Abstract
Objectives:
Opioid use disorder (OUD) impacts millions of people worldwide. The prevalence and debilitating effects of OUD present a pressing need to understand its neural mechanisms to provide more targeted interventions. Prior studies have linked altered functioning in large-scale brain networks with clinical symptoms and outcomes in OUD. However, these investigations often do not consider how brain responses change over time. Time-varying brain network engagement can convey clinically relevant information not captured by static brain measures.
Methods:
We investigated brain dynamic alterations in individuals with OUD by applying a new multivariate computational framework to movie-watching (i.e., naturalistic; N=76) and task-based (N=70) fMRI. We further probed the associations between cognitive control and brain dynamics during a separate drug cue paradigm in individuals with OUD.
Results:
Compared to healthy controls (N=97), individuals with OUD showed decreased variability in the engagement of recurring brain states during movie-watching. We also found that worse cognitive control was linked to decreased variability during the rest period when no opioid-related stimuli were present.
Conclusions:
These findings suggest that individuals with OUD may experience greater difficulty in effectively engaging brain networks in response to evolving internal or external demands. Such inflexibility may contribute to aberrant response inhibition and biased attention toward opioid-related stimuli, two hallmark characteristics of OUD. By incorporating temporal information, the current study introduces novel information about how brain dynamics are altered in individuals with OUD and their behavioral implications.
Introduction
Opioid use disorder (OUD) is a chronic condition impacting millions of people globally (1). Individuals with OUD face increased risks of injuries, incarceration, and premature mortality (2,3). Given OUD’s profound burden on the individual and the community, there is an urgent need to elucidate its etiology to develop more targeted preventions and interventions (4).
Recent work using functional magnetic resonance imaging (fMRI) has greatly expanded our understanding of OUD (5–7). Task-based fMRI collected during drug cue and response inhibition paradigms has been widely utilized to investigate OUD’s neural mechanisms. These studies implicated the involvement of several canonical functional brain networks, including the default mode (DMN), reward, and cognitive control networks (5,8–10). Specifically, individuals with OUD expressed hyperactivation in the striatum, amygdala, and dorsolateral prefrontal cortex (PFC) in response to opioid-related stimuli (7,11). Anterior cingulate and lateral and medial PFC hypoactivation were additionally observed in individuals with OUD when the need to suppress automatic response arose (7,12,13).
However, extant fMRI studies adopt a static approach, where brain activity is averaged over a minutes-long scan. Therefore, these methods are unable to track brain activation fluctuations across time. How individuals dynamically engage brain networks contains crucial information about psychopathology. Notably, dynamic brain markers reveal insights about clinical symptoms not captured by static brain measures computed from the average activity patterns over a single scan (14,15). Thus, not considering the dynamic changes in brain activity over time may contribute to an incomplete understanding of OUD.
To address this gap, we leveraged a new multivariate computational framework to investigate brain dynamics in individuals with OUD stabilized on medication for OUD (MOUD). Our framework has the advantage of simultaneously tracking multiple brain states (i.e., recurring brain activation patterns) at the resolution of individual time points. As brain states can overlap temporally (16,17), examining multiple brain states concurrently addresses the caveat of potentially neglecting less dominant brain states with important behavioral relevance. A helpful analogy to consider here is color (Figure 1). If blue (representing the most dominant brain state) contributes the most to purple (representing a time point of interest) and only blue is examined, information on how other colors like red (representing the less dominant brain states) also contribute to forming purple is lost. This framework allows us to assess variability in brain state engagement over time by extracting moment-to-moment engagement information for multiple brain states simultaneously.
Figure 1. Study pipeline.
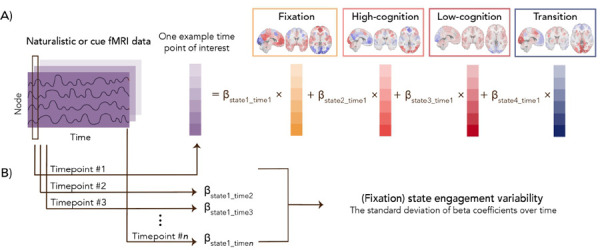
Four recurring brain states were identified using task-based fMRI from the Human Connectome Project dataset. Based on the prominent task conditions associated with each brain state, we characterized these four brain states as fixation, high-cognition, low-cognition, and transition (Supplementary Materials). For example, the fixation brain state mainly includes time points from the fixation condition across task paradigms. In contrast, the low-cognition brain state consists of time points from the motor task, the 0-back working memory task condition, and the neutral emotion task condition (Supplementary Table 1). Moment-to-moment state engagement was assessed in the naturalistic and drug cue paradigms using non-negative least squares regression. For a given time point, the framework can assess the engagement (indicated by the beta coefficients) of multiple brain states concurrently. A demonstrates this process for one example time point of interest. After engagement values are extracted from all time points, variability is computed as the standard deviation of moment-to-moment engagement across time. As an example, B shows how variability is computed for the fixation state.
