Abstract
Background
Guided bronchoscopy is increasingly used to diagnose peripheral pulmonary lesions (PPLs). A meta-analysis published in 2012 demonstrated a pooled diagnostic yield of 70%; however, recent publications have documented yields as low as 40% and as high as 90%.
Research Question
Has the diagnostic yield of guided bronchoscopy in patients with PPLs improved over the past decade?
Study Design and Methods
A comprehensive search was performed of studies evaluating the diagnostic yield of differing bronchoscopic technologies used to reach PPLs. Study quality was assessed using the Quality assessment of diagnostic accuracy of studies (QUADAS-2) assessment tool. Number of lesions, type of technology used, overall diagnostic yield, and yield by size were extracted. Adverse events were recorded. Meta-analytic techniques were used to summarize findings across all studies.
Results
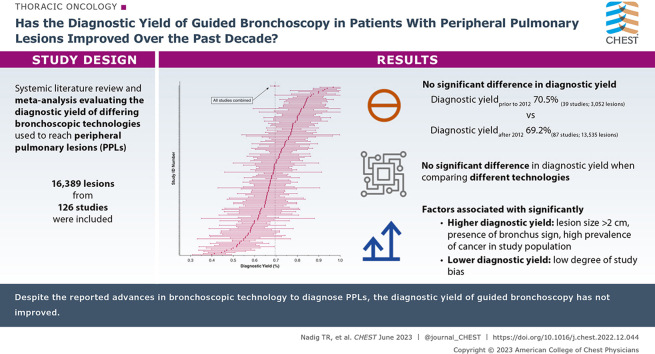
A total of 16,389 lesions from 126 studies were included. There was no significant difference in diagnostic yield prior to 2012 (39 studies; 3,052 lesions; yield 70.5%) vs after 2012 (87 studies; 13,535 lesions; yield 69.2%) (P > .05). Additionally, there was no significant difference in yield when comparing different technologies. Studies with low risk of overall bias had a lower diagnostic yield than those with high risk of bias (66% vs 71%, respectively; P = .018). Lesion size > 2 cm, presence of bronchus sign, and reports with a high prevalence of malignancy in the study population were associated with significantly higher diagnostic yield. Significant (P < .0001) between-study heterogeneity was also noted.
Interpretation
Despite the reported advances in bronchoscopic technology to diagnose PPLs, the diagnostic yield of guided bronchoscopy has not improved.
Key Words: bronchoscopy, lung cancer, navigational bronchoscopy, pulmonary nodule, radial endobronchial ultrasound, robotic bronchoscopy
Graphical Abstract
Take-home Points.
Study Question: Has the diagnostic yield of guided bronchoscopic techniques for peripheral lung lesions improved over the last decade?
Results: A total of 16,389 lesions from 126 studies were included. There was no significant difference in diagnostic yield prior to 2012 (39 studies; 3,052 lesions; yield 70.5%) vs after 2012 (87 studies; 13,535 lesions; yield 69.2%) (P > .05) There was no significant difference in yield when comparing different bronchoscopic technologies to one another. The diagnostic yield was significantly higher in patients with larger lesions and in those with the presence of bronchus sign, and significantly lower in studies which had low degree of study bias.
Interpretation: Comparing the decade prior to and after 2012, the diagnostic yield of guided bronchoscopy for the evaluation of peripheral lung lesions has not improved despite reported advancements in technology and thousands of additional patients added to the medical literature.
Because of the ubiquitous use of diagnostic CT scan and the implementation of lung cancer screening, the number of pulmonary nodules detected yearly continues to increase. A 2015 study showed that approximately 5 million US adults undergo chest CT scan annually, with roughly 1.5 million pulmonary nodules detected on those scans.1 Only a fraction (5.2%) of these patients received a diagnosis of lung cancer within 2 years of identification.1 Therefore, most lesions identified are benign and may not require invasive procedures, highlighting the importance of appropriate diagnosis. The approach to evaluation of a pulmonary nodule begins with the assessment of the pretest probability of cancer (pCA) that the nodule represents a lung cancer. Low-risk nodules with a pCA < 5% can be managed with watchful waiting using serial CT scan, and high-risk nodules (pCA > 65%) can be managed with tissue biopsy or surgical excision.2 Nodules with intermediate risk (pCA between 5% and 65%) of lung cancer are considered for further evaluation with PET scan or nonsurgical biopsy including transthoracic needle biopsy or bronchoscopy.2
We published a meta-analysis in 2012 which included 39 studies, involving 3,004 patients with 3,052 peripheral pulmonary lesions (PPLs). We documented a diagnostic yield of 70% for guided bronchoscopy with a pooled risk for pneumothorax of 1.5%.3 Over the past 10 years, newer technologies have emerged, and established technologies have been used with increasing frequency in both academic and community centers. We undertook this study to update our meta-analysis of guided bronchoscopy for PPLs and to determine if advances in technology and widespread utilization have had an effect on diagnostic yield.
Study Design and Methods
Materials and Methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies statement.4
Literature Search
A database search using a predetermined search strategy (Table 1) was performed by review authors (T. R. N., N. A. T., and G. A. S.) using the following databases: PubMed (MEDLINE), Embase, ScienceDirect (www.sciencedirect.com), LILACS (www.scielo.org), Clinical Trials (ClinicalTrials.gov), Cochrane Central Register of Controlled Trials, and Scirus (www.scirus.com/srsapp). The literature review was stopped on August 15, 2021, but was updated to include the 24-month follow up of the NAVIGATE trial.5 Bibliographies of included studies and review articles were abstracted manually for additional relevant studies to ensure that all articles were captured.
