Abstract
Alzheimer’s disease (AD) is a major progressive neurodegenerative disorder of the aging population. High post–menopausal levels of the pituitary gonadotropin follicle–stimulating hormone (FSH) are strongly associated with the onset of AD, and we have shown recently that FSH directly activates the hippocampal Fshr to drive AD–like pathology and memory loss in mice. To establish a role for FSH in memory loss, we used female 3xTg;Fshr+/+, 3xTg;Fshr+/− and 3xTg;Fshr−/− mice that were either left unoperated or underwent sham surgery or ovariectomy at 8 weeks of age. Unoperated and sham–operated 3xTg;Fshr−/− mice were implanted with 17β-estradiol pellets to normalize estradiol levels. Morris Water Maze and Novel Object Recognition behavioral tests were performed to study deficits in spatial and recognition memory, respectively, and to examine the effects of Fshr depletion. 3xTg;Fshr+/+ mice displayed impaired spatial memory at 5 months of age; both the acquisition and retrieval of the memory were ameliorated in 3xTg;Fshr−/− mice and, to a lesser extent, in 3xTg;Fshr+/− mice– –thus documenting a clear gene–dose–dependent prevention of hippocampal–dependent spatial memory impairment. At 5 and 10 months, sham–operated 3xTg;Fshr−/− mice showed better memory performance during the acquasition and/or retrieval phases, suggesting that Fshr deletion prevented the progression of spatial memory deficits with age. However, this prevention was not seen when mice were ovariectomized, except in the 10–month–old 3xTg;Fshr−/− mice. In the Novel Object Recognition test performed at 10 months, all groups of mice, except ovariectomized 3xTg;Fshr−/− mice showed a loss of recognition memory. Consistent with the neurobehavioral data, there was a gene–dose–dependent reduction mainly in the amyloid β40 isoform in whole brain extracts. Finally, serum FSH levels < 8 ng/mL in 16–month–old APP/PS1 mice were associated with better retrieval of spatial memory. Collectively, the data provide compelling genetic evidence for a protective effect of inhibiting FSH signaling on the progression of spatial and recognition memory deficits in mice, and lay a firm foundation for the use of an FSH–blocking agent for the early prevention of cognitive decline in postmenopausal women.
INTRODUCTION
Alzheimer’s disease (AD) poses a major global health crisis in an increasingly aged population, constituting around 60 to 80% of dementia cases. The neuropathology typically includes the presence of amyloid β (Aβ) plaques, neurofibrillary tangles, neuronal and synaptic loss, and neuroinflammation. Marked by progressive memory loss, profound physical disability, and impaired quality of life, women constitute ~ 70% of the AD population1, and compared with men, have a higher life–time risk2, ~ 3–fold higher progression rate3, and a broader spectrum of dementia–related symptoms4. However, mechanism(s) underpinning the preponderance of AD in women remain unclear. Post–menopausal reductions in estrogen have been considered causal, but there is evidence that estrogen upregulates, rather than suppresses, certain AD genes, such as APOE45. Furthermore, depending upon the estrogenic compound used in a series of clinical trials, the data with hormone replacement have been mixed with improvement, no change, or even worsening of cognition6–9.
In contrast, there is increasing evidence that high post–menopausal levels of gonadotropins, notably FSH, are associated with established AD10–13. More importantly, certain neuropathologic features, including neuritic plaques, neurofibrillary tangles, and chronic gliosis, often begin prior to the last menstrual period––namely during the menopausal transition (ages 45 to 50 years)14. During this period, when a steady rise of serum FSH coincides with unperturbed estrogen levels, women show a sharp decline in memory function and increased risk of mild cognitive impairment (MCI) and dementia15–17. This phase also coincides with bone loss, obesity, dysregulated energy balance, and reduced physical activity18–22.
We and others have shown that FSH directly causes bone loss and increases body fat23–27. Prompted by these data, we asked the question: does FSH contribute to AD––and, if so, do the sharp, up to 10–fold increases in serum FSH across and beyond the menopausal transition account for the disproportionately high incidence of AD in aging women versus men, who display only a 3.5% annual rise in serum FSH28? Using AD–prone 3xTg mice, we found that recombinant FSH or ovariectomy (high serum FSH) induce Aβ and phosphorylated TAU (pTAU), inflammation, neuronal apoptosis, and spatial and recognition memory loss29. Downregulating the hippocampal Fshr inhibits ovariectomy–induced AD pathology29. To block FSH action, we designed and generated a panel of polyclonal and monoclonal antibodies to a small FSHR–binding epitope of FSHβ that block FSH action25, 30–32. Injected into 3xTg mice, our polyclonal antibody prevented ovariectomy–induced AD–like pathology and spatial memory loss29––thus, providing further, more compelling evidence that FSH is a disease driver for AD. More recently, we found that FSH interacts with the Apoe4 gene, and not the Apoe3 gene, in mice to promote AD–like features33. It also stimulates the transcription factor C/EBPβ to upregulate asparagine endopeptidase (AEP) that acts as a δ-secretase to cleave amyloid precursor protein (APP) and TAU29, 33.
Here, we explore whether the global deletion of the Fshr, which we find is expressed predominantly in AD–vulnerable brain regions29, 34, namely the granular layer of the hippocampal dentate gyrus and the entorhinal cortex, can prevent the onset and severity of the memory impairment in AD–prone mice. For this, we generated Fshr haploinsufficient and null mice on a 3xTg background. Using the Morris Water Maze test, we documented an impressive gene–dose–dependent prevention of both the acquisition and retrieval of spatial memory loss. However, in 10–month–old ovariectomized 3xTg;Fshr−/− mice, prevention was restricted to the retrieval of consolidated spatial and recognition memory. In a second experimental prong, we used 15–month–old APP/PS1 mice to demonstrate a clear effect of low serum FSH levels (< 8 ng/mL) in improving the retrieval of spatial memory. Given that we now have an FSH–blocking antibody that prevents memory loss in 3xTg mice, these genetic prevention data provide a firm framework for testing our humanized monoclonal antibody, MS-Hu630, for the prevention of AD and MCI in people.
RESULTS
Here, we report, in loss–of–function studies, that the genetic deletion of Fshr globally in mice results in a gene–dose–dependent prevention of impairments in spatial and recognition memory. For this, we first crossed Fshr+/− mice with 3xTg mice to obtain 3xTg;Fshr+/− mice, which were then crossed to generate 3xTg;Fshr+/+, 3xTg;Fshr+/− and 3xTg;Fshr−/− mice. Of note is that the 3xTg background is homozygous for four mutations, namely APPK670N, M671L, MAPTP301L and Psen1M146V. As female Fshr−/− mice are known to be hypogonadal, we implanted 90–day, slow–release 17β–estradiol pellets into 8–week–old mice; this, we have found, normalizes serum estradiol levels29. We also confirmed a near–complete loss and an ~ 50% reduction of Fshr in 3xTg;Fshr−/− and 3xTg;Fshr+/− mice, respectively, on digital PCR (Fig. S1A).
