Abstract
The interplay of stochastic and ecological processes that govern the establishment and persistence of host-associated microbial communities is not well understood. Here we illustrate the conceptual and practical advantages of fitting stochastic population dynamics models to multi-species bacterial time series data. We show how the stability properties, fluctuation regimes and persistence probabilities of human vaginal microbial communities can be better understood by explicitly accommodating three sources of variability in ecological stochastic models of multi-species abundances: 1) stochastic biotic and abiotic forces, 2) ecological feedback and 3) sampling error. Rooting our modeling tool in stochastic population dynamics modeling theory was key to apply standardized measures of a community’s reaction to environmental variation that ultimately depends on the nature and intensity of the intra-specific and inter-specific interaction strengths. Using estimates of model parameters, we developed a Risk Prediction Monitoring (RPM) tool that estimates temporal changes in persistence probabilities for any bacterial group of interest. This method mirrors approaches that are often used in conservation biology in which a measure of extinction risks is periodically updated with any change in a population or community. Additionally, we show how to use estimates of interaction strengths and persistence probabilities to formulate hypotheses regarding the molecular mechanisms and genetic composition that underpin different types of interactions. Instead of seeking a definition of “dysbiosis” we propose to translate concepts of theoretical ecology and conservation biology methods into practical approaches for the management of human-associated bacterial communities.
Keywords: stochastic population dynamics of the microbiome, environmental stochasticity, stochastic stability, persistence probability, population viability monitoring, multivariate autoregressive model of population dynamics, stochastic community population dynamics
INTRODUCTION
For decades now, inferring the interplay between stochastic processes and the ecological and evolutionary conditions that permit the establishment and persistence of host-associated microbial communities has remained a topic laden with controversies and unresolved conceptual and practical issues (Zaoli and Grilli 2021; Grilli 2020; Zhou and Ning 2017; Ferguson and Ponciano 2014; Gudelj et al. 2010; Robinson, Bohannan, and Young 2010; Ponciano et al. 2007). The paucity of studies connecting extensive time-series data with population dynamics models rooted in ecological principles has been at the center of the problems faced when inferring processes from patterns in this area of research (Zhou and Ning 2017). This knowledge gap is exemplified here for the human vaginal microbiome. Work done to characterize these bacterial communities using experimental and quantitative analytical approaches (Ravel et al. 2011) has shown that idiosyncratic changes in species composition and wide temporal fluctuations in the relative abundances of the different species are undeniably associated with specific environmental variables like pH. However, even a basic understanding of the mechanisms leading to these fluctuations remains elusive. Given that the structure and composition of an ecological community often alternates between distinct, widely different states (Shade et al. 2012; Gonze et al. 2017; 2018; Bardgett and Caruso 2020), the chances of dramatic community shifts are better predicted using mechanistic, stochastic population dynamics models (Schooler et al. 2011; Ives et al. 2003; Ponciano 2018; Ponciano, Taper, and Dennis 2018; Auger‐Méthé et al. 2021). Illustrating the conceptual and practical advantages of fitting stochastic population dynamics models to multi-species bacterial time series data is the focus of this paper.
Here we developed and tested a multi-species stochastic population modeling approach (Ludwig 1976; Nisbet and Gurney 2003; Cushing et al. 2003; L. J. Allen 2010; Dennis et al. 2006; Ovaskainen and Meerson 2010; Dennis and Ponciano 2014; Ponciano 2018; Ponciano, Taper, and Dennis 2018) to better understand how fluctuations in the environment ultimately contribute to changes in species composition and abundances as well as to the overall community stability. Our central hypothesis is that stability properties, diversity and fluctuation regimes of human vaginal microbial communities can be better understood by explicitly accommodating the following three sources of variability in time series models of multi-species abundances: 1) stochastic biotic and abiotic forces, 2) ecological feedbacks and 3) sampling error. This modeling framework translates tentative explanations of the sources of the temporal variation in bacterial abundances into testable hypotheses that describe the interplay between ecological processes and the dynamics of abiotic factors while taking sampling variability into account. This translation was achieved by combining time series data of bacterial species composition with stochastic models derived from basic ecological principles. This probabilistic approach results in a practical statistical connection between biological hypotheses and time series data (Ponciano, Taper, and Dennis 2018). Here we exemplify this process using 135 time series of human vaginal microbial communities (Ravel et al. 2011).
In recent years considerable efforts have been made to characterize the composition of vaginal bacterial communities found in healthy reproductive age women and to understand interruptions to the homeostasis of this microbiome. Community compositions that widely differ from these “normal” states are thought to be in a state of ‘dysbiosis’. Indigenous bacterial populations that reside in and on the human body constitute the first line of defense against infection by preventing non-indigenous organisms from causing disease. In the context of the vaginal microbiome, dysbiosis can reflect changes in the absolute numbers of microbes, the species composition, or changes in the relative abundances of bacterial taxa or some combination thereof. The bacterial communities of reproductive age women often vary over time in a seemingly haphazard way, and investigators assert that certain community states reflect an ‘imbalance’ in the vaginal microbiome, and these are ‘unhealthy’ states. Some of these states, like those depleted of Lactobacillus species are said to reflect ‘dysbiosis’ despite persisting for extended periods of time in women who are asymptomatic and otherwise healthy.
The concept of biological community stability has motivated significant theoretical advances and large empirical research efforts in ecology (McCann 2000; Loreau et al. 2001; May 2019; Ives and Carpenter 2007; Little et al. 2008; Loreau 2010). The disparity between the theoretical predictions and empirical evidence concerning diversity-stability relationships has generated historical controversies that remain unresolved (Loreau 2010). These can in part be attributed to the multiple definitions of stability that have been used (Ives and Carpenter 2007), and partly because diversity per se is rarely a primary driver of stability. Rather than being immediately linked to stability, diversity commonly acts as a secondary driver, itself being subject to the same anthropogenic and environmental drivers that affect stability via a variety of mechanisms (Ives et al. 2008; Altizer et al. 2006). Studies are needed that reveal the ecological processes and abiotic factors that link diversity to stability, particularly in microbial communities (Arumugam et al. 2011; Faith et al. 2011; 2013; Gajer et al. 2012).
Combining mathematical, statistical and stochastic process tools to explicitly model the mechanisms that underlie community dynamics on a temporal scale has long proved to be a fruitful approach to fill knowledge gaps regarding the functioning of ecological communities (Cushing et al. 2003; Ives et al. 2003). This approach has also been shown to reliably reproduce the regular waxing and waning of natural population densities in single and multi-species systems (Zeng et al. 1998; Ponciano et al. 2005; E. J. Allen, Allen, and Schurz 2005; Dennis et al. 2006; Barger and Bunge 2008; Taper and Ponciano 2016; Ponciano 2018; Ponciano, Taper, and Dennis 2018; Dennis et al. 2019). Here we approached the problem of estimating bacterial community stability by explicitly modeling this property as resulting from the interaction of ecological feedback and stochastic (randomly fluctuating) abiotic factors (Ives et al. 2003).
Biological communities are continuously buffeted by changing environments and abiotic factors that induce temporal fluctuations in the growth rates of each species in the system (Dennis 1989; Dennis and Taper 1994; Grenfell, Bjørnstad, and Kappey 2001; Ives et al. 2003). Environmental changes are likely to affect the availability of resources and hence the rate at which bacteria replicate. Furthermore, changes in the availability of limiting resources are expected to be concomitant with changes in the nature and intensities of intra-specific and inter-specific competition processes. Ultimately, these environmental changes are expected to be translated into changes in population sizes. In the face of unpredictable environmental changes, equilibrial states of ecological communities are better characterized by means, variances and other statistical quantities instead of point equilibria derived from deterministic, Lotka-Volterra like models (Ives et al. 2003; Grilli 2020).
Mathematical characterizations of how the mean and variance of population sizes change over time can be obtained by formulating multi-species population growth as stochastic processes (L. J. Allen 2010; Ferguson and Ponciano 2014; Ponciano 2018; 2018). These mathematical expressions reveal the links between the patterns of population variation, environmental variation and key ecological quantities like intrinsic growth rates and inter-specific and intra-specific competition coefficients. In this work, we bring these mathematical characterizations to life by fitting stochastic ecological population models to a large data set consisting of 135 time series data sets that each spanned 70 days with daily samples. From the abundance time series, we explicitly estimate the strength of intra- and inter-specific competition and use these estimates to compute dynamic stability metrics that describe the system’s behavior. Furthermore, we show that it is possible to link changes in the persistence (or extinction) probabilities of any given bacterial type of interest with changes in intra-specific and inter-specific competition coefficients. By understanding the relationship between these coefficients and the population fluctuations of vaginal bacteria of clinical interest, our analysis constitutes the first step towards assessing the risk to diseases linked to either the fast growth, invasion, or extinction of different species in vaginal bacterial communities. Our approach makes fundamental restoration principles and modeling techniques accessible to applied research programs that focus on predicting the tendencies of microbial communities.
STOCHASTIC MODELS OF POPULATION ABUNDANCE
Past decades have seen the theory and practice of statistical ecology merge into a unified, coherent, and robust framework for scientific inquiry using time series of animal abundances (Newman et al. 2014, Murray and Sandercock 2020). Stochastic models of the temporal fluctuations of species’ abundances aim to translate fundamental concepts in ecology and evolution into testable hypotheses and predictions that can be confronted with abundance time series datasets. These models decompose the changes in abundances of one, two or more species over time into four main components (Lewontin and Cohen 1969; Athreya and Karlin 1971; Ludwig 1976; Tier and Hanson 1981; Dennis and Taper 1994; Engen, Bakke, and Islam 1998; Ives et al. 2003; Dennis and Ponciano 2014; Ferguson and Ponciano 2014; 2015; Ponciano 2018; Ponciano, Taper, and Dennis 2018). These four components are: 1) basic demographic processes of the study organisms (here bacteria) like reproduction and the effects of density dependence and inter-specific interactions, all of which may depend on current and past abundances of the species in the system; 2) chance variation and individual heterogeneities affecting births and deaths, known as “demographic stochasticity” effects; 3) environmental stochasticity or temporal variation in vital rates (e.g., birth and death rates) that reflect variation in environmental conditions; and 4) observation error and sampling noise. If sampling error is not accounted for then dynamics and processes may be grossly misrepresented. This caveat is particularly relevant in microbial systems (Kareiva, Parker, and Pascual 1996; Dennis et al. 2006; Ferguson and Ponciano 2014; Grilli 2020).
One of the most widespread applications of these stochastic population dynamics models is the characterization of extinction processes and persistence dynamics of species of interest (Lande and Orzack 1988; Dennis, Munholland, and Scott 1991; Boyce 1992; Lande 1993; Foley 1994; Staples, Taper, and Shepard 2005; Chaudhary and Oli 2020). Of particular interest for studies of bacterial population dynamics are recent efforts that explicitly incorporate the effects of interspecific interactions on extinction or persistence processes in experimental microcosms (Ferguson and Ponciano 2014). By including the interactions between species, these models can quickly become intractable. However, if the effects of environmental stochasticity are included, simple models that forego some of the biological complexities can still provide accurate characterizations of the fates of species in a community (Ferguson and Ponciano 2014).