Table 1.
Brief Literature Search Strategy
|
|
|
|
|
|
Selection of Studies
All articles identified by our search strategy were independently assessed by two authors (T. R. N. and G. A. S.) for inclusion in this meta-analysis. Discordance was resolved by consensus. Both prospective and retrospective studies were evaluated for inclusion. All studies that reported the use of any of the following technologies to evaluate PPLs were considered for inclusion: robotic bronchoscopy, electromagnetic navigational bronchoscopy (ENB), virtual bronchoscopy (VB), radial endobronchial ultrasound (R-EBUS) ± guide sheath (GS), and thin/ultrathin bronchoscope ± GS. All included studies documented the diagnostic yield of guided bronchoscopy. Case reports, studies with < 10 patients, studies that included linear EBUS, review articles, letters, papers not available in English, or studies in which data to calculate diagnostic yield were insufficient were excluded. When two or more studies were published by the same author(s), the methods sections were reviewed to ensure study dates were not overlapping. If dates were overlapping or the authors stated explicitly that data was derived from a previously published study, the study with the largest sample size was included.
Data Extraction
All data were reviewed and extracted by review authors (T. R. N. and N. T.). The following information was collected: first author, publication year, publication type (retrospective or prospective), type of technology used, number of participants, number of nodules, diagnosis (malignant or benign), nodule size, number of lesions with a bronchus sign, sensitivity of malignancy, and complications of the procedure. The diagnostic yield was calculated from the extracted data and compared with the reported diagnostic yield. Although most diagnoses were malignant (primary lung or metastatic disease), other benign causes of the PPLs (eg, TB, sarcoidosis) were also considered diagnostic. The yield by size (≤ 20 or > 20 mm) was recorded where available.
Quality Assessment
The Quality assessment of diagnostic accuracy of studies (QUADAS-2) tool was used to evaluate the degree of bias of the included studies. The tool consists of 14 signaling questions which assess two aspects of study quality: risk of bias and applicability of the study. Risk of bias is scored across four domains: patient selection, reference standard, index test, and flow and timing. We tailored the QUADAS-2 questions to our study question (Table 2). We used this approach to categorize the included studies as either low risk (ie, low on each of the four domains) or high risk of bias (ie, high on at least one of the four domains). Each study was reviewed independently by two reviewers (T. R. N., J. L., N. A. T., N. T. T., N. J. P., and J. S. W. M.). If there was discordance in the QUADAS-2 results between the two reviewers, it was discussed with a third reviewer (G. A. S.) until consensus was reached. e-Appendix 1 details the QUADAS-2 findings of each study.
Table 2.
Modified Quality Assessment of Diagnostic Accuracy of Studies Questions
| Domain | Original Signaling Question | Customized for the Purpose of Our Study |
|---|---|---|
| Risk of bias | ||
| Patient selection | Was a consecutive or random sample of patients enrolled? | Not changed |
| Was a case-control design avoided? | Not changed | |
| Did the study avoid inappropriate exclusions? | If study had specific inclusion criteria (eg, size of lesion, characteristic of lesion [ground-glass opacities]) or included only known malignant lesions which could alter the yield, they were considered high risk of bias | |
| Index text | Were the index test results interpreted without knowledge of the results of the reference standard? |
Answer was always yes |
| If a threshold was used, was it prespecified? | Not applicable for this study | |
| Reference standard | Is the reference standard likely to correctly classify the target condition? |
We included the following tests as reference standard for corroboration of the bronchoscopy results:
|
| Were the reference standard results interpreted without knowledge of the results of the index test? |
Not applicable for this study | |
| Flow and timing | Was there an appropriate interval between index tests and reference standard? |
Not applicable for this study |
| Did all patients receive a reference standard? | If nonmalignant lesions underwent a second test (CT scan-guided biopsy/surgery/radiologic surveillance for at least 1 y), they were considered low risk of bias | |
| Did all patients receive the same reference standard? | Not applicable for this study | |
| Were all patients included in the analysis? | If all the patients who underwent the procedure were included in the analysis, then it was considered low risk. If a patient was lost to follow up, or was excluded in the main study after being initially included, then it was high risk of bias. Exclusion of patients with endobronchial disease was deemed acceptable. | |
| Concerns about applicability | ||
| Patient selection | Are there concerns that the included patients do not match the review question? |
Not changed |
| Index test | Are there concerns that the index test, its conduct, or its interpretation differ from the review question? |
If they used technology which is not commonly used, then there was high concern for applicability |
| Reference standard | Are there concerns that the target condition as defined by the reference standard does not match the review question? |
If at least 12 mo follow up was used as reference standard for negative bronchoscopy, then it was considered low concern. If no follow up was mentioned, then it was deemed unclear. |
Statistical Analysis
The meta-analysis methodology was similar to that described in our previous meta-analysis; the reported diagnostic yields from each study were pooled together using a weighting scheme based on the inverse variance of the diagnostic yield.6 Between-study heterogeneity was assessed via the Q statistic.6 Weighted mean diagnostic yields and their 95% CIs were reported for the entire group of publications, and for subgroups of publications and patient populations, including whether the publication was included or not included in our prior meta-analysis from 2012; whether it was deemed to have low vs high bias based on its QUADAS-2 assessment; whether it was retrospective, prospective/nonrandomized, or prospective/randomized; whether individual nodules were < 20 or ≥ 20 mm; and what type of technology was reportedly used in the study (ie, R-EBUS ± GS, ultrathin/thin ± GS, VB, ENB, robotic, ENB + R-EBUS, VB + R-EBUS, ultrathin/thin + R-EBUS ± GS, ultrathin/thin + VB, ultrathin/thin +VB + R-EBUS, other combinations). Comparisons in diagnostic yield between subgroups of studies were made using inverse variance-weighted two-sample t tests. A paired t test was used to compare diagnostic yields among nodules < 20 mm in diameter and on nodules > 20 mm, for studies that reported yields for both nodule size groups. A paired t test was also used to compare yields among nodules in patients with bronchus present vs absent. The proportion of studies which were deemed to be of high bias according to the QUADAS-2 assessment was also reported across all studies and for each technology grouping.