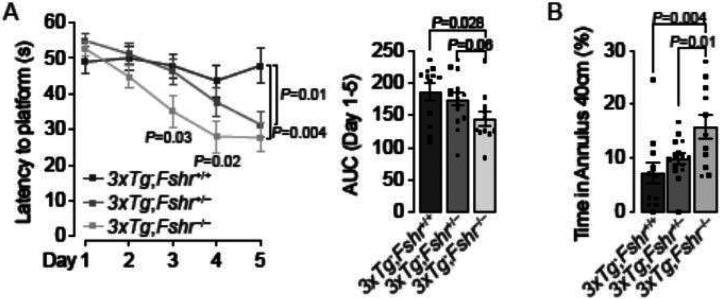
We first studied the effects of depleting Fshr on the 3xTg background on spatial acquisition and memory using the Morris Water Maze Test. 5–month–old female 3xTg;Fshr+/+ mice were expectedly impaired during the spatial acquisition phase (platform submerged, Fig. 1A), indicative of an impaired spatial learning, as well as during the retention phase (platform removed, Fig. 1B), indicating impaired spatial memory retrieval. The complete loss of the Fshr in 3xTg;Fshr−/− mice resulted in a remarkable reduction of latency to locate the platform in the acquisition phase, statistically significant at days 3, 4 and 5, as well as in the retention phase. Partial depletion of the Fshr in 3xTg;Fshr+/− mice also resulted in prevention of impaired learning at day 5, and a trend in the retention phase. No effect on motor activity (swim speed) was noted for any experimental group in both spatial acquisition and retention tests (Fig. S1B). Together, the data demonstrate unequivocally an effect of FSHR signaling on both spatial acquisition and memory retrieval, further substantiating the effects of pharmacologic inhibition by our FSH–blocking antibody29.
Figure 1.
Morris Water Maze to evaluate the effect of genetic Fshr depletion in 5–month–old 3xTg females on acquisition and retrieval of spatial memory. (A) In 5–month–old 3xTg female mice, latency to find the hidden platform (seconds, s) was significantly shorter for 3xTg;Fshr−/− on days 3, 4 and 5 of the acquisition trials compared with 3xTg;Fshr+/+ mice. The effect was also significant on day 5 in 3xTg;Fshr+/− mice compared with 3xTg;Fshr+/+ mice. Both time courses and area under the curve (AUC, days 1 to 5) are shown. Mice that showed repeated episodes of extensive floating (>10 s per trial or >25% of trial time across 5 days) were excluded from the analysis (B) The effect of Fshr depletion in the same mouse groups at day 7 in the retention trial which platform was removed and time spent around the 40–cm platform center point (platform zone) was determined. Mice that showed repeated episodes of extensive oating (>25% of trial time) were excluded from the analysis. N=8 to 13 mice per group; Mean ± SEM; P values as shown, significant at P≤0.05; repeated measures ANOVA followed by Fisher’s Least Significant Difference post–hoc analyses).
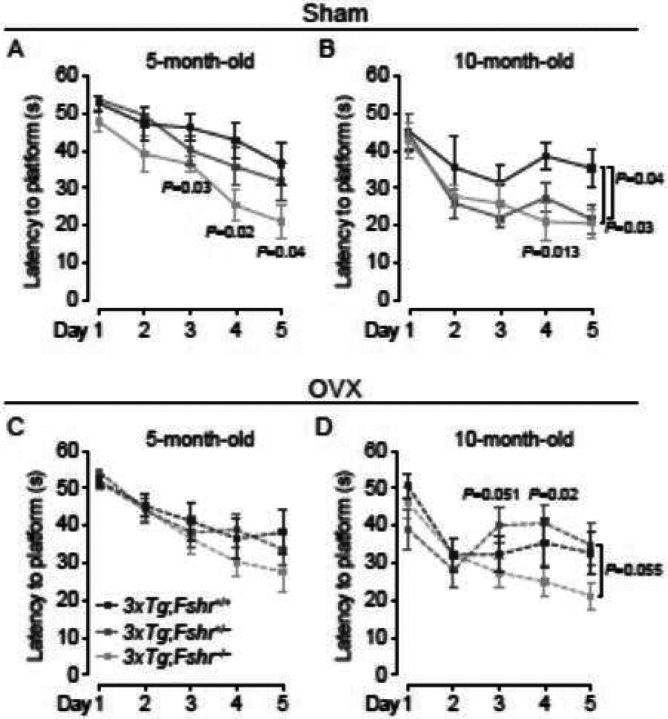
We next evaluated the effect of Fshr depletion on the progression of spatial acquisition impairment with age in mice that had been ovariectomized or sham–operated at 8 weeks of age. Sham–operated 10–month–old 3xTg;Fshr+/+ mice expectedly displayed greater latency than at 5 months. Furthermore, 5– and 10–month–old sham–operated 3xTg;Fshr−/− mice showed reduced latency compared with 3xTg;Fshr+/+ mice on training days 3 to 5 (Fig. 2A) and on days 4 and 5 (Fig. 2B), respectively. This indicates that Fshr deletion in sham–operated mice ameliorates the spatial acquisition impairment, as in Fig. 1, and its progression with age. While sham–operated heterozygotic 3xTg;Fshr+/− mice showed no effect on the latency at 5 months (Fig. 2A), at 10 months, there was a significantly shorter escape latency on training day 5 (Fig. 2B). In contrast, and surprisingly, ovariectomy masked the effect of Fshr gene depletion, resulting in no latency differences between groups during all training days at 5 months of age (Fig. 2C). However, at 10 months, there was a significantly shorter latency in ovariectomized 3xTg;Fsh+/− mice at day 4, and a trend toward significance trend toward significance on days 3 and 5 compared with 3xTg;Fshr+/+ mice (Fig. 2D). No effect on motor activity (swim speed) was noted during any training day for all experimental groups (Fig. S1C).
Figure 2.