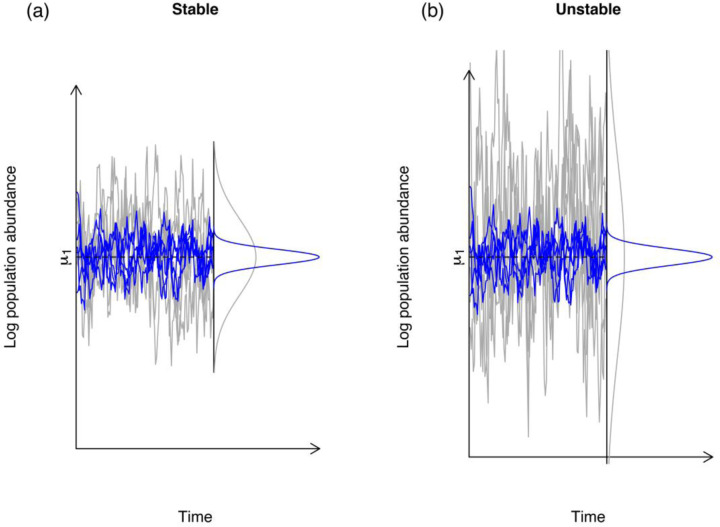
The interaction between environmental stochasticity and intra-specific and inter-specific competition coefficients determine how much population sizes will fluctuate over time (Ives et al. 2003). Changes in the quality of the environment have historically been cast as agents that change population growth rates in mathematical models of population dynamics. These are directly expressed as increases in the mean of the progeny distribution in a population of interest, with a concomitant improvement in the maximum growth rate. Simultaneously, temporal fluctuations in the environment are modeled as a time dependent random variable that will randomly improve or reduce the growth rates of a population (Lewontin and Cohen 1969; Ludwig 1976). However, stochastic contributions to the quality of an environment and overall population dynamics have been found to interact in important ways with ecological processes, such as density-dependence and inter-specific competition (Tier and Hanson 1981; Engen, Bakke, and Islam 1998). Ives et al (2003) showed that the overall effect of environmental fluctuations in the growth of a population is modulated by ecological processes. These authors showed that the growth rate of a population characterized by weak density-dependence was easily affected by fluctuations in the quality of the environment whereas those populations characterized by strong density-dependence were not. When presented with the same temporal regime of environmental variation a population with strong density-dependence will fluctuate much less than a population with weak density-dependence (see Figure 1). Ives et al (2003) went on to show that for a single population, stability could be measured and conceptualized as the ratio of the magnitude of environmental variation to the strength of density dependence. This finding allows for a direct comparison of the reactions of two different populations to the same environmental noise regime. This insight that was brought about by Ives et al. (2003) in the context of community ecology, made it possible to compare different populations and communities on the same level playing field.
Figure 1.
The abundances in stable (a) vs. unstable (b) populations. In both panels the grey lines represent the log-population abundances at stationarity were simulated under the stochastic Gompertz model of Ponciano et al. (2018) under the same environmental noise regime that are shown in blue. The variance of the long run log-population abundances is equal to the ratio of the environmental noise variance (here 0.11) to one minus the squared strength of density-dependence c. This coefficient is stronger on the left than on the right. On the left c = 0.75 and so the log-population size variance is 0.11/(1−0.752) = 0.2514. On the right panel, density dependence is much weaker, with a coefficient equal to 0.93. (Coefficients closer to 1 are close to density-independence.) The variance of the population abundances under the same environmental noise variance is approximately three times higher: 0.11/(1 − 0.932) = 0.8142. The magnitude of the response of a population to environmental noise, in terms of variability, is modulated by c.
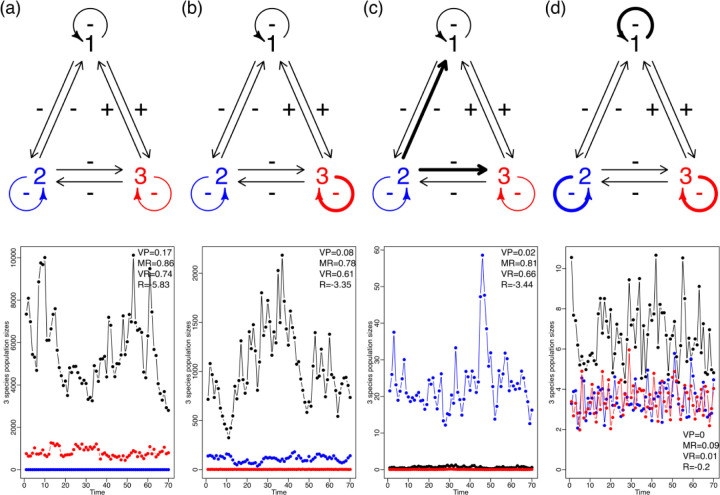
In an ecological community, the influence of environmental noise variance is modulated by the density dependence and the inter-specific interaction coefficients (Ives et al. 2003; Ferguson and Ponciano 2015). This is illustrated in Figure 2. Here we show four different scenarios in which the strength of intra-specific and inter-specific interactions varied while environmental noise remained constant. In each of these scenarios, three species (1, 2 and 3) interact in the following ways: species 1 and 2 and 2 and 3 are competitors and thus have a negative effect on each other. Species 1 and 3 are mutualists and hence have a positive effect on each other (Figure 1). Finally, all the species show negative density dependence. In the first scenario, all the interactions including intraspecific density dependence are weak. In the second scenario only species 3 had strong density dependence while the rest of the interactions were weak. In the third scenario species 2 has a strong negative effect over 1 and 3 but the rest of the interactions were kept weak. Finally, in the fourth scenario we made intraspecific interactions strong while keeping interspecific interactions weak. The coefficients used for each scenario are shown in Table S1.
Figure 2.
This figure extends the simulation shown in Figure 1 to an instance with two or more species for 70 days. It shows that changes in the nature and intensity of community interactions directly affect the stochastic stability, measured using Ives’ et al. four stability metrics: VP,MR,VR and R which are defined and explained in the main text. In this case, the modulation of a variable environment depends on the structure, as well as the nature and intensity of the intraspecific and interspecific interactions. This figure shows the fluctuation in population sizes of four different community structures (a-d) with three species, subject to the same environmental noise regime. The upper row represents the four community structure types. The intraspecific interactions (looped arrows) and interspecific interactions (straight arrows) change in magnitude with weak interactions shown as thin arrows and strong interactions shown as thick arrows. In the row directly below each of these interaction graphs we show the resulting temporal dynamics of the abundances of each species. Since all four simulations were done under the exact same environmental noise regime the differences in magnitude and fluctuation of population abundances across community types can be directly attributed to differences in structure. From left to right, it is shown that weaker interaction strengths lead to larger fluctuations in populations under the same environmental variance. Note the different values of the Y-axis.
We show how the same amount of environmental variance may result in either large or small growth rate variation, depending on the maximum growth rates and those specified interaction strengths (Figure 2). In a community, the strength of the inter-specific and intra-specific interactions and the overall architecture of its assembly is what ultimately modulates the response to environmental variation. Just as in single species population dynamics, the same level of observed variation in the growth rate can result from the populations in a community over-reacting to mild exogenous fluctuations, or alternatively, from a community dampening considerably unusually large environmental variability. Ives et al. (2003) showed that it was possible, through the analysis of multi-species time series, to estimate four different statistics or “stability metrics” (called VP, MR, VR and R in Figure 2 (and as explained below) that would allow the comparison of multiple communities in the face of the same magnitude of environmental noise. In essence, and without entering into mathematical details, these authors showed that it was possible through these metrics to obtain a standardized measure of the reaction of a community to such noise. These findings also imply that deeming a particular set of time series as representative of “stable” or “unstable” dynamics just by its overall variability might be misleading and conflate the fundamental processes governing the dynamics of an ensemble of interacting populations.
THE “MAR” MULTI-SPECIES STOCHASTIC POPULATION DYNAMICS MODEL
Twenty years have elapsed since Ives et al. proposed their modeling approach (the “MAR” model) yet its use in microbiology has seldom been considered. We believe that the MAR model provides benefits in terms of understanding, classifying, and predicting the dynamics of bacterial abundances that have seldom been clearly presented in the context of microbial communities. What follows is an effort to explain these benefits.
The MAR model is a discrete-time Markov process that is deeply rooted in stochastic population dynamics modeling theory (Ives et al. 2003). It jointly models three processes that determine the variation in abundance of the species in a community through time: 1) a deterministic density-dependent population growth for every species in the system on a log-scale, 2) the effect of every species on the growth rate of any other species and 3) the effects of environmental variation on the growth rate (see Ives et al 2003 for a full model description). This stochastic model has as its deterministic counterpart, the multispecies Gompertz density-dependent model, which has been widely applied to estimate bacterial growth (see Dennis and Ponciano 2014 and citations therein). The MAR model is amenable to simulations via recursion because the total abundance of any species in one time step only depends on the abundance of all the species in the previous time step. Thus, the time series data can be modeled using its linear, multivariate recursion and representation
is a vector of the log-population abundances at time , is a vector whose elements give the intrinsic rate of increase for each species in the system, is a squared matrix, whose elements denote the effect of the abundance of species on the growth rate of species . Finally, represents a vector of stochastic, environmental factors varying independently from one time step to the next. These factors are modeled with a multivariate normal distribution with mean 0 and variance-covariance matrix . Through this variance-covariance matrix the modeler can specify whether the response to environmental variation is independent from one species to the next or not, and if not, any covariance structure could be added. In macro-ecological communities for instance the response to the environment from one species to the next might be phylogenetically constrained. In bacterial communities, these phylogenetic constraints likely directly translate into explicit functional constraints, since any given strain might be better at doing something the others cannot do (Ma et al 2020). The MAR model can be viewed as a linear, first approximation to a complex, multi-species population dynamics process of the form , where the species population abundances at time are given by some transformation of the abundances on the previous time step. Specifically, it can be shown that the community matrix of such a complex process has eigenvalues that are identical to the matrix of the MAR model. The diagonal elements of , , which represent the intra-specific, density-dependent effects also satisfy the three existing theoretical definitions of the strength of density dependence (see Ponciano, Taper, and Dennis 2018): the marginal effect on the per capita growth rate of an increase in density (Holt 1985; Holt and Barfield 2012), the derivative of the recruitment map at equilibrium (Holt and Barfield 2012) and the negative elasticity at equilibrium of the per capita population growth rate with respect to change in the population (Lande et al. 2002). The latter measure is readily extendable to scenarios dealing with more complex life histories (Lande et al. 2002).