Using fMRI data collected during a movie-watching paradigm, we first compared brain state engagement variability between individuals with OUD and healthy controls (HCs). Naturalistic stimuli closely mimic the time-varying interactions in real life (18), potentially presenting a more ecologically valid understanding of brain dynamics in OUD. Increased brain network recruitment variability supports greater flexibility in addressing current demands (19,20). As decreased cognitive flexibility has been observed in individuals with OUD (21,22), we hypothesized that they would show decreased state engagement variability compared to HCs during movie-watching. Next, taking advantage of task-based fMRI’s increased power in detecting brain-behavior associations (23), we correlated brain dynamics during a drug cue paradigm with Stroop-assessed cognitive control. We hypothesized that lower variability in the drug cue paradigm, particularly during rest block, would be associated with worse cognitive control. Additionally, despite notable sex differences in the mortality rate and risk for OUD (24,25), an understanding of sex differences in OUD at the neurobiological level is lacking (7). To address this gap, we explored sex effects on brain dynamics and their associations with behaviors.
Methods
Participants
Data from two datasets were analyzed. The Yale University Institutional Review Board and the Yale MRRC Protocol Review committee approved both studies. Informed consent was obtained from all participants. The OUD sample was recruited as a part of an NIH-funded study from Yale School of Medicine and the APT foundation (Collaboration Linking Opioid Use Disorder and Sleep). It included individuals who met DSM-5 criteria for OUD and who were stabilized on MOUD (<24 weeks), an evidence-based treatment for OUD. Naturalistic and drug cue fMRI data were examined here. During the 6-minute naturalistic paradigm, participants watched three movie clips presented in the same order without a break (Inside Out, The Princess Bride, and Up). In the drug cue paradigm, participants were presented with either a rest (a white crosshair presented in the center of a black screen) or a cue block (a picture showing opioid-related stimuli; e.g., a needle or a bottle of pills). There were nine alternating rest and cue blocks, each lasting 16 seconds.
HCs from a separate transdiagnostic study were also studied. Participants were only included if they had no neurological or mental health diagnoses based on the Mini-International Neuropsychiatric Interview for DSM-5. Naturalistic fMRI data were collected using the same paradigm described above. No drug cue data were collected from HCs.
FMRI data preprocessing
Detailed acquisition and preprocessing information can be found in Supplementary Materials. In brief, a standard preprocessing pipeline described in previous studies was applied to the structural and functional data. Statistical Parametric Mapping (SPM12) performed slice time and motion correction for both datasets on the functional data. Additional data cleaning was carried out in BioImage Suite. Covariates of no interest were regressed out, including linear and quadratic drift, white matter, cerebrospinal fluid, gray matter, and a 24-parameter motion model. The functional data were temporally smoothed (cutoff frequency around 0.12Hz). A combination of nonlinear and linear transformations aligned the Shen-268 atlas to the functional data before timeseries data was parcellated.
Additional quality control included removing participants with mean framewise displacement over 0.2mm, missing time points, or brain coverage (see Supplementary Materials for more details). After these criteria, 97 HCs (Table 1) and 76 individuals with OUD (Table 1) were included in the naturalistic fMRI analysis; 70 individuals with OUD with drug cue fMRI data (Table 1) were analyzed.
Table 1.
Demographic information for both datasets. OUD duration was computed as the difference between their current and age at first use. OUD severity was measured using SCID-V based on DSM-5 (mild: 2–3 symptoms, moderate: 4–5 symptoms, severe: 6 or more symptoms). HC, healthy control. OUD, individuals with opioid use disorder.