Morris Water Maze to evaluate the effect of age, ovariectomy, and genetic Fshr depletion in 3xTg females on acquisition of spatial memory. In 5–month–old sham–operated 3xTg mice (A), latency to find the hidden platform (seconds, s) was significantly shorter for 3xTg;Fshr−/− on days 3, 4 and 5 of the acquisition trials compared with 3xTg;Fshr+/+ mice. At 10 months (B), the effect was significant on days 4 and 5 in 3xTg;Fshr−/− mice and on day 5 in 3xTg;Fshr+/− mice. In ovariectomized mice, there was no difference between the groups at 5 months of age (C), but at 10 months (D), the 3xTg;Fshr−/− mice showed shorter latency compared with the 3xTg; Fshr+/− group at days 4 and a trend toward significance on days 3 and 5. Mice that showed repeated episodes of extensive floating (>10 s per trial or >25% of trial time across 5 days) were excluded from the analysis. N=10 to 14 mice per group; Mean ± SEM; P values as shown, significant at P≤0.05; repeated measures ANOVA followed by Fisher’s Least Significant Difference post–hoc analyses).
We also studied the effect of Fshr depletion on retrieval of consolidated memory using the retention trial of the Morris Water Maze test in the 3xTg;Fshr genotypes. Memory retrieval, assessed by the percent of time spent in the platform zone, was more impaired at 10 months of age compared with 5 months in both sham–operated and ovariectomized 3xTg;Fshr+/+ mice (Fig. 3). However, both 5– and 10–month–old 3xTg; Fshr−/− mice displayed improved memory retrieval compared with 3xTg;Fshr+/+ mice; this effect was gene–dose–dependent (Figs. 3A and 3B). The data suggest a protective effect of graduated Fshr depletion in ameliorating the memory retrieval decline with age. Yet again, in 5–month–old ovariectomized mice, the beneficial effect of Fshr depletion on memory retrieval was absent (Fig. 3C); however, better memory retrieval was found at 10 months of age in ovariectomized 3xTg;Fshr−/− mice compared with 3xTg;Fshr+/− mice (Fig. 3D)—this suggests that absence of FSHR signaling does have an effect in attenuating the progression of memory retrieval decline with age in ovariectomized 3xTg mice. No effect on motor activity (swim speed) was found during the memory retention trial for any experimental group at both ages (Figs. S1B and S1C, day 7)
Figure 3.
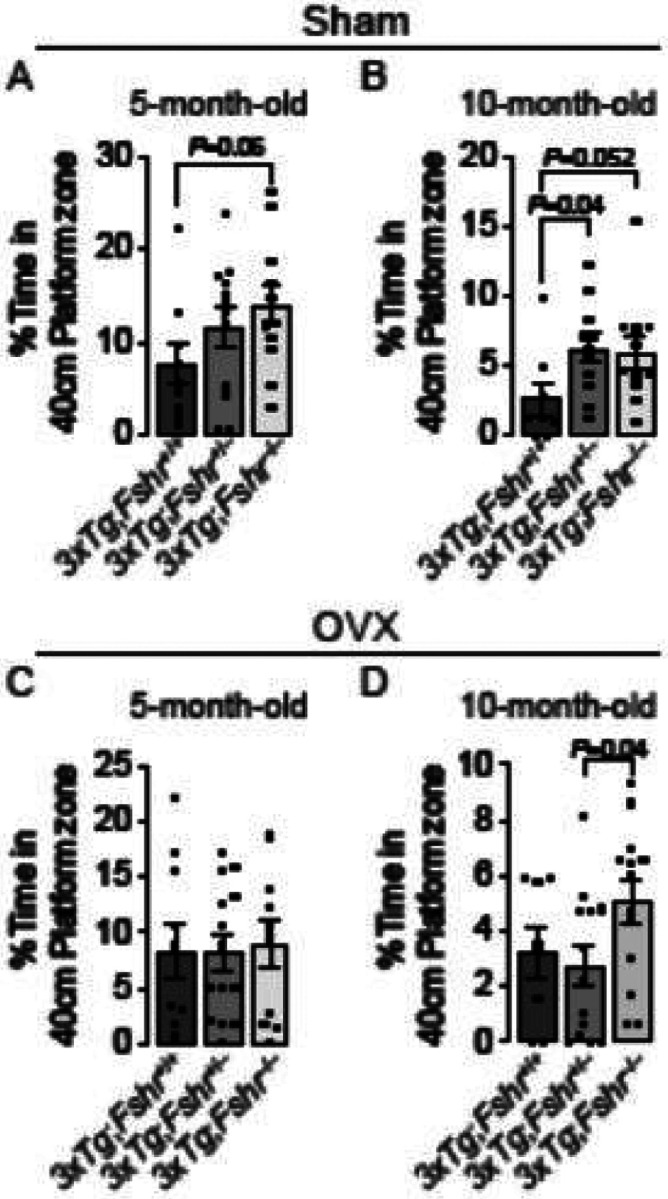
Morris Water Maze to evaluate the effect of age, ovariectomy, and genetic Fshr depletion in 3xTg females on retrieval of spatial memory. In 5–month–old sham–operated 3xTg mice (A), time spent in the 40–cm platform zone in the absence of the platform showed a trend to significance for 3xTg;Fshr−/− mice compared with 3xTg;Fshr+/+ mice. At 10 months (B), the effect was significant for 3xTg;Fshr+/− compared with 3xTg;Fshr+/+ mice and showed a trend toward significance in 3xTg;Fshr−/− mice compared with 3xTg;Fshr+/+ mice. In ovariectomized mice, there was no difference between the groups at 5 months of age (C), but at 10 months (D), the 3xTg;Fshr−/− mice showed longer time spent in the platform zone compared with the 3xTg;Fshr+/− group. Mice that showed repeated episodes of extensive floating (>25% of trial time) were excluded from the analysis. N=10 to 14 mice per group; Mean ± SEM; P values as shown, significant at P≤0.05; repeated measures ANOVA followed by Fisher’s Least Significant Difference post–hoc analyses).
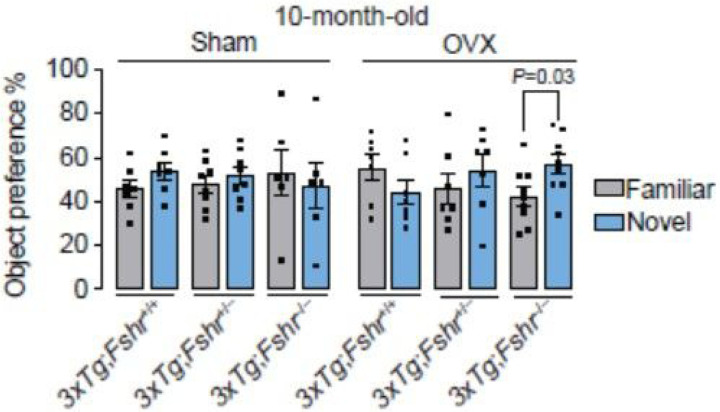
To further explore whether graduated Fshr loss benefited other memory domains in 10–month–old 3xTg mice, we tested recognition memory using the Novel Object Recognition test, which is based on the inherent ability of rodents to explore and recognize a novel object in the environment over a familiar one24. We observed no differences between the interaction with familiar and novel objects in 10–month–old sham–operated mice of any genotype, consistent with our prior data using an FSH–blocking antibody in 9–month–old male APP/PS1 mice29. However, significantly increased interaction with the novel over the familiar object was noted in ovariectomized 3xTg; Fshr−/− group, confirming a protective effect of absent FSHR signaling on recognition memory in ovariectomized aged mice (Fig. 4). The latter finding is also consistent with the prevention of consolidated spatial memory retrieval in these mice (Fig. 3D).
