With the development of disease-modifying drugs and biologics, remission has become the primary treatment goal for rheumatologic and inflammatory bowel diseases. In asthma, the concept of remission is nascent, although gaining in popularity. Recently, the Global Initiative for Asthma emphasized remission as a treatment goal for asthma. A 2020 expert consensus panel defined clinical remission as ≥ 12 months with (1) absence of significant symptoms based on a validated instrument, (2) lung function optimization or stabilization, (3) patient and provider agreement regarding remission, and (4) no use of systemic corticosteroids.1 Complete remission was defined as clinical remission in combination with objective resolution of asthma-related inflammation and, if appropriate, a negative bronchial hyperresponsiveness test. Remission off treatment required no asthma treatment for ≥ 12 months.
The term remission does not necessarily imply disease modification, nor does it imply cure, because modifying or curing disease requires reverting the disease process to a normal pathologic state and having no evidence of disease based on the absence of signs and symptoms without ongoing treatment. Asthma remission also differs from disease control. Although control generally implies minimal recent symptoms and management of exacerbation risk and lung function decline, remission is a more durable absence of symptoms, with elimination of exacerbations requiring systemic corticosteroids and stabilization of lung function, ideally coupled with elimination of inflammation.1 Remission can have a bidirectional relationship with control because patients with well-controlled asthma are deemed to be in remission over time. Recent innovations in asthma therapeutics, particularly regarding biologics, have raised the bar for successful treatment from achieving control to the increasing possibility of inducing remission.
Advances in our understanding of asthma phenotypes and inflammatory mechanisms have led to the advent of targeted therapies that cause significant clinical improvements in moderate to severe asthma, with a proportion of patients achieving control that can be categorized reasonably as remission. Importantly, the therapeutic effects of biologics largely are dependent on the presence of active type 2 inflammation, for which the treatment benefits of biologics are three-fold to five-fold greater in those with severe airways disease and elevated biomarkers (ie, a raised blood eosinophil count, fractional exhaled nitric oxide, or both) than in those without type 2 inflammation.2
Control and remission occur on a continuum. In recent years, remarkable outcomes have been reported in patients receiving biologics defined as a “super response” or a “complete response.”3 A super response is accomplished in approximately one-third of patients with severe asthma treated with biologics and likely is the precursor to achieving remission.4 The challenge is how to consolidate and clarify this overlapping terminology and furthermore to identify the clinical usefulness of these terms and whether they describe different entities or simply varying thresholds of optimal control. The critical tenets of both definitions focus on the elimination of exacerbations and oral corticosteroids as well as concomitant improvements in asthma control. None of the definitions include measures of asthma quality of life nor requirements for changes in background inhaled corticosteroid (ICS) therapies. Moreover, neither the remission nor the super response definition has been adopted into widespread clinical practice to date, and it remains to be seen how these terms will be used to define response in the future, both in real-world and research settings.
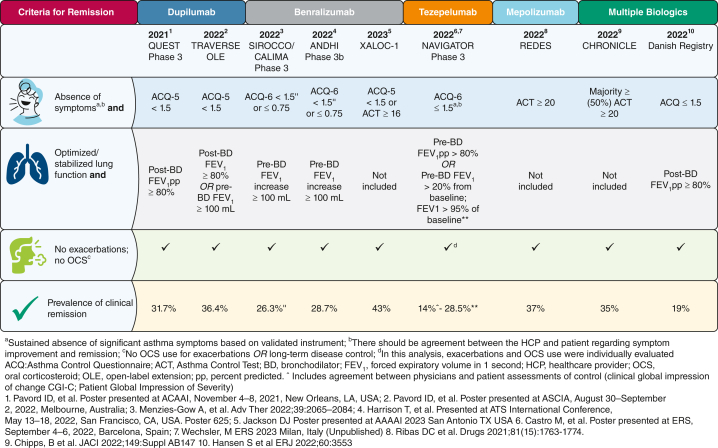
Recently, remission was assessed in post hoc analyses of both clinical trials and clinical cohorts with an aim of identifying the prevalence of remission in patients receiving biologics (Fig 1). The criteria used to define remission in this inherently more severe population included elimination of exacerbations, lack of use of oral corticosteroids, and achieving optimal asthma control. Lung function criteria were used variably, with a focus on either achieving normal lung function (FEV1 > 80%) or an increase in FEV1 of 100 mL, the minimally clinically important difference. Studies varied between randomized controlled trials and real-world studies lasting 6 to 12 months, questioning whether remission was being defined over a shorter duration than has been recommended. Between 15% and 37% of patients achieved remission, although remission was defined independently and thus heterogeneously (Fig 1). Predictors of remission include the presence of a shorter duration of illness, younger age, preserved lung function at initiation of biological therapy, and either lower or no maintenance oral corticosteroids. The presence of markedly elevated blood eosinophil levels (> 0.5-0.7 × 109/L for anti-IL-4R, anti-IL-5, and anti-thymic stromal lymphopoietin) and fractional exhaled nitric oxide (> 40-50 parts per billion for anti-IL-4R and anti-thymic stromal lymphopoietin) are important predictors of remission,2 suggesting that earlier prediction (using clinical characteristics and biomarkers) and action (using type 2-targeting antiinflammatory therapy) may increase the odds of remission. Finally, although maintenance oral steroids historically have been used to achieve asthma control, studies including oral steroid-dependent populations still demonstrate a high level of morbidity as indicated by a high frequency of exacerbations, poor control, and increased prevalence of comorbidities. The use of maintenance oral steroids to achieve optimal control and even remission is not encouraged.