Jointly, the model matrices and hold the key to formulate standardized measures of how a community reacts to environmental variation. These measures ultimately depend on the nature and intensity of the intra- and inter-specific interaction coefficients (Ives et al. 2003; Dennis et al. 2006; Ferguson and Ponciano 2015; Ponciano, Taper, and Dennis 2018). Ives et al. (2003) derived four standardized metrics based on the and matrices and their eigenvalues. Variance Proportion (VP) quantifies how the long-run variance of the population compares to the variance of the environmental noise process. It is a summary of how the environmental noise distribution in blue in Figure 1 compares to the population size distribution in gray in Figure 1. As Figure 2 shows, differences in variability in the multi-species time series can be directly attributed to species interactions. In a stable system the interactions among species that modulate changes in population sizes in a community from one generation to the next will be such that they cause the variance of the population abundances to be only slightly larger than the variance of the environmental noise (see Figure 1 for an example with a one-species system). On the other hand, in a less stable system the species interactions greatly amplify the environmental variability thereby generating large population fluctuations (Ives et al. 2003). This amplification can be directly measured by the eigenvalues of the matrix , namely, by where is the number of species in the system (see Ives et al. 2003 eq. 24 and subsequent paragraph). In the face of environmental variation, the growth rate of a population will react. This reaction is modulated, or filtered, by the intra-specific and inter-specific competition coefficients (Ives et al. 2003).
The Mean Return time (MR) and the Variance Return time (VR) refer to the amount of time that it takes the system to return to its stationary distribution. It’s the stochastic equivalent of the deterministic return time (Ives et al 2003). Specifically, it refers to the rate at which the transition distribution of the system converges to its stationary distribution. The shorter the time, the more stable the community is. Finally, Reactivity (R) is a measurement of how far the system pushes away from its equilibrium after it is perturbed and as Ives et al. argue, can be computed in two different ways, giving a total of four metrics of stochastic stability.
METHODS
Fitting the MAR model to extensive time series of microbial abundances presents at least three major methodological challenges: The first is determining whether there exists enough information in the data to estimate the MAR model parameters. This question boils down to determining which time series length is sufficient to provide statistically sound parameter estimates. The second methodological task is separating the environmental process variability from sampling noise. The third one is dealing with missing data points: incomplete time series are commonplace in these ecological studies. In what follows, we detail our approach to these three problems.
Minimal sample size to fit a MAR model
The quality of the statistical fit of the MAR model depends on the amount of information present in the multi-species data set. This information can be measured through the statistical properties and diagnostics related to the model parameter estimates. The statistical quality of the parameter estimates is in turn related to how many data points per parameter, or “degrees of freedom” one has available to do model fitting. Another way to think about quantifying this information is by computing the ratio of data to the number of unknown parameters. Ives et al. (2003) model is, however, quite data-hungry: Let be the number of species in the data set. The vector of maximum growth rates has unknown parameters. The matrix of interactions and the variance-covariance matrix of the environmental fluctuations have each unknown parameters, thus, the total number of model unknowns is . With 13 species this number is 351. This number can be compared to the available number of independent data points in order to gauge if one has enough “degrees of freedom” for estimation.
Because this model is Markovian, every time-step transition (change in population abundance) is an independent data point. The likelihood function of the MAR model, from where its parameter estimates are derived, is therefore computed as the product of all the observed transitions (Ives et al. 2003). This likelihood is maximized to obtain the parameter estimates. If is the length of the time series (70 in our case, see below), then the number of transitions that can be used for the maximization of the likelihood function is . If is the number of replicated samples per species per time point, then the number of data points available for parameter estimation is simply . Consequently, for the estimation to be feasible, one needs to verify that . On the other hand, solving for in this inequality gives the minimum sample size (time series length and/or number of replicates per time step) needed to ensure estimability as , which is equal to in the common case where . For example, with 13 species and one replicated time series with no observation error, and the ratio of observations to number of parameters is . Finally, this thinking can be extended by including the parameters needed to decompose biological (process) variation from sampling error variation.
Statistical decomposition of the sources of temporal variation
In this study, we decompose the changes in abundances of species over time into three of the four main components mentioned above (Dennis et al. 2006; Dennis and Ponciano 2014; Ferguson and Ponciano 2014): 1) Population growth, density dependence and inter-specific interactions, or predictable changes in births and death due to current and past abundances of the species in the system 2) environmental stochasticity or (random) temporal variation in vital rates representing variation in environmental conditions (good/bad times for survival and reproduction and 3) observation error and/or sampling noise which if left unaccounted can lead to grossly misrepresented dynamics (Kareiva, Parker, and Pascual 1996; Dennis et al. 2006; Dennis and Ponciano 2014). Demographic stochasticity, the fourth component, although not included in this first phase of our studies can be accommodated in time series estimation methods (Newman et al. 2014).
State-space models, widely known as statistical hierarchical models, allow decomposing the biological and sampling sources of variation using a one pass statistical fit (Ponciano 2004, Dennis et al 2006, Ponciano et al 2009, Newman et al 2014). Stochastic population models with added observation error are just one example of this wide class of models. Although these models are routinely used (Auger‐Méthé et al. 2021), it has long been known that their fitting isn’t without statistical difficulties due to parameter identifiability problems, among others (Ponciano et al. 2005, 200; Dennis et al. 2006; Knape 2008; Lele, Nadeem, and Schmuland 2010). Recently, statistical ecologists have extensively documented and demonstrated such challenges (Lele 2020; Auger‐Méthé et al. 2021). Further studying the statistical and scientific merits of different computer intensive approaches to obtain either the maximum likelihood estimates (via Data Cloning, the Laplace approximation, the Geyer-Thompson likelihood ratio algorithm, Monte Carlo integration to name a few) or the Bayesian posteriors as well as Bayes Factors for these state-space models is a task that merits its own, separate efforts and goes well beyond the conceptual scope of this manuscript. In any the case, our research group in collaboration with microbial ecologists and evolutionary biologists has extensively compared experimentally derived population dynamics parameter estimates with those obtained via maximum likelihood fitting of multi-species/types models and thus verified experimentally and theoretically the reliability of this approach (De Gelder et al 2004, 2007, Ponciano et al 2007, 2009, Loftie-Eaton et al 2016, 2017).
Our present approach to fit a multi-species population model to a bacterial community time series data set was as follows: first, we estimated the most likely location of the true, unobserved abundances with sampling error removed, along with their confidence intervals using the Kalman estimation methodology developed by Dennis and Ponciano (2014). This methodology simultaneously accounts for sampling error and missing data points in the time series of abundances. The resulting observation-noise filtered time series of abundances were then used to fit the MAR stochastic population dynamics model for the entire community. While doing this second fit, the statistical uncertainty resulting from the first observation error step was propagated via parametric bootstrap (Taper et al. 2021). Separating the estimation of the observation error from the biological process error allowed us to be sure at each step that the Mean Squared Error (MSE) of the model parameters were adequate via extensive simulations (github.com/jmponciano/StochasticMicrobiome).
RESULTS
From statistical ecology theory to practice: insights from a case study
In what follows, we applied the theoretical insights described above to an extensive data set of dynamic vaginal microbial communities. We then contrasted the resulting inference with the traditional practice of using the presence of a particular bacterial species at certain abundances from a snapshot of a bacterial community to imply etiology. We contend that such practice may in the end obscure, rather than illuminate our understanding the effects of different bacterial community compositions simply because population abundances can and often do vary widely over time. Additionally, we show how the concepts explained above contribute to answer questions of practical interest. For example: under which ecological scenarios (i.e,. set of inter-specific and intra-specific interactions) will the abundances of species in a community quickly return from their current state to one where variation and composition regimes imply low health risks. How can the concept and measurement of “stochastic stability” contribute to estimate persistence probabilities?
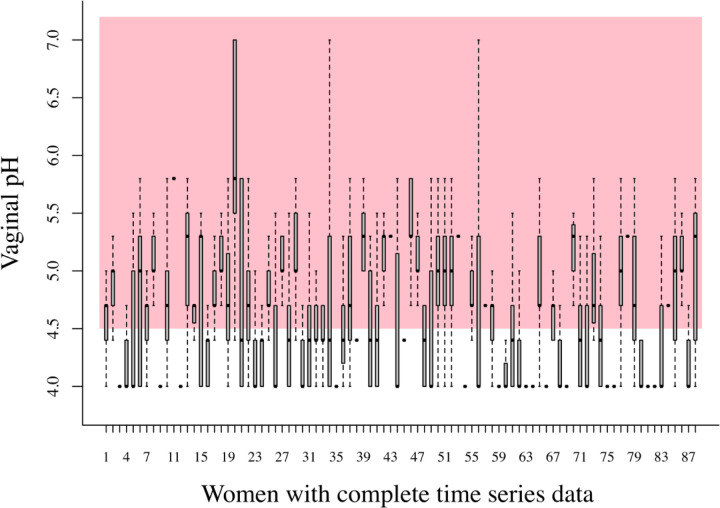
The data we analyzed to exemplify the application of statistical ecology concepts were part of the Human Microbiome Project funded by the National Institutes of Health in which 135 women (see Clinical Study Methods in Supplementary Material). Women enrolled in this study self-collected daily mid-vaginal swabs for 10 weeks. We examined temporal changes in the composition of vaginal communities established using 16S rRNA gene sequencing. Every day after swab collection each participant also measured vaginal pH (see Ravel et al. 2011 for pH measurement methods). A simple examination of the temporal variation of pH in these samples (Figure 3) clearly illustrate the fact that ample temporal variation in the dynamics of bacterial populations and their metabolic activities were the rule, rather than the exception. Important feedback loops between pH levels and bacterial metabolic activity are expected (Ravel et al. 2011) and these processes can be examined with our theoretical approach, as we explain below.
Figure 3.
Boxplots of all the pH measurements taken over 70 days for 88 women in our bacterial community time series data which were complete enough to estimate the MAR model parameters, as illustrated below. The boxplots here showcase the fact that the vaginal pH of 88 healthy, asymptomatic women varied widely over 70 days, inside and outside what is considered to be a healthy vaginal pH region (shaded pink and region < 4.5).
Minimal sample sizes to fit a MAR model
Using the diagnostic tool presented in the Methods section, we determined which time series data sets had enough data to be able to estimate the MAR model parameters. Computing the ratio of available data to the number of parameters to be estimated, we determined that 88 community time series out of the 135 total available could be reliably used for a full, multi-species population model-fitting analysis. The rest of the analyses presented here are based on these 88 community time series data sets.
