of prenatal stress exposure and adverse birth outcomes. Stress. 2015;18:49–56. [DOI] [PubMed] [Google Scholar]
- 27.Melamed N, Yogev Y and Glezerman M. Fetal gender and pregnancy outcome. J Matern Fetal Neonatal Med. 2010;23:338–44. [DOI] [PubMed] [Google Scholar]
- 28.Al-Qaraghouli M and Fang YMV. Effect of Fetal Sex on Maternal and Obstetric Outcomes. Front Pediatr. 2017;5:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Armson BA, Attenborough R, Carson GD, da Silva O, Heaman M, Janssen P, Murphy PA, Pasquier JC, Sauve R, Von Dadelszen P, Walker M and Lee SK. Survey of Mode of Delivery and Maternal and Perinatal Outcomes in Canada. J Obstet Gynaecol Can. 2022;44:960–971. [DOI] [PubMed] [Google Scholar]
- 30.Heidari T, Batavani RA, Malekinejad H and Hobbenaghi R. Evaluation of di-n-butyl phthalate reproductive toxicity in pregnant rats and their offspring and assessment of vitamin E administration in reducing toxicity. Vet Res Forum. 2022;13:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craig ZR, Hannon PR, Wang W, Ziv-Gal A and Flaws JA. Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol Reprod. 2013;88:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Research Council Committee for the Update of the Guide for the C and Use of Laboratory A. The National Academies Collection: Reports funded by National Institutes of Health Guide for the Care and Use of Laboratory Animals Washington (DC): National Academies Press (US) Copyright © 2011, National Academy of Sciences.; 2011. [Google Scholar]
- 33.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T and Würbel H. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dranse HJ, Waise TMZ, Hamr SC, Bauer PV, Abraham MA, Rasmussen BA and Lam TKT. Physiological and therapeutic regulation of glucose homeostasis by upper small intestinal PepT1-mediated protein sensing. Nat Commun. 2018;9:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisler CE, Ghimire S, Bruggink SM, Miller KE, Weninger SN, Kronenfeld JM, Yoshino J, Klein S, Duca FA and Renquist BJ. A critical role of hepatic GABA in the metabolic dysfunction and hyperphagia of obesity. Cell Rep. 2021;35:109301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JG, Kim G, Park SG, Yon JM, Yeom J, Song HE, Cheong SA, Lim JS, Sung YH, Kim K, Yoo HJ, Hong EJ, Nam KH, Seong JK, Kim CJ, Nam SY and Baek IJ. Lipid signatures reflect the function of the murine primary placentation†. Biol Reprod. 2022;106:583–596. [DOI] [PubMed] [Google Scholar]
- 37.Brown SH, Eather SR, Freeman DJ, Meyer BJ and Mitchell TW. A Lipidomic Analysis of Placenta in Preeclampsia: Evidence for Lipid Storage. PLoS One. 2016;11:e0163972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou C, Zou QY, Li H, Wang RF, Liu AX, Magness RR and Zheng J. Preeclampsia Downregulates MicroRNAs in Fetal Endothelial Cells: Roles of miR-29a/c-3p in Endothelial Function. The Journal of clinical endocrinology and metabolism. 2017;102:3470–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou C, Yan Q, Zou QY, Zhong XQ, Tyler CT, Magness RR, Bird IM and Zheng J. Sexual Dimorphisms of Preeclampsia-Dysregulated Transcriptomic Profiles and Cell Function in Fetal Endothelial Cells. Hypertension (Dallas, Tex: 1979). 2019;74:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia B, Zhu Q, Zhao Y, Ge W, Zhao Y, Song Q, Zhou Y, Shi H and Zhang Y. Phthalate exposure and childhood overweight and obesity: Urinary metabolomic evidence. Environment international. 2018;121:159–168. [DOI] [PubMed] [Google Scholar]
- 41.Harley KG, Berger K, Rauch S, Kogut K, Claus Henn B, Calafat AM, Huen K, Eskenazi B and Holland N. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatric research. 2017;82:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian Y, Shao H, Ying X, Huang W and Hua Y. The Endocrine Disruption of Prenatal Phthalate Exposure in Mother and Offspring. Frontiers in public health. 2020;8:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radke EG, Galizia A, Thayer KA and Cooper GS. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environ Int. 2019;132:104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM and Urbina EM. Urinary phthalates are associated with higher blood pressure in childhood. The Journal of pediatrics. 2013;163:747–53.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutrition reviews. 2013;71 Suppl 1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bodnar LM, Ness RB, Markovic N and Roberts JM. The risk of preeclampsia rises with increasing prepregnancy body mass index. Annals of Epidemiology. 2005;15:475–482. [DOI] [PubMed] [Google Scholar]