Figure 4.
The effect of Fshr depletion in 10–month–old 3xTg females following sham operation (Sham) or ovariectomy (OVX) on recognition memory in the Novel Object Recognition Test. Significant preference of a novel (N) object over a familiar (F) object represents intact recognition memory. While recognition memory is expectedly impaired in all groups at 10 months of age, ovariectomized 3xTg;Fshr−/− mice showed a significant preference towards novel object, suggesting intact recognition memory. Mice that showed a total object interaction of <5% during training or testing trials were excluded from the analysis of the entire experiment. Sham: N=6–8/group, OVX: N=7–9/group; Mean ± SEM; *P<0.05; Student’s t-test.
For validation25, we first tested whether there was a preference to which side of the box two identical objects were placed. We found no left or right preference in the identical object trial irrespective of genotype (Fig. S1D). Further validation of our dataset required that mice interact with both objects at a minimal threshold of 5% during both training and testing trials; mice that explored below this threshold were excluded a priori. Object interactions ranged from 25.3–37.8% in the training trial and from 9.2–14.4% in the testing trial. There was no main effect of genotype or operation in the training and testing trials (Table S1). The observed increase in novel over familiar object interaction in ovariectomized 3xTg;Fshr−/− mice is also unlikely to be due to increased general locomotor activity, since ovariectomy perse reduced general activity during training trials, and the testing trial had no effect on locomotion (Table S1).
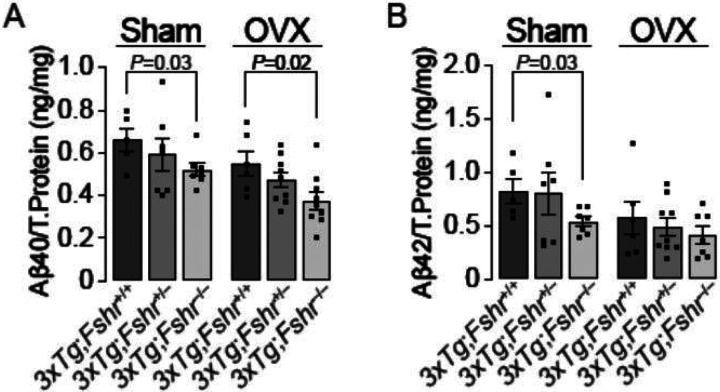
We measured Aβ40 and Aβ42 isoforms in whole brain extracts using ELISA in 10–month old mice. There was a clear Fshr gene–dose–dependent reduction of Aβ40 levels in sham–operated and ovariectomized 3xTg mice (Fig. 5), consistent with the prevention of memory retrieval deficit (Fig. 3D). However, only sham–operated 3xTg;Fshr−/− mice showed a significant reduction in Aβ42 compared with 3xTg;Fshr+/+ mice.
Figure 5.
The effect of Fshr depletion in 10–month–old 3xTg females following sham operation (Sham) or ovariectomy (OVX) on the accumulation of amyloid b (b) isoforms, Ab40 (A) and Ab42 (B) in whole brain extracts. There was a gene–dose–dependent reduction of Ab40 accumulation in both sham–operated and ovariectomized groups, but reductions in Ab42 were significant only in the sham–operated 3xTg;Fshr−/−group. Sham: N=6–8/group, OVX: N=7–9/group; Mean ± SEM; P values as shown, significant at P≤0.05, Student’s t-test.
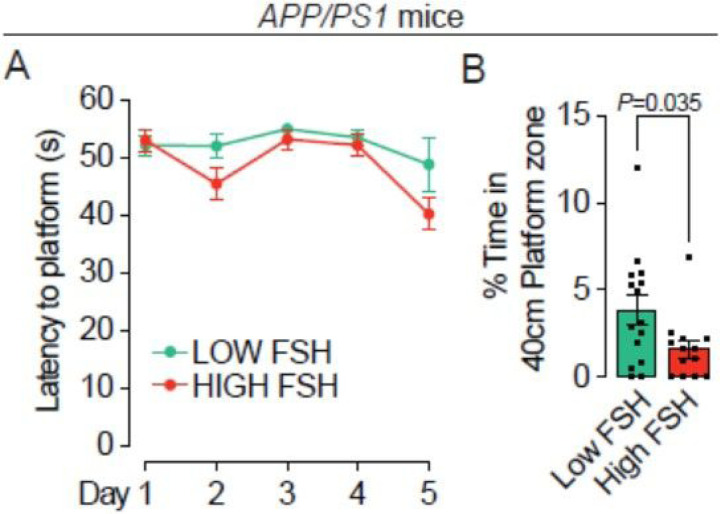
Lastly, to complement the effects of deleting the Fshr genetically in 3xTg mice, we studied the effect of circulating FSH levels on acquisition and retrieval of spatial memory in 16–month–old APP/PS1 mice–– which, unlike 3xTg mice, represent a less aggressive, single pathology model involving Aβ accumulation. These mice develop spatial memory impairments around 15 months35, 36. For this, we pooled two cohorts of APP/PS1 mice and separated the combined group by an arbitrary cut–off of serum FSH at 8 ng/mL to reflect broadly pre– and post–menopausal FSH levels (Fig. S1E). The learning trials of the Morris Water Maze test revealed no difference in spatial acquisition between the low FSH (< 8 ng/mL) and high FSH (> 8 ng/mL) group (Fig. 6A). However, using the retention trial, we found an impressive difference between the two groups in the time spent in platform zone favoring the low–FSH group (Fig. 6B). These data provide additional evidence that low circulating FSH levels in mice are directly associated with better retrieval of consolidated memory.
Figure 6.