STATISTICAL DECOMPOSITION OF THE SOURCES OF TEMPORAL VARIATION
Estimating the interaction coefficients: compositional data vs abundance data
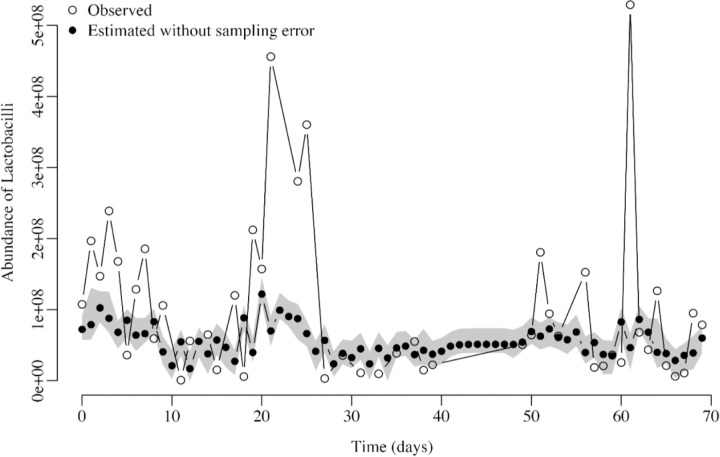
We proceeded to fit the MAR model using the estimated population dynamics time trajectories without sampling error for all species and all data sets (Fig 4). While doing this second fit, the statistical uncertainty from the first step was propagated via parametric bootstrap. However, for this second step microbiologists usually face a key practical decision: should they only work with relative abundances of species or work with both, relative and estimated total abundances. For our case, the total abundance is the number of 16S rRNA gene copies per sample established using quantitative PCR. To make an informed decision as to which approach to undertake, here we simulated time series community abundances under the four scenarios shown in Figure 2. We then verified which interaction strength estimates were less biased (whether those resulting from using the compositional data or those resulting from using the total abundances). Although the relative abundances of species in a community (i.e., compositional time series data) are sometimes the only time series data available, our simulations showed that using compositional data leads to biased estimates of the interaction strengths specified in the matrix of the MAR model.
Figure 4.
Observed Lactobacillus species abundances with error (empty circles) vs. estimated abundances (black circles) after accounting for sampling error for one time series. The gray area shows the 95% confidence interval of the estimated true abundances.
Our simulation approach was as follows: first, we selected the four community scenarios described in Figure 2 and simulated for each case 1000 time series of the abundance of the three taxa A, B and C. We then estimated the interaction coefficients using the MAR model described above fitted to both the relative abundance time series and the absolute abundance time series. The absolute abundances were estimated by anchoring the proportions into total abundances at each time step. Next, we fitted the MAR model to estimate the interaction coefficients using both the 1000 time series of relative abundances and the 1000 time series of total abundances. Then, we calculated the ratio between the estimated and the true interaction strength in each case. When the interaction coefficients were estimated appropriately, a boxplot centered at 1 with a small variance resulted. We estimated if each ratio between estimated and true coefficients departed from an expected value of 1. The results of this simulation experiment (see Figures S1–S4) clearly show that when the total abundances are used, the relative bias boxplots are centered around one. When compositional data is used, those boxplots have a much wider interquartile range and most of the time, are not even centered around one. Thus, fitting the MAR model to compositional data tends to lead to severely biased estimates of the interaction strengths. Therefore, the best approach to estimate the interaction coefficients is to use total abundance data.
In longitudinal studies of microbiomes, the number of 16S rRNA gene copies only provides estimates of the absolute abundance of taxa and not the true abundance of each bacterial species. Our simulations demonstrated that estimating the strength of intra-specific and inter-specific interactions based on relative abundance data results in biased estimates of the interaction strengths. Hence, we performed a pan-bacterial qPCR assay to quantify the total 16S rRNA gene copies in each of the samples, which estimates the absolute bacterial abundance in each sample. Estimates of true abundance were then calculated for each taxon by multiplying relative abundance by total 16S rRNA gene copies. The qPCR assays were done in triplicate for each of the 135 women to document the variability in the abundances of species due to observation error.
We fitted the three different multi-species population dynamics assemblies/model variants using the MAR model of Ives et al. (2003) and the de-noised time series data sets. The first model variant consisted of using all 13 species mentioned above. The second model variant required fitting a three-species model where we grouped all four Lactobacillus species into a single ecological species, Gardnerella vaginalis as the second species and the other eight species grouped into a third species. For the third variant we fitted a simple 2-species model with all four Lactobacillus species grouped as the first species and the other nine species grouped as the second taxon.
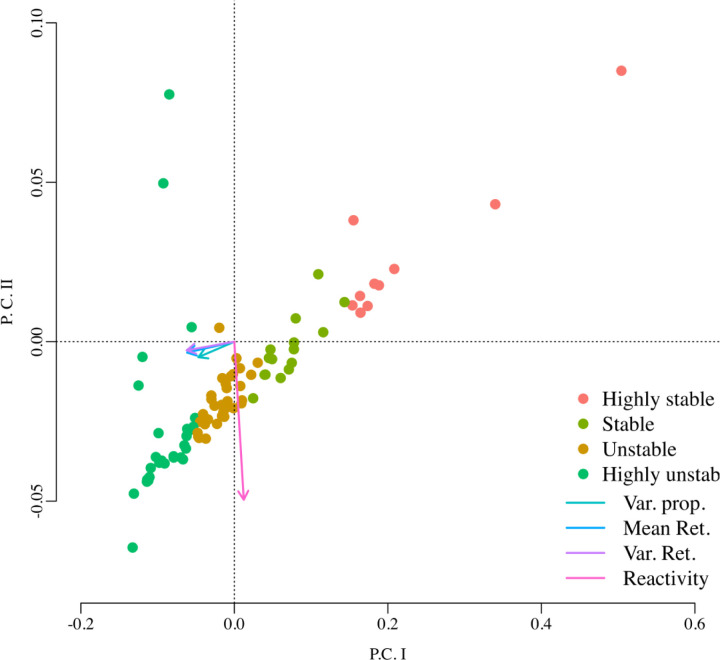
With the MAR model parameter estimates for each model variant (13 species, 3 species and 2 species models), we computed Ives et al. stochastic stability metrics. For each one of the three cases, we then classified the 88 vaginal bacterial communities into four different stability categories using a Principal Components Analysis (PCA) on their estimated stability metrics. Using k-means clustering on the resulting PCA scores for these women and the fact that for all these metrics, lower values indicate higher stability, the 88 bacterial communities were classified into four different categories: Highly stable, stable, unstable, highly unstable. The best classification scheme out of the three different multi-species models corresponded to the two-species MAR model where we pooled all the 4 Lactobacillus species into the first taxa and the other 9 species into the second species (the performance criterion to pick a best classification scheme was the amount of variance explained by the analysis). This classification scheme is shown in Figure 5.
Figure 5.
Principal component analysis (PCA) performed on the four stochastic stability metrics estimated for the vaginal bacterial community time series data of 88 women. In this analysis the samples (rows) correspond to each woman and the four columns (variables in the PCA analysis) correspond to the four stability metrics estimated by fitting the MAR model of Ives et al. The arrows’ lengths and direction represent the strength of association of each one of these four metrics with the principal component axes: the variance proportion, the mean return time and the variance return time are highly associated with the first principal component while the reactivity is highly associated with the second principal component. Using k-means clustering, the PCA scores of these 88 bacterial communities were classified into four groups. Because lower values in these stability metrics indicate higher stochastic stability, an examination of the magnitude of these four metrics in each one of these four groups suggested the labeling of highly stable, stable, unstable and highly unstable dynamics (see Supplementary material for details).
Estimation of persistence dynamics from the MAR model parameter MLEs
Alone, our stability classification scheme is an insufficient approach to understand multi-species population dynamics because the “stable” and “unstable” attributes are given here to a community without regard for the health risks associated with its composition. Stability, which is a property of dynamic systems, should not be equated with desirable or undesirable behavior in terms of health outcomes because one community can have stable population dynamics but sustain a low relative abundance of a strain, thus bringing high health risk. Thus, considering overall abundance and composition in addition to stability is needed in order to assess the desirability of a particular community dynamics. Indeed, Klatt et al (2017) show that when the relative abundance of Lactobacillus dwindles down below a 0.5 proportion, the bacterial community is under a high risk of infection by HIV. On the other hand, as the relative abundance of Lactobacillus moves above 0.5, the risk of infection decreases. Seeking to elucidate which type and magnitude of ecological interactions would lead to desirable dynamics (i.e. fluctuations in relative abundance of Lactobacillus above 0.5) is a reachable target under our analysis using the MAR model. If attaining a sustained high relative abundance of Lactobacillus over time is a health-management target as in Klatt et al 2017 (see Figure S5), then we contend that our approach described next should be used.
We developed a Risk Prediction Monitoring (RPM) tool that estimates the temporal changes in persistence probabilities. This method mirrors conservation biology approaches for population monitoring in which a metric measuring extinction risks is periodically updated with any change in the population called Population Viability Monitoring, or VPM (Staples, Taper, and Shepard 2005). Before explaining and implementing our RPM tool, we first explain how the well-known VPM method from Staples et al. works and apply it directly to one of our 88 data sets to exemplify it. Immediately afterwards, we fully develop and implement our RPM method.
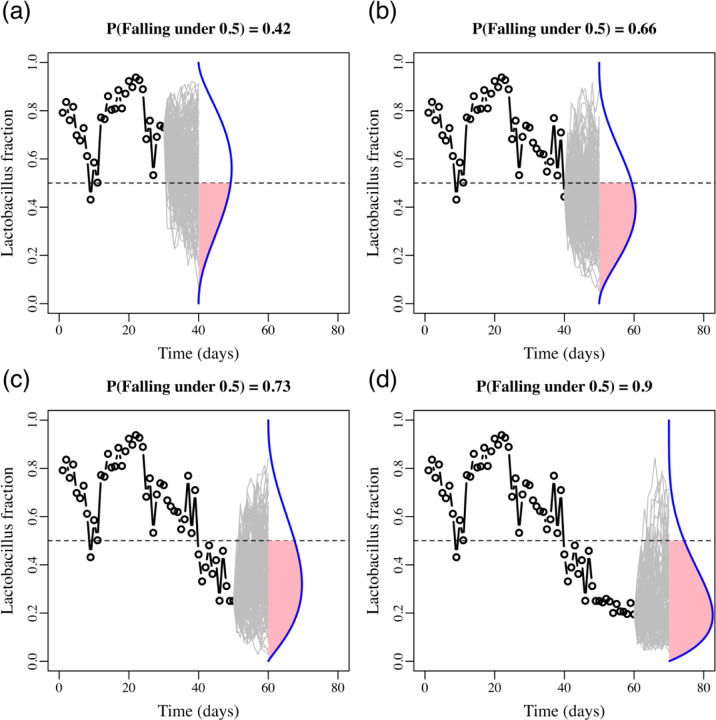
The VPM method consists of serially estimating the persistence probabilities with every data point added to the current length of the time series of population abundances. For annually reproducing species, with every year that passes a new total abundance is recorded. With it, an updated estimate of extinction risk is computed. Repeating the same process for multiple years yields a temporal trend of extinction risks. Consider the following example from conservation biology: if population abundances of a threatened species are available for the past 30 years, and if managers want to check whether as of late (e.g., for the past 10 years), the extinction risk of the population has been increasing or decreasing, then the following is done (Staples, Taper, and Shepard 2005): First, a stochastic population dynamics model is fit using the first 20 years of the data. With the model parameter estimates and the data up to year 20, the probability that the population will crash below a critical threshold within the near future, e.g., during next 5 years, is computed. The resulting probability is recorded. Next, the observed population size for year 21 is added to the time series. The model parameters are then re-estimated and the probability that the population will crash sometime during the next 5 years after year 21 is computed. That probability is also recorded. Iterating this process for 10 more years yields a time series of the extinction risks for the last 30 years.
