Abstract
Objective
Olanzapine has already been used to treat schizophrenia patients; however, the initial dosage recommendation when multiple drugs are used in combination, remains unclear. The purpose of this study was to explore the drug–drug interaction (DDI) of multiple drugs combined with olanzapine and to recommend the optimal administration of olanzapine in schizophrenia patients.
Methods
In this study, we obtained olanzapine concentrations from therapeutic drug monitoring (TDM) database. In addition, related medical information, such as physiological, biochemical indexes, and concomitant drugs was acquired using medical log. Sixty-five schizophrenia patients were enrollmented for analysis using population pharmacokinetic model by means of nonlinear mixed effect (NONMEM).
Results
Weight and combined use of aripiprazole significantly affected olanzapine clearance. Without aripiprazole, for once-daily olanzapine administration dosages, 0.6, 0.5 mg/kg/day were recommended for 40–70, and 70–100 kg schizophrenia patients, respectively; for twice-daily olanzapine administration dosages, 0.6, 0.5 mg/kg/day were recommended for 40–60, and 60–100 kg schizophrenia patients, respectively. With aripiprazole, for once-daily olanzapine administration dosages, 0.4, 0.3 mg/kg/day were recommended for 40–53, and 53–100 kg schizophrenia patients, respectively; for twice-daily olanzapine administration dosages, 0.4 mg/kg/day was recommended for 40–100 kg schizophrenia patients, respectively.
Conclusion
Aripiprazole significantly affected olanzapine clearance, and when schizophrenia patients use aripiprazole, the olanzapine dosages need adjust. Meanwhile, we firstly recommended the optimal initial dosages of olanzapine in schizophrenia patients.
Keywords: aripiprazole, olanzapine, population pharmacokinetics, initial dosage optimization, schizophrenia patients
Introduction
Schizophrenia affects about 1% of the global population, which is a debilitating disease that deserves the world’s attention,1–3 whose characteristics are manifold but typically include any or all of changes in behaviour, thought, perception, and cognition.2 The etiologies of schizophrenia are complex, and the environment also plays an important role.2,4–6 The origin of schizophrenia may come from utero: obstetric complications such as bleeding, asphyxia, low birth weight, gestational diabetes, and emergent caesarean section have been connected with the progression of schizophrenia.7 In addition, social isolation, childhood trauma, urban dwelling, and ethnicity may also contribute.2
Drugs are often the foundation of effective treatment and are the cornerstone of schizophrenia treatment. The most common prescription drugs are antipsychotics, which control symptoms by affecting dopamine.8 The goal of drug therapy is to effectively manage symptoms at the optimized dosage possible.9 First-generation antipsychotics are typical or traditional antipsychotics, such as chlorpromazine, haloperidol, perphenazine, et al, but may cause elevated prolactin levels, affecting libido, mood, menstrual cycles, and breast tissue in both men and women.10 Newer antipsychotics are known as second-generation or atypical antipsychotics, such as aripiprazole, clozapine, olanzapine, quetiapine, risperidone, amisulpride et al11,12 Second-generation antipsychotics are often clinically preferred because of their lower serious adverse reactions compared to first-generation antipsychotics.11,13
Olanzapine, and aripiprazole are atypical antipsychotics widely used for schizophrenia treatment,14 where olanzapine is an atypical antipsychotic for patients with schizophrenia who respond to initial treatment and maintain their clinical efficacy during maintenance therapy.15 Therapeutic drug monitoring (TDM) technique is often used to monitor the blood concentration of olanzapine in clinical practice to evaluate the drug efficacy and adverse reactions, and the therapeutic range of olanzapine in schizophrenia patients was 20–80 ng/mL,16 where lower drug concentrations lead to poor efficacy and higher drug concentrations are associated with adverse reactions.17–20 As is known to all, cytochrome P450 (CYP) 2D6 is one of the metabolic enzymes of olanzapine,21,22 meanwhile CYP2D6 also metabolizes aripiprazole.23–25 Namely, olanzapine and aripiprazole both are CYP2D6 substrates, there may be competing interactions, influencing olanzapine concentration and clinical treatment outcome such as efficacy and adverse reactions.
In addition, clinically olanzapine is usually used in combination with multiple drugs.26–28 However, the initial dosage recommendation of olanzapine based on concentration who is closely related to efficacy and adverse reactions when multiple drugs are used in combination remains unclear. The purpose of this study was to explore the drug–drug interaction (DDI) of multiple drugs combined with olanzapine, especially aripiprazole, and to recommend the optimal administration of olanzapine in schizophrenia patients.
Methods
Data Collection
Schizophrenia patients treated with olanzapine from Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University between July 2020 and October 2022 were collected, retrospectively. In this study, we obtained olanzapine concentrations from therapeutic drug monitoring (TDM) database. In addition, related medical information, such as physiological, biochemical indexes, and concomitant drugs was acquired using medical log. The above research was approved by the Research Ethics Committee of the Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University (No.20220725011), where the requirement for written informed consent could be waived since the data were retrospectively collected without patient identifiers. The Helsinki Declaration has been followed for human subjects in the study.
Modeling
Olanzapine population pharmacokinetic model was established using the non-linear mixed effect modeling (NONMEM, edition 7, ICON Development Solutions, Ellicott City, MD, USA) software with a first-order conditional estimation method with interaction (FOCE-I method). In the modeling process, apparent oral clearance (CL/F), volume of distribution (V/F), and absorption rate constant (Ka, fixed at 0.861/h29) were taken into consideration.