| Characteristic | |||
|---|---|---|---|
| Naturalistic paradigm (HC) | N | 97 | |
| Self-reported sex assigned at birth | Female | 54 | |
| Male | 43 | ||
| Age (years) | Mean | 31.660 | |
| Standard deviation | 12.178 | ||
| Self-reported race | Asian | 16 | |
| Black | 23 | ||
| More than one race | 5 | ||
| Unknown | 1 | ||
| White | 52 | ||
| Naturalistic paradigm (OUD) | N | 76 | |
| Self-reported sex assigned at birth | Female | 31 | |
| Male | 45 | ||
| Self-reported race | Asian | 0 | |
| Black | 2 | ||
| More than one race | 6 | ||
| Unknown | 3 | ||
| White | 65 | ||
| OUD duration | Mean | 14.816 | |
| Standard deviation | 9.927 | ||
| OUD severity | Moderate | 4 | |
| Severe | 72 | ||
| Methadone dose at baseline (milligram) | Mean | 81.947 | |
| Standard deviation | 22.644 | ||
| Drug cue paradigm | N | 70 | |
| Self-reported sex assigned at birth | Female | 31 | |
| Male | 39 | ||
| Age (years) | Mean | 39.2 | |
| Standard deviation | 10.691 | ||
| Self-reported race | Asian | 0 | |
| Black | 2 | ||
| More than one race | 6 | ||
| Unknown | 3 | ||
| White | 59 | ||
| OUD duration | Mean | 14.686 | |
| Standard deviation | 9.909 | ||
| OUD severity | Moderate | 3 | |
| Severe | 67 | ||
| Methadone dose at baseline (milligram) | Mean | 81.5 | |
| Standard deviation | 22.847 | ||
Brain dynamic measures
We leveraged a new multivariate computational framework to assess brain dynamics at the whole-brain level (26; Supplementary Materials; Figure 1). Briefly, four recurring brain states were identified by applying nonlinear manifold learning to task-based fMRI data from the Human Connectome Project (HCP) dataset (27). These brain states were later characterized as fixation, high-cognition, low-cognition, and transition based on the task conditions and their associated cognitive load. For instance, the time points in the high-cognition brain state are from complex cognitive paradigms requiring higher cognitive load (e.g., working memory, gambling, and emotion). The transition brain state consists of time points when individuals switched from one task condition to another. The fixation brain state includes time points where individuals were presented with a crosshair while resting in the scanner (Supplementary Table 1). The rich HCP dataset allowed us to identify brain states underlying a range of cognitive processes while circumventing circular analysis. While HCP consists of healthy individuals, previous work has indicated that similar brain states can be identified in individuals with psychopathology, suggesting that the way brain states are recruited, instead of the brain states themselves, is more likely to drive group differences (17,28,29).
Non-negative least squares regression next extended these four brain states to our two datasets, evaluating each state’s engagement at each time point during both the naturalistic and drug cue paradigms. Our approach permits temporal overlap in brain states and allows for the joint contributions of all brain states to a given time point. This flexibility is crucial, as the activation pattern observed at a single time point might not always match exactly onto a specific brain state. A summary measure was then extracted for each individual and each brain state by computing the standard deviation of moment-to-moment engagement across time (i.e., state engagement variability). Variability was calculated across the entire run for the naturalistic paradigm. As prior work has revealed significant differences in variability during rest and task (26), we opted to compute variability within the rest and cue condition separately for the drug cue paradigm. As a validation analysis, we compared state engagement variability between conditions in the drug cue paradigm to test whether our brain dynamic measures were sensitive to changes induced by task demands. The more constrained nature of the cue condition likely reduces the likelihood of mind wandering. If our measures are sensitive to brain dynamic changes in response to variations in demand, we may observe lower variability during cue in contrast to the rest condition.
Stroop-assessed cognitive control
We extracted cognitive control measures from a Stroop task to evaluate the behavioral implications of alterations in state engagement variability. Both accuracy (ACC; i.e., control trial ACC - incongruent trial ACC) and response time (RT; i.e., incongruent trial RT - control trial RT) interference scores were computed. Individuals with missing or outlier behavioral scores were excluded. We included 62 and 61 participants with OUD in the final ACC and RT interference score analysis (see exclusion criteria in Supplementary Materials), respectively.
Statistical Analysis
Multivariate group differences in state engagement variability during movie-watching were examined with Hotelling’s T-square test. Age and sex were included as covariates. Sex-by-group and age-by-group interactions were explored. We additionally correlated Stroop interference scores with state engagement variability during the drug cue paradigm using Spearman correlation. There may be lasting effects of opioid-related stimuli in the rest block, thereby making this period more sensitive to flexibility in the brain. To probe this possibility, the first rest block was excluded when computing variability for consistency since no prior stimuli were presented. We also examined the association between cognitive control and state engagement variability during the cue condition for completeness. Results for these analyses were FDR corrected for multiple comparisons.
Results
Demographic data
Demographic information for healthy controls and individuals with OUD is included in Table 1. All individuals with OUD were stabilized on methadone and met DSM-5 criteria for either moderate or severe OUD (see Supplementary Table 3 for additional clinical characteristics). Among individuals included in the naturalistic paradigm analyses, HCs were significantly younger than individuals with OUD (two-sample t-test; t(169.49)=−4.472; p<0.001). The two groups did not differ in sex (Chi-squared; p=0.073).