Results
Literature Search and Study Selection
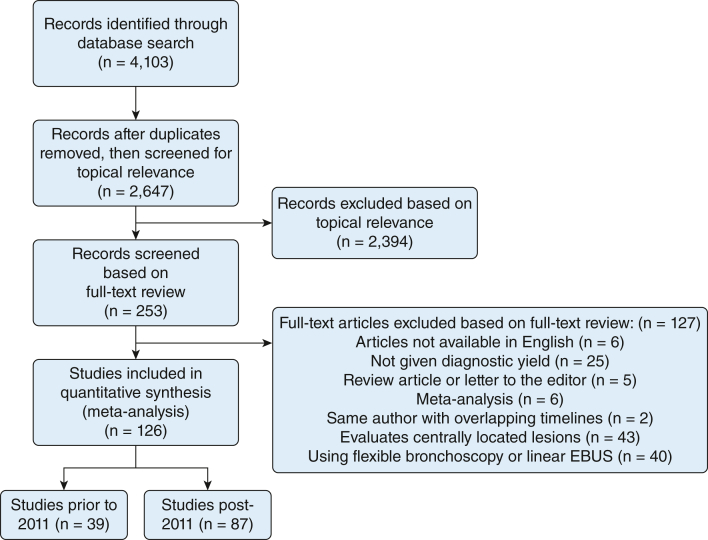
Using the search algorithm, we identified 4,103 studies for consideration. After thorough review, 126 studies met the inclusion criteria. The included studies were published between 2002 and August 2021, except for the NAVIGATE trial 24-month results, which were published in April 2022.5 A summary of the literature search following the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies statement is shown in Figure 1.
Figure 1.
Literature search and selection. EBUS = endobronchial ultrasound.
Study Characteristics
A total of 16,077 patients with 16,389 lesions from the 126 studies were included in this meta-analysis. Of those, 39 studies with 3,052 lesions were included prior to 2011 and 87 studies with 13,535 lesions were included post-2011. Of these, 60 studies were retrospective, 55 were prospective nonrandomized, and 11 were randomized controlled trials. Nineteen studies had two or more arms, which resulted in a total of 149 arms included to calculate yield. The average nodule size ± SD was 24.4 ± 5.53 mm. e-Appendix 2 lists the study characteristics and summarizes the findings for each of the studies.
Overall Diagnostic Yield and Yield by Technology
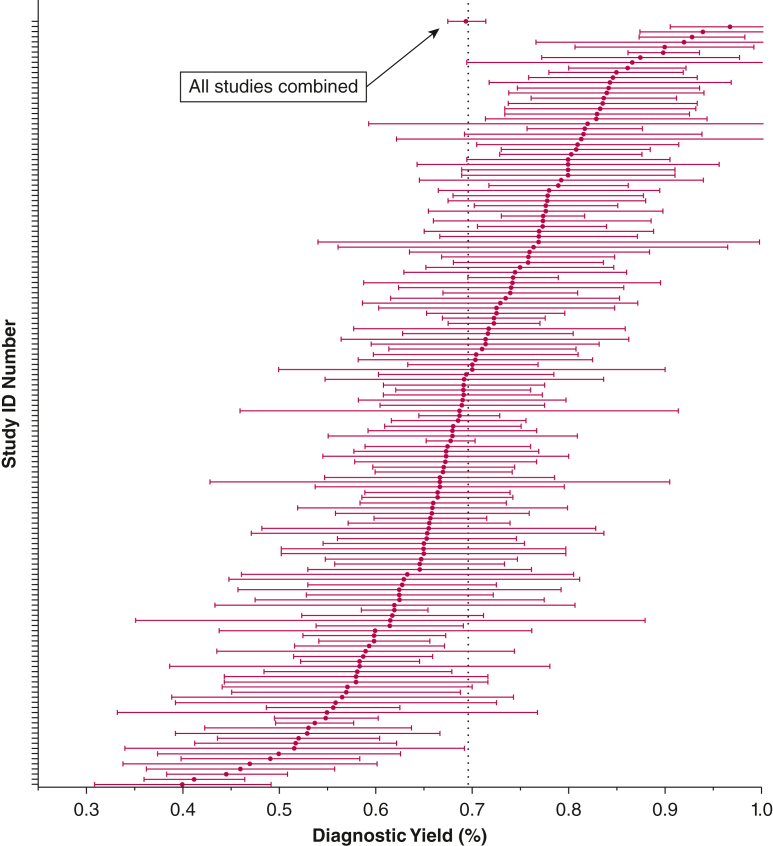
The inverse variance weighted pooled diagnostic yield of 126 studies was 69.4% (95% CI, 0.67-0.71). Across the studies, the diagnostic yield varied from 40% to 96.8% (Fig 2). The Q statistic, a measure of between-study heterogeneity was 1,177.56, with 148 df (P < .0001), indicating a high degree of heterogeneity.
