Abstract
Specialized cellular protrusions facilitate local intercellular communications in various species, including mammals. Among these, airinemes play a crucial role in pigment pattern formation in zebrafish by mediating long-distance Notch signaling between pigment cells. Remarkably, airinemes exhibit large vesicle-like structure at their tips, which are pulled by a macrophage subpopulation and delivered to target cells. The interaction between macrophages and Delta-ligand carrying airineme vesicles is essential for initiating airineme-mediated signaling, yet the molecular detail of this interaction remains elusive. Through high-resolution live imaging and genetic in vivo manipulations, we found that adhesive interactions via the extracellular domain of CD44, a class I transmembrane glycoprotein, between macrophages and airineme vesicles are critical for airineme signaling. Mutants lacking the extracellular domain of CD44 lose their adhesiveness, resulting in a significant reduction in airineme extension and pigment pattern defects. Our findings provide valuable insights into the role of adhesive interactions between signal-sending cells and macrophages in long-range intercellular signaling.
Introduction
Proper signal delivery between cells is essential in development and homeostasis in multicellular organisms. Even single-celled organisms communicate each other for their survival in specific environments (Combarnous and Nguyen, 2020). Consequently, understanding the mechanisms of intercellular signaling has been a central topic in Biology and Medicine for decades. Although several cell-to-cell communication modalities have been identified, recent advancements in microscopy and techniques enabled us to uncover previously unappreciated mechanisms across various species and contexts. Specialized cellular protrusions or also known as signaling filopodia are one of them. These are long, thin cellular extensions similar to neuronal axons or dendrites but are present in non-neuronal cells, spanning from sea urchin to mice in vivo (Daly et al., 2022; Hall et al., 2024; Kornberg and Roy, 2014a). This suggests that signaling filopodia may represent a general mechanism of intercellular communication in living organisms.
Distinguished by their cytoskeletal composition, morphology, and signaling mode, these can be categorized into several types with different terms, including cytonemes, airinemes, tunneling nanotubes, and others (Daly et al., 2022; Eom, 2020; Zhang and Scholpp, 2019). All of them are extended by either signal-sending or signal-receiving cells or both, establishing physical contact with their specific target cells. Signaling molecules, including major morphogens, are moved along the protrusions, or packaged into the vesicles and delivered (Kornberg and Roy, 2014b; Roy et al., 2011; Yamashita et al., 2018).
Airinemes were initially identified in pigment cells in developing zebrafish (Eom et al., 2015). Airinemes are frequently extended by unpigmented yellow pigment cells called xanthoblasts, particularly during metamorphic stages when pigment pattern development takes place (Fig. 1A, B). Unlike fully differentiated xanthophores, xanthoblasts display bleb-like bulged membrane structures at their surface, serving as the origin of airineme-vesicles at the tips of the airinemes (Fig. 2A, yellow arrowheads). The initial step of airineme-mediated signaling involves the interaction of a specific skin-resident macrophage subpopulation, termed metaphocytes, with these blebs (Bowman et al., 2023; Eom and Parichy, 2017). This subset of macrophages (henceforth called metaphocytes) plays an essential role in airineme-mediated intercellular communication, a unique feature not reported in other signaling cellular protrusions that typically involve signal-sending and -receiving cells for their signaling events. Live imaging experiments have shown that metaphocytes engulf the blebs, pulling them as they migrate, with filaments extending behind the migrating metaphocytes (Bowman et al., 2023; Eom and Parichy, 2017) (Fig. 2A, white arrowhead). Subsequently, metaphocytes release the blebs (now referred to as airineme vesicles) onto the surface of target melanophores (Eom, 2020).
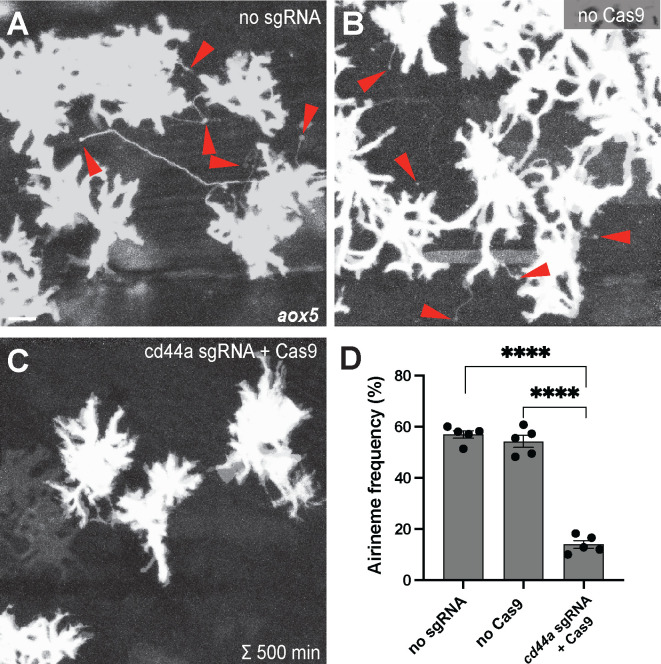
Figure 1. Gene knock-out of cd44a results in significant reduction in airineme extension.