Individuals with OUD demonstrated lower state engagement variability
We observed a significant group effect on state engagement variability during movie-watching (F(4,164)=9.967; p<0.001; Figure 2) when including age and sex as covariates. There was no sex main effect (F(4,164)=1.323; p=0.264) or sex-by-group interaction (F(4,164)=0.327, p=0.860). Consistent with previous work (26), age showed a significant effect on variability (F(4,164)=6.329, p<0.001), but there was no age-by-group interaction (F(4,164)=2.295, p=0.061).
Figure 2. Group differences in state engagement variability.
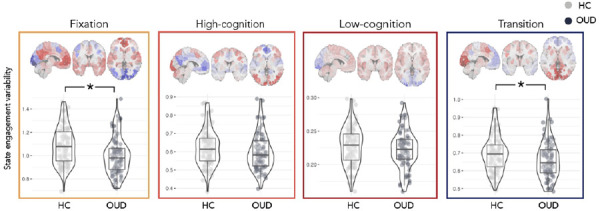
During naturalistic fMRI, individuals with OUD (N=76) showed lower state engagement variability than HCs (N=97). This was prominent in the fixation and transition brain states. OUD, opioid use disorder; HC, healthy control. Scatter plots created using the ggstatsplot R package (30).
Post-hoc ANOVAs were performed to explore group differences in each brain state separately (Supplementary Table 4). Fixation (F(1,167)=12.343; p<0.001) and transition state engagement variability (F(1,167)=6.667; p=0.011) were significantly lower in individuals with OUD compared to HCs (fixation: HC: 1.085±0.159, OUD: 1.000±0.160; transition: HC: 0.698±0.103, OUD: 0.658±0.104). High-cognition (F(1,167)=2.455; p=0.119) and low-cognition state engagement variability (F(1,167)=1.270; p=0.261) did not differ significantly between groups.
Lower state engagement variability during rest condition was associated with worse cognitive control
As expected, our validation analysis revealed that state engagement variability decreased during cue compared to rest across all four states (fixation: t(69)=2.014, p=0.048; high-cognition: t(69)=2.104, p=0.039; low-cognition: t(69)=4.623, p<0.001; transition: t(69)=2.136, p=0.036). These patterns remained consistent even when we introduced lags to task time indices (Supplementary Table 5), indicating that our measures are sensitive to brain dynamic changes following demand changes in the environment.
Notably, while state engagement variability during the cue condition was not linked to cognitive control (i.e., ACC or RT interference scores; Table 2), cognitive control was significantly correlated with variability assessed during the rest condition. Specifically, decreased transition and low-cognition state engagement variability was linked to worse ACC (ρ(60)=−0.405; p=0.001; q=0.016) and RT interference (ρ(59)=−0.383; p=0.002; q=0.016), respectively (Figure 3; Table 3). These associations remained largely consistent when lags were added to task time indices (Supplementary Tables 6 & 7).
Table 2.
State engagement variability during cue and cognitive control
| ACC Interference | RT Interference | |||||
|---|---|---|---|---|---|---|
| ρ | p | q | ρ | p | q | |
| Fixation | 0.022 | 0.868 | 0.888 | −0.100 | 0.443 | 0.885 |
| High-cognition | 0.023 | 0.858 | 0.888 | −0.032 | 0.808 | 0.888 |
| Low-cognition | 0.030 | 0.820 | 0.888 | −0.018 | 0.888 | 0.888 |
| Transition | 0.020 | 0.876 | 0.888 | 0.020 | 0.880 | 0.888 |
Figure 3. State engagement variability and cognitive control.
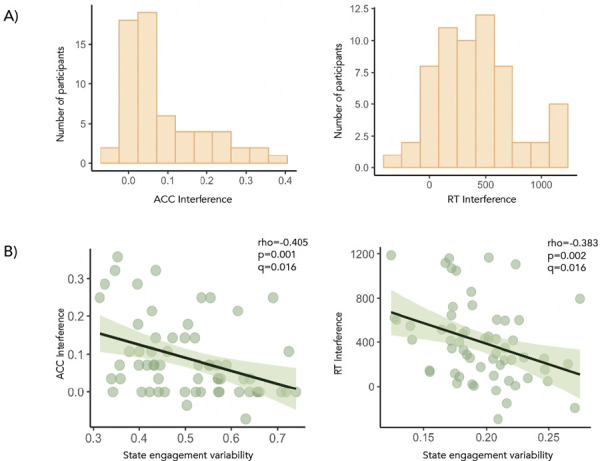
ACC and RT interference score distributions were shown in A. B During the rest condition of the drug cue paradigm, decreased transition state engagement variability and low-cognition state engagement variability were associated with worse cognitive control performance (i.e., higher ACC and RT interference scores), respectively.