The inter-individual variability was described using (Equation 1):
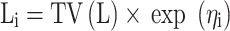 |
(1) |
Li represented the individual parameter value. TV(L) represented the typical individual parameter value. ηi represented symmetrical distribution, which was random term with zero mean and variance omega^2 (ω2).
The random residual variability was described by (Equation 2):
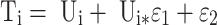 |
(2) |
Ti represented the observed concentration. Ui represented the individual predicted concentration. εn represented symmetrical distribution, which was random term with zero mean and variance sigma^2 (σ2).
The relationship of pharmacokinetic parameters with weight was described with Equation (3):
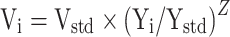 |
(3) |
Vi represented the i-th individual parameter. Yi represented the i-th individual weight. Ystd represented the standard weight of 70 kg. Vstd represented the typical individual parameter, whose weight was Ystd. Z represented the allometric coefficient: 0.75 for the CL/F and 1 for the V/F.30
The pharmacokinetic parameters between continuous covariates or categorical covariates were described via Formulas (4) and (5), respectively:
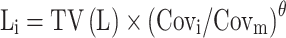 |
(4) |
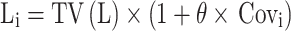 |
(5) |
Li represented the individual parameter value. TV(L) represented the typical individual parameter value. θ represented the parameter to be estimated; Covi represented the covariate of the i-th individual. Covm represented the population median for the covariate.
The stepwise way was used to built up covariate model. The physiological, biochemical indexes and concomitant drugs were considered as potential covariates, which were examined, where concomitant drugs mainly included alprazolam tablets, amisulpride tablets, aripiprazole orally disintegrating tablets, benzhexol hydrochloride tablets, bezafibrate tablets, buspirone hydrochloride tablets, clonazepam tablets, clopidogrel hydrogen sulphate tablets, docusate sodium tablets, enteric-coated aspirin, escitalopram oxalate tablets, finasteride tablets, irbesartan and hydrochlorothiazide tablets, lamotrigine tablets, lorazepam tablets, metformin hydrochloride tablets, metoprolol tartrate tablet, nimodipine tablets, omeprazole enteric-coated capsules, paliperidone sustained-release tablets, perospirone hydrochloride tablets, perphenazine tablets, propranolol hydrochloride tablets, pyrazinamide tablets, risperidone tablets, sertraline hydrochloride tablets, valsartan capsule, zaleplon dispersion tablets, ziprasidone hydrochloride capsules, zopiclone tablets. The objective function value (OFV) changes were deemed to the covariate inclusion criteria. A decrease of OFV >6.63 (P<0.01) defined the inclusion standard and an increase of OFV >10.8 (P<0.001) defined the exclusion standard.
Model Validation
Observations vs population predictions, absolute value of weighted residuals of individual (│iWRES│) vs individual predictions, observations vs individual predictions, weighted residuals vs time, density vs weighted residuals, quantilies of weighted residuals vs quantilies of normal, visual predictive check (VPC) of model and individual plot were used to evaluate final model. Furthermore, the medians and 2.5th-97.5th percentiles of bootstrap (n = 1000) were used to compare with final model parameters.
Simulation
Monte Carlo simulation was used to forecast olanzapine initial dosage optimization, where the therapeutic range of olanzapine in schizophrenia patients was 20–80 ng/mL.16 Each condition included 1000 virtual patients with schizophrenia, ten dosages (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0 mg/kg/day) for seven weight groups (40, 50, 60, 70, 80, 90, 100 kg), respectively. The evaluation criterion was the probability for achieving target concentration.
Results
Patient Information
There were 65 schizophrenia patients included for study, where 36 men and 29 women, whose ages were 45.92 ± 15.79 years old, weights were 63.16 ± 10.57 kg. Demographic data of schizophrenia patients and drug combination were shown in Table 1 and Table 2, respectively.
Table 1.
Demographic Data of Patients with Schizophrenia (n = 65)
| Characteristic | Mean ± SD |
|---|---|
| Gender (men/women) | 36/29 |
| Age (years) | 45.92 ± 15.79 |
| Weight (kg) | 63.16 ± 10.57 |
| Albumin (g/L) | 41.53 ± 3.65 |
| Globulin (g/L) | 27.32 ± 3.99 |
| Alanine transaminase (IU/L) | 23.52 ± 22.73 |
| Aspartate transaminase (IU/L) | 23.10 ± 13.20 |
| Creatinine (μmol/L) | 66.16 ± 13.79 |
| Urea (mmol/L) | 4.54 ± 1.39 |
| Total protein (g/L) | 68.86 ± 6.07 |
| Total cholesterol (mmol/L) | 4.15 ± 0.99 |
| Triglyceride (mmol/L) | 1.54 ± 1.08 |
| Direct bilirubin (μmol/L) | 4.14 ± 1.94 |
| Total bilirubin (μmol/L) | 10.56 ± 4.59 |
| Hematocrit (%) | 39.30 ± 4.67 |
| Hemoglobin (g/L) | 128.66 ± 16.53 |
| Mean corpuscular hemoglobin (pg) | 30.52 ± 2.02 |
| Mean corpuscular hemoglobin concentration (g/L) | 327.24 ± 9.98 |
Abbreviation: SD, standard deviation.
Table 2.