Morris Water Maze to evaluate the effect of elevated FSHin 15–month–old APPIPS1 females on acquisition and retrieval of spatial memory. (A) In 16–month–old APP/PS1female mice, latency to find the hidden platform (seconds, s) was not different among the groups. Mice that showed repeated episodes of extensive floating (>10 s per trial or >25% of trial time across 5 days) were excluded from the analysis. (B) The effect of elevated FSH in the same mouse groups at day 7 in the retention trial which the platform was removed and time spent around the 40–cm platform center point (platform zone) was determined. Mice with low FSH levels showed significantly greater time spent in the platform zone. Mice that showed repeated episodes of extensive floating (>25% of trial time) were excluded from the analysis. N=13 to 15 mice per group; Mean ± SEM; P values as shown, significant at P≤0.05; repeated measures ANOVA followed by Fisher’s Least Significant Difference post–hoc analyses).
DISCUSSION
There is little information on circuitry through which glycoprotein hormones from the anterior pituitary regulate central functions37. We recently discovered that FSH, hitherto considered solely a fertility hormone but with a number of newly–discovered somatic functions25–27, acts on FSH receptors on neurons29. Furthermore, our comprehensive analysis through RNAscope at the single transcript level revealed Fshr transcripts in 353 regions of the brain, albeit without ascribed functions34. High Fshr expression was noted selectively on neurons in AD–vulnerable regions, namely on the granular layer of the dentate gyrus of the hippocampus and the entorhinal cortex29, 34. This allowed us to interrogate the hippocampal Fshr through siRNA knockdown, which revealed a clear attenuation of both spatial memory acquisition and retrieval in ovariectomized 3xTg mice29. Furthermore, FSH injections caused spatial memory impairment, whereas our FSH–blocking antibody attenuated the loss of both acquisition and retrieval of memory29. Here, we provide intriguing genetic data that unequivocally establish a role for FSH in regulating spatial memory impairment in models of AD mouse model. We report that the global loss of Fshr expression produces a gene–dose–dependent amelioration of defects in both the acquisition and retrieval of memory in 3xTg mice, and that, equally importantly, APP/PS1 mice with serum FSH levels ≤ 8 ng/mL display improved retrieval of memory.
We find an expected impairment of acquisition and retrieval of spatial memory with age in unoperated or sham–operated 3xTg mice. Furthermore, Fshr depletion on a 3xTg background causes a gene–dose–dependent attenuation of both spatial acquisition and retrieval of memory at 5 and 10 months of age. Consistent with this, Aβ40 and Aβ42 were reduced in Fshr–deficient mice in a gene–dose–dependent manner. This provides compelling evidence that the age–associated impairment of the two components of spatial memory––acquisition and retrieval––as well as Aβ isoform accumulation are rescued upon Fshr depletion.
However, in contrast to unoperated or sham–operated mice, the effect of age on memory impairment in ovariectomized was surprisingly restricted to memory retrieval, but not to the acquisition of spatial memory. Thus, we found that while memory retrieval was impaired with age, there was no difference between sham–operated and ovariectomized groups, likely due to a ceiling effect at 10 months. However, the impaired memory retrieval at 10 months showed improvement upon complete deletion of the Fshr, when compared with Fshr haploinsufficiency. This was consistent with a significant reduction in Aβ40 in Fshr–depleted ovariectomized 3xTg mice. These findings are further concordant with the rescue of recognition memory––another form of hippocampus–dependent consolidated memory––in 10–month– old, Fshr–null mice. The results also support data showing that low basal serum FSH in a different mouse model, the APP/PS1 mouse, is associated with better consolidated memory retrieval, but not with improved acquisition of spatial memory.
In addition to FSH, rising post–menopausal levels of LH have also been implicated in the pathogenesis of memory deficits38. Earlier reports show that LHβ transgenic mice or mice receiving human chorionic gonadotropin (hCG) are cognitively impaired39, 40. Furthermore, similarly to the Fshr, Lhcgr transcripts are expressed in AD–vulnerable regions, such as the dentate gyrus of the hippocampus and entorhinal cortex34. Although we recently discovered that absent LH signaling in Lhcgr−/− mice prevents the anxiety phenotype that develops with aging, further loss–of–function studies using cognitively impaired mice should shed light on the potential effects of LH on memory functions.
With that said, our findings provide a unique genetics–based framework for FSH inhibition to prevent AD–like features in people, particularly in women across the menopausal transition where deficits in memory and MCI are associated with rapid bone loss and the onset of visceral obesity15, 18, 19. Towards targeting a rising FSH in these women, as well as post–menopausal women of advanced ages, we recently produced a first–in–class humanized FSH–blocking antibody, MS-Hu6, which targets a short FSHR–binding epitope of FSHβ, and, in doing so, blocks FSH action30. We have shown that MS-Hu6 has an acceptable affinity to FSH, with a KD of 7.2 nM that approaches trastuzumab; a long half–life of 7.8 days in humanized Tg32 mice; limited, but measurable accumulation in the brain upon subcutaneous injection; and thermal, colloidal, monomeric, structural and accelerated stability in formulation, as evidence of durability and manufacturability30, 41, 42. However, supporting our core concept of inhibiting FSH to prevent MCI is a recent study in women between ages 40 to 65, of which 35% were peri–menopausal––documenting a strong positive correlation between serum FSH levels and Aβ load (measured on PET scans) and gray matter volume in AD–vulnerable regions43.
METHODS
Mouse Models
3xTg mice were sourced from Jackson Laboratory (strain: 034830). The mice carry a transgene containing mutated human APPK670N/M671L and MAPTP301L, as well as a knock–in mutation Psen1M146V, on a heterozygous C57BL/6;129 background44. The mice exhibit AD–like neuropathology and a decline in long–term memory around 3 to 4 months of age26. Fshr mutants were bred and maintained at the Icahn School of Medicine at Mount Sinai (ISMMS), with heterozygotes on 129T2svEmsJ27. The two strains were crossed to produce viable F1 hybrid 3xTg+/−;Fshr+/− littermates. The latter were subsequently crossed with 3xTg+/+ mice to generate compound 3xTg+/+;Fshr+/− mice, which were then crossed to create 3xTg+/+;Fshr+/+; 3xTg+/+; Fshr+/− and 3xTg+/+;Fshr−/− mice (hitherto termed 3xTg;Fshr+/+, 3xTg;Fshr+/−, and 3xTg;Fshr−/− mice). Half of the animals in each group underwent either ovariectomy or sham operation. 90–day, slow–release pellets containing 0.36 mg 17β-estradiol were inserted in the unoperated and sham–operated 3xTg;Fshr−/− to normalize their estrogen level, as before29. APP/PS1 mice were obtained from Jackson Laboratory (strain: 34829) and maintained at ISMMS. The mice carry a human transgene containing APPK670N/M671Land PSEN1ΔE9 mutations36. All experimental mice were grouped–housed to reduce single–house stress, under a 12–hour light/dark cycle with food and water ad libitum. Behavioral tests were performed at ages 5 and 10 months for the Fshr;3xTg mutants, and at 15 months for APP/PS1 mice. All tests were conducted in the light phase, and the order of behavioral tests was the same for each mouse. The protocols were reviewed and approved by the ISMMS Institutional Animal Care and Use Committee.