(A, B) Merged time-lapse frames over 500 mins display airinemes. Red arrowheads mark airineme vesicles. (C, D) The extension of airinemes was significantly decreased in embryos injected with cd44a sgRNA/Cas9, (F2, 12) = 172.8, P<0.0001, 5 larvae each). Statistically significant results were evaluated using a One-way ANOVA, followed by a Tukey’s HSD post hoc test. Scale bars represent 20μm. Error bars denote mean ± SEM.
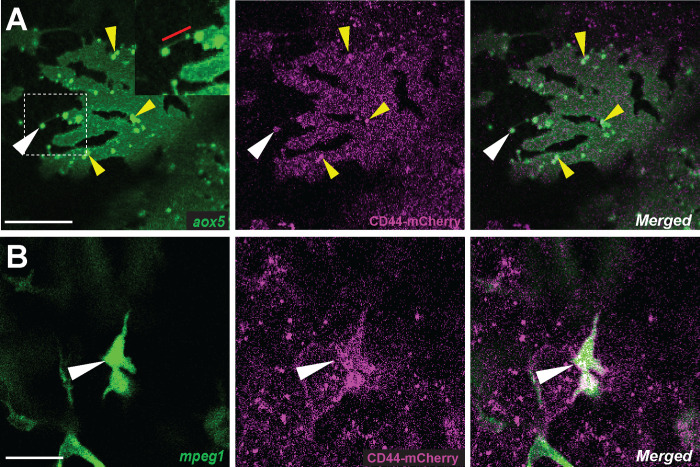
Figure 2. Localization of CD44a protein in xanthoblasts and metaphocytes.
(A) CD44-mCherry expression across the xanthoblasts cell membrane. CD44 is also notably present in the airineme vesicle (white arrowhead) and in the airineme blebs (yellow arrowheads). (B) CD44-mCherry expression in metaphocytes recognized by their amoeboid morphology, in contrast to the non-displayed dendritic population (white arrowheads) (Bowman et al., 2023). Scale bars represent 20μm (A, B).
The target cells for airinemes are embryonic melanophores and newly differentiating melanophores, rather than fully differentiated melanophores. These target melanophores are situated in the developing interstripe of the metamorphic zebrafish and receive Notch signals through airineme vesicles containing DeltaC ligand. It has been suggested that the Notch signal activated by airinemes subsequently triggers Kita signaling, which is essential for melanophore migration and survival (Eom et al., 2015). Consequently, target melanophores within the interstripe gradually migrate out of the interstripe and coalesce into the stripes. Inhibiting airineme extension or depleting metaphocytes leads to the melanophore retention in the interstripe, emphasizing the critical role of airinemes and metaphocytes in orchestrating pigment patterning during zebrafish development (Bowman et al., 2023; Eom et al., 2015).
In the initial step of airineme/metaphocyte-mediated signaling, it is probable that mechanisms exist for metaphocytes to recognize and adhere to airineme blebs. Previous study showed that airineme blebs express a high level of phosphatidylserine, a phospholipid used as an ‘eat-me’ signal for macrophages to initiate phagocytosis (Davies et al., 2013; Eom and Parichy, 2017; Ginhoux and Guilliams, 2016). However, the necessity and molecular nature of adhesive interactions between these remain unknown.
CD44 is a class I transmembrane adhesion protein with broad expression across various cell types, including lymphocytes, immune cells, fibroblasts, neuronal cells, and more (Ponta et al., 2003). Notably, its overexpression in several cancer stem cells suggests a significant role in cancer development and progression (Chen et al., 2018; Weng et al., 2022). Different CD44 isoforms exhibit distinct functions in interacting with ligands and other interacting molecules, emphasizing their role in diverse tumor progression in humans (Gunthert et al., 1995; Wang et al., 2009).
Like Notch receptors, CD44 can undergo cleavage by membrane type 1 matrix metalloprotease and subsequent cleavage by γ-secretase. This sequential cleavage releases the intracellular domain (ICD) of CD44 into cytosol, allowing its translocation into nucleus. The CD44-ICD functions as a transcription factor that induce genes associated with cell survival, migration, metastasis, and others (Miletti-Gonzalez et al., 2012; Skandalis, 2023). Meanwhile, extracellular domain (ECD) of CD44 facilitates adhesion with other cells expressing CD44 or interact with several ligands in the extracellular matrix (ECM). One of such ligand is hyaluronic acid (HA), and CD44-HA interaction is known to regulate cytoskeleton dynamics and tumor progression in some contexts. Furthermore, CD44-ECD-mediated homophilic cell-to-cell adhesion is well-documented in tumors that can facilitate tumor cell aggregation and metastasis (Kawaguchi et al., 2020; Liu et al., 2019). The multifaceted roles of CD44 highlights its significance in both physiological and pathological cellular processes, making it a crucial focus in cancer research and therapeutic development.
In this study, we demonstrate that CD44, through its extracellular domain, plays a crucial role in the adhesive interaction between the blebs of airineme-producing xanthoblasts and airineme-pulling metaphocytes. This interaction is critical for airineme-mediated intercellular communication and the formation of pigment pattern in zebrafish.