Drug Combination in Patients with Schizophrenia (n = 65)
| Drug | Category | N | Drug | Category | N |
|---|---|---|---|---|---|
| Alprazolam Tablets | 0 | 61 | Metformin Hydrochloride Tablets | 0 | 59 |
| 1 | 4 | 1 | 6 | ||
| Amisulpride Tablets | 0 | 62 | Metoprolol Tartrate Tablet | 0 | 62 |
| 1 | 3 | 1 | 3 | ||
| Aripiprazole Orally Disintegrating Tablets | 0 | 61 | Nimodipine Tablets | 0 | 63 |
| 1 | 4 | 1 | 2 | ||
| Benzhexol Hydrochloride Tablets | 0 | 53 | Omeprazole Enteric-Coated Capsules | 0 | 63 |
| 1 | 12 | 1 | 2 | ||
| Bezafibrate Tablets | 0 | 62 | Paliperidone Sustained-Release Tablets | 0 | 61 |
| 1 | 3 | 1 | 4 | ||
| Buspirone Hydrochloride Tablets | 0 | 61 | Perospirone Hydrochloride Tablets | 0 | 63 |
| 1 | 4 | 1 | 2 | ||
| Clonazepam Tablets | 0 | 58 | Perphenazine Tablets | 0 | 61 |
| 1 | 7 | 1 | 4 | ||
| Clopidogrel Hydrogen Sulphate Tablets | 0 | 63 | Propranolol Hydrochloride Tablets | 0 | 58 |
| 1 | 2 | 1 | 7 | ||
| Docusate Sodium Tablets | 0 | 63 | Pyrazinamide Tablets | 0 | 63 |
| 1 | 2 | 1 | 2 | ||
| Enteric-Coated Aspirin | 0 | 61 | Risperidone Tablets | 0 | 50 |
| 1 | 4 | 1 | 15 | ||
| Escitalopram Oxalate Tablets | 0 | 62 | Sertraline Hydrochloride Tablets | 0 | 62 |
| 1 | 3 | 1 | 3 | ||
| Finasteride Tablets | 0 | 62 | Valsartan Capsule | 0 | 63 |
| 1 | 3 | 1 | 2 | ||
| Irbesartan and Hydrochlorothiazide Tablets | 0 | 63 | Zaleplon Dispersion Tablets | 0 | 63 |
| 1 | 2 | 1 | 2 | ||
| Lamotrigine Tablets | 0 | 63 | Ziprasidone Hydrochloride Capsules | 0 | 61 |
| 1 | 2 | 1 | 4 | ||
| Lorazepam Tablets | 0 | 56 | Zopiclone Tablets | 0 | 57 |
| 1 | 9 | 1 | 8 |
Notes: Category, 0: without drug, 1: with drug; N: number of patients.
Modeling
Weight and combined use of aripiprazole significantly affected olanzapine clearance. Results of hypothesis testing of concomitant drugs in the model development procedure was show in Table S1.Thus, the final model of olanzapine in schizophrenia patients was as follow:
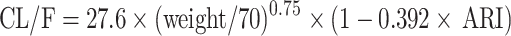 |
(6) |
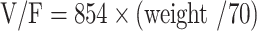 |
(7) |
CL/F represented apparent oral clearance. V/F represented apparent volume of distribution. ARI was aripiprazole, when patients took aripiprazole, ARI was 1, otherwise ARI was 0.
Evaluation
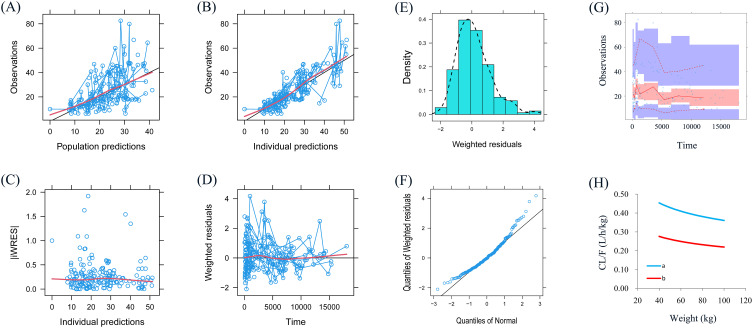
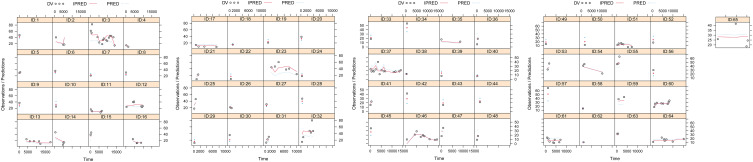
Figure 1A–G showed the model evaluation, including observations vs population predictions, absolute value of weighted residuals of individual (│iWRES│) vs individual predictions, observations vs individual predictions, weighted residuals vs time, density vs weighted residuals, quantiles of weighted residuals vs quantiles of normal, visual predictive check (VPC) of model, respectively, hinting the olanzapine concentrations were well predicted by our model. Figure 1 H showed at the same weight, the olanzapine clearance rates were 0.608:1 in patients with or without aripiprazole, respectively. Figure 2 showed the individual plot, suggesting that the final model may predict the olanzapine concentrations of patients well at the individual level. The parameter estimates of the final model and bootstrap validation were shown in Table 3, in which the median values of bootstraps were near the respective parameter values of final model, indicating the final model was accurate and reliable.
Figure 1.
Model evaluation.