Table 3.
State engagement variability during rest and cognitive control
| ACC Interference | RT Interference | |||||
|---|---|---|---|---|---|---|
| ρ | p | q | ρ | p | q | |
| Fixation | −0.302 | 0.017 | 0.091 | −0.290 | 0.024 | 0.096 |
| High-cognition | −0.247 | 0.053 | 0.170 | 0.182 | 0.160 | 0.366 |
| Low-cognition | −0.088 | 0.498 | 0.885 | −0.383 | 0.002 | 0.016 |
| Transition | −0.405 | 0.001 | 0.016 | −0.225 | 0.081 | 0.216 |
Exploratory analysis was conducted to investigate whether these significant associations varied by sex. Interestingly, ACC interference was only significantly correlated with transition state engagement variability in male (ρ(30)=−0.516; p=0.003) but not female participants (ρ(28)=−0.228; p=0.226). In contrast, RT interference was significantly linked to low-cognition state engagement variability in female (ρ(29)=−0.580; p<0.001) but not male participants (ρ(28)=−0.174; p=0.357). Group comparison of these correlations were not significant (two-tailed; ACC: z=1.22, p=0.223; RT: z=−1.74, p=0.082).
Control analyses
Our framework assessed variability in the moment-to-moment engagement of recurring whole-brain brain states. As a control analysis, we extracted variability in brain node activation to investigate whether similar results can be obtained (31; Supplementary Materials). Briefly, node activation variability is computed as the average of variability in activation timeseries across all brain nodes. Node activation variability did not differ significantly between groups (ANOVA; F(1,180)=1.602, p=0.207) or correlate with ACC interference scores (rest node activation variability: ρ=−0.084, p=0.517; cue node activation variability: ρ=0.044, p=0.735). RT interference scores correlated significantly with node activation variability during rest (ρ=−0.349, p=0.006) and cue (ρ=v0.279, p=0.030). These preliminary results indicate that compared to variability in node activation across time, our state engagement variability measures may be more sensitive to detecting group differences in brain dynamics. While significant associations between variability and RT interference aligned with previous literature (32,33), the effect sizes found here were smaller than those observed with our brain dynamic measures.
We additionally explored whether brain responses during the drug cue paradigm were related to cognitive control. A general linear model analysis was performed (Supplementary Materials; Supplementary Figure 1). Beta coefficients were extracted from each brain node and correlated with ACC and RT interference scores. After correction for multiple comparisons, none of the brain nodes demonstrated responses significantly correlated with cognitive control (Supplementary Table 8).
Discussion
We leveraged a new multivariate computational framework to investigate alterations in brain dynamics in individuals receiving MOUD for OUD. During movie-watching, individuals with OUD demonstrated reduced state engagement variability compared to HCs. Decreased variability during the rest condition of a drug cue paradigm was further associated with worse cognitive control in individuals with OUD. The novelty of the current study is twofold. First, it served as an initial investigation of brain dynamic alterations in OUD. By employing naturalistic fMRI in our exploration, we built a more ecologically valid understanding of brain dynamics and contributed new insights about OUD. Second, lower variability in brain state engagement was linked to worse cognitive control. By analyzing brain activity across time, we found that a task classically used to study one hallmark characteristic of OUD (i.e., aberrant response to opioid-related stimuli) could provide information on another symptom (i.e., impaired cognitive control). Notably, a traditional brain activation analysis did not reveal an association between cognitive control and brain responses during the drug cue paradigm.
Broadly, state engagement variability is a form of neural variability. Once considered noise, neural variability is now recognized to support behavioral flexibility. Increased variability allows the brain to flexibly recruit various brain networks to call on different processes in response to current needs (19,20,34). Thus, decreased state engagement variability may reflect an impairment in effectively engaging different brain states to address evolving demands. By incorporating time information, our approach provides information about how network recruitment over time may be different in OUD.
The interpretation that altered variability reflects disrupted brain network communications in OUD aligns with the reduced structural and functional brain network connections found in mice and humans dependent on opioids (35,36). We observed significantly reduced variability in the fixation and transition brain states in individuals with OUD. The fixation brain state predominantly recruits the DMN (Supplementary Table 2), which is relevant for relapse risk and withdrawal symptoms in individuals using heroin (37,38). The transition brain state captures activation patterns that often appear when individuals respond to a change in task demand (Supplementary Table 1). Taken together, the inability to effectively recruit these two brain states may lead to greater difficulty switching away from self-relevant thoughts supported by the DMN. This possibility can have important clinical implications if these self-related thoughts revolve around the urge or motivation for opioid use (e.g., the physical or mental pain one is experiencing). These perseverative thoughts may further contribute to craving or substance-seeking behaviors.