Here we exemplify the conservation biology method with one of our 88 bacterial community datasets. For our vaginal bacteria data set, our target was to track the probability that the proportion of all the Lactobacillus species in a vaginal bacterial community drop below 50%. Our time unit in this case is days, as new swabs were collected daily. The abundances for all bacterial taxa, as well as for the proportion of Lactobacillus species were available for 70 time-steps. In Figure 6, upper left panel, we first fitted the stochastic multi-species Gompertz model of Ives et al. (2003) to a single time series of observed abundances and proportions of Lactobacillus up to day 30 (black empty circles). We did so by placing all Lactobacillus taxa as one type in the model and all other species were pooled together as a second type. We then used the model parameters to project in the next ten days the Lactobacillus abundances and their proportion in the population 50,000 times (grey lines). The proportion of such projected trajectories that dropped below 50%, which was 0.4 in the upper left panel, is an estimate of the Lactobacillus persistence probability above 50% during those ten days. With every passing day, this estimate was updated. In the next three panels (upper right, lower left and then lower right) we show these simulations for only days 40, 50 and 60, but daily changes in persistence probabilities for days 30 to 70 were computed.
Figure 6.
Population viability monitoring and estimating the temporally varying chances of Lactobacillus persistence. Illustrated is an example of Risk Prediction Monitoring (RPM) using stochastic population dynamics models.
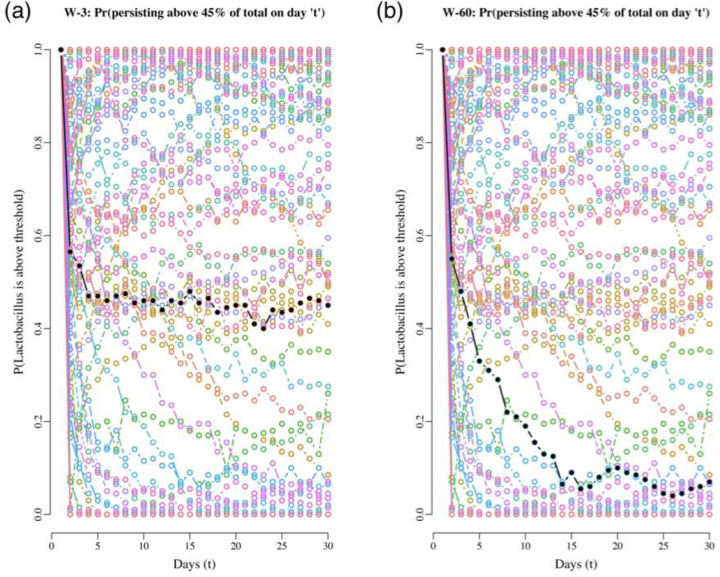
The VPM method illustrated above for our data set is essentially retrospective but here we devised a prospective modified version of it, one that allows comparing the dynamics of multiple communities in the near future. Furthermore, we switched the estimation focus from tracking the probabilities of crashing below a population size or proportion threshold to follow their complement, persistence probabilities. This modified method links our stability metrics with the risk assessment task and is what we call the RPM tool. We developed the RPM tool because we faced the problem of assessing the risk dynamics for all 88 communities and being able to evaluate these under the same level playing field. To do such comparisons, we chose to evaluate the risk dynamics all while answering the question: How would the risk of Lactobacillus spp. falling below 45% change over the next 20 days if all communities were started with the same proportion (50%) and then monitored over the next 20 days? We answered this question by implementing these steps: First, we retrieved the MAR model parameter estimates for all 88 communities. Using these estimates, we computed the MAR model predicted mean abundance of Lactobacillus at stationarity for every case. We then set these mean abundances as the starting abundances for a 20-day projection in each case. Additionally, we assumed that the starting total abundances for the non-Lactobacillus taxa in all these projections were equal to these abundances. Thus, if in one case the mean abundance at stationarity of Lactobacillus was predicted to be 3.5×108 16S rRNA gene copies per swab, the starting mean abundance of the non-Lactobacillus species were assumed to be identical, 3.5×108 16S rRNA gene copies per swab. With these starting values, we computed the mean projected abundances for the next 20-day trajectories and used these to numerically estimate via simulations the probability that the Lactobacillus taxa would remain above 45% on day for . The resulting trends in Lactobacillus persistence probabilities are shown in Figure 7.
Figure 7.
Projecting the probability of Lactobacillus persisting above 45% for two women, starting at 50/50 from their carrying capacities for 20 days. The black dots are the projected probabilities of persisting for woman 3 (on the left) and woman 60 (on the right), for 30 days. As a background and in different colors, the same trend for all the other women in the study is shown. The wide array of trajectories of all the trends for the other women emphasizes the wide variability in predicted community dynamics.
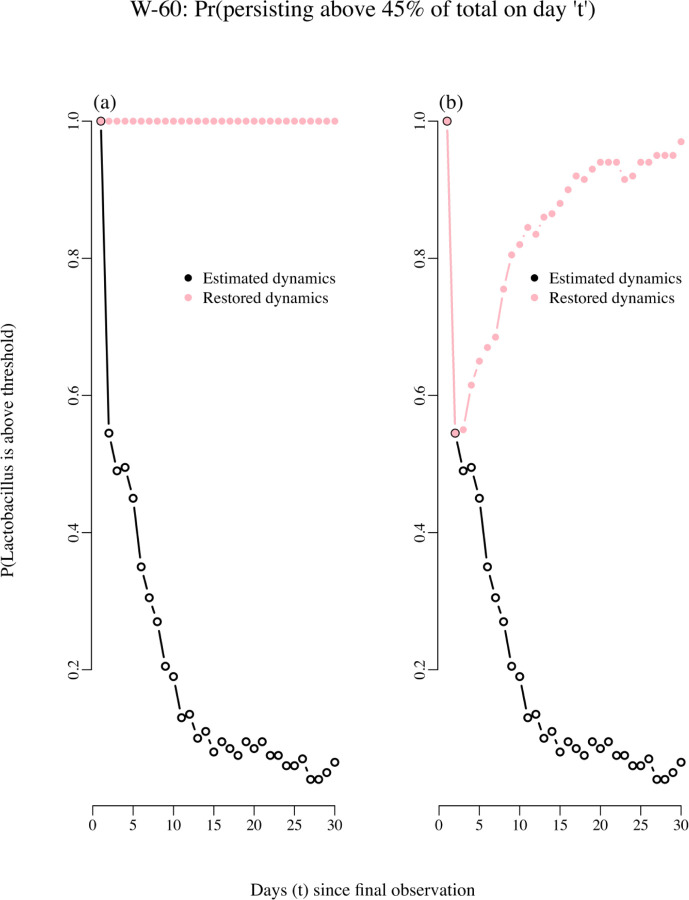
Our RPM tool was used in conjunction with the estimated matrix to identify which interaction coefficient drove each persistence trend. We found that a decaying persistence trend of a bacterial type of interest was explained by whether other bacteria impacted negatively or positively its growth, which is the information contained in . We illustrated this finding using the resulting decaying RPM trend for woman 60 (right panel of Figure 7). The estimated two-by-two matrix of interactions for woman 60 is as follows: the one-step total effect of non-Lactobacillus species on the per capita growth rate of Lactobacillus species had a negative coefficient, −0.39. On the other hand, Lactobacillus species had a small positive effect on non-Lactobacillus, 0.001. Therefore, while the presence of non-Lactobacillus taxa had a negative density dependence effect on the growth of Lactobacillus, while Lactobacillus had a positive effect on the growth rate of non-Lactobacillus. In the end this asymmetry negatively affected the growth of Lactobacillus. In both cases, the strength of the intra-specific density dependence was weak (0.86 for Lactobacillus and 0.76 for non-Lactobacillus species). To verify whether that asymmetry in the inter-specific growth rate effects was what drove the decay in persistence probabilities for the Lactobacillus taxa, we did two numerical experiments: for the first experiment, we simply switched the sign of the effect of one group on the other, so that non-Lactobacillus had a positive effect of 0.39 in the growth rate of Lactobacillus and in turn, Lactobacillus had a negative effect of −0.001 on the growth rate of non-Lactobacillus taxa. Next, we re-computed the RPM trend in persistence probabilities using this modified matrix of interactions. The resulting RPM trend of persistence probabilities, plotted in pink in Figure 8, remained at 1 for the next 20 days. The second numerical experiment consisted of artificially increasing the maximum growth rate of the Lactobacillus taxa while leaving the matrix of interactions unchanged. Then, a restored trend in persistence probabilities was also obtained (Figure 8).
Figure 8.
Probability of persisting above 45% of total abundances “t” days into the future. As explained in the text, the changes in the estimated coefficients of the interactions matrix results in restored dynamics and persistence probabilities. In panel A the effect of Lactobacillus on non-Lactobacillus was changed from positive to negative and the effect of non-Lactobacillus on Lactobacillus was switched from negative to positive. In panel B only the maximum growth rate of the Lactobacillus species was increased by 25%.
DISCUSSION
This study constitutes an unprecedented integration of ecological, mathematical, statistical, and conservation biology principles to understand and predict the dynamics of an extensive microbial community’s time series data set. In the past few years, the complex nature of microbiome data has brought together an ever-growing number of multi-disciplinary research teams (Qian, Lan, and Venturelli 2021). Yet, the fast pace of modern methodological research in microbiome studies contrasts sharply with the paucity of population dynamics studies seeking to understand from basic principles the benefits, or perhaps shortcomings, of novel data analysis techniques. The main motivation of this study was the fact that by and large, variability in microbial time-series data is still perceived as “statistical noise” rather than as an intrinsic property of the growth of bacterial communities. Phrasing through the MAR model variability over time as an intrinsic property of a growing population allows linking concepts like the strength of intra-specific and inter-specific competition to the qualitative response of a population in the face of uncertain environments. Not only can these competition coefficients be estimated, and the stability of the system assessed by fitting the MAR model, but the chance of persistence of bacteria taxa can be further assessed. To our knowledge, this is the first study that demonstrates how persistence probabilities of bacteria of medical and ecological interest can be estimated and even manipulated by identifying which interaction coefficient strengths are their main drivers. We thus demonstrate how the apparently simple stochastic multi-species time series model of Ives et al. (2003) can be used beyond its original applications to approach some of the most pressing questions regarding the monitoring of bacterial communities (Bardgett and Caruso 2020).