Notes: (A) Observations vs population predictions. (B) Observations vs individual predictions. (C) absolute value of weighted residuals of individual (│iWRES│) vs individual predictions. (D) Weighted residuals vs time. (E) Density vs weighted residuals. (F) Quantiles of weighted residuals vs quantiles of normal. (G) Visual predictive check (VPC) of model. (H) Olanzapine clearance. a: without aripiprazole, b: with aripiprazole.
Figure 2.
Individual plot.
Abbreviations: ID, patient ID number; DV, measured concentration value; IPRED, individual predictive value; PRED, population predictive value.
Table 3.
Parameter Estimates and Bootstrap Validation in Patients with Schizophrenia
| Parameter | Estimate | SE (%) | Bootstrap | Bias (%) | |
|---|---|---|---|---|---|
| Median | 95% Confidence Interval | ||||
| CL/F (L/h) | 27.6 | 5.6 | 27.3 | [24.7, 30.4] | −1.09 |
| V/F (L) | 854 | 25.5 | 858 | [600, 2530] | 0.47 |
| Ka (h−1) | 0.861 (fixed) | -- | -- | -- | -- |
| θARI | −0.392 | 27.8 | −0.377 | [−0.535, −0.194] | −3.83 |
| ωCL/F | 0.316 | 13.4 | 0.321 | [0.223, 0.395] | 1.58 |
| σ1 | 0.288 | 10.1 | 0.278 | [0.219, 0.348] | −3.47 |
| σ2 | 3.701 | 17.4 | 3.742 | [0.703, 4.822] | 1.11 |
Notes: 95% confidential interval was displayed as the 2.5th, 97.5th percentile of bootstrap estimates. CL/F, apparent oral clearance (L/h); V/F, apparent volume of distribution (L); Ka, absorption rate constant (h−1); θARI was the coefficient of aripiprazole; ωCL/F, inter-individual variability of CL/F; σ1, residual variability, proportional error; σ2, residual variability, additive error; Bias, prediction error, Bias = (Median-Estimate)/Estimate×100%. --, Not reported.
Simulation
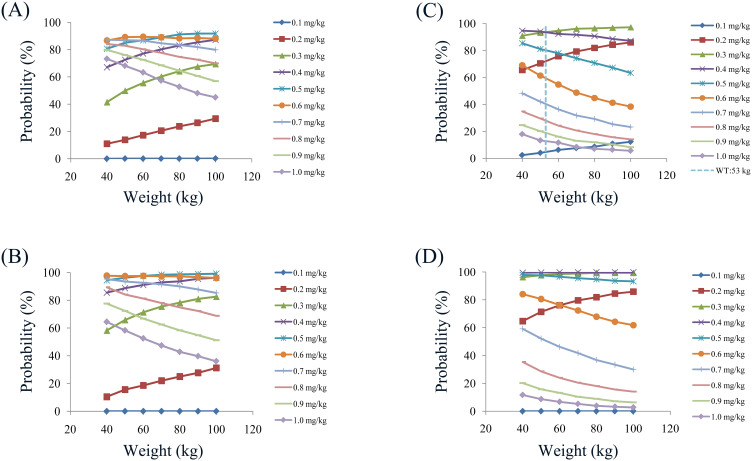
In the final model, weight and combined use of aripiprazole significantly affected olanzapine clearance. Therefore, based on whether aripiprazole was used in combination and a once-daily or a twice-daily olanzapine administration dosages, the present study simulated four different conditions: (1) Once-daily olanzapine administration dosages without aripiprazole. (2) Twice-daily olanzapine administration dosages without aripiprazole. (3) Once-daily olanzapine administration dosages with aripiprazole. (4) Twice-daily olanzapine administration dosages with aripiprazole, which were shown in Figure 3 Ai-3Dvii. The lower and upper red dashed lines were therapeutic ranges from 20 to 80 ng/mL. The probabilities for achieving the target concentrations were shown in Figure 4, among which Figure 4A–D were once-daily olanzapine administration dosages without aripiprazole, twice-daily olanzapine administration dosages without aripiprazole, once-daily olanzapine administration dosages with aripiprazole, twice-daily olanzapine administration dosages with aripiprazole, respectively.
Figure 3.
Simulated olanzapine concentrations.
Notes: (A) Once-daily olanzapine administration dosages without aripiprazole. (B) Twice-daily olanzapine administration dosages without aripiprazole. (C) Once-daily olanzapine administration dosages with aripiprazole. (D) Twice-daily olanzapine administration dosages with aripiprazole. (i): schizophrenia patients with 40 kg, (ii): schizophrenia patients with 50 kg, (iii): schizophrenia patients with 60 kg, (iv): schizophrenia patients with 70 kg, (v): schizophrenia patients with 80 kg, (vi): schizophrenia patients with 90 kg, (vii): schizophrenia patients with 100 kg. The lower and upper red dashed lines were 20 and 80 ng/mL, respectively.
Figure 4.
Probabilities for achieving therapeutic window.
Notes: (A) Once-daily olanzapine administration dosages without aripiprazole. (B) Twice-daily olanzapine administration dosages without aripiprazole. (C) Once-daily olanzapine administration dosages with aripiprazole. (D) Twice-daily olanzapine administration dosages with aripiprazole.