Results
Gene knock-out of cd44a using CRISPR/Cas9 results in a substantial decrease in airineme extension
To identify candidate genes involved in adhesive interaction between metaphocytes and airineme vesicles, we conducted gene expression profiling between xanthophores and airineme-producing xanthoblasts. Among several adhesion proteins analyzed, cd44a exhibited the most significant gene expression difference between these two cell types. To investigate the role of cd44a in airineme-mediated signaling, we designed single-guide RNA (sgRNA) against cd44a and injected it into one-cell-stage embryos with Cas9 protein, along with an aox5:palmEGFP construct to label cell membrane and airinemes of xanthophore-lineages. Control groups included embryos that receive cd44a sgRNA injection without Cas9 protein and embryos injected only with Cas9 protein into wild-type embryos. The fish were raised until metamorphic stages (SSL 7.5), where airineme extension is most frequent (Eom et al., 2015). Our results showed that embryos injected with cd44a sgRNA/Cas9 had a significant reduction in airineme extension as compared to the two controls, suggesting that cd44a may be required for proper airineme signaling (Fig. 1).
CD44 protein expression in metaphocytes and xanthophore-lineages
The role of CD44 in cell-cell or cell-ECM interactions is well-established in various contexts (Chen et al., 2018; Ponta et al., 2003; Senbanjo and Chellaiah, 2017). Given this, we predicted that CD44 is expressed in either airineme producing xanthoblasts or metaphocytes, or potentially both. To investigate the localization of CD44 protein under native regulatory elements, we recombineered a 82 kb BAC containing zebrafish cd44a coding sequence and regulatory elements to generate an mCherry fusion, TgBAC(cd44a:cd44a-mCherry). To assess CD44 protein expression in xanthoblasts, we injected aox5:palmEGFP construct into TgBAC(cd44a:cd44a-mCherry). The CD44-mCheery signal was detected in various cell types, including xanthophore lineages (Fig. 2A). Interestingly, we were able to detect enriched CD44 expression in the airineme vesicles (Fig. 2A, white arrowhead) and airineme blebs (Fig. 2A, yellow arrowheads) which are the precursors of airineme vesicles. To examine CD44 expression in metaphocytes, we injected mpeg1:palmEGFP construct, which label all macrophages. Metaphocytes were easily distinguishable by their amoeboid morphology compared to the dendritic population in the zebrafish skin (Bowman et al., 2023; Kuil et al., 2020; Lin et al., 2020; Lin et al., 2019). We confirmed that metaphocytes also express CD44 (Fig. 2B, arrowheads) (Vachon et al., 2006). CD44-mCherry expression was not confined to specific subcellular structures except airineme blebs but was distributed throughout the cells in xanthophore-lineages. Given the fact that CD44 is a membrane protein, we assumed its expression at least in the cell membrane. Although, metaphocytes exhibited strong CD44 expression at the center of the cell, it was also expressed throughout the cells. Thus, our findings confirm that CD44 protein is expressed in both xanthoblasts and metaphocytes (Fig. 2).
Extracellular domain of CD44 is essential for airineme extension
As CD44 is a multifunctional protein that can promote adhesion between cells or extracellular matrix (ECM) through interactions with various ligands (Senbanjo and Chellaiah, 2017). One well-known ligand for CD44 is hyaluronic acid (HA). Given the fact that airinemes are actin- and tubulin-based structures, and the CD44-HA interaction can activate the cytoskeleton in certain contexts, we aimed to investigate whether CD44 controls airineme extension through its interaction with HA (Eom, 2020; Eom et al., 2015; Ponta et al., 2003). We anticipated observing HA expression in CD44-expressing xanthoblasts if CD44 acts through HA. However, we did not detect any overlapping expression of HA in xanthoblasts, suggesting that the CD44-HA interaction does not appear to be involved in airineme signaling (Fig. S1).
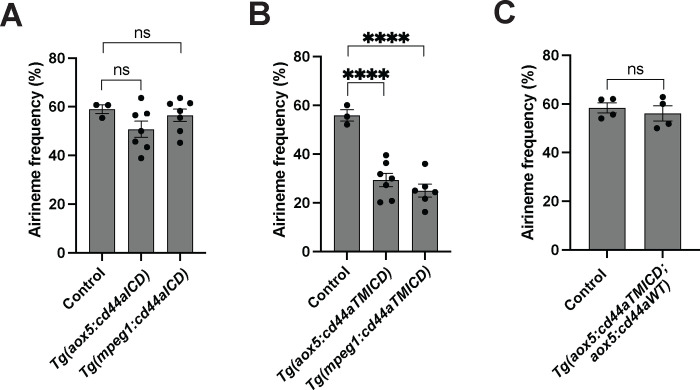
Domain studies of CD44 suggested that its intracellular domain (ICD) functions as a transcription factor, influencing cell migration, angiogenesis, invasion and other behaviors (Chen et al., 2018; Skandalis, 2023). To investigate whether CD44-ICD plays a role in airineme extension, we generated transgenic lines overexpressing cd44-ICD in either xanthophore-lineages or macrophages, Tg(aox5:cd44aICD) and Tg(mpeg1:cd44aICD). In both transgenic lines, we did not observe any significant changes in airineme extension frequency, suggesting that CD44-ICD doesn’t seem to be involved in airineme extension (Fig. 3A).
Figure 3. The crucial role of the extracellular domain of CD44 in airineme extension.