Figure 2.
Overall summary of the diagnostic yields of the studies included in this meta-analysis. Error bars reflect 95% CIs. ID = identification.
There was no difference in diagnostic yield when comparing studies before and after 2011 (69% vs 71%, respectively; P > .05). Similarly, there was no significant difference when comparing study design: retrospective (60 studies) vs prospective nonrandomized (55 studies) vs prospective randomized trials (11 studies) (71% vs 68.5% vs 66.5%, respectively; P > .05). Eighty-one studies evaluated the effect of size > 20 mm (5,948 lesions) or ≤ 20 mm (4,707 lesions) on diagnostic yield. The weighted diagnostic yield of lesions > 20 mm was significantly greater than those ≤ 20 mm (78.7%; 95% CI, 76.2-81.3 vs 58.7%; 95% CI, 55.1-62.3, respectively; P < .001). The presence of bronchus sign was reported in 40 studies which included 4,291 lesions. The pooled diagnostic yield for lesions with the presence of bronchus sign was 78.6% (95% CI, 75.2-82.0), which was significantly greater (P < .0001) than among lesions with no bronchus sign present (51.2%; 95% CI, 45.0%-57.4%). In our meta-analysis, diagnostic yield was strongly correlated with prevalence of malignancy in the study population (correlation coefficient ρ = 0.57; P < .0001).
Diagnostic Yield by Technology
R-EBUS ± GS was the most prevalent technology (5,494 lesions) followed by ENB (1,952 lesions); VB + R-EBUS (1,048 lesions) with robotic bronchoscopy was the least studied technology to date (483 lesions) (Table 3). The weighted diagnostic yield of the different technologies when compared with each other and with the total pooled diagnostic yield was not significantly different Table 4).
Table 3.
Study Characteristics and Diagnostic Yield, Summarized Across Relevant Subgroups
| Publication /Patient Population | No. of Study Arms | Total No. of Nodules Included | No. of Nodules Per Study, Median (range) |
Diagnostic Yield, Mean (95% CI) |
|---|---|---|---|---|
| All publications | 149 | 16,389 | 65 (11-1,329) | 69.4% (67.5%-71.4%) |
| Included in prior meta-analysis | 39 | 2,854 | 53 (13-279) | 70.5% (67.3%-73.7%) |
| Not included | 110 | 13,535 | 72 (11-1,329) | 69.2% (66.9%-71.6%) |
| QUADAS-2: low bias | 43 | 5,695 | 99 (13-581) | 66.1% (62.5%-69.7%) |
| QUADAS-2: high bias | 106 | 10,694 | 56 (11-1,329) | 71.0% (68.8%-73.3%)a |
| Retrospective | 69 | 7,979 | 84 (11-760) | 71.0% (67.9%-74.0%) |
| Prospective, not RCT | 59 | 5,548 | 54 (13-1,329) | 68.5% (65.8%-71.3%) |
| Prospective, RCT | 21 | 2,862 | 102 (20-340) | 66.5% (61.0%-72.0%) |
| Nodule diameter, mm | ||||
| < 20 | 81 | 4,707 | 81 (4-661) | 62.5% (58.3%-66.7%) |
| ≥ 20 | 75 | 5,948 | 86 (9-679) | 79.7% (77.1%-82.4%)b |
| Bronchus sign present | 40 | 4,291 | 179 (7-777) | 78.6% (75.2%-82.0%)c |
| Bronchus sign absent | 36 | 2,223 | 64 (3-255) | 51.2% (45.0%-57.4%) |
QUADAS-2 = Quality assessment of diagnostic accuracy of studies; RCT = randomized controlled trial.
P = .018 when compared with the yield among publications with QUADAS-2 low bias.
P < .0001 when compared with the yield when assessing nodules < 20 mm.
P < .0001 when compared with the yield when bronchus sign was absent.
Table 4.