Vaginal communities dominated by species of Lactobacillus have been associated with health and a reduced risk to diseases such as bacterial vaginosis or sexually transmitted infections. The notion that dominance of Lactobacillus is associated with health is deeply engrained in the field of women’s urogenital health and strongly supported by the findings of numerous studies (Chee, Chew, and Than 2020; Witkin and Linhares 2022). Regrettably, the converse — that low proportions or the absence of Lactobacillus is unhealthy — has also permeated the field’s lexicon. This is a logical fallacy of denying the antecedent (Gaul 2018), that essentially argues that if healthy women have vaginal communities dominated by Lactobacillus, then the absence of Lactobacillus in vaginal communities is, of itself, unhealthy. This claim is refuted by the findings of numerous studies on the species composition of healthy, asymptomatic women that have shown that a significant proportion of healthy asymptomatic women have vaginal communities with low proportions of Lactobacillus (Saraf et al. 2021; Gosmann et al. 2017; Anahtar et al. 2018). Instead, they are dominated by various species of strictly and facultatively anaerobic bacteria such as Gardnerella vaginalis, Mobiluncus, Prevotella, Brevibacterium, Peptoniphilus and others (Onderdonk et al 2016). With that said, it should also be recognized that low proportions of Lactobacillus in vaginal communities are associated with an increased risk to disease (France et al 2022) though it is not a disease state per se. Nonetheless, investigators have often referred to these communities as being either abnormal (Green, Zarek, and Catherino 2015), out of balance (Olesen and Alm 2016), or in a state of dysbiosis (Hooks and O’Malley 2017) that somehow needs to be corrected. We posit that except for symptomatic bacterial infections, all other states are ‘healthy’ and in many instances they are ‘normal’ (meaning they are often observed) though they may differ in terms of risk to disease.
Most studies on the species composition of vaginal bacterial communities have employed cross-sectional designs that yield point estimates of community composition. It seems to be assumed that the species composition of communities is rather invariant over time in the absence of some sort of natural or unnatural environmental disturbances such as menstruation or the use of lubricants (Gajer et al. 2012; Wilkinson et al. 2019; O’Hanlon et al. 2021; Łaniewski et al. 2021). Contrary to this assumption, longitudinal studies have shown that the vaginal microbiota of many women is dynamic and often transition through states in which Lactobacillus spp. are lacking (Gajer et al. 2012; Lewis, Bernstein, and Aral 2017). These states vary in frequency and duration and are therefore associated with varying levels of risk for urogenital infections and other maladies. One could reasonably consider these to be windows of elevated risk that can open and close, sometimes over very short periods of time.
Our PCA and MAR model-based stability classification scheme (Figure 5) takes a first, admittedly imperfect step toward process-based management of bacterial community dynamics and rigorous use of the term “stability”. Although previous community classification schemes using PCA relied on patterns of abundances, our approach relies on inferred ecological processes from the time series of abundances. As theory and current practice in conservation biology show (Murray and Sandercock 2020), the longer the multi-species time series data, the better the information regarding species interactions in a community can be better teased apart. Here we went one step further and estimated how these inferred interactions ultimately govern the community response to environmental variability. The nature of such response was quantified with Ives et al.’s (2003) four stability metrics and the PCA in Figure 5 separates bacterial communities according to these metrics (see Supplementary material). Thus, the position of each bacterial community in PCA space is determined by the strength of ecological interactions. If one community is found to be largely unstable, an analyst can peer into the nature and intensity of those estimated interactions and change them one by one to move the community in PCA space from an unstable group into another classification group. In other words, an investigator can test statistical hypotheses regarding which interactions are responsible for one or another stability classification result. Identifying interactions that render a community stochastically stable can be the first step in a research agenda that seeks to understand how to guarantee such stability by modulating the strengths of interactions. Our RPM approach is a natural extension of our stochastic stability inferences. It is an easy-to-understand approach to approximate the time-dependent persistence probabilities of the bacterial species of interest. As Olesen and Alm (2016) have argued, tools like our RPM approach that focus on prediction rather than simply the detection of differences are needed, and here we deliver on that particular need.
Using this persistence probability methodology in studies of the vaginal microbiome would mirror an approach called Population Viability Analyses that has been successfully used in conservation biology for many years (Chaudhary and Oli 2020; Ponciano, Taper, and Dennis 2018). Unlike the majority of cases in conservation biology our model choice (the multivariate, stochastic Gompertz with environmental stochasticity and added sampling error; Ives et al. 2003; Dennis et al. 2006; Dennis, Ponciano, and Taper 2010) has been extensively tested in a recent theoretical-simulation study (Ponciano, Taper, and Dennis 2018). Estimates of the strengths of interactions can be used to formulate hypotheses regarding the molecular mechanisms and genetic composition that underpin different types of interactions. By plotting the variability in the sign (positive or negative) and intensity of interaction coefficients (for example, the effect of Lactobacillus on the growth rate of Gardnerella or some other species) one can locate and isolate cases where the sign of species interaction relations flip (say from positive to negative) and eventually guide the laboratory determination of the genetic composition of strains associated with interaction relationships in every quadrant (Figure S6).
Adopting statistical ecology theory and concepts reveals the inconsistencies of using terms like “dysbiosis” to characterize a microbial community. Dysbiosis is commonly defined as a change in the composition and function of a human microbial community that is typically driven by environmental and host-related factors that exceed a community’s resistance and resilience (Kriss et al. 2018; Kindinger et al. 2016; Borgdorff et al. 2016; Levy et al. 2017). But this definition doesn’t seem to fully fit with what theoreticians in ecology understand as resilience and resistance. Resilience, in one hand, is the rate at which a community returns to a state that existed prior to a change. Resistance, on the other hand, is the magnitude of a community’s response to a given disturbance (Begon and Townsend 2021). Both, resilience, and resistance are built into Ives’ et al stability metrics. Instead of trying to frame a dysbiosis definition into these concepts, it seems much more straightforward to use Ives’ stability metrics directly to classify the stability dynamics of a community, just as we do here. Additionally, in current practice, investigators will often state that ‘healthy’ communities are ‘in balance’ (White et al. 2011; Olesen and Alm 2016; Gupta 2021). This terminology reflects an erroneous assumption that the composition of bacterial communities in healthy individuals is essentially invariant and that changes in the relative abundances of species are necessarily bad and, in some cases, constitute sufficient evidence to classify these variants as disease states. This classification is often done based on pairwise comparisons of a microbiome at two points in time. Except for symptomatic bacterial infections, it seems that all other states are ‘healthy’ and in many instances they are ‘normal’ (meaning they are often observed). These words and phrases are loosely defined and inconsistently used, and this leads to confusion among non-experts. The literature is peppered with examples (White et al. 2011; Olesen and Alm 2016; Gupta 2021). Instead of seeking a definition of dysbiosis we assert that it might be better to translate concepts of theoretical ecology into practical approaches for the management of human-associated bacterial communities. This can be accomplished using concepts and methods that have come to be well known in the fields of population dynamics and conservation biology.
CONCLUSION
Population dynamics as a field in ecology has long touted the theoretical and practical advantages of jointly modeling demography and the influence of the environment and sampling error (Cushing et al. 2003; Dennis et al. 2006), while conservation biology has taken advantage of these ideas and modeling approaches to predict population persistence probabilities (Staples, Taper, and Shepard 2005; Chaudhary and Oli 2020). Here we have shown that the same sort of stochastic population dynamics equations can be used to re-phrase the concept of stability as the magnitude of the reaction to a variable environment. Our work represents the first comprehensive integration of theoretical stochastic population dynamics, unusually long time series of bacterial community abundances and conservation biology principles. This integrated approach resulted in two major steps towards a better understanding of human-associated bacterial communities. First, through the estimation of each bacterial community’s reaction to exogenous variability we achieved a stability-based ecological community classification. Second, we provide for the first time, estimates of the short-term persistence probabilities of bacterial types of medical interest. This result is important because our estimated temporal trend in persistence probabilities can be used to construct an evidence-based inference regarding the fate of a pathogen, for example. Finally, we conclude that a comprehensive examination of the reach of stochastic population dynamics modeling in the field of microbial community ecology is beginning to take shape as a body of work. Our efforts provide a theoretical framework that can very well represent microbial phenomena of interest in a simpler and unified way as effects of a common cause: an alteration of the growth rate of a population by itself, by another population or by the environment.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the valuable input and advice at various stages of this work from Drs Mark L Taper, Brian Dennis, and Robert D. Holt. Funding for this research was provided by the National Institute of General Medical Sciences of the National Institutes of Health, under grant number 1R01GM103604 to University of Florida, with JMP as PI. JMP was also supported by NSF grant No. 2052372 to University of Florida.
Footnotes
Open Research statement: the data used here is available at Ravel et al (2013). The same data is also available at the computer code repository, github.com/jmponciano/StochasticMicrobiome. Upon acceptance, the data will be uploaded to Dryad and the code to Zenodo.
CONFLICT OF INTEREST STATEMENT
JR is the cofounder of LUCA Biologics, a biotechnology company focusing on translating microbiome research into live biotherapeutics drugs for women’s health.