(A) Airineme extension frequency did not significantly altered in transgenic embryos that overexpressed the intracellular domain of cd44 (cd44aICD) specifically in the xanthophore-lineages or macrophages, (F(2, 14)=1.736, P=0.2121, 17 embryos in total). (B) Overexpression of CD44a with a truncated extracellular domain (cd44aTMICD) exhibited significant reduction in airineme extension frequency in both the xanthophore-lineages and macrophages, (F(2, 13) = 23.62, P<0.0001, 16 embryos in total). (C) Airineme extension frequency was restored when the WT CD44 was overexpressed in embryos already expressing cd44aTMICD in the xanthophore lineages, (P=0.5767, 4 embryos each). Statistically significant results were evaluated using a One-way ANOVA, followed by a Tukey’s HSD post hoc test or a Student’s t test. Error bars indicate mean ± SEM.
Subsequently, we explored the potential role of the extracellular domain (ECD) of CD44 in airineme extension. CD44-ECD is known to interact with the extracellular matrix (ECM) or CD44-ECD from other cells (Senbanjo and Chellaiah, 2017). To test this, we generated transgenic lines overexpressing an ECD-truncated form of cd44 (cd44TMICD), specifically in xanthophore-lineages, Tg(aox5:cd44aTMICD) or macrophages, Tg(mpeg1:cd44aTMICD). These transgenic lines did not impact cell viability or other noticeable cellular behaviors in either xanthophore-lineages or macrophages (Fig. S2). Interestingly, we observed a significant decrease in airineme extension frequency in the transgenic lines overexpressing cd44aTMICD (Fig. 3B). We hypothesized that the overexpressed CD44aTMICD might out-compete the wild-type CD44 in the transgenic zebrafish. To test this, we investigated whether the additional supply of WT CD44 could rescue the phenotype in Tg(aox5:cd44aTMICD) by generating a rescue line Tg(aox5:cd44aTMICD; aox5:cd44aWT). This rescue fish exhibited almost full recovery of airineme extension frequency. Together, these results suggest that CD44-ECD is critical for airineme extension (Fig. 3C).
Trans-adhesive interaction via CD44ECD between xanthoblasts and metaphocytes is critical in airineme extension
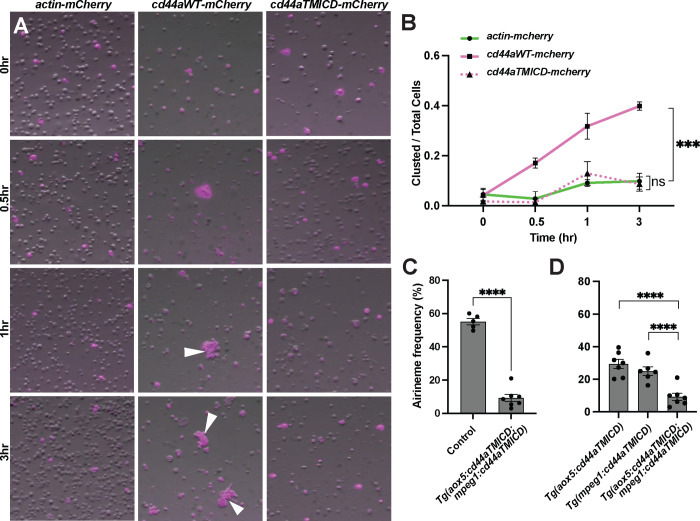
We investigated whether extracellular domain of zebrafish CD44 can mediate adhesion between cells. To test this, we expressed constructs that encode wild-type zebrafish cd44a or ECD-truncated cd44a (cd44aTMICD), both C-terminally fused with mCherry, in Drosophila S2 cells (Schneider, 1972). Actin-mCherry fusion construct was used as a control. Transfected S2 cells expressing mCherry were monitored, and after several hours of culture in a rotary incubator, we assessed whether CD44 expressing S2 cells adhere to each other. The results showed that, unlike the actin control and cd44aTMICD-expressing S2 cells, wild-type cd44a-expressing S2 cells began forming aggregates 30 minutes after incubation. The degree of aggregation increased over time, reaching a significant level at 3 hours. This indicates that zebrafish CD44 mediates trans-adhesion via its ECD between cells (Fig. 4A, B).
Figure 4. Importance of trans-adhesive interaction via CD44ECD between xanthoblasts and metaphocytes in airineme extension.
(A) S2 cells were transfected with constructs to express actin-mCherry (control), wild-type cd44a-mCherry, or cd44aTMICD-mCherry. Within 1 hour of rotary culture, cell aggregates were formed by those expressing the wild-type cd44a (white arrowheads). (B) Quantification of the transfected cells found within clusters relative to the total numbers of transfected cells in 3 replicate cultures, (F(2, 6)=45.50, P=0.0002). (C) Overexpressing cd44aTMICD in both xanthophore lineages and macrophages resulted in a significant reduction in airineme extension frequency. (D) The graph combines data from Fig. 3B and 4C to illustrate the statistical significant between cd44aTMICD overexpression in either of cell type and both simultaneously, (F(2, 17)=18.04, P<0.0001). Statistically significant results were evaluated using a One-way ANOVA, followed by a Tukey’s HSD post hoc test or a Student’s t test. Scale bars: 20μm. Error bars indicate mean ± SEM.