Our findings in the naturalistic paradigm indicated that lower state engagement variability might be a characteristic of OUD. Further investigations with variability in a drug cue paradigm demonstrated that this decreased variability could have important behavioral relevance. Consistent with prior literature suggesting that neural variability supports behavioral performance (32,33,39), we found that decreased state engagement variability was associated with worse cognitive control in individuals with OUD. An elevated ACC interference score was linked to lower transition engagement variability, suggesting that the flexible recruitment of a brain state relevant to task demand change supports cognitive control. Further, a higher RT interference score was linked to decreased low-cognition state engagement variability. The low-cognition brain state recruits the motor and medial frontal networks (Supplementary Table 2). Notably, the activity of these two networks during a Stroop task predicts opioid abstinence (6). These findings indicate that more effective engagement of these brain networks may contribute to better cognitive control over the automatic or habitual motor-based responses associated with substance-seeking behaviors (40,41). Future research can probe whether the flexible engagement of the low-cognition brain state relates to abstinence and whether individual variations in cognitive control may mediate this relationship.
Interestingly, significant associations between cognitive control and state engagement variability were found in the rest but not cue condition. This finding dovetails with previous work suggesting that—in addition to the initial response to salient stimuli (such as opioid-related images)—the lingering or recovery from such stimuli also conveys important information (42–44). Drug cue paradigms are a popular approach to investigate the neural mechanisms underpinning OUD. While brain responses to opioid-related stimuli relate to treatment adherence and withdrawal symptoms (45,46), less work has focused on the rest period with no cue presentation. Our results indicate that this period may serve as a novel window into studying stickiness (i.e., residual engagement or decreased flexibility) in the brain. The lower variability observed during naturalistic stimuli in individuals with OUD might reflect more restricted brain network recruitment (19) and a general tendency to linger in a particular thought pattern. This characteristic may become even more prominent in the rest period after opioid-related stimuli are presented. Interestingly, variability during the rest period before presentation of opioid-related stimuli was not related to cognitive control (Supplementary Table 9). Altogether, these results suggest that following exposure to opioid-related stimuli, brain responses to these stimuli may linger due to decreased variability, potentially contributing to perseverative thoughts about opioids and inducing stronger cravings.
However, lingering opioid-related processing is only one of many interpretations of what lower variability during rest reflects. Rest also appears before a cue block. Instead of altered inhibition leading to more persistent processing, biased attention towards opioid-related stimuli may also constrain thoughts and reduce variability during rest. Both response inhibition and biased attention for substance-related stimuli are hallmark characteristics of OUD (7,8,10,47,48) and substance use disorders (49,50). Since we did not instruct participants to disengage from the opioid-related pictures they saw, it is unclear whether there was an actual attempt to inhibit such thoughts during rest. We additionally did not assess how much perseveration participants experienced. Thus, understanding what contributes to lower variability during rest and whether it is linked to more intense craving or drive for substance-seeking behaviors remains a future research direction.
Sex is an important factor in OUD. We did not find significant sex-by-group interaction on state engagement variability during movie watching. However, the strength of the associations between cognitive control and variability varied by sex. While our sample size allows us to study sex differences, power was likely low. Further work on sex differences in brain dynamics in OUD is urgently needed. Opioid use and overdose deaths increase at a more rapid rate in women than men (25). A faster progression to substance misuse has also been consistently observed in women (51). The possibility that similar brain dynamic alterations may contribute to varying behavioral profiles in different groups is critical to consider. Future studies investigating this topic can contribute to more targeted interventions.
The current study also has several limitations. Individuals with OUD present a heterogeneous sample, with remarkable interindividual variations in risk, susceptibility, disorder profile, and progression. More understanding of how comorbidities or polysubstance use may interact with opioid use to influence brain dynamics is needed. Additionally, all participants analyzed in this study were on methadone treatment. As there are multiple treatment options available for OUD (52), future investigations should explore whether brain dynamics may be altered in similar ways across individuals with OUD on different medications (e.g., buprenorphine, extended-release naltrexone) or at different stages of intervention. Abstinence time, treatment time, and time since last dose have all been shown to affect brain responses to cues and cognitive control performance in individuals with OUD (46,53,54). Future work should examine whether different time scales interact with each other. For instance, would individuals abstinent for longer (i.e., relatively longer time scale) show different brain dynamics alterations (i.e., relatively shorter time scale)? Future studies can additionally select naturalistic stimuli specific to substance-use (e.g., scenes of opioid use) to probe brain dynamic alterations more precisely in OUD. Furthermore, as the OUD sample in this study consists predominantly of white individuals, additional research in a more diverse sample is needed to replicate the findings here.