Study Characteristics and Diagnostic Yield, Summarized Across Types of Technology Used
| Technology Used | No. of Study Arms | Proportion of Study Arms With High Bias, % | Total No. of Nodules Included | No. of Nodules Per Study, Median (range) |
Diagnostic Yield, Mean (95% CI) |
|---|---|---|---|---|---|
| R-EBUS ± GS | 51 | 78.4 | 5,494 | 83 (11-760) | 70.9% (67.9%-73.9%) |
| ENB | 24 | 75.0 | 1,952 | 53.5 (13-279) | 74.0% (68.6%-79.4%) |
| ENB + R-EBUS | 15 | 73.3 | 2,913 | 56 (26-1,329) | 66.5% (59.8%-73.3%) |
| VB + R-EBUS | 13 | 76.9 | 1,048 | 55 (12-334) | 76.4% (72.7%-80.1%) |
| Ultrathin or thin + VB | 10 | 80.0 | 795 | 63 (25-167) | 69.9% (62.4%-77.3%) |
| Ultrathin or thin + R-EBUS | 7 | 42.9 | 1,133 | 101 (20-467) | 62.6% (55.3%-70.0%) |
| Other combination | 7 | 57.1 | 771 | 63 (31-245) | 64.4% (49.0%-79.9%) |
| Ultrathin or thin | 6 | 50.0 | 770 | 104 (20-340) | 50.2% (37.3%-63.2%) |
| Ultrathin + VB + R-EBUS | 6 | 16.7 | 737 | 152.5 (32-179) | 67.3% (58.4%-76.2%) |
| Robotic | 6 | 66.7 | 483 | 56.5 (15-167) | 77.6% (70.4%-84.8%) |
| VB | 4 | 100.0 | 293 | 60.5 (50-122) | 72.4% (55.1%-89.7%) |
ENB = electromagnetic navigational bronchoscopy; GS = guide sheath; R-EBUS = radial endobronchial ultrasound; VB = virtual bronchoscopy.
Study Quality and Diagnostic Yield
Studies with low risk of bias had a pooled diagnostic yield significantly lower than those with a high risk of bias (66.1%; 95% CI, 62.5%-69.7% vs 71.0%; 95% CI, 68.8%-73.3%, respectively; P = .018). Table 3 summarizes the diagnostic yields of the various subgroups that were analyzed in this meta-analysis. Table 4 shows the proportion of studies which were deemed to be of high bias according to the QUADAS-2 assessment and were reported across all studies and for each technology grouping.
Safety
Complications (eg, pneumothorax, minor or major bleeding, respiratory failure, death) were reported in 111 of 126 studies including 14,683 patients. The overall adverse event rate was 3.9% ± 3.4% with most being pneumothorax (n = 295, 2.1% ± 1.9%). No episodes of death were reported (e-Appendix 2).
Discussion
There has been widespread adoption of guided bronchoscopy using differing technologies, all with a singular goal—to accurately diagnose peripheral pulmonary nodules. Here we update our previous meta-analysis and report several findings. First, since the publication of the previous meta-analysis, an additional 87 studies with 13,535 lesions biopsied have been added to the literature. However, despite the additional cases and another decade of experience with these technologies, the diagnostic yield has not improved. Second, size still matters and is an important predictor of diagnostic yield with a statistically significant 20% increase in yield for lesions > 2 cm. Third, studies with a low risk of bias reported a lower diagnostic yield compared with those with a high risk of bias, suggesting that how study end points are defined impacts the diagnostic yield reported. Finally, guided bronchoscopy remains a safe procedure with a total adverse event rate of 4% and pneumothorax rate of 2%, making bronchoscopy an attractive alternative to transthoracic needle biopsy in the appropriate clinical scenario.
Although the pooled diagnostic yield in this study was 69%, there was substantial variation among studies from a low of 40% to a high of 97%. The reasons for this are not entirely clear; however, when analyzing the data, some possible explanations emerge. First, the prevalence of cancer in the study population has a significant impact on diagnostic yield. In studies reporting higher yields, the prevalence of lung cancer is as high as 87%,7, 8, 9, 10 whereas when the prevalence of cancer was low, so too was the diagnostic yield.11 Further illustrating this point, in a single large prospective study analyzing different bronchoscopic approaches in a population with a cancer prevalence of 77%, the physician assessed pCA prior to the procedure impacted the diagnostic yield.12 Patients with pCA < 10%, those with 10% to 60%, and those with > 60% had diagnostic yields of 44%, 42%, and 77%, respectively (P < .001).12 Therefore, a high physician pCA prior to the procedure can, in essence, act as a proxy for high prevalence of disease. Second, the reported diagnostic yield from some studies may overestimate the true yield by using poor definitions for benign disease (eg, designating a true negative when pathology reported nonspecific inflammation or normal alveoli).13, 14, 15, 16, 17, 18, 19 By using the tailored QUADAS-2 tool, we were able to categorize studies as high and low risk of bias.20 Most of the studies with high risk of bias had poorly defined reference standards or poor flow and timing. In the 33 low bias studies, predefined criteria for a positive diagnosis and a well-defined reference standard are documented. Some of the most conservative (ie, low risk of bias) studies did not consider nonspecific inflammation as a positive diagnosis,11,21, 22, 23, 24, 25, 26, 27, 28, 29, 30 whereas others considered nonspecific inflammation as positive when resolution or stability was confirmed with a minimum follow up of 12 months.31, 32, 33, 34, 35 These studies report diagnostic yields significantly lower than studies with a high risk of bias and call into question whether diagnostic yield has been overreported. One could argue that by taking the conservative approach of considering only low bias studies, the benchmark for the pooled diagnostic yield for guided bronchoscopy should be reported out at 66%.