This led us to ask whether trans-adhesive interactions between xanthoblasts and metaphocytes could play a role in airineme extension in zebrafish. We hypothesized that a greater reduction in airineme extension frequency would be observed if CD44a function is compromised simultaneously in both cell types, compared to its expression in only one cell type. To test this, we generated a double transgenic line that overexpresses cd44aTMICD in both xanthophore-lineages and macrophages, Tg(aox5:cd44aTMICD; mpeg1:cd44aTMICD). Indeed, we observed a dramatic decrease in airineme extension in this double transgenic zebrafish compared to single manipulations (Fig. 3B, Fig. 4C, D), suggesting, at least in part, a requirement for CD44ECD by both xanthoblasts and metaphocytes for proper airineme signaling as CD44 is expressed in airineme vesicles and metaphocytes (Fig. 2A, B). However, we cannot rule out the possibility that there are other adhesion molecules may trans-interact with CD44 in both xanthophore lineages and macrophages.
Next, we asked whether CD44-mediated adhesion is critical when metaphocytes contact airineme blebs (equivalent to airineme vesicle) and or when they drag the airineme vesicles. We hypothesized that if such adhesive interaction is important to maintain the attachment of airineme vesicles to metaphocyte while dragging, a reduction in airineme length would be observed. This is because the detachment of metaphocytes from the airineme vesicle would stop the extension of airineme filaments (Bowman et al., 2023; Eom and Parichy, 2017). However, we did not detect any statistically significant change in airineme length in embryos overexpressing CD44aTMICD in either cell type or both simultaneously (Fig. S3). This finding suggests that the trans-adhesive interaction mediated by CD44 plays a more significant role when metaphocytes are contacting airineme blebs as opposed to pulling the airineme vesicles.
CD44-mediated airineme signaling is crucial for pigment pattern formation
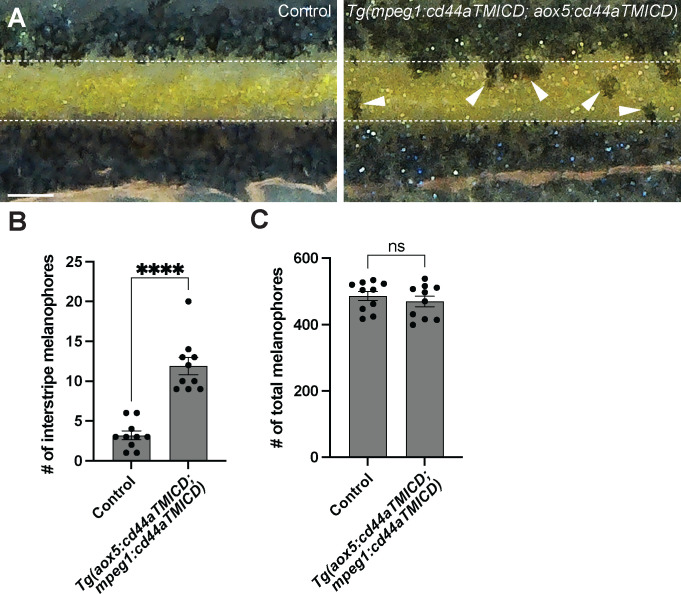
Airineme-mediated intercellular signaling is indispensable for pigment pattern formation in zebrafish. Our results indicating the importance of CD44a in airineme extension led us to anticipate pigment pattern defects in embryos overexpressing cd44aTMICD. However, noticeable pigment pattern defects were not detected in either transgenic line overexpressing cd44aTMICD in xanthophore lineages or macrophages. This suggests that residual airineme extensions may still be sufficient to deliver the necessary signals for generating a stripe pattern, or subtle difference could be challenging to detect (Fig. 2B). Nevertheless, when CD44a function was compromised by overexpressing cd44aTMICD in both cell types, a significant number of melanophores were observed to be retained in the interstripe compared to the control, although the total number of melanophores remained unchanged (Fig. 5A, B, C).
Figure 5. CD44-mediated airineme extension contributes to zebrafish pigment pattern formation.
(A) Unlike the control, melanophores failed to coalesce into stripe and remained in the interstripe zone (white arrowheads) at SSL11 (Parichy et al., 2009). The white dotted lines demarcate stripes and interstripe. (B) In embryos overexpressing cd44aTMICD simultaneously in both xanthophore-lineages and macrophage, the count of interstripe melanophores was significantly higher, (P<0.0001, 20 embryos in total). (C) However, the total number of melanophores was not differ in the experimental group as compared to the controls, (P=0.4525, 20 embryos in total). Statistical significance was assessed using a Student’s t test. Scale bars represent 200μm. Error bars indicate mean ± SEM.
Together, these findings collectively suggest that CD44-mediated trans-adhesive interaction between airineme blebs on xanthoblasts and metaphocytes play an essential role in airineme-mediated signaling during pigment pattern formation in zebrafish.