Based on simulation results, the present study recommended the optimal olanzapine initial dosages in schizophrenia patients, as shown in Table 4. Without aripiprazole, for once-daily olanzapine administration dosages, 0.6, 0.5 mg/kg/day were recommended for 40–70, and 70–100 kg schizophrenia patients, respectively, simultaneously, the probabilities for achieving the target concentrations for the dosages of 0.6, 0.5 mg/kg/day were 86.7–89.4%, 89.0–91.8%, respectively; For twice-daily olanzapine administration dosages, 0.6, 0.5 mg/kg/day were recommended for 40–60, and 60–100 kg schizophrenia patients, respectively, simultaneously the probabilities for achieving the target concentrations for the dosages of 0.6, 0.5 mg/kg/day were 97.4–97.7%, 97.6–99.2%, respectively. With aripiprazole, for once-daily olanzapine administration dosages, 0.4, 0.3 mg/kg/day were recommended for 40–53, and 53–100 kg schizophrenia patients, respectively, simultaneously the probabilities for achieving the target concentrations for the dosages of 0.4, 0.3 mg/kg/day were 93.5–94.7%, 93.5–97.3%, respectively; For twice-daily olanzapine administration dosages, 0.4 mg/kg/day were recommended for 40–100 kg schizophrenia patients, respectively, simultaneously the probabilities for achieving the target concentrations for the dosages of 0.4 mg/kg/day were 99.5–99.9%.
Table 4.
Initial Dosage Recommendation of Olanzapine in Schizophrenic Patients Without or with Aripiprazole
| Without Aripiprazole | With Aripiprazole | ||||
|---|---|---|---|---|---|
| Once a Day | Once a Day | ||||
| Body weight (kg) | Dose (mg/kg/day) | Probability to achieve the target concentrations (%) | Body weight (kg) | Dose (mg/kg/day) | Probability to achieve the target concentrations (%) |
| (40–70) | 0.6 | 86.7–89.4 | (40–53) | 0.4 | 93.5–94.7 |
| [70–100] | 0.5 | 89.0–91.8 | [53–100] | 0.3 | 93.5–97.3 |
| Split evenly into two doses a day | Split evenly into two doses a day | ||||
| Body weight (kg) | Dose (mg/kg/day) | Probability to achieve the target concentrations (%) | Body weight (kg) | Dose (mg/kg/day) | Probability to achieve the target concentrations (%) |
| (40–60) | 0.6 | 97.4–97.7 | [40–100] | 0.4 | 99.5–99.9 |
| [60–100] | 0.5 | 97.6–99.2 | |||
Discussion
Olanzapine is a second-generation antipsychotic approved for treating schizophrenia and bipolar I disorder (manic or mixed episodes), which mainly prevents 5-hydroxytryptamine2A (5-HT2A) serotonin receptors and expresses a low affinity for dopamine D2 receptors, making it more effective than first-generation antipsychotics for negative schizophrenia symptoms.31 To some extent, olanzapine works better compared to other second-generation atypical antipsychotics.32 In addition, the inhibition of 5-HT2A receptors induces an increase in dopamine release, attenuating the D2 receptor-blocking action and ultimately eliciting minimal extrapyramidal symptoms.33,34
However, due to high pharmacokinetic variability and narrow therapeutic window of olanzapine,35 it is difficult to make the best drug delivery scheme. Existing researches reported that the clearance of olanzapine among individuals varied from 4-fold to 10-fold,36,37 leading to a considerable variation in olanzapine concentration after taking the same dosage. From a clinical point of view, low levels of exposure to olanzapine concentrations results in diminished drug effects and poor control of psychiatric disorders. On the contrary, high levels of exposure to olanzapine concentrations leads to weight gain, diabetes, and somnolence.38,39 In addition, in clinical practice, whether there is an impact of patient combination of drugs on dosage formulation of olanzapine also needs to be further explored.
In clinical practice, the adjustment of olanzapine dose mainly relies on therapeutic drug monitoring to adjust the next dose. However, for the first administration of olanzapine, there is no reference for monitoring therapeutic concentrations to adjust. Fortunately, population pharmacokinetics and Monte Carlo simulation can solve this problem, and many successful cases have been carried out. For example, Shen et al reported pharmacogenetics-based population pharmacokinetic analysis and dose optimization of valproic acid in Chinese southern children with epilepsy: Effect of ABCB1 gene polymorphism.40 Thyssen et al reported population pharmacokinetics and Monte Carlo simulations demonstrated similar pharmacokinetics of risperidone in children, adolescents and adults.41 Chen et al reported population pharmacokinetics and dose simulation of oxcarbazepine in Chinese paediatric patients with epilepsy.42 Wang et al reported effects of comedication and genetic factors on the population pharmacokinetics of lamotrigine: a prospective analysis in Chinese patients with epilepsy and recommended dose regimens for patients with different gene polymorphisms and comedications were estimated on the basis of Monte Carlo simulations and the established model, which was valuable for developing individualized dosage regimens in adult and adolescent Chinese patients 13–65 years of age.43 Based on the above research, the purpose of this study was to explore the DDI of multiple drugs combined with olanzapine and to recommend the optimal administration of olanzapine in schizophrenia patients based on population pharmacokinetics and Monte Carlo simulation.