Surgery
For ovariectomy and sham operation, mice were anesthetized using ketamine/xylazine and received a prophylactic dose of meloxicam to alleviate potential post-surgical pain or distress. After anesthesia, the lower back was shaved, cleaned with 70% ethanol, and washed with povidone prior to the surgery. A ~1 cm skin incision was made, followed by an incision through the muscle layer to access the peritoneal cavity. The ovaries were identified, extracted one at a time through the incision, tied off, and removed. The muscle layer was sutured, and the external incision was closed using wound clips. For pellet implantation, a 0.5 cm incision was created in the skin at the nape of the neck of the 3xTg;Fshr−/− mice. A small pocket was carefully dissected towards the caudolateral area behind the ear, where the 90–day, slow–release pellet containing 0.36 mg 17β-estradiol was placed using tweezers. The incision was closed with a wound clip. Each mouse was placed in a clean cage, allowed to recover from anesthesia, and returned to the home cage after exhibiting normal behavior and ambulation. Meloxicam was continued 24, 48, and 72 hours after surgery.
Reagents
ELISA kits for human Aβ40 (Cat. #KHB3481) and Aβ42 (Cat. #KHB3544) were purchased from Invitrogen and FSH (Cat. #MPTMAG-49K) were purchased from Millipore. The 90–day, slow–release pellets containing 0.36 mg 17β-estradiol were purchased from Innovative Research of America (Cat. #NE121).
Digital PCR
Fshr mRNA levels were quantified using droplet digital PCR (ddPCR). In brief, RNA was isolated by TRIzol (Life Technologies). Reverse transcription was performed using SuperScript III reverse transcriptase (Life Technologies). Isolated RNA was used to perform ddPCR to determine Fshr mRNA levels using FSHR Probe (Life Technologies, Cat. #4331182). Droplets containing the cDNA were generated using a Biorad Droplet generator (QX200) by mixing with droplet generator oil (Cat. #D9161172A), and the formed droplets were amplified using a thermocycler (Applied Biosystems) and analyzed using droplet reader (Biorad).
Behavioral Tests
Behavioral testing, described by us previously45, consisted of two memory tests conducted in the order of increasing invasiveness in the following order: Novel Object Recognition and Morris Water Maze. Mice received 3 days of resting time between tests to decrease carryover effects from prior tests. Mice were habituated to the testing room for 30 minutes at the beginning of each test day. The order of tests in which mice were tested was the same across all mice. Each mouse was tested once per test. The behavioral room wall cues remained the same for all tasks and the same experimenter conducted all of the tests. All test trials were video–recorded, tracked, and analyzed with ANY-maze tracking software (v7.2; Stoelting, Wood Dale, IL). Locomotor activity data for each test are summarized in Table S1.
Novel Object Recognition Test
The Novel Object Recognition test was performed in square test boxes (40×40×35 cm) with even lighting conditions (30 ± 5 lux). Each test box consisted of grey steel bottom plate, white Perspexunder a camera mounted above all boxes. A tower of Lego bricks and Falcon tissue culture flask filled with sand were used as objects27. Prior to the experiments, both objects were tested with a separate cohort of mice to exclude that mice showed a preference for either object or side preference due to the behavioral room conditions. Sample object and the novel object placement followed a counterbalanced design between trials to control for order and location effects.
The test consisted of two trials––training and testing––separated by 6 hours. In the training trial, mice were placed into the test box containing two equal sample objects (e.g., flasks), in front of the south wall facing away from the objects. Each mouse was allowed to explore the objects for 10 minutes before it was returned to its home cage. After 6 hours, the testing was conducted by placing the mouse into the same test box again, but containing one sample (familiar) object and one unfamiliar object (a flask and a Lego bricks tower) and object interaction was recorded for 10 minutes. After each trial, the objects and boxes were cleaned with a Quatricidedilution to eliminate odor cues. The maze was cleaned using 70% ethanol between each trial. Object interaction was defined as an event where a mouse’s head was within 2 cm of the object and directed towards the object, excluding sitting on the objects28,29. For the training trial, object Interaction [%] was calculated as [sample object interaction time]/[total test time] × 100%. For the testing trial, object interaction [%] was calculated as [novel object interaction time]/[total object interaction time] × 100%30. Mice with less than 5% of total object interaction in either trial were excluded from the analysis31.
Morris Water Maze
To test spatial memory accusation and retrieval, we used the Morris Water Maze test (adapted from Vorhees et al.)32. This utilized a circular pool (150 cm diameter) filled with water (26 ± 1°C; 10 cm distance from water surface to wall rim) made opaque with non–toxic tempera paint. A circular rescue platform (diameter: 11 cm; distance between platform center point and pool wall: 27 cm) was submerged 1–1.5 cm below the water surface and the testing area was illuminated with indirect lighting (150 ± 10 lux) to avoid reflections. To monitor animals during trials, a camera was mounted to the ceiling centrally above the pool. The water maze was surrounded by black–and–white extra–maze cues on the walls of the room. Repeated episodes of excessive floating (>10 seconds and/or ≥25% of trial across five days of training) was rare and found in only 6 mice during the entire study. These mice were excluded from the analysis a priori32.
For spatial acquisition trials, a submerged rescue platform, invisible to the mice was used. To locate the escape platform, mice used the extra–maze cues. The platform location remained the same for all trials, whereas the starting location was varied between trials. Mice had 60 seconds to find the rescue platform, after which they were guided there. Each mouse performed four trials per day over 5 days with an inter– trial interval of 15 to 20 minutes. Mice that failed to locate the platform during the 60 second trial, were placed on the platform for 15 seconds immediately after the end of the trial. At the end spatial acquisition day 5, mice were housed back in home cage for 24 hours (day 6). The retention trial was conducted on day 7 without additional training. For the retrieval trials, the rescue platform was removed from the pool and the mouse was allowed to swim for 60 seconds. Mice with extensive floating of >25% of the trial time were removed from the entire analysis a priori. For the spatial acquisition trials, the mean latency to reach the platform was calculated for each test. For the retention trials, the percent of the time spent in the platform zone (40 cm diameter surrounding the platform center point) was analyzed32 (See Supplementary Videos 1 and 2 for examples of unimpaired and impaired 3xTg mice in the Morris Water Maze testing).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism v.10. For molecular analyses, the tests were either unpaired two-tailed Student’s t-test (two-group comparison) or one–way ANOVA followed by Fisher’s least significant difference post hoc test (more than two groups). Differences with P≤0.05 were considered significant. P values are annotated in the figures and are provided in the Source Data Files. For behavioral analyses, repeated measures two–way ANOVA followed by Fisher’s Least Significant Difference post–hoc test (more than two groups), one–way ANOVA or two–tailed Student’s t-test (two–group comparison) were utilized. Differences with P ≤0.05 were considered significant.