Discussion
In this study, we demonstrated that CD44 protein is expressed in xanthoblasts, particularly in the airineme vesicles (Fig. 2A, yellow arrowheads). Intriguingly, though, not every airineme bleb expresses CD44 but only some of them do. This observation leads to a few possible explanations. One explanation could be that CD44 expression determines which blebs are used for airineme signaling. It is possible that some blebs do not express CD44 and serve a different function beyond airineme signaling. In addition, our observation may suggest that there is a mechanism by which CD44 protein in the cell membrane becomes further enriched in the airineme blebs. Similar to CD44, we observed that DeltaC, one of signaling molecule found in the airineme vesicles, is not expressed in some of the blebs before their extraction by metaphocytes (Eom et al., 2015). It is conceivable that there are mechanisms that sort proteins required for airineme signaling into the blebs and ultimately into the airineme vesicle. These might be used to adhere to the metaphocyte membrane and activate Notch signal on the target cell surface. It would be interesting to study the underlying molecular mechanisms involved in sorting out those proteins essential for airineme-mediated intercellular signaling into the airineme blebs.
Furthermore, CD44 has previously been reported as a signal for phagocytosis in macrophages (Vachon et al., 2007; Vachon et al., 2006). Therefore, alongside phosphatidylserine (PtdSer), CD44 may serve as an additional recognition signal for metaphocytes to identify airineme blebs, as well as acting as an adhesive signal between airineme blebs and metaphocytes (Eom and Parichy, 2017).
This raises an interesting question as to why we did not observe any differences in airineme filament length when the adhesive interaction mediated by CD44 was reduced (Fig. S3A). Our interpretation is that this adhesive interaction would be crucial during the initial interaction between metaphocytes and the airineme blebs, rather than during the process of dragging them. Thus, it seems plausible that CD44 functions as both a recognition and adhesion element at the time when metaphocytes interact with airineme blebs, but future experiments would be required to verify this hypothesis.
On a related note, our previous research, using high-resolution 3D confocal image reconstruction, has shown that airineme vesicles are found inside the dragging metaphocytes (Eom and Parichy, 2017). This implies that airineme vesicles are physically entrapped within the metaphocytes during the pulling process, suggesting that adhesive interaction between the membranes of those two signaling components might not be strictly necessary while pulling.
We observed residual airineme extension even when we overexpressed cd44aTMICD in both xanthophore lineages and macrophages (Fig. 4C). This residual airineme extension may indicate that either remaining wild-type CD44 continues to mediate adhesion or other adhesion proteins and players are involved in the process. To explore this further, we considered the studied interaction of Matrix Metalloproteinase-9 (MMP9) with CD44 (Senbanjo and Chellaiah, 2017). Cell surface expression of MMP9, along with CD44, is known to facilitate cell migration and invasion in tumors and PC3 cells (Desai et al., 2008; Gupta et al., 2013; Yu and Stamenkovic, 1999). Given our previous findings that airineme-pulling metaphocytes express high-levels of mmp9, crucial for metaphocyte migration and penetration into the hypodermis in zebrafish skin (Bowman et al., 2023), we explored whether CD44 also plays a role in metaphocyte migration speed (=airineme extension speed) through its interaction with MMP9. However, our analysis did not reveal a significant difference in airineme extension speed in cd44aTMICD overexpressed macrophages (Fig. S3B). Although further investigations are necessary, this suggests that CD44 may function independently in metaphocyte migration during airineme extension.
Taken together, our study discovered that extracellular domain of CD44 in both airineme-extending xanthoblasts and airineme-pulling metaphocytes facilitates a trans-adhesive interaction, which appears to be critical in initiating airineme extension. This study provides evidence for the requirement of cellular adhesion between signaling-sending cell and relaying cells in airineme-mediated intercellular signaling. It is also conceivable that similar mechanisms could be conserved in other cellular contexts.
Materials Availability:
This study generated several zebrafish transgenic lines, Tg(mpeg1:cd44aICD-v2a-mCherry), Tg(aox5:cd44aICD-v2a-mCherry), Tg(mpeg1:cd44aTMICD-v2a-mCherry), Tg(aox5:cd44aTMICD-v2a-mCherry), Tg(aox5:cd44aFL-v2a-mCherry), Tg(mpeg1:cd44aFL-v2a-mCherry) and TgBAC(cd44a:cd44amCherry). They are available from the lead contact without restriction.
Experimental Model and Subject Details:
Zebrafish:
Fish were maintained at 28.5C, 16:8 L:D. Zebrafish were wild-type ABWP or its derivative WT(ABb), as well as Tg(mpeg1:BrainbowW201), which expresses tdTomato in the absence of Cre-mediated recombination, and Tg(aox5:palmEGFP). Experiments were performed prior to the development of secondary sexual characteristics, so the number of males and females in the study could not be determined, however, all stocks generated approximately balanced sex ratios, so the experiments likely sampled similar numbers of males and females. All animal work in this study was conducted with the approval of the University of California Irvine Institutional Animal Care and Use Committee (Protocol #AUP-22–023) in accordance with institutional and federal guidelines for the ethical use of animals.