- 47.Bodnar LM, Catov JM, Klebanoff MA, Ness RB and Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology (Cambridge, Mass). 2007;18:234–239. [DOI] [PubMed] [Google Scholar]
- 48.Catov JM, Ness RB, Kip KE and Olsen J. Risk of early or severe pre-eclampsia related to pre-existing conditions. International journal of epidemiology. 2007;36:412–419. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Aranguren LC, Prada CE, Riano-Medina CE and Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Frontiers in physiology. 2014;5:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray LE Jr., Ostby J, Furr J, Price M, Veeramachaneni DN and Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–65. [DOI] [PubMed] [Google Scholar]
- 51.Boberg J, Christiansen S, Axelstad M, Kledal TS, Vinggaard AM, Dalgaard M, Nellemann C and Hass U. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol. 2011;31:200–9. [DOI] [PubMed] [Google Scholar]
- 52.Li H, Li J, Qu Z, Qian H, Zhang J, Wang H, Xu X and Liu S. Intrauterine exposure to low-dose DBP in the mice induces obesity in offspring via suppression of UCP1 mediated ER stress. Sci Rep. 2020;10:16360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venturelli AC, Meyer KB, Fischer SV, Kita DH, Philipsen RA, Morais RN and Martino Andrade AJ. Effects of in utero and lactational exposure to phthalates on reproductive development and glycemic homeostasis in rats. Toxicology. 2019;421:30–40. [DOI] [PubMed] [Google Scholar]
- 54.Jauregui EJ, McSwain M, Liu X, Miller K, Burns K and Craig ZR. Human-relevant exposure to di-n-butyl phthalate tampers with the ovarian insulin-like growth factor 1 system and disrupts folliculogenesis in young adult mice. Toxicol Sci. 2023;195:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sen N, Liu X and Craig ZR. Short term exposure to di-n-butyl phthalate (DBP) disrupts ovarian function in young CD-1 mice. Reprod Toxicol. 2015;53:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao KW, Kuo PL, Huang HB, Chang JW, Chiang HC and Huang PC. Increased risk of phthalates exposure for recurrent pregnancy loss in reproductive-aged women. Environ Pollut. 2018;241:969–977. [DOI] [PubMed] [Google Scholar]
- 57.Biezenski JJ. Role of placenta in fetal lipid metabolism. I. Injection of phospholipids double labeled with C14-glycerol and P32 into pregnant rabbits. Am J Obstet Gynecol. 1969;104:1177–89. [DOI] [PubMed] [Google Scholar]
- 58.Coleman RA. Placental metabolism and transport of lipid. Fed Proc. 1986;45:2519–23. [PubMed] [Google Scholar]
- 59.Lorentzen B and Henriksen T. Plasma lipids and vascular dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:33–9. [DOI] [PubMed] [Google Scholar]
- 60.Lewis RM, Wadsack C and Desoye G. Placental fatty acid transfer. Curr Opin Clin Nutr Metab Care. 2018;21:78–82. [DOI] [PubMed] [Google Scholar]
- 61.Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM and Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond). 2010;119:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simmons DG, Natale DR, Begay V, Hughes M, Leutz A and Cross JC. Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development. 2008;135:2083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cross JC, Simmons DG and Watson ED. Chorioallantoic morphogenesis and formation of the placental villous tree. Ann N Y Acad Sci. 2003;995:84–93. [DOI] [PubMed] [Google Scholar]
- 64.Saillenfait AM, Payan JP, Fabry JP, Beydon D, Langonne I, Gallissot F and Sabate JP. Assessment of the developmental toxicity, metabolism, and placental transfer of Di-n-butyl phthalate administered to pregnant rats. Toxicol Sci. 1998;45:212–24. [DOI] [PubMed] [Google Scholar]
- 65.Clewell RA, Kremer JJ, Williams CC, Campbell JL Jr., Andersen ME and Borghoff SJ. Tissue exposures to free and glucuronidated monobutylyphthalate in the pregnant and fetal rat following exposure to di-n-butylphthalate: evaluation with a PBPK model. Toxicol Sci. 2008;103:241–59. [DOI] [PubMed] [Google Scholar]
- 66.Fennell TR, Krol WL, Sumner SC and Snyder RW. Pharmacokinetics of dibutylphthalate in pregnant rats. Toxicol Sci. 2004;82:407–18. [DOI] [PubMed] [Google Scholar]
- 67.Thompson CJ, Ross SM and Gaido KW. Di(n-butyl) phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology. 2004;145:1227–37. [DOI] [PubMed] [Google Scholar]