In summary, applying a multivariate framework to naturalistic and task-based fMRI data unveiled significant alterations in brain dynamics in individuals with OUD compared to HCs. This aberrant ability to engage brain networks flexibly and effectively over time can have implications for cognitive control, which may be particularly important to consider during exposure to opioid-related stimuli. The current study presented a novel understanding of altered brain dynamics in OUD and their behavioral consequences.
Supplementary Material
Acknowledgements:
We would like to thank the participants who took part in the study.
Funding Statement
Gruber Science Fellowship, R01 MH121095, U01 HL150596
References
- 1.Strang J. et al. Opioid use disorder. Nat. Rev. Dis. Primer 6, 1–28 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Paulozzi L. J. Prescription drug overdoses: A review. J. Safety Res. 43, 283–289 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Bahji A., Cheng B., Gray S. & Stuart H. Mortality Among People With Opioid Use Disorder: A Systematic Review and Meta-analysis. J. Addict. Med. 14, e118 (2020). [DOI] [PubMed] [Google Scholar]
- 4.McCarty D., Priest K. C. & Korthuis P. T. Treatment and Prevention of Opioid Use Disorder: Challenges and Opportunities. Annu. Rev. Public Health 39, 525–541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ieong H. F. & Yuan Z. Resting-State Neuroimaging and Neuropsychological Findings in Opioid Use Disorder during Abstinence: A Review. Front. Hum. Neurosci. 11, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichenstein S. D., Scheinost D., Potenza M. N., Carroll K. M. & Yip S. W. Dissociable neural substrates of opioid and cocaine use identified via connectome-based modelling. Mol. Psychiatry 26, 4383–4393 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moningka H. et al. Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacology 44, 259–273 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart J. L., May A. C., Aupperle R. L. & Bodurka J. Forging Neuroimaging Targets for Recovery in Opioid Use Disorder. Front. Psychiatry 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Z., Langleben D. D., O’Brien C. P., Childress A. R. & Wiers C. E. Multivariate pattern analysis links drug use severity to distributed cortical hypoactivity during emotional inhibitory control in opioid use disorder. NeuroImage Clin. 32, 102806 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y. et al. Association of Cortico-Striatal Engagement During Cue Reactivity, Reappraisal, and Savoring of Drug and Non-Drug Stimuli With Craving in Heroin Addiction. Am. J. Psychiatry appi.ajp. 20220759 (2023) doi: 10.1176/appi.ajp.20220759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courtney K. E., Schacht J. P., Hutchison K., Roche D. J. O. & Ray L. A. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict. Biol. 21, 3–22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forman S. D. et al. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol. Psychiatry 55, 531–537 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Fu L. et al. Impaired response inhibition function in abstinent heroin dependents: An fMRI study. Neurosci. Lett. 438, 322–326 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Rashid B. et al. Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. NeuroImage 134, 645–657 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen T. T. et al. Dynamic functional connectivity in bipolar disorder is associated with executive function and processing speed: A preliminary study. Neuropsychology 31, 73–83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karahanoğlu F. I. & Van De Ville D. Transient brain activity disentangles fMRI resting-state dynamics in terms of spatially and temporally overlapping networks. Nat. Commun. 6, 7751 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piguet C., Karahanoğlu F. I., Saccaro L. F., Van De Ville D. & Vuilleumier P. Mood disorders disrupt the functional dynamics, not spatial organization of brain resting state networks. NeuroImage Clin. 32, 102833 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonkusare S., Breakspear M. & Guo C. Naturalistic Stimuli in Neuroscience: Critically Acclaimed. Trends Cogn. Sci. 23, 699–714 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Garrett D. D., Kovacevic N., McIntosh A. R. & Grady C. L. The Importance of Being Variable. J. Neurosci. 31, 4496–4503 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uddin L. Q. Bring the Noise: Reconceptualizing Spontaneous Neural Activity. Trends Cogn. Sci. 24, 734–746 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirastu R. et al. Impaired decision-making in opiate-dependent subjects: Effect of pharmacological therapies. Drug Alcohol Depend. 83, 163–168 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Baldacchino A., Armanyous M., Balfour D. J. K., Humphris G. & Matthews K. Neuropsychological functioning and chronic methadone use: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 73, 23–38 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Greene A. S., Gao S., Scheinost D. & Constable R. T. Task-induced brain state manipulation improves prediction of individual traits. Nat. Commun. 9, 2807 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetherington C. L. Sex-gender differences in drug abuse: A shift in the burden of proof? Exp. Clin. Psychopharmacol. 