Aripiprazole is a second-generation antipsychotic with a low incidence of side effects and metabolic adverse events.44,45 From the point of view of mechanism, aripiprazole is a partial agonist with a high affinity for dopamine D2 (R-D2) and R-D3 receptors and the serotonin receptor R-5-HT1A, combined with an antagonistic activity on the R-5-HT2A receptor.46 Additionally, aripiprazole also has a moderate affinity for R-5-HT747 and R-5-HT2C receptors, playing a role in them as a partial agonist.48
In the present study, 65 schizophrenia patients were enrollmented for analysis using nonlinear mixed effect (NONMEM), whose results showed weight and combined use of aripiprazole significantly affected olanzapine clearance. At the same weight, the olanzapine clearance rates were 0.608:1 in patients with or without aripiprazole, respectively. As is known to all, cytochrome P450 (CYP) 2D6 is one of the metabolic enzymes of olanzapine,21,22 meanwhile CYP2D6 also metabolizes aripiprazole.23–25 Namely, olanzapine and aripiprazole both are CYP2D6 substrates, there be competing interactions. From the present results, we can conclude that aripiprazole competes with olanzapine metabolism, and then reduces olanzapine clearance to a certain extent. In our final model, the CL/F was 27.6 L/h, and the V/F was 854 L.
In order to provide an individualized dosing regimen of olanzapine in schizophrenia patients for the clinic, this study further explored the optimal initial dosing regimen with or without aripiprazole. At the same time, innovative exploration was carried out in the frequency of administration, including once-daily and twice-daily dosing design. Without aripiprazole, for once-daily olanzapine administration dosages, 0.6, 0.5 mg/kg/day were recommended for 40–70, and 70–100 kg schizophrenia patients, respectively; For twice-daily olanzapine administration dosages, 0.6, 0.5 mg/kg/day were recommended for 40–60, and 60–100 kg schizophrenia patients, respectively. With aripiprazole, for once-daily olanzapine administration dosages, 0.4, 0.3 mg/kg/day were recommended for 40–53, and 53–100 kg schizophrenia patients, respectively; For twice-daily olanzapine administration dosages, 0.4 mg/kg/day were recommended for 40–100 kg schizophrenia patients. The present study was the first time for exploring the effects of aripiprazole on olanzapine population pharmacokinetics and initial dosage optimization in schizophrenia patients.
Conclusion
Aripiprazole significantly affected olanzapine clearance, and when schizophrenia patients use aripiprazole, the olanzapine dosages need adjust. Meanwhile, we firstly recommended the optimal initial dosages of olanzapine in schizophrenia patients.
Funding Statement
This work was supported by National Natural Science Foundation of China (No. 82104296), The Xuzhou Special Fund for Promoting Scientific and Technological Innovation (No. KC23217, No. KC23254), The Medical Research Project of Jiangsu Provincial Health Commission (No. Z2023010), The Initializing Fund of Xuzhou Medical University (No. RC20552111, No. RC20552222), The Fusion Innovation Project of Xuzhou Medical University (No. XYRHCX2021011, No. XYRHCX2022005), Jiangsu Province Education Science Planning Project (No. C/2022/01/36), Xuzhou Medical University Labor Education Special Project (No. X1d202209), Jiangsu Province Higher Education Informatization Research Topic (No. 2023JSETKT136), Xuzhou Medical University Research Topic of Higher Education Teaching Reform (No. Xjyzrd202304).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Cun Zhang, Lei Jiang, and Ke Hu are co-first authors for this study. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399(10323):473–486. doi: 10.1016/s0140-6736(21)01730-x [DOI] [PubMed] [Google Scholar]
- 2.Srivastava A, Dada O, Qian J, et al. Epigenetics of schizophrenia. Psychiatry Res. 2021;305:114218. doi: 10.1016/j.psychres.2021.114218 [DOI] [PubMed] [Google Scholar]
- 3.Messias EL, Chen CY, Eaton WW. Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin North Am. 2007;30(3):323–338. doi: 10.1016/j.psc.2007.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowalski K, Zebrowska-Rozanska P, Karpinski P, et al. Profiling gut microbiota signatures associated with the deficit subtype of schizophrenia: findings from a case-control study. Prog Neuropsychopharmacol Biol Psychiatry. 2023;127:110834. doi: 10.1016/j.pnpbp.2023.110834 [DOI] [PubMed] [Google Scholar]
- 5.Xiong Z, Wang H, Qu Y, et al. The mitochondria in schizophrenia with 22q11.2 deletion syndrome: from pathogenesis to therapeutic promise of targeted natural drugs. Prog Neuropsychopharmacol Biol Psychiatry. 2023;127:110831. doi: 10.1016/j.pnpbp.2023.110831 [DOI] [PubMed] [Google Scholar]
- 6.Zhilyaeva TV, Kasyanov ED, Rukavishnikov GV, et al. Pterin metabolism, inflammation and oxidative stress biochemical markers in schizophrenia: factor analysis and assessment of clinical symptoms associations. Prog Neuropsychopharmacol Biol Psychiatry. 2023;127:110823. doi: 10.1016/j.pnpbp.2023.110823 [DOI] [PubMed] [Google Scholar]
- 7.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20(3):201–225. doi: 10.1016/S0893-133X(98)00060-8 [DOI] [PubMed] [Google Scholar]