In a modeling exercise to assess diagnostic yield under differing conditions, Vachani et al36 generated a hypothetical cohort of 1,000 patients and applied various definitions of yield. They defined nonmalignant lesions on biopsy as either a documented specific benign diagnosis (SBD), a nonspecific benign finding (eg, inflammation), or a nondiagnostic result. Yield was characterized as strict, intermediate, or liberal. Modeling for strict criteria included malignant lesions + SBD for yield calculation, intermediate defined yield using malignant lesions + SBD + nonspecific benign finding, and liberal criteria included all of the aforementioned definitions plus nondiagnostic results. The diagnostic yields were 66.7% vs 74.1% vs 79.8% for strict, intermediate, and liberally defined, respectively. Therefore, the use of variable definitions of diagnostic yield may influence the interpretation of study results and limit the ability to compare findings across studies.36
It is worth commenting on two published meta-analyses which focused on single technologies, either R-EBUS or ENB.37,38 Unfortunately, it is difficult to compare those studies with this analysis because they have moved away from calculating diagnostic yield and instead report the sensitivity and specificity of malignancy as end points.37,38 The R-EBUS meta-analysis included 51 studies with 7,601 patients and had a pooled sensitivity for malignancy of 72% (95% CI, 70%-75%).37 Folch et al38 performed a meta-analysis on ENB which included 40 studies with 3,342 patients and had a pooled sensitivity for malignancy of 77% (95% CI, 72%-82%). Both meta-analyses noted a high degree of between-study heterogeneity. How to reconcile their findings with ours is difficult because the end points were different. Diagnostic yield remains the most frequently used metric reported in the literature to assess the performance of bronchoscopy; however, there remains no standardized approach to measurement of this outcome in these studies. A uniformed approach to how diagnostic yield is defined and used in comparative studies of bronchoscopic technologies is needed, and work is underway by professional societies to fill this gap.
This study has limitations. Some studies reported diagnostic yield in best- and worst-case scenarios based on strict and liberal inclusion of nonspecific inflammation in diagnostic yield calculations.5,39 We selected the stricter criteria for inclusion in yield calculation which provides a more conservative estimate of diagnostic yield. In addition, studies could have been classified as high bias because length of follow up was not documented after nonspecific inflammation was reported. Finally, our study methodology excluded non-English articles. This study focused on bronchoscopic technologies and not necessarily the adjuncts to it. Studies using additional imaging tools (eg, cone beam CT scan) have been reported; although we have included 5 studies which used cone beam CT scan as an adjunct imaging tool, there were not enough data to formally review for inclusion as a separate additional technology.17,27,40, 41, 42 This study also has several strengths. It includes > 16,000 patients, making it the largest meta-analysis assessing diagnostic yield of guided bronchoscopy for PPLs in the medical literature. Additionally, it covers 20 years of published data and compares differences between two decades of work. Finally, this study explores predictors of yield.
Placing these findings into clinical context is challenging. We have previously suggested that improvements in diagnostic yield will depend on three likely interrelated factors: technique, technology, and patient selection.12 The proportional contribution of each is impossible to discern; however, this study continues to inform the topic. We have no way of knowing how much physician technique influences yield from this study because physician volume or training is not recorded; however, previous studies in the surgical literature have shown a volume outcome relationship.43,44 This study did compare five technologies either alone or in combination and none outperformed the other. Furthermore, despite thousands of additional cases, four of the five procedure types showed no improvement in yield over time, suggesting that the technology has not significantly improved. The fifth and most recently added modality, robotic bronchoscopy, is too new to the field to assess improvements in yield over time. Several findings in this study do support the notion that patient selection remains an important factor in whether or not the diagnostic yield will be high. Those with larger lesions, those with a bronchus sign leading to the lesion, and bronchoscopies performed in populations where the prevalence of malignancy is high are more likely to yield a positive result as opposed to when some or all of those parameters are absent.
Interpretation
As we look toward the next decade with an attempt to move the needle (pun intended) for guided bronchoscopy, this study provides a roadmap by highlighting the importance of study design and execution. Well-designed comparative effectiveness trials with standardized definitions of diagnostic yield are needed. Other end points are similarly important but have yet to be captured in a systematic way. These include, but are not limited to, patient-level outcomes (eg, anxiety), need for second biopsy procedures, delay in diagnosis, and total cost. The diagnostic yield for bronchoscopy remains stuck at 70% despite an additional 10 years of advancements and thousands of additional cases. Doing more of the same is unlikely to improve this result.
Funding/Support
P. J. N.’s time on this study was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health [Grant UL1 TR001450].
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: N. T. T., N. J. P., and G. A. S. have received research funding from Olympus, Inc. G. A. S. has also received consulting from Olympus, Inc. N. J. P. and G. A. S. have received research funding from Auris inc, Johnson and Johnson, and G. A. S. has received consulting fees from Auris. None declared (T. R. N., N. T., P. J. N., J. L., J. S. W. M.).
Acknowledgments
Author contributions: T. R. N. and G. A. S. are the guarantors of the paper. T. R. N., N. A. T., and G. A. S. contributed to the manuscript conception, literature search, data extraction, QUADAS analysis, and manuscript editing. T. R. N., P. J. N., N. T. T., and G. A. S. contributed to manuscript conception, data analysis, manuscript writing, final editing, and review. T. R. N., N. A. T., J. L., N. T. T., N. J. P., and J. S. W. M. contributed to QUADAS analysis, manuscript writing, final editing, and review. All the authors approved the final version of the manuscript.
Role ofsponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank Teri Lynn Herbert, MS, MLIS, Associate Professor, Library, Medical University of South Carolina, Charleston, SC, for her contributions in literature search for this meta-analysis.
Additional information: The e-Appendixes are available online under "Supplementary Data."