15, 411–417 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Marsh J. C., Park K., Lin Y.-A. & Bersamira C. Gender differences in trends for heroin use and nonmedical prescription opioid use, 2007–2014. J. Subst. Abuse Treat. 87, 79–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye J. et al. Altered Brain Dynamics Across Bipolar Disorder and Schizophrenia During Rest and Task Switching Revealed by Overlapping Brain States. Biol. Psychiatry 94, 580–590 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Essen D. C. et al. The WU-Minn Human Connectome Project: An overview. NeuroImage 80, 62–79 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao S., Mishne G. & Scheinost D. Nonlinear manifold learning in functional magnetic resonance imaging uncovers a low-dimensional space of brain dynamics. Hum. Brain Mapp. 42, 4510–4524 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D. et al. Altered temporal, but intact spatial, features of transient network dynamics in psychosis. Mol. Psychiatry 26, 2493–2503 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patil I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 6, 3167 (2021). [Google Scholar]
- 31.Parkes L. & Bassett D. S. Tracking Disordered Brain Dynamics in Psychiatry. Biol. Psychiatry 94, 528–530 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Garrett D. D. et al. Moment-to-moment brain signal variability: a next frontier in human brain mapping? Neurosci. Biobehav. Rev. 37, 610–624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grady C. L. & Garrett D. D. Understanding variability in the BOLD signal and why it matters for aging. Brain Imaging Behav. 8, 274–283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen J. R. The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. NeuroImage 180, 515–525 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brynildsen J. K. et al. Gene coexpression patterns predict opiate-induced brain-state transitions. Proc. Natl. Acad. Sci. 117, 19556–19565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upadhyay J. et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 133, 2098–2114 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W. et al. Dysfunctional Default Mode Network in Methadone Treated Patients Who Have a Higher Heroin Relapse Risk. Sci. Rep. 5, 15181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J. et al. Methadone maintenance treatment alters couplings of default mode and salience networks in individuals with heroin use disorder: A longitudinal self-controlled resting-state fMRI study. Front. Psychiatry 14, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutchison R. M. & Morton J. B. Tracking the Brain’s Functional Coupling Dynamics over Development. J. Neurosci. 35, 6849–6859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garavan H. & Hester R. The Role of Cognitive Control in Cocaine Dependence. Neuropsychol. Rev. 17, 337–345 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Yalachkov Y., Kaiser J. & Naumer M. J. Sensory and motor aspects of addiction. Behav. Brain Res. 207, 215–222 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Schuyler B. S. et al. Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Soc. Cogn. Affect. Neurosci. 9, 176–181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapate R. C. & Heller A. S. Context matters for affective chronometry. Nat. Hum. Behav. 4, 688–689 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Puccetti N. A., Villano W. J., Fadok J. P. & Heller A. S. Temporal dynamics of affect in the brain: Evidence from human imaging and animal models. Neurosci. Biobehav. Rev. 133, 104491 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang A.-L. et al. Neural correlates of adherence to extended-release naltrexone pharmacotherapy in heroin dependence. Transl. Psychiatry 5, e531–e531 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lou M., Wang E., Shen Y. & Wang J. Cue-Elicited Craving in Heroin Addicts at Different Abstinent Time: An fMRI Pilot Study. Subst. Use Misuse 47, 631–639 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Field M., Munafò M. R. & Franken I. H. A. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol. Bull. 135, 589–607 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang M. W. B. et al. Cognitive Biases in Cannabis, Opioid, and Stimulant Disorders: A Systematic Review. Front. Psychiatry 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldstein R. Z. & Volkow N. D. Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. Am. J. Psychiatry 159, 1642–1652 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldstein R. Z. & Volkow N. D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 12, 652–669 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernandez-Avila C. A., Rounsaville B. J. & Kranzler H. R. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 74, 265–272 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Wang S. Historical Review: Opiate Addiction and Opioid Receptors. Cell Transplant. 28, 233–238 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao D.-L. et al. Cognitive Control in Opioid Dependence and Methadone Maintenance Treatment. PLOS ONE 9, e94589 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langleben D. D. et al. Acute Effect of Methadone Maintenance Dose on Brain fMRI Response to Heroin-Related Cues. Am. J. Psychiatry 165, 390–394 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