- 8.de Bartolomeis A, Ciccarelli M, De Simone G, Mazza B, Barone A, Vellucci L. Canonical and non-canonical antipsychotics’ dopamine-related mechanisms of present and next generation molecules: a systematic review on translational highlights for treatment response and treatment-resistant schizophrenia. Int J Mol Sci. 2023;24(6). doi: 10.3390/ijms24065945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Z, Zhang Y, Sun Y, et al. Therapeutic outcomes wide association scan of different antipsychotics in patients with schizophrenia: randomized clinical trials and multi-ancestry validation. Psychiatry Clin Neurosci. 2023;77(9):486–496. doi: 10.1111/pcn.13567 [DOI] [PubMed] [Google Scholar]
- 10.Bahta M, Ogbaghebriel A, Russom M, Tesfamariam EH, Berhe T. Impact of adverse reactions to first-generation antipsychotics on treatment adherence in outpatients with schizophrenia: a cross-sectional study. Ann Gen Psychiatry. 2021;20(1):27. doi: 10.1186/s12991-021-00348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutwalli H, Keeler JL, Bektas S, Dhopatkar N, Treasure J, Himmerich H. Eating cognitions, emotions and behaviour under treatment with second generation antipsychotics: a systematic review and meta-analysis. J Psychiatr Res. 2023;160:137–162. doi: 10.1016/j.jpsychires.2023.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251–267. doi: 10.2165/00023210-200418040-00005 [DOI] [PubMed] [Google Scholar]
- 13.Cepaityte D, Siafis S, Papazisis G. Safety of antipsychotic drugs: a systematic review of disproportionality analysis studies. Behav Brain Res. 2021;404:113168. doi: 10.1016/j.bbr.2021.113168 [DOI] [PubMed] [Google Scholar]
- 14.Soria-Chacartegui P, Villapalos-Garcia G, Zubiaur P, Abad-Santos F, Koller D. Genetic polymorphisms associated with the pharmacokinetics, pharmacodynamics and adverse effects of olanzapine, aripiprazole and risperidone. Front Pharmacol. 2021;12:711940. doi: 10.3389/fphar.2021.711940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Liu H, Wang W, Wang Y, Xiu M, Li S. Carnitine metabolites and cognitive improvement in patients with schizophrenia treated with olanzapine: a prospective longitudinal study. Front Pharmacol. 2023;14:1255501. doi: 10.3389/fphar.2023.1255501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding J, Zhang Y, Zhang Y, et al. Effects of age, sex, and comedication on the plasma concentrations of olanzapine in Chinese patients with schizophrenia based on therapeutic drug monitoring data. J Clin Psychopharmacol. 2022;42(6):552–559. doi: 10.1097/JCP.0000000000001618 [DOI] [PubMed] [Google Scholar]
- 17.Karwautz A, Zeiler M, Schwarzenberg J, et al. Therapeutic drug monitoring in adolescents with anorexia nervosa for safe treatment with adjunct olanzapine. Eur Eat Disord Rev. 2023. doi: 10.1002/erv.3022 [DOI] [PubMed] [Google Scholar]
- 18.Miroshnichenko II, Pozhidaev IV, Ivanova SA, Baymeeva NV. Therapeutic drug monitoring of olanzapine and cytochrome P450 genotyping in nonsmoking subjects. Ther Drug Monit. 2020;42(2):325–329. doi: 10.1097/FTD.0000000000000695 [DOI] [PubMed] [Google Scholar]
- 19.Xiao T, Wang Z, Li G, et al. What to do about missed doses? A retrospective study of olanzapine in the elderly. Drug Des Devel Ther. 2021;15:3411–3423. doi: 10.2147/DDDT.S316110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu X, Hu J, Xiao T, Huang S, Wen Y, Shang D. An interpretable stacking ensemble learning framework based on multi-dimensional data for real-time prediction of drug concentration: the example of olanzapine. Front Pharmacol. 2022;13:975855. doi: 10.3389/fphar.2022.975855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwahashi K. Olanzapine metabolism by CYP1A2/CYP2D6 and hyperglycaemia. Acta Neuropsychiatr. 2004;16(4):229–230. doi: 10.1111/j.0924-2708.2004.00089.x [DOI] [PubMed] [Google Scholar]
- 22.Rojas-Macetas A, Medalla-Garro G, Saravia M, et al. Potential polymorphic CYP1A2 and CYP2D6-mediated pharmacokinetic interactions between risperidone or olanzapine and selected drugs intended to treat COVID-19. Drug Metab Bioanal Lett. 2022. doi: 10.2174/1872312815666221125112724 [DOI] [PubMed] [Google Scholar]
- 23.Xin Y, Gao L, Tuo Y, et al. Understanding inter-individual variability in pharmacokinetics/pharmacodynamics of aripiprazole in children with tic disorders: individualized administration based on physiological development and CYP2D6 genotypes. Front Pharmacol. 2022;13:1048498. doi: 10.3389/fphar.2022.1048498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islam F, Marshe VS, Magarbeh L, et al. Effects of CYP2C19 and CYP2D6 gene variants on escitalopram and aripiprazole treatment outcome and serum levels: results from the CAN-BIND 1 study. Transl Psychiatry. 2022;12(1):366. doi: 10.1038/s41398-022-02124-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kneller LA, Zubiaur P, Koller D, Abad-Santos F, Hempel G. Influence of CYP2D6 phenotypes on the pharmacokinetics of aripiprazole and dehydro-aripiprazole using a physiologically based pharmacokinetic approach. Clin Pharmacokinet. 2021;60(12):1569–1582. doi: 10.1007/s40262-021-01041-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiu M, Zhao L, Sun Q, Lang X. Efficacy of low-dose olanzapine in combination with sertraline on negative symptoms and psychosocial functioning in schizophrenia: a randomized controlled trial. Curr Neuropharmacol. 2023. doi: 10.2174/1570159X21666230913152344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brothwood PL, Husain M, Pinson J, Oloyede E, Davey P, Whiskey E. Clozapine in combination with olanzapine long-acting injection: the intersection of treatment-resistant schizophrenia and poor medication adherence-a case report. J Clin Psychopharmacol. 2023;43(5):472–474. doi: 10.1097/JCP.0000000000001746 [DOI] [PubMed] [Google Scholar]