Methods:
Transgenesis and transgenic line production
To examine how loss of extracellular domain (ECD) of CD44 adhesion molecule may impact interactions between macrophages and xanthoblasts, we generated mpeg1:cd44aTMICD-v2a-mCherry and aox5:CD44aTMICD-v2a-mCherry constructs using Gateway assembly into Tol2 backbone and injected into WT(ABb). Truncated version of CD44a (CD44TMICD) was obtained by extracting CD44aTMICD cDNA from WT(ABb) cDNA. To examine whether interactions between macrophages and xanthoblasts were gene expression dependent via CD44a-ICD, we generated aox5:CD44aICD-v2a-mCherry and mpeg1:CD44aICD-v2a-mCherry transgenic lines, which were likewise generated using Gateway assembly into Tol2 backbone and injected into WT(ABb). Similarly, CD44aICD cDNA was isolated from WT(ABb) cDNA. To further examine how ECD of CD44a may impact interactions between macrophages and xanthoblasts, we generated Tg(aox5:CD44aFL-v2a-mCherry) to see if we could rescue the phenotypes seen in Tg(aox5:CD44aTMICD-v2a-mCherry).
To visualize CD44 localization under native regulatory elements, we inserted mCherry C-terminally using BAC CH211–102L7 with 82 kb 5’ and 92 kb 3’ to the open reading frame.
Hyaluronic Acid Binding Protein (HABP) assay
Zebrafish embryos (SSL7.5) were incubated in a fish water diluted biotin-HABP (1:150, amsbio) along with streptavidin-Alexa546 for 1 hr. Controls incubated solely with streptavidin-Alexa546. Fish were then washed two times with fish water and imaged under a confocal microscope.
Time-lapse imaging and still imaging
Ex-vivo imaging of pigment cells and macrophages was done using Leica TCS SP8 confocal microscope equipped with a resonant scanner and two HyD detectors. Time-lapse images were taken at 5-min intervals over a span of 10h. Overnight time-lapse imaging was performed when larvae reached SSL (Standardized Standard Length) 7.5 (Parichy et al., 2009).
S2 Cell Adhesion Assay
S2 cells were obtained from the Drosophila Genomics Resource Center (DGRC, S2-DGRC (DGRC Stock 6 ; https://dgrc.bio.indiana.edu//stock/6 ; RRID:CVCL_TZ72)) and cultured in Schneider’s Drosophila Medium (Catalog No. 21720024, Gibco, USA) supplemented with 10% Fetal Bovine Serum (FBS) (Catalog No. 25–514H, Genclone, USA) and 1% Penicillin-Streptomycin (Catalog No. 15140148, Gibco, USA). For transfection, S2 cells were transfected with three different plasmids: pAW-actin-mcherry, pAW-cd44a-FL-mcherry, and pAW-cd44a-TMICD-mcherry. Lipofectamine™ LTX Reagent (Catalog No. 15338030, Invitrogen, USA) was used for transfection following the manufacturer’s instructions. After 72 hours of transfection, the cells were counted, and each type of cell was seeded into 35mm glass-bottom petri dishes at a density of 5000 cells per dish (Catalog No. 706011, Nest Scientific, USA). The dishes were then subjected to rotating incubation for different time intervals, including 30 minutes, 1 hour, and 3 hours. Cellular imaging was performed using a Zeiss microscope equipped with a 599mm filter to capture the cellular mCherry signal and bright field images.
Pigment pattern and melanophore counts
Fish were imaged upon reaching SSL 11.0 using a Koolertron LCD digital microscope. Images were taken of the entire trunk and were later cropped to include only the area underneath the dorsal fin. ImageJ cell counter plugin was used to count interstripe and total numbers of melanophores. Numbers for each group were averaged.
Quantification and statistical analysis
Statistical analyses were performed using GraphPad Prism.
Supplementary Material
Acknowledgements
We thank Parichy lab for their support in the preliminary stages of this study. Additionally, we thank Zach Waller for his contribution to generating and managing some of the transgenic lines used for this study. We acknowledge funding from NIH R35GM142791 to D.S.E. and support from the Drosophila Genomics Resource Center (NIH Grant 2P40OD010949).
References
- Bowman R.L., Wang D., Eom D.S., 2023. A macrophage subpopulation promotes airineme-mediated intercellular communication in a matrix metalloproteinase-9 dependent manner. Cell Rep 42, 112818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhao S., Karnad A., Freeman J.W., 2018. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol 11, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combarnous Y., Nguyen T.M.D., 2020. Cell Communications among Microorganisms, Plants, and Animals: Origin, Evolution, and Interplays. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C.A., Hall E.T., Ogden S.K., 2022. Regulatory mechanisms of cytoneme-based morphogen transport. Cell Mol Life Sci 79, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R., 2013. Tissue-resident macrophages. Nat Immunol 14, 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai B., Ma T., Chellaiah M.A., 2008. Invadopodia and matrix degradation, a new property of prostate cancer cells during migration and invasion. J Biol Chem 283, 13856–13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom D.S., 2020. Airinemes: thin cellular protrusions mediate long-distance signalling guided by macrophages. Open Biol 10, 200039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom D.S., Bain E.J., Patterson L.B., Grout M.E., Parichy D.M., 2015. Long-distance communication by specialized cellular projections during pigment pattern development and evolution. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom D.S., Parichy D.M., 2017. A macrophage relay for long-distance signaling during postembryonic tissue remodeling. Science 355, 1317–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Guilliams M., 2016. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 44, 439–449. [DOI] [PubMed] [Google Scholar]