REFERENCES
- Allen Edward J, Allen Linda JS, and Schurz Henri. 2005. “A Comparison of Persistence-Time Estimation for Discrete and Continuous Stochastic Population Models That Include Demographic and Environmental Variability.” Mathematical Biosciences 196 (1): 14–38. [DOI] [PubMed] [Google Scholar]
- Allen Linda JS. 2010. An Introduction to Stochastic Processes with Applications to Biology. CRC press. [Google Scholar]
- Altizer Sonia, Dobson Andrew, Hosseini Parviez, Hudson Peter, Pascual Mercedes, and Rohani Pejman. 2006. “Seasonality and the Dynamics of Infectious Diseases.” Ecology Letters 9 (4): 467–84. [DOI] [PubMed] [Google Scholar]
- Anahtar Melis N, Gootenberg David B, Mitchell Caroline M, and Kwon Douglas S. 2018. “Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity.” Cell Host & Microbe 23 (2): 159–68. [DOI] [PubMed] [Google Scholar]
- Arumugam Manimozhiyan, Raes Jeroen, Pelletier Eric, Paslier Denis Le, Yamada Takuji, Mende Daniel R, Fernandes Gabriel R, Tap Julien, Bruls Thomas, and Batto Jean-Michel. 2011. “Enterotypes of the Human Gut Microbiome.” Nature 473 (7346): 174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athreya Krishna B, and Karlin Samuel. 1971. “On Branching Processes with Random Environments: I: Extinction Probabilities.” The Annals of Mathematical Statistics 42 (5): 1499–1520. [Google Scholar]
- Auger‐Méthé Marie, Newman Ken, Cole Diana, Empacher Fanny, Gryba Rowenna, King Aaron A, Leos‐Barajas Vianey, Flemming Joanna Mills, Nielsen Anders, and Petris Giovanni. 2021. “A Guide to State–Space Modeling of Ecological Time Series.” Ecological Monographs 91 (4): e01470. [Google Scholar]
- Bardgett Richard D, and Caruso Tancredi. 2020. “Soil Microbial Community Responses to Climate Extremes: Resistance, Resilience and Transitions to Alternative States.” Philosophical Transactions of the Royal Society B 375 (1794): 20190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger Kathryn, and Bunge John. 2008. “Bayesian Estimation of the Number of Species Using Noninformative Priors.” Biometrical Journal: Journal of Mathematical Methods in Biosciences 50 (6): 1064–76. [DOI] [PubMed] [Google Scholar]
- Begon Michael, and Townsend Colin R. 2021. Ecology: From Individuals to Ecosystems. John Wiley & Sons. [Google Scholar]
- Borgdorff Hanneke, Gautam Raju, Armstrong Stuart D, Xia Dong, Ndayisaba Gilles F, van Teijlingen Nienke H, Geijtenbeek Teunis BH, Wastling Jonathan M, and van de Wijgert Janneke HHM. 2016. “Cervicovaginal Microbiome Dysbiosis Is Associated with Proteome Changes Related to Alterations of the Cervicovaginal Mucosal Barrier.” Mucosal Immunology 9 (3): 621–33. [DOI] [PubMed] [Google Scholar]
- Boyce Mark S. 1992. “Population Viability Analysis.” Annual Review of Ecology and Systematics 23 (1): 481–97. [Google Scholar]
- Chaudhary Vratika, and Oli Madan K. 2020. “A Critical Appraisal of Population Viability Analysis.” Conservation Biology 34 (1): 26–40. [DOI] [PubMed] [Google Scholar]
- Chee Wallace Jeng Yang, Chew Shu Yih, and Than Leslie Thian Lung. 2020. “Vaginal Microbiota and the Potential of Lactobacillus Derivatives in Maintaining Vaginal Health.” Microbial Cell Factories 19 (1): 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing Jim Michael, Costantino Robert F, Dennis Brian, Desharnais Robert, and Henson Shandelle Marie. 2003. Chaos in Ecology: Experimental Nonlinear Dynamics. Vol. 1. Elsevier. [Google Scholar]
- De Gelder L., Ponciano J.M., Abdo Z., Joyce P., Forney L.J. and Top E.M., 2004. Combining mathematical models and statistical methods to understand and predict the dynamics of antibiotic-sensitive mutants in a population of resistant bacteria during experimental evolution. Genetics, 168(3): 1131–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gelder L., Ponciano J.M., Joyce P. and Top E.M., 2007. Stability of a promiscuous plasmid in different hosts: no guarantee for a long-term relationship. Microbiology, 153(2): 452–463. [DOI] [PubMed] [Google Scholar]
- Dennis Brian. 1989. “Allee Effects: Population Growth, Critical Density, and the Chance of Extinction.” Natural Resource Modeling 3 (4): 481–538. [Google Scholar]
- Dennis Brian, Munholland Patricia L, and Scott J Michael. 1991. “Estimation of Growth and Extinction Parameters for Endangered Species.” Ecological Monographs 61 (2): 115–43. [Google Scholar]
- Dennis Brian, and Ponciano José Miguel. 2014. “Density‐dependent State‐space Model for Population‐abundance Data with Unequal Time Intervals.” Ecology 95 (8): 2069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis Brian, Ponciano José Miguel, Lele Subhash R, Taper Mark L, and Staples David F. 2006. “Estimating Density Dependence, Process Noise, and Observation Error.” Ecological Monographs 76 (3): 323–41. [Google Scholar]
- Dennis Brian, Ponciano José Miguel, and Taper Mark L. 2010. “Replicated Sampling Increases Efficiency in Monitoring Biological Populations.” Ecology 91 (2): 610–20. [DOI] [PubMed] [Google Scholar]
- Dennis Brian, Ponciano José Miguel, Taper Mark L, and Lele Subhash R. 2019. “Errors in Statistical Inference under Model Misspecification: Evidence, Hypothesis Testing, and AIC.” Frontiers in Ecology and Evolution, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis Brian, and Taper Mark L. 1994. “Density Dependence in Time Series Observations of Natural Populations: Estimation and Testing.” Ecological Monographs 64 (2): 205–24. [Google Scholar]
- Engen Steinar, Bakke Øyvind, and Islam Aminul. 1998. “Demographic and Environmental Stochasticity-Concepts and Definitions.” Biometrics, 840–46.
- Faith Jeremiah J, Guruge Janaki L, Charbonneau Mark, Subramanian Sathish, Seedorf Henning, Goodman Andrew L, Clemente Jose C, Knight Rob, Heath Andrew C, and Leibel Rudolph L. 2013. “The Long-Term Stability of the Human Gut Microbiota.” Science 341 (6141): 1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith Jeremiah J, McNulty Nathan P, Rey Federico E, and Gordon Jeffrey I. 2011. “Predicting a Human Gut Microbiota’s Response to Diet in Gnotobiotic Mice.” Science 333 (6038): 101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson Jake M, and Ponciano José Miguel. 2014. “Predicting the Process of Extinction in Experimental Microcosms and Accounting for Interspecific Interactions in Single‐species Time Series.” Ecology Letters 17 (2): 251–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson Jake M, and Ponciano José Miguel. 2015. “Evidence and Implications of Higher-Order Scaling in the Environmental Variation of Animal Population Growth.” Proceedings of the National Academy of Sciences 112 (9): 2782–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley Patrick. 1994. “Predicting Extinction Times from Environmental Stochasticity and Carrying Capacity.” Conservation Biology 8 (1): 124–37. [Google Scholar]
- Gajer Pawel, Brotman Rebecca M, Bai Guoyun, Sakamoto Joyce, Schütte Ursel ME, Zhong Xue, Koenig Sara SK, Fu Li, Ma Zhanshan, and Zhou Xia. 2012. “Temporal Dynamics of the Human Vaginal Microbiota.” Science Translational Medicine 4 (132): 132ra52–132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul Brett. 2018. “Denying the Antecedent.” In Bad Arguments, 46–47. John Wiley & Sons, Ltd. 10.1002/9781119165811.ch3. [DOI] [Google Scholar]
- Gonze Didier, Coyte Katharine Z, Lahti Leo, and Faust Karoline. 2018. “Microbial Communities as Dynamical Systems.” Current Opinion in Microbiology 44: 41–49. [DOI] [PubMed] [Google Scholar]
- Gonze Didier, Lahti Leo, Raes Jeroen, and Faust Karoline. 2017. “Multi-Stability and the Origin of Microbial Community Types.” The ISME Journal 11 (10): 2159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosmann Christina, Anahtar Melis N, Handley Scott A, Farcasanu Mara, Abu-Ali Galeb, Bowman Brittany A, Padavattan Nikita, Desai Chandni, Droit Lindsay, and Moodley Amber. 2017. “Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women.” Immunity 46 (1): 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green Katherine A, Zarek Shvetha M, and Catherino William H. 2015. “Gynecologic Health and Disease in Relation to the Microbiome of the Female Reproductive Tract.” Fertility and Sterility 104 (6): 1351–57. [DOI] [PubMed] [Google Scholar]
- Grenfell Bryan T, Bjørnstad Ottar N, and Kappey Jens. 2001. “Travelling Waves and Spatial Hierarchies in Measles Epidemics.” Nature 414 (6865): 716–23. [DOI] [PubMed] [Google Scholar]
- Grilli Jacopo. 2020. “Macroecological Laws Describe Variation and Diversity in Microbial Communities.” Nature Communications 11 (1): 4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelj Ivana, Joshua S Weitz Tom Ferenci, Horner‐Devine M Claire, Marx Christopher J, Meyer Justin R, and Forde Samantha E. 2010. “An Integrative Approach to Understanding Microbial Diversity: From Intracellular Mechanisms to Community Structure.” Ecology Letters 13 (9): 1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PD. 2021. “The Human Vaginal Microbiota: Boon or Bane.” J of Obstetrics Gynecology and Reproductive Sciences 5 (1).
- Holt Robert D. 1985. “Population Dynamics in Two-Patch Environments: Some Anomalous Consequences of an Optimal Habitat Distribution.” Theoretical Population Biology 28 (2): 181–208. [Google Scholar]
- Holt Robert D, and Michael Barfield. 2012. “Trait-Mediated Effects, Density Dependence and the Dynamic Stability of Ecological Systems.” Trait-Mediated Indirect Interactions: Ecological and Evolutionary Perspectives 89.