Supplementary Data
References
- 1.Gould M.K., Tang T., Liu I.A., et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208–1214. doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 2.Gould M.K., Donington J., Lynch W.R., et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Memoli J.S., Nietert P.J., Silvestri G.A. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142(2):385–393. doi: 10.1378/chest.11-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McInnes M.D.F., Moher D., Thombs B.D., et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 5.Folch E.E., Bowling M.R., Pritchett M.A., et al. NAVIGATE 24-month results: electromagnetic navigation bronchoscopy for pulmonary lesions at 37 centers in Europe and the United States. J Thorac Oncol. 2022;17(4):519–531. doi: 10.1016/j.jtho.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Hedges L.V., Olkin I. Academic Press; 1985. Statistical Methods for Meta-Analysis. [Google Scholar]
- 7.Kurimoto N., Miyazawa T., Okimasa S., et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004;126(3):959–965. doi: 10.1378/chest.126.3.959. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa M., Sukoh N., Yamazaki K., et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest. 2007;131(6):1788–1793. doi: 10.1378/chest.06-2506. [DOI] [PubMed] [Google Scholar]
- 9.Yamada N., Yamazaki K., Kurimoto N., et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest. 2007;132(2):603–608. doi: 10.1378/chest.07-0637. [DOI] [PubMed] [Google Scholar]
- 10.Iwano S., Imaizumi K., Okada T., Hasegawa Y., Naganawa S. Virtual bronchoscopy-guided transbronchial biopsy for aiding the diagnosis of peripheral lung cancer. Eur J Radiol. 2009;79(1):155–159. doi: 10.1016/j.ejrad.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Ost D.E., Ernst A., Lei X., et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. results of the AQuIRE registry. Am J Respir Crit Care Med. 2016;193(1):68 77. doi: 10.1164/rccm.201507-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvestri G.A., Bevill B.T., Huang J., et al. An evaluation of diagnostic yield from bronchoscopy: the impact of clinical/radiographic factors, procedure type, and degree of suspicion for cancer. Chest. 2020;157(6):1656–1664. doi: 10.1016/j.chest.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Triller N., Dimitrijevic J., Rozman A. A comparative study on endobronchial ultrasound-guided and fluoroscopic-guided transbronchial lung biopsy of peripheral pulmonary lesions. Respir Med. 2011;105(Suppl 1):S74–S77. doi: 10.1016/S0954-6111(11)70015-4. [DOI] [PubMed] [Google Scholar]
- 14.Brownback K.R., Quijano F., Latham H.E., Simpson S.Q. Electromagnetic navigational bronchoscopy in the diagnosis of lung lesions. J Bronchology Interv Pulmonol. 2012;19(2):91–97. doi: 10.1097/LBR.0b013e31824dd9a1. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee S., Chacey M. Diagnostic yield of electromagnetic navigation bronchoscopy using a curved-tip catheter to aid in the diagnosis of pulmonary lesions. J Bronchology Interv Pulmonol. 2017;24(1):35–39. doi: 10.1097/LBR.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikezawa Y., Shinagawa N., Sukoh N., et al. Usefulness of endobronchial ultrasonography with a guide sheath and virtual bronchoscopic navigation for ground-glass opacity lesions. Ann Thorac Surg. 2016;103(2):470–475. doi: 10.1016/j.athoracsur.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Ali E.A., Takizawa H., Kawakita N., et al. Transbronchial biopsy using an ultrathin bronchoscope guided by cone-beam computed tomography and virtual bronchoscopic navigation in the diagnosis of pulmonary nodules. Respiration. 2019;98(4):321–328. doi: 10.1159/000500228. [DOI] [PubMed] [Google Scholar]
- 18.Ma L., Fang Y., Zhang T., et al. Original article comparison in efficacy and safety of forceps biopsy for peripheral lung lesions guided by endobronchial ultrasound-guided sheath (EBUS-GS) and electromagnetic navigation bronchoscopy combined with EBUS (ENB-EBUS) Am J Transl Res. 2020;12(8):4604. [PMC free article] [PubMed] [Google Scholar]
- 19.Cherian S., Kaur S., Karanth S., Xian J., Estrada-Y-Martin R. Diagnostic yield of electromagnetic navigational bronchoscopy: a safety net community-based hospital experience in the United States. Ann Thorac Med. 2021;16(1):102–109. doi: 10.4103/atm.ATM_388_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiting P.F., Rutjes A.W., Westwood M.E., et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 21.Eberhardt R., Anantham D., Ernst A., Feller-Kopman D., Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176(1):36–41. doi: 10.1164/rccm.200612-1866OC. [DOI] [PubMed] [Google Scholar]
- 22.Huang C., Ho C., Tsai Y., Yu C., Yang P. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology. 2009;14(6):859–864. doi: 10.1111/j.1440-1843.2009.01585.x. [DOI] [PubMed] [Google Scholar]
- 23.Oki M., Saka H., Kitagawa C., Kogure Y., Mori K., Kajikawa S. Endobronchial ultrasound-guided transbronchial biopsy using novel thin bronchoscope for diagnosis of peripheral pulmonary lesions. J Thorac Oncol. 2009;4(10):1274–1277. doi: 10.1097/JTO.0b013e3181b623e1. [DOI] [PubMed] [Google Scholar]