- Gunthert U., Stauder R., Mayer B., Terpe H.J., Finke L., Friedrichs K., 1995. Are CD44 variant isoforms involved in human tumour progression? Cancer Surv 24, 19–42. [PubMed] [Google Scholar]
- Gupta A., Cao W., Sadashivaiah K., Chen W., Schneider A., Chellaiah M.A., 2013. Promising noninvasive cellular phenotype in prostate cancer cells knockdown of matrix metalloproteinase 9. ScientificWorldJournal 2013, 493689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E.T., Dillard M.E., Cleverdon E.R., Zhang Y., Daly C.A., Ansari S.S., Wakefield R., Stewart D.P., Pruett-Miller S.M., Lavado A., Carisey A.F., Johnson A., Wang Y.D., Selner E., Tanes M., Ryu Y.S., Robinson C.G., Steinberg J., Ogden S.K., 2024. Cytoneme signaling provides essential contributions to mammalian tissue patterning. Cell 187, 276–293 e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M., Dashzeveg N., Cao Y., Jia Y., Liu X., Shen Y., Liu H., 2020. Extracellular Domains I and II of cell-surface glycoprotein CD44 mediate its trans-homophilic dimerization and tumor cluster aggregation. J Biol Chem 295, 2640–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg T.B., Roy S., 2014a. Communicating by touch--neurons are not alone. Trends Cell Biol 24, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg T.B., Roy S., 2014b. Cytonemes as specialized signaling filopodia. Development 141, 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuil L.E., Oosterhof N., Ferrero G., Mikulasova T., Hason M., Dekker J., Rovira M., van der Linde H.C., van Strien P.M., de Pater E., Schaaf G., Bindels E.M., Wittamer V., van Ham T.J., 2020. Zebrafish macrophage developmental arrest underlies depletion of microglia and reveals Csf1r-independent metaphocytes. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Zhou Q., Lin G., Zhao C., Wen Z., 2020. Endoderm-Derived Myeloid-like Metaphocytes in Zebrafish Gill Mediate Soluble Antigen-Induced Immunity. Cell Rep 33, 108227. [DOI] [PubMed] [Google Scholar]
- Lin X., Zhou Q., Zhao C., Lin G., Xu J., Wen Z., 2019. An Ectoderm-Derived Myeloid-like Cell Population Functions as Antigen Transporters for Langerhans Cells in Zebrafish Epidermis. Dev Cell 49, 605–617 e605. [DOI] [PubMed] [Google Scholar]
- Liu X., Taftaf R., Kawaguchi M., Chang Y.F., Chen W., Entenberg D., Zhang Y., Gerratana L., Huang S., Patel D.B., Tsui E., Adorno-Cruz V., Chirieleison S.M., Cao Y., Harney A.S., Patel S., Patsialou A., Shen Y., Avril S., Gilmore H.L., Lathia J.D., Abbott D.W., Cristofanilli M., Condeelis J.S., Liu H., 2019. Homophilic CD44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models. Cancer Discov 9, 96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletti-Gonzalez K.E., Murphy K., Kumaran M.N., Ravindranath A.K., Wernyj R.P., Kaur S., Miles G.D., Lim E., Chan R., Chekmareva M., Heller D.S., Foran D., Chen W., Reiss M., Bandera E.V., Scotto K., Rodriguez-Rodriguez L., 2012. Identification of function for CD44 intracytoplasmic domain (CD44-ICD): modulation of matrix metalloproteinase 9 (MMP-9) transcription via novel promoter response element. J Biol Chem 287, 18995–19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parichy D.M., Elizondo M.R., Mills M.G., Gordon T.N., Engeszer R.E., 2009. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn 238, 2975–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H., Sherman L., Herrlich P.A., 2003. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4, 33–45. [DOI] [PubMed] [Google Scholar]
- Roy S., Hsiung F., Kornberg T.B., 2011. Specificity of Drosophila cytonemes for distinct signaling pathways. Science 332, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I., 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol 27, 353–365. [PubMed] [Google Scholar]
- Senbanjo L.T., Chellaiah M.A., 2017. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front Cell Dev Biol 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skandalis S.S., 2023. CD44 Intracellular Domain: A Long Tale of a Short Tail. Cancers (Basel) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon E., Martin R., Kwok V., Cherepanov V., Chow C.W., Doerschuk C.M., Plumb J., Grinstein S., Downey G.P., 2007. CD44-mediated phagocytosis induces inside-out activation of complement receptor-3 in murine macrophages. Blood 110, 4492–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon E., Martin R., Plumb J., Kwok V., Vandivier R.W., Glogauer M., Kapus A., Wang X., Chow C.W., Grinstein S., Downey G.P., 2006. CD44 is a phagocytic receptor. Blood 107, 4149–4158. [DOI] [PubMed] [Google Scholar]
- Wang S.J., Wong G., de Heer A.M., Xia W., Bourguignon L.Y., 2009. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope 119, 1518–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng X., Maxwell-Warburton S., Hasib A., Ma L., Kang L., 2022. The membrane receptor CD44: novel insights into metabolism. Trends Endocrinol Metab 33, 318–332. [DOI] [PubMed] [Google Scholar]
- Yamashita Y.M., Inaba M., Buszczak M., 2018. Specialized Intercellular Communications via Cytonemes and Nanotubes. Annu Rev Cell Dev Biol 34, 59–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Stamenkovic I., 1999. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev 13, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Scholpp S., 2019. Cytonemes in development. Curr Opin Genet Dev 57, 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.