ACKNOWLEDGEMENTS
Work at Icahn School of Medicine at Mount Sinai carried at the Center for Translational Medicine and Pharmacology was supported by R01 AG071870, R01 AG074092 and U01 AG073148 to T.Y. and M.Z.; and U19 AG060917 and R01 DK113627 to M.Z.
Footnotes
COMPETING FINANCIAL INTERESTS
M.Z. is inventor on issued and pending patients on the use of FSH as a target for osteoporosis, obesity and Alzheimer’s disease. The patents will be held by Icahn School of Medicine at Mount Sinai, and M.Z. would be recipient of royalties, per institutional policy. The other authors declare no competing financial interests.
Supplementary Files
Contributor Information
Tal Frolinger, Icahn School of Medicine at Mount Sinai.
Funda Korkmaz, Icahn School of Medicine at Mount Sinai.
Steven Sims, Icahn School of Medicine at Mount Sinai.
Fazilet Sen, Icahn School of Medicine at Mount Sinai.
Farhath Sultana, Icahn School of Medicine at Mount Sinai.
Victoria Laurencin, Icahn School of Medicine at Mount Sinai.
Liam Cullen, Icahn School of Medicine at Mount Sinai.
Anusha Rani Pallapati, Icahn School of Medicine at Mount Sinai.
Avi Liu, Icahn School of Medicine at Mount Sinai.
Satish Rojekar, Icahn School of Medicine at Mount Sinai.
Georgii Pevnev, Icahn School of Medicine at Mount Sinai.
Uliana Cheliadinova, Icahn School of Medicine at Mount Sinai.
Darya Vasilyeva, Icahn School of Medicine at Mount Sinai.
Guzel Burganova, Icahn School of Medicine at Mount Sinai.
Mansi Saxena, Icahn School of Medicine at Mount Sinai.
Ki Goosens, Icahn School of Medicine.
Orly Barak, Icahn School of Medicine at Mount Sinai.
Daria Lizneva, Icahn School of Medicine at Mount Sinai.
Keqiang Ye, Shenzhen Institute of Advanced Technology.
Vitaly Ryu, Icahn School of Medicine at Mount Sinai.
Mone Zaidi, Icahn School of Medicine at Mount Sinai.
References
- 1.Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology 1999; 53(9): 1992–1997. [DOI] [PubMed] [Google Scholar]
- 2.Fisher DW, Bennett DA, Dong H. Sexual dimorphism in predisposition to Alzheimer’s disease. Neurobiol Aging 2018; 70: 308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laws KR, Irvine K, Gale TM. Sex differences in cognitive impairment in Alzheimer’s disease. World J Psychiatry 2016; 6(1): 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koran MEI, Wagener M, Hohman TJ, Alzheimer’s Neuroimaging I. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav 2017; 11(1): 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratnakumar A, Zimmerman SE, Jordan BA, Mar JC. Estrogen activates Alzheimer’s disease genes. Alzheimers Dement (N Y) 2019; 5: 906–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vina J, Lloret A. Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis 2010; 20 Suppl 2: S527–533. [DOI] [PubMed] [Google Scholar]
- 7.Matyi JM, Rattinger GB, Schwartz S, Buhusi M, Tschanz JT. Lifetime estrogen exposure and cognition in late life: the Cache County Study. Menopause 2019; 26(12): 1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC, Cache County Memory Study I. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 2002; 288(17): 2123–2129. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien J, Jackson JW, Grodstein F, Blacker D, Weuve J. Postmenopausal hormone therapy is not associated with risk of all-cause dementia and Alzheimer’s disease. Epidemiol Rev 2014; 36(1): 83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Short RA, Bowen RL, O’Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc 2001; 76(9): 906–909. [DOI] [PubMed] [Google Scholar]
- 11.Bowen JD, Malter AD, Sheppard L, Kukull WA, McCormick WC, Teri L, Larson EB. Predictors of mortality in patients diagnosed with probable Alzheimer’s disease. Neurology 1996; 47(2): 433–439. [DOI] [PubMed] [Google Scholar]
- 12.Casadesus G, Atwood CS, Zhu X, Hartzler AW, Webber KM, Perry G, Bowen RL, Smith MA. Evidence for the role of gonadotropin hormones in the development of Alzheimer disease. Cell Mol Life Sci 2005; 62(3): 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meethal SV, Smith MA, Bowen RL, Atwood CS. The gonadotropin connection in Alzheimer’s disease. Endocrine 2005; 26(3): 317–326. [DOI] [PubMed] [Google Scholar]
- 14.Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12(2): 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 2009; 72(21): 1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randolph JF Jr., Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 2003; 88(4): 1516–1522. [DOI] [PubMed] [Google Scholar]
- 17.Randolph JF Jr., Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, Vuga M. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab 2011; 96(3): 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greendale GA, Sowers M, Han W, Huang MH, Finkelstein JS, Crandall CJ, Lee JS, Karlamangla AS. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res 2012; 27(1): 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greendale GA, Sternfeld B, Huang M, Han W, Karvonen-Gutierrez C, Ruppert K, Cauley JA, Finkelstein JS, Jiang SF, Karlamangla AS. Changes in body composition and weight during the menopause transition. JCI Insight 2019; 4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sowers MR, Finkelstein JS, Ettinger B, Bondarenko I, Neer RM, Cauley JA, Sherman S, Greendale GA, Study of Women’s Health Across the N. The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int 2003; 14(1): 44–52. [DOI] [PubMed] [Google Scholar]
- 21.Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM, Ettinger B. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int 2003; 14(3): 191–197. [DOI] [PubMed] [Google Scholar]
- 22.Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab 2006; 91(4): 1261–1267. [DOI] [PubMed] [Google Scholar]
- 23.Geng W, Yan X, Du H, Cui J, Li L, Chen F. Immunization with FSHbeta fusion protein antigen prevents bone loss in a rat ovariectomy-induced osteoporosis model. Biochem Biophys Res Commun 2013; 434(2): 280–286. [DOI] [PubMed] [Google Scholar]