- Hooks Katarzyna B, and O’Malley Maureen A. 2017. “Dysbiosis and Its Discontents.” MBio 8 (5): e01492–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives Anthony R, and Carpenter Stephen R. 2007. “Stability and Diversity of Ecosystems.” Science 317 (5834): 58–62. [DOI] [PubMed] [Google Scholar]
- Ives Anthony R, Dennis Brian, Cottingham Kathryn L, and Carpenter Stephen R. 2003. “Estimating Community Stability and Ecological Interactions from Time‐series Data.” Ecological Monographs 73 (2): 301–30. [Google Scholar]
- Ives Anthony R, Einarsson Árni, Jansen Vincent AA, and Gardarsson Arnthor. 2008. “High-Amplitude Fluctuations and Alternative Dynamical States of Midges in Lake Myvatn.” Nature 452 (7183): 84–87. [DOI] [PubMed] [Google Scholar]
- Jordt H., Stalder T., Kosterlitz O., Ponciano J.M., Top E.M. and Kerr B., 2020. Coevolution of host–plasmid pairs facilitates the emergence of novel multidrug resistance. Nature ecology & evolution, 4(6): 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareiva Peter, Parker Ingrid M, and Pascual Miguel. 1996. “Can We Use Experiments and Models in Predicting the Invasiveness of Genetically Engineered Organisms?” Ecology 77 (6): 1670–75. [Google Scholar]
- Kindinger Lindsay M, MacIntyre David A, Lee Yun S, Marchesi Julian R, Smith Ann, McDonald Julie AK, Terzidou Vasso, Cook Joanna R, Lees Christoph, and Israfil-Bayli Fidan. 2016. “Relationship between Vaginal Microbial Dysbiosis, Inflammation, and Pregnancy Outcomes in Cervical Cerclage.” Science Translational Medicine 8 (350): 350ra102–350ra102. [DOI] [PubMed] [Google Scholar]
- Knape Jonas. 2008. “Estimability of Density Dependence in Models of Time Series Data.” Ecology 89 (11): 2994–3000. [DOI] [PubMed] [Google Scholar]
- Kriss Michael, Hazleton Keith Z, Nusbacher Nichole M, Martin Casey G, and Lozupone Catherine A. 2018. “Low Diversity Gut Microbiota Dysbiosis: Drivers, Functional Implications and Recovery.” Current Opinion in Microbiology 44: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande Russell. 1993. “Risks of Population Extinction from Demographic and Environmental Stochasticity and Random Catastrophes.” The American Naturalist 142 (6): 911–27. [DOI] [PubMed] [Google Scholar]
- Lande Russell, Engen S, Sæther B-E, Filli, Matthysen E, and Weimerskirch H. 2002. “Estimating Density Dependence from Population Time Series Using Demographic Theory and Life-History Data.” The American Naturalist 159 (4): 321–37. [DOI] [PubMed] [Google Scholar]
- Lande Russell, and Orzack Steven Hecht. 1988. “Extinction Dynamics of Age-Structured Populations in a Fluctuating Environment.” Proceedings of the National Academy of Sciences 85 (19): 7418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łaniewski Paweł, Owen Kimberley A., Khnanisho Michael, Brotman Rebecca M., and Herbst-Kralovetz Melissa M.. 2021. “Clinical and Personal Lubricants Impact the Growth of Vaginal Lactobacillus Species and Colonization of Vaginal Epithelial Cells: An in Vitro Study.” Sexually Transmitted Diseases 48 (1): 63. 10.1097/OLQ.0000000000001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele Subhash R. 2020. “How Should We Quantify Uncertainty in Statistical Inference?” Frontiers in Ecology and Evolution 8: 35. [Google Scholar]
- Lele Subhash R, Nadeem Khurram, and Schmuland Byron. 2010. “Estimability and Likelihood Inference for Generalized Linear Mixed Models Using Data Cloning.” Journal of the American Statistical Association 105 (492): 1617–25. [Google Scholar]
- Levy Maayan, Kolodziejczyk Aleksandra A, Thaiss Christoph A, and Elinav Eran. 2017. “Dysbiosis and the Immune System.” Nature Reviews Immunology 17 (4): 219–32. [DOI] [PubMed] [Google Scholar]
- Lewis Felicia MT, Bernstein Kyle T, and Aral Sevgi O. 2017. “Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases.” Obstetrics and Gynecology 129 (4): 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewontin Richard C, and Cohen Daniel. 1969. “On Population Growth in a Randomly Varying Environment.” Proceedings of the National Academy of Sciences 62 (4): 1056–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little Ainslie EF, Robinson Courtney J, Peterson S Brook, Raffa Kenneth F, and Handelsman Jo. 2008. “Rules of Engagement: Interspecies Interactions That Regulate Microbial Communities.” Annu. Rev. Microbiol. 62: 375–401. [DOI] [PubMed] [Google Scholar]
- Loftie-Eaton W., Yano H., Burleigh S., Simmons R.S., Hughes J.M., Rogers L.M., Hunter S.S., Settles M.L., Forney L.J., Ponciano J.M. and Top E.M., 2016. Evolutionary paths that expand plasmid host-range: implications for spread of antibiotic resistance. Molecular biology and evolution, 33(4): 885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftie-Eaton W., Bashford K., Quinn H., Dong K., Millstein J., Hunter S., Thomason M.K., Merrikh H., Ponciano J.M. and Top E.M., 2017. Compensatory mutations improve general permissiveness to antibiotic resistance plasmids. Nature ecology & evolution, 1(9): 1354–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau Michel. 2010. “Linking Biodiversity and Ecosystems: Towards a Unifying Ecological Theory.” Philosophical Transactions of the Royal Society B: Biological Sciences 365 (1537): 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau Michel, Naeem Shahid, Inchausti Pablo, Bengtsson Jan, Philip Grime J, Hector Andrew, Hooper DU, Huston MA, Raffaelli David, and Schmid Bernhard. 2001. “Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges.” Science 294 (5543): 804–8. [DOI] [PubMed] [Google Scholar]
- Ludwig Donald. 1976. “A Singular Perturbation Problem in the Theory of Population Extinction.” In, 10:87–104. [Google Scholar]
- May Robert M. 2019. Stability and Complexity in Model Ecosystems. Princeton university press. [Google Scholar]
- McCann Kevin Shear. 2000. “The Diversity–Stability Debate.” Nature 405 (6783): 228–33. [DOI] [PubMed] [Google Scholar]
- Murray Dennis L, and Sandercock Brett K. 2020. Population Ecology in Practice. John Wiley & Sons. [Google Scholar]
- Newman KB, Buckland ST, Morgan BJT, King R, Borchers DL, Cole DJ, Besbeas P, Gimenez O, and Thomas L. 2014. “State-Space Models.” In Modelling Population Dynamics, 39–50. Springer. [Google Scholar]
- Nisbet Roger M, and Gurney William. 2003. Modelling Fluctuating Populations: Reprint of First Edition (1982). Blackburn Press. [Google Scholar]
- O’Hanlon D. Elizabeth, Brown Sarah E., He Xin, Stennett Christina A., Robbins Sarah J., Johnston Elizabeth D., Wnorowski Amelia M., et al. 2021. “Observational Cohort Study of the Effect of a Single Lubricant Exposure during Transvaginal Ultrasound on Cell-Shedding from the Vaginal Epithelium.” PLOS ONE 16 (5): e0250153. 10.1371/journal.pone.0250153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen Scott W, and Alm Eric J. 2016. “Dysbiosis Is Not an Answer.” Nature Microbiology 1 (12): 1–2. [DOI] [PubMed] [Google Scholar]
- Ovaskainen Otso, and Meerson Baruch. 2010. “Stochastic Models of Population Extinction.” Trends in Ecology & Evolution 25 (11): 643–52. [DOI] [PubMed] [Google Scholar]
- Ponciano José Miguel. 2018. “A Parametric Interpretation of Bayesian Nonparametric Inference from Gene Genealogies: Linking Ecological, Population Genetics and Evolutionary Processes.” Theoretical Population Biology 122: 128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponciano José Miguel, Gelder Leen De, Top Eva M, and Joyce Paul. 2007. “The Population Biology of Bacterial Plasmids: A Hidden Markov Model Approach.” Genetics 176 (2): 957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponciano José Miguel, Taper Mark L, and Dennis Brian. 2018. “Ecological Change Points: The Strength of Density Dependence and the Loss of History.” Theoretical Population Biology 121: 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponciano José Miguel, Vandecasteele Frederik PJ, Hess Thomas F, Forney Larry J, Crawford Ronald L, and Joyce Paul. 2005. “Use of Stochastic Models to Assess the Effect of Environmental Factors on Microbial Growth.” Applied and Environmental Microbiology 71 (5): 2355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Yili, Lan Freeman, and Venturelli Ophelia S. 2021. “Towards a Deeper Understanding of Microbial Communities: Integrating Experimental Data with Dynamic Models.” Current Opinion in Microbiology 62: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel Jacques, Gajer Pawel, Abdo Zaid, Schneider G Maria, Koenig Sara SK, McCulle Stacey L, Karlebach Shara, Gorle Reshma, Russell Jennifer, and Tacket Carol O. 2011. “Vaginal Microbiome of Reproductive-Age Women.” Proceedings of the National Academy of Sciences 108 (supplement_1): 4680–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel Jacques, Brotman Rebecca M., Gajer Pawel, Ma Bing, Nandy Melissa, Fadrosh Douglas W., Sakamoto Joyce, et al. 2013. “Daily Temporal Dynamics of Vaginal Microbiota before, during and after Episodes of Bacterial Vaginosis.” Microbiome 1 (1): 29. 10.1186/2049-2618-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson Courtney J, Bohannan Brendan JM, and Young Vincent B. 2010. “From Structure to Function: The Ecology of Host-Associated Microbial Communities.” Microbiology and Molecular Biology Reviews 74 (3): 453–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraf Viqar Sayeed, Sheikh Sohail Aslam, Ahmad Aftab, Gillevet Patrick M, Bokhari Habib, and Javed Sundus. 2021. “Vaginal Microbiome: Normalcy vs Dysbiosis.” Archives of Microbiology 203: 3793–3802. [DOI] [PubMed] [Google Scholar]
- Schooler Shon S, Salau Buck, Julien Mic H, and Ives Anthony R. 2011. “Alternative Stable States Explain Unpredictable Biological Control of Salvinia Molesta in Kakadu.” Nature 470 (7332): 86–89. [DOI] [PubMed] [Google Scholar]
- Shade Ashley, Peter Hannes, Allison Steven D, Baho Didier L, Berga Mercè, Bürgmann Helmut, Huber David H, Langenheder Silke, Lennon Jay T, and Martiny Jennifer BH. 2012. “Fundamentals of Microbial Community Resistance and Resilience.” Frontiers in Microbiology 3: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples David F, Taper Mark L, and Shepard Bradley B. 2005. “Risk‐based Viable Population Monitoring.” Conservation Biology 19 (6): 1908–16. [Google Scholar]
- Taper Mark L., Lele Subhash R., José Miguel Ponciano Brian Dennis, and Jerde Christopher L.. 2021. “Assessing the Global and Local Uncertainty of Scientific Evidence in the Presence of Model Misspecification.” Frontiers in Ecology and Evolution 9. 10.3389/fevo.2021.679155. [DOI]
- Taper Mark L, and Ponciano José Miguel. 2016. “Evidential Statistics as a Statistical Modern Synthesis to Support 21st Century Science.” Population Ecology 58: 9–29. [Google Scholar]
- Tier Charles, and Hanson Floyd B. 1981. “Persistence in Density Dependent Stochastic Populations.” Mathematical Biosciences 53 (1–2): 89–117. [Google Scholar]
- White Bryan A, Creedon Douglas J, Nelson Karen E, and Wilson Brenda A. 2011. “The Vaginal Microbiome in Health and Disease.” Trends in Endocrinology & Metabolism 22 (10): 389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson Ellen M, Łaniewski Paweł, Herbst-Kralovetz Melissa M, and Brotman Rebecca M. 2019. “Personal and Clinical Vaginal Lubricants: Impact on Local Vaginal Microenvironment and Implications for Epithelial Cell Host Response and Barrier Function.” The Journal of Infectious Diseases 220 (12): 2009–18. 10.1093/infdis/jiz412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin Steven S, and Linhares Iara Moreno. 2022. “The Medium Is the Message: Defining a ‘Normal’ Vaginal Microbiome in Healthy Reproductive-Age Women.” Reproductive Sciences, 1–6. [DOI] [PubMed]
- Zaoli Silvia, and Grilli Jacopo. 2021. “A Macroecological Description of Alternative Stable States Reproduces Intra-and Inter-Host Variability of Gut Microbiome.” Science Advances 7 (43): eabj2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Zheng, Nowierski Robert M, Taper Mark L, Dennis Brian, and Kemp William P. 1998. “Complex Population Dynamics in the Real World: Modeling the Influence of Time‐ varying Parameters and Time Lags.” Ecology 79 (6): 2193–2209. [Google Scholar]
- Zhou Jizhong, and Ning Daliang. 2017. “Stochastic Community Assembly: Does It Matter in Microbial Ecology?” Microbiology and Molecular Biology Reviews 81 (4): e00002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.