- 28.Liu JL, Tan ZM, Jiao SJ. Repetitive transcranial magnetic stimulation combined with olanzapine and amisulpride for treatment-refractory schizophrenia. World J Psychiatry. 2023;13(7):453–460. doi: 10.5498/wjp.v13.i7.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L, Mills R, Sadler BM, Rege B. Population pharmacokinetics of olanzapine and samidorphan when administered in combination in healthy subjects and patients with schizophrenia. J Clin Pharmacol. 2021;61(11):1430–1441. doi: 10.1002/jcph.1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708 [DOI] [PubMed] [Google Scholar]
- 31.Horacek J, Bubenikova-Valesova V, Kopecek M, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20(5):389–409. doi: 10.2165/00023210-200620050-00004 [DOI] [PubMed] [Google Scholar]
- 32.Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(3):CD006654. doi: 10.1002/14651858.CD006654.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ennis C, Kemp JD, Cox B. Characterisation of inhibitory 5-hydroxytryptamine receptors that modulate dopamine release in the striatum. J Neurochem. 1981;36(4):1515–1520. doi: 10.1111/j.1471-4159.1981.tb00594.x [DOI] [PubMed] [Google Scholar]
- 34.Matsui-Sakata A, Ohtani H, Sawada Y. Pharmacokinetic-pharmacodynamic analysis of antipsychotics-induced extrapyramidal symptoms based on receptor occupancy theory incorporating endogenous dopamine release. Drug Metab Pharmacokinet. 2005;20(3):187–199. doi: 10.2133/dmpk.20.187 [DOI] [PubMed] [Google Scholar]
- 35.Mao JH, Han L, Liu XQ, Jiao Z. Significant predictors for olanzapine pharmacokinetics: a systematic review of population pharmacokinetic studies. Expert Rev Clin Pharmacol. 2023;16(6):575–588. doi: 10.1080/17512433.2023.2219055 [DOI] [PubMed] [Google Scholar]
- 36.Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine: pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet. 1999;37(3):177–193. doi: 10.2165/00003088-199937030-00001 [DOI] [PubMed] [Google Scholar]
- 37.Bigos KL, Pollock BG, Coley KC, et al. Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol. 2008;48(2):157–165. doi: 10.1177/0091270007310385 [DOI] [PubMed] [Google Scholar]
- 38.Beasley CM, Sanger T, Satterlee W, Tollefson G, Tran P, Hamilton S. Olanzapine versus placebo: results of a double-blind, fixed-dose olanzapine trial. Psychopharmacology. 1996;124(1–2):159–167. doi: 10.1007/BF02245617 [DOI] [PubMed] [Google Scholar]
- 39.Solmi M, Fornaro M, Ostinelli EG, et al. Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects. World Psychiatry. 2020;19(2):214–232. doi: 10.1002/wps.20765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen X, Chen X, Lu J, et al. Pharmacogenetics-based population pharmacokinetic analysis and dose optimization of valproic acid in Chinese southern children with epilepsy: effect of ABCB1 gene polymorphism. Front Pharmacol. 2022;13:1037239. doi: 10.3389/fphar.2022.1037239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thyssen A, Vermeulen A, Fuseau E, Fabre MA, Mannaert E. Population pharmacokinetics of oral risperidone in children, adolescents and adults with psychiatric disorders. Clin Pharmacokinet. 2010;49(7):465–478. doi: 10.2165/11531730-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 42.Chen CY, Zhou Y, Cui YM, Yang T, Zhao X, Wu Y. Population pharmacokinetics and dose simulation of oxcarbazepine in Chinese paediatric patients with epilepsy. J Clin Pharm Therapeutics. 2019;44(2):300–311. doi: 10.1111/jcpt.12792 [DOI] [PubMed] [Google Scholar]
- 43.Wang ZZ, Zhang YF, Huang WC, et al. Effects of comedication and genetic factors on the population pharmacokinetics of lamotrigine: a prospective analysis in Chinese patients with epilepsy. Front Pharmacol. 2019;10:832. doi: 10.3389/fphar.2019.00832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirose T, Mamiya N, Yamada S, Taguchi M, Kameya T, Kikuchi T. The antipsychotic drug aripiprazole (abilify). Nihon Yakurigaku Zasshi. 2006;128(5):331–345. doi: 10.1254/fpj.128.331 [DOI] [PubMed] [Google Scholar]
- 45.Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763–771. doi: 10.4088/jcp.v63n0903 [DOI] [PubMed] [Google Scholar]
- 46.Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164(9):1411–1417. doi: 10.1176/appi.ajp.2007.06091479 [DOI] [PubMed] [Google Scholar]
- 47.Sarkisyan G, Roberts AJ, Hedlund PB. The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior. Behav Brain Res. 2010;209(1):99–108. doi: 10.1016/j.bbr.2010.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fraguas D, Almenta Gallego D, Arques-Egea S, et al. Aripiprazole for the treatment of schizophrenia: recommendations of a panel of Spanish experts on its use in clinical practice. Int J Psychiatry Clin Pract. 2022:1–10. doi: 10.1080/13651501.2022.2064308 [DOI] [PubMed] [Google Scholar]