- 24.Han X, Guan Z, Xu M, Zhang Y, Yao H, Meng F, Zhuo Y, Yu G, Cao X, Du X, Bu G, Kong F, Huang A, Zeng X. A novel follicle-stimulating hormone vaccine for controlling fat accumulation. Theriogenology 2020; 148: 103–111. [DOI] [PubMed] [Google Scholar]
- 25.Ji Y, Liu P, Yuen T, Haider S, He J, Romero R, Chen H, Bloch M, Kim SM, Lizneva D, Munshi L, Zhou C, Lu P, Iqbal J, Cheng Z, New MI, Hsueh AJ, Bian Z, Rosen CJ, Sun L, Zaidi M. Epitope-specific monoclonal antibodies to FSHbeta increase bone mass. Proc Natl Acad Sci U S A 2018; 115(9): 2192–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, Abu-Amer W, Izadmehr S, Zhou B, Shin AC, Latif R, Thangeswaran P, Gupta A, Li J, Shnayder V, Robinson ST, Yu YE, Zhang X, Yang F, Lu P et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 2017; 546(7656): 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell 2006; 125(2): 247–260. [DOI] [PubMed] [Google Scholar]
- 28.Araujo AB, Wittert GA. Endocrinology of the aging male. Best Pract Res Clin Endocrinol Metab 2011; 25(2): 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong J, Kang SS, Wang Z, Liu X, Kuo TC, Korkmaz F, Padilla A, Miyashita S, Chan P, Zhang Z, Katsel P, Burgess J, Gumerova A, Ievleva K, Sant D, Yu SP, Muradova V, Frolinger T, Lizneva D, Iqbal J et al. FSH blockade improves cognition in mice with Alzheimer’s disease. Nature 2022; 603(7901): 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gera S, Sant D, Haider S, Korkmaz F, Kuo TC, Mathew M, Perez-Pena H, Xie H, Chen H, Batista R, Ma K, Cheng Z, Hadelia E, Robinson C, Macdonald A, Miyashita S, Williams A, Jebian G, Miyashita H, Gumerova A et al. First-in-class humanized FSH blocking antibody targets bone and fat. Proc Natl Acad Sci U S A 2020; 117(46): 28971–28979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, Tourkova IL, Li J, Davies TF, Sun L, Bian Z, Rosen C, Zallone A, New MI, Zaidi M. Blocking antibody to the beta-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci U S A 2012; 109(36): 14574–14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu LL, Tourkova I, Yuen T, Robinson LJ, Bian Z, Zaidi M, Blair HC. Blocking FSH action attenuates osteoclastogenesis. Biochem Biophys Res Commun 2012; 422(1): 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong J, Kang SS, Wang M, Wang Z, Xia Y, Liao J, Liu X, Yu SP, Zhang Z, Ryu V, Yuen T, Zaidi M, Ye K. FSH and ApoE4 contribute to Alzheimer’s disease-like pathogenesis via C/EBPbeta/delta-secretase in female mice. Nat Commun 2023; 14(1): 6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryu V, Gumerova A, Korkmaz F, Kang SS, Katsel P, Miyashita S, Kannangara H, Cullen L, Chan P, Kuo T, Padilla A, Sultana F, Wizman SA, Kramskiy N, Zaidi S, Kim SM, New MI, Rosen CJ, Goosens KA, Frolinger T et al. Brain atlas for glycoprotein hormone receptors at single-transcript level. Elife 2022; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webster SJ, Bachstetter AD, Van Eldik LJ. Comprehensive behavioral characterization of an APP/PS-1 double knock-in mouse model of Alzheimer’s disease. Alzheimers Res Ther 2013; 5(3): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minkeviciene R, Ihalainen J, Malm T, Matilainen O, Keksa-Goldsteine V, Goldsteins G, Iivonen H, Leguit N, Glennon J, Koistinaho J, Banerjee P, Tanila H. Age-related decrease in stimulated glutamate release and vesicular glutamate transporters in APP/PS1 transgenic and wild-type mice. J Neurochem 2008; 105(3): 584–594. [DOI] [PubMed] [Google Scholar]
- 37.Kannangara H, Cullen L, Miyashita S, Korkmaz F, Macdonald A, Gumerova A, Witztum R, Moldavski O, Sims S, Burgess J, Frolinger T, Latif R, Ginzburg Y, Lizneva D, Goosens K, Davies TF, Yuen T, Zaidi M, Ryu V. Emerging roles of brain tanycytes in regulating blood-hypothalamus barrier plasticity and energy homeostasis. Ann N Y Acad Sci 2023; 1525(1): 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palm R, Chang J, Blair J, Garcia-Mesa Y, Lee HG, Castellani RJ, Smith MA, Zhu X, Casadesus G. Down-regulation of serum gonadotropins but not estrogen replacement improves cognition in aged-ovariectomized 3xTg AD female mice. J Neurochem 2014; 130(1): 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol 2007; 269(1–2): 107–111. [DOI] [PubMed] [Google Scholar]
- 40.Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav 2008; 54(1): 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gera S, Kuo TC, Gumerova AA, Korkmaz F, Sant D, DeMambro V, Sudha K, Padilla A, Prevot G, Munitz J, Teunissen A, van Leent MMT, Post T, Fernandes JC, Netto J, Sultana F, Shelly E, Rojekar S, Kumar P, Cullen L et al. FSH-blocking therapeutic for osteoporosis. Elife 2022; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojekar S, Pallapati AR, Gimenez-Roig J, Korkmaz F, Sultana F, Sant D, Haeck CM, Macdonald A, Kim SM, Rosen CJ, Barak O, Meseck M, Caminis J, Lizneva D, Yuen T, Zaidi M. Development and biophysical characterization of a humanized FSH-blocking monoclonal antibody therapeutic formulated at an ultra-high concentration. Elife 2023; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nerattini M, Rubino F, Jett S, Andy C, Boneu C, Zarate C, Carlton C, Loeb-Zeitlin S, Havryliuk Y, Pahlajani S, Williams S, Berti V, Christos P, Fink M, Dyke JP, Brinton RD, Mosconi L. Elevated gonadotropin levels are associated with increased biomarker risk of Alzheimer’s disease in midlife women. Frontiers in Dementia 2023; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 2003; 39(3): 409–421. [DOI] [PubMed] [Google Scholar]
- 45.Sims S, Barak O, Ryu V, Miyashita S, Kannangara H, Korkmaz F, Wizman S, Macdonald A, Gumerova A, Goosens K, Zaidi M, Yuen T, Lizneva D, Frolinger T. Absent LH signaling rescues the anxiety phenotype in aging female mice. Mol Psychiatry 2023; 28(8): 3324–3331. [DOI] [PubMed] [Google Scholar]