- 24.Oki M., Saka H., Kitagawa C., et al. Randomized study of endobronchial ultrasound-guided transbronchial biopsy: thin bronchoscopic method versus guide sheath method. J Thorac Oncol. 2012;7(3):535–541. doi: 10.1097/JTO.0b013e3182417e60. [DOI] [PubMed] [Google Scholar]
- 25.Chee A., Stather D.R., MacEachern P., et al. Diagnostic utility of peripheral endobronchial ultrasound with electromagnetic navigation bronchoscopy in peripheral lung nodules. Respirology. 2013;18(5):784–789. doi: 10.1111/resp.12085. [DOI] [PubMed] [Google Scholar]
- 26.Asano F., Shinagawa N., Ishida T., et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy a randomized clinical trial. Am J Respir Crit Care Med. 2013;188(3):327–333. doi: 10.1164/rccm.201211-2104OC. [DOI] [PubMed] [Google Scholar]
- 27.Pritchett M.A., Schampaert S., Groot De, Joris A.H., Schirmer C.C., Van Der Bom I. Cone-beam CT with augmented fluoroscopy combined with electromagnetic navigation bronchoscopy for biopsy of pulmonary nodules. J Bronchology Interv Pulmonol. 2018;25(4):274. doi: 10.1097/LBR.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bo L., Li C., Pan L., et al. Diagnosing a solitary pulmonary nodule using multiple bronchoscopic guided technologies: a prospective randomized study. Lung Cancer. 2019;129:48–54. doi: 10.1016/j.lungcan.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Oki M., Saka H., Asano F., et al. Use of an ultrathin vs thin bronchoscope for peripheral pulmonary lesions. Chest. 2019;156(5):954–964. doi: 10.1016/j.chest.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 30.Chen A.C., Pastis N.J., Mahajan A.K., et al. Robotic bronchoscopy for peripheral pulmonary lesions. Chest. 2021;159(2):845–852. doi: 10.1016/j.chest.2020.08.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oki M., Saka H., Kitagawa C., et al. Novel thin bronchoscope with a 1.7-mm working channel for peripheral pulmonary lesions. Eur Respir J. 2008;32(2):465–471. doi: 10.1183/09031936.00169107. [DOI] [PubMed] [Google Scholar]
- 32.Bertoletti L., Robert A., Cottier M., Chambonniere M.L., Vergnon J. Accuracy and feasibility of electromagnetic navigated bronchoscopy under nitrous oxide sedation for pulmonary peripheral opacities: an outpatient study. Respiration. 2009;78(3):293–300. doi: 10.1159/000226128. [DOI] [PubMed] [Google Scholar]
- 33.Pearlstein D.P., Quinn C.C., Burtis C.C., Ahn K.W., Katch A.J. Electromagnetic navigation bronchoscopy performed by thoracic surgeons: one center's early success. Ann Thorac Surg. 2012;93(3):944–950. doi: 10.1016/j.athoracsur.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Chan A., Devanand A., Low S.Y., Koh M.S. Radial endobronchial ultrasound in diagnosing peripheral lung lesions in a high tuberculosis setting. BMC Pulm Med. 2015;15:90. doi: 10.1186/s12890-015-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinfort D.P., Bonney A., See K., Irving L.B. Sequential multimodality bronchoscopic investigation of peripheral pulmonary lesions. Eur Respir J. 2016;47(2):607–614. doi: 10.1183/13993003.00786-2015. [DOI] [PubMed] [Google Scholar]
- 36.Vachani A., Maldonado F., Laxmanan B., Kalsekar I., Murgu S. The impact of alternative approaches to diagnostic yield calculation in studies of bronchoscopy. Chest. 2022;161(5):1426–1428. doi: 10.1016/j.chest.2021.08.074. [DOI] [PubMed] [Google Scholar]
- 37.Sainz Zuñiga P.V., Vakil E., Molina S., Bassett J., Roland L., Ost D.E. Sensitivity of radial endobronchial ultrasound-guided bronchoscopy for lung cancer in patients with peripheral pulmonary lesions: an updated meta-analysis. Chest. 2020;157(4):994–1011. doi: 10.1016/j.chest.2019.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Folch E.E., Labarca G., Ospina-Delgado D., et al. Sensitivity and safety of electromagnetic navigation bronchoscopy for lung cancer diagnosis. Chest. 2020;158(4):1753–1769. doi: 10.1016/j.chest.2020.05.534. [DOI] [PubMed] [Google Scholar]
- 39.Chaddha U., Kovacs S.P., Manley C., et al. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med. 2019;19(1):243. doi: 10.1186/s12890-019-1010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casal R.F., Sarkiss M., Jones A.K., et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis. 2018;10(12):6950–6959. doi: 10.21037/jtd.2018.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kheir F., Thakore S.R., Uribe Becerra J.P., et al. Cone-beam computed tomography-guided electromagnetic navigation for peripheral lung nodules. Respiration. 2021;100(1):44–51. doi: 10.1159/000510763. [DOI] [PubMed] [Google Scholar]
- 42.Benn B.S., Romero A.O., Lum M., Krishna G. Robotic-assisted navigation bronchoscopy as a paradigm shift in peripheral lung access. Lung. 2021;199(2):177–186. doi: 10.1007/s00408-021-00421-1. [DOI] [PubMed] [Google Scholar]
- 43.Birkmeyer J.D., Warshaw A.L., Finlayson S.R.G., Grove M.R., Tosteson A.N.A. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126(2):178–183. [PubMed] [Google Scholar]
- 44.Bach P.B., Cramer L.D., Schrag D., Downey R.J., Gelfand S.E., Begg C.B. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345(3):181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.