Figure 1.
Early attempts to define clinical remission include composite end points.
It is notable that a substantial proportion of patients receiving placebo in these studies were able to achieve remission criteria while receiving medium and high doses of ICSs or long-acting β-agonists. This highlights both the critical importance of good asthma care and also the potential impact of ICSs on mitigating inflammation and increasing the likelihood of achieving remission. This is not surprising because spontaneous remission has been observed in asthma and typically occurs during the transition from childhood to adulthood, with up to 20% of children experiencing complete remission. Although spontaneous remission has been reported in 5% to 18% of adult-onset asthma, several cohorts have higher levels of remission,5 particularly those with mild disease, allergies, preserved lung function, better asthma control, younger age, early onset asthma, shorter duration of illness, less bronchial hyperresponsiveness, fewer comorbidities, and former tobacco use or never tobacco use status. Hence, aggressive management of disease should occur at an earlier stage in those at greatest risk of poor outcomes and disease progression.
Interestingly, none of the current definitions of remission take into consideration the risks associated with exposure to high-dose ICSs over time and have not included reduction of ICS therapy as a criterion for remission. Studies are ongoing that will determine whether remission can be maintained using biologics while reducing ICS exposure, including the Multicentre, Randomised, Open-Label, Parallel-Group, Active-Controlled, Phase IV Study to Assess the Reduction of Daily Maintenance ICS/LABA Treatment Towards Anti-Inflammatory Reliever Treatment in Patients With Severe Eosinophilic Asthma Treated With Benralizumab (SHAMAL) study.6
Clinical remission with treatment now is an achievable goal for patients with severe asthma, and more work needs to be carried out to identify additional therapies or strategies to increase the likelihood of achieving remission. As a respiratory community, we need to embrace the idea of disease remission in asthma, to work toward its standardization, and to share this aspirational goal with patients. More research is needed to define the populations that stand to benefit the most from earlier introduction of biologic therapies, and these findings need to be replicated in other real-world cohorts with longer duration of follow-up. Although current therapies can achieve on-treatment remission, it is yet unknown whether these therapies or new therapies under development can induce off-treatment remission in selected patients, bringing us one step closer to the possibility of disease modification and cure.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: N. L. L. received consulting fees from Amgen, AstraZeneca, Avillion, Genentech, GlaxoSmithKline (GSK), Novartis, Regeneron, Sanofi, and Teva; honoraria for nonspeakers bureau presentations from GSK and AstraZeneca; and travel support from AstraZeneca; her institution received research support from Amgen, AstraZeneca, Avillion, Evidera, Gossamer Bio, Genentech, GSK, Regeneron, Sanofi, Novartis and Teva. She is an honorary faculty member of Observational and Pragmatic Research Institute (OPRI) but does not receive compensation for this role. P. A. is a research-industry/investigator initiated consultant for AstraZeneca, GSK, Regeneron, Sanofi, National Institutes of Health, and American Partnership for Eosinophilic Disorders; P. A. also receives royalties from UpToDate. S. C. has received nonrestricted research grants from the National Institute for Health and Care Research Oxford Biomedical Research Centre, AstraZeneca, bioMérieux, Sanofi-Genyme-Regeneron, and the Quebec Respiratory Health Research Network; he is the holder of the Association Pulmonaire du Québec’s Research Chair in Respiratory medicine; he received speaker honoraria from AstraZeneca, GSK, Sanofi-Regeneron, and Valeo Pharma; he received consultancy fees for FirstThought, AstraZeneca, GSK, and Sanofi-Regeneron; he has received sponsorship to attend/speak at international scientific meetings by/for AstraZeneca. He is an advisory board member and will have stock options for Biometry Inc – a company which is developing a FeNO device (myBiometry). M. E. W. has received consulting, advisory, or speaking honoraria from Amgen, AstraZeneca, Avalo Therapeutics, Boehringer Ingelheim, Cerecor, Cohero Health, Cytoreason, Eli Lilly, Equillium, GSK, Incyte, Kinaset, Novartis, Om Pharma, Phylaxis, Pulmatrix, Rapt Therapeutics, Regeneron, Restorbio, Roche/Genentech, Sanofi/Genzyme, Sentien, Sound Biologics, Tetherex Pharmaceuticals, Teva, and Upstream Bio. None declared (A. M., S. R.).
References
- 1.Menzies-Gow A., Bafadhel M., Busse W.W., et al. An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol. 2020;145(3):757–765. doi: 10.1016/j.jaci.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Couillard S., Côté A. Predicting on-biologic remission in asthma: insight from the airways. Chest. 2023;163(6):1341–1343. doi: 10.1016/j.chest.2023.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Upham J.W., Le Lievre C., Jackson D.J., et al. Defining a severe asthma super-responder: findings from a Delphi process. J Allergy Clin Immunol Pract. 2021;9(11):3997–4004. doi: 10.1016/j.jaip.2021.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Harvey E.S., Langton D., Katelaris C., et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.02420-2019. [DOI] [PubMed] [Google Scholar]
- 5.Harvey C.J., Thimmulappa R.K., Sethi S., et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med. 2011;3(78) doi: 10.1126/scitranslmed.3002042. 78ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institutes of Health Clinical Center . National Institutes of Health; 2019. A study to assess the reduction of daily maintenance ICS/LABA treatment towards anti-inflammatory reliever treatment in patients with severe eosinophilic asthma treated with Benralizumab. NCT04159519. ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT04159519 Updated February 16, 2023. [